Abstract
The fabrication of biodegradable 3-D scaffolds enriched with multipotent stem cells seems to be a promising strategy for the repair of irreversibly injured tissues. The fine mechanisms of the interaction of rat mesenchymal stem cells (rMSCs) with a hyaluronan-based scaffold, i.e. HYAFF®11, were investigated to evaluate the potential clinical application of this kind of engineered construct. rMSCs were seeded (2 × 106 cells cm−2) on the scaffold, cultured up to 21 days and analysed using appropriate techniques. Light (LM), scanning (SEM) and transmission (TEM) electron microscopy of untreated scaffold samples showed that scaffolds have a highly porous structure and are composed of 15-µm-thick microfibres having a rough surface. As detected by trypan blue stain, cell adhesion was high at day 1. rMSCs were viable up to 14 days as shown by CFDA assay and proliferated steadily on the scaffold as revealed by MTT assay. LM showed rMSCs in the innermost portions of the scaffold at day 3. SEM revealed a subconfluent cell monolayer covering 40 ± 10% of the scaffold surface at day 21. TEM of early culture showed rMSCs wrapping individual fibres with regularly spaced focal contacts, whereas confocal microscopy showed polarized expression of CD44 hyaluronan receptor; TEM of 14-day cultures evidenced fibronexus formation. Immunohistochemistry of 21-day cultures showed that fibronectin was the main matrix protein secreted in the extracellular space; decorin and versican were seen in the cell cytoplasm only and type IV collagen was minimally expressed. The expression of CD90, a marker of mesenchymal stemness, was found unaffected at the end of cell culture. Our results show that HYAFF®11 scaffolds support the adhesion, migration and proliferation of rMSCs, as well as the synthesis and delivery of extracellular matrix components under static culture conditions without any chemical induction. The high retention rate and viability of the seeded cells as well as their fine modality of interaction with the substrate suggest that such scaffolds could be potentially useful when wide tissue defects are to be repaired as in the case of cartilage repair, wound healing and large vessel replacement.
Keywords: electron microscopy, hyaluronan-based scaffold, mesenchymal stem cells, tissue engineering
Introduction
In recent years, tissue engineering emerged as a novel promising field of science aimed at overcoming organ and tissue shortages in transplantation medicine. The possibility of generating ‘in vitro’ bioartificial tissues from cells cultured or embedded within an artificial support system is particularly appealing; examples of tissue-engineered skin (Tonello et al. 2005; Wong et al. 2007), blood vessels (Stankus et al. 2007) and heart valve leaflets (Sodian et al. 2000) have already been provided, raising great expectations for their clinical use. Besides this type of substitutive strategy, the artificial support can be also useful for targeting multipotent stem cell release in vivo (Radice et al. 2000). Both these approaches are highly desirable for substituting or regenerating irreversibly damaged tissues in the recipient. An added value of the stem cell delivery approach is that the scaffold itself can be selected to augment tissue repair through its chemical and/or physical characteristics.
To be usable in this strategy, the biomaterials must be non-toxic and highly biocompatible. Moreover, they should be degraded in vivo as they support the loaded stem cells to proliferate, differentiate, and secrete beneficial bioactive molecules, e.g. growth factors and extracellular matrix components. In this way, the long-term presence of foreign materials and the consequent inflammatory responses can be prevented (Langer & Vacanti, 1993). As outlined above, building an ideal scaffold requires that multiple, even conflicting, criteria be met. Up to now no biomaterial has yet satisfied all the properties which are necessary to generate such a clinically usable stem cell delivery system.
In this study, a microfibrous three-dimensional scaffold based on hyaluronic acid was used to investigate its fine interaction with rat mesenchymal stem cells using different microscopic techniques, i.e. light, confocal laser, fluorescence, transmission and scanning electron microscopy. This scaffold was chosen because the hyaluronic acid esters possess both the processability of completely synthetic polymers and the advantages related to the use of a highly purified natural polysaccharide (Campoccia et al. 1998; Mori et al. 2004). In particular, HYAFF®11, the hyaluronan ester used in the present study, appears to be an inert material for up to 1 month and its degradation begins gradually to start just after this period, as soon as the hydrolysis of ester bonds takes place. Moreover, in vivo long-term implantation studies have never shown local or systemic effects of any importance associated with HYAFF®11 material (Wragg et al. 1996).
Materials and methods
Biomaterial
The biomaterial used in this study was a hyaluronic acid-based polymer, HYAFF®11, developed by Fidia Advanced Biopolymer – FAB – s.r.l. (Abano Terme, Italy). HYAFF®11 is derived through the total esterification with benzyl alcohol of the carboxyl groups along the polymeric backbone of sodium hyaluronate. The experiments have been performed on a non-woven HYAFF®11 construct. The chemistry of the biomaterial here employed is detailed elsewhere (Campoccia et al. 1998).
