Abstract
Background
The homeodomain transcription factors Engrailed-1 and Engrailed-2 are required for the survival of mesencephalic dopaminergic (mesDA) neurons in a cell-autonomous and gene-dose-dependent manner. Homozygote mutant mice, deficient of both genes (En1-/-;En2-/-), die at birth and exhibit a loss of all mesDA neurons by mid-gestation. In heterozygote animals (En1+/-;En2-/-), which are viable and fertile, postnatal maintenance of the nigrostriatal dopaminergic system is afflicted, leading to a progressive degeneration specific to this subpopulation and Parkinson's disease-like molecular and behavioral deficits.
Results
In this work, we show that the dose of Engrailed is inversely correlated to the expression level of the pan-neurotrophin receptor gene P75NTR (Ngfr). Loss of mesDA neurons in the Engrailed-null mutant embryos is caused by elevated expression of this neurotrophin receptor: Unusually, in this case, the cell death signal of P75NTR is mediated by suppression of Erk1/2 (extracellular-signal-regulated kinase 1/2) activity. The reduction in expression of Engrailed, possibly related to the higher levels of P75NTR, also decreases mitochondrial stability. In particular, the dose of Engrailed determines the sensitivity to cell death induced by the classic Parkinson-model toxin MPTP and to inhibition of the anti-apoptotic members of the Bcl-2 family of proteins.
Conclusion
Our study links the survival function of the Engrailed genes in developing mesDA neurons to the regulation of P75NTR and the sensitivity of these neurons to mitochondrial insult. The similarities to the disease etiology in combination with the nigral phenotype of En1+/-;En2-/- mice suggests that haplotype variations in the Engrailed genes and/or P75NTR that alter their expression levels could, in part, determine susceptibility to Parkinson's disease.
Background
Mesencephalic dopaminergic (mesDA) neurons are the main source of dopamine in the mammalian central nervous system. They are located in three distinct nuclei, substantia nigra pars compacta, ventral tegmentum and retrorubral field. Their main innervation targets are the basal ganglia, where they play key roles in the control of emotion, motivation and motor behavior, documented by their connection to schizophrenia, addiction and, most prominently, to Parkinson's disease (PD) [1,2]. The main characteristic of PD is the slow progressive loss of dopaminergic neurons in the substantia nigra pars compacta, causing diminished release of dopamine in the caudate putamen and debilitating motor deficits. Although the molecular causes for the selective vulnerability of this neuronal population are hardly understood, multiple lines of evidence suggest mitochondrial dysfunction as a major contributing factor [3] and apoptosis as the executioner of cell death [4]. Several mutations associated with familial forms of PD encode mitochondrial proteins [5] and neurotoxins specific to the nigrostriatal system – MPTP (1-methyl-4-phenyl-,1,3,6-tetrahydropyridine), 6-hydroxydopamine and rotenone – cause mitochondrial damage as inhibitors of complex-I of the electron transport chain [6]. Mitochondrial insult can cause oxidative stress by production of reactive oxygen species, leading to increased permeability of the mitochondrial membrane, release of pro-apoptotic molecules, including cytochrome-C, into the cytoplasm and, subsequently, to activation of caspases and induction of apoptosis [7].
Neuronal cell death can be a result of neurotrophin deficiency. Action of the neurotrophins, consisting of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)4/5 and NT3, is mediated via a set of specific tyrosine kinase (Trk) receptors (TrkA, B, C) and a common receptor, P75NTR (Ngfr). While the Trk receptors signal survival, P75NTR can relay a survival or cell death signal, depending on the cellular context and the molecular form of the ligand [8]. The pro-survival function of neurotrophins and their receptors has been mainly attributed to the downstream effect of phosphotidyl inositol-3 kinase (PI3K) and the extracellular-signal-regulated kinase 1/2 (Erk1/2) pathways [9], whereas cell death signaling via P75NTR is mediated by phosphorylation of c-Jun N-terminal kinase (JNK) and BH3-only members of the Bcl-2 family [10].
The homeodomain transcription factors Engrailed-1 (En1) and Engrailed-2 (En2) are required for the survival and maintenance of mesDA neurons in a cell-autonomous and gene-dose-dependent manner, demonstrated by in vitro cell mixing experiments, RNA interference (RNAi) and analysis of chimeric mice [11-13]. In Engrailed double mutant mice (En1-/-;En2-/-; from here on EnDM), the mesDA neurons are generated and begin to express their neurotransmitter phenotype but then die by apoptosis between embryonic day (E)12 and E14, the stage when Engrailed expression starts in their wild-type counterparts [11,12]. The intermediate genotypes between EnDM and wild type show various degrees of cell loss in the mesDA system. Most interestingly, mice heterozygous null for En1 and homozygous null for En2 (En1+/-;En2-/-; from here on EnHT), which are viable and fertile, exhibit a slow progressive loss of nigral dopaminergic neurons within the first two months after birth, resulting in diminished storage and release of dopamine in the striatum and in PD-like motor deficiencies [14].
We show here that Engrailed-deficiency in mesDA neurons leads to elevated P75NTR expression that is causal for cell death if the neurons are null for En1 and En2. The death signal is mediated by the suppression of Erk1/2 activity. Probably linked to the P75NTR elevation, the dose of the Engrailed genes also determines the sensitivity to mitochondrial insult.
Materials and methods
Animals
The generation of the En2 null mutant and the En1tau-LacZ 'knock-in' mice have been previously described [15,16]. The En2 mutants with an original mixed genetic background of 129 and Swiss Webster were crossed three times into a C57/BL6 background. The line was bred as En1+/tlZ;En2-/- at the central animal facility, University of Heidelberg.
Quantitative RT-PCR
Quantitative RT-PCR reactions were performed according to the manufacture in a 7000 Sequence Detection System from Applied Biosystems (Foster City, CA, USA) using pre-formulated 'assays on-demand' and calculating the results with the comparative cycle time (CT) method. The pre-formulated 'assays on demand' had the following identification tag: Mm00446294_m1 for Ngfr (P75NTR); as standard control Mm00507222_s1 ribosomal protein S18; Mm00435617_m1 for phosphoglycerate kinase 1 (Pgk1); and Mm00446973_m1 for TATA box binding protein (Tbp). The dissected ventral midbrains were homogenized, the RNA isolated and reverse transcribed using random hexamers to initiate transcription. Each of the individual PCR reactions was done in triplicate and at least two of three standard controls were run in parallel. Each of the experimental sets consisted of RNA from different pools of mutant and littermate control as well as RNA from Tet-On induced Engrailed expressing N2A cells and control.
