Abstract
Background
Sepsis caused by Staphylococcus aureus constitutes an important cause of morbidity and mortality in humans, and the incidence of this disease-entity is increasing. In this paper we describe the initial microbial dynamics and lesions in pigs experimentally infected with S. aureus, with the aim of mimicking human sepsis and pyemia.
Methods
The study was conducted in anaesthetized and intravenously inoculated pigs, and was based on bacteriological examination of blood and testing of blood for IL-6 and C-reactive protein. Following killing of the animals and necropsy bacteriological and histological examinations of different organs were performed 4, 5 or 6 h after inoculation.
Results
Clearance of bacteria from the blood was completed within the first 2 h in some of the pigs and the highest bacterial load was recorded in the lungs as compared to the spleen, liver and bones. This probably was a consequence of both the intravenous route of inoculation and the presence of pulmonary intravascular macrophages. Inoculation of bacteria induced formation of acute microabscesses in the lungs, spleen and liver, but not in the kidneys or bones. No generalized inflammatory response was recorded, i.e. IL-6 was not detected in the blood and C-reactive protein did not increase, probably because of the short time course of the study.
Conclusion
This study demonstrates the successful induction of acute pyemia (microabscesses), and forms a basis for future experiments that should include inoculation with strains of S. aureus isolated from man and an extension of the timeframe aiming at inducing sepsis, severe sepsis and septic shock.
Background
Sepsis constitutes an important cause of morbidity and mortality in humans, and the incidence of this disease-entity is increasing. At present, 660,000 cases of sepsis occur in the USA each year and combined with the high mortality, this ranks sepsis as a leading cause of death in this country. Staphylococci, including methicillin resistant Staphylococcus aureus (MRSA), have become the most frequently isolated bacteria in nosocomial infections giving rise to more than 50% of the cases [1]. Similar observations have been made in other countries, including Denmark [2,3]. Staphylococcal seeding to e.g. endocardium, skeleton and lungs resulting in the development of pyemic lesions (i.e., infective endocarditis, pyogenic osteomyelitis and lung abscesses) are serious complications to sepsis [4-6].
Models of bacteraemia, sepsis and pyemia caused by S. aureus have been established primarily in small laboratory animals (mice, rats, guinea pigs, and rabbits), while studies of experimental blood stream infection with S. aureus in pigs are few. Some of these studies in pigs aimed at the characterization of pulmonary intravascular macrophages such as the study by Winkler [7], some were models of prosthetic device infections exemplified by that reported by Paget et al. [8], and some investigated the pulmonary haemodynamics and function such as those by Walther et al. [9,10]. A few extensive studies modelling the pathogenesis of human sepsis and the ensuing shock have been performed with group A streptococci in pigs [11-14], but most of this type of research has been performed with Gram-negative bacteria or endotoxin [15].
Pigs may spontaneously develop pyemia and based on records from the post mortem meat inspection, approximately 125,000 pigs (0.4% of the total number of slaughtered pigs) are each year diagnosed with pyemia in Denmark (Ministry of Food, Agriculture and Fisheries, Danish Veterinary and Food Administration, 2007, unpublished data). In a study of pyemic lung lesions in pigs, S. aureus was found in monoculture in 46% of the cases [16]. Pig farming is a risk factor for nasal S. aureus colonization in man and sequence typing and phylogenetic comparisons of isolates have suggested a high rate of strain exchange between pigs and pig farmers [17]. Similar studies on MRSA showed an increased prevalence rate of nasal colonization in persons in contact with pigs [18] and infections in humans with MRSA have been related to a domestic animal source that included pigs [19,20]. The reported pig-associated subtype of MRSA, i.e. clonal complex (CC) 398, also has been identified in pigs and man from Denmark [21], and the paper draws similar epidemiological and zoonotical conclusions as reported by others.
The aim of the present study was to study the initial microbial dynamics and lesions in pigs inoculated intravenously with S. aureus. With the increasing use of the pig in biomedical research, a model of S. aureus sepsis and pyemia could prove useful to the study of the disease in man, but also to the study of the disease in pigs, as spontaneous generalized S. aureus infections are of major concern in both species.
Methods
Animals and housing
Nine clinically healthy Yorkshire-Landrace-Duroc crossbreed pigs (nos. 1–9), body weight (BW) of 20–25 kg corresponding to 9–10 weeks of age, were obtained from a commercial pig herd. The pigs were allowed to acclimatize for 5–10 days before entering the trial. Food was withdrawn 12 h before the start of the experiment, and immediately before the start the pigs underwent a clinical examination and measurement of body temperature, to secure absence of clinical signs of disease.
