Abstract
Background: Phenylketonuria (PKU) requires a lifelong low-phenylalanine diet that provides the majority of protein from a phenylalanine-free amino acid (AA) formula. Glycomacropeptide (GMP), an intact protein formed during cheese production, contains minimal phenylalanine.
Objective: The objective was to investigate the effects of substituting GMP food products for the AA formula on acceptability, safety, plasma AA concentrations, and measures of protein utilization in subjects with PKU.
Design: Eleven subjects participated in an inpatient metabolic study with two 4-d treatments: a current AA diet (AA diet) followed by a diet that replaced the AA formula with GMP (GMP diet) supplemented with limiting AAs. Plasma concentrations of AAs, blood chemistries, and insulin were measured and compared in AA (day 4) and GMP diets (day 8).
Results: The GMP diet was preferred to the AA diet in 10 of 11 subjects with PKU, and there were no adverse reactions to GMP. There was no significant difference in phenylalanine concentration in postprandial plasma with the GMP diet compared with the AA diet. When comparing fasting with postprandial plasma, plasma phenalyalanine concentration increased significantly with the AA but not with the GMP diet. Blood urea nitrogen was significantly lower, which suggests decreased ureagenesis, and plasma insulin was higher with the GMP diet than with the AA diet.
Conclusions: GMP, when supplemented with limiting AAs, is a safe and highly acceptable alternative to synthetic AAs as the primary protein source in the nutritional management of PKU. As an intact protein source, GMP improves protein retention and phenylalanine utilization compared with AAs.
INTRODUCTION
Phenylketonuria (PKU) is an inborn error of metabolism caused by a defect in phenylalanine hydroxylase (PAH; EC 1.14.16.1), which metabolizes the indispensable amino acid (AA) phenylalanine to tyrosine. The resulting elevated phenylalanine concentrations adversely affect the developing central nervous system, which causes profound mental retardation and neurologic impairment (1, 2). Lifelong treatment with a low-phenylalanine diet results in reversal of this devastating phenotype (3–5). The PKU diet includes 2 major modalities. First, an AA-based medical food (formula) provides the major source of phenylalanine-free protein equivalents, energy, and micronutrients in the diet. Second, intake of intact protein is restricted to naturally low-protein foods and specialty low-protein bread and pasta products made from starch. This allows for an adequate, but not excessive, supply of phenylalanine for growth and protein turnover (1). Dietary products for treatment of PKU have improved; however, poor compliance remains a problem, especially in adolescents and young adults (6–9). New treatment modalities are needed to improve the palatability, variety, and convenience of this diet.
Glycomacropeptide (GMP) provides an alternative to AAs as a source of low-phenylalanine protein for the PKU diet. GMP is a 64-AA glycophosphopeptide formed during cheese production when bovine κ-casein is cleaved by chymosin into para-κ-casein, which remains with the curd, and GMP, which remains with the whey (10). Pure GMP contains no aromatic AAs (phenylalanine, tryptophan, and tyrosine) and no arginine, cysteine, and histidine. However, GMP contains a 2- to 3-fold greater concentration of the large neutral AAs (LNAAs) threonine and isoleucine than do reference proteins (10). Isolation of GMP from cheese whey results in contamination with other whey proteins that contain phenylalanine and other AAs not found in pure GMP. Commercially available GMP contains 2.5–5.0 mg phenylalanine/g of protein (11, 12). GMP requires supplementation with limiting AAs to provide a complete source of protein for individuals with PKU (13, 14).
Studies in the PKU mouse model indicate that intake of GMP supplemented with limiting AAs provides a nutritionally adequate source of protein and significantly reduces concentrations of phenylalanine in plasma and brain compared with an AA diet (15). Similar reductions in phenylalanine concentration in plasma or brain have been reported in subjects with PKU given supplementation with threonine (16) or mixtures of LNAAs (17–19). Competition among LNAAs, including threonine and isoleucine, with phenylalanine for intestinal absorption and transport across the blood-brain barrier may explain the observed reductions in phenylalanine (19, 20).
To further evaluate the potential benefits of GMP in the PKU diet, an 8-d clinical investigation was conducted in individuals with PKU. The objective was to investigate the effects of substituting GMP food products for the AA formula on acceptability, safety, plasma AA concentrations, and measures of protein utilization in subjects with PKU.
SUBJECTS AND METHODS
Subjects
Twelve subjects with PKU who were routinely monitored at the Biochemical Genetics Program, the Waisman Center, the University of Wisconsin-Madison, participated in this study between March 2006 and June 2008. One subject (age: 10 y) withdrew from the study because she was unable to complete the protocol. Thus, data from 11 subjects (age range: 11–31 y; 7 males and 4 females) are reported (Table 1). The University of Wisconsin-Madison Health Sciences Institutional Review Board approved this study.
TABLE 1.
Individual characteristics of 11 subjects with phenylketonuria
| Subject no./sex | Age at study initiation | Height/weight | Phenylalanine concentration at diagnosis1 | Age at diagnosis1 | Mutation | Baseline plasma phenylalanine2 | Dietary phenylalanine allowance per day | Dietary phenylalanine allowance per kg |
| y | cm/kg | μmol/L | d | μmol/L | mg | mg | ||
| 1/M | 27 | 173/73 | 1270 | 35 | R408W | 640 | 1151 | 15.8 |
| IVS12nt1g→a | ||||||||
| 2/M | 29 | 170/67 | 2208 | 15 | R408W | 1011 | 1793 | 26.7 |
| R261Q | ||||||||
| 3/M | 14 | 164/52 | 1210 | 8 | R408W | 1009 | 673 | 13.0 |
| IVS10nt-11g→a | ||||||||
| 4/M | 11 | 137/35 | 2051 | 7 | IVS4nt5g→t | 767 | 372 | 10.7 |
| IVS12nt1g→a | ||||||||
| 5/M | 12 | 148/45 | 2154 | 7 | R408W | 690 | 979 | 21.6 |
| Y356N | ||||||||
| 6/F | 23 | 94/51 | 1488 | 10 | R408W | 536 | 545 | 10.6 |
| IVS12nt1g→a | ||||||||
| 7/F | 28 | 159/64 | 2632 | 11 | R408W | 603 | 545 | 8.3 |
| L242F | ||||||||
| 8/M | 27 | 170/76 | 1924 | 11 | R261Q | 810 | 971 | 12.8 |
| E280K | ||||||||
| 9/F | 20 | 93/64 | 1016 | 10 | L48S | 331 | 378 | 5.9 |
| F299C | ||||||||
| 10/F | 31 | 157/70 | 1876 | 15 | R408W | 192 | 408 | 5.8 |
| F299C | ||||||||
| 11/M | 28 | 180/91 | 3122 | 13 | IVS1nt5g→t | 392 | 711 | 7.8 |
| IVS12nt1g→a |
Age and phenylalanine concentrations at diagnosis represent values when diet treatment was initiated during infancy.
Values represent concentrations of phenylalanine in plasma 2.5 h after eating breakfast on day 3 while consuming the prescribed amino acid diet.
Criteria for participation included a diagnosis of classical or variant PKU and a willingness to consume ≥50% of the prescribed volume of the AA formula. Optimal control of plasma phenylalanine concentrations, however, was not a prerequisite for participation. Optimal control includes maintenance of phenylalanine concentrations between 120 and 360 μmol/L for neonates through age 12 y, between 120 and 600 μmol/L for adolescents, and <900 μmol/L for adults (5). The diagnosis of PKU was based on concentration of phenylalanine measured before initiation of dietary treatment during infancy; those with classical PKU show phenylalanine concentrations ≥1200 μmol/L (Table 1) (1). All subjects in this study were diagnosed with classical PKU, except for one subject who was determined to have a variant form of PKU (subject 1).
