Abstract
Background: Overweight children have greater circulating concentrations of markers of inflammation (MOI) than do lean children. Whether adiposity influences the postprandial MOI response is unknown.
Objective: We aimed to evaluate the relations of fasting and postprandial MOI with total and regional adiposity and insulin sensitivity in children.
Design: Fifty-nine children aged 7–12 y were assessed for C-reactive protein (CRP), interleukin-6 (IL-6), and soluble tumor necrosis factor receptor-2 (sTNF-R2) in the fasted state and after a mixed meal. Insulin sensitivity, body composition, and abdominal adipose tissue distribution were assessed with a frequently sampled intravenous-glucose-tolerance test, dual-energy X-ray absorptiometry, and computed tomography, respectively.
Results: Central adipose measures were not independently associated with fasting MOI, although they were independently inversely associated with the postprandial sTNF-R2 response (r = −0.30 to −0.37, P = 0.02–0.006). The inverse association between intraabdominal adipose tissue and the postprandial CRP response was nearly significant (r = −0.27, P = 0.05). Insulin sensitivity was not associated with fasting or postprandial CRP or sTNF-R2; however, there was a positive relation between insulin sensitivity and fasting IL-6 (r = 0.27, P = 0.03), which was attenuated after adjustment for lean body mass (r = 0.25, P = 0.08).
Conclusions: Excess adiposity is associated with both fasting and postprandial MOI. The postprandial MOI response may be influenced by central adiposity in children. The positive association of insulin sensitivity with IL-6 warrants further study.
INTRODUCTION
Increasing rates of obesity in the pediatric population are likely to result in premature chronic disease, such as coronary heart disease and type 2 diabetes mellitus (1, 2). Factors underlying the association between adiposity and chronic disease deserve attention. Excess adiposity is strongly associated with elevated concentrations of circulating markers of inflammation (MOI), such as C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and TNF receptors (3–6). Importantly, systemic inflammation may underlie the development of obesity-related chronic diseases (7, 8). Central adiposity, in particular, has been shown to be strongly associated with both systemic inflammation and chronic disease risk in adults (9–12). However, the relation between inflammation and central adiposity has not been thoroughly defined in children. CRP was positively associated with visceral adipose tissue in a group of overweight and obese prepubertal children, although it is not known whether this relation was independent of total body fat (13).
Low-grade systemic inflammation may be a causal factor in the development of insulin resistance (14). Few have reported on the association between MOI and directly measured insulin sensitivity in children, and results from these studies suggest no association (15, 16); however, the confounding effects of body composition were not taken into account. Robust techniques to measure insulin sensitivity and body composition are required to determine the true relation between MOI and insulin sensitivity in children.
Postprandial metabolism may influence disease risk (17). The concentrations of circulating inflammatory cytokines and CRP have been shown to change in response to a meal in healthy (18–23) and overweight (24) adults. However, published data have been inconsistent regarding the direction of the specific postprandial MOI responses (ie, increase, decrease, or no change). Findings by Manning et al (25) suggest that obesity augments the postprandial MOI response. Alternatively, the postprandial MOI response may depend on the extent of the subjects' insulin resistance (24). The associations of postprandial MOI with adiposity and insulin sensitivity have not been investigated in a pediatric population.
The aim of this study was to investigate relations of fasting and postprandial MOI with adiposity and insulin sensitivity in children aged 7–12 y by using robust measures of total and regional fat distribution and insulin sensitivity. Specifically, we hypothesized the following: 1) overweight children have greater fasting MOI concentrations and a greater postprandial MOI response than do lean children; 2) central body fat is positively associated with fasting and postprandial MOI, independent of total body fat; and 3) insulin sensitivity is inversely associated with fasting and postprandial MOI.
SUBJECTS AND METHODS
Subjects
Subjects were 59 healthy children aged 7–12 y (≈64% boys and 63% African Americans) recruited as part of a larger cross-sectional study and agreed to undergo a mixed-meal tolerance test (MMTT), which was not required for the parent study. All subjects were in pubertal stage 1–3, as determined by a pediatrician according to the method of Marshall and Tanner (26, 27). Participants were recruited from January 2005 to September 2005 via newspaper advertisements, postings at local churches and community centers, flyers sent home from school, and word-of-mouth. Exclusion criteria included a current diagnosis of type 1 or type 2 diabetes, genetic disorders known to affect body composition or fat distribution, glucose or lipid metabolism disturbances, and/or use of medications known to affect body composition or physical activity. This study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board for Human Use. Consent and assent were obtained from a parent and the subject, respectively, before the study began.