Cell culture
All primary cell cultures were performed using E12.5 mouse embryos. EnDM embryos were distinguished from the other genotypes in the litter by their midbrain/hindbrain morphology [11], which was occasionally verified by PCR. Distinction between En2-/- and En1+/tlZ;En2-/- embryos was achieved by incubation of the limb buds in X-Gal solution at 37°C (40 mg/ml X-Gal (Sigma-Aldrich; Munich, Germany) in 5 mM K3Fe(CN), 5 mM K4Fe(CN)6x3H2O, 1 mM MgCl2 in phosphate-buffered saline). For the cell culture, the neural tubes were dissected and ventral midbrains were isolated. The tissue was then dissociated using trypsine (Invitrogen; Karlsruhe, Germany). The preparation of laminin (Sigma) coated coverslips was described elsewhere [11]. The medium was DMEM-F12 supplemented with 5% fetal calf serum, 0.25% bovine serum albumin (Sigma), 33 mM glucose, 50 U/ml penicillin, 50 U/ml streptomycin, and 1% Fungizone (Invitrogen). The cells were seeded at approximately 150,000 per cover slip and incubated at 37°C. After 36 hours, EnDM mutant cells were fixed in 4% paraformaldehyde and processed for immunostaining. The typical numbers of tyrosine hydroxylase (TH)-positive cells per cover slip were between 100 and 300 cells if wild-type or heterozygote mutant tissue was dissociated. The numbers of TH-positive neurons was always significantly lower if the dissociated ventral midbrains were derived from E12 EnDM embryos. To obtain comparable numbers for mutant and wild-type experiments, all cell counts were normalized against each of the controls. Numbers presented in the results section (n) refer to the number of experiments. Each of the experimental conditions is represented by at least three cover slips in each experiment. The optimum concentration for the toxic substances was determined by titration and checking for an intermediate rate of survival (between 30% and 60%) in cultures of mixed genotypes (En2-/- and En1+/-;En2-/-). For induction of cell death, serum was withdrawn from the medium after 48 hours and the cultures were treated with the compounds HA14-1, chelerythrine chloride (Axxora, San Diego, CA)), prima-1, Apoptosis Activator-2, tumor necrosis factor (TNF)α and 1-methyl-4-phenylpyridinium (MPP+). Used concentrations, solvents, durations of treatment and vendor sources are provided in Additional file 1[17-33]. The number 'n' corresponds to the number of individual experiments conducted.
Design and transfection of siRNA oligos
The design of the 21-mer RNAi oligonucleotides was carried out at Biomers.net (Ulm, Germany) and in accordance with the protocol by Ebashir et al. [34]. Both RNA duplexes targeted the coding sequence of P75NTR (A, sense ACAGAACACAGUGUGUGAA(dTdT) and anti-sense UUCACACACUGUGUUCUGU(dTdT); B, sense CAUUCCGACCGCUGAUGUUCU(dTdT) and anti-sense AACAUCAGCGGUCGGAAUGUG(dTdT)). For coupling with Penetratin-One (QBiogene; Strasbourg, France), the sense strand was modified with a thiol group on the 5' end. Tris-2-carboxyethylphosphine (TCEP; 1 μl; Pierce; Bonn, Germany) was added to 224 μl of the small interfering RNA (siRNA; stock solution, 900 μM) and incubated for 15 minutes at room temperature. Then, 25 μl of Penetratin-1 was added, mixed and incubated for 5 minutes at 65°C following by 1 hour at 37°C. Penetratin-1 solution was reconstituted to 2 mg/ml (≈0.8 mM) in sterile water. A stock solution of 20 mM TCEP in sterile RNAse/DNAse free water was made. The aliquots were frozen at -80°C. The Penetratin-coupled siRNA oligos were heated to 65°C for 15 minutes and 3 μl of the mix was dissolved in complete growth medium. As controls, we used Penetratin-1 coupled double-stranded RNA oligos directed against Maged1 (Nrage) (UAACUUGAAUGUGGAAGAG(dTdT) and CUCUUCCACAUUCAAGUUA(dTdT)) and the randomly generated Scramble I Duplex (Dpharmacon; Heidelberg, Germany) sense CAGTCGCGTTTGCGACTGG and antisense CCAGTCGCAAACGCGACTG. Both had no effect on the survival rate of control or EnDM mesDA neurons. Alternatively, uncoupled double-stranded RNA oligos with the same sequences were transfected using HiPerfect (Qiagen; Hilden, Germany) in accordance with the manufacturer's protocol.
Immunohistochemistry and western blot analysis
The immunohistochemistry and western blot analysis was done according to the protocol described elsewhere [35]. All the phospho-specific antibodies were purchased from Cell Signaling Technology, the neurotrophin and TH antibodies from Chemicon (Molsheim, France) and the antibodies for neurotrophin receptors from Santa Cruz Biotechnology (Heidelberg, Germany).
Statistical analysis
Values are expressed as mean ± standard error. Differences between means were analyzed by using a paired, two-tailed Student t-test. All shown p-values are rounded up at the third or fourth digit.
Results
Elevated P75NTR expression in absence of Engrailed genes
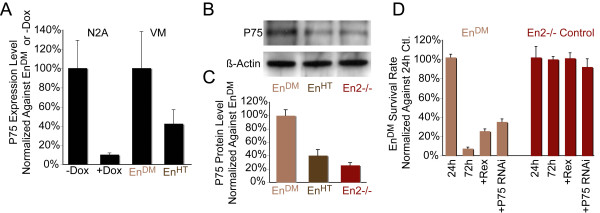
A genome-wide expression analysis, using microarrays on En1 inducible N2A cell line, identified the NGF receptor P75NTR as downstream of the Engrailed transcription factors (data not shown). To confirm the microarray, we examined by quantitative RT-PCR the levels of P75NTR in the cell line as well as in ventral midbrain tissue in relationship to En1 expression. As a result of En1 induction in N2A cells, the endogenous expression of P75NTR decreased by 10-fold (9.9 ± 1.9%, p < 0.001, n = 8). Likewise, the ventral midbrain tissue, derived from control littermates (En2-/-) expressed almost 2.5-fold less P75NTR (42% ± 14.0%, p < 0.001, n = 6) than EnDM mutants (Figure 1A). The western blot analysis confirmed the latter results; ventral midbrain from En2-/- and EnHT embryos contained 74.4 ± 4.4% (p < 0.001, n = 3) and 60.2 ± 8.8% (p = 0.002, n = 3) less P75NTR protein, respectively, than the same tissue from EnDM littermates (Figure 1B,C).
Figure 1.
