Abstract
l-Glutamate is one of the major excitatory neurotransmitters in the mammalian central nervous system, but recently it has been shown to have a role also in the transduction of sensory input at the periphery, and in particular in the nociceptive pathway. An excess of glutamate is implicated in cases of peripheral neuropathies as well. Conventional therapeutic approaches for treating these diseases have focused on blocking glutamate receptors with small molecules or on reducing its synthesis of the receptors through the inhibition of glutamate carboxypeptidase II (GCPII), the enzyme that generates glutamate. In vivo studies have demonstrated that the pharmacological inhibition of GCPII can either prevent or treat the peripheral nerve changes in both BB/Wor and chemically induced diabetes in rats. In this study, we characterized the expression and distribution of glutamate transporters GLT1, GLAST, EAAC1 and of the enzyme GCPII in the peripheral nervous system of female Wistar rats. Immunoblotting results demonstrated that all glutamate transporters and GCPII are present in dorsal root ganglia (DRG) and the sciatic nerve. Immunofluorescence localization studies revealed that both DRG and sciatic nerves were immunopositive for all glutamate transporters and for GCPII. In DRG, satellite cells were positive for GLT1 and GCPII, whereas sensory neurons were positive for EAAC1. GLAST was localized in both neurons and satellite cells. In the sciatic nerve, GLT1 and GCPII were expressed in the cytoplasm of Schwann cells, whereas GLAST and EAAC1 stained the myelin layer. Our results give for the first time a complete characterization of the glutamate transporter system in the peripheral nervous system. Therefore, they are important both for understanding glutamatergic signalling in the PNS and for establishing new strategies to treat peripheral neuropathies.
Keywords: glutamate, glutamate transporters, immunoblotting, immunohistochemistry, peripheral nervous system
Introduction
l-Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system (CNS) that contributes to fast synaptic neurotransmission. It is involved in complex physiological processes such as memory, learning and plasticity. Recently, glutamate has been shown to have a role also in the transduction of sensory input at the periphery, and in particular in nociceptive transmission (Carlton, 2001). An excess of glutamate, however, is frequently correlated with neurotoxicity and neuronal cell death (Ozawa et al. 1998; Dingledine et al. 1999) and, consequently, it is also implicated in a number of neurological disorders including peripheral neuropathies (Watkins, 2000). For these reasons, therapeutic approaches for treating these diseases have attempted to block glutamate receptors with small antagonist molecules. The mechanism of action of these drugs is believed to be the inhibition of the effects of excitatory aminoacids and intracellular calcium accumulation (Kawamata & Omote, 1996). However, severe side effects have limited the clinical use of such glutamate antagonists (Yashpal et al. 2001; Fisher et al. 2002; Cvrcek, 2008). On the other hand, reducing glutamate synthesis through the inhibition of glutamate carboxypeptidase (GCPII), the enzyme that generates glutamate from N-acetyl-aspartyl-glutamate (NAAG), protects against neurodegeneration and disease progression in multiple in vitro and in vivo models of neurological disorders, including peripheral neuropathies (Bacich et al. 2005). In addition, it appears that GCPII is exclusively recruited to provide a source of glutamate in hyperglutamatergic, excitotoxic conditions and would, therefore, be devoid of the side effects of glutamate antagonist drugs (Zhang et al. 2006). This has been clinically confirmed by exposing patients to GCPII inhibitors at presumed therapeutic doses (van der Post et al. 2005). In animal models, GCPII inhibitors reduce neuropathic pain and ectopic discharges from injured nerves but with slight central sensitization in inflammatory and injury-induced neuropathies (Yamamoto et al. 2001; Carpenter et al. 2003; Yamamoto et al. 2004). These data are in agreement with previous reports that show the ability of GCPII inhibitors to prevent pain, nerve conduction velocity reduction and nerve degeneration in diabetic BB/Wor rats (Zhang et al. 2002). Despite all these therapeutic attempts, little is known about the basic features of the glutamatergic system in the peripheral nervous system (PNS). There is now growing evidence that glutamate may have a signaling function together with acetylcholine at the vertebrate neuromuscular junction (NMJ) (Rinholm et al. 2007). Some glutamate transporters (i.e. GLAST and GLT1) are located in the postsynaptic muscle membrane of the NMJ of skeletal muscle and their presence could also be hypothesized in the Schwann cells, although with a lower density than in the postsynaptic sarcolemma. In agreement with the well-known CNS localization, the expression of GLAST, GLT1 and the neuronal glutamate transporter EAAC1 has also been detected in rat optic nerve (Choi & Chiu, 1997). In addition, GLAST and EAAC1 seemed to be expressed in the glial and neuronal component, respectively, of dorsal root ganglia (DRG), although these results are not conclusive (Berger & Hediger, 2000; Tao et al. 2004).