Protocol
All data were collected during 2 overnight visits, which were no more than 30 d apart. At the first visit, height and weight were measured, a physical examination was conducted, and a computed tomography (CT) scan was performed at the UAB Hospital. After a 12-h overnight fast, a frequently sampled intravenous-glucose-tolerance test (FSIGT) was administered at the General Clinical Research Center (GCRC) to assess insulin secretion and insulin sensitivity (28). For the second visit, the subjects reported back to the GCRC, where they fasted overnight for 12 h and then underwent an MMTT. This was followed by a dual-energy X-ray absorptiometry (DXA) scan in the Metabolism Core Laboratory of the UAB Clinical Nutrition Research Center.
Frequently sampled intravenous-glucose-tolerance test
To administer the FSIGT, flexible intravenous catheters were placed in the antecubital vein of both arms. Two fasting blood samples were drawn over a 15-min period for the measurement of basal concentrations of insulin, glucose, and MOI. An intravenous infusion of dextrose [300 mg/kg body weight using a 25% (weight per volume) solution] was given at time zero. Twenty minutes after dextrose administration, insulin (0.02 U/kg body wt) was infused over a 5-min period. A total of 26 blood samples were drawn, including the 2 fasting/baseline samples and 24 samples up to 240 min. Sera were stored at −85°C until analyzed. Glucose and insulin values over the 240-min FSIGT were entered into the millennium version of the Bergman minimal model (Minmod millennium 5.18, 2000; University of Southern California, Los Angeles, CA) for determination of the insulin sensitivity index (SI) and the acute insulin response to glucose (AIRg).
Mixed-meal tolerance test
An MMTT was used to assess MOI and insulin concentrations after consumption of a meal containing carbohydrate, fat, and protein. To perform the test, a single flexible intravenous catheter was placed in the antecubital vein of the left arm. Two blood samples taken over a 15-min period were used to determine fasting/baseline concentrations of insulin, glucose, and MOI. A standard mixed meal (one can of Ensure enteral supplement; Ross Laboratories, Abbott Park, IL) was given to the subjects at time zero. This meal consisted of 250 kcal, 6 g fat, 40 g carbohydrate, and 9 g protein. The children were required to consume the meal within 5 min. Twelve additional blood samples were drawn up to 180 min after ingestion for a total of 14 blood samples. Sera were stored at −85° until analyzed.
Measurement of glucose and insulin
Glucose was measured in 10 μL sera with a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX). The mean intraassay CV for this analysis was 1.2%, and the mean interassay CV was 1.9%. Insulin was assayed by using a double-antibody radioimmunoassay with 100-μL serum aliquots in duplicate (Linco Research Inc, St. Charles, MO). The mean intra- and interassay CVs for the insulin assay were 3.7% and 6.5%, respectively, and the mean assay sensitivity was 3.35 μIU/mL. The insulin response to the MMTT was defined as the incremental insulin area under the curve (AUC), which was calculated by using the trapezoidal method (29, 30).
Measurement of inflammation markers
Concentrations of serum CRP, IL-6, and soluble tumor necrosis factor receptor type 2 (sTNF-R2) were measured in pooled sera from 2 fasting blood samples from the FSIGT (used for analyses of the fasted state) and blood samples taken at baseline and at 60, 120, and 180 min of the MMTT (used for postprandial analyses). High- sensitivity enzyme-linked immunosorbent assays were used to determine CRP (ALPCO Diagnostics, Windham, NH) and IL-6 concentrations (R&D Systems, Minneapolis, MN). The mean inter- and intraassay CVs for CRP and IL-6 were 10.2%, 10.0%, 15.9%, and 10.1%, respectively. An enzyme-amplified sensitivity immunoassay (EASIA) was used to determine sTNF-R2 concentrations (BioSource Europe, Nivelles, Belgium). The mean inter- and intraassay CVs for TNF-R2 were 8.7% and 6.5%, respectively. Samples were not available for all MMTT time points for every subject. Therefore, the postprandial MOI response was expressed as the incremental AUC per minute where appropriate in statistical analyses. The AUC was calculated by using the trapezoidal method (30).
Determination of body composition and fat distribution
Total body fat, percentage body fat, and lean body mass were determined by DXA with a Lunar Prodigy densitometer (software version 6.10.029; GE-Lunar Corp, Madison, WI). Subjects were scanned in the supine position with their hands placed at their sides. Total abdominal adipose tissue, intraabdominal adipose tissue (IAAT), and subcutaneous abdominal adipose tissue were determined via CT with a GE HiLight/Advantage scanner (General Electric, Milwaukee, WI). Subjects were scanned in the supine position with their arms stretched above the head. A 5-mm single slice scan was taken at the level of the umbilicus, and a cross-sectional area analysis of adipose tissue (cm2) was performed by using a density contour computer program (31).