Elevated P75NTR expression is causal for cell death. (A) Quantitative RT-PCR of ventral midbrain tissue (VM) derived from EnDM (En1-/-;En2-/-) and EnHT E12 embryos, and of En1-expressing N2A cells inducible by doxycycline (Dox). P75NTR expression is inversely correlated with En1 expression levels in tissues and cell lines. (B, C) Western blot analysis of the ventral midbrain tissue shows the same relationship between P75NTR protein levels and En1 expression. Each active En1 allele decreases the P75NTR expression level (n = 3, p = 0.002). (D) Ventral midbrain cultures derived from EnDM and En2-/- embryos. Silencing of P75NTR by double-stranded RNA oligos and application of P75NTR-inhibiting antibody (Rex) increases the survival rate of EnDM mesDA neurons compared to untreated control (Ctl) or after treatment with scrambled RNA oligos (n ≥ 6, p < 0.01). Error bars indicate standard error.
Since P75NTR can mediate cell death in neurons [8], we began to investigate whether its elevated expression is causal for the death of mesDA neurons in EnDM embryos. In order to functionally interfere with P75NTR, we applied an activity-blocking antibody (Rex) [28] to primary ventral midbrain cell cultures. This antibody increased the survival rate from 7.5 ± 1.24% to 34.8 ± 4.6% (p < 0.001, n = 6; Figure 1D). Furthermore, to lower P75NTR expression levels in the mutant neurons, we applied specific Penetratin-coupled siRNA duplexes [36]; 72 hours after transfection, the total P75NTR protein was reduced by 83.2 ± 6.3% (p = 0.05, n = 3; western blot not shown) and the survival rate increased from 7.5 ± 1.24% to 25.1 ± 2.1% (p < 0.001 n = 16) (Figure 1D). These data suggested that elevated expression of P75NTR is the direct cause of the induction of apoptosis in Engrailed-deficient mesDA neurons.
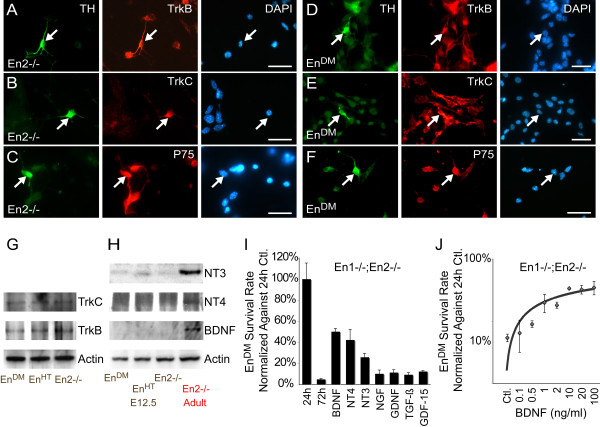
P75NTR mediates dual, opposing functions of cell survival and death, controlled by the presence or absence of neurotrophins. For the anti-apoptotic function, neurotrophins require their cognate Trk receptors as heterodimerization partners for P75NTR [8]. In order to assess a potential role of the Trk/P75NTR system during the course of cell loss, we determined the expression of the Trk-receptors in E12 mesDA neurons. TrkC and TrkB, but not TrkA, were detectable by immunohistochemistry and western blot at equal levels in wild type and EnDM mutants (Figure 2A–G).
Figure 2.
Loss of Engrailed induces neurotrophin requirement in mesDA neurons. (A-F) Double immunohistochemistry on dissociated cells derived from En2-/- (A-C) and En1-/-;En2-/- (EnDM) (D-F) E12 ventral midbrain using antibodies against tyrosine kinase (Trk)B (A, D), TrkC (B, E), P75NTR(C, F) and TH (green) counterstained with DAPI. TrkB, TrkC and P75NTR are expressed by TH+ cells from both genotypes; however, the immunohistochemistry is not sensitive enough to detect differences in P75NTR expression between genotypes. (G, H) Western blot of ventral midbrain tissue derived from different Engrailed genotypes. The two Trk receptors do not depend on Engrailed expression (G). Brain-derived neurotrophic factor (BDNF), neurotrophin (NT)4 and NT3 are not expressed in E12 ventral midbrain tissue, but they are in the adult (H). (I) Treatments (>10 ng/ml) for 72 hours with TrkB/C-specific neurotrophins – BDNF, NT4 and NT3 – greatly increases the survival rate of EnDM mesDA neurons (n ≥ 6; p < 0.001), whereas nerve growth factor (NGF), glial cell line-derived neurotrophic factor (GDNF), transforming growth factor (TGF)-β and growth differentiation factor (GDF)-15 do not significantly alter survival rate. (J) Dose response curve: BDNF concentration plotted against survival rate showing saturation at approximately the 10 ng/ml. Scale bars: 25 μm. Error bars indicate standard error. Ctl, control.
The up-regulation of P75NTR and the presence of Trk receptors suggested that Engrailed deficiency introduces a neurotrophin requirement to the E12 mesDA neurons that cannot be satisfied at this age, since the neurotrophins specific to TrkB and TrkC – that is, BDNF, NT4 and NT3 – are not expressed in the E12 ventral midbrain as they are in the adult (Figure 2H). To test this hypothesis, we applied saturating concentrations of BDNF, NT4 and NT3 to ventral midbrain cultures. After 72 hours, 50.2 ± 2.9% (p < 0.0001, n = 27), 42.3 ± 10.1% (p < 0.001, n = 9) and 26.0 ± 3.5% (p < 0.001, n = 9), respectively, of the otherwise dying EnDM mesDA neurons were still present in the cultures (Figure 2I). The addition of BDNF to the control littermate cultures demonstrated that this was due to an elevated survival rate and not attributable to a higher rate of precursor cell proliferation (Figure 3L). As expected from the lack of TrkA, application of its ligand, NGF, did not change the survival rate significantly. To test the specificity of BNDF, NT3 and NT4, we applied glial cell line-derived neurotrophic factor (GDNF), growth differentiation factor (GDF)-15 and transforming growth factor (TGF)-β to the mutant cultures, all known survival factors for mesDA neurons [37-39]. Similar to NGF, none of them prevented the death of the Engrailed-deficient mesDA neurons (Figure 2I). Furthermore, the linear dose-response trend-line of the survival effect of BDNF (Figure 2J) indicated a Kd value of approximately 2.5 nM, which corresponds with the reported affinity of BDNF for the TrkB/P75NTRcomplex [40].