A deeper knowledge of the pattern of expression and localization of glutamate transporters and GCPII in normal PNS would allow these molecules to be used as a target for pharmacological compounds. The aim of this study is to characterize the expression and the distribution of glutamate transporters GLT1, GLAST, EAAC1 (also known as EAAT3) and of the enzyme GCPII in rat PNS, in particular in DRG and in myelinated fibres of the sciatic nerve.
Materials and methods
All the experiments involving animal care and personnel safety were conducted according to the relevant standard operating procedures of the University of Milano-Bicocca, which were predefined based on good laboratory practice (GLP) conditions.
Animal care and husbandry
The care and husbandry of animals were in conformity with the institutional guidelines in compliance with national (D.L. no. 116, Gazzetta Ufficiale della Repubblica Italiana, suppl. 40, Feb. 18, 1992) and international laws and policies (EEC Council Directive 86/609, OJ L 358, 1, Dec.12, 1987; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996).
Animals
Eight adult female Wistar rats (200–220 g, Harlan Italy, Correzzana, Italy) were housed in a limited-access animal facility where room temperature and relative humidity were set at 22 ± 2 °C and 55 ± 10% respectively. Artificial lighting provided a 24-h cycle of 12 h light/12 h dark (7 a.m.–7 p.m.).
Antibodies
Primary antibodies
Rabbit anti-rat GLT1 (diluted 1 : 100 and 1 : 500 for immunofluorescence analysis and immunoblotting analysis, respectively) polyclonal antibody against the N-terminus region of the protein (Immunological Sciences, Rome, Italy), guinea pig anti-rat GLAST (diluted 1 : 100 and 1 : 1000 for immunofluorescence analysis and immunoblotting analysis, respectively) polyclonal antibody (Chemicon International, Hampshire, UK) against the C-terminus region of the protein, rabbit anti-rat EAAC1 (EAAT3) (diluted 1 : 100 and 1 : 500 for immunofluorescence analysis and immunoblotting analysis, respectively) polyclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany) and polyclonal antibody anti-rat glial fibrillary acidic protein (GFAP, Sigma Aldrich, Milan, Italy), (diluted 1 : 100 for immunofluorescence analysis) were used. Non-commercial mouse anti-rat GCPII (diluted 1 : 150 and 1 : 5000 for immunofluorescence analysis and immunoblotting analysis, respectively) polyclonal antibody was used (Bařinka et al. 2004).
Anti-actin primary antibody (Santa Cruz Biotechnology, Heidelberg, Germany) diluted 1 : 500 was used for immunoblotting analysis.
Secondary antibodies for immunofluorescence analysis
For the detection of GCPII, GLT1, EAAC1 and GLAST, anti-mouse, anti-rabbit and anti-guinea pig secondary antibodies (diluted 1 : 100), tetra-methyl-rhodamine isothiocyanate (TRITC 568)-conjugated (Alexa Fluor, Invitrogen, Paisley, UK) were used. For the detection of GFAP signal, anti-rat secondary antibody fluorescein isothiocyanate (FITC 488)-conjugated (Alexa Fluor) was used.
Secondary antibodies for immunoblotting analysis
For the detection of GCPII and GLAST, respectively, anti-mouse and anti-guinea pig (diluted 1 : 1000) secondary antibodies (Chemicon International) were used. Anti-goat and anti-rabbit (diluted 1 : 1000) secondary antibodies (GE Healthcare, Milan, Italy) were used for the detection of EAAT2 and of EAAC1, respectively.