Statistical analyses
Subjects were dichotomized by percentage body fat according to sex-specific cutoffs suggested by Williams et al (32). Girls with <30% body fat and boys with <25% body fat were classified as lean; children exceeding these cutoffs were classified as overweight. Descriptive statistics (mean ± SD) for all variables of interest were determined for each percentage fat group. Comparisons between groups for continuous variables were performed by using the 2-group t test or analysis of covariance, and comparisons for categorical data were performed by using the 2-group chi-square test or Fisher's exact test as necessary. Two-factor mixed-model repeated-measures analysis of variance was performed to evaluate changes in circulating MOI after the mixed meal. Pearson and partial correlations were performed to examine associations among continuous variables. The MOI response was expressed as the incremental AUC per minute in all postprandial correlation analyses. Ethnic differences in insulin sensitivity and secretion have been reported (33). Thus, all analyses involving SI and AIRg included ethnicity as a covariate. Continuous variables that deviated from a normal distribution (MOI, total body fat, lean body mass, total abdominal adipose tissue, IAAT, subcutaneous abdominal adipose tissue, fasting insulin, and AIRg) were log-transformed before statistical analyses. All statistical analyses were 2-sided with a 0.05 significance level and were performed by using SAS (version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Descriptive characteristics and outcome measures are presented in Table 1 by percentage fat status. Compared with lean children, overweight children had a significantly higher fasting insulin (P = 0.002, 2-group t test) and a lower SI (P = 0.03, analysis of covariance). Overweight children also had higher fasting CRP (P < 0.001), IL-6 (P = 0.005), and sTNF-R2 (P = 0.02) concentrations than did lean children, as determined by 2-group t tests.
TABLE 1.
Descriptive statistics and metabolic outcomes by percentage body fat status1
| Variable | Lean (n = 37) | Overweight (n = 22) | P |
| Male [n (%)]2 | 23 (62) | 15 (68) | 0.64 |
| African American [n (%)]2 | 25 (68) | 12 (55) | 0.32 |
| Tanner stage 1/2/3 (n)3 | 25/7/2 | 11/7/3 | 0.31 |
| Age (y) | 9.8 ± 1.64 | 10.1 ± 1.4 | 0.49 |
| Height (cm) | 139.9 ± 9.6 | 144.4 ± 10.3 | 0.10 |
| Weight (kg) | 33.1 ± 5.7 | 48.4 ± 13.7 | <0.001 |
| BMI percentile | 46.3 ± 27.1 | 87.5 ± 14.1 | <0.001 |
| BMI (kg/m2) | 16.8 ± 1.7 | 22.8 ± 4.1 | <0.001 |
| Total body fat (kg) | 5.6 ± 2.3 | 17.8 ± 7.7 | <0.001 |
| Lean body mass (kg) | 25.4 ± 4.5 | 28.3 ± 6.6 | 0.07 |
| Percentage body fat (%) | 17.2 ± 6.2 | 36.2 ± 7.3 | <0.001 |
| Fasting glucose (mg/dL) | 91.8 ± 6.3 [31] | 93.9 ± 7.6 [18] | 0.31 |
| Fasting insulin (μIU/mL) | 87.7 ± 27.0 [36] | 118.6 ± 38.1 [19] | 0.002 |
| SI [× 10−4min−1/(μIU/mL)]5 | 4.8 ± 2.7 [36] | 3.4 ± 0.4 [19] | 0.03 |
| AIRg5 | 701.2 ± 444.5 [33] | 1002.8 ± 624.8 [19] | 0.04 |
| TAAT (cm2) | 77.4 ± 36.6 [36] | 223.2 ± 91.4 [22] | <0.001 |
| IAAT (cm2) | 26.5 ± 13.3 [36] | 50.3 ± 20.1 [22] | <0.001 |
| SAAT (cm2) | 50.9 ± 26.2 [36] | 172.9 ± 75.9 [22] | <0.001 |
| Fasting CRP (mg/L) | 0.17 ± 0.21 [34] | 1.10 ± 1.11 [19] | <0.001 |
| Fasting IL-6 (pg/mL) | 0.76 ± 0.52 [36] | 1.42 ± 1.22 [21] | 0.005 |
| Fasting sTNF-R2 (ng/mL) | 4.21 ± 0.66 [34] | 4.80 ± 1.03 [21] | 0.02 |
n values in brackets. Girls with ≥30% body fat and boys with ≥25% body fat were classified as overweight. SI, insulin sensitivity index; AIRg, acute insulin response to glucose; TAAT, total abdominal adipose tissue; IAAT, intraabdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; CRP, C-reactive protein; IL-6, interleukin-6; sTNF-R2, soluble tumor necrosis factor receptor type 2. Differences between groups were determined by using a 2-group t test. Statistical analyses were performed on log-transformed data for total body fat, lean body mass, fasting insulin, AIRg, TAAT, IAAT, SAAT, fasting CRP, fasting IL-6, and fasting sTNF-R2.