rMSC isolation
Bone marrow cells were harvested from femurs of adult rats (body weight 450–550 g). The rats were housed in identical cages and were allowed access to water and a standard rodent diet ad libitum. The animals received care in accordance with Italian law (DL-116, 27 January 1992), which complies with the Guide for the Care and Use of Laboratory Animals by the US National Research Council. The animals were anaesthetized with diethylether. Marrow cells were obtained by inserting a 21-gauge needle into the upper end of the femur and flushing into the shaft 5 mL of complete α-modified Eagle's medium (αMEM) containing 20% fetal bovine serum (FBS), 2 mm L-glutamine, 100 U mL−1 penicillin and 100 µg mL−1streptomycin. Cell suspensions (10 mL of final volume from each rat) were dripped from the lower end of both femurs into a 50-mL sterile tube containing 40 mL of complete medium. The cells were then filtered through a 70-µm nylon filter (Falcon, Franklin Lakes, NJ, USA) and plated into one 75-cm2 flask. They were grown in complete αMEM containing 10% FBS, 2 mm L-glutamine, 100 U mL−1 penicillin and 100 µg mL−1streptomycin at 37 °C and 5% CO2 for 3 days. The medium was then replaced with fresh medium and the adherent cells were grown to 90% confluence to obtain samples here defined as mesenchymal stem cells (rMSCs) at passage zero (P0). The P0 rMSCs were washed with phosphate-buffered saline (PBS) and detached by incubation with 0.25% trypsin and 0.1% EDTA (Sigma, St. Louis, MO, USA) for 5–10 min at 37 °C. Complete medium was added to inactivate the trypsin. The cells were centrifuged at 450 g for 10 min, resuspended in 10 mL complete medium, counted manually in duplicate using a Bürker's chamber, and plated as P1 on 58-cm2 plates at densities of 2000 cells cm2. Complete medium was replaced every 3–4 days over the 18–24-day period of culture. The basic morphological and immunophenotypical (CD44+, CD90+, CD105+) properties of rMSCs were consistent with those reported in previous studies (Raimondo et al. 2006; Gallo et al. 2007).
HYAFF®11 morphological analysis
Samples from non-woven HYAFF®11 were initially examined under an inverted light microscope and then processed for scanning (SEM) and transmission (TEM) electron microscopy. For SEM the samples were directly mounted on aluminium stubs and then coated with a thin layer of gold before examination. For TEM, both glutaraldehyde-fixed and -unfixed 2 × 2 mm3 samples were embedded in Epoxy resin. Before examination, thin sections were stained with lead citrate and uranyl acetate.
rMSC seeding on HYAFF®11
rMSCs were resuspended in 150 µL of complete medium and slowly seeded at the density of 2 × 106 cell cm−2 on the surface of non-woven HYAFF®11 samples in a 12-well plate for 4 h at 37 °C. Complete medium 1 mL was then added to all samples. Interaction between rMSCs and HYAFF®11 was investigated on days 1, 3, 7, 14, and 21 using appropriate techniques. Cell culture media was changed twice a week. At least three scaffolds were analysed for each experimental condition and at each time-point.
Cell adhesion on HYAFF®11
The trypan blue exclusion staining method was used to quantify rMSC adhesion onto the biopolymer. rMSCs were seeded on the scaffold as described above. After 24 h, the number of cells bound to the fibres was calculated by subtracting from the known number of total seeded cells the amount of cells which adhered to the well bottom and those still present in the supernatant, determined by Bürker hemocytometry chamber counting:
[no. of HYAFF®-adherent cells = no. of seeded cells – (no. of well-adherent cells + no. of suspended cells)].
rMSC viability and proliferation into HYAFF®11
rMSC viability onto HYAFF®11 was analysed at 1, 7, and 14 days by using the vital dye carboxyfluorescein diacetate, succinimidyl ester (Vybrant CFDA-SE Cell Tracer Kit, Molecular Probes, Eugene, OR, USA) following the manufacturer's instructions.
rMSC proliferation was determined by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay at days 4, 7 and 14 as previously reported (Lisignoli et al. 2001). After removing the supernatant, the scaffold was washed with PBS, transferred into a new well containing 1 mL of MTT solution (1 mg mL−1) and incubated for 3 h at 37 °C. The scaffold was then transferred into an Eppendorf tube containing 1 mL of solubilization solution (0.01 m HCl in isopropanol) and vortexed for 5 min. This provoked the release from the scaffold of MTT which was actively reduced by viable cells acquiring a yellow colouring. Each sample was centrifuged at 15 000 × g for 5 min and the supernatant was read at 595 nm using a multiwell spectrophotometer (Victor 2 microplate reader, Perkin Elmer, Wellesley, MA, USA). A linear calibration curve was performed using standard rMSCs (from 105 to 106 cells) and the corresponding values of absorbance were calculated after blank (without cells) subtraction (not shown). Each value of the standard curve was obtained in quadruplicate.
Light, transmission and scanning electron microscopy analysis
On days 1, 3, 7, 14 and 21, HYAFF®11-rMSC cultures were processed for light and electron microscopy.
For light microscopy (LM), the samples were fixed in formalin and embedded in paraffin; the samples were stained with hematoxylin and eosin.
For TEM, the samples were fixed in 2.5% buffered glutaraldehyde, post-fixed with 1% osmium tetroxide and embedded in Epoxy resin; semithin sections were stained with toluidine blue and thin sections with uranyl citrate and lead citrate; the thin sections were observed in a Philips 400T transmission electron microscope.
For SEM, following fixation the samples were dehydrated and critical point-dried. The samples were mounted on aluminium stubs and then coated with a thin layer of gold using a sputtering device. The samples were observed in a Philips 505 scanning electron microscope at 15 kV.