Immunoblotting study
Tissue preparation
Brain, sciatic nerves and DRG were separately collected from healthy rats at sacrifice under deep CO2 anaesthesia, frozen at −80 °C in liquid nitrogen and then homogenized/resuspended in Tripure Isolation Kit (Roche, Monza, Italy) according to the manufacturer's instructions. Total proteins were measured with the Coomassie® Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). Protein aliquots (40 µg) were solubilized in Laemmli buffer 5X, boiled for 5 min and run onto 11% SDS-polyacrylamide gels (SDS-PAGE).
Incubation protocol
For each primary antibody, immunoblotting analysis was performed according to the manufacturer's instructions. The immunoreactive proteins were visualized using the ECL chemiluminescence system (Euroclone, Pero, Italy).
Quantification
Actin immunoblotting analysis was used as a control for equal loading. The densitometric analysis was performed using the Gel Logic 100 Imaging System (Eastman Kodak Co., Cinisello Balsamo, Italy).
Immunofluorescence study
Tissue preparation
L4–L5 DRG and sciatic nerves were collected at sacrifice and fixed in 4% paraformaldehyde for 2 h. Samples were infiltrated with increasing concentrations of sucrose (7%, 15% and 30%) and cryoprotected in Optimal Cutting Temperature (OCT) before freezing.
Incubation protocol
Cryostat sections 10 µm thick were thaw-mounted on poly-l-lysine-coated microscope slides. All incubations were carried out at room temperature in a humidified container, sections were washed with PBS between each step, and all primary and secondary antibodies were diluted in PBS containing 5% bovine serum albumin and 10% Triton X-100. Samples were incubated overnight with specific primary antibodies at appropriate dilutions and subsequently for 1 h at room temperature with the appropriate secondary antibodies.
Confocal imaging
Samples were examined with a Radiance 2100 confocal laser microscope (Bio-Rad, Hercules, CA, USA) equipped with a krypton/argon laser. For FITC, excitation and emission wavelengths were 488 nm and 520 nm, respectively; for TRITC the wavelengths were 568 nm and 603 nm, respectively. The thickness of z slices was 0.4 µm and noise reduction was achieved by Kalman filtering during acquisition.
Results
Analysis of expression: immunoblotting studies
In western blot, 40 µg per lane of total protein from a pool of a DRG or sciatic nerves of healthy adult Wistar rats was analysed.
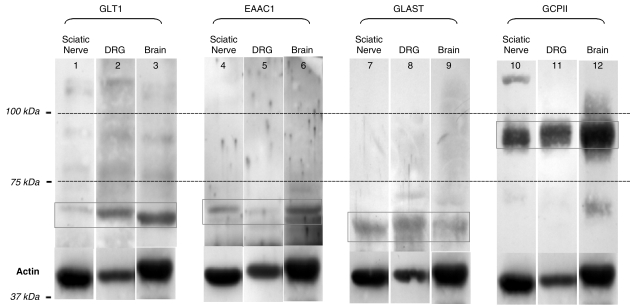
DRG and the sciatic nerve expressed all glutamate transporters and GCPII. As shown in Fig. 1, affinity-purified polyclonal antibodies against the corresponding peptides of the glutamate transporters showed labelled protein bands at 68 kDa for GLT1, 70 kDa for EAAC1, 60 kDa for GLAST and 85 kDa for GCPII. Similar molecular weight bands were shown for the total brain, representing positive controls.
Fig. 1.
Immunoblotting (11% SDS-PAGE). Affinity-purified primary antibody against GLT1 (lanes 1–3), EAAC1 (lanes 4–6), GLAST (lanes 7–9) and GCPII (lanes 10–12) were used. The amount of total protein samples loaded per lane was 40 µg. All three glutamate transporters and GCPII were expressed in both the sciatic nerve and DRG, at the same expected molecular weight as the positive control represented by brain homogenate (GLT1 = 68 kDa, EAAC1 = 70 kDa, GLAST = 60 kDa and GCPII = 85 kDa). Actin (42 kDa) was used for the normalized quantitative densitometric analysis of the bands.