Differences between groups were determined by using the 2-group chi-square test.
Differences between groups were determined by using Fisher's exact test.
Mean ± SD (all such values).
Reported as least-squares means ± SDs. Differences between groups were determined by using ANCOVA, with race as a covariate.
Postprandial response
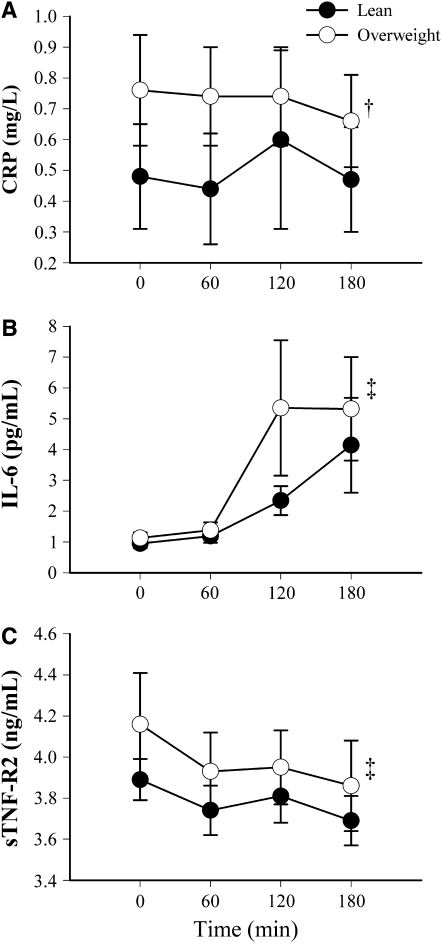
To evaluate changes in postprandial circulating MOI within and between the percentage fat groups, 2-factor mixed-model repeated-measures analysis of variance was performed. CRP concentrations did not change significantly in either group (Figure 1A), and overweight children consistently had significantly higher postprandial CRP concentrations than did lean children (P = 0.003). In both lean and overweight children, IL-6 concentrations increased after the meal (P < 0.001; Figure 1B), and there was no difference in postprandial IL-6 between the groups. Postprandial sTNF-R2 concentrations decreased significantly in both groups (P < 0.001; Figure 1C) and were not significantly different between the groups. Time-by-group interaction terms were not statistically significant for any MOI. The incremental insulin AUC did not differ between the percentage fat groups (37,536.6 ± 19331.8 compared with 40,130.8 ± 17,380.6 for lean and overweight groups, respectively; P = 0.61, 2-group t test)
FIGURE 1.
Mean (±SEM) postprandial markers of inflammation (MOI) by time and percentage fat status. Two-factor mixed-model repeated-measures analysis of variance was used for the statistical analysis. (A) C-reactive protein (CRP): P for time = 0.98, P for group = 0.003, P for time × group = 0.93; sample sizes for overweight children were 20, 20, 20, and 18 for time points 0, 60, 120, and 180 min, respectively; sample sizes for lean children were 36, 35, 33, and 36 for time points 0, 60, 120, and 180 min, respectively. (B) Interleukin-6 (IL-6): P for time < 0.001, P for group = 0.15, P for time × group = 0.95; sample sizes for overweight children were 22, 22, 19, and 20 for time points 0, 60, 120, and 180 min, respectively; sample sizes for lean children were 36, 36, 31, and 35 for time points 0, 60, 120, and 180 min, respectively. (C) Soluble tumor necrosis factor receptor type 2 (sTNF-R2): P for time < 0.001, P for group = 0.11, P for time × group = 0.88; sample sizes for overweight children were 21, 21, 20, and 18 for time points 0, 60, 120, and 180 min, respectively; sample sizes for lean children were 36, 35, 31, and 34 for time points 0, 60, 120, and 180 min, respectively. Analyses were performed on log-transformed data for each MOI time point. †Significant group effect (P = 0.003). ‡Significant time effect (P < 0.001).