Experimental design
The pigs were sedated by intramuscular injection of a solution containing a mixture of zolazepam, tiletamine, xylazine and ketamine (0.83 mg/kg BW of each of the drugs), and butorphanol (0.17 mg/kg BW). A catheter (22 G) was then inserted in the right ear vein and used for infusion of anaesthetics, which consisted of a solution containing a mixture of xylazine (1 mg/mL), ketamine (2 mg/mL), butorphanol (0.1 mg/mL) and guaifenesine (48 mg/mL).
A catheter (22 G) was inserted in the left ear vein and eventually used for the administration of bacteria or mock followed by flushing with 10 mL sterile isotonic saline. After this procedure the catheter was removed. Another catheter (diameter of 2.6 mm) was surgically inserted into the left external jugular vein, adjusted to sit in the bi-jugular trunk, fixed to the skin with stitches, and flushed with 10 mL sterile isotonic saline followed by 2 mL of sterile 18 EI heparin solution. This catheter was used for blood sampling. The samples were secured free of heparin solution by discarding the first 5 mL of blood. All surgical procedures, insertion of catheters, injections, withdrawal of blood, aliqotation of blood etc. were performed strictly aseptically using 70% ethanol as disinfectant.
Eight pigs (nos. 1–6, 8 and 9) were inoculated with S. aureus, and one (no. 7) was mock-inoculated with sterile isotonic saline. Examination of blood included bacteriology on full blood and measurement of IL-6 in plasma and C-reactive protein in serum. The blood samples were taken at regular intervals until killing of the pigs 4 h after inoculation (PI) (nos. 1–4 and 7), 5 h PI (nos. 6 and 9) or 6 h PI (nos. 5 and 8), and included samples taken 2 min before inoculation (-2 min). Sampling timepoints for bacteriology were -2, 2, 30, 60, 120, 240, 300 and 360 min as indicated in Table 1, and timepoints for the testing of IL-6 and C-reactive protein were -2, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 min. The post mortem examination included bacterial culture from organs, histopathology, and fluorescent in situ hybridisation for bacteria. The study was conducted in accordance with the EU directive 86/609 and the Danish Animal Experimentation Act.
Table 1.
Viable count of Staphylococcus aureus in blood
| Blood sampling timepoints (min) | ||||||||||
| Pig no. | Innoculationa | Pig killed (h PIb) |
-2c | 2d | 30 | 60 | 120 | 240 | 300 | 360 |
| 1 | S. aureus | 4 | 0 | 2600e | 29 | 13 | 0 | 0 | ||
| 2 | S. aureus | 4 | 0 | 2200 | 25 | 8 | 5 | 0 | ||
| 3 | S. aureus | 4 | 0 | 6500 | 47 | 10 | 2 | 4 | ||
| 4 | S. aureus | 4 | 0 | 400 | 47 | 11 | 0 | 0 | ||
| 5 | S. aureus | 6 | 0 | 850 | 50 | 18 | 2 | 1 | NTf | -g |
| 6 | S. aureus | 5 | 0 | 1100 | 100 | 57 | 14 | 4 | 2 | |
| 8 | S. aureus | 6 | 0 | 480 | 34 | 12 | 5 | 2 | NT | 0 |
| 9 | S. aureus | 5 | 0 | 520 | 88 | 46 | 21 | 2 | 1 | |
| Mean | S. aureus | 4 – 6 | 0 | 1800 | 53 | 22 | 6 | 2 | ||
| 7 | Mock | 4 | 0 | 0 | 0 | 0 | 0 | 0 | ||
a 1 mL of 108 CFU were inoculated intravenous per kg of body weight and 1 control pig was injected with and equal volume of sterile isotonic saline (mock)
b PI, after inoculation
c "-2" indicates that blood was collected 2 min before inoculation
d Blood was collected 2 min PI
e Numbers indicate viable counts per ml of blood
f NT, not tested
g Result missing
Staphylococcus aureus suspension
Staphylococcus aureus, isolate no. S54F9 was obtained from a chronic embolic pulmonary abscess in a Danish slaughter pig (Department of Veterinary Pathobiology journal no. 36444). The isolate was identified using Api ID 32 Staph (Biomerieux, Inc., Marcy-l'Etoile, France) and was propagated in 100 mL of Luria-Bertani (LB) broth [22] for 18 h at 37°C, sedimented by centrifugation and re-suspended in sterile isotonic saline. The viable count was determined by counting the number of colonies formed on LB agar medium inoculated with 10 μL volumes of ten fold dilutions and incubated at 37°C for 24 h. The suspension was diluted with sterile isotonic saline to obtain a suspension containing 108 colony-forming units (CFU)/mL. This was used for intravenous inoculation at a dose of 108 CFU/kg BW.