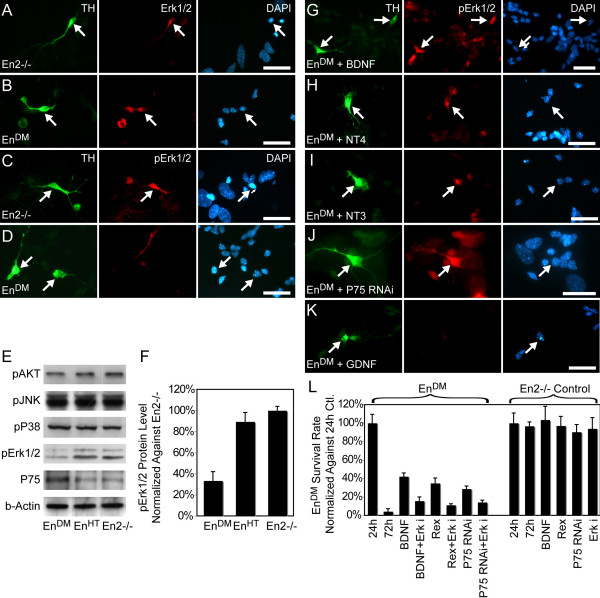
Figure 3.
Differential activation of Erk1/2 in mesDA neurons. (A-D, G-K) Immunohistochemistry of E12 ventral midbrain cell culture stained against TH (green), total Erk1/2 protein (red) (A, B) and phosphorylated Erk1/2 (red) (C-D, G-K). (A-D) While Erk1/2 protein is present in mesDA neurons of both genotypes (A, B), it is only phosphorylated in En2-/- mesDA neurons (C) and not in the En1-/-;En2-/- (EnDM) counterparts (D). (E-I) Erk1/2 becomes activated in EnDM mesDA neurons after treatment with the survival-inducing neurotrophins, brain-derived neurotrophic factor (BDNF), neurotrophin (NT)4 and NT3, or after silencing of P75NTR (RNA interference (RNAi)) (G-J), but not when glial cell line-derived neurotrophic factor (GDNF) is applied (I). (E) Western blot of E12 ventral midbrain tissue confirms the immunohistochemical finding of differential phosphorylation between genotypes and shows that neither AKT, part of the phosphotidyl inositol-3 kinase pathway, nor other mitogen-activated protein kinases, such as JNK and P38, are differentially activated. (F) Quantification of phosphorylated Erk1/2 in western blot normalized against En2-/- tissue. (L) Number of TH-positive cells in EnDM and En2-/- ventral midbrain cultures after 72 hours, treated with the 400 nM Mek inhibitor U0126 in conjunction with BDNF, Penetratin-coupled P75NTR double-stranded RNA oligonucleotides and the P75NTR inhibiting antibody (Rex). Numbers are normalized against untreated cultures at 24 hours. The rescue effect is significantly reduced when the EnDM cultures are treated with the Erk1/2 inhibitor. Scale bars: 25 μm. Error bars indicate standard error. Ctl, control.
The survival-mediating role of Erk1/2
The survival function of neurotrophins has been mainly attributed to Erk1/2 and PI3K pathways [41]. As the next step, we examined whether these pathways play a role in arbitrating the effect of the neurotrophins on Engrailed-deficient mesDA neurons and whether they intersect with the molecular events regulated by Engrailed expression. Therefore, we studied activation of the two pathways, using antibodies against the phosphorylated forms of Erk1/2 and AKT [41]. Immunohistochemistry showed that while total Erk1/2 protein was present in both En2-/- and EnDM mesDA neurons, it was activated only in the wild-type-like cells and not in EnDM mesDA neurons (Figure 3A–D). Western blot analysis of ventral midbrain tissue showed a similar effect; Erk1/2 activity in E12 EnDM ventral midbrain was reduced by 66.8 ± 18.2% (p = 0.02, n = 3) in comparison to the same tissue derived from their En2-/- littermates (Figure 3E,F). In contrast to Erk1/2, we did not detect differential activation of AKT in the western blot (Figure 3E). Additionally, we could not detect signs of differential activation of other components of the mitogen-activated protein kinase (MAPK) pathways, such as JNK or P38 (Figure 3E).
The loss of Erk1/2 phosphorylation suggested that this MAPK pathway was differentially activated in EnDM mesDA neurons in response to a survival or death signal. To investigate this hypothesis, we examined the state of activation of Erk1/2 after application of the survival factors. The addition of BDNF, NT4 and NT3, and the knock-down of P75NTR by siRNA oligonucleotides all caused phosphorylation of Erk1/2 (Figure 3G–J); however, MAPK was not activated after application of NGF, GDNF, TGF-β or GDF-15 (for example, see Figure 3K). If the loss of Erk1/2 activity is the primary cause of cell death, when P75NTR is elevated in mesDA neurons, and this correlation is not accidental, then inhibition of the Erk1/2 pathway should interfere with the rescue by the neurotrophins and by P75NTR inhibition or silencing. To test this, we concurrently treated the cultures with U0126 [42], an inhibitor of the MAPK kinase upstream of Erk1/2, MEK1/2 [32], at a concentration (400 nM) not toxic to the wild-type (En2-/-) neurons. The inhibition of Erk1/2 by U0126 significantly reduced the rescue effect of all three survival factors; from 42.2 ± 3.1% to 11.7 ± 3.5% (p = 0.002) for BDNF, from 34.7 ± 4.7% to 8.3 ± 2.7% (p < 0.0001) for Rex and from 25.4 ± 2.7% to 10.8 ± 1.9% (p = 0.009) after P75NTR silencing (control mutant 4.2 ± 2.2%, p = 0.01, n = 4 for all experiments) (Figure 3L).
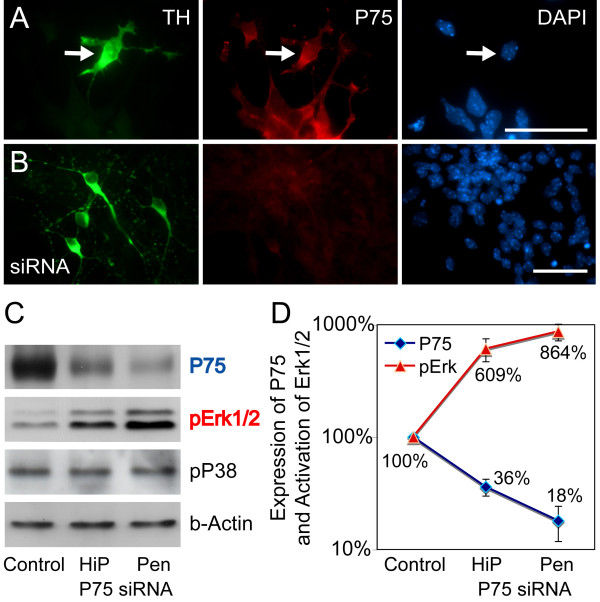
To elaborate further on the correlation between the expression of P75NTR and the state of phosphorylation of Erk1/2, we silenced the P75NTR expression in EnHT ventral midbrain cultures by RNAi, using two methods with different transfection efficiencies (the lipophilic transfection reagent HiPerfect, and Penetratin-coupled oligos). The former reduced P75NTR expression levels, on average, by 63.3 ± 2.0% (p = 0.011, n = 3) and the latter by 82.2 ± 6.3% (p = 0.05, n = 3). This, in turn, caused 6.09 ± 1.40-fold (p = 0.01, n = 3) and 8.64 ± 1.45-fold (p = 0.008, n = 3) increases, respectively, in the phosphorylation of Erk1/2 (Figure 4A–D).