Fasting and postprandial MOI compared with total and regional adiposity
Pearson correlation analyses showed that fasting MOI were significantly associated with most indexes of adiposity (Table 2). None of the fasting MOI were significantly associated with lean body mass. Adjustment for total body fat showed no significant relations between central fat deposition and fasting MOI (Table 3). All measures of adiposity were inversely associated with the incremental AUC for CRP (r = −0.36–0.50, P < 0.01 for all), such that the greater the adiposity, the smaller the postprandial CRP response (Table 2). Likewise, there was a trend toward a significant inverse association between IAAT and postprandial sTNF-R2 (r = −0.26, P = 0.05). None of the measures of adiposity were significantly associated with postprandial IL-6. The relations of all central adipose measures with postprandial sTNF-R2 became statistically significant (r = −0.30 to −0.37, P = 0.02–0.006) after adjustment for total body fat. A trend toward a significant inverse relation appeared between postprandial CRP and IAAT after adjustment for total body fat (r = −0.27, P = 0.05).
TABLE 2.
Pearson correlations (r) of fasting and postprandial markers of inflammation (MOI) with total and regional adiposity1
| Fasting MOI |
Postprandial MOI |
|||||
| Adipose measure | CRP | IL-6 | sTNF-R2 | CRP | IL-6 | sTNF-R2 |
| BMI2 | 0.513 | 0.21 | 0.294 | −0.365 | 0.19 | –0.005 |
| BMI percentile2 | 0.523 | 0.21 | 0.284 | −0.473 | 0.21 | 0.03 |
| Total body fat2 | 0.673 | 0.274 | 0.425 | −0.453 | 0.20 | –0.01 |
| Lean body mass2 | 0.02 | −0.18 | 0.04 | −0.12 | 0.10 | 0.08 |
| Percentage body fat2 | 0.713 | 0.375 | 0.443 | −0.473 | 0.18 | –0.03 |
| TAAT6 | 0.643 | 0.324 | 0.425 | −0.503 | 0.13 | –0.15 |
| IAAT6 | 0.593 | 0.267 | 0.385 | −0.493 | 0.01 | –0.267 |
| SAAT6 | 0.633 | 0.324 | 0.415 | −0.473 | 0.15 | –0.12 |
CRP, C-reactive protein; IL-6, interleukin-6; sTNF-R2, soluble tumor necrosis factor receptor type 2; TAAT, total abdominal adipose tissue; IAAT, intraabdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue. Statistical analyses were performed on log-transformed data for fasting CRP, fasting IL-6, fasting sTNF-R2, total body fat, lean body mass, TAAT, IAAT, and SAAT.
n = 53, 57, and 55 for fasting CRP, IL-6, and sTNF-R2, respectively; n = 53, 53, and 58 for postprandial CRP, IL-6, and sTNF-R2, respectively.
P < 0.001.
P < 0.05.
P < 0.01.
n = 52, 59, and 54 for fasting CRP, IL-6, and sTNF-R2, respectively; n = 52, 52, and 57 for postprandial CRP, IL-6, and sTNF-R2, respectively.
Trend toward statistical significance (P < 0.10).
TABLE 3.
Partial correlations (r) of fasting and postprandial markers of inflammation (MOI) with regional adiposity, adjusted for total body fat1
| Fasting MOI2 |
Postprandial MOI3 |
|||||
| Adipose measure | CRP | IL-6 | sTNF-R2 | CRP | IL-6 | sTNF-R2 |
| TAAT | 0.10 | 0.16 | 0.09 | −0.23 | −0.15 | −0.364 |
| IAAT | 0.19 | 0.08 | 0.11 | −0.275 | −0.20 | −0.374 |
| SAAT | 0.03 | 0.17 | 0.08 | −0.14 | −0.10 | −0.306 |
CRP, C-reactive protein; IL-6, interleukin-6; sTNF-R2, soluble tumor necrosis factor receptor type 2; TAAT, total abdominal adipose tissue; IAAT, intraabdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue. Statistical analyses were performed on log-transformed data for fasting CRP, fasting IL-6, fasting sTNF-R2, TAAT, IAAT, and SAAT.
n = 52, 56, and 54 for CRP, IL-6, and sTNF-R2, respectively.
n = 52, 52, and 57 for CRP, IL-6, and sTNF-R2, respectively.
P < 0.01.
P = 0.05.
P < 0.05.