As part of another study, the strain was typed by tandem repeat analysis of the staphylococcal protein A (spa) gene, a standard method for molecular typing of S. aureus [23]. The spa type observed in this strain (t1333) is one of the two most common types among porcine clinical S. aureus isolates in Denmark and is associated with clonal complex 30 according to the classification based on multi-locus sequence typing (Bent Aalbaek and Luca Guardabassi, unpublished data).
Bacteriological examination of blood and organs
Heparin-stabilized blood samples of 10 mL were taken aseptically and kept at 5°C for a maximum of 4 h until being processed. Blood in volumes of 1 mL and 1 mL of decimal dilutions (using sterile isotonic saline) were added to empty Petri dishes and mixed with melted LB agar medium. Viable count was determined after incubation for 48 h at 37°C and presented as counts/mL blood.
Quantitative bacteriological examination was performed on the lung (left diaphragmatic lobe), spleen (dorsal half) and liver (left lateral lobe) from all nine pigs upon euthanasia. In addition, bone tissue from the metaphysis/physis region of the left femur was cultured in 4 pigs (nos. 5, 6, 8, 9). The samples were kept at 5°C for a maximum of 12 h before being processed. Approximately 1 g of tissue was removed aseptically from the organs, cut into minor pieces with a scalpel, weighed and homogenized in 9 mL of sterile isotonic saline using a stomacher. Ten fold dilutions in sterile isotonic saline of the homogenized tissues were prepared. From each of these preparations 10 μL were inoculated on the surface of an LB agar medium and incubated for 48 h at 37°C before counting the colonies. The counts/g tissue were then calculated. Colony morphology was evaluated and representative colonies were subcultured on blood agar (Blood agar base, CM55; Oxoid, Basingstoke, Hampshire, England) containing 5% sterile bovine blood and phenotypically characterized using Api ID 32 Staph.
Assays for plasma IL-6 and serum C-reactive protein
Plasma was generated by centrifugation of ethylenediaminetetraacetic acid (EDTA) stabilised blood sampled in endotoxin free vials. Centrifugation was performed immediately after blood had been collected. The plasma samples were kept at 5°C for maximum 1 h before storing at -80°C. The IL-6 content was determined in plasma (diluted 1/2) by an R & D Systems DuoSet ELISA (R & D Systems, Abingdon, UK, catalog no. DY686), using ELISA plates from Nunc (Roskilde, Denmark, type: Macrosorp), and using goat anti porcine IL-6 for coating (0.8 μg/mL in PBS), biotinylated goat anti porcine IL-6 (0.1 μg/mL in PBS with 1% bovine serum albumin (BSA), Sigma St. Louis, MO, catalog no. A2153) and peroxidase-conjugated streptavidin (from the DuoSet kit, diluted 1/200) for detection, and finally TMB Plus from Kem-En-Tec (Taastrup, Denmark) as chromogen. A standard preparation of recombinant porcine IL-6 (from the DuoSet kit) was applied in double determination as a two-fold dilution row from 8000 pg/mL to 125 pg/mL. Two wells were used for buffer controls. Sample values for IL-6 were calculated from the curve fitted to the readings of the standard (using Ascent software v. 2.6).
Serum was generated by centrifugation of blood samples left to coagulate for no longer than 1 h at 22°C in plain, endotoxin free vials. The serum samples were kept at 5°C for maximum 1 h before storing at -80°C. The C-reactive protein (CRP) content in serum was determined on the ADVIA 1650 (Bayer), using a procedure that included loading undiluted serum into the machine [24].
Post mortem examination and histopathology
The post mortem examination included sagittal sections of the bones. Tissue samples of the lungs (dorsal part of the diaphragmatic lobes), the spleen, the liver, the kidneys, and the metaphysis/physis region of the right femur, tibia, radius, ulna, sacral bone, thoracic vertebrae nos. 8 and 9 plus the costochondral junction of the 8th and 9th ribs were fixed for 24 h in PBS buffered 4% formaldehyde. After fixation, bone tissues were decalcified for 6 days in a solution of EDTA and sodium hydroxide (280 g EDTA and 30 g NaOH dissolved in 2000 mL of water). The tissue specimens were then processed through graded concentrations of ethanol and xylene, embedded in paraffin wax, cut at 3–5 μm, rehydrated, and stained with haematoxylin and eosin (HE) [25]. Tissue sections for in situ hybridisation were mounted on Super Frost Plus glass slides (Gerhard Menzel, Braunschweig, Germany) and processed as stated below.