Immunohistochemical analysis of matrix protein synthesis
Matrix protein analysis for fibronectin, decorin, versican and type IV collagen was performed by immunostaining 4-µm-thick dewaxed histological sections. The following antibodies were used; rabbit polyclonal antibody to fibronectin, a glycoprotein which is involved in cell adhesion and migration processes (1 : 1000, Abcam, Cambridge, UK); rabbit polyclonal antibody to decorin, a small proteoglycan which binds to matrix proteins such as collagen types I, II, and IV as well as fibronectin (1 : 500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); rabbit polyclonal antibody to versican, a member of the family of the large aggregating proteoglycans which is able to interact with hyaluronan, tenascin-R, fibulin-1, fibulin-2 and fibronectin (1 : 700, Abcam); rabbit polyclonal antibody to external lamina type IV collagen (1 : 200, Santa Cruz Biotechnology, Inc.). The antigen–antibody reaction was revealed using the Novolink Polymer Detection System (Novocastra Laboratories, Newcastle, UK). Negative controls were performed by omitting the primary antibodies. Additional experiments were performed to verify the ability of rMSCs to produce the investigated matrix components under different culture conditions. Briefly, rMSCs were cultured on glass slides in complete medium up to 21 days. The samples were then rapidly washed and fixed in formalin without using any permeabilizing agent. Matrix protein production was investigated by using the same panel of polyclonal antibodies described above; the Novolink Polymer Detection System was used to visualize the immunological reaction.
Confocal microscopy analysis of CD44 expression
Dewaxed histological sections 4-µm-thick were incubated overnight with the anti-CD44 monoclonal antibody (1 : 100, Chemicon, Temecula, CA, USA), which specifically recognizes the extracellular domain of the hyaluronate receptor. After washing in PBS, immunolabelling was carried out by incubating sections for 1 h with a goat anti-mouse IgG Alexa-Fluor-488-conjugate (1:200, Molecular Probes). The sections were finally mounted with the ProLong® Gold Antifade Reagent (Molecular Probes) and analysed using an LSM 510 confocal laser microscopy system (Zeiss, Jena, Germany), which incorporates two lasers (Argon and HeNe). Negative controls were performed by omitting the primary monoclonal antibody.
Immunofluorescence detection of CD90 expression
HYAFF®11-rMSC cultures were performed on 12-well plates, fixed at day 21 at room temperature for 15 min with 3% paraformaldehyde in PBS, and rinsed twice with PBS after blocking with 4% bovine serum albumin (BSA) dissolved in 0.2% Tween-20/PBS. A primary mouse monoclonal antibody against the CD90 (Thy-1) glycoprotein (Armingen, San Diego, CA, USA), a member of the immunoglobulin superfamily, was used because it is commonly expressed on the surface of mesenchymal stem cells. The antibody was diluted 1 : 100 in blocking buffer for 1 h at room temperature and washed three times in PBS. The secondary Cy3-conjugated polyclonal anti-mouse antibody (Sigma) was diluted 1 : 2000 in blocking buffer and washed three times in PBS. Nuclei were stained with DAPI diluted 1 : 1000 for 15 min and washed three times in PBS. The samples were observed using an IX50-Olympus inverted microscopy (Olympus, Tokyo, Japan). Negative controls were obtained with the Cy3-conjugated secondary antibody alone.
Results
HYAFF®11 structural analysis
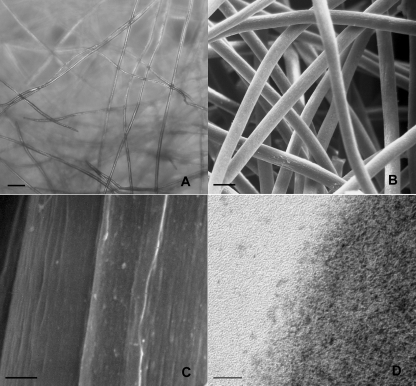
At LM the HYAFF®11 scaffold was composed of randomly distributed layers of translucid fibres in the micron dimensional range (Fig. 1A). The overall morphology of the scaffold was better appreciated under SEM (Fig. 1B); here the scaffold fibres had a solid appearance with interconnected voids between them such that an evident gross porous structure was recognizable; the fibre diameter ranged from 12 to 16 µm with an average of 15 µm diameter. At higher magnification the fibre surface was slightly irregular. At TEM the fibres were moderately electron dense and were composed of a tight mesh of nanofibrils which bulged out from the free edge of the fibre surface (Fig. 1C,D).
Fig. 1.
HYAFF®11 morphological analysis. Inverted LM (A) showing layers of translucid fibres which SEM reveals as having a micron dimensional range (B). At higher magnification, SEM (C) shows that the fibre surface is rough. TEM (D) of an individual fibre composed of a tight mesh of nanofibrils bulging out at the free border edge. Scale bars: (A) 100 µm; (B) 25 µm; (C) 1 µm; (D) 20 nm.