Mutation analysis was completed for each subject by DNA sequencing of the PAH gene (Laboratory Service Section, Texas Department of State Health Services, Austin, TX) using primers designed by Guldberg et al (21). All subjects were compound heterozygous for PAH mutations (Table 1). Five subjects showed 2 copies of mutations considered to express primarily a classical phenotype and 6 subjects showed a classical mutation and a mutation observed in PKU patients with variant and/or non-PKU hyperphenylalaninemia mutations (http://www.pahdb.mcgill.ca/).
Because a formal evaluation of each subject's dietary prescription had not been completed within 2 y of study recruitment, a phenylalanine allowance for all subjects was determined before study initiation. For this study, phenylalanine allowance was defined as the amount of dietary phenylalanine intake that allowed for a constant plasma phenylalanine concentration (±5% variance) as determined by sequential increases in phenylalanine intake with frequent monitoring of blood phenylalanine concentrations in blood spots. Each subject's dietary phenylalanine allowance was verified by completing one or more “dry runs” in which all food, beverages, and formula were provided for a 5-d period with measurement of phenylalanine concentrations in blood spots before and at the end of each dry run. Plasma phenylalanine concentrations at the initiation of the study ranged from 192 μmol/L (subject 10) to 1011 μmol/L (subject 2; Table 1). To maintain these plasma phenylalanine concentrations, the dietary phenylalanine allowance for the subjects ranged from 5.8 mg/kg (subject 10) to 26.7 mg/kg (subject 2).
Study protocol
Each subject served as his or her own control in this metabolic study, which included 2 dietary treatments of 4 d each: the AA diet (days 1–4) and the GMP diet (days 5–8). One 24-h menu was designed for the AA diet and another for the GMP diet; the same menu was repeated on all days of each diet treatment (Table 2). In each diet, the AA formula or GMP products were divided equally in each of 3 meals during the day. Distribution of protein equivalents throughout the day improves protein utilization and can lower plasma phenylalanine concentrations (22). During the study, all food, beverages, snacks, formula, and GMP products were weighed in grams by trained dietary staff at the Waisman Center or the University of Wisconsin Clinical and Translational Research Core (UW-CTRC). To ensure identical intake on all days of the study, subjects were encouraged to consume all foods and beverages. No subject failed to do this.
TABLE 2.
Comparison of typical menus for the amino acid (AA) and the glycomacropeptide (GMP) diets1
| AA diet | GMP diet |
| Breakfast (103 mg phenylalanine, 14 g protein) | Breakfast (102 mg phenylalanine, 14 g protein) |
| 177 mL PKU formula2 (0 mg phenylalanine) | 296 mL GMP chocolate beverage (51 mg phenylalanine) |
| 30 g Cold cereal (51 mg phenylalanine) | 30 g Cold cereal (51 mg phenylalanine) |
| 11 g Pretzels (52 mg phenylalanine) | |
| Lunch (124 mg phenylalanine, 15 g protein) | Lunch (124 mg phenylalanine, 13 g protein) |
| 177 mL PKU formula2 (0 mg phenylalanine) | 148 mL GMP chocolate beverage (24 mg phenylalanine) |
| 12.5 g Cinnamon toast (62 mg phenylalanine) | 113 g (1/2 cup) GMP chocolate pudding (38 mg phenylalanine) |
| Cheese sandwich (45 mg phenylalanine; 64 g low-protein bread, 1 Slice low-protein cheese, 8.7 g butter) | Cheese sandwich (45 mg phenylalanine; 64 g low-protein bread, 1 Slice low-protein cheese, 8.7 g butter) |
| 125 g Peaches (17 mg phenylalanine) | 125 g Peaches (17 mg phenylalanine) |
| Dinner (220 mg phenylalanine, 18 g protein) | Dinner (226 mg phenylalanine, 18 g protein) |
| 177 mL PKU formula2 (0 mg phenylalanine) | 1 GMP bar (33 mg phenylalanine) |
| 9 g Bowtie pasta (53 mg phenylalanine) | 237 mL GMP sports beverage (19 mg phenylalanine) |
| Pasta Alfredo (61 mg phenylalanine; 60 g low-protein pasta, 5 g Regular bowtie pasta, 88 g low-protein Alfredo sauce) | Pasta Alfredo (61 mg phenylalanine; 60 g low-protein pasta, 5 g Regular bowtie pasta, 88 g low-protein Alfredo sauce) |
| 92 g Broccoli, 50 g carrots and 14 g butter (105 mg phenylalanine) | 92 g Broccoli, 50 g carrots and 14 g butter (105 mg phenylalanine) |
| 140 g Pears (7 mg phenylalanine) | 140 g Pears (7 mg phenylalanine) |
| 237 mL Lemonade (0 mg phenylalanine) | 237 mL Lemonade (0 mg phenylalanine) |
All foods were measured on a gram scale by trained staff. Since this study was completed, improved recipes have been developed to further lower the phenylalanine content of all of the GMP products shown in this typical menu (12). For example, a GMP bar can now be produced with only 14 mg phenylalanine compared with 33 mg phenylalanine, and the GMP chocolate pudding now contains 21 mg phenylalanine compared with 38 mg phenylalanine in the original formulation used for this research. PKU, phenylketonuria.
The PKU formula used in this menu is 40 g Phenex 2 (Abbott Laboratories, Columbus, OH).
Each subject was provided with all food and formula to consume at home for 2 d before initiation of the study and for days 1 and 2 of the AA diet. Before dinner on day 2, each subject was admitted to the UW-CTRC for continuation of the AA diet (days 3 and 4) and for 4 d of the GMP diet (days 5–8). A physical exam was completed on all days of the UW-CTRC admission. All subjects were required to walk or complete physical activity 2–3 times/d to allow for an activity level consistent with their usual routine. Timing of meals and snacks was also similar to each subject's usual routine.
On days 1 and 2, each subject collected a blood spot on filter paper for phenylalanine and tyrosine analysis. During the UW-CTRC admission, blood was drawn daily for plasma AA and automated chemistry panel analysis to measure serum concentrations of prealbumin, albumin, total protein, electrolytes, glucose, blood urea nitrogen (BUN), creatinine, calcium, magnesium, phosphate, uric acid, total and direct bilirubin, alkaline phosphatase, and liver enzymes (γ-glutamyltranspeptidase, alanine aminotransferase, aspartate aminotransferase, and lactic dehydrogenase). All postprandial blood samples were drawn daily 3 h after the start of breakfast or 2.5 h after eating breakfast (days 3–8).
After the first 5 subjects had completed the protocol, the Data Safety and Monitoring Board evaluated the protocol and study progress. As a result of the board's suggestions, blood draws for chemistry panels were eliminated on the first 2 d of the GMP diet (days 5 and 6), and an additional fasting blood sample was added before breakfast on the last 2 d of the AA diet (days 3 and 4) and on the last 2 d of the GMP diet (days 7 and 8) for the remaining 6 subjects. All fasting samples were analyzed for plasma AAs. The mean age of the 6 subjects for whom both fasting and postprandial blood samples were obtained was 26 ± 2 y and included 4 females and 2 males (subjects 6–11; Table 1).
Because the GMP food products were not supplemented with vitamins and minerals, all subjects were given a complete multivitamin with mineral supplement (Phlexy-Vits; Nutritia North America, Gaithersburg, MD) or a combination of Theragran M (Walgreen Co, Deerfield, IL) and Target-Mins (Country Life, Hauppauge, NY) during the GMP diet. Any subject consuming a formula or formulas that did not contain vitamins and minerals was given the same supplements provided for the GMP diet during the AA diet. Additional calcium was given, if needed, to meet Dietary Reference Intake (DRI) recommendations for age (23).