Fluorescent in situ hybridisation
Fluorescent in situ hybridisation (FISH) was applied on selected tissue sections using an Alexa 555 5'-labeled oligonucleotide probe (EUB 338) targeting a 16S rRNA sequence specific for the Domain Bacteria [26]. The procedure was modified after Boye et al. [27] and included a 10 min pre-treatment at 20°C of the sections with 3 mg/mL of lysozyme (cat. no. L-6876, Sigma Aldrich, USA) dissolved in a Tris/EDTA-buffer (100 mM Tris and 50 mM EDTA (pH 6.5)). The sections were rinsed in water and hybridised for 16 h in a moist chamber at 40°C with 5 ng/mL of probe dissolved in a hybridisation buffer (700 mM NaCl, 100 mM Tris (pH 8) and 0.1% sodium dodecyl sulphate (SDS)). Washing of the sections was performed 2 times with 2 × standard saline citrate (SSC) for 1 min, with hybridisation buffer prewarmed to 45°C for 20 min, and finally 2 times with 2 × SSC for 1 min.
Results
Bacteriological examination of blood and organs
The viable counts obtained from blood and tissue samples are given in Table 1 and 2. All colonies had a morphology identical to that of the inoculation strain. Representative isolates showed the same reaction pattern in API Staph as the strain used for inoculation.
Table 2.
Viable count of Staphylococcus aureus in organs
| Organsc | ||||||
| Pig no. | Innoculationa | Pig killed (h PIb) |
Lung | Spleen | Liver | Metaphysis/physis region of bone |
| 1 | S. aureus | 4 | 140000 | 2800 | 93000 | NTd |
| 2 | S. aureus | 4 | 130000 | 18000 | 7700 | NT |
| 3 | S. aureus | 4 | 290000 | 11000 | 3800 | NT |
| 4 | S. aureus | 4 | 110000 | 2700 | 9600 | NT |
| 5 | S. aureus | 6 | 78000 | 34000 | 3300 | 1400 |
| 6 | S. aureus | 5 | 26000 | 43000 | 34000 | 2700 |
| 8 | S. aureus | 6 | 50000 | 16000 | 1200 | 730 |
| 9 | S. aureus | 5 | 47000 | 63000 | 15000 | 4300 |
| Mean | S. aureus | 4 – 6 | 110000 | 24000 | 21000 | 2300 |
| 7 | Mock | 4 | 0 | 0 | 0 | NT |
a 1 mL of 108 CFU were inoculated intravenous per kg of body weight and 1 control pig was injected with and equal volume of sterile isotonic saline (mock)
b PI, after inoculation
c Numbers indicate viable counts per g of tissue
d NT, not test
Plasma IL-6 and serum C-reactive protein
On each of the time points tested, the content of IL-6 was under the detection limit of the assay (250 pg/mL). Also, CRP measurements did not reveal any significant increases or variations.
Gross pathology, histopathology and FISH
All pigs showed atelectasis of the dorsal part of the diaphragmatic lobes. This probably was related to the dorsal recumbency of the pigs during anaesthesia, and thus a result of the experimental design.