Analysis of cell adhesion and proliferation in HYAFF®11
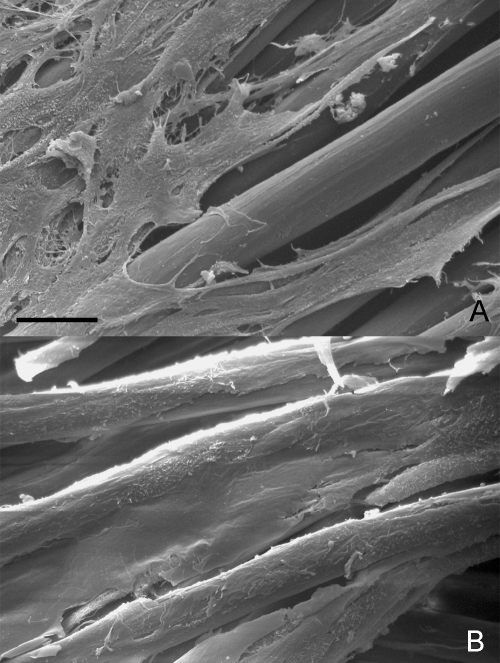
As detected using the trypan blue exclusion assay, the rMSC adhesion on the scaffold was high. After 24 h of rMSC seeding, the number of cells entrapped in the scaffold was similar to the number of cells which had been previously seeded, i.e. 96 ± 0.4% of the overall number of the initially seeded cells. As viewed with SEM, rMSCs adhered on the HYAFF®11 surface, efficiently forming extremely thin cell sheets from the early days of culture. At day 1, the cells covered an area of about 12 ± 2% of the entire seeded scaffold. Cell adhesion on the scaffold steadily increased and after 21 days the rMSCs covered an area of 40 ± 10% (Fig. 2A,B).
Fig. 2.
Surface analysis of cell adhesion on the scaffold. At day 1 (A), rMSCs cover small areas of the seeded scaffold as thin cell layers, whereas after 21 days of culture (B), rMSCs spread over the surface forming large confluent cell layers. SEM. Scale bars: (A,B) = 20 µm.
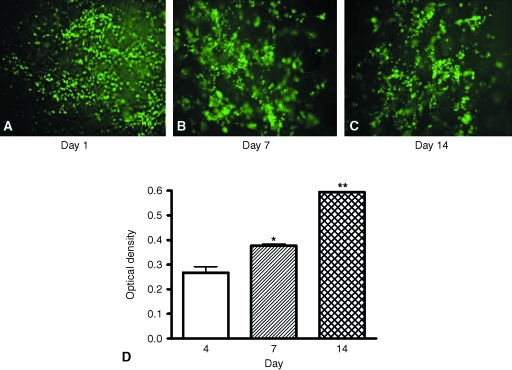
As shown in Fig. 3, MSCs not only remained viable during their growth in the scaffold but also progressively proliferated during incubation times.
Fig. 3.
rMSC viability and proliferation into HYAFF®11 non-woven scaffold. Micrographs (A–C; magnification 10×) show representative CFDA staining of rMSCs at different times of culture onto HYAFF®11. Proliferation of rMSCs up to 14 days (D) was estimated by the MTT assay as described in Materials and methods. Values are expressed as mean ± SEM (n = 3). One-way anova was performed as statistical analysis (P < 0.01), followed by Dunnett's comparison test. *P < 0.05, **P < 0.01 vs. day 4.
Morphological analysis of rMSC interaction with and migration through HYAFF®11 fibres
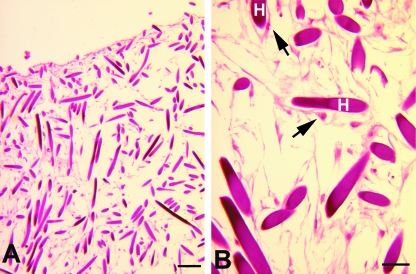
LM showed that rMSCs were able to migrate throughout the entire thickness of the scaffold; cells permeating the inner regions of the scaffold were observed after 3 days of culture even though rMSCs only appeared more homogeneously distributed in the scaffold thickness from day 7 of culture (Fig. 4A,B).
Fig. 4.
rMSC migration through HYAFF®11 fibres. LM showing rMSC migration in the inner portions of the scaffold at day 3 (A). At higher magnification (B) cells are spindle-shaped and viable. The arrows indicate rMSCs. H, HYAFF®11 fibre. Scale bars: (A) 200 µm; (B) 50 µm.
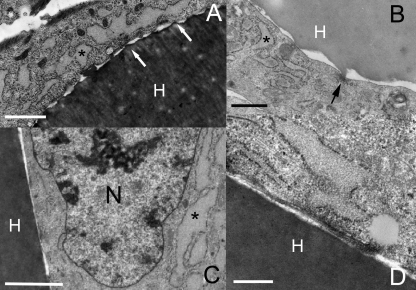
At day 7 of culture, TEM showed the rMSCs tightly adhered to the fibres through well-spaced focal contacts (Fig. 5A,B); nuclear chromatin was finely dispersed and the cytoplasm was rich in dilated cisternae of rough endoplasmic reticulum (Fig. 5C). After 14 days, a fibronexus was found in the free narrow space located between cell membrane and biomaterial surface (Fig. 5D). At this time, TEM revealed that rMSCs still had a synthetic phenotype and no significant degenerative changes, e.g. vacuoles, secondary lysosomes, lipid inclusions, were observed.
Fig. 5.
rMSC interaction with HYAFF®11 fibres. TEM of 7-day culture. (A,B) rMSCs wrapping individual scaffold fibres; the arrows indicate regularly spaced focal contacts and the asterisks show dilated cisternae of rough endoplasmic reticulum in the cytoplasm. (C) rMSCs have nuclei (N) with finely dispersed chromatin and dilated cisternae of rough endoplasmic reticulum (asterisk) in the cytoplasm. TEM of 14-day culture. (D) At high magnification a distinct fibronexus is seen connecting the contractile cell cytoskeleton with the outer portion of a scaffold fibre. TEM. H, HYAFF®11 fibre. Scale bars: (A,B) 2 µm; (C) 1 µm; (D) 0.5 µm.
