Abstract
Background: The quality of nutrition-related systematic reviews (SRs) is an unstudied but important factor affecting their usefulness.
Objectives: The objectives were to evaluate the reporting quality of published SRs and to identify areas of improvement.
Design: Descriptive and exploratory analyses of the reporting quality (7 nutrition items and 28 SR reporting items) of all English-language SRs published through July 2007 linking micronutrients and health outcomes in humans were conducted. Factors that may be associated with reporting quality were also evaluated.
Results: We identified 141 eligible SRs of 21 micronutrients. Ninety SRs that included only interventional studies met a higher proportion of our reporting criteria (median: 62%; interquartile range: 51%, 72%) than did 31 SRs with only observational studies (median: 53%; interquartile range: 47%, 60%) or 20 SRs with both study designs (median: 47%; interquartile range: 39%, 52%) (P < 0.001). SRs published after consensus reporting standards (since 2003) met a higher proportion of the reporting criteria than did earlier SRs (median: 59% compared with 50%; P = 0.01); however, the reporting of nutrition variables remained unchanged (median: 38% compared with 33%; P = 0.7). The least-reported nutrition criteria were baseline nutrient exposures (28%) and effects of measurement errors from nutrition exposures (24%). Only 58 SRs (41%) used quality scales or checklists to assess the methodologic quality of the primary studies included.
Conclusions: The reporting quality of SRs has improved 3 y after publication of SR reporting standards, but the reporting of nutrition variables has not. Improved adherence to consensus methods and reporting standards should improve the utility of nutrition SRs.
INTRODUCTION
Leading nutrition organizations are using systematic reviews (SRs) to develop evidence-based nutrition and research agendas, revise dietary guidelines, formulate public health policies, and support dietetic practice guidelines with the goal of improving patient outcomes and practitioner effectiveness (1). The Office of Dietary Supplements in collaboration with other institutes and centers of the National Institutes of Health use SRs to identify research needs and set research priorities (2, 3). In 2001, the American Dietetic Association began carrying out SRs on a wide range of nutrition-related diseases (Evidence Analysis Library, http://adaevidencelibrary.com/). Evidence-based guidelines are being developed to provide an additional tool for food and nutrition professionals to apply the best research results to their practice, with the goal of improving patient outcomes and practitioner effectiveness (4, 5). In addition, the Food and Drug Administration developed a draft guidance document of an evidence-based review system to evaluate publicly available scientific evidence for health claims on food and supplement products (6). The US Preventive Services Task uses SRs to develop clinical practice recommendations on preventive and counseling interventions, including recommendations on nutrition topics (http://ahrq.gov/clinic/USpstfix.htm).
The complexity of relations between nutrition and health and the lack of widely accepted guidance on how to address nutrition issues have impeded the transfer of evidence-based methodologies from medicine to the field of nutrition. Whereas the concepts and methods of evidence-based medicine can be applied to nutrition questions, there are important differences between evaluations of drug therapies and nutrient-related health outcomes (7, 8). For SRs of medical interventions, there exist checklists to improve SR reporting quality (ie, clarity and transparent reporting of SR methods and results) such as MOOSE (Meta-analysis of Observational Studies in Epidemiology) (9) and QUOROM (Quality Of Reporting Of Meta-analyses) (10). Whereas these checklists represent consensus guidelines to improve the quality of SRs in general, they do not provide guidance for reporting or analyses of variables unique to the field of nutrition. Standardized guidance for researchers conducting SRs on nutrition-related topics could benefit the users of these reviews (11, 12).
Our aim was to examine the reporting quality of existing SRs linking micronutrients and health outcomes and to identify areas for improvement. We also performed exploratory analyses to evaluate factors that may be associated with reporting quality, such as the designs of primary studies (interventional compared with observational studies), years of publications, methods of evidence syntheses (meta-analyses or qualitative synthesis), and impact factors of journals that published these reviews.
METHODS
Literature search
We searched Medline from its inception through July 2007 using keywords for micronutrients, multivitamins, and antioxidants. We also searched for SRs, evidence-based reviews, and meta-analyses (see supplementary Table under “Supplemental data” in the online issue). Citations of SRs were reviewed for additional relevant articles. The essential micronutrients included in the analysis were fat-soluble vitamins A, D, E, and K; water-soluble B vitamins (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folate, and B-12); vitamin C; macrominerals (calcium, chloride, magnesium, phosphorous, potassium, sodium, and sulfur); and trace minerals (chromium, copper, fluoride, iodide, iron, manganese, molybdenum, selenium, and zinc). Multivitamins or minerals and antioxidant supplements were also included. Potentially relevant reviews included those whose abstracts described searches or eligibility criteria for study identification or included terms such as “systematic,” “evidence,” “evidence-based,” “meta-analysis,” or “pooled analysis.”
Eligibility criteria
Full-text articles of screened-in abstracts were retrieved and examined to confirm their eligibility according to predetermined criteria. For the purpose of this study, we defined an SR as a study that contained 3 components: a statement of the research questions (aims or objectives), a description of the literature search, and a listing of the study eligibility criteria. A review that lacked any of these components was excluded. We did not attempt to contact authors for clarification. The following types of reviews were excluded: reviews of foods or diets that did not quantify micronutrient intake, reviews including nonoral routes of nutrient delivery, reviews that did not relate nutrients to health outcomes, reviews of nonhuman data, and pooled analyses of primary databases (ie, secondary database analyses of multiple cohorts) that did not include an SR.
Data abstraction and collection
The unit of analysis was the SR article. We did not analyze the primary studies within the SRs. The following data were collected from the full-text articles of eligible SRs: topics covered (exposures and outcomes), whether meta-analyses were performed, specific journal, publication date, and number of citations per SR. We categorized the outcomes examined as either clinical outcomes or intermediate outcomes. A clinical outcome was defined as a measurement of how a person feels, functions, or survives; the severity of an existing disease; or the incidence of a new diagnosis. Intermediate disease outcomes included laboratory measurements or physical signs used as surrogates for a clinical endpoint (eg, plasma cholesterol concentrations or blood pressure for cardiovascular disease or dark adaptation for night blindness).
A standardized form was used for data collection. From published guidelines for reporting of the meta-analyses, such as MOOSE (9) and QUOROM (10), we collected and evaluated 28 reporting items regarding the search and study selection criteria; methods for assessing methodologic quality of the included primary studies, methods for quantitative syntheses, and protocols for reporting of results. The primary goal of guidelines for SR reporting is to encourage authors to provide clear and transparent reporting of the factors relating to the literature review and evidence syntheses they carried out. Most widely recognized reporting guidelines reflect consensus opinion of groups of experts in a particular field, including research methodologists and journal editors (13). Because there is no widely accepted guidance for reporting or analyses of variables unique to the field of nutrition in SRs, we included 7 items in addition to those identified in MOOSE and QUOROM specific to nutrition or diet variables based on the concern that failure to adhere to the items could lead to biased syntheses and/or interpretation of results in nutrition-related SRs. The definitions and the reasons for selecting these 35 reporting items are described in Table 1.
TABLE 1.