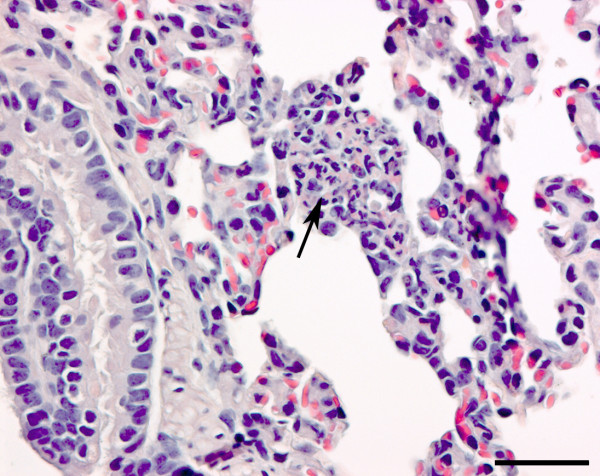
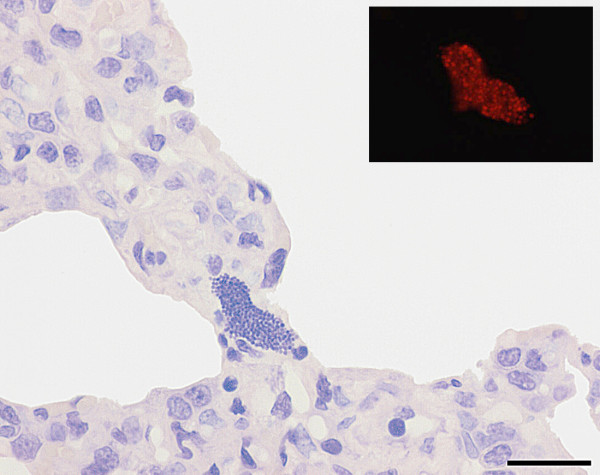
The HE stained section of the lungs revealed presence of acute microabscesses (Figure 1) and aggregates of spherical, basophilic organisms in three of the four S. aureus-inoculated pigs killed 5 or 6 h PI (nos. 5, 6, 8). These aggregates were identified as bacterial colonies by FISH and bacterial colonies were often present without any ensuing inflammatory reaction (Figure 2). Lung lesions were absent in all S. aureus-inoculated pigs killed 4 h PI, one pig killed 5 h PI and the mock-inoculated pig.
Figure 1.
Microabscess in the lung. Section of lung from a Staphylococcus aureus infected pig killed 6 h after inoculation (pig no. 5) showing a microabscess (arrow) located to an alveolar septum. Haematoxylin- and eosin stain. Bar = 50 μm.
Figure 2.
Bacterial colony in the lung. Section of lung from a Staphylococcus aureus infected pig killed 5 h after inoculation (pig no. 6) showing a bacterial colony without any inflammatory reaction and identified by fluorescent in situ hybridisation (insert). The in situ hybridisation was performed first and the bacterial colony photographed. Then the section was stained with haematoxylin and the same colony identified and a new photo taken. Bar = 20 μm.
Acute microabscesses were present in the marginal zone of the spleen (the zone between the red and white pulpa) in the S. aureus-inoculated animals killed 5 or 6 h PI (nos. 5, 6, 8, 9). In two of the S. aureus-inoculated pigs killed 4 h PI (nos. 1, 3) neutrophils seemed to accumulate in the marginal zone without forming true microabscesses. The remaining pigs (two S. aureus-inoculated and the mock-inoculated) were without histological lesions. In the liver, acute microabscesses were detected in pigs from the staphylococcus group killed 4 h PI (nos. 1, 2, 4), 5 h PI (nos. 6, 9), and 6 h PI (no. 8). The rest of the pigs, including the mock-inoculated, were without histological lesions. The kidneys and the metaphyses of all bones from both inoculated and control animals had no lesions.
Discussion
The quantitative bacteriological examination of blood and tissues (Table 1 and 2) showed that blood samples taken 2 min after intravenous injection of S. aureus contained an initial mean viable count of 1800 CFU/mL. The subsequent blood samples showed decreasing numbers of bacteria reaching a mean of 2 CFU/mL 4 h PI and with 3 animals being culture negative. These results reflect both dilution of the inoculated bacteria within the blood compartment and clearance.
In the organs, which were examined 4, 5 or 6 h PI, mean viable counts largely exceeded the initial viable count obtained in the blood indicating a considerable capacity of the organs for withholding bacteria from the circulation. The mean viable count per g of spleen and liver tissue were of the same magnitude, 24,000 CFU/g and 21,000 CFU/g, respectively in contrast to a higher mean viable count from lung tissue, 110,000 CFU/g, suggesting that the porcine lung has a high capacity for retaining bacteria from the circulation. This finding is in agreement with previous reports [28,29] and is closely linked to the clearing action of pulmonary intravascular macrophages (PIM), present in swine and many other animal species but not in man [7]. When comparing the content of bacteria in different organs, the volume of blood and the blood-load of bacteria entering the organs should be taken into account [29]. Thus, the lungs will receive the total volume of blood and load of bacteria (total right ventricular outflow) whereas the spleen, for example, will only receive a fraction of the blood and only bacteria that is not withheld by the lungs or other organs. Also, proliferation or destruction of the bacteria within the organs would influence the level. Thus destruction of bacteria in the lungs could explain the seemingly lower levels in the lungs of pigs examined 5 and 6 h PI as opposed to pigs examined 4 h PI. The mean viable count from the metaphysis/physis region was 2300 CFU/g, the lowest recorded, but compared to the 0 – 2 CFU/mL present in the blood still indicates some capacity for the retention of bacteria by bone tissue.