Morphological and immunohistochemical analysis of rMSC matrix protein synthesis
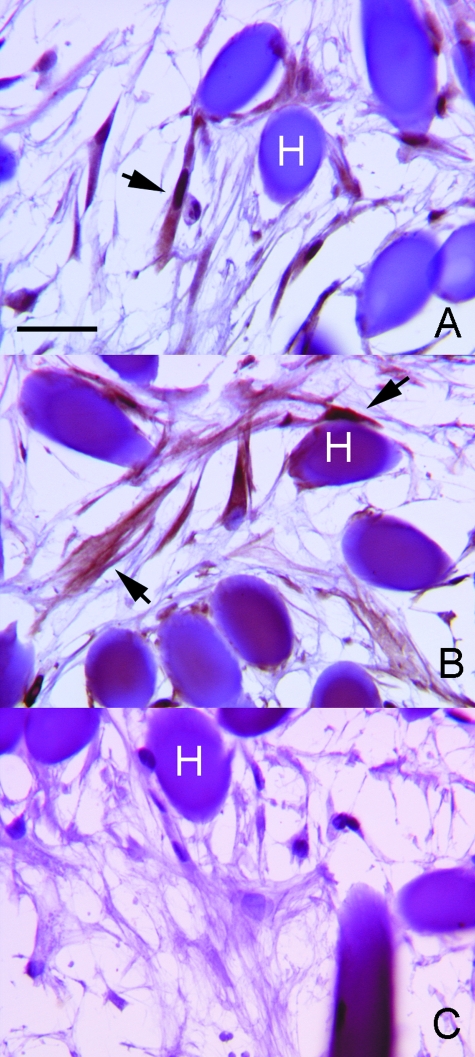
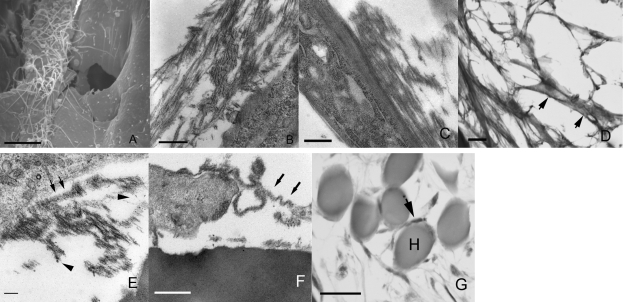
After 21 days, immunohistochemical analysis showed that rMSCs expressed the matrix proteins decorin (Fig. 6A) and versican (Fig. 6B), and combined ultrastructural investigations evidenced the presence of extracellular matrix filamentous (Fig. 7A) and amorphous-fibrillary (Fig. 7B) material in close contact with the rMSC plasma membrane. In particular, TEM (Fig. 7C) showed prominent thin filaments aligned along the rMSC long axis; these filaments possibly corresponded to the adhesive protein fibronectin, which immunohistochemistry revealed to be intensely expressed at the rMSC cell periphery (Fig. 7D); at higher magnification TEM showed that a few collagen fibres and granulofilamentous proteoglycans (Fig. 7E) were also present. An additional finding was the presence of ribbon-like, undulating, moderately electron-dense matrix structures that partially followed the contours of the cell membrane (Fig. 7F); this characteristic along with the observed immunohistochemical positivity for type IV collagen (Fig. 7G) was believed to be consistent with external lamina formation.
Fig. 6.
rMSC extracellular matrix synthesis under culture conditions with HYAFF®11 fibres. After 21 days of culture, immunohistochemistry shows intracellular rMSC synthesis of the ECM components decorin (A) and versican (B). (C) Control experiment performed by omitting the primary antibody. Immunohistochemistry. The arrows indicate cells positive for specific antibodies. H, HYAFF®11 fibre. Scale bars: (A–C) 20 µm.
Fig. 7.
rMSC extracellular matrix delivery under culture with HYAFF®11 fibres. Extracellular filaments (A) and amorphous-fibrillary (B) material are seen using SEM and TEM, respectively. TEM shows thin filaments aligned along the rMSC long axis (C) possibly corresponding to the intense pericellular fibronectin positivity (short arrows) seen at immunohistochemistry (D). (E) Individual collagen fibres (arrows), and granulofilamentous proteoglycans (arrowheads) are focally seen by TEM. (F) Ribbon-like moderately electron-dense structures (arrows) reminiscent of external lamina and weak linear positivities for type IV collagen (arrow) (G) are observed using TEM and IHC, respectively. H, HYAFF®11 fibre. Scale bars: (A) 10 µm; (B) 1 µm; (C) 0.5 µm; (D) 20 µm; (E) 100 nm; (F) 0.5 µm; (G) 20 µm.
Confocal and immunofluorescence analysis of CD44 and CD90 expression
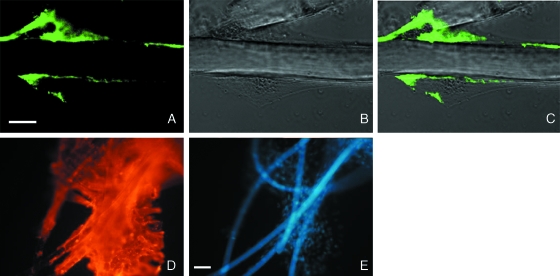
At day 3, confocal images of MSCs cultured on non-woven HYAFF®11 showed a polarized cell membrane expression of CD44 molecule (Fig. 8A–C). After 21 days, the abundance of CD90, a marker of MSC stemness, was not negatively influenced by cell–scaffold interaction, showing a homogeneous membranous pattern (Fig. 8D,E).