Reporting items for nutrition-related systematic reviews1
| Reporting item | Definition for adequate reporting | Rationale for inclusion |
| With or without meta-analyses | ||
| Search terms | Keywords for identifying relevant studies for the research questions [ie, PI(E)COS], or complete search strategy (eg, keywords and medical subject headings) were described or referred to elsewhere. | In QUOROM and MOOSE |
| Searches in multiple databases | Search was conducted in more than one electronic database. | In QUOROM and MOOSE |
| Search years | Time period of the articles searched and included was described. | In QUOROM and MOOSE |
| Searches in multiple languages | Search was conducted in English and other languages. | In QUOROM and MOOSE |
| Searching for unpublished data | Authors explicitly stated the efforts to include unpublished data (eg, contact with authors, meeting abstracts or conference preceding, dissertations, or gray literature search). | In QUOROM and MOOSE |
| Inclusion or exclusion criteria | Definitions of at least 2 of the PI(E)COS criteria (eg, randomized controlled trials of vitamin E were included) were reported. | In QUOROM and MOOSE |
| Baseline nutrition status of the population | Nutrition status of the population at baseline (ie, malnutrition, normal, or mixed). Acceptable data include data from nutrition assessments, explicit interpretations or discussions of the nutrition status of the locations where the study were conducted, and inclusion/exclusion criterion for the nutrition status of the study population. | Malnutrition is associated with vitamins and/or mineral deficiencies. Under- or over-nutrition is associated with mechanisms that affect health outcomes (14). Therefore, baseline nutrition status is an important covariate in any studies concerning the associations between micronutrients and health. |
| Types of interventions/exposures | Nutrient interventions or exposures were described (must include dose/level and type). | In QUOROM and MOOSE |
| Types of comparators | Comparators were described (must include dose/level and type). | In QUOROM and MOOSE |
| Types of outcomes | Outcomes or endpoints were defined. | In QUOROM and MOOSE |
| Types of study designs | Design of the included studies was described. | In QUOROM |
| Number of included and excluded studies | Number of eligible and ineligible studies identified from the search was reported. | In QUOROM |
| Reasons for exclusion | Reasons for exclusions were described. | In QUOROM and MOOSE |
| Use of specific checklist for quality items | The list of quality items for the validity (or quality) assessment of studies were applied and reported for each included study. | In QUOROM and MOOSE |
| Overall rating of the study given | An overall rating of study quality was assessed (eg, A, B, C or good, fair, poor). | In QUOROM and MOOSE |
| Models for meta-analyses2 | The methods of combining estimates (eg, fixed- and random-effects models) were reported. | In QUOROM and MOOSE |
| Assessment for heterogeneity | Heterogeneity across studies was assessed (ie, statistical methods) or discussed (ie, qualitative analyses). | In QUOROM and MOOSE |
| Dose-response relation of the nutrient-outcome associations/effects | Dose-response relations were examined by using dose-response statistical models, meta-regression, or subgroup analyses by different doses (ie, quantitative assessments), or examined qualitatively (ie, discussions). | In MOOSE |
| Assessment of publication bias | Quantitative assessment of publication bias (eg, funnel plot and Begg and Egger tests) were used. | In QUOROM and MOOSE |
| Discussion of publication bias | Issue of publication bias was raised in Discussion. | In MOOSE |
| Data sufficient to calculate the effect size2 | Data needed to calculate the effect size (eg, 2 × 2 table or mean change within group) for each study were presented in the tables or figures. | In QUOROM and MOOSE |
| Flow diagram for the number of included and excluded studies | A flow diagram showing the progress of study selection was presented. | In QUOROM |
| The total number of primary studies included in the systematic review/meta-analysis | The total number of studies that met inclusion criteria was reported in the text, tables, or figures. | In QUOROM and MOOSE |
| Graphical presentation of the results | Graphics summarizing individual study estimates and overall estimates were presented. | In MOOSE |
| Strength (eg, effect size) of nutrient-outcome associations/effects | The principal measures of effect (eg, relative risk, odds ratio, risk difference, or absolute difference) were reported. | In QUOROM and MOOSE |
| Uncertainty of nutrient-outcome associations/effects | Indication of statistical uncertainty of findings (eg, CI), and/or description on the ranges of estimates (eg, SD) was reported. | In QUOROM and MOOSE |
| Analysis (qualitatively or quantitatively) for potential confounding or interactions of the nutrient-outcome association | Assessment of confounding and/or interactions (eg, comparability of study groups) was reported, or analyzing crude and adjusted effect sizes separately. | In MOOSE |
| Specific future research recommendations | Specific suggestions for future research agenda (ie, other than “more future research is needed”). | In QUOROM and MOOSE |
| Including intervention studies | ||
| Sources of the nutrient interventions | Brand names or components (or formulation) of the nutrient supplements, or foods (or recipes) in the nutrition interventions were reported. | Different forms of nutrients (eg, all-rac-α-tocopherol (chemically synthesized form), RRR-α-tocopherol (naturally occurring form), or γ-tocopherol) may have different health benefits and/or bioavailability in the body. |
| Doses of the nutrient interventions | The amount of nutrients (or the doses) in the interventions and intervention regimens (eg, the number of times per day) were reported. | High dose of nutrient supplementations may have harmful health effect (15). Also, the dose is necessary to understand what the intervention was. |
| Baseline nutrient exposures in the study population | Baseline nutrient exposures or the background diet (ie, baseline dietary intake levels or the levels of the biomarker of intakes) in the study population were reported. | Data suggest differential effects of nutrient supplementations on the prevention of chronic diseases depending on the background nutrient exposures (16–19). |
| Including observational studies | ||
| Methods/instruments for assessing intakes of nutrient exposures | Methods or instruments for assessing intakes of nutrient exposures [ie, dietary assessments (FFQ, 24-h recall, diet record, or diet recall) and/or biomarkers of intakes] were reported. | There are known errors associated with different methods or instruments for assessing dietary intakes. The ideal method or instrument for assessing intakes of nutrient exposures depends on the research question being asked. |
| Ranges or distributions of the nutrient exposures | Ranges or distributions of the nutrient exposures (ie, quartiles, mean and SD, or ranges) in the study population were reported. | Ranges or distributions of the nutrient exposures represent the ranges of doses of the nutrients in relation to the health outcomes. |
| Errors in assessing nutrient exposures | Measurement errors of the dietary assessments or biomarkers of intakes were reported or discussed. | Dietary intake cannot be estimated without errors. Some of these errors can be dealt with by analytic techniques (20). Some of these errors can introduce bias. |
| Potential impacts of the errors from assessing the nutrient exposures on the nutrient-outcome association | Potential impacts of the errors from assessing the nutrient exposures on the nutrient-outcome association were reported or discussed. | The impact of particular type of errors in measuring the nutrient exposures depends on the research question being asked and the analytic methodology used to address it (21). |
PI(E)COS, Population, Intervention (Exposure), Comparator, Outcome, and Study design; QUOROM, Quality Of Reporting Of Meta-analyses; MOOSE, Meta-analysis of Observational Studies in Epidemiology; FFQ, food-frequency questionnaire.
Data were collected for systematic reviews with meta-analyses only.
Additional data elements collected included the number of primary studies, instruments or methods used to assess the quality of the primary studies, and the types of primary studies (interventional or observational studies). An interventional study was defined as a study with an active intervention, such as randomized or nonrandomized controlled trials, crossover trials, quasi-interventional studies (or community trials), and before-and-after studies. Observational studies included cohort, case-control, cross-sectional and ecological studies, case series, and case reports, where the intervention was not dictated by the investigator.
For each SR, we also collected citation counts of the SRs and impact factors of the journals that published these reviews from the Science Citation Index and the Institute for Scientific Information Journal Citation Reports edition 2006. The impact factor of a journal is calculated based on a 3-y period and can be considered to be the average number of times articles published in the journal are cited up to 2 years after publication. The citation count is the number of times an article was cited by other articles published in journals indexed in Journal Citation Reports. Citation counts were collected in February 2008. The mean yearly citation number for each SR was calculated [citation count of SR/(2008-publication year of SR)].
Statistical analyses
Descriptive analyses and summary statistics were performed on the reporting characteristics of SRs, including whether the reporting followed published standards such as MOOSE (9) and QUOROM (10), reporting of nutrition variables, number and types of primary studies analyzed, whether quality assessment of primary studies were performed, and what instruments were used to assess quality or susceptibility to biases. Fisher's exact test was used to examine differences in the proportion of SRs reporting each item and to compare the SRs that included observational studies with those that included interventional studies.
We used the Mann-Whitney U test to examine differences in the proportion of reporting criteria met by SRs of different study types (interventional studies, observational studies, or both designs), to compare SRs published before with those published 3 y after QUOROM and MOOSE standards, and to compare SRs with those without meta-analyses. A correlation analysis was conducted to examine the association between journal impact factors and citation numbers and the proportion of reporting criteria met among SRs. The maximal number of reporting criteria is 29 (26 SR-reporting factors and 3 nutrition variables) for SRs of interventional studies alone, 30 (26 + 4) for SRs of observational studies alone, and 33 (26 + 7) for SRs of both designs. Two reporting items for SRs containing meta-analyses (reporting of models for meta-analyses and data needed to calculate the effect size) were excluded from these calculations. Medians and interquartile ranges (IQRs) are reported when the distributions were skewed. All P values are 2-tailed and were considered significant when P < 0.05.
RESULTS
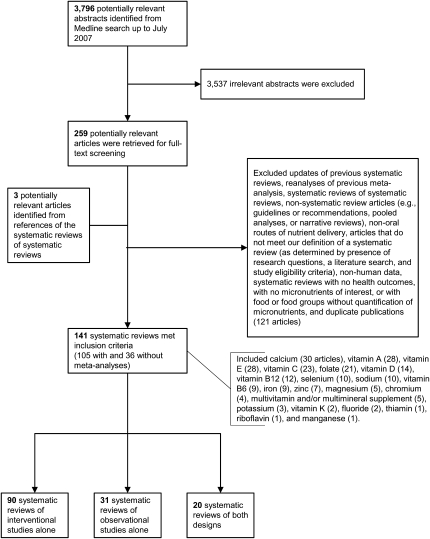
The Medline search identified 3796 citations, of which 259 full-text articles were retrieved and examined to confirm their eligibility. Three additional articles were identified from citations in the retrieved SRs. A total of 141 SRs (105 with and 36 without meta-analyses) were eligible (15, 22–161). Of these, 90 included interventional studies alone, 31 included observational studies alone, and 20 included both types of study designs (Figure 1). Of the reviews that did not meet eligibility criteria, 9 publications stated they were an SR and/or meta-analysis or an evidence-based review but they did not meet the criteria of our predetermined definition, mostly because the authors did not state the eligibility criteria for primary studies reviewed (162–170). Among the eligible reviews, alternative names used for SRs included evidence-based review, evidence review, critical review, qualitative overview, overview, in-depth review of the evidence, and review.
FIGURE 1.
Selection process and the number of the included and excluded systematic reviews.