Study diets
GMP (Bio-Pure GMP; Davisco, Le Sueur, MN) was analyzed for AA content at the University of Missouri Experimental Station Chemical Laboratory (24). The phenylalanine content for the commercial GMP was 0.4 g phenylalanine/100 g GMP with a protein content of 86.0 g/100 g GMP. This GMP was used for 3 subjects with a higher phenylalanine tolerance. For 9 subjects with a lower phenylalanine tolerance, the original stock of GMP was further purified to reduce the phenylalanine content to an average of 0.21 ± 0.01 g phenylalanine/100 g GMP with an average protein content of 75.0 ± 0.7 g/100 g GMP (25). Purification of the GMP decreased only the phenylalanine content; the proportion of the other AAs remained unchanged in the purified GMP compared with the commercial GMP.
The GMP was supplemented with 4 limiting AAs, expressed as the final concentration in milligrams AA per gram GMP protein: histidine, 23; leucine, 72; methionine, 28; and tryptophan, 9. This is equivalent to 130% of estimated needs on the basis of the 2002 DRIs (13). Because tyrosine is an indispensable AA in PKU, tyrosine was supplemented at 150% of estimated needs for a final concentration of 71 mg/g GMP protein (13, 25). For the GMP diet, no attempt was made to duplicate the concentration of supplemental tyrosine found in the various formulas consumed by each subject because, in most cases, the tyrosine content of the formula was substantially greater than the estimated needs. Thus, for all subjects, tyrosine intake in the AA diet was greater than tyrosine consumed when GMP products were substituted.
Low-phenylalanine food products made with GMP as the protein source were developed for this study by the Wisconsin Center for Dairy Research, the University of Wisconsin-Madison. Before initiation of the study, each subject tasted a variety of food products made with GMP and selected 2 to 3 products that would be included in menus for the GMP diet. GMP beverages and foods included an orange-flavored sports beverage, a chocolate-flavored or caramel-flavored beverage, chocolate or strawberry pudding, and a cinnamon crunch bar (12). The range of phenylalanine content in the GMP food products varied with the purity of the GMP and the additional ingredients used to produce these foods and beverages, but, in general, a serving of GMP food products provided 5–10 g protein and 15–30 mg phenylalanine. Since this study was completed, improved recipes have been developed to further lower the phenylalanine content of all the GMP products used for the study (12).
Diet composition
The AA and GMP diets were calculated on the basis of a prestudy evaluation of each subject's phenylalanine allowance and were controlled for energy, protein, phenylalanine, and fat (Table 3). The AA diet (days 1–4) included a subject's usual AA formula, which was different for each subject. For the GMP diet (days 5–8), GMP products were substituted for a subject's entire daily intake of AA formula. The phenylalanine content of foods used to plan the menus was determined by AA analysis of selected foods and by calculation of phenylalanine content for the remaining foods (26, 27). Foods that were not analyzed were matched in quantity, brand, and packing lot in both diets, whereas foods analyzed for phenylalanine content were used in variable amounts to account for the phenylalanine content of the GMP products.
TABLE 3.
Nutrient composition of amino acid (AA) and glycomacropeptide (GMP) diets1
| AA diet | GMP diet | |
| Energy (kcal/kg) | ||
| <18 y old | 56 ± 6 | 57 ± 5 |
| ≥18 y old | 35 ± 1 | 35 ± 2 |
| Energy from protein (%)2 | 11 ± 1 | 10 ± 1 |
| Energy from fat (%)3 | 24 ± 1 | 23 ± 1 |
| Phenylalanine intake (mg · kg−1 · d−1) | 13 ± 2 | 13 ± 2 |
| Tyrosine intake (mg · kg−1 · d−1) | 85 ± 9 | 51 ± 54 |
Values are means ± SEMs and are based on calculated dietary intake; n = 11.
Protein from synthetic AAs represents 75% of the total protein in the AA diet and only 10% of the total protein in the GMP diet (from supplementing the GMP with limiting indispensable AAs). All other protein in the AA and GMP diets is from natural sources of intact protein.
Total fat intake ranged from 18% to 31% of total energy. A low fat intake is typical in those with phenylketonuria, given their selection of carbohydrate-based foods and the low fat content of many AA formulas designed for older individuals with this disorder (28).
Significantly different from the AA diet, P <0.0001 (paired t test, pairing on subject).
Because of the limitations in data to quantitate the phenylalanine content of foods, dietary composites were collected for phenylalanine analysis to verify calculations of phenylalanine content. Thus, a duplicate of all food, formula, and GMP food products consumed by each subject during a 24-h period was collected for 2 d during both the AA diet and the GMP diet. Each duplicate was ground and freeze-dried, and an aliquot of each composite was sent to the University of Missouri for AA analysis (24). When comparing the composite analyses for each subject, phenylalanine content in the AA diet and the GMP diet was not significantly different (P = 0.061).
Measurements
The blood spots collected by each subject to establish their phenylalanine allowance and on prestudy days 1 and 2 were analyzed for phenylalanine and tyrosine by tandem mass spectrometry (MS/MS; data not shown) (29). An AA profile was completed on all fasting and postprandial plasma samples collected on days 3–8 by using a Beckman 6300 amino acid analyzer (Beckman-Coulter Inc, Fullerton, CA) equipped with an ion chromatography system that uses postcolumn ninhydrin derivatization (30). The samples were deproteinized with sulfosalicylic acid, centrifuged (14,000 × g; 5 min) and passed through a 0.2-μm syringe filter before adding an internal standard and injecting it into the column.
Serum chemistry profiles were analyzed by using standard techniques at the Clinical Laboratory, the University of Wisconsin-Madison Hospital and Clinics. Plasma insulin was measured in postprandial samples by using an radioimmunoassay specific for human insulin (Linco Research, St Charles, MO) on samples pooled within subjects for days 3 + 4 and days 7 + 8. Insulin-like growth factor I (IGF-I) was measured in postprandial plasma samples for days 4 and 8 after removal of IGF-binding proteins by HPLC; the recovery of IGF-1 was 85–90% (31).
Statistical analysis
All statistical analysis was conducted with the statistical program R for Mac OS X version 1.12 (R Project for Statistical Computing, Wirtschaftsuniversität, Vienna, Austria; http://www.r-project.org). After dietary composites were analyzed, AA values within each diet were averaged for each subject (n = 2), and then values between both diets were compared by using paired t tests. Also, paired t tests, pairing on subject, were conducted to compare plasma AA values from the last day of the AA diet (day 4) to the last day of the GMP diet (day 8) for both postprandial and fasting samples. Changes in the chemistry panel and liver function tests were compared by using the same method. In addition, paired t tests were conducted to compare fasting and postprandial AA concentrations within each diet in the subset of 6 subjects from whom fasting plasma was available. All comparisons were considered statistically significant if P ≤ 0.05. On the basis of the primary endpoint comparing plasma phenylalanine concentration on the last day of the AA diet (day 4) with the last day of the GMP diet (day 8), the achieved sample size (n = 11) was sufficient to provide 80% power at P = 0.05 if the change in plasma phenylalanine concentration was 150 μmol/L.
RESULTS
Diet acceptability and AA composition
After consuming the GMP diet for 4 d, 10 of 11 subjects claimed that the GMP products were superior in sensory qualities to their usual AA formula. Moreover, at the conclusion of the study, 6 of the 7 adult subjects expressed a strong preference to consume GMP products rather than their usual AA formula, if GMP became available to them as a dietary option.
Compared with current recommendations, the analyzed intake (mg amino acid/g of dietary protein) of all indispensable AAs met requirements for both the AA and GMP diets (13, 14). However, AA analysis of the dietary composites indicated several significant differences in AA intake with ingestion of the AA diet compared with the GMP diet (Table 4). Because GMP contains a high concentration of the LNAAs threonine and isoleucine, mean intakes of both of these AAs were significantly higher with the GMP than the AA diet. Despite supplementation of GMP with tyrosine at 150% of DRI and leucine, histidine, tryptophan, and methionine at 130% of the DRI, the intake of these AAs, with the exception of methionine, was significantly lower with the GMP than the AA diet. The intakes of other AAs that were significantly lower with ingestion of the GMP diet compared with the AA diet included the indispensable AA lysine and the dispensable AAs arginine, alanine, glycine, and taurine.