Figure 4.
Erk1/2 activation is inversely correlated with P75NTR expression. (A, B) Immunohistochemistry on E12 dissociated ventral midbrain cultures derived from EnHT E12 embryos stained against TH (green) and P75NTR. P75NTR expression is absent in cultures treated with Penetratin-coupled double-stranded RNA oligos. (C) Western blot of ventral midbrain cultures using two methods of RNA transfection with different efficiencies: HiPerfect (HiP) and Penetratin-coupled RNA oligos show increase in phosphorylated Erk1/2 after silencing of P75NTR, but no changes in phosphorylation of P38. (D) Quantification of P75NTR expression and Erk1/2 phosphorylation after transfection with P75NTR double-stranded RNA oligonucleotides, normalized against untreated EnHT cultures, showing the inverse correlation between the two parameters. Scale bars: 25 μm. Error bars indicate standard error.
Sensitivity to mitochondrial dysfunction and Engrailed expression level
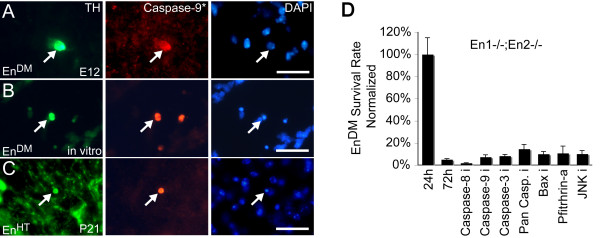
P75NTR signaling often mediates cell death via induction of the mitochondrial (intrinsic) pathway of apoptosis [43,44]. To further assess whether the elevated level of P75NTR expression in the Engrailed-deficient mesDA neurons is causal for demise of the cells, we investigated the dying neurons for signs of this pathway. We had previously reported that loss of Engrailed expression in mesDA neurons causes activation of caspase-3 [11,14], an effector caspase, triggered by the intrinsic or extrinsic pathways of apoptosis [45,46]. The intrinsic death pathway involves release of cytochrome C from the mitochondria, which participates in formation of apoptosome, which is required for activation of caspase-9. We detected small, rounded TH-positive cells with active caspase-9, and pyknotic, fragmented nuclei in the ventral midbrain of Engrailed-deficient E13 embryos as well as in EnHT mice during the postnatal stages of nigral cell loss (postnatal day 20; Figure 5A–C) confirming our hypothesis that the pathway downstream of P75NTR signaling must be the cause of the demise of these neurons in the absence of the Engrailed genes [43].
Figure 5.
Mitochondrial apoptosis in Engrailed-deficient mesDA neurons. (A-C) TH and activated caspase-9 immunohistochemistry on ventral midbrain coronal sections of E13 En1-/-;En2-/- (EnDM) embryos (A), of En1+/-;En2-/- (EnHT) postnatal day 20 brain (C), and of EnDM E12 cell culture (B) counterstained with the nuclear marker DAPI. Dying TH-positive neurons (arrows) exhibit small rounded cell bodies and signs of apoptosis, that is, activated caspase-9 and pyknotic nuclei (DAPI). (D) EnDM ventral midbrain cultures treated for 72 hours with inhibitors for caspases-3, -8 and -9, a pan-caspase inhibitor, z-vad-fmk, the BAX inhibitor Ku70, the P53 inhibitor Pfithrin-α, and the JNK inhibitor SP600125. None of the treatments significantly changed the survival rate of EnDM mesDA neurons. The number of surviving TH-positive cells was normalized in each case against untreated cultures 24 hours after dissociation. Scale bars: 25 μm. Error bars indicate standard error.
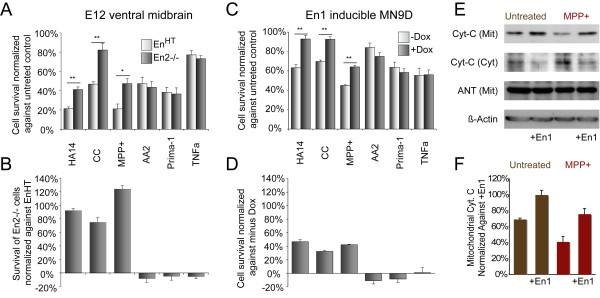
P75NTR expression in tumor cells induces a dose-dependent increase of the pro-apoptotic members (Bad, Bax and Bak) and a decrease of the anti-apoptotic members (Bcl-2 and Bcl-XL) of the Bcl-2 family [47]. Since we were unable to directly measure the relative amounts of these proteins in mesDA neurons themselves, we followed up on this possibility by application of HA14-1 and chelerythrine chloride, inhibitors of Bcl-2 and Bcl-XL, respectively. HA14-1 was discovered as an inhibitor of Bcl-2 using a computer screening strategy based on its predicted structure [24]. Chelerythrine chloride was identified by a high-throughput screening of natural compounds [48]. Both are highly specific for their targets. If the levels of the two anti-apoptotic members of the Bcl-2 family of proteins are lowered, as suggested by these previous tumor cell-experiments [47], the sensitivity to HA14-1 and chelerythrine chloride should be higher when the level of Engrailed protein decreases. Twenty-four hours after treatment with HA14-1 and chelerythrine chloride, the rate of survival of mesDA neurons, derived from E12 En2-/- embryos, was, on average, 75.3 ± 6.8% and 92.7 ± 2.7% (p < 0.001, n = 7 for both compounds) higher, respectively, than their counterparts derived from EnHT littermates (Figure 6A,B), suggesting that reduced Engrailed and elevated P75NTR expression lowers the threshold at which the intrinsic pathway of apoptosis is triggered.
Figure 6.
The sensitivity to induction of the intrinsic pathway of apoptosis correlates with En1 expression. (A-D) Ventral midbrain cultures 24 hours after application of apoptosis-inducing compounds. Charts of EnHT (En1+/-;En2-/-) and En2-/- E12 cultures depicting the number of surviving TH-positive cells (A, B) and cultures of En1-inducible MN9D cells depicting cell survival measured by cell proliferation assay (C, D). Surviving cells were normalized against untreated EnHT cultures (A) or untreated non-induced MN9D cells (C), treated EnHT cultures (B) or treated non-induced MN9D cells (D). Higher Engrailed expression reduced the cell death rate after MPP+, HA14-1 and chelerythrine chloride (CC) treatment, whereas the rate of cell survival after application of the tumor necrosis factor alpha (TNFa), Prima-1 and Apoptosis Activator-2 (AA2) does not correlate with the level of En1 expression. Dox, doxycycline. (E) Western blot analysis of mitochondrial and cytoplasmic protein fractions of MN9D cells 72 hours after En1 induction and 24 hours after MPP+ treatment. (F) Proportion of cytochrome C (Cyt-C) in cytosol is lower in En1-expressing MN9D cells before and after MPP+ treatment. Scale bars: 25 μm. Error bars indicate standard error. 6 ≤ n ≤ 27, *p ≤ 0.001, **p ≤ 0.0001. Cyt, cytosolic; Mit, mitochondrial; ANT, adenine nucleotide transporter; Dox, doxycycline.