Plasma IL-6 was not detected in any of the pigs and no increase in serum CRP was observed. IL-6 together with IL-1 and tumor necrosis factor-α (TNF-α) are some of the major proinflammatory cytokines produced in monocytes and other cells as an immediate response to infection and other stimuli. The cytokines have a range of local and systemic effects, including the recruitment of neutrophils and the induction of acute phase proteins from the liver. The systemic effects rely on the presence of cytokines in the blood and their presence is linked to a range of different factors. For example, endotoxin caused production of IL-1, IL-6 and TNFα within 1–5 h in cellular in vitro assays, whereas Gram-positive toxins induced a peak response of lymphotoxin-α and interferon-γ 50–75 h after challenge [30]. Infusion of endotoxin to pigs caused TNF-α and IL-6 to peak in the blood 1–4 h later [31]. In experimental aerogenous infection studies in pigs with the Gram-negative pulmonary pathogen Actinobacillus pleuropneumoniae, blood IL-6 was detected within the first 10–14 h PI [32,33]. Increase in blood CRP has been demonstrated in several studies as reviewed by Petersen et al. [34]. The absence of a systemic IL-6 and CRP response in our study could have a variety of causes, including the short duration of the experiment and the bacterial strain used. However, IL-6 was detected in the blood only 1 h after the intravenous inoculation of S. aureus in mice [35] and was produced in response to in vitro challenge of human endothelial cells by S. aureus [36]. Also, TNF-α was detected in blood only 3 h after the intravenous inoculation of serogroup A streptococci in pigs [13].
The histological examination revealed presence of acute microabscesses and bacterial colonies while evidence of thrombosis was absent. Microabscesses were seen in all S. aureus-inoculated animals except for one pig (no. 3) and were detected in the lung, spleen and liver, but not in the kidney and the metaphysis/physis of bones. These lesions represent acute pyemia. S. aureus colonies were present in only the lungs of 3 S. aureus-inoculated pigs. The bacterial colonies may represent trapping of bacterial emboli or local proliferation.
The presence of microabscesses in the marginal zone of the spleen has been linked to sepsis [37]. Presence of microabscesses in the lungs and the liver probably reflects the presence of PIM in the lungs and Kupffer's cells in the liver [38]. Naturally occurring pyemia in pigs is often associated with lesions in the lungs and the skeleton (Ministry of Food, Agriculture and Fisheries, Danish Veterinary and Food Administration, 2007, unpublished data). Frequently isolated bacteria from lung lesions are S. aureus [16] and Arcanobacterium pyogenes from skeleton abscesses [39]. Bacteria, including S. aureus are isolated from cases of osteomyelitis, and different predisposing factors, including the presence of receptors to bone surface proteins in S. aureus, have been suggested to explain the frequent occurrence of acute osteomyelitis localized to the metaphysial or the equivalent epiphysial regions [39]. The lack of osteomyelitis in our study could be a result of the short timeframe of the study or the rather light colonization of the skeleton.
Conclusion
In conclusion, we were able to induce acute pyemia (the formation of acute microabscesses) in pigs 4 to 6 h after the intravenous inoculation of S. aureus. Microabscesses were present in the lungs, spleens and livers, but not in the kidneys or bones. Presence of IL-6 or increased levels of CRP in the blood were not seen and a septic stage, defined by the presence of these biomarkers [40], was thus not reached. Future experiments should include inoculation with strains of S. aureus isolated from man and an extension of the timeframe aiming at inducing sepsis, severe sepsis and septic shock, thus modelling the human disease syndromes in pig.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
OLN, TI, PSL, JSA and HEJ designed the study, participated in the execution of the study and supervised the histopathology. PH, ALJ and MK-H contributed substantially to designing the study. BA performed the bacteriology, including characterization of the S. aureus strain, preparation of the inoculum and culture from blood and organs. SS, KBJ, SC, KM, AKB, ERB, MHJ, DKG and MB made substantial contributions to the acquisition, analysis and interpretation of data. PH performed the blood analysis for IL-6. MK-H performed the blood analysis for C-reactive protein. OLN drafted the manuscript, which was reviewed and commented by TI, PSL, JSA and HEJ. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by Danish Medical Research Council (Ministry of Science, Technology and Innovation) grant no. 271-07-0417.
Contributor Information
Ole L Nielsen, Email: ole@life.ku.dk.
Tine Iburg, Email: tib@life.ku.dk.
Bent Aalbaek, Email: baal@life.ku.dk.