TABLE 4.
Analyzed profile of amino acids (AAs) from 24-h composites of AA and glycomacropeptide (GMP) diets1
| AA | AA diet | GMP diet | P value2 |
| g amino acid/24-h diet | |||
| Alanine | 4.08 ± 0.32 | 3.15 ± 0.07 | 0.039 |
| Arginine | 4.07 ± 0.23 | 0.80 ± 0.09 | <0.0001 |
| Aspartic acid | 6.09 ± 0.37 | 5.08 ± 0.25 | 0.059 |
| Cysteine | 1.43 ± 0.09 | 0.50 ± 0.05 | <0.0001 |
| Glutamic acid | 10.3 ± 0.90 | 11.6 ± 0.64 | 0.219 |
| Glycine | 3.52 ± 0.26 | 0.99 ± 0.07 | <0.0001 |
| Histidine | 1.89 ± 0.10 | 1.27 ± 0.09 | 0.001 |
| Isoleucine | 3.70 ± 0.16 | 4.75 ± 0.26 | 0.004 |
| Leucine | 6.51 ± 0.35 | 4.12 ± 0.28 | <0.0001 |
| Lysine | 4.53 ± 0.21 | 3.09 ± 0.17 | <0.0001 |
| Methionine | 1.24 ± 0.06 | 1.28 ± 0.08 | 0.605 |
| Phenylalanine | 0.79 ± 0.09 | 0.74 ± 0.08 | 0.061 |
| Proline | 5.27 ± 0.27 | 5.91 ± 0.32 | 0.198 |
| Serine | 3.27 ± 0.26 | 3.30 ± 0.18 | 0.883 |
| Taurine | 0.54 ± 0.08 | 0.25 ± 0.03 | 0.019 |
| Threonine | 3.00 ± 0.10 | 7.12 ± 0.41 | <0.0001 |
| Tryptophan | 1.07 ± 0.07 | 0.57 ± 0.05 | <0.0001 |
| Tyrosine | 4.40 ± 0.17 | 2.63 ± 0.21 | <0.0001 |
| Valine | 4.50 ± 0.14 | 4.13 ± 0.21 | 0.105 |
| BCAA | 14.72 ± 0.62 | 13.00 ± 0.72 | 0.034 |
Values are means ± SEMs; n = 22. BCAA, sum of leucine, isoleucine, and valine.
Represents the difference between the AA and the GMP diets by paired t test.
Physical examination and blood chemistry
There were no physical concerns detected on exam or expressed by any subject to indicate any negative effect on health status when subjects consumed GMP as the primary protein source for a 4-d period. There were no significant differences among serum concentrations of albumin, prealbumin, or total protein as indicators of protein status or creatinine as an indicator of renal status measured on the last day of the AA diet (day 4) compared with the GMP diet (day 8; Table 5). However, BUN as an indicator of hepatic ureagenesis was significantly lower with ingestion of the GMP diet on both day 7 and day 8 than with the AA diet on day 4 (Figure 1). Plasma concentration of IGF-I was not significantly different with the AA and GMP diets, which suggests adequate protein nutrition in both diets (32). Plasma insulin concentration was higher and marginally significant with the GMP diet compared with the AA diet (P = 0.053), and serum glucose concentration was not significantly different. Serum carbon dioxide content, which is primarily bicarbonate, was significantly lower with the GMP diet compared with the AA diet, which is consistent with a lower systemic acid content. The mean concentrations of other standard chemistries, including electrolytes and liver function tests, remained within the normal range with both diets (data not shown). The exception was elevated concentrations of various liver function tests (alanine aminotransferase and γ-glutamyltranspeptidase) measured in subject 2, who was on anticonvulsant medications for his seizure disorder. However, further increases in these liver function tests were not detected with ingestion of the GMP diet compared with the elevations measured at admission to the study.
TABLE 5.
Effect of amino acid (AA) and glycomacropeptide (GMP) diets on postprandial indexes of protein and glucose metabolism1
| Test | AA diet | GMP diet | P value2 |
| Blood urea nitrogen (mmol/L) | 4.2 ± 0.3 | 3.4 ± 0.2 | 0.02 |
| Creatinine (μmol/L) | 73 ± 5.5 | 73 ± 4.6 | 1.00 |
| Total protein (g/L) | 68 ± 1.4 | 67 ± 1.4 | 0.27 |
| Albumin (g/L) | 44 ± 0.9 | 44 ± 0.8 | 0.84 |
| Prealbumin (g/L) | 317 ± 7.5 | 310 ± 7.3 | 0.22 |
| Insulin-like growth factor I (nmol/L) | 13.5 ± 1.3 | 13.7 ± 1.5 | 0.14 |
| Insulin (pmol/L) | 84 ± 22 | 116 ± 34 | 0.05 |
| Glucose (mmol/L) | 4.5 ± 0.1 | 4.8 ± 0.1 | 0.14 |
| CO2 content (mmol/L) | 26 ± 0.6 | 28 ± 0.6 | 0.01 |
Values are means ± SEMs; n = 11, except total protein and insulin for which n = 10; all values are within normal range. Values are for serum except those for insulin-like growth factor I and insulin, which used plasma.
Difference between the last day of the AA diet (day 4) and the GMP diet (day 8) by paired t test, pairing on subject.
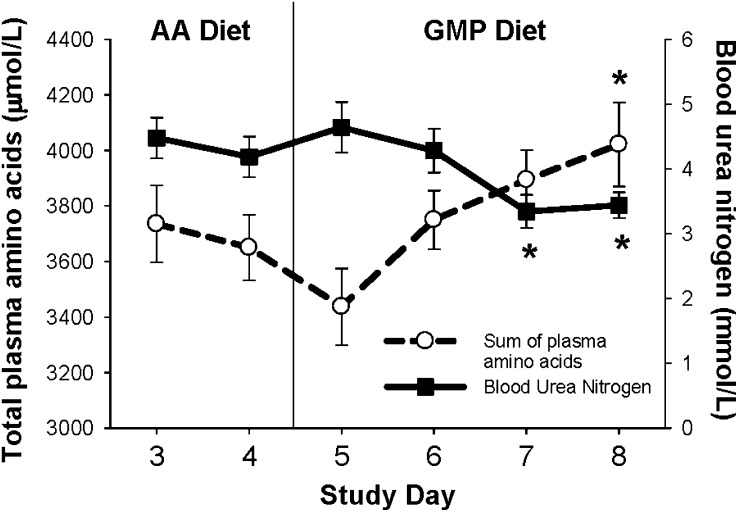
FIGURE 1.
The concentration of total amino acids (AAs) and blood urea nitrogen in postprandial plasma with ingestion of the glycomacropeptide (GMP) or the AA diet. Plasma was obtained 2.5 h after eating breakfast; n = 11 with the exception of blood urea nitrogen on study days 5 and 6 for which n = 6. Total plasma AAs indicate the sum of all AAs measured in plasma. Values are means ± SEMs. Total plasma AAs increased and blood urea nitrogen decreased with ingestion of the GMP diet when compared with day 4 of the AA diet. There was a significant effect of time in the repeated-measures ANOVA. *Significantly different from the AA diet on day 4, P < 0.05 (paired t test, pairing on subject).
Plasma AA concentrations
The concentration of total AAs in plasma was significantly greater, and the concentration of BUN was significantly lower, with the GMP diet compared with the AA diet when measured 2.5 h after eating breakfast (Figure 1). This is consistent with slower absorption of AAs from an intact source of protein compared with synthetic AAs (33) and higher insulin concentrations with ingestion of GMP (34–36).