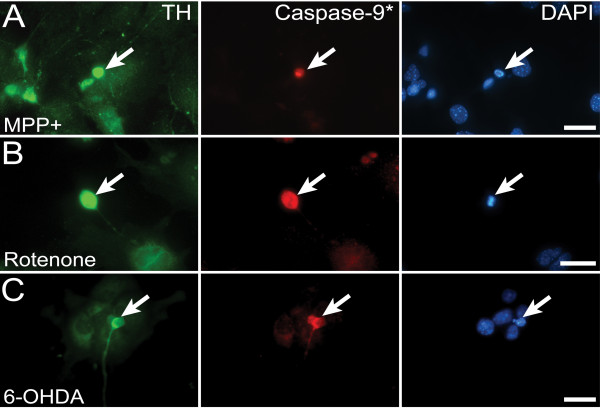
These results could indicate that the reduction in Engrailed expression increases the vulnerability of mesDA neurons to mitochondrial dysfunction in a general manner, since the three most commonly used reagents to model PD – MPP+, 6-Hydroxydopamine (6-OHDA) and rotenone [17,29,49,50] – also cause cell death by induction of this pathway of apoptosis, evident from activation of caspase-9 [51-53] (Figure 7A–C). To test this hypothesis directly, we treated EnHT mesDA neurons with MPP+, the metabolite of MPTP, an inhibitor of complex-I of the mitochondrial electron transport chain [49]. After 48 hours in culture, the rate of survival of En2-/- mesDA neurons was, on average, 124.5 ± 5.0% (p < 0.001, n = 12) higher than for their En1 heterozygote (EnHT) littermates, suggesting that MPTP and Engrailed may act upon the same molecular pathway upstream of caspase-9 (Figure 6A,B).
Figure 7.
Mitochondrial apoptosis after complex I inhibition. (A-C) Immunohistochemistry of E12 ventral midbrain cell cultures. Caspase-9 is activated in wild-type mesDA neurons (arrows), treated with MPP+, rotenone, or 6-OHDA.
To determine whether this sensitivity to cell death is specifically related to instability of the mitochondria, we also employed Apoptosis Activator-2 [19], Prima-1 [27], and TNFα [31]. The first induces cell death by triggering apoptosome formation, the second by activation of p53 and the third, as a ligand of the TNF receptors, induces the extrinsic, receptor-mediated pathway of apoptosis. In all three cases, the level of En1 expression did not have a significant influence on the survival rates of these neurons (-8.1 ± 6.0% (p = 0.67) for Apoptosis Activator-2, 4.6 ± 6.2% (p = 0.82) for Prima-1, and 5.1 ± 3.1% (p = 0.48) for TNFα; n = 7 for all three experiments; Figure 6A,B).
Gain of function in a mesDA cell line
To confirm the Engrailed dose-dependent sensitivity of mesDA neurons to mitochondrial insult, we performed a gain-of-function experiment using the inducible Tet-On system to express En1 in the dopaminergic cell line MN9D [54]. As in the primary cultures, En1 was protective against cell death if induced by administration of MPP+ (42.4 ± 0.7%, p < 0.0001, n = 23), chelerythrine chloride (46.7 ± 1.3%, p < 0.0001, n = 10) and HA14-1 (32.4 ± 1.3%, p < 0.0001, n = 10). Accordingly, survival rate was not significantly altered if the other three reagents were employed (-10.7 ± 4.7% (p = 0.26) for Apoptosis Activator-2, -8.3 ± 5.2% (p = 0.50) for Prima-1, and 1.0 ± 7.4% (p = 0.95) for TNFα; n = 12 for all experiments; Figure 6C,D).
Functional inhibition of Bcl-2 and Bcl-XL and application of MPP+ induces apoptosis by release of cytochrome C from the mitochondrial inter-membrane space into the cytosol [55,56]. The protective effect of En1 against HA14-1, chelerythrine chloride and MPP+ may be attributable to higher mitochondrial stability. To test this hypothesis, we compared the cytosolic and mitochondrial protein fractions of En1-expressing MN9D cells to non-expressing cells. The proportion of cytochrome C in the mitochondria was always significantly higher after induction of En1, either in the presence of MPP+ or in untreated control cultures (76.1 ± 7.3%, p = 0.03, n = 3, and 41.6 ± 7.0%, p = 0.005, n = 3, respectively), whereas the total amount of cellular cytochrome C was unaltered (Figure 6F,H). In contrast, the proportional levels in mitochondria and cytosol of the apoptosis inducing factor, which causes caspase-independent mitochondrial apoptosis [57], were independent of the level of En1 expression, suggesting that Engrailed participates in the regulation of mitochondrial stability via the cytochrome C/caspase-dependent pathway of apoptosis rather than the caspase-independent pathway, represented by apoptosis inducing factor.
Discussion
In this study, we provide evidence that cell death in Engrailed-deficient mesDA neurons is a result of higher P75NTR expression and the loss of Erk1/2 activity. Furthermore, we show that the dose of Engrailed is part of the molecular mechanism that determines the sensitivity of these neurons to mitochondrial insult.
P75NTR can cause apoptosis in various neuronal populations by mere high expression [10] or as a mediator of a (pro-)neurotrophin death signal [8]. Alternatively, since TrkB and C are expressed by the mesDA neurons, the abnormal increase in the expression of P75NTR could introduce a neurotrophin dependency, which does not occur in the wild type at this age. The latter is more likely in mesDA neurons deprived of the Engrailed genes, since the death signal could be counteracted by addition of neurotrophins (BDNF, NT3, NT4) [58]. Furthermore, the Kd calculated from the dose response of BDNF, corresponds to the known affinity of neurotrophins for P75NTR and Trk receptors [40], demonstrating that the survival effect of the neurotrophins is attributable to direct binding to the receptors on the surface of mesDA neurons, as opposed to a survival signal that originates from the surrounding cells, which would be reflected in a different shape of the dose response curve. A direct interaction of the neurotrophins with receptors on mesDA neurons is also consistent with our previous findings that the Engrailed genes are cell-autonomously required for the survival of these cells [11]. The ineffectiveness of Ngf can be readily explained by the lack of expression of TrkA. It is noteworthy that neither neurotrophins nor inhibition of P75NTR completely (that is, 100%) rescue mesDA neurons deprived of Engrailed genes, and it is thus possible that P75NTR is only one of many factors contributing to the death of mesDA neurons in the absence of the Engrailed genes. However, since the in vitro timeframe of loss of EnDM mesDA neurons is 72 hours, it is also possible that those cells, which died anyway, had already been committed to cell death before the knock-down of P75NTR by the RNA duplexes was sufficiently high or the neurotrophin effect set in.