Páll S Leifsson, Email: ple@life.ku.dk.
Jørgen S Agerholm, Email: jager@life.ku.dk.
Peter Heegaard, Email: pmhh@vet.dtu.dk.
Mette Boye, Email: mbo@vet.dtu.dk.
Sofie Simon, Email: snoffer@dsr.kvl.dk.
Kristine B Jensen, Email: kbje@life.ku.dk.
Sophie Christensen, Email: sop@dsr.kvl.dk.
Karin Melsen, Email: kamel@dsr.kvl.dk.
Anne K Bak, Email: annebak@dsr.kvl.dk.
Elín R Backman, Email: ellar@dsr.kvl.dk.
Mia H Jørgensen, Email: miahn@dsr.kvl.dk.
Désirée K Groegler, Email: desiree@dsr.kvl.dk.
Asger L Jensen, Email: alj@life.ku.dk.
Mads Kjelgaard-Hansen, Email: mjkh@life.ku.dk.
Henrik E Jensen, Email: helj@life.ku.dk.
References
- Bearman GM, Wenzel RP. Bacteremias: a leading cause of death. Arch Med Res. 2005;36:646–659. doi: 10.1016/j.arcmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Benfield T, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhøj P. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–263. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen MS, Espersen F, Frimodt-Moller N, Jensen AG, Larsen AR, Pallesen LV, Skov R, Westh H, Skinhøj P, Benfield T. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J. 2007;26:398–405. doi: 10.1097/01.inf.0000261112.53035.4c. [DOI] [PubMed] [Google Scholar]
- Schoen FJ. The Heart. In: Kumar V, Abbas AK, Fausto N, editor. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2005. pp. 555–618. [Google Scholar]
- Husain AN, Kumar V. The Lung. In: Kumar V, Abbas AK, Fausto N, editor. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2005. pp. 711–772. [Google Scholar]
- Rosenberg AE. Bones, Joints, and Soft Tissue Tumors. In: Kumar V, Abbas AK, Fausto N, editor. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2005. pp. 1273–1324. [Google Scholar]
- Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181:217–234. doi: 10.1002/aja.1001810302. [DOI] [PubMed] [Google Scholar]
- Paget DS, Bukhari RH, Zayyat EJ, Lohr JM, Roberts WH, Welling RE. Infectibility of endovascular stents following antibiotic prophylaxis or after arterial wall incorporation. Am J Surg. 1999;178:219–224. doi: 10.1016/s0002-9610(99)00114-2. [DOI] [PubMed] [Google Scholar]
- Walther S, Jansson I, Gunnarsson M, Lennquist S. Nebulized corticosteroid improves pulmonary-function and outcome in experimental porcine septicemia. Acta Anaesthesiol Scand. 1991;35:635–641. doi: 10.1111/j.1399-6576.1991.tb03363.x. [DOI] [PubMed] [Google Scholar]
- Walther S, Berg S, Jansson I, Lennquist S. Activity of serum angiotensin converting enzyme in septic pigs treated with intrapulmonary corticosteroid. Eur J Surg. 1994;160:3–7. [PubMed] [Google Scholar]
- Saetre T, Lindgaard AK, Lyberg T. Systemic activation of coagulation and fibrynolysis in a porcine model of serogroup A streptococcal shock. Blood Coagul Fibrinolysis. 2000;11:433–438. doi: 10.1097/00001721-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Saetre T, Hoiby EA, Aspelin T, Lermark G, Egeland T, Lyberg T. Aminoethyl-isothiourea, a nitric oxide synthase inhibitor and oxygen radical scavenger, improves survival and counteracts hemodynamic deterioration in a porcine model of streptococcal shock. Crit Care Med. 2000;28:2697–2706. doi: 10.1097/00003246-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Saetre T, Hoiby EA, Aspelin T, Lermark G, Lyberg T. Acute serogroup A streptococcal shock: A porcine model. J Infect Dis. 2000;182:133–141. doi: 10.1086/315692. [DOI] [PubMed] [Google Scholar]
- Middelveld RJM, Alving K. Synergistic septicemic action of the Gram-positive bacterial cell wall components peptidoglycan and lipoteichoic acid in the pig in vivo. Shock. 2000;13:297–306. doi: 10.1097/00024382-200004000-00008. [DOI] [PubMed] [Google Scholar]
- Goldfarb RD, Dellinger RP, Parrillo JE. Porcine models of severe sepsis: emphasis on porcine peritonitis. Shock. 2005;24(Suppl 1):75–81. doi: 10.1097/01.shk.0000191337.01036.b7. [DOI] [PubMed] [Google Scholar]
- Liljegren CH, Aalbaek B, Nielsen OL, Jensen HE. Some new aspects of the pathology, pathogenesis, and aetiology of disseminated lung lesions in slaughter pigs. APMIS. 2003;111:531–538. doi: 10.1034/j.1600-0463.2003.1110501.x. [DOI] [PubMed] [Google Scholar]