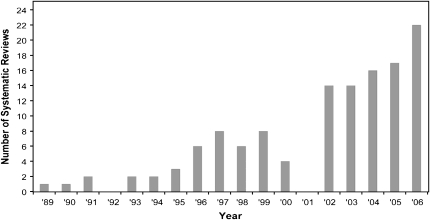
The earliest SR identified was published in 1989 (51). Half of the SRs were published since 2003. There has been a steady increase in the number of SRs published annually; the number of published SRs tripled from 1999 to 2006 (Figure 2). The number of primary studies included in each SR ranged from 1 to 264; 60% of the SRs included <20 primary studies. A wide variety of potential relations between micronutrients and health outcomes were examined (Table 2). Of 141 SRs, 88 (62%) evaluated clinical outcomes, 35 (25%) intermediate outcomes, and 18 (13%) both types of outcomes. Cardiovascular disease and cancers were the most common outcomes reported.
FIGURE 2.
Annual publication of systematic reviews of micronutrients and health (search ended week 2, July 2007).
TABLE 2.
Topics covered in the 141 qualifying systematic reviews linking micronutrients and health outcomes1
| No. of systematic reviews | Health outcomes |
|||||||||||||
| Micronutrient (references) | Clinical outcomes | Intermediate outcomes | Both | Age-related/neurologic2 | Bone3 | Cancer4 | CVD5 | Death6 | DM7 | Eye8 | Infection9 | Pregnancy/birth10 | Other11 | |
| n (%) | n (%) | n (%) | ||||||||||||
| Calcium (15, 22–51) | 30 | 14 (46) | 11 (37) | 5 (17) | 0 | 15 | 5 | 7 | 2 | 0 | 0 | 0 | 2 | 3 |
| Vitamin A and β-carotene (15, 26, 32, 52–76) | 28 | 23 (82) | 3 (11) | 2 (7>) | 1 | 0 | 8 | 6 | 9 | 0 | 0 | 5 | 0 | 7 |
| Vitamin E (15, 32, 52–56, 59–61, 66, 70–72, 76–89) | 28 | 22 (79) | 2 (7) | 4 (14) | 3 | 0 | 6 | 11 | 12 | 1 | 2 | 1 | 2 | 5 |
| Vitamin C (15, 32, 52–56, 60, 66, 67, 69–72, 76–78, 85, 87, 90–93) | 23 | 16 (70) | 4 (17) | 2 (9) | 0 | 0 | 5 | 8 | 9 | 0 | 0 | 4 | 1 | 2 |
| Folic acid (26, 54, 67, 85, 94–110) | 21 | 14 (67) | 4 (19) | 3 (14) | 2 | 0 | 7 | 7 | 2 | 0 | 0 | 0 | 4 | 2 |
| Vitamin D (22, 29, 30, 32–34, 36, 38, 39, 41, 111–114) | 14 | 10 (71) | 1 (7) | 3 (21) | 0 | 9 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Vitamin B-12 (54, 67, 85, 97, 101, 104, 105, 107, 108, 115–117) | 12 | 5 (42) | 4 (33) | 3 (25) | 2 | 0 | 1 | 6 | 1 | 1 | 0 | 0 | 1 | 2 |
| Selenium (15, 32, 52, 53, 56, 84, 118–121) | 10 | 10 (100) | 0 | 0 | 0 | 0 | 6 | 1 | 5 | 0 | 0 | 0 | 0 | 1 |
| Sodium (122–131) | 10 | 0 | 8 (80) | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 2 |
| Vitamin B-6 (26, 54, 85, 97, 101, 105, 108, 115, 132) | 9 | 5 (56) | 3 (33) | 1 (11) | 1 | 0 | 2 | 4 | 1 | 1 | 0 | 0 | 0 | 1 |
| Iron (57, 67, 133–139) | 9 | 4 (44) | 3 (33) | 2 (22) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 7 |
| Zinc (15, 56, 84, 85, 87, 140, 141) | 7 | 5 (71) | 0 | 2 (29) | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 3 |
| Magnesium (86, 142–145) | 5 | 2 (40) | 3 (60) | 0 | 0 | 0 | 0 | 4 | 1 | 2 | 0 | 0 | 0 | 0 |
| Chromium (86, 146–148) | 4 | 2 (50) | 2 (50) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Multivitamin and/or multimineral supplements (149–153) | 5 | 5 (100) | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 0 |
| Potassium (124, 154, 155) | 3 | 0 | 3 (100) | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin K (156, 157) | 2 | 1 (50) | 0 | 1 (50) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fluoride (38, 158) | 2 | 2 (100) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Iodine (159, 160) | 2 | 1 (50) | 0 | 1 (50) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Thiamin (115) | 1 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Riboflavin (56) | 1 | 1 (100) | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Manganese (161) | 1 | 1 (100) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | — | 88 (62) | 35 (22) | 18 (13) | ||||||||||
One systematic review may have more than one micronutrient and health outcome. CVD, cardiovascular disease; DM, diabetes mellitus.
Age-related or neurologic outcomes include Alzheimer disease, Parkinson disease, tardive dyskinesia, cognitive function testing, and epilepsy.
Bone outcomes include the prevalence or incidence of fracture, osteoporosis, and bone mineral density or content.
Cancer outcomes include the prevalence, incidence, or recurrence of cancers or malignant tumors, precursors of malignant tumors (eg, cervical squamous neoplasia and colorectal adenoma), and cancer mortality.
Cardiovascular disease outcomes include the prevalence or incidence of cardiovascular diseases (eg, heart diseases, vascular disease, and cerebrovascular disease), blood pressure, lipid profiles, and homocysteine concentrations, intima media thickness, arrhythmia, and cardiovascular disease mortality.
Death outcomes include all-cause or total mortality, infant mortality, and fetal neural tube defects.
Diabetes outcomes include the prevalence or incidence of diabetes, glycemic control, diabetic neuropathy, and glucose or insulin concentrations.
Eye outcomes include cataracts, infant eye outcomes, and age-related macular disease.
Infection outcomes include infectious diseases, common cold or respiratory infections, pneumococcal colonization, immune markers, and pneumonia-specific mortality.
Pregnancy or birth outcomes include preeclampsia, preterm delivery or prematurity, infant growth retardation, low birth weight, retinopathy of prematurity, small-for-gestational age, oral cleft birth, placental abruption or infarction, congenital anomalies, and spontaneous abortion.
Other outcomes include falls, diarrhea, hemoglobin concentration, any morbidity, growth, healing of chronic wound, toxicity, twinning, strength or physical performance, body weight, depressive symptoms, symptoms of vitamin B-12 deficiency, environment-associated health disorders, premenstrual syndrome, anemia, loss of renal function, hormone concentrations (eg, renin, aldosterone, and catecholamines), hemorrhagic disease of newborns, dental fluorosis, goiter, thyroid-stimulating hormone, and endothelial dysfunction.
Reporting characteristics of the 141 SRs linking micronutrients and health outcomes are summarized in Table 3. Items that SRs commonly did not report or include were as follows: whether literature searches in multiple languages (30% of SRs), whether unpublished data were included (28%), descriptions of the nutrition status of the population at baseline (32%), use of quality scales or items to assess validity (29%), dose-response relations of the nutrient-outcome association (35%), assessments or discussions of publication bias (40%), use of a flow diagram for the number of studies included and excluded (26%), evaluations of potential confounding or interactions of the nutrient-outcome association (49%), specific future research recommendations (35%), sources of the nutrient interventions (ie, brand names, components or formulation of the nutrient supplements, or foods or recipes) (46%), baseline nutrient exposures in the study population (28%), ranges of the nutrient exposures (33%), errors from assessing nutrient exposures (ie, errors from dietary assessments or biomarker assays) (31%), and potential impacts of the errors from assessing the nutrient exposures on the findings (24%). The definitions of adequate reporting of the 35 reporting items are described in Table 1.
TABLE 3.