Fig. 8.
Confocal and immunofluorescence microscopy of CD44 and CD90 expression of rMSCs grown in the scaffold. Confocal images of immunohistochemical analysis of CD44 antigen (A–C) in rMSCs at day 3. (A) CD44 expression (green); (B) phase contrast; (C) merged image. CD44 expression is polarized in correspondence with the scaffold contact. Scale bars: (A–C) = 10 µm. Immunofluorescence detection of CD90 (D,E) at day 21. (D) CD90-related Cy3 fluorescence (red) was extended to a large portion of the sample. (E) Nuclear staining with DAPI (blue) shows the high number of MSCs which are present in the sample. In the background the hyaluronan fibres are weakly stained with DAPI. Scale bars: (D,E) = 50 µm.
Table 1 presents a scheme of the major findings of the present study.
Table 1.
Major findings of the interaction between rMSCs and non-woven HYAFF®11. SEM, scanning electron microscopy; TEM, transmission electron microscopy; LM, light microscopy; IHC, immunohistochemistry; CFDA, carboxyfluorescein diacetate; MTT: (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
| Investigated characteristic | Technique | Result |
|---|---|---|
| Cell adhesion (quantitative) | Trypan blue stain | 96 ± 0.4% (at day 1) |
| Cell adhesion (qualitative) | SEM and TEM | Sheets of spindle cells; focal contacts; fibronexus |
| Cell viability | CFDA stain, LM, TEM | High, unaffected by culture conditions; degeneration not a significant morphological finding |
| Cell proliferation | MTT stain | Steady growth during culture time |
| Scaffold surface coverage | SEM | 40 ± 10% (at day 21) |
| Migration | LM | rMSCs seen below the scaffold surface at day 3; in the entire thickness at day 7 |
| ECM synthesis and delivery | IHC and TEM | Decorin and versican cytoplasmic positivity; prominent fibronectin extracellular deposition; scant collagen fibres and granulofilamentous proteoglycans; external lamina and collagen type IV at day 21 |
Discussion
HYAFF® is a biomaterial derived from the chemical modification, i.e. esterification, of native hyaluronan, which is an abundant non-sulfated glycosaminoglycan component of synovial fluid and extracellular matrices ubiquitous in the body. At higher percentages of esterification, the resulting HYAFF® materials become insoluble in water and can be extruded to produce fibres in the micron scale range. The esterification process provides a natural biomaterial with adequate biomechanical properties but still retaining its native biocompatibility and biodegradability (Campoccia et al. 1998; Mori et al. 2004). In appropriate experimental settings HYAFF®11 scaffolds have been demonstrated to have properties which are interesting for the field of tissue engineering. HYAFF®11 promotes the adhesion and growth of several cytotypes, including keratinocytes and skin fibroblasts (Tonello et al. 2005), endothelial and smooth muscle cells (Turner et al. 2004; Lepidi et al. 2006) and chondrocytes (Mori et al. 2004; Facchini et al. 2006). In vivo HYAFF®11 is biocompatible (Campoccia et al. 1996) and resorbed within 15–120 days after implantation in relation to the degree of its esterification (Radice et al. 2000; Lepidi et al. 2006). HYAFF®11 is already used by clinicians as scaffold material for the reconstruction of cartilage defects in trauma patients (Marcacci et al. 2002; Pavesio et al. 2003).
The possibility of combining such biological properties of HYAFF®11 with those offered by multipotent stem cells has been previously investigated by others with the aim at improving cartilage repair (Radice et al. 2000; Lisignoli et al. 2001; Rhodes et al. 2004; Cristino et al. 2005; Facchini et al. 2006).
In the present study we focused on the basic abilities of HYAFF®11 to facilitate the adhesion, migration and proliferation of rMSCs under static culture conditions without any chemical induction.
The plain morphological analysis of the non-woven HYAFF®11 scaffold confirms its microfibrous nature and gross porous structure and its three-dimensional architecture, which could be essential in facilitating the migration of the seeded rMSCs within its entire thickness. In confirmation, starting 3 days after the initial cell seeding, we found cells permeating the innermost region of the scaffold. This finding mimics one of the pivotal in vivo properties of hyaluronate, i.e. facilitating cell migration.
The presence of a rough surface dramatically expands the sites of possible chemical interactions between cells and substrate; this previously unrecognized finding, which we documented by both SEM and TEM, could contribute to early effective cell adhesion on the HYAFF®11 surface. Coherent with this view, the rMSC retention in the substrate was high at day 1 and the number of rMSCs entrapped within HYAFF®11 was found to be comparable to the number of the originally seeded cells using the trypan blue exclusion stain method. However, it should be emphasized that a scaffold having a nanoscaled fibrous composition should be even more effective for many bioengineering applications where there is the necessity to mimic the nanoscaled structures found in natural extracellular matrices (Zong et al. 2005). Accordingly, the HYAFF®11 construct investigated here could have a greater indication for repair of wide tissue defects, as in the case of cartilage repair, wound healing and large vessel replacement.