Using actin (42 kDa) as a normalizer in densitometric analysis (Table 1), the intensities of expression of our target molecules were different in DRG and in the sciatic nerve. GLT1, GLAST and GCPII were more intensely expressed in DRG than in sciatic nerve (0.308 vs. 0.053; 0.578 vs. 0.301; 1.470 vs. 0.876), whereas EAAC1 was equally expressed in DRG and in sciatic nerve (0.128 vs. 0.100).
Table 1.
Densitometric quantitative analysis (Gel logic 100 Imaging System). The values in the table show the ratio between the densitometric analysis of the labelled band of our target molecules and the labelled band of actin. GLT1, GLAST and GCPII were expressed more in DRG then in the sciatic nerve (0.308 vs. 0.053; 0.578 vs. 0.301; 1.470 vs. 0.876, respectively). EAAC1 was equally expressed in DRG and in the sciatic nerve (0.128 vs. 0.100)
| Ratios | ||||
|---|---|---|---|---|
| Target organs | GLT1/actin | EAAC1/actin | GLAST/actin | GCPII/actin |
| Sciatic nerve CTRL | 0.053 | 0.128 | 0.301 | 0.876 |
| Dorsal root ganglia CTRL | 0.308 | 0.100 | 0.578 | 1.470 |
| Brain | 2.114 | 0.231 | 0.329 | 1.146 |
Analysis of localization: immunofluorescence studies
DRG
L4-L5 DRG of Wistar rats were immunopositive for all glutamate transporters and for GCPII (Fig. 2), although the pattern of immunostaining was different for each molecule.
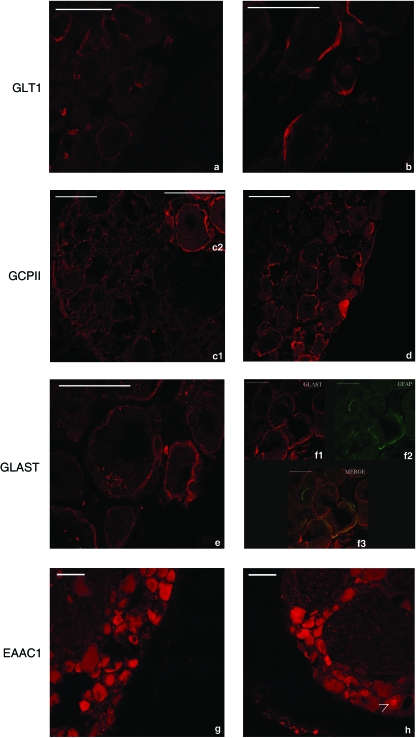
Fig. 2.
Immunofluorescence analysis of dorsal root ganglia with affinity-purified primary antibody against GLT1, GCPII, GLAST and EAAC1. GLT1 (a,b) and GCPII (c,d) immunolabelling is outside the profile of sensory neuron plasma membrane marking the cytoplasmic portions of satellite cells (arrows). GLAST (e,f) immunoreaction was localized in the cytoplasm both of sensory neurons and of satellite cells (arrow). Strong immunolabelling was observed in the cytoplasm of satellite cells, and discrete GLAST-positive spots were seen in sensory neuron cytoplasm. A double staining study (f1-GLAST, f2-GFAP, f3-MERGE) confirmed that GLAST was mainly located in satellite cells. Anti-EAAC1 immunostaining (g,h) strongly labelled the cytoplasm of sensory neurons and, very rarely, the nucleus was also labelled (arrow in h). Bar: 50 µm.
GLT1 immunolabelling was not present in neuronal cell body and, at higher magnification, a strong cytoplasmic labelling was evident in satellite cells (Fig. 2a,b). GCPII immunolabelling was clearly present in the cytoplasm of satellite cells (Fig. 2c,d). Anti-GLAST immunoreaction was localized in the cytoplasm of both sensory neurons and satellite cells (Fig. 2e). Strong immunolabelling was observed in the cytoplasm of satellite cells, whereas discrete GLAST positive spots were seen in sensory neurons. These spots were unevenly distributed in the sensory neuron cytoplasm. As both neurons and satellite cells were immunolabelled, a double-staining experiment, performed with anti-GLAST and anti-GFAP (glial marker for satellite cells) primary antibodies, confirmed that GLAST was mainly located in satellite cells (Fig. 2f). A large number of sensory neurons were immunoreactive for EAAC1 (Fig. 2g,h). EAAC1 strongly labelled the cytoplasm of sensory neurons and, very rarely, the nucleus was also labelled (as shown by the arrow in Fig. 2h). No EAAC1-positive satellite cells were present.