Phenylalanine and tyrosine
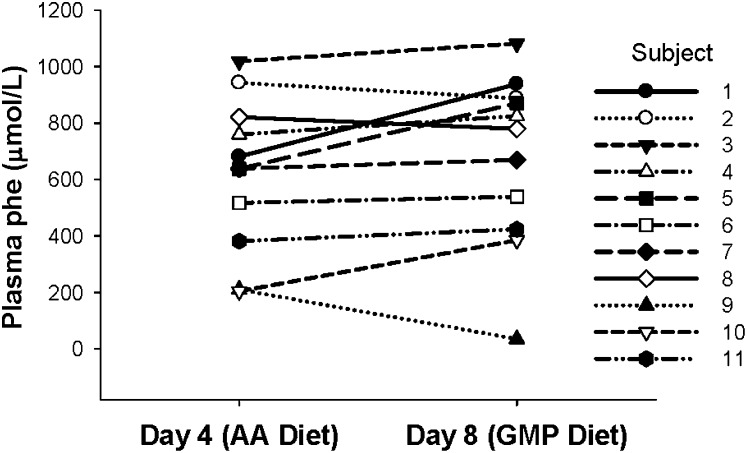
There was no significant difference (P = 0.173) in the mean postprandial concentration of phenylalanine in plasma with ingestion of the AA diet (day 4) compared with the GMP diet (day 8; Figure 2). The mean change in the concentration of phenylalanine in plasma was 57 ± 52 μmol phenylalanine/L. Among individual subjects, the response of plasma phenylalanine concentration to ingestion of the GMP diet was heterogeneous, ranging from a decrease of 175 μmol phenylalanine/L to an increase of 257 μmol phenylalanine/L. Overall, there was no consistent association between a change in the concentration of phe in plasma with ingestion of the AA diet compared with the GMP diet and sex, genotype, and age.
FIGURE 2.
Concentrations of phenylalanine in plasma of individual subjects with phenylketonuria (n = 11) after consuming the amino acid (AA) diet or the glycomacropeptide (GMP) diet for 4 d. Blood was obtained 2.5 h after eating breakfast, and plasma was isolated for analysis of the complete AA profile. Subjects showed a range of plasma phenylalanine concentrations after consuming the AA diet or the GMP diet for 4 d. There was no significant difference in the concentration of phenylalanine in plasma when the last day of the AA diet (day 4) was compared with the last day of the GMP diet (day 8); P = 0.173 by paired t test, pairing on subject. Group mean ± SEM was 619 ± 82 μmol/L (AA diet) and 676 ± 92 μmol/L (GMP diet). The mean change in the concentration of phenylalanine in plasma was 57 ± 52 μmol/L. phe, phenylalanine.
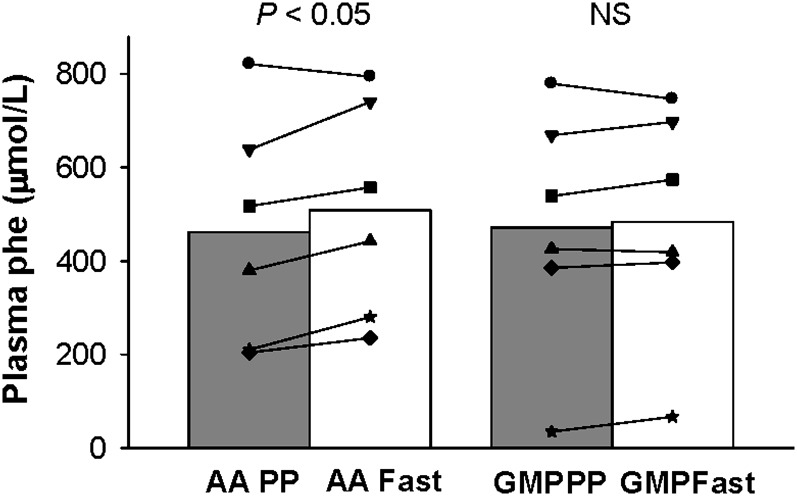
Concentrations of phenylalanine in both fasting and postprandial plasma were available on day 4 (AA diet) and on day 8 (GMP diet) for a subset of 6 adult subjects. The postprandial response to the GMP diet was not significantly different with this subset (n = 6) than with the first 5 subjects. Ingestion of the AA diet for 4 d resulted in a significant 10% increase in the concentration of phenylalanine in plasma obtained after an overnight fast compared with the concentration of phenylalanine in postprandial plasma obtained 2.5 h after eating breakfast (P = 0.048; Figure 3). In contrast, ingestion of the GMP diet for 4 d resulted in no significant change in the concentration of phenylalanine in plasma when comparing plasma obtained in a fasting state to plasma obtained in a postprandial state.
FIGURE 3.
The concentration of phenylalanine in postprandial (PP; 2.5 h after eating breakfast) compared with fasting (fast, overnight fast) plasma in subjects with phenylketonuria fed glycomacropeptide (GMP) compared with the amino acid (AA) diet for 4 d. Group means and the response of individual subjects are shown; n = 6 (day 4 compared with day 8). There was no significant change in plasma phenylalanine concentration comparing fasting with PP concentrations when consuming the GMP diet (P = 0.349); however, the AA diet showed a significant increase in plasma phenylalanine (P = 0.048) by paired t test, pairing on subject. phe, phenylalanine.
Tyrosine is an important AA in the PKU diet because it is indispensable and a precursor of adrenaline, norepinephrine, melanin, and thyroxine (1). Concentrations of tyrosine in plasma obtained in the postprandial or fasting samples were not significantly different with ingestion of the GMP or AA diets (Table 6). Concentrations of tyrosine in plasma after an overnight fast were decreased compared with postprandial concentrations with ingestion of both the GMP and the AA diet; however, the GMP diet resulted in a mean fasting tyrosine concentration that was below the normal range.
TABLE 6.
Effect of amino acid (AA) and glycomacropeptide (GMP) diets on fasting and postprandial (PP) concentrations of AAs in plasma1
| AA diet | GMP diet | Response to diet fasting compared with PP | |||||
| AA | PP2 | Fasting2 | P value3 | PP2 | Fasting2 | P value1 | P value4 |
| μmol/L | μmol/L | ||||||
| Alanine | 455 ± 52 | 356 ± 25 | 0.029 | 514 ± 45 | 401 ± 50 | 0.001 | 0.743 |
| Arginine | 62 ± 14 | 57 ± 5 | 0.694 | 47 ± 5 | 47 ± 6 | 0.845 | 0.551 |
| Citrulline | 37 ± 4 | 26 ± 5 | 0.138 | 23 ± 3 | 26 ± 4 | 0.084 | 0.063 |
| Cystine | 43 ± 1 | 43 ± 2 | 0.899 | 37 ± 2 | 41 ± 3 | 0.019 | 0.062 |
| Glutamic acid | 40 ± 8 | 50 ± 10 | 0.207 | 43 ± 10 | 49 ± 9 | 0.556 | 0.692 |
| Glutamine | 635 ± 29 | 628 ± 15 | 0.747 | 659 ± 33 | 623 ± 28 | 0.025 | 0.095 |
| Glycine | 415 ± 62 | 399 ± 55 | 0.473 | 346 ± 40 | 371 ± 47 | 0.099 | 0.112 |
| Histidine | 85 ± 9 | 78 ± 5 | 0.206 | 82 ± 6 | 75 ± 4 | 0.066 | 0.681 |
| Isoleucine | 57 ± 6 | 49 ± 4 | 0.352 | 119 ± 15 | 54 ± 5 | 0.015 | 0.004 |
| Leucine | 120 ± 20 | 96 ± 3 | 0.313 | 86 ± 11 | 83 ± 4 | 0.823 | 0.264 |
| Lysine | 210 ± 19 | 181 ± 9 | 0.062 | 191 ± 24 | 171 ± 16 | 0.138 | 0.311 |
| Methionine | 24 ± 2 | 24 ± 1 | 0.832 | 31 ± 4 | 25 ± 3 | 0.150 | 0.144 |
| Ornithine | 74 ± 7 | 60 ± 8 | 0.004 | 50 ± 4 | 62 ± 18 | 0.460 | 0.129 |
| Phenylalanine | 462 ± 100 | 508 ± 95 | 0.048 | 472 ± 106 | 483 ± 101 | 0.349 | 0.037 |
| Proline | 200 ± 15 | 144 ± 12 | 0.001 | 234 ± 37 | 160 ± 20 | 0.012 | 0.322 |
| Serine | 131 ± 21 | 122 ± 16 | 0.123 | 127 ± 14 | 120 ± 11 | 0.517 | 0.821 |
| Taurine | 73 ± 19 | 82 ± 18 | 0.154 | 73 ± 13 | 63 ± 11 | 0.145 | 0.027 |
| Threonine | 158 ± 22 | 135 ± 17 | 0.013 | 354 ± 44 | 265 ± 29 | 0.030 | 0.064 |
| Tryptophan | 48 ± 5 | 44 ± 3 | 0.269 | 34 ± 3 | 42 ± 2 | 0.048 | 0.006 |
| Tyrosine | 81 ± 8 | 38 ± 3 | 0.001 | 56 ± 10 | 29 ± 3 | 0.027 | 0.155 |
| Valine | 240 ± 9 | 194 ± 13 | 0.015 | 241 ± 21 | 187 ± 5 | 0.048 | 0.521 |
| BCAA | 417 ± 28 | 347 ± 14 | 0.084 | 445 ± 45 | 324 ± 8 | 0.048 | 0.062 |
Values are means ± SEMs; n = 6, except for cystine for which n = 5. BCAA, sum of leucine, isoleucine, and valine.
