Abstract
Glial cell line-derived neurotrophic factor (GDNF) acts through RET receptor tyrosine kinase and its co-receptor GFRalpha1. In an effort to better understand the possible biological contribution of the GDNF and GFRalpha1/RET complex in pancreatic development, in this study we report the cellular localization of these proteins in the pancreas of domestic cat embryos and fetuses by immunocytochemical methods. In early embryos, GDNF, GFRalpha and RET immunoreactivity (IR) was localized in closely intermingled cells. GDNF and RET immunoreactive cells displayed chromogranin (an endocrine marker) and PGP 9.5 (a neuronal marker) IR, respectively. GFRalpha IR was present in both a few GDNF/chromogranin and RET/PGP 9.5 immunoreactive cells. In elderly fetuses, GDNF and GFRalpha IR were co-localized in glucagon cells and RET IR was detected in few neurons and never co-localized with GFRalpha or GDNF IR. In early embryos, the presence of GDNF IR in chromogranin immunoreactive cells and GFRalpha1/RET complex IR in PGP9.5 immunoreactive cells seems to suggest a paracrine action of GDNF contained in endocrine cell precursors on neuronal cell precursors expressing its receptor complex. The presence in different cell populations of RET and its co-receptor GFRalpha1 IR could be due to independent signaling of GRFalpha1. Thus, the co-presence of GDNF and GFRalpha1 in chromogranin and glucagon cells could lead to the hypothesis that GDNF can act in an autocrinal manner. In fetuses, RET IR was detected only in intrapancreatic ganglia. Because of the lack of GFRalpha1 IR in pancreatic innervation, RET receptor could be activated by other GFR alphas and ligands of GDNF family. In conclusion, these findings suggest that in differently aged embryos and fetuses the GDNF signal is differently mediated by RET and GFRalpha1.
Keywords: digestive system, embryos, neurotrophic-factors
Introduction
Glial cell line-derived neurotrophic factor (GDNF), together the related growth factors neurturin, artemin and persephin, acts through RET receptor tyrosine kinase. RET is alternatively spliced, producing at least two isoforms: a short RET isoform, or RET9, and a long RET isoform, or RET51. The ligand-binding specificity is determined by a glycosyl phosphatidylinositol (GPI)-linked ligand-binding subunit known as GDNF family receptor alpha (GFRalpha). GFRalpha1, GFRalpha2, GFRalpha3 and GFRalpha4 receptors bind to GDNF, neurturin, artemin and persephin, respectively. Some putative cross-talks were described between GDNF/GFRalpha2 and artemin/GFRalpha1 (for a review see Sariola & Saarma, 2003).
GDNF is a growth factor of many neuronal populations in the central, peripheral and autonomous nervous system. Outside of the nervous system, it induces branching of the ureteric bud in vitro and appears to be involved in the regulation of the spermatogenesis (for a review see Costantini, 2006; Huleihel et al. 2007; Runeberg-Roos & Saarma, 2007).
In the pancreas of adult rats, GDNF is a critical component of the response to experimentally induced pancreatitis in rat (Toma et al. 2002). In man, GDNF appears to promote pancreatic cancer cell proliferation and intrapancreatic neural invasion through its receptors (Ito et al. 2005). Transgenic mice overexpressing GDNF in glia pancreas showed increased beta-cell mass, and insulin content (Mwangi et al. 2008).
In the pancreas of embryos, however, there are no studies concerning the presence and role of GDNF. In an effort to better understand the possible biological contribution of the GDNF and GFRalpha1/RET complex in the development of the pancreas, in this study we report the cellular localization of these proteins in the developing pancreas of the domestic cat. Although the majority of the studies on pancreatic development were conducted in rat and mouse, other mammalian species could be considered more suitable models for pancreatic studies because of the higher similarity to human pancreas (for a review see Case, 2006). Moreover, domestic cat is a species used for embryological studies today because its gestation is short and it is easy to care for (Knospe, 2002).
Materials and methods
Animals
Fetuses aged according to Knospe (2002) were taken from ovariohysterectomized pregnant queens. At 54 min before surgery, cats were premedicated with atropine sulfate (ATI) 0.025 mg kg−1 SC and Rimadyl (carprofen, Pfizer Inc., New York, NY, USA) 2 mg kg−1 SC. Some minutes later, cats were sedated with Domitor (medetomidine hydrochloride, Pfizer Inc.) 0.05 mL kg−1 IM and Altadol (tramadol chlorohydrate Formevet Animal Health) 2 mg kg−1 IM. Anaesthesia was induced with Rapinovet 0.4 mL kg−1 (propofol 4 mg kg−1, Schering-Plough Spa) and maintained after tracheal intubation with 1.5% isoflurane in 1 L min−1 of oxygen.
Eight embryos belonging to stage 11 (17–18 days), 14 (21–23 days), 16 (25–28 days) were fixed in toto and three fetuses of stage 19 (38–44 days) were fixed after decapitation. Two fetuses belonging to stage 22 were excised and the pancreas removed. Fixation was by immersion in Bouin's fluid for 12–48 h at room temperature (RT). They were then dehydrated in an ethanol series and embedded in paraffin wax. Sagittal, transversal and horizontal 7-µm-thick sections were cut.
Single immunocytochemical staining
Immunocytochemical staining was performed using the peroxidase-antiperoxidase (PAP) method (Sternberger, 1986). After dewaxing in xylene, sections were rinsed in distilled water and subjected to microwave oven treatment to unmask the antigens (0.01 m sodium citrate buffer, pH 6.0, for 10 min at 750 W) (Reynolds et al. 1994). Then, sections were rinsed in distilled water and treated with 3% H2O2 (20 min) to block endogenous peroxidase activity. After rinsing in phosphate-buffered saline (PBS), pH 7.4, containing 0.2% Triton X-100 and 0.1% bovine serum albumin (BSA), background blocking was achieved by incubating the sections with 1 : 5 normal serum of the species in which the primary antiserum was raised (rabbit serum: 011-000-120, goat serum: 005-000