Reporting characteristics in systematic reviews (with or without meta-analyses) of micronutrients and health outcomes1
| Systematic reviews of study
types |
|||||||
| Topic | Reporting item | QUOROM | MOOSE | Intervention (n = 90) | Observational (n = 31) | Both (n = 20) | Total (n = 141) |
| n (%) | n (%) | ||||||
| Search | Search terms were described or referred to elsewhere | √ | √ | 67 (74) | 24 (77) | 13 (65) | 104 (74) |
| Multiple databases were searched | √ | √ | 58 (64) | 16 (52) | 11 (55) | 85 (60) | |
| Years searched were described | √ | √ | 76 (84) | 27 (87) | 15 (75) | 118 (84) | |
| Multiple languages were included in search | √ | √ | 27 (30) | 10 (32) | 5 (25) | 42 (30) | |
| Authors explicitly stated searching for unpublished data | √ | √ | 36 (40)2 | 1 (3)2 | 2 (10) | 39 (28) | |
| Selection | Inclusion or exclusion criteria were stated3 | √ | √ | 90 (100) | 31 (100) | 20 (100) | 141 (100) |
| Nutrition status of the population at baseline was reported | 29 (32) | 7 (23) | 9 (45) | 45 (32) | |||
| Interventions or exposures were described | √ | √ | 88 (98) | 30 (97) | 19 (95) | 137 (97) | |
| Comparators were described | √ | √ | 73 (81) | 25 (83) | 15 (75) | 113 (81) | |
| Outcomes were described | √ | √ | 87 (97) | 31 (100) | 20 (100) | 138 (98) | |
| Types of studies included were reported | √ | 90 (100) | 31 (100) | 20 (100) | 141 (150) | ||
| Number of studies included and excluded were reported | √ | 62 (69) | 19 (61) | 9 (45) | 90 (64) | ||
| Reasons for exclusion were described | √ | √ | 58 (64)4 | 13 (42) 4 | 10 (50) | 81 (57) | |
| Validity | Quality rating was used (eg, A, B, C or good, fair, poor) | √ | √ | 31 (342 | 0 (0)2 | 6 (30) | 37 (26) |
| Quality items or checklists were applied and reported | √ | √ | 35 (39)2 | 1 (3)2 | 5 (25) | 41 (29) | |
| Quantitative or qualitative synthesis | Models for meta-analyses were reported5 | √ | √ | 66 (89) | 18 (86) | 7 (70) | 91 (87) |
| Heterogeneity was assessed or discussed? | √ | √ | 71 (79) | 27 (87) | 13 (65) | 111 (79) | |
| Dose-response relation of the nutrient-outcome association/effect was examined | √ | 28 (31) | 14 (45) | 7 (35) | 49 (35) | ||
| Publication bias was assessed | √ | √ | 32 (36) | 13 (42) | 3 (15) | 48 (34) | |
| Publication bias was discussed | √ | 33 (37) | 16 (52) | 8 (40) | 57 (40) | ||
| Data needed to calculate the effect size were given5 | √ | √ | 54 (73) | 16 (73) | 7 (70) | 77 (73) | |
| Results | A flow diagram for the number of studies included and excluded was used | √ | 33 (37)4 | 2 (6)4 | 1 (5) | 36 (26) | |
| The total number of primary studies included in the systematic review/meta-analysis was reported | √ | √ | 89 (99) | 31 (100) | 20 (100) | 140 (99) | |
| Results were presented graphically | √ | 61 (68) | 18 (58) | 8 (40) | 87 (62) | ||
| Strength (eg, effect size) of nutrient-outcome associations/effects were described | √ | √ | 81 (90) | 30 (97) | 19 (95) | 130 (92) | |
| Uncertainty of nutrient-outcome associations/effects was described | √ | √ | 77 (86) | 27 (87) | 15 (75) | 119 (84) | |
| Potential confounding or interactions of the nutrient-outcome association/effect were analyzed (qualitatively or quantitatively) | √ | 33 (37)4 | 22 (71)4 | 14 (70) | 69 (49) | ||
| Specific future research recommendations were made | √ | √ | 26 (29)4 | 16 (52)4 | 7 (35) | 49 (35) | |
| Nutrition variables (interventional studies) | Sources of the nutrient interventions were described | 46 (51) | NA | 5 (25) | NA | ||
| Doses of the nutrient interventions were described | 84 (93) | NA | 16 (80) | NA | |||
| Baseline nutrient exposures in the study population were described | 24 (27) | NA | 7 (35) | NA | |||
| Nutrition variables (observational studies) | Methods/instruments for assessing intakes of nutrient exposures were reported | NA | 24 (77) | 10 (50) | NA | ||
| Ranges or distributions of the nutrient exposures were described | NA | 14 (45) | 3 (15) | NA | |||
| Errors from assessing nutrient exposures were described or discussed | NA | 11 (35) | 5 (25) | NA | |||
| Potential impacts of the errors from assessing the nutrient exposures on the nutrient-outcome association were described or discussed | NA | 9 (29) | 3 (15) | NA | |||
QUOROM, Quality Of Reporting Of Meta-analyses; MOOSE, Meta-analysis of Observational Studies in Epidemiology; NA, not available.
P < 0.001, Fisher's exact test for the difference between intervention and observational studies.
Inclusion or exclusion criteria must be stated to be included in the analyses.
P < 0.05, Fisher's exact test for the difference between intervention and observational studies.
Data were collected for systematic reviews with meta-analyses only (n = 104).
Factors associated with the reporting quality
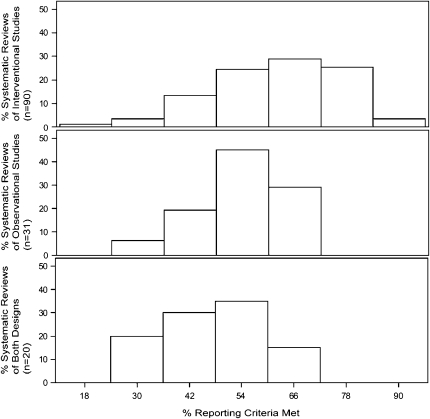
On average, SRs that linked micronutrients and health outcomes met 57% (IQR: 48%, 66%) of our reporting criteria. SRs that included only interventional studies met a higher proportion of reporting criteria (median: 62%; IQR: 51%, 72%) than those with only observational studies (median: 53%; IQR: 47%, 60%) or both study designs (median: 47%; IQR: 39%, 52%) (P < 0.001) (Figure 3). There were significantly more SRs of interventional than observational studies that reported a search for unpublished studies (40% compared with 3%), described the reasons for study exclusions (64% compared with 42%), used quality scales or items to assess validity (39% compared with 3%), and included a flow diagram of the number of studies included and excluded (37% compared with 6%). There were significantly fewer SRs of interventional than observational studies that analyzed the potential confounding or interactions of the nutrient-outcome associations (37% compared with 71%) and that made specific future research recommendations (29% compared with 52%).
FIGURE 3.
Proportion of reporting criteria met among 141 systematic reviews of micronutrients and health.
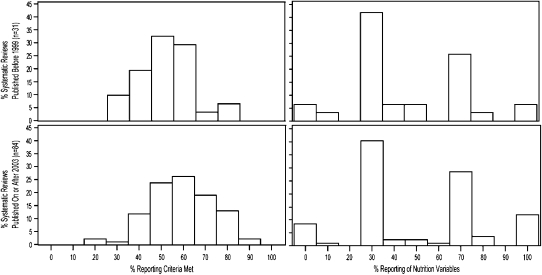
We examined the association between the reporting quality and publication of the MOOSE and QUOROM reporting standards for SRs by testing the difference in reporting quality comparing those published before publication of these standards and SRs published 3 y after. One-hundred fifteen SRs were published before 1999 (n = 31) or since 2003 (n = 84); articles published between 1999 and 2002 were excluded for being conducted too close in time to the publication of the reporting standards. Before the reporting standards, SRs met a lower proportion of our reporting criteria than after the publication of the standards (median: 50% compared with 59%; P = 0.01), which suggests that the overall reporting quality of SRs linking micronutrients and health outcomes has improved since publication of the reporting standards. In contrast, the reporting of nutrition variables remained unchanged (median: 33% compared with 38%; P = 0.7) (Figure 4).
FIGURE 4.
Proportion of reporting criteria met comparing systematic reviews published before 1999 and 3 y after publication of QUOROM (Quality Of Reporting Of Meta-analyses) and MOOSE (Meta-analysis of Observational Studies in Epidemiology).
Of the 141 SRs, 128 were published in 84 journals with impact factors that ranged from 0.3 to 25.8; 13 SRs (8 with meta-analyses) were published in journals not indexed in the Journal Citation Reports; therefore, they were excluded from the relevant analyses. There was a positive correlation between the proportion of reporting criteria met and the journals' impact factors (r = 0.35, P < 0.001), which indicates that SRs published in higher impact journals were more likely to have met a high proportion of our reporting criteria. The median yearly number of citations attributable to the SRs was 4, ranging from 0 to 100 [excluding an outlier (109) that has had 2128 citations since 1995]. The proportion of reporting criteria met was not significantly correlated with the yearly number of citations (r = 0.11, P = 0.18), but both the correlation coefficient and statistical significance improved after the outlier SR was excluded (r = 0.26, P = 0.003).
SRs containing meta-analyses (n = 105) met a higher proportion of our reporting criteria compared with the 31 SRs without meta-analyses (median: 62% compared with 48%; P < 0.001). SRs containing meta-analyses were also published in journals with higher impact factors (median: 4.3 compared with 2.8; P = 0.001) and received more yearly citations (median: 16 compared with 6; P = 0.001).
Quality assessment of the primary studies
There were 58 SRs (49 of interventional studies, 1 of observational studies, and 8 of both designs) that used quality scales or checklists to assess the methodologic quality of the primary studies. The most commonly used were Jadad (171) and Schulz (172) quality scores or checklists, which were designed to assess the adequacy of the RCTs. The one SR of observational studies used a modified quality checklist, which was originally developed to evaluate the quality of interventional studies (an unpublished thesis). Of the 8 SRs of both interventional and observational studies, 8 different quality scales or checklists were used. Seven of the 8 SRs used single quality scales (eg, good, fair, or poor) for both intervention and observational studies. The definitions (or the quality items considered) of these quality scales varied. One SR used separate quality checklists for intervention (Jadad) and observational studies.