PP plasma concentrations of ornithine (P = 0.019) and tryptophan (P = 0.003) were significantly lower, but within the normal range, and isoleucine (P = 0.003) and threonine (P = 0.004) were significantly higher with ingestion of the GMP diet than with the AA diet and above the normal range. The only significant differences in fasting AA concentrations were a decrease in arginine (P = 0.008) and an increase in threonine (P = 0.001) with ingestion of the GMP diet compared with the AA diet.
There was a significant effect of time in the repeated-measures ANOVA. Statistical analysis by paired t test, pairing on subject, is from data collected on the last day of the AA diet (day 4) and the last day of the GMP diet (day 8).
The response to a diet is calculated first by finding the difference between fasting and PP AA concentrations for each subject on the AA diet and on the GMP diet and then by comparing the differences by paired t test, pairing on subject.
Additional AAs
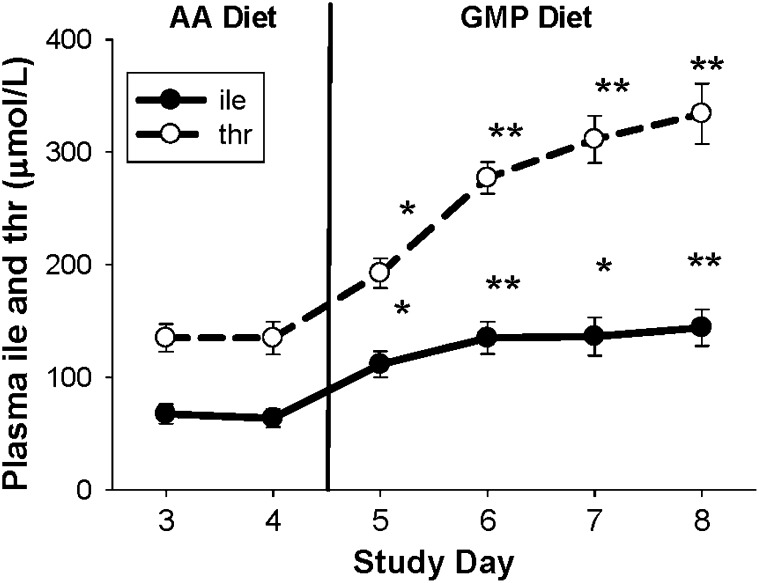
The most dramatic change in the profile of AAs in plasma with ingestion of the GMP compared with the AA diet was the 2.25- to 2.47-fold increase in postprandial concentrations of the nontoxic LNAA isoleucine and threonine (37, 38), which places these values above the normal clinical range (Table 6). A significant increase in plasma concentration of isoleucine and threonine with the GMP diet occurred within 24 h of ingesting the GMP diet and was consistent with the high concentrations of these AAs in GMP (Figure 4). However, there was no further significant increase in plasma concentration of isoleucine and threonine after days 5 and 7, respectively. The concentration of isoleucine was not different in plasma obtained after an overnight fast, whereas the concentration of threonine in fasting plasma remained ≈2-fold greater with ingestion of the GMP compared with the AA diet.
FIGURE 4.
Concentrations of threonine and isoleucine in postprandial plasma after consuming the glycomacropeptide (GMP) diet for 4 d (days 5–8). Values are mean ± SEM; n = 11, of plasma obtained 2.5 h after breakfast. For study days 3 and 4, all subjects consumed an amino acid (AA) diet; on days 5–8, all AA formula was replaced with GMP food products. There was a significant effect of time in the repeated-measures ANOVA. *Significantly different from the last day of the AA diet (day 4), P < 0.05 (paired t test, pairing on subject). **Significantly different from the last day of the AA diet (day 4), P < 0.0001. There was no further significant increase in plasma concentration of isoleucine and threonine after days 5 and 7, respectively. ile, isoleucine; thr, threonine.
Consistent with the AA profile of the GMP and AA dietary composites, there were significantly lower postprandial concentrations of ornithine and tryptophan in plasma and significantly higher concentrations of isoleucine and threonine in plasma with consumption of the GMP compared with the AA diet. After an overnight fast, plasma concentration of arginine was significantly lower and concentration of threonine was significantly higher with the GMP compared with the AA diet (Table 6).
DISCUSSION
This is the first clinical trial to investigate the efficacy of substituting intact protein from GMP food products for synthetic AA formulas that are currently required for nutritional management of PKU. No adverse health problems were found, and blood chemistries remained normal when subjects with PKU consumed GMP as their primary protein source for 4 d in this controlled metabolic diet study (Table 5). Furthermore, the GMP products were preferred by the subjects, which confirms the results of blind taste tests comparing GMP to AA products in those with PKU (11). Thus, foods and beverages made with GMP are both safe and highly acceptable for use in the phenylalanine-restricted diet for PKU.
Over a period of 4 d, there was no significant change in plasma phenylalanine concentrations with ingestion of GMP products compared with the AA formula (Figure 2). Previous studies have shown that supplementation with LNAAs for 1–2 wk (17, 18) or with threonine for 8 wk (16) reduces plasma phenylalanine concentration, possibly via reduced intestinal phenylalanine absorption through the common LNAA transporter (20). Similar effects could be expected with administration of GMP because of its elevated threonine and isoleucine content (10, 11). Indeed, PKU mice fed GMP for 4–7 wk showed an 11–20% reduction in phenylalanine concentrations in plasma and brain compared with an AA diet (15). Furthermore, one individual with PKU who replaced his entire AA formula prescription with GMP food products for 10 wk at home showed significantly lower circulating concentrations of phenylalanine within 1–2 wk of initiation of the GMP diet (12). These findings suggest that longer-term consumption of GMP products will reduce concentrations of circulating phenylalanine and that the current 4-d study period was not sufficient to show this positive effect.