Sciatic nerve
The sciatic nerves of Wistar rats were positive for the immunostaining of all glutamate transporters and of GCPII (Fig. 3).
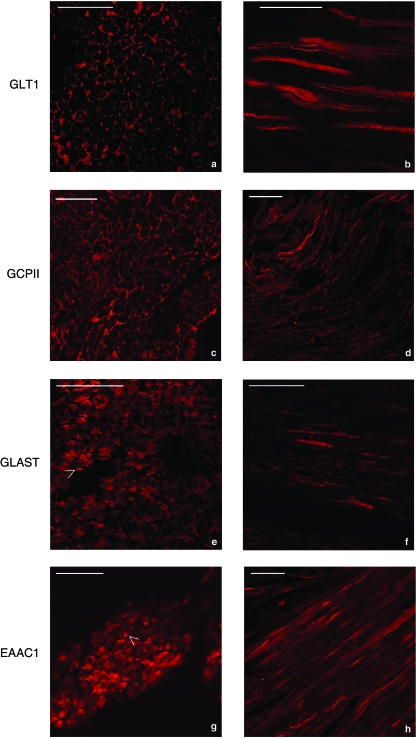
Fig. 3.
Immunofluorescence analysis of the sciatic nerve with affinity-purified primary antibody against GLT1, GCPII, GLAST and EAAC1. In transverse and longitudinal sections of the sciatic nerve (a,b), GLT1 immunolabelling was stronger in the outer cytoplasm of Schwann cells around nerve fibres (arrows). In some cases, the axon also showed positive, although weak, immunoreactivity for anti-GLT1 antibodies (a). In transverse section of the sciatic nerve (c), GCPII immunolabelling was confined to the area around axons, probably in the outer cytoplasm of Schwann cells (arrow). In longitudinal section (d), anti-GCPII immunoreactivity was localized only on the surface of the nerve fibres with no axonal labelling. Anti-GLAST and anti-EAAC1 immunoreactivities (e,f and g,h) were present in the myelin layer immediately around the axon. As shown by the arrows in (e) and (g), spots of strong labelling were visible on the external periphery of the myelin itself. Bar: 50 µm.
In transverse sections of the sciatic nerve, GLT1 immunolabelling was stronger in the outer cytoplasm of Schwann cells (Fig. 3a). In some cases the axon also showed positive but weak immunoreactivity for GLT1. In longitudinal sections of the sciatic nerve, GLT1 was clearly localized in Schwann cells around the nerve fibres (Fig. 3b). In transverse sections of the sciatic nerve, GCPII immunolabelling was confined to the outer cytoplasm of Schwann cells (Fig. 3c). In longitudinal section (Fig. 3d), GCPII was localized only on the surface of the nerve fibres, without any sign of immunolabelling in the axonal internal compartment. GLAST and EAAC1 immunoreactivity (Fig. 3e–h) were present in the myelin layer immediately around the axon. Spots of strong labelling (arrows in Fig. 3e and 3g) were visible on the external periphery of the myelin itself and were due to the positivity to GLAST and EAAC1 of the cytoplasm of glial cells around the axons.