DISCUSSION
The number of SRs relating micronutrient intake to health outcomes has grown rapidly in recent years. These reviews have been published in a broad range of journals, many with relatively high citation impacts. These trends suggest an increasing acceptance of SRs as a useful way to summarize the data by the nutrition community. SRs of the literature serve as the core of evidence-based guideline development. Dietary guidance issued without prespecified and transparent evidentiary support may be more prone to errors (173) because of their greater reliance on expert opinion and the potential for omitting important data unknown to the experts. Because of the complex nature of how nutrients are handled and function in the human body, a large number of linked questions are often required for the development of nutrition guidelines. Incorporating currently existing SRs into a new SR can be a cost-effective use of resources but also has potential risks associated with doing so (174). To ensure that future nutrition-related SRs will be of maximal value, the highest standards in their conduct and reporting must be used. Good-quality SRs should minimize the likelihood of bias or misinterpretation. SRs are also helpful in identifying knowledge gaps for which specific research agenda or recommendations are needed.
Because of deficiencies in the conducting and reporting of SRs in the medical literature, expert panels convened to develop guidelines for SRs. The resulting QUOROM and MOOSE lists have been adopted by SR methodologists and medical journals as standards (13). However, several factors are important to interpreting nutrition research, and thus nutrition SRs, that are not included in the SR quality checklists designed for the medical literature. Thus, we developed a list of 35 items that included the potentially relevant items from QUOROM and MOOSE, along with new nutrition-specific items following the rationale described in Table 1.
Our analysis of a large cohort of nutrition SRs found that 14 of the 35 items commonly were not reported or considered in the SRs; of these, 6 concerned variables that are unique to the field of nutrition. Moreover, we identified deficiencies in reporting of 8 (of 28) items on the clarity or transparency of methods and results (Table 3). Whereas there is currently no consensus on nutrition quality rating issues, the reporting items used in this analysis were selected because of the likelihood that they would have generic utility across SRs conducted for different purposes. It is, however, also recognized that exceptions to generic reporting standards for nutrition SRs may be needed for specific SR applications (eg, regulatory applications). In these cases, justification for the exceptions could be noted in the design and reporting of the SR. This standardization and transparency would clarify the applicability of an SR for purposes other than those for which it was designed and enhance comparisons of results across SRs on similar topics.
Some generic quality issues are applicable to all SRs. For example, a comprehensive and transparent search strategy, with adequate justifications for inclusion or exclusion of specific studies, is needed to ensure an unbiased selection of studies for SRs and to improve understanding of how the SR was conducted. Furthermore, searching for unpublished data and comparing them with published data could shed some insights on the potential impact of publication bias (175). There is an underlying suspicion of publication bias against studies having either null or negative outcomes (176). It is important to note that there are no reliable methods to measure publication bias. Studies have shown that the most frequently used method to assess publication bias (funnel plots) can be misleading (177–179). Quality assessment of the primary studies is essential for the evaluation of validity and the overall strength of the conclusions in an SR.
The strength of SRs and meta-analyses relies not only on the validity of the included primary studies, but also on the clarity of the reporting of the SR itself. Although good reporting does not necessarily equate valid results, good reporting provides useful information for evaluating the validity of the findings. Our analyses showed that more SRs of interventional studies than those of observational studies (54% compared with 3%, respectively) used quality scales or checklists to assess the methodologic quality of the primary studies included. Without quality assessments, the validity of the included primary studies is unclear and the impact of the potential biases in the primary studies on the conclusions of an SR cannot be assessed. Furthermore, SRs of interventional studies met more quality criteria than did SRs of observational studies. This finding could be explained in part by the lack of reporting standards for observational studies. This is in contrast with RCTs, many of which have adopted the CONSORT reporting standards (180, 181). Recently, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (182) was developed to improve the reporting quality of observational studies. It is important to note that CONSORT and STROBE are aimed at guiding authors to report the findings of the primary studies; they were not designed as tools to assess the quality of the primary studies included in the SRs or meta-analyses. Our analyses also showed that the proportion of reporting criteria met was significantly, positively correlated with both the journals' impact factors and yearly citation numbers. This suggests that SRs of higher reporting quality are more likely to be published on higher impact journals and had wider research dissemination.
In summary, our findings suggest that the reporting quality of SRs has improved since publication of the reporting standards, but the reporting of nutrition or diet variables has not. This limits their potential value to help in formulating nutrition-related guidelines, recommendations, or research agendas. Reporting standards of SRs should be tailored for specific types of research to help the users of these SRs interpret the results. An improvement in the reporting quality of meta-analyses of RCTs in the critical care literature was documented after the publication of QUOROM (183). Our analysis documents the lack of consistent standards in conducting and reporting SRs of nutrition-related topics. It also provides useful insights on key reporting items for nutrition SRs. In addition to study design features that are important in reducing bias in all studies, for nutrition-related interventional studies it is critical to report the source and dose of the intervention, such as brand names or components (or formulation) of the nutrient supplements, or foods (or recipe) in the nutrition interventions, and the amount of nutrients (or the doses) in the interventions and intervention regimens (eg, the number of times per day). It is also important to report the baseline nutrient exposures or the background diet (ie, baseline dietary intake levels or the levels of the biomarker of intakes) in the study population, because the background diet could be one source of heterogeneity (ie, differential effects of nutrient supplementations on health outcomes) in an SR or meta-analysis. For the nutrition epidemiologic studies, it is important to report the methods or instruments for assessing intakes of nutrient exposures, ranges or distributions of the nutrient exposures, measurement errors of the diet or nutrient variables, and the potential impact of the errors from assessing the nutrient exposures on the nutrient-outcome association.
Improving the methodologic and reporting quality of nutrition SRs ought to produce more accurate, less biased summaries of the evidence and will allow users of the SRs—general readers, guideline developers, policy makers, and others—to have a better understanding of what evidence the SRs summarize and what biases may exist. Whereas there is room for revision of the quality items for nutrition SRs based on expert consensus, better adherence to the quality items analyzed here is likely to improve the usefulness and acceptance of nutrition SRs.
Note Added in Proof: When this article was in press, we found one qualified SR (184) that was not included in our original analyses. Adding this article, the number of SRs of interventional studies alone changed from 90 to 91, and the number of SRs with meta-analyses changed from 105 to 106. However, our findings and conclusions did not change.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—MC, EMB, TAT, AHL, EAY, and JL: conception and design; MC, EMB, TAT, AHL, and JL: analysis and interpretation of the data; MC: draft of the article; MC, EMB, SI, GR, WWY, and TAT: collection and assembly of data; JL and EAY: obtained funding and provided technical and logistic support; and all authors: critical revision of the article for important intellectual content and final approval of the article. None of the authors reported a conflict of interest.