GMP, as an intact protein source, may delay absorption of AAs and improve utilization of phenylalanine and other AAs for protein synthesis when compared with a synthetic AA source. In this study, the AA diet showed a significantly higher mean fasting phenylalanine concentration compared with the postprandial phenylalanine concentration (Figure 3), whereas there was no significant difference in fasting and postprandial phenylalanine concentrations apparent with the GMP diet (Table 6). This suggests that the GMP diet induced less variation and potentially lower mean concentrations of phenylalanine in plasma over a 24-h period. Gropper and Acosta (33) suggested that protein retention improved with a lower maximal plasma concentration of total AAs and a slower decrease in these concentrations when the intact protein cottage cheese, rather than its component AAs, was ingested by healthy adults as their sole protein source. Moreover, Metges et al (39) showed significantly increased net protein synthesis and decreased leucine oxidation over 8 h by using an oral l-[1-13C]leucine and an intravenous [2H3]leucine tracer in human subjects fed intrinsically labeled casein than those fed extrinsically labeled AAs simulating casein. Similar findings were reported using a single meal that contained either casein or free AAs mimicking casein (40). Thus, evidence is consistent with increased protein retention and decreased oxidation of AAs in association with a slower rate of absorption of AAs when the dietary protein source is an intact protein, such as GMP, compared with a free AA source, such as AA formula.
Evidence of improved protein retention with the GMP diet was also shown by a lower serum BUN and higher plasma insulin and total AA concentrations when measured 2.5 h after eating a breakfast containing GMP compared with one containing AAs (Table 5, Figure 1). Urea is produced linearly in response to plasma AA concentrations, and control of nitrogen balance is primarily regulated by urea production (41). BUN, as a measure of hepatic utilization of AAs for urea synthesis, would be expected to remain lower with slower splanchnic AA release (40, 42). Thus, a slower, more gradual and sustained elevation in plasma AA concentration with an intact protein source (33), in conjunction with a lower BUN concentration, suggests that fewer AAs are degraded for urea production and instead are retained for protein synthesis when GMP is substituted for synthetic AAs as the primary protein source. Postabsorptive AAs, including isoleucine and threonine (35, 36), are known to stimulate insulin release with subsequent stimulation of protein synthesis and inhibition of protein degradation (34). Because GMP induces a slower and more prolonged release of amino acids, the insulin response and stimulus of net protein synthesis may be potentiated. In addition, whey protein has been shown to increase insulin concentration to a greater extent than other milk protein fractions or other intact protein sources (43). Thus, the ability of GMP to slow AA catabolism and ureagenesis may reflect increased postprandial concentrations of threonine and isoleucine acting as insulin secretagogues as well as delayed absorption of AAs.
Arginine has multiple functions, which include serving as a substrate for synthesis of protein, urea, and nitric oxide. The cofactor for PAH, tetrahydrobiopterin, is also the cofactor for nitric oxide synthetase (44, 45). Arginine is synthesized in the kidney from intestinal citrulline derived from glutamine, but it is considered to be a conditionally indispensable AA in severely stressed patients (46). Recent evidence in healthy adults fed a diet free of both arginine and precursors of arginine for 4 wk shows that arginine is a nutritionally dispensable AA; however, the functional requirement for arginine is unknown (42). Consistent with minimal arginine in GMP, plasma arginine concentrations were significantly lower with ingestion of the GMP compared with the AA diet. Taken together, we conclude that it is prudent to supplement GMP with arginine for utilization in the PKU diet.
For this study, GMP was supplemented with the following 5 limiting AAs on the basis of the 2002 DRI recommendations (13): histidine, leucine, methionine, tryptophan, and tyrosine. Plasma concentrations of histidine, leucine, and tryptophan remained within the normal range, which suggests adequate supplementation of these AAs in the GMP diet (Table 6). Recent findings of a lower minimum requirement for methionine plus cysteine for school-age children (47) and adults (48) suggest that methionine supplementation is not required in GMP. In contrast, plasma concentration of tyrosine was below the normal range when measured in the fasting state (Table 6), which suggests that additional tyrosine supplementation may be required in GMP. Indeed, additional tyrosine from a supplement providing 1000 mg/d allowed for plasma tyrosine concentrations to remain within the normal range for one subject who consumed GMP as his primary protein source for 10 wk (12). In summary, our data suggest that GMP must be supplemented with arginine, histidine, leucine, tryptophan, and tyrosine to provide a complete source of dietary protein in the PKU diet.
Lifelong adherence to the PKU diet is very difficult, often resulting in poor compliance and the neuropsychological consequences of hyperphenylalaninemia (1, 6–8). This research shows a new, improved paradigm for the PKU diet through the use of palatable foods and beverages made with the intact, low-phenylalanine protein GMP instead of synthetic AAs. When supplemented with limiting indispensable AAs, GMP appears to be a safe and acceptable alternative to synthetic AAs as the primary protein source for nutritional management of PKU. As an intact protein, GMP delays absorption of AAs and improves protein retention and phenylalanine utilization compared with a diet that provides the majority of nitrogen from AAs. Further research is required to investigate the long-term nutritional safety of GMP and its ability to reduce concentrations of phenylalanine in blood and brain and to improve compliance with the PKU diet.
Acknowledgments
We thank Michael J Grahn and Phillip Williams for expert technical assistance; Gregory Rice for providing medical care to the subjects; Caitlin LaClair for purifying the GMP; Kathy Nelson and KJ Burrington from the Wisconsin Center for Dairy Research for development and production of GMP products; Lisa Davis for assistance with the research diets at the UW-CTRC; and finally, clients with PKU from the UW Biochemical Genetics Program who participated as subjects in this study.
The authors' responsibilities were as follows—SCvC, ELM, STG, and DMN: involved in the design, implementation, and analysis of the study; SCvC, ELM, and DMN: drafted the manuscript; MRE: involved in the design and implementation of the study; and MKC and JAW: contributed to the interpretation of the data. All authors contributed to the final version of the manuscript. There was no conflict of interest for any of the authors.