Fasting and postprandial MOI compared with insulin sensitivity
Partial correlations, adjusted for ethnicity and total body fat, showed a significant positive association between fasting IL-6 and SI (r = 0.30, P = 0.03; Table 4). Further adjustment for lean body mass attenuated the association to nonsignificance (r = 0.25, P = 0.08). Fasting circulating MOI concentrations were not significantly associated with fasting insulin or AIRg, and there were no significant associations between the postprandial MOI response and SI or postprandial incremental insulin AUC (data not shown).
TABLE 4.
Partial correlations (r) of the insulin sensitivity index with fasting markers of inflammation (MOI), adjusted for 1) ethnicity and total body fat and 2) ethnicity, total body fat, and lean body mass1
| CRP (n = 51) |
IL-6 (n = 54) |
sTNF-R2 (n = 52) |
||||
| Partial variables | r | P | r | P | r | P |
| Ethnicity, total body fat | 0.01 | 0.93 | 0.30 | 0.03 | 0.06 | 0.69 |
| Ethnicity, total body fat, lean body mass | −0.05 | 0.74 | 0.25 | 0.08 | 0.03 | 0.84 |
CRP, C-reactive protein; IL-6, interleukin-6; sTNF-R2, soluble tumor necrosis factor receptor type 2. Statistical analyses were performed on log-transformed data for CRP, IL-6, sTNF-R2, total body fat, and lean body mass.
DISCUSSION
We sought to examine the relations between MOI, total and regional adiposity, and insulin sensitivity in lean and overweight children in both the fasted and postprandial states using robust measures of body composition, fat distribution, and insulin sensitivity. Although measures of central adiposity were not independently associated with MOI in the fasted state, both total body and central adiposity were found to be associated with the postprandial MOI response. In addition, there was an unexpected positive relation between insulin sensitivity and fasting IL-6.
Overweight children (5, 34, 35) and adults (36, 37) have been shown to have higher concentrations of fasting MOI than normal-weight individuals. Likewise, we found fasting CRP, IL-6, and sTNF-R2 to be higher in overweight than in lean children. The assumed mechanism is that adipose tissue secretes various MOI, including IL-6, TNF-α, and TNF receptors (38), and IL-6, in turn, induces the hepatic synthesis of CRP (39).
To our knowledge, the postprandial inflammatory response has not previously been examined in healthy children. Consistent with previous reports in adults (21–25), IL-6 increased after the meal in both lean and overweight children. Although obesity status influenced the postprandial IL-6 response among adult females (25), we found no influence of obesity in our subjects. Because CRP and IL-6 are mechanistically linked (39), one would expect their postprandial changes to be similar. In our subjects, however, CRP did not change significantly in either percentage fat group. Dfferent postprandial responses of CRP and IL-6 were also found in healthy, lean men (23). The observed disassociation between the IL-6 and CRP postprandial responses suggests that regulation of CRP is multifactorial and that CRP synthesis/secretion is not tightly coupled to acute change in IL-6. Indeed, CRP concentrations do not appear to be influenced by diurnal variations in IL-6 (40).
Contrary to the IL-6 response, sTNF-R2 decreased after the mixed meal in both the lean and overweight children. Postprandial decreases in the TNF system have been observed in adults (23, 24, 41), although this has not been a consistent finding (20–22, 25). It has been hypothesized that TNF-α concentrations decrease after a meal to augment insulin-mediated nutrient uptake (24, 41). Because sTNF-R2 may reflect the autocrine/paracrine actions of TNF-α (42), perhaps the decrease in sTNF-R2 we observed reflects a compensatory decrease in TNF-α activity. Alternatively, binding of TNF-α to circulating sTNF-R2 may inhibit the actions of TNF-α (43), which include the promotion of lipolysis and inhibition of lipogenesis (44). Hence, it may be that the decrease in sTNF-R2 enables greater TNF-α activity.
Although central adiposity has been found to be associated with circulating MOI in adults and adolescents (9–11, 45), our results in children showed that central adipose deposition was not independently associated with fasting MOI (Table 3). This observation confirmed the findings of Maffeis et al (13). In contrast, we found a previously unreported independent association between central adiposity and postprandial CRP and sTNF-R2 responses. Unlike previous findings in adults (25), we observed no association with postprandial IL-6. Our findings suggest that, in children, whereas total adiposity influences fasting MOI, central fat deposition influences postprandial CRP and sTNF-R2 responses. It is likely that there are different physiologic processes mediating the relations of adiposity with fasting MOI and with postprandial MOI responses. Alternatively, it is possible that the early metabolic effects of central obesity are more readily visualized in the postprandial state, and, with age and further accumulation of fat in the abdominal area, metabolic perturbations become apparent in the fasted state.