REFERENCES
- 1.Lichtenstein AH, Yetley EA, Lau J. Application of systematic review methodology to the field of nutrition. J Nutr 2008;138:2297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brannon PM, Yetley EA, Bailey RL, Picciano MF. Overview of the conference “Vitamin D and health in the 21st century: an update.” Am J Clin Nutr 2008;88:483S–90S [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health and National Heart Lung and Blood Institute Working group report on future clinical research directions on omega-3 fatty acids and cardiovascular disease. June 2, 2004. Available from: http://www.nhlbi.nih.gov/meetings/workshops/omega-3/omega-3-rpt.htm (cited 1 Dec 2008)
- 4.Blumberg-Kason S, Lipscomb R. Evidence-based nutrition practice guidelines: a valuable resource in the evidence analysis library. J Am Diet Assoc 2006;106:1935–6 [DOI] [PubMed] [Google Scholar]
- 5.Myers E. Systems for evaluating nutrition research for nutrition care guidelines: do they apply to population dietary guidelines? J Am Diet Assoc 2003;103:S34–41 [DOI] [PubMed] [Google Scholar]
- 6.CFSAN Office of Nutrition Labeling and Dietary Supplements Evidence-based review system for the scientific evaluation of health claims. July 2007. Available from: http://www.cfsan.fda.gov/∼dms/hclmgui6.html (cited 17 Feb 2009)
- 7.Balk EM, Horsley TA, Newberry SJ, et al. A collaborative effort to apply the evidence-based review process to the field of nutrition: challenges, benefits, and lessons learned. Am J Clin Nutr 2007;85:1448–56 [DOI] [PubMed] [Google Scholar]
- 8.De Lorgeril M, Salen P. Fish and N-3 fatty acids for the prevention and treatment of coronary heart disease: nutrition is not pharmacology. Am J Med 2002;112:316–9 [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354:1896–900 [DOI] [PubMed] [Google Scholar]
- 11.Lodge M, Becker L, van Binsbergen J, van Weel C, Rosser W. Organisation of a proposed Cochrane Diet and Nutrition Field. Eur J Clin Nutr 2005;59(suppl):S162–6 [DOI] [PubMed] [Google Scholar]
- 12.Becker LA, van Binsbergen JJ. How can a proposed Cochrane diet and nutrition field work effectively? Eur J Clin Nutr 2005;59(suppl 1):S167–71 [DOI] [PubMed] [Google Scholar]
- 13.The EQUATOR Network Enhancing the quality and transparency of health research. July 2008. Available from: http://www.equator-network.org (cited 1 Dec 2008)
- 14.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 2007;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37–46 [DOI] [PubMed] [Google Scholar]
- 16.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev 2002;11:630–9 [PubMed] [Google Scholar]
- 17.Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev 2002;11:1285–91 [PubMed] [Google Scholar]
- 18.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335–42 [DOI] [PubMed] [Google Scholar]
- 19.Wright ME, Lawson KA, Weinstein SJ, et al. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 2006;84:1200–7 [DOI] [PubMed] [Google Scholar]
- 20.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr 1997;65:1179S–86S [DOI] [PubMed] [Google Scholar]
- 21.Beaton GH. Approaches to analysis of dietary data: relationship between planned analyses and choice of methodology. Am J Clin Nutr 1994;59:253S–61S [DOI] [PubMed] [Google Scholar]
- 22.Izaks GJ. Fracture prevention with vitamin D supplementation: considering the inconsistent results. BMC Musculoskelet Disord 2007;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trumbo PR, Ellwood KC. Supplemental calcium and risk reduction of hypertension, pregnancy-induced hypertension, and preeclampsia: an evidence-based review by the US Food and Drug Administration. Nutr Rev 2007;65:78–87 [DOI] [PubMed] [Google Scholar]
- 24.van Mierlo LA, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens 2006;20:571–80 [DOI] [PubMed] [Google Scholar]
- 25.Winzenberg T, Shaw K, Fryer J, Jones G. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ 2006;333:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies AA, Davey SG, Harbord R, et al. Nutritional interventions and outcome in patients with cancer or preinvasive lesions: systematic review. J Natl Cancer Inst 2006;98:961–73 [DOI] [PubMed] [Google Scholar]
- 27.Trowman R, Dumville JC, Hahn S, Torgerson DJ. A systematic review of the effects of calcium supplementation on body weight. Br J Nutr 2006;95:1033–8 [DOI] [PubMed] [Google Scholar]
- 28.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 2005;97:1768–77 [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257–64 [DOI] [PubMed] [Google Scholar]
- 30.Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif Tissue Int 2005;76:176–86 [DOI] [PubMed] [Google Scholar]
- 31.Shaukat A, Scouras N, Schunemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol 2005;100:390–4 [DOI] [PubMed] [Google Scholar]
- 32.Dagnelie PC, Schuurman AG, Goldbohm RA, van den Brandt PA. Diet, anthropometric measures and prostate cancer risk: a review of prospective cohort and intervention studies. BJU Int 2004;93:1139–50 [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA 2004;291:1999–2006 [DOI] [PubMed] [Google Scholar]
- 34.Richy F, Ethgen O, Bruyere O, Reginster JY. Efficacy of alphacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int 2004;15:301–10 [DOI] [PubMed] [Google Scholar]
- 35.Xu L, McElduff P, D'Este C, Attia J. Does dietary calcium have a protective effect on bone fractures in women? A meta-analysis of observational studies. Br J Nutr 2004;91:625–34 [DOI] [PubMed] [Google Scholar]
- 36.Latham NK, Anderson CS, Reid IR. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc 2003;51:1219–26 [DOI] [PubMed] [Google Scholar]
- 37.Hofmeyr GJ, Roodt A, Atallah AN, Duley L. Calcium supplementation to prevent pre-eclampsia—a systematic review. S Afr Med J 2003;224–8 [PubMed] [Google Scholar]
- 38.Meunier PJ. Evidence-based medicine and osteoporosis: a comparison of fracture risk reduction data from osteoporosis randomised clinical trials. Int J Clin Pract 1999;53:122–9 [PubMed] [Google Scholar]
- 39.Papadimitropoulos E, Wells G, Shea B, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 2002;23:560–9 [DOI] [PubMed] [Google Scholar]
- 40.Shea B, Wells G, Cranney A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev 2002;23:552–9 [DOI] [PubMed] [Google Scholar]
- 41.Amin S, LaValley MP, Simms RW, Felson DT. The role of vitamin D in corticosteroid-induced osteoporosis: a meta-analytic approach. Arthritis Rheum 1999;42:1740–51 [DOI] [PubMed] [Google Scholar]
- 42.Cumming RG, Nevitt MC. Calcium for prevention of osteoporotic fractures in postmenopausal women. J Bone Miner Res 1997;12:1321–9 [DOI] [PubMed] [Google Scholar]
- 43.Bergsma-Kadijk JA. van't Veer P, Kampman E, Burema J. Calcium does not protect against colorectal neoplasia. Epidemiology 1996;7:590–7 [DOI] [PubMed] [Google Scholar]
- 44.Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Ann Intern Med 1996;124:825–31 [DOI] [PubMed] [Google Scholar]
- 45.Bucher HC, Cook RJ, Guyatt GH, et al. Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomized controlled trials. JAMA 1996;275:1016–22 [DOI] [PubMed] [Google Scholar]
- 46.Welten DC, Kemper HC, Post GB, van Staveren WA. A meta-analysis of the effect of calcium intake on bone mass in young and middle aged females and males. J Nutr 1995;125:2802–13 [DOI] [PubMed] [Google Scholar]
- 47.Cappuccio FP, Elliott P, Allender PS, Pryer J, Follman DA, Cutler JA. Epidemiologic association between dietary calcium intake and blood pressure: a meta-analysis of published data. Am J Epidemiol 1995;142:935–45 [DOI] [PubMed] [Google Scholar]
- 48.Carroli G, Duley L, Belizan JM, Villar J. Calcium supplementation during pregnancy: a systematic review of randomised controlled trials. Br J Obstet Gynaecol 1994;101:753–8 [DOI] [PubMed] [Google Scholar]
- 49.Cumming RG. Calcium intake and bone mass: a quantitative review of the evidence. Calcif Tissue Int 1990;47:194–201 [DOI] [PubMed] [Google Scholar]
- 50.Mackerras D, Lumley T. First- and second-year effects in trials of calcium supplementation on the loss of bone density in postmenopausal women. Bone 1997;21:527–33 [DOI] [PubMed] [Google Scholar]
- 51.Cappuccio FP, Siani A, Strazzullo P. Oral calcium supplementation and blood pressure: an overview of randomized controlled trials. J Hypertens 1989;7:941–6 [DOI] [PubMed] [Google Scholar]
- 52.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007;297:842–57 [DOI] [PubMed] [Google Scholar]
- 53.Bjelakovic G, Nagorni A, Nikolova D, Simonetti RG, Bjelakovic M, Gluud C. Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma. Aliment Pharmacol Ther 2006;24:281–91 [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Closas R, Castellsague X, Bosch X, Gonzalez CA. The role of diet and nutrition in cervical carcinogenesis: a review of recent evidence. Int J Cancer 2005;117:629–37 [DOI] [PubMed] [Google Scholar]
- 55.Etminan M, Gill SS, Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol 2005;4:362–5 [DOI] [PubMed] [Google Scholar]
- 56.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004;364:1219–28 [DOI] [PubMed] [Google Scholar]
- 57.Ramakrishnan U, Aburto N, McCabe G, Martorell R. Multimicronutrient interventions but not vitamin a or iron interventions alone improve child growth: results of 3 meta-analyses. J Nutr 2004;134:2592–602 [DOI] [PubMed] [Google Scholar]
- 58.Myhre AM, Carlsen MH, Bohn SK, Wold HL, Laake P, Blomhoff R. Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr 2003;78:1152–9 [DOI] [PubMed] [Google Scholar]
- 59.Gray M. Does oral supplementation with vitamins A or E promote healing of chronic wounds? J Wound Ostomy Continence Nurs 2003;30:290–4 [DOI] [PubMed] [Google Scholar]