It is generally accepted that cells attach and organize better around fibres with diameters smaller than the diameter of cells (Laurencin et al. 1999). However, the present study demonstrates that even the microfibres of HYAFF®11 provide adequate anchorage for cell adhesion. SEM analysis clearly demonstrated that rMSCs firmly adhered to the substrate as thin sheets, thus recovering their phenotypic shape rapidly. More importantly, TEM revealed that the adhesion was achieved through regularly spaced adhesion structures, namely focal contacts, which are specifically involved in the interaction of MSCs with extracellular matrix (ECM). Focal contacts represent the ultrastructural mirror of a complex, highly regulated process where integrins in the plasma membrane are clustered with other molecules, pushing cells toward many adhesion-mediated signalling pathways. Schematically, many fundamental cellular functions including motility, proliferation, differentiation, and apoptosis are regulated through cell–ECM interactions (Arnold et al. 2004). A dramatic consequence of functional failure of cell–ECM attachment is anoikis – the subset of apoptosis triggered by inadequate or inappropriate cell–matrix contacts (Frisch & Screaton, 2001). In the present experimental setting no significant cell loss and/or degeneration was found up to 21 days of cell culture on the substrate as detected by CFDA assay and morphological investigations. Moreover, MTT analysis demonstrated that rMSCs proliferated steadily during culture and SEM revealed a subconfluent surface monolayer at the end of culture. As a logical consequence, the interaction between rMSCs and HYAFF®11 was functional and this was also supported by the development of fibronexus, a distinct ultrastructural structure which documents anchorage of the cell contractile cytoskeleton to extracellular bundles of thin filaments possibly represented by fibronectin (Singer, 1979; Eyden, 1993). This morphological observation was confirmed by the presence of an intense pericellular immunohistochemical positivity for the adhesive protein fibronectin. Also, an additional finding supporting a functional interaction is the demonstration by confocal microscopy that the CD44 molecule, the native receptor for hyaluronan acid, was preferentially expressed in correspondence with the material–cell contact.
An additional point of interest is that the rMSC growth on the HYAFF®11 was accompanied by ultrastructural and immunohistochemical documentation of protein synthesis and extracellular matrix production. Evidence of protein synthesis was provided by TEM analysis of early and late cultures showing rMSCs with euchromatic nuclei and numerous rough endoplasmic reticulum cisternae in the cytoplasm. In agreement with the ultrastructural features, at day 21 immunohistochemistry showed cytoplasmic positivities for structural components of the interstitial ECM. In particular, rMSCs expressed decorin, a small leucin-rich proteoglycan which promotes stability of interstitial matrix through the binding of various types of collagens and fibronectin, as well as versican, a versatile molecule that is able to interact with a number of other matrix components, including hyaluronan and fibronectin. In addition to their most typical functions, these proteoglycans have been recently found to have a role as repositories of growth factor and cytokine (Macri et al. 2007); for example, decorin differentially binds and modulates transforming growth factor-beta, thereby controlling the availability of this growth factor for cell activation (Droguett et al. 2006). As a result, the proteoglycan expression we observed during rMSC growth on the HYAFF®11 is expected to have an active role in the remodelling of the engineered scaffold once implanted in vivo.
Although a quantitative analysis of the matrix production was not performed, the ultrastructural and immunohistochemical results showed that late extracellular matrix production occurred in the present experimental model; distinct strands of extracellular material mainly composed of fibronectin filaments, scant collagen fibres and granulofilamentous proteoglycans were seen; moreover, small foci of type IV collagen deposition possibly corresponding to pericellular ribbons of external-like lamina were also found. The immunohistochemical staining pattern, similar to that observed when rMSCs were cultured on plain glass substrates (results not shown), is consistent with the mesenchymal nature of the cell model utilized here. In consonance with this view, others reported extracellular matrix production following hyaluronan-based scaffolds cultures with human mesenchymal stromal cells under plain (Radice et al. 2000) or differentiating conditions (Lisignoli et al. 2005).
In the present study the basic ability of HYAFF®11 to facilitate cell differentiation has not been fully investigated. Overall, the results suggest that prolonged rMSC interaction with the substrate did not modify the multipotency of the investigated cell model. In fact we found that the CD90 surface antigen, a stemness molecule which belongs to the basic phenotype of mesenchymal stem cells, was retained up to the end of the culture. The ultrastructural analysis also revealed that cells preserved their mesenchymal morphology during the experimental setting. This characteristic is desirable for two main reasons. First, specific cell differentiations can be promoted in vitro when appropriate growth factors are utilized; this is the case in osteo-chondrogenesis achieved by culturing human MSCs in a similar polymer scaffold under bFGF (Lisignoli et al. 2001) or TGFβ1 exposure (Lisignoli et al. 2005). Alternatively, cell differentiation of multipotent stem cells could be achieved by implanting a scaffold loaded with multipotent stem cells in an injured tissue; in this case cell differentiation could be primed in vivo by microenvironmental paracrine factors that are known to drive locally specific cell differentiation programs.
Conclusion
The HYAFF®11 non-woven scaffold has many characteristics which makes it attractive for tissue engineering. In the present study we have demonstrated HYAFF®11 supports adhesion, migration, and proliferation of rMSCs as well as the synthesis and delivery of autologous extracellular matrix components under static culture conditions without any chemical induction. The high retention rate and viability of the seeded cells, as well as the fine mechanisms of cell interaction with the substrate, suggest that such a scaffold could be potentially useful when wide tissue defects are to be repaired, as in the case of cartilage repair, wound healing, and large vessel replacement.