Despite the hypothesis that chronic inflammation can induce insulin resistance (14), we showed using robust measures that insulin sensitivity was not inversely associated with fasting or postprandial MOI in children. In contrast with our findings, Blackburn et al (24) found a significant positive association between postprandial insulin AUC and postprandial TNF-α in abdominally obese men. Adiposity, however, was not addressed as a potential confounder. Our data in children suggest that the postprandial MOI response is linked to adiposity rather than to insulin sensitivity.
In our population, fasting IL-6 was positively associated with insulin sensitivity in a manner that was dependent on lean body mass (Table 4). Skeletal muscle contraction produces IL-6, which is associated with postexercise peripheral glucose uptake (46). In addition, insulin-mediated glucose uptake is enhanced by acute IL-6 infusion (47). Thus, IL-6 may have a protective, rather than a detrimental, influence on insulin sensitivity. Additional research is necessary to fully understand IL-6 and its physiologic effects.
The strengths of this study included robust measures of insulin sensitivity, body composition, and fat distribution and the use of healthy children. A limitation of the study was the unequal distribution of lean and overweight children, which may have limited statistical power in subgroup analyses, as well as the unequal distribution of African American and white children, which inhibited our ability to investigate potential ethnic influences in our findings. The cross-sectional design of the study did not allow for cause and effect relations to be determined; thus, experimental studies are required to probe potential cause and effect relations. In addition, results reflected the response to only one type of meal and to a dose that was uniform across subjects, regardless of body mass or composition; use of a meal with a different composition (21) or a mass-specific dose may yield different results.
Our findings suggest that, even in children, excess adiposity influences both fasting and postprandial MOI, which may have implications for disease risk. Because of the association of inflammation with risk of chronic disease, research is needed on the effects of weight loss (35) and diet modification (21, 48) on the reduction of inflammation in overweight children. Further research is needed to determine whether the observed positive association between IL-6 and insulin sensitivity is mediated by skeletal muscle and whether skeletal muscle–derived IL-6 is beneficial to carbohydrate metabolism in children.
Acknowledgments
We gratefully acknowledge Maryellen Williams and Cindy Zeng for laboratory support, Robert Petri for technical assistance, and the GCRC staff and AMERICO team for their invaluable assistance.
The authors' responsibilities were as follows—JAA, PBH, JRF, and BAG: study design and development of hypotheses; JAA, PBH, and BED: collection of data; JAA and RAO: statistical analyses; JAA and BAG: interpretation of results; JAA, PBH, and BAG: drafting of the manuscript; PBH, RAO, JRF, and BAG: critical review of manuscript; and BED: editorial review and proofreading of manuscript. All authors reviewed and approved the final manuscript. None of the authors had any conflicts of interest to disclose.
REFERENCES
- 1.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002;288:1728–32 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 3.Cook DG, Mendall MA, Whincup PH, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis 2000;149:139–50 [DOI] [PubMed] [Google Scholar]
- 4.Moon YS, Kim DH, Song DK. Serum tumor necrosis factor-α levels and components of the metabolic syndrome in obese adolescents. Metabolism 2004;53:863–7 [DOI] [PubMed] [Google Scholar]
- 5.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics 2001;107:E13. [DOI] [PubMed] [Google Scholar]
- 6.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972–8 [DOI] [PubMed] [Google Scholar]
- 7.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 2003;52:1799–805 [DOI] [PubMed] [Google Scholar]
- 8.de Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Pathol 2007;16:14–21 [DOI] [PubMed] [Google Scholar]
- 9.Festa A, D'Agostino R, Jr, Williams K, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 2001;25:1407–15 [DOI] [PubMed] [Google Scholar]
- 10.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract 2005;69:29–35 [DOI] [PubMed] [Google Scholar]
- 11.Tsigos C, Kyrou I, Chala E, et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism 1999;48:1332–5 [DOI] [PubMed] [Google Scholar]
- 12.Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990;10:497–511 [DOI] [PubMed] [Google Scholar]
- 13.Maffeis C, Manfredi R, Trombetta M, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab 2008;93:2122–8 [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–80 [DOI] [PubMed] [Google Scholar]