- 60.Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;139:56–70 [DOI] [PubMed] [Google Scholar]
- 61.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017–23 [DOI] [PubMed] [Google Scholar]
- 62.Grotto I, Mimouni M, Gdalevich M, Mimouni D. Vitamin A supplementation and childhood morbidity from diarrhea and respiratory infections: a meta-analysis. J Pediatr 2003;142:297–304 [DOI] [PubMed] [Google Scholar]
- 63.Gupta P, Indrayan A. Effect of vitamin A supplementation on childhood morbidity and mortality: critical review of Indian studies. Indian Pediatr 2002;39:1099–118 [PubMed] [Google Scholar]
- 64.D'Souza RM, D'Souza R. Vitamin A for the treatment of children with measles—a systematic review. J Trop Pediatr 2002;48:323–7 [DOI] [PubMed] [Google Scholar]
- 65.D'Souza RM, D'Souza R. Vitamin A for preventing secondary infections in children with measles–a systematic review. J Trop Pediatr 2002;48:72–7 [DOI] [PubMed] [Google Scholar]
- 66.Asplund K. Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Intern Med 2002;251:372–92 [DOI] [PubMed] [Google Scholar]
- 67.Sloan NL, Jordan E, Winikoff B. Effects of iron supplementation on maternal hematologic status in pregnancy. Am J Public Health 2002;92:288–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinmaus CM, Nunez S, Smith AH. Diet and bladder cancer: a meta-analysis of six dietary variables. Am J Epidemiol 2000;151:693–702 [DOI] [PubMed] [Google Scholar]
- 69.Gandini S, Merzenich H, Robertson C, Boyle P. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer 2000;36:636–46 [DOI] [PubMed] [Google Scholar]
- 70.Marchioli R. Antioxidant vitamins and prevention of cardiovascular disease: laboratory, epidemiological and clinical trial data. Pharmacol Res 1999;40:227–38 [DOI] [PubMed] [Google Scholar]
- 71.Law MR, Morris JK. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? Eur J Clin Nutr 1998;52:549–56 [DOI] [PubMed] [Google Scholar]
- 72.Lonn EM, Yusuf S. Is there a role for antioxidant vitamins in the prevention of cardiovascular diseases? An update on epidemiological and clinical trials data. Can J Cardiol 1997;13:957–65 [PubMed] [Google Scholar]
- 73.Giles G, Ireland P. Diet, nutrition and prostate cancer. Int J Cancer 1997;(suppl 10):13–7 [DOI] [PubMed] [Google Scholar]
- 74.Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ 1993;306:366–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality: a meta-analysis. JAMA 1993;269:898–903 [PubMed] [Google Scholar]
- 76.Aminbakhsh A, Mancini J. Chronic antioxidant use and changes in endothelial dysfunction: a review of clinical investigations. Can J Cardiol 1999;15:895–903 [PubMed] [Google Scholar]
- 77.Polyzos NP, Mauri D, Tsappi M, et al. Combined vitamin C and E supplementation during pregnancy for preeclampsia prevention: a systematic review. Obstet Gynecol Surv 2007;62:202–6 [DOI] [PubMed] [Google Scholar]
- 78.Coulter ID, Hardy ML, Morton SC, et al. Antioxidants vitamin C and vitamin E for the prevention and treatment of cancer. J Gen Intern Med 2006;21:735–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pham DQ, Plakogiannis R. Vitamin E supplementation in Alzheimer's disease, Parkinson's disease, tardive dyskinesia, and cataract: Part 2. Ann Pharmacother 2005;39:2065–72 [DOI] [PubMed] [Google Scholar]
- 80.Pham DQ, Plakogiannis R. Vitamin E supplementation in cardiovascular disease and cancer prevention: part 1. Ann Pharmacother 2005;39:1870–8 [DOI] [PubMed] [Google Scholar]
- 81.Alkhenizan AH, Al-Omran MA. The role of vitamin E in the prevention of coronary events and stroke. Meta-analysis of randomized controlled trials. Saudi Med J 2004;25:1808–14 [PubMed] [Google Scholar]
- 82.Eidelman RS, Hollar D, Hebert PR, Lamas GA, Hennekens CH. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med 2004;164:1552–6 [DOI] [PubMed] [Google Scholar]
- 83.Shekelle PG, Morton SC, Jungvig LK, et al. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med 2004;19:380–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lacour M, Zunder T, Restle A, Schwarzer G. No evidence for an impact of selenium supplementation on environment associated health disorders—a systematic review. Int J Hyg Environ Health 2004;207:1–13 [DOI] [PubMed] [Google Scholar]
- 85.Bleys J, Miller ER, III, Pastor-Barriuso R, Appel LJ, Guallar E. Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2006;84:880–7 [DOI] [PubMed] [Google Scholar]
- 86.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003;26:1277–94 [DOI] [PubMed] [Google Scholar]
- 87.Moreira A, Kekkonen RA, Delgado L, Fonseca J, Korpela R, Haahtela T. Nutritional modulation of exercise-induced immunodepression in athletes: a systematic review and meta-analysis. Eur J Clin Nutr 2007;61:443–60 [DOI] [PubMed] [Google Scholar]
- 88.Barak Y, Swartz M, Shamir E, Stein D, Weizman A. Vitamin E (alpha-tocopherol) in the treatment of tardive dyskinesia: a statistical meta-analysis. Ann Clin Psychiatry 1998;10:101–5 [DOI] [PubMed] [Google Scholar]
- 89.Raju TN, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr 1997;131:844–50 [DOI] [PubMed] [Google Scholar]
- 90.Hemila H. Vitamin C supplementation and respiratory infections: a systematic review. Mil Med 2004;169:920–5 [DOI] [PubMed] [Google Scholar]
- 91.Ness AR, Chee D, Elliott P. Vitamin C and blood pressure—an overview. J Hum Hypertens 1997;11:343–50 [DOI] [PubMed] [Google Scholar]
- 92.Hemila H. Vitamin C intake and susceptibility to the common cold. Br J Nutr 1997;77:59–72 [DOI] [PubMed] [Google Scholar]
- 93.Ness AR, Powles JW, Khaw KT. Vitamin C and cardiovascular disease: a systematic review. J Cardiovasc Risk 1996;3:513–21 [PubMed] [Google Scholar]
- 94.Muggli EE, Halliday JL. Folic acid and risk of twinning: a systematic review of the recent literature, July 1994 to July 2006. Med J Aust 2007;186:243–8 [DOI] [PubMed] [Google Scholar]
- 95.Badovinac RL, Werler MM, Williams PL, Kelsey KT, Hayes C. Folic acid-containing supplement consumption during pregnancy and risk for oral clefts: a meta-analysis. Birth Defects Res A Clin Mol Teratol 2007;79:8–15 [DOI] [PubMed] [Google Scholar]
- 96.Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 2007;99:64–76 [DOI] [PubMed] [Google Scholar]
- 97.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med 2007;167:21–30 [DOI] [PubMed] [Google Scholar]
- 98.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006;296:2720–6 [DOI] [PubMed] [Google Scholar]
- 99.Lewis SJ, Harbord RM, Harris R, Smith GD. Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J Natl Cancer Inst 2006;98:1607–22 [DOI] [PubMed] [Google Scholar]
- 100.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology 2006;131:1271–83 [DOI] [PubMed] [Google Scholar]
- 101.Homocysteine Lowering Trialists' Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 2005;82:806–12 [DOI] [PubMed] [Google Scholar]
- 102.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005;113:825–8 [DOI] [PubMed] [Google Scholar]
- 103.Taylor MJ, Carney SM, Goodwin GM, Geddes JR. Folate for depressive disorders: systematic review and meta-analysis of randomized controlled trials. J Psychopharmacol 2004;18:251–6 [DOI] [PubMed] [Google Scholar]
- 104.Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet 2004;17:371–83 [DOI] [PubMed] [Google Scholar]
- 105.de Bree BA, Mennen LI, Hercberg S, Galan P. Evidence for a protective (synergistic?) effect of B-vitamins and omega-3 fatty acids on cardiovascular diseases. Eur J Clin Nutr 2004;58:732–44 [DOI] [PubMed] [Google Scholar]
- 106.Diculescu M, Ciocirlan M, Ciocirlan M, et al. Folic acid and sulfasalazine for colorectal carcinoma chemoprevention in patients with ulcerative colitis: the old and new evidence. Rom J Gastroenterol 2003;12:283–6 [PubMed] [Google Scholar]
- 107.Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: a systematic review. Placenta 1999;20:519–29 [DOI] [PubMed] [Google Scholar]
- 108.Homocysteine Lowering Trialists' Collaboration Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 1998;316:894–8 [PMC free article] [PubMed] [Google Scholar]
- 109.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. JAMA 1995;274:1049–57 [DOI] [PubMed] [Google Scholar]
- 110.Canadian Task Force on the Periodic Health Examination Periodic health examination, 1994 update: 3. Primary and secondary prevention of neural tube defects. Can Med Assoc J 1994;151:159–66 [PMC free article] [PubMed] [Google Scholar]
- 111.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 2007;32:210–6 [DOI] [PubMed] [Google Scholar]
- 112.Gorham ED, Garland CF, Garland FC, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97:179–94 [DOI] [PubMed] [Google Scholar]
- 113.Grant WB, Garland CF. A critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer 2004;48:115–23 [DOI] [PubMed] [Google Scholar]
- 114.Weatherall M. A meta-analysis of 25 hydroxyvitamin D in older people with fracture of the proximal femur. N Z Med J 2000;113:137–40 [PubMed] [Google Scholar]
- 115.Sun Y, Lai MS, Lu CJ. Effectiveness of vitamin B12 on diabetic neuropathy: systematic review of clinical controlled trials. Acta Neurol Taiwan 2005;14:48–54 [PubMed] [Google Scholar]
- 116.Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract 2006;23:279–85 [DOI] [PubMed] [Google Scholar]
- 117.Ray JG, Blom HJ. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM 2003;96:289–95 [DOI] [PubMed] [Google Scholar]
- 118.Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer 2006;42:2463–71 [DOI] [PubMed] [Google Scholar]
- 119.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 2006;84:762–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Etminan M, FitzGerald JM, Gleave M, Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control 2005;16:1125–31 [DOI] [PubMed] [Google Scholar]