Discussion
In the present study, we characterized the expression and localization of the specific membrane-bound glutamate transporters GLT1, EAAC1, GLAST and of the enzyme GCPII in some areas of the adult rat PNS. So far, very few studies have addressed this issue and therefore only limited information is available as to their expression and localization in the PNS. On the other hand, some studies (Oliveira et al. 2003; Brumovsky P et al. 2007) demonstrated that vesicular glutamate transporters (VGLUTs), representing specific markers for neurons using glutamate as neurotransmitter, are expressed in distinct subpopulations of DRG neurons, indicating that glutamate is involved in the PNS. Moreover, it is well known that inhibition of the enzyme GCPII is neuroprotective, not only in cases of cerebral ischemia (Slusher et al. 1999), schizophrenia (Olszewski et al. 2004), amyotrophic lateral sclerosis (Ghadge et al. 2003) and stroke (Bacich et al. 2005), but also in several peripheral neuropathies (Bacich et al. 2005). The inhibition of GCPII decreases the level of toxic extracellular glutamate and increases the level of NAAG, which acts as an agonist to the group II metabotropic glutamate receptor mGluR3. This result is in accordance with the hypothesis of Berger (Berger et al. 1999) who previously demonstrated the involvement of GCPII in the glutamatergic signalling between Schwann cells and axons. However, on the basis of our immunofluorescence analysis, we could not exclude a slight labelling of the neuronal component of DRG as well. It is also well known that, under normal conditions, the extracellular concentration of glutamate in the CNS strongly depends on its re-uptake into cells through specific transporters located in neurons (EAAC1) and on the membrane of glial cells (GLAST and GLT1). Alterations in the expression and activity of glutamate transporters have been reported in some neurological disorders such as experimental autoimmune encephalomyelitis (EAE) (Ohgoh et al. 2002), motor neuron disease (Banner et al. 2002), neuropathic pain (Sung et al. 2003), amyotrophic lateral sclerosis (Aoki et al. 1998), epilepsy (Miller et al. 1997) and ischemia (Labrande et al. 2006), but no clear information is available about their changes in peripheral neuropathies or about their distribution in normal PNS. Nevertheless, glutamate is now considered to be the principal neurotransmitter in many sensory neurons including DRG and spinal cord neurons. This evidence is based on pharmacological and electrophysiological studies (Yoshimura & Jessell, 1990; Dickenson, 1997; Carlton, 2001; Huettner et al. 2002; Garry et al. 2004; Willis & Coggeshall, 2004) as well as histochemical analyses using antibodies against glutamate (De Biasi & Rustioni, 1988; Westlund et al. 1989; Keast & Stephensen, 2000). Analysis of RT-PCR, western blot and immunocytochemistry (Tao et al. 2004) have provided new evidence that the neuronal transporter EAAC1 is expressed in small sensory neurons of DRG and in nociceptive primary afferent fibres terminating within the superficial dorsal horn of the spinal cord. Starting from these observations, in the present study we looked at the expression of EAAC1 in DRG, and we narrowed down its localization in the cytoplasm of sensory neurons, with a sporadic labelling also of their nuclei. Our immunoblotting and immunofluorescence analysis also demonstrated the expression and localization of EAAC1 in the sciatic nerve. Labelling was confined to the area immediately around axons, probably marking the myelin layer. Some spots of labelling were clearly visible in the periphery of the layer itself. These strongly labelled spots could be due to the positivity to EAAC1 of the cytoplasm of satellite cells around the axons. No previous data were available about the expression and localization of this molecule in the sciatic nerve. A recent study (Kugler & Beyer, 2003) demonstrated that the cellular expression of glutamate transporters in the optic nerve was almost the same as in other white matter tracts of the CNS. In particular, the neuronal transporter EAAC1 was localized by immunocytochemistry in the axoplasm of nerve fibres. Moreover, in our study, we localized GLAST both in the satellite and in the neuronal cells of DRG with a stronger signal from the glial component. In the sciatic nerve, GLAST labelling was mainly confined to the area immediately around axons, staining the myelin layer in the same way as EAAC1. In accordance with our results, previous data demonstrated that GLAST mRNA was expressed by satellite cells in DRG, outlining neuronal cell bodies (Berger & Hediger, 2000), and by astrocytes in the optic nerve of rat (Otori et al. 1994; Choi & Chiu, 1997; Kugler & Beyer, 2003). However, GLAST and GLT1 mRNA expression was not reported in satellite cells, although occasional DRG neurons appeared to express appreciable levels of GLT1 mRNA (Berger & Hediger, 2000), whereas in our study we demonstrated that GLT1 strongly labelled the cytoplasm of satellite cells in DRG and the outer cytoplasm of Schwann cells in the nerve fibres of the sciatic nerve. We determined the expression of GCPII both in the sciatic nerve and in DRG by immunoblotting analysis. Moreover, our immunofluorescence analysis demonstrated GCPII localization in the satellite cell compartment of DRG and in Schwann cells of the sciatic nerve. However, on the basis of our immunofluorescence analysis, we could not exclude a slight labelling also of neuronal components of DRG.