Neurotrophin-induced Trk/P75NTR interaction, or lack thereof, can provoke intracellular activation of the MAPK and PI3K pathways. Among MAPKs, JNK and p38 participate in stress responses and often trigger apoptosis, while Erk1/2 signaling regulates cell proliferation, differentiation and survival [59]. Alternatively, the pro-survival role of neurotrophins can be mediated by PI3K signaling. This is in contrast to our findings. The activity of JNK, p38 or PI3K is independent of the Engrailed genes and the level of P75NTR expression in mesDA neurons. The lack of Erk1/2 activity in EnDM mesDA neurons, which is reversed under any of the rescue conditions, demonstrates that the level of Erk1/2 phosphorylation in mesDA neurons is correlated with Engrailed expression and inversely to the level of P75NTR, suggesting that the P75NTR death signal is mediated in these neurons by suppression of Erk1/2 activity. Although the association of P75NTR and the Erk proteins has been shown in PC12 cells [60], regulation of the activity of the Erk1/2 signaling pathway as a result of death signaling by P75NTR is a novel mechanism, which signifies both the role of sustained activity of Erk1/2 in the survival and/or of P75NTR in the demise of mesDA neurons. Our data also suggest that the survival effect of neurotrophins may be the result of dis-inhibition of a death signal (high expression of P75NTR) that is triggered in the absence of the proper transcriptional regulation (by the Engrailed genes) during the course of development or possibly throughout life.
The pro-apoptotic role of P75NTR has been established in the animal models of neurodegenerative disorders, other than PD, including beta-amyloid peptide-dependent cell death [61], or stress conditions, including ischemia [62]. Although function of the ligands and the co-receptors of P75NTR, TrkA/B/C, have been investigated in mesDA neurons [63-65], the role of P75NTR, itself, has not been reported in this system. Our work provides the first evidence for the pivotal role of P75NTR in mesDA neurons, as a negative mediating factor for the lifelong survival function of the Engrailed genes in this neuronal population.
The sensitivity to neurotoxin-induced cell death by MPTP and to inducers of mitochondrial instability, such as the Bcl-2 and Bcl-XL inhibitors, is inversely correlated with the dose of Engrailed expression in mesDA neurons. Mitochondrial dysfunction was first implicated in the pathogenesis of PD after accidental administration of MPTP by drug abusers and a consequent Parkinsonian syndrome. Lowered complex I activity and reduced mitochondrial stability [3] are believed to be key factors in the etiology of PD. In postmortem brains of PD patients, reduced activity of complex-I (NADH/ubiquinone oxidoreductase) of the mitochondrial electron transport chain [49] and elevated levels of the pro-apoptotic members of the Bcl-2 family have been observed in the substantia nigra [66]. Then, three genes, DJ1, PINK1 and OMI/HTRA2, mutations of which have been associated with familiar forms of PD, were discovered to have a function in the mitochondria [5]. Furthermore, the toxicity of MPTP is conferred by inhibition of complex I of the electron transport chain, triggering the release of cytochrome-C from the mitochondrial intermembrane space into the cytosol, which involves Bcl-2 family members [7]. Here, we demonstrate that the sensitivity to neurotoxin-induced cell death and to inducers of mitochondrial apoptosis, such as the Bcl-2 and Bcl-XL inhibitors, is inversely correlated to the dose of Engrailed expression in mesDA neurons. This, the previous findings of other groups, and the PD-like slow progressive loss of nigral dopaminergic neurons in EnHT mice [14] suggest that the mode of action of the Engrailed genes converges with MPTP toxicity and possibly also with the disease mechanism on the mitochondria. Intriguingly, a recent association study into PD indicated a single nucleotide polymorphism in the intron of En1 as a potential risk factor for sporadic forms of this disease [67].
Abbreviations
BDNF: brain-derived neurotrophic factor; E: embryonic day; En: Engrailed; EnDM: Engrailed double mutant mice (En1-/-;En2-/-); EnHT: heterozygous null for En1 and homozygous null for En2 (En1+/-;En2-/-); Erk: extracellular-signal-regulated kinase; GDF: growth differentiation factor; GDNF: glial cell line-derived neurotrophic factor; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; mesDA: mesencephalic dopaminergic; MPTP: 1-methyl-4-phenyl-,1,3,6-tetrahydropyridine; NGF: nerve growth factor; NT: neurotrophin; PD: Parkinson's disease; PI3K: phosphotidyl inositol-3 kinase; RNAi: RNA interference; siRNA: small interfering RNA; TGF: transforming growth factor; Trk: tropomyosin-receptor-kinase.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KNA and HHS conceived of, designed and discussed the studies, carried out the experiments, analyzed and interpreted the data and wrote the draft and the final version of the manuscript. PS and LA made the stable MN9D cell lines. SS helped with immunoassays. The manuscript was approved by all authors.
Supplementary Material
List of compounds and concentrations. Compounds employed for the cell culture experiments: Final concentrations, solvents, duration of application, activity, source and literature.
Acknowledgments
Acknowledgements
This work was supported by grants from the Federal Secretary for Education and Research BMBF Biofuture 98, the German Research Council SI 752/3-1 and the Michael J Fox Foundation. We thank Martyn Goulding for the En1/tauLacZ, Alex Joyner for the En2 mutant mice, Louis Reichardt for the Rex antibody, and Jutta Fey for technical assistance.
Contributor Information
Kambiz N Alavian, Email: kambiz.alavian@gmail.com.
Paola Sgadò, Email: paola.sgado@gmail.com.
Lavinia Alberi, Email: lalberi2@jhmi.edu.
Srinivasa Subramaniam, Email: ssubram9@jhmi.edu.
Horst H Simon, Email: hsimon@mac.com.