REFERENCES
- 1.Donlon J, Levy HL, Scriver CR. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. Scriver CR, Beaudet AL, Sly WS, Valle D. Online metabolic and molecular basis of inherited disease. Chapter 77. New York, NY: McGraw-Hill, 2007. Available from: http://www.ommbid.com [Google Scholar]
- 2.Kölker S, Sauer SW, Hoffmann GF, Müller I, Morath MA, Okun JG. Pathogenesis of CNS involvement in disorders of amino and organic acid metabolism. J Inherit Metab Dis 2008;31:194–204 [DOI] [PubMed] [Google Scholar]
- 3.Bickel H, Gerrard J, Hickmans EM. The influence of phenylalanine intake on the chemistry and behaviour of a phenylketonuric child. Acta Paediatr 1954;43:64–77 [DOI] [PubMed] [Google Scholar]
- 4.O'Flynn ME, Hotzman NA, Blaskovics M, Azen C, Williamson ML. The diagnosis of phenylketonuria: a report from the Collaborative Study of Children Treated for Phenylketonuria. Am J Dis Child 1980;134:769–74 [DOI] [PubMed] [Google Scholar]
- 5.NIH Consensus Statement Phenylketonuria (PKU): screening and management. Washington, DC: National Institutes of Health, 2000 [PubMed] [Google Scholar]
- 6.Koch R, Burton B, Hoganson G, et al. Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 2002;25:333–46 [DOI] [PubMed] [Google Scholar]
- 7.MacDonald A, Daly A, Davies P, Asplin D, Hall SK, Booth IW. Protein substitutes for PKU: what's new? J Inherit Metab Dis 2004;27:363–71 [DOI] [PubMed] [Google Scholar]
- 8.MacDonald A. Diet and compliance in phenylketonuria. Eur J Pediatr 2000;159(suppl 2):S136–41 [DOI] [PubMed] [Google Scholar]
- 9.Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health 2004;16:41–5 [DOI] [PubMed] [Google Scholar]
- 10.Etzel MR. Manufacture and use of dairy protein fractions. J Nutr 2004;134(suppl):996S–1002S [DOI] [PubMed] [Google Scholar]
- 11.Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM. Acceptable low-phenylalanine foods and beverages can be made from glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab 2007;92:176–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ney DM, Gleason ST, van Calcar SC, et al. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis 2009;32:32–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine Dietary reference intakes for energy, carbohydrates, fiber, fat, protein and amino acids. Washington, DC: National Academy Press, 2002 [Google Scholar]
- 14.World Health Organization Protein and amino acid requirements in human nutrition. Geneva, Switzerland: United Nations University, 2007 [PubMed] [Google Scholar]
- 15.Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr 2008;138:316–22 [DOI] [PubMed] [Google Scholar]
- 16.Sanjurjo P, Aldamiz L, Georgi G, Jelinek J, Ruiz JI, Boehm G. Dietary phenylalanine reduces plasma phenylalanine levels in patients with hyperphenylalaninemia. J Pediatr Gastroenterol Nutr 2003;36:23–6 [DOI] [PubMed] [Google Scholar]
- 17.Matalon R, Michals-Matalon K, Bhatia G, et al. Double blind placebo control trial of large neutral amino acids in treatment of PKU: effect on blood phenylalanine. J Inherit Metab Dis 2007;30:153–8 [DOI] [PubMed] [Google Scholar]
- 18.Schindeler S, Ghosh-Jarath S, Thompson S, et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol Genet Metab 2007;91:48–54 [DOI] [PubMed] [Google Scholar]
- 19.Pietz J, Kreis R, Rupp A, et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 1999;103:1169–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo IJ, Borchart RT. Transport of a large neutral amino acid (phenylalanine) in human intestinal epithelial cell line: Caco-2. Biochim Biophys Acta 1990;1028:25–30 [DOI] [PubMed] [Google Scholar]
- 21.Guldberg P, Romano V, Ceratto N, et al. Mutational spectrum of phenylalanine hydroxylase deficiency in Sicily: implications for diagnosis of hyperphenylalaninemia in southern Europe. Hum Mol Genet 1993;2:1703–7 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald A, Rylance G, Davies P, Asplin D, Hall SK, Booth IW. Administration of protein substitute and quality of control in phenylketonuria: a randomized study. J Inherit Metab Dis 2003;26:319–26 [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academies Press, 1997 [PubMed] [Google Scholar]
- 24.Association of Official Analytical Chemists Official methods of analysis of Association of Official Analytical Chemists. 18th ed Gaithsburg, MD: Association of Official Analytical Chemists, International, 2005 [Google Scholar]
- 25.LaClair CE. Purification and use of whey proteins for improved health. Ph.D. dissertation. University of Wisconsin, Madison, WI, 2008 [Google Scholar]
- 26.US Department of Agriculture Nutrient database for standard reference. Washington, DC: Agriculture Research Station, 2005 [Google Scholar]
- 27.Schuett VE. Low protein food list for PKU. 2nd ed Seattle, WA: National PKU News, 2002 [Google Scholar]
- 28.Rose HJ, White F, MacDonald A, Rutherford PJ, Favre E. Fat intakes of children with PKU on low phenylalanine diets. J Hum Nutr Diet 2005;18:395–400 [DOI] [PubMed] [Google Scholar]
- 29.Rashad MS, Ozand PT, Bucknall MP, Little D. Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electospray tandem mass spectrometry. Pediatr Res 1995;38:324–31 [DOI] [PubMed] [Google Scholar]
- 30.Slocum RH, Cummings JG. Amino acid analysis of physiological samples. Hommes FA. Techniques in diagnostic human biochemical genetics: a laboratory manual. New York, NY: Wiley-Liss, 1991:87–126 [Google Scholar]
- 31.Ney DM, Yang H, Smith SM, Unterman TG. High-calorie total parenteral nutrition reduces hepatic insulin-like growth factor I mRNA and alters serum levels of insulin-like growth factor-binding protein-1,-3,-5 and -6 in the rat. Metabolism 1995;44:152–60 [DOI] [PubMed] [Google Scholar]
- 32.Houston MS. The insulin-like growth factors and assessment of nutritional status. Houston MS, Holly JMP, Feldman EL. IGF and nutrition in health and disease. Totowa, NY: Humana Press, 2005:75–103 [Google Scholar]
- 33.Gropper SS, Acosta PB. Effect of simultaneous ingestion of L-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. JPEN J Parenter Enteral Nutr 1991;15:48–53 [DOI] [PubMed] [Google Scholar]
- 34.Schmid R, Schulte-Frohlinde E, Schusdziarra V, et al. Contribution of postprandial amino acid levels to stimulation of insulin, glucagon, and pancreatic polypeptide in humans. Pancreas 1992;7:698–704 [DOI] [PubMed] [Google Scholar]
- 35.van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acids or protein mixtures with carbohydrate. Am J Clin Nutr 2000;72:96–105 [DOI] [PubMed] [Google Scholar]
- 36.Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr 2002;132:2174–82 [DOI] [PubMed] [Google Scholar]
- 37.Darling PB, Grunow J, Rafii M, Brookes S, Ball RO, Pencharz PB. Threonine dehydrogenase is a minor degradative pathway of threonine catabolism in adult humans. Am J Physiol Endocrinol Metab 2000;278:E877–84 [DOI] [PubMed] [Google Scholar]
- 38.Zhao XH, Wen ZM, Meridith CN, Matthews DE, Bier DM, Young VR. Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr 1986;43:795–802 [DOI] [PubMed] [Google Scholar]
- 39.Metges CC, El-Khoury AE, Selvaraj AB, et al. Kinetics of L-[1-13C] leucine when ingested with free amino acids, unlabeled or intrinsically labeled casein. Am J Physiol Endocrinol Metab 2000;278:E1000–9 [DOI] [PubMed] [Google Scholar]
- 40.Dangin M, Boirie Y, Garcia-Rodenas C, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 2001;280:E340–8 [DOI] [PubMed] [Google Scholar]
- 41.Young VR, El-Khoury AE, Raguso CA, Forslund AH, Hambraus L. Rates of urea production and hydrolysis and leucine oxidation change linearly over widely varying protein intakes in healthy adults. J Nutr 2000;130:761–6 [DOI] [PubMed] [Google Scholar]
- 42.Tharakan JF, Yu YM, Zurakowski D, Roth RM, Young VR, Castillo L. Adaptation to a long-term (4 weeks) arginine- and precursor (glutamine, proline and aspartate)-free diet. Clin Nutr 2008;27:513–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IME. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004;80:1246–53 [DOI] [PubMed] [Google Scholar]
- 44.Nagatsu T, Ichinose H. Regulation of pterine-requiring enzymes by cofactor tetrahydrobiopterin. Mol Neurobiol 1999;19:79–96 [DOI] [PubMed] [Google Scholar]
- 45.Settergren M, Bohm F, Malmström RE, Channon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis 2008; Sept 4 (Epub ahead of print; DOI:10.1016/j.atherosclerosis.2008.08.034) [DOI] [PubMed] [Google Scholar]
- 46.Yu YM, Ryan CM, Castillo L, et al. Arginine and ornithine kinetics in severely burned patients: increased rate of arginine disposal. Am J Physiol Endocrinol Metab 2001;280:E509–17 [DOI] [PubMed] [Google Scholar]
- 47.Turner JM, Humayan MA, Elango R, et al. Total sulfur amino acid requirement of healthy school-age children as determined by indicator amino acid oxidation technique. Am J Clin Nutr 2006;83:619–23 [DOI] [PubMed] [Google Scholar]
- 48.Ball RO, Courtney-Martin G, Pencharz PB. The in vivo sparing of methionine by cysteine in sulfur amino acid requirements in animal models and adult humans. J Nutr 2006;136(suppl 2):1682S–93S [DOI] [PubMed] [Google Scholar]