Concluding remarks
In this study, we revealed the expression and the localization of glutamate transporters and GCPII in the PNS. This knowledge is fundamental for the understanding of glutamatergic signalling in the PNS and may be the first step towards using glutamate transporters and GCPII as new targets for pharmacologic compounds able to contrast glutamate toxicity in peripheral neuropathies.
Acknowledgments
Thanks to Dr E. Genton for language assistance and to Drs Rena Lapidus and Krystina Wozniak (MGI Pharma, Baltimore, MD) for the helpful discussion of the results.
References
- Aoki M, Lin CL, Rothstein JD, et al. Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Ann Neurol. 1998;43:645–653. doi: 10.1002/ana.410430514. [DOI] [PubMed] [Google Scholar]
- Bacich DJ, Wozniak KM, May Lu XC, et al. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–323. doi: 10.1111/j.1471-4159.2005.03361.x. [DOI] [PubMed] [Google Scholar]
- Banner SJ, Fray AE, Ince PG, Steward M, Cookson MR, Shaw PJ. The expression of the glutamate re-uptake transporter excitatory amino acid transporter 1 (EAAT1) in the normal human CNS and in motor neuron disease: an immunohistochemical study. Neuroscience. 2002;109:27–44. doi: 10.1016/s0306-4522(01)00437-7. [DOI] [PubMed] [Google Scholar]
- Bařinka C, Mlčochová P, Šácha P, et al. Amino acids at the N- and C-termini of human glutamate carboxypeptidase II are required for enzymatic activity and proper folding. Eur J Biochem. 2004;271(13):2782–2790. doi: 10.1111/j.1432-1033.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J Comp Neurol. 2000;421:385–399. doi: 10.1002/(sici)1096-9861(20000605)421:3<385::aid-cne7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, et al. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Watanabe M, Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:469–490. doi: 10.1016/j.neuroscience.2007.02.068. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral excitatory amino acids. Curr Opin Pharmacol. 2001;1:52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Carpenter KJ, Sen S, Matthews EA, et al. Effects of GCP-II inhibition on responses of dorsal horn neurones after inflammation and neuropathy: an electrophysiological study in the rat. Neuropeptides. 2003;37:298–306. doi: 10.1016/j.npep.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Choi I, Chiu SY. Expression of high affinity neuronal and glial glutamate transporters in rat optic nerve. Glia. 1997;20:184–192. [PubMed] [Google Scholar]
- Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain. 2008;9:253–257. doi: 10.1111/j.1526-4637.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci USA. 1988;85:7820–7824. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH. NMDA receptor antagonists: interactions with opioids. Acta Anaesthesiol Scand. 1997;41:112–115. doi: 10.1111/j.1399-6576.1997.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channel. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Garry EM, Jones E, Fleetwood-Walker SM. Nociception in vertebrates: key receptors participating in spinal mechanisms of chronic pain in animals. Brain Res Brain Res Rev. 2004;46:216–224. doi: 10.1016/j.brainresrev.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ghadge GD, Slusher BS, Bodner A, et al. Glutamate carboxypeptidase II inhibition protects motor neurons from death in familial amyotrophic lateral sclerosis models. Proc Natl Acad Sci USA. 2003;100(16):9554–9559. doi: 10.1073/pnas.1530168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Kerchner GA, Zhuo M. Glutamate and the presynaptic control of spinal sensory transmission. Neuroscientist. 2002;8:89–92. doi: 10.1177/107385840200800204. [DOI] [PubMed] [Google Scholar]
- Kawamata M, Omote K. Involvement of increased excitatory amino acids and intracellular calcium concentration in the spinal dorsal horn in an animal model of neuropathic pain. Pain. 1996;68:85–96. doi: 10.1016/S0304-3959(96)03222-8. [DOI] [PubMed] [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424:577–587. [PubMed] [Google Scholar]