Acknowledgments
We thank Dr Cristina Longinotti, Fidia Advanced Biopolymer – FAB – s.r.l., Abano Terme, Italy, for kindly providing HYAFF®11. This research was supported by a grant from Compagnia di San Paolo, Turin, Italy and from Regione Piemonte, Italy (P.P., S.G.).
References
- Arnold M, Cavalcanti-Adam EA, Glass R, et al. Activation of integrin function by nanopatterned adhesive interfaces. Chem Phys Chem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- Campoccia D, Hunt JA, Doherty PJ, et al. Quantitative assessment of the response to films of hyaluronan derivatives. Biomaterials. 1996;17:963–975. doi: 10.1016/0142-9612(96)84670-9. [DOI] [PubMed] [Google Scholar]
- Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, Williams DF. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials. 1998;19:2101–2127. doi: 10.1016/s0142-9612(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Cristino S, Grassi F, Toneguzzi S, et al. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF-11-based prototype ligament scaffold. J Biomed Mater Res A. 2005;73:275–283. doi: 10.1002/jbm.a.30261. [DOI] [PubMed] [Google Scholar]
- Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-beta bioavailability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332–341. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Eyden BP. A brief review of the fibronexus and its significance for myofibroblastic differentiation and tumor diagnosis. Ultrastruct Pathol. 1993;17:613–624. doi: 10.3109/01913129309027797. [DOI] [PubMed] [Google Scholar]
- Facchini A, Lisignoli G, Cristino S, et al. Human chondrocytes and mesenchymal stem cells grown onto engineered scaffold. Biorheology. 2006;43:471–480. [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Gallo MP, Ramella R, Alloatti G, et al. Limited plasticity of mesenchymal stem cells cocultured with adult cardiomyocytes. J Cell Biochem. 2007;100:86–99. doi: 10.1002/jcb.21012. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA., Jr Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- Lepidi S, Abatangelo G, Vindigni V, et al. In vivo regeneration of small-diameter (2 mm) arteries using a polymer scaffold. FASEB J. 2006;20:103–105. doi: 10.1096/fj.05-4802fje. [DOI] [PubMed] [Google Scholar]
- Lisignoli G, Zini N, Remiddi G, et al. Basic fibroblast growth factor enhances in vitro mineralization of rat bone marrow stromal cells grown on non-woven hyaluronic acid based polymer scaffold. Biomaterials. 2001;22:2095–2105. doi: 10.1016/s0142-9612(00)00398-7. [DOI] [PubMed] [Google Scholar]
- Lisignoli G, Cristino S, Piacentini A, et al. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials. 2005;26:5677–5686. doi: 10.1016/j.biomaterials.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Del Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc. 2002;10:154–159. doi: 10.1007/s00167-001-0275-6. [DOI] [PubMed] [Google Scholar]
- Mori M, Yamaguchi M, Sumitomo S, Takai Y. Hyaluran-based biomaterials in tissue engineering. Acta Histochem Cytochem. 2004;37:1–5. [Google Scholar]
- Pavesio A, Abatangelo G, Borrione A, et al. Hyaluronan-based scaffolds (Hyalograft C) in the treatment of knee cartilage defects: preliminary clinical findings. Novartis Found Symp. 2003;249:203–217. [PubMed] [Google Scholar]
- Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Abatangelo G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000;50:101–109. doi: 10.1002/(sici)1097-4636(200005)50:2<101::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Raimondo S, Penna C, Pagliaro P, Geuna S. Morphological characterization of GFP stably transfected adult mesenchymal bone marrow stem cells. J Anat. 2006;208(3):12. doi: 10.1111/j.1469-7580.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes NP, Srivastava JK, Smith RF, Longinotti C. Metabolic and histological analysis of mesenchymal stem cells grown in 3-D hyaluronan-based scaffolds. J Mat Sci: Mat Med. 2004;15:391–395. doi: 10.1023/b:jmsm.0000021108.74004.7e. [DOI] [PubMed] [Google Scholar]
- Singer II. The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell. 1979;16:675–685. doi: 10.1016/0092-8674(79)90040-0. [DOI] [PubMed] [Google Scholar]
- Sodian R, Sperling JS, Martin DP, et al. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 2000;6:183–188. doi: 10.1089/107632700320793. [DOI] [PubMed] [Google Scholar]
- Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28:2738–2746. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonello C, Vindigni V, Zavan B, et al. In vitro reconstruction of an endothelialized skin substitute provided with a microcapillary network using biopolymer scaffolds. FASEB J. 2005;19:1546–1548. doi: 10.1096/fj.05-3804fje. [DOI] [PubMed] [Google Scholar]
- Turner NJ, Kielty CM, Walker MG, Canfield AE. A novel hyaluronan-based biomaterial (Hyaff-11®) as a scaffold for endothelial cells in tissue engineered vascular grafts. Biomaterials. 2004;25:5955–5964. doi: 10.1016/j.biomaterials.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wong T, McGrath JA, Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- Wragg MS, Brooks PN, Doleman N. Biodegradation/toxicity study in the rat. 1996. pp. 1–158. Safepharm Ltd. Project no. 551/27.
- Zong X, Biena H, Chung C-Y, et al. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]