- 121.Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev 2004;13:771–8 [PubMed] [Google Scholar]
- 122.He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension 2006;48:861–9 [DOI] [PubMed] [Google Scholar]
- 123.Jones-Burton C, Mishra SI, Fink JC, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol 2006;26:268–75 [DOI] [PubMed] [Google Scholar]
- 124.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 2003;17:471–80 [DOI] [PubMed] [Google Scholar]
- 125.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens 2002;16:761–70 [DOI] [PubMed] [Google Scholar]
- 126.Hooper L, Bartlett C, Davey SG, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ 2002;325:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Graudal NA, Galloe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 1998;279:1383–91 [DOI] [PubMed] [Google Scholar]
- 128.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA 1996;275:1590–7 [DOI] [PubMed] [Google Scholar]
- 129.Cutler JA, Follmann D, Elliott P, Suh I. An overview of randomized trials of sodium reduction and blood pressure. Hypertension 1991;17:I27–33 [DOI] [PubMed] [Google Scholar]
- 130.Alam S, Johnson AG. A meta-analysis of randomised controlled trials (RCT) among healthy normotensive and essential hypertensive elderly patients to determine the effect of high salt (NaCl) diet of blood pressure. J Hum Hypertens 1999;13:367–74 [DOI] [PubMed] [Google Scholar]
- 131.Ebrahim S, Smith GD. Lowering blood pressure: a systematic review of sustained effects of non-pharmacological interventions. J Public Health Med 1998;20:441–8 [DOI] [PubMed] [Google Scholar]
- 132.Wyatt KM, Dimmock PW, Jones PW. Shaughn O'Brien PM. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ 1999;318:1375–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gera T, Sachdev HP, Nestel P, Sachdev SS. Effect of iron supplementation on haemoglobin response in children: systematic review of randomised controlled trials. J Pediatr Gastroenterol Nutr 2007;44:468–86 [DOI] [PubMed] [Google Scholar]
- 134.Gera T, Sachdev HP, Nestel P. Effect of iron supplementation on physical performance in children and adolescents: systematic review of randomized controlled trials. Indian Pediatr 2007;44:15–24 [PubMed] [Google Scholar]
- 135.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutr 2006;9:904–20 [DOI] [PubMed] [Google Scholar]
- 136.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 2006;84:1261–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 2005;8:117–32 [DOI] [PubMed] [Google Scholar]
- 138.Gera T, Sachdev HP. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ 2002;325:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tran T, Wax JR, Philput C, Steinfeld JD, Ingardia CJ. Intentional iron overdose in pregnancy—management and outcome. J Emerg Med 2000;18:225–8 [DOI] [PubMed] [Google Scholar]
- 140.Gray M. Does oral zinc supplementation promote healing of chronic wounds? J Wound Ostomy Continence Nurs 2003;30:295–9 [DOI] [PubMed] [Google Scholar]
- 141.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2002;75:1062–71 [DOI] [PubMed] [Google Scholar]
- 142.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 2006;23:1050–6 [DOI] [PubMed] [Google Scholar]
- 143.Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart 2005;91:618–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jee SH, Miller ER, III, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens 2002;15:691–6 [DOI] [PubMed] [Google Scholar]
- 145.Mizushima S, Cappuccio FP, Nichols R, Elliott P. Dietary magnesium intake and blood pressure: a qualitative overview of the observational studies. J Hum Hypertens 1998;12:447–53 [DOI] [PubMed] [Google Scholar]
- 146.Pittler MH, Stevinson C, Ernst E. Chromium picolinate for reducing body weight: meta-analysis of randomized trials. Int J Obes 2003;27:522–9 [DOI] [PubMed] [Google Scholar]
- 147.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol 2003;94:651–9 [DOI] [PubMed] [Google Scholar]
- 148.Althuis MD, Jordan NE, Ludington EA, Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr 2002;76:148–55 [DOI] [PubMed] [Google Scholar]
- 149.Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther 2007;81:685–91 [DOI] [PubMed] [Google Scholar]
- 150.Stephen AI, Avenell A. A systematic review of multivitamin and multimineral supplementation for infection. J Hum Nutr Diet 2006;19:179–90 [DOI] [PubMed] [Google Scholar]
- 151.Huang HY, Caballero B, Chang S, et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med 2006;145:372–85 [DOI] [PubMed] [Google Scholar]
- 152.Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis. J Obstet Gynaecol Can 2006;28:680–9 [DOI] [PubMed] [Google Scholar]
- 153.El-Kadiki A, Sutton AJ. Role of multivitamins and mineral supplements in preventing infections in elderly people: systematic review and meta-analysis of randomised controlled trials. BMJ 2005;330:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Whelton PK, He J, Cutler JA, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997;277:1624–32 [DOI] [PubMed] [Google Scholar]
- 155.Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens 1991;9:465–73 [DOI] [PubMed] [Google Scholar]
- 156.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256–61 [DOI] [PubMed] [Google Scholar]
- 157.Brousson MA, Klein MC. Controversies surrounding the administration of vitamin K to newborns: a review. Can Med Assoc J 1996;154:307–15 [PMC free article] [PubMed] [Google Scholar]
- 158.Ismail AI, Bandekar RR. Fluoride supplements and fluorosis: a meta-analysis. Community Dent Oral Epidemiol 1999;27:48–56 [DOI] [PubMed] [Google Scholar]
- 159.Kotwal A, Priya R, Qadeer I. Goiter and other iodine deficiency disorders: a systematic review of epidemiological studies to deconstruct the complex web. Arch Med Res 2007;38:1–14 [DOI] [PubMed] [Google Scholar]
- 160.Clar C, Wu T, Liu G, Li P. Iodized salt for iodine deficiency disorders: a systematic review. Endocrinol Metab Clin North Am 2002;31:681–98 [DOI] [PubMed] [Google Scholar]
- 161.Gonzalez-Reyes RE, Gutierrez-Alvarez AM, Moreno CB. Manganese and epilepsy: a systematic review of the literature. Brain Res Rev 2007;53:332–6 [DOI] [PubMed] [Google Scholar]
- 162.Myers VH, Champagne CM. Nutritional effects on blood pressure. Curr Opin Lipidol 2007;18:20–4 [DOI] [PubMed] [Google Scholar]
- 163.Dennehy CE. The use of herbs and dietary supplements in gynecology: an evidence-based review. J Midwifery Womens Health 2006;51:402–9 [DOI] [PubMed] [Google Scholar]
- 164.Trumbo PR, Ellwood KC. Chromium picolinate intake and risk of type 2 diabetes: an evidence-based review by the United States Food and Drug Administration. Nutr Rev 2006;64:357–63 [DOI] [PubMed] [Google Scholar]
- 165.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr 2006;26:1–16 [DOI] [PubMed] [Google Scholar]
- 166.Rios J, Passe MM. Evidenced-based use of botanicals, minerals, and vitamins in the prophylactic treatment of migraines. J Am Acad Nurse Pract 2004;16:251–6 [DOI] [PubMed] [Google Scholar]
- 167.Zeegers MP, Kellen E, Buntinx F, van den Brandt PA. The association between smoking, beverage consumption, diet and bladder cancer: a systematic literature review. World J Urol 2004;21:392–401 [DOI] [PubMed] [Google Scholar]
- 168.Dickey RA, Janick JJ. Lifestyle modifications in the prevention and treatment of hypertension. Endocr Pract 2001;7:392–9 [DOI] [PubMed] [Google Scholar]
- 169.Kraemer K, Koch W, Hoppe PP. Is all-rac-alpha-tocopherol different from RRR-alpha-tocopherol regarding cardiovascular efficacy? A meta-analysis of clinical trials. Ann N Y Acad Sci 2004;1031:435–8 [DOI] [PubMed] [Google Scholar]
- 170.The Vitamin A and Pneumonia Working Group Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality.. Bull World Health Organ 1995;73:609–19 [PMC free article] [PubMed] [Google Scholar]
- 171.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 172.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408–12 [DOI] [PubMed] [Google Scholar]
- 173.Marantz PR, Bird ED, Alderman MH. A call for higher standards of evidence for dietary guidelines. Am J Prev Med 2008;34:234–40 [DOI] [PubMed] [Google Scholar]
- 174.Whitlock EP, Lin JS, Chou R, Shekelle P, Robinson KA. Using existing systematic reviews in complex systematic reviews. Ann Intern Med 2008;148:776–82 [DOI] [PubMed] [Google Scholar]
- 175.McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228–31 [DOI] [PubMed] [Google Scholar]
- 176.Dickersin K, Rennie D. Registering clinical trials. JAMA 2003;290:516–23 [DOI] [PubMed] [Google Scholar]
- 177.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol 2005;58:894–901 [DOI] [PubMed] [Google Scholar]
- 179.Pham B, Platt R, McAuley L, Klassen TP, Moher D. Is there a “best” way to detect and minimize publication bias? An empirical evaluation. Eval Health Prof 2001;24:109–25 [DOI] [PubMed] [Google Scholar]
- 180.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Clin Oral Investig 2003;7:2–7 [DOI] [PubMed] [Google Scholar]
- 181.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med 2006;144:364–7 [DOI] [PubMed] [Google Scholar]
- 182.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7 [DOI] [PubMed] [Google Scholar]
- 183.Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB, Doig CJ. A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit Care 2005;9:R575–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bucher HC, Guyatt GH, Cook RJ, et al. Effect of calcium supplementation on pregnancy-induced hypertension and preeclampsia: a meta-analysis of randomized controlled trials. JAMA 1996;275:1113–7 (Published erratum appears in JAMA 1996;276:1388.) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.