- Kugler P, Beyer A. Expression of Glutamate transporters in human and rat retina and rat optic nerve. Histochem Cell. 2003;120:199–212. doi: 10.1007/s00418-003-0555-y. [DOI] [PubMed] [Google Scholar]
- Labrande C, Velly L, Canolle B, et al. Neuroprotective effects of tacrolimus (FK506) in a model of ischemic cortical cell cultures: role of glutamate uptake and FK506 binding protein 12 kDa. Neuroscience. 2006;137:231–239. doi: 10.1016/j.neuroscience.2005.08.080. [DOI] [PubMed] [Google Scholar]
- Miller H, Levey AI, Rothstein JD, Tzingounis AV, Conn PJ. Alterations in glutamate transporter protein levels in kindling-induced epilepsy. J Neurochem. 1997;68:1564–1570. doi: 10.1046/j.1471-4159.1997.68041564.x. [DOI] [PubMed] [Google Scholar]
- Ohgoh M, Hanada T, Smith T, et al. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;125:170–178. doi: 10.1016/s0165-5728(02)00029-2. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, et al. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Olszewski RT, Buchari N, Zhou J. NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem. 2004;89:876–885. doi: 10.1111/j.1471-4159.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- Otori Y, Shimada S, Tanaka K, Ishimoto I, Tano Y, Tohyama M. Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retina ischemia. Brain Res Mol Brain Res. 1994;27:310–314. doi: 10.1016/0169-328x(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Slettaløkken G, Marcaggi P, et al. Subcellular localization of the glutamate transporters GLAST and GLT at the neuromuscular junction in rodents. Neuroscience. 2007;16:579–591. doi: 10.1016/j.neuroscience.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Liaw WJ, Zhang B. Evidence of neuronal excitatory amino acid carrier 1 expression in rat dorsal root ganglion neurons and their central terminals. Neuroscience. 2004;123:1045–1051. doi: 10.1016/j.neuroscience.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Van der Post JP, de Visser SJ, de Kam ML, et al. The central nervous system effects, pharmacokinetics and safety of the NAALADase-inhibitor GPI 5693. Br J Clin Pharmacol. 2005;2:128–136. doi: 10.1111/j.1365-2125.2005.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC. L-glutamate as a central neurotransmitter: looking back. Biochem Soc Trans. 2000;28:297–309. [PubMed] [Google Scholar]
- Westlund KN, McNeill DL, Coggeshall RE. Glutamate immunoreactivity in rat dorsal root axons. Neurosci Lett. 1989;96:13–17. doi: 10.1016/0304-3940(89)90235-8. [DOI] [PubMed] [Google Scholar]
- Willis WDJ, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 3rd edn. New York: Plenum; 2004. [Google Scholar]
- Yamamoto T, Nozalis-Tagushi N, Sakashita Y. Inhibition of spinal N-acetylated-alpha-linked acidic dipeptidase produces an antinociceptive effect in the rat formalin test. Neuroscience. 2001;102:473–479. doi: 10.1016/s0306-4522(00)00502-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hirasawa S, Wroblewska B. Antinociceptive effects of N-acetylaspartylglutamate (NAAG) peptidase inhibitors ZJ-11, ZJ-17 and ZJ-43 in the rat formalin test and in the rat neuropathic pain model. Eur J Neurosci. 2004;20:483–494. doi: 10.1111/j.1460-9568.2004.03504.x. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Fisher K, Chabot JG, Coderre TJ. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 2001;94:17–29. doi: 10.1016/S0304-3959(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Murakawa Y, Wozniak KM, Slusher BS, Sima AA. The preventive and therapeutic effects of GCPII (NAALADase) inhibition on painful and sensory diabetic neuropathy. J Neurol Sci. 2006;247:217–223. doi: 10.1016/j.jns.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Zhang W, Slusher BS, Murakawa Y, et al. GCPII (NAALADase) inhibition prevents long-term diabetic neuropathy in type 1 diabetic BB/Wor rats. J Neurol Sci. 2002;194:21–28. doi: 10.1016/s0022-510x(01)00670-0. [DOI] [PubMed] [Google Scholar]