- Armand-Lefevre L, Ruimy R, Andremont A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg Infect Dis. 2005;11:711–714. doi: 10.3201/eid1105.040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11:1965–1966. doi: 10.3201/eid1112.050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W, Strommenger B, Stanek C, Cuny C. Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg Infect Dis. 2007;13:255–258. doi: 10.3201/eid1302.060924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck ME, Pluister GN, Voss A, Wannet WJ, de Neeling AJ. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob. 2006;5:26. doi: 10.1186/1476-0711-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HC, Molbak K, Reese C, Aarestrup FM, Selchau M, Sorum M, Skov RL. Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg Infect Dis. 2008;14:1383–1389. doi: 10.3201/eid1409.071576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning A laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard-Hansen M, Martinez-Subiela S, Petersen HH, Jensen AL, Ceron JJ. Evaluation and comparison of two immunoturbidimetric assays for the heterologous determination of porcine serum C-reactive protein. Vet J. 2007;173:571–577. doi: 10.1016/j.tvjl.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. 4. New York: Churchill Livingstone; 1996. [Google Scholar]
- Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye M, Feenstra AA, Tegtmeier C, Andresen LO, Rasmussen SR, Bille-Hansen V. Detection of Streptococcus suis by in situ hybridization, indirect immunofluorescence, and peroxidase-antiperoxidase assays in formalin-fixed, paraffin-embedded tissue sections from pigs. J Vet Diagn Invest. 2000;12:224–232. doi: 10.1177/104063870001200305. [DOI] [PubMed] [Google Scholar]
- Brain JD, Molina RM, DeCamp MM, Warner AE. Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species. Am J Physiol. 1999;276:L146–L154. doi: 10.1152/ajplung.1999.276.1.L146. [DOI] [PubMed] [Google Scholar]
- Dehring DJ, Crocker SH, Wismar BL, Steinberg SM, Lowery BD, Cloutier CT. Comparison of live bacteria infusions in a porcine model of acute respiratory failure. J Surg Res. 1983;34:151–158. doi: 10.1016/0022-4804(83)90054-9. [DOI] [PubMed] [Google Scholar]
- Andersson J, Nagy S, Bjork L, Abrams J, Holm S, Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Ebdrup L, Krog J, Granfeldt A, Larsen PO, Vestergaard C, Hokland M, Tønnesen E. Leukocyte, plasma, and organ-associated cytokine profiles in an animal model of acute inflammation. APMIS. 2008;116:352–360. doi: 10.1111/j.1600-0463.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen B, Lykkesfeldt J, Skaanild MT, Angen O, Nielsen JP, Friis C. Putative biomarkers for evaluating antibiotic treatment: an experimental model of porcine Actinobacillus pleuropneumoniae infection. Res Vet Sci. 2003;74:261–270. doi: 10.1016/s0034-5288(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Fossum C, Wattrang E, Fuxler L, Jensen KT, Wallgren P. Evaluation of various cytokines (IL-6, IFN-alpha, IFN-gamma, TNF-alpha) as markers for acute bacterial infection in swine – a possible role for serum interleukin-6. Vet Immunol Immunopathol. 1998;64:161–172. doi: 10.1016/s0165-2427(98)00126-3. [DOI] [PubMed] [Google Scholar]
- Petersen HH, Nielsen JP, Heegaard PMH. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Yao L, Berman JW, Factor SM, Lowy FD. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun. 1997;65:3889–3895. doi: 10.1128/iai.65.9.3889-3895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Bengualid V, Lowy FD, Gibbons JJ, Hatcher VB, Berman JW. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli VEOT. Hematopoietic System. In: Maxie MG, editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Philadelphia: Elsevier Saunders; 2007. pp. 107–324. [Google Scholar]
- Stalker MJ, Hayes MAT. Liver and Biliary System. In: Maxie MG, editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Philadelphia: Elsevier Saunders; 2007. pp. 297–388. [Google Scholar]
- Thompson K. Bones and Joints. In: Maxie MG, editor. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Philadelphia: Elsevier Saunders; 2007. pp. 1–184. [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]