References
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003;53:S61–70. doi: 10.1002/ana.10489. discussion S70-62. [DOI] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K. TGF-beta and the regulation of neuron survival and death. J Physiol Paris. 2002;96:25–30. doi: 10.1016/S0928-4257(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci. 2003;23:11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131:3229–3236. doi: 10.1242/dev.01128. [DOI] [PubMed] [Google Scholar]
- Simon HH, Saueressig H, Wurst W, Goulding MD, O'Leary DD. Fate of midbrain dopaminergic neurons controlled by the Engrailed genes. J Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HH, Bhatt L, Gherbassi D, Sgado P, Alberi L. Midbrain dopaminergic neurons: determination of their developmental fate by transcription factors. Ann NY Acad Sci. 2003;991:36–47. [PubMed] [Google Scholar]
- Sgado P, Alberi L, Gherbassi D, Galasso SL, Ramakers GM, Alavian KN, Smidt MP, Dyck RH, Simon HH. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proc Natl Acad Sci USA. 2006;103:15242–15247. doi: 10.1073/pnas.0602116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Fink DW, Horn PT, Mirkin BL. Differential effects of 6-hydroxydopamine-induced ablation of peripheral and central adrenergic axons on C-1300 murine neuroblastoma tumor growth and catecholamine concentration in the A/J mouse. J Pharmacol Exp Ther. 1992;262:1070–1075. [PubMed] [Google Scholar]
- Mocanu MM, Baxter GF, Yellon DM. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol. 2000;130:197–200. doi: 10.1038/sj.bjp.0703336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci USA. 2003;100:7533–7538. doi: 10.1073/pnas.1031631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol. 2003;5:320–329. doi: 10.1038/ncb950. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Fadeel B, Hassan Z, Hellstrom-Lindberg E, Henter JI, Orrenius S, Zhivotovsky B. Cleavage of Bcl-2 is an early event in chemotherapy-induced apoptosis of human myeloid leukemia cells. Leukemia. 1999;13:719–728. doi: 10.1038/sj/leu/2401411. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Honda M, Takabatake T. Anti-apoptotic effect of atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, on cardiac myocytes through protein kinase C activation. Clin Exp Pharmacol Physiol. 2004;31:360–364. doi: 10.1111/j.1440-1681.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Langston EB, Forno LS. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neurosci Lett. 1984;48:87–92. doi: 10.1016/0304-3940(84)90293-3. [DOI] [PubMed] [Google Scholar]
- Hoagland MS, Hoagland EM, Swanson HI. The p53 inhibitor pifithrin-alpha is a potent agonist of the aryl hydrocarbon receptor. J Pharmacol Exp Ther. 2005;314:603–610. doi: 10.1124/jpet.105.084186. [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- Kruttgen A, Moller JC, Heymach JV, Jr, Shooter EM. Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci USA. 1998;95:9614–9619. doi: 10.1073/pnas.95.16.9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M. SP60 a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson's disease. Neurosci Res. 0125;48:195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Adams JM. Ways of dying/multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs FW, Olson RE. MEK inhibitors: the chemistry and biological activity of U its analogs, and cyclization products. Bioorg Med Chem Lett. 0126;8:2839–2844. doi: 10.1016/S0960-894X(98)00522-8. [DOI] [PubMed] [Google Scholar]
- Martin DA, Siegel RM, Zheng L, Lenardo MJ. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. J Biol Chem. 1998;273:4345–4349. doi: 10.1074/jbc.273.8.4345. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Thuret S, Bhatt L, O'Leary DD, Simon HH. Identification and developmental analysis of genes expressed by dopaminergic neurons of the substantia nigra pars compacta. Mol Cell Neurosci. 2004;25:394–405. doi: 10.1016/j.mcn.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelau J, Sullivan A, Bottner M, Lingor P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J, Krieglstein K, Unsicker K. Growth/Differentiation factor-15/Macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci. 2000;20:8597–8603. doi: 10.1523/JNEUROSCI.20-23-08597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M. A null mutation in TGF-alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nat Neurosci. 1998;1:374–377. doi: 10.1038/1584. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Wars) 2000;60:531–545. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Wang X, Bauer JH, Li Y, Shao Z, Zetoune FS, Cattaneo E, Vincenz C. Characterization of a p75(NTR) apoptotic signaling pathway using a novel cellular model. J Biol Chem. 2001;276:33812–33820. doi: 10.1074/jbc.M010548200. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Jürgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ, Salvesen GS. Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- Tabassum A, Khwaja F, Djakiew D. The p75(NTR) tumor suppressor induces caspase-mediated apoptosis in bladder tumor cells. Int J Cancer. 2003;105:47–52. doi: 10.1002/ijc.11038. [DOI] [PubMed] [Google Scholar]
- Chan SL, Lee MC, Tan KO, Yang LK, Lee AS, Flotow H, Fu NY, Butler MS, Soejarto DD, Buss AD, Yu VC. Identification of chelerythrine as an inhibitor of BclXL function. J Biol Chem. 2003;278:20453–20456. doi: 10.1074/jbc.C300138200. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease/mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins/an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Liang Q, Liou AK, Ding Y, Cao G, Xiao X, Perez RG, Chen J. 6-Hydroxydopamine induces dopaminergic cell degeneration via a caspase-9-mediated apoptotic pathway that is attenuated by caspase-9dn expression. J Neurosci Res. 2004;77:747–761. doi: 10.1002/jnr.20198. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther. 2004;311:948–953. doi: 10.1124/jpet.104.071381. [DOI] [PubMed] [Google Scholar]
- Choi HK, Won L, Roback JD, Wainer BH, Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci USA. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM., Jr Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron. 1998;21:695–705. doi: 10.1016/S0896-6273(00)80587-5. [DOI] [PubMed] [Google Scholar]
- Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998;1366:139–149. doi: 10.1016/S0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- Davey F, Davies AM. TrkB signalling inhibits p75-mediated apoptosis induced by nerve growth factor in embryonic proprioceptive neurons. Curr Biol. 1998;8:915–918. doi: 10.1016/S0960-9822(07)00371-5. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Volente C, Angelastro JM, Greene LA. Association of protein kinases ERK1 and ERK2 with p75 nerve growth factor receptors. J Biol Chem. 1993;268:21410–21415. [PubMed] [Google Scholar]
- Costantini C, Rossi F, Formaggio E, Bernardoni R, Cecconi D, Della-Bianca V. Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. J Mol Neurosci. 2005;25:141–156. doi: 10.1385/JMN:25:2:141. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/S0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploin-sufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. Faseb J. 2005;19:1740–1742. doi: 10.1096/fj.05-3845fje. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192:226–234. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, Ernfors P, Alberch J. Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington's disease. J Neurochem. 2005;93:1057–1068. doi: 10.1111/j.1471-4159.2005.03047.x. [DOI] [PubMed] [Google Scholar]
- Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Mueller JC, Lichtner P, Schulte C, Munz M, Berg D, Wullner U, Illig T, Sharma M, Gasser T. The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of compounds and concentrations. Compounds employed for the cell culture experiments: Final concentrations, solvents, duration of application, activity, source and literature.