- 15.Maffeis C, Silvagni D, Bonadonna R, Grezzani A, Banzato C, Tatò L. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr 2007;151:647–52 [DOI] [PubMed] [Google Scholar]
- 16.Moran A, Steffen LM, Jacobs DR, Jr, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care 2005;28:1763–8 [DOI] [PubMed] [Google Scholar]
- 17.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr 2005;93:3–9 [DOI] [PubMed] [Google Scholar]
- 18.Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor κB and decrease in inhibitor κB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr 2004;79:682–90 [DOI] [PubMed] [Google Scholar]
- 19.Greenfield JR, Samaras K, Hayward CS, Chisholm DJ, Campbell LV. Beneficial postprandial effect of a small amount of alcohol on diabetes and cardiovascular risk factors: modification by insulin resistance. J Clin Endocrinol Metab 2005;90:661–72 [DOI] [PubMed] [Google Scholar]
- 20.Hansen K, Sickelmann F, Pietrowsky R, Fehm HL, Born J. Systemic immune changes following meal intake in humans. Am J Physiol 1997;273:R548–53 [DOI] [PubMed] [Google Scholar]
- 21.Nappo F, Esposito K, Cioffi M, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 2002;39:1145–50 [DOI] [PubMed] [Google Scholar]
- 22.Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and β-adrenergic stimulation on adipose-derived hormones and cytokines in man. J Clin Endocrinol Metab 1999;84:2126–33 [DOI] [PubMed] [Google Scholar]
- 23.Poppitt SD, Keogh GF, Lithander FE, et al. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-α, and C-reactive protein to a high-fat dietary load. Nutrition 2008;24:322–9 [DOI] [PubMed] [Google Scholar]
- 24.Blackburn P, Després JP, Lamarche B, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006;14:1747–54 [DOI] [PubMed] [Google Scholar]
- 25.Manning PJ, Sutherland WHF, McGrath MM, de Jong SA, Walker RJ, Williams MJA. Postprandial cytokine concentrations and meal composition in obese and lean women. Obesity 2008;16:2046–52 [DOI] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve: methodological aspects. Diabetes Care 1990;13:172–5 [DOI] [PubMed] [Google Scholar]
- 30.Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kekes-Szabo T, Hunter GR, Nyikos I, Nicholson C, Snyder S, Berland L. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res 1994;2:450–7 [DOI] [PubMed] [Google Scholar]
- 32.Williams DP, Going SB, Lohman TG, et al. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health 1992;82:358–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab 2002;87:2218–24 [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Ten S, Anhalt H. Serum levels of soluble tumor necrosis factor-α receptor 2 are linked to insulin resistance and glucose intolerance in children. J Pediatr Endocrinol Metab 2005;18:75–82 [DOI] [PubMed] [Google Scholar]
- 35.Reinehr T, Stoffel-Wagner B, Roth CL, Andler W. High-sensitive C-reactive protein, tumor necrosis factor α, and cardiovascular risk factors before and after weight loss in obese children. Metabolism 2005;54:1155–61 [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–5 [DOI] [PubMed] [Google Scholar]
- 37.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr 2004;28:410–5 [DOI] [PubMed] [Google Scholar]
- 38.Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 2006;14(suppl 5):242S–9S [DOI] [PubMed] [Google Scholar]
- 39.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem 2004;279:48487–90 [DOI] [PubMed] [Google Scholar]
- 40.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem 2001;47:426–30 [PubMed] [Google Scholar]
- 41.Manning PJ, Sutherland WH, Hendry G, de Jong SA, McGrath M, Williams SM. Changes in circulating postprandial proinflammatory cytokine concentrations in diet-controlled type 2 diabetes and the effect of ingested fat. Diabetes Care 2004;27:2509–11 [DOI] [PubMed] [Google Scholar]
- 42.Schröder J, Stüber F, Gallati H, Schade FU, Kremer B. Pattern of soluble TNF receptors I and II in sepsis. Infection 1995;23:143–8 [DOI] [PubMed] [Google Scholar]
- 43.Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor α in vitro and in vivo. Proc Natl Acad Sci USA 1992;89:4845–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warne JP. Tumour necrosis factor α: a key regulator of adipose tissue mass. J Endocrinol 2003;177:351–5 [DOI] [PubMed] [Google Scholar]
- 45.Barbeau P, Litaker MS, Woods KF, et al. Hemostatic and inflammatory markers in obese youths: effects of exercise and adiposity. J Pediatr 2002;141:415–20 [DOI] [PubMed] [Google Scholar]
- 46.Pedersen BK, Steensberg A, Keller P, et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch 2003;446:9–16 [DOI] [PubMed] [Google Scholar]
- 47.Carey AL, Steinberg GR, Macaulay SL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006;55:2688–97 [DOI] [PubMed] [Google Scholar]
- 48.Katcher HI, Legro RS, Kunselman AR, et al. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 2008;87:79–90 [DOI] [PubMed] [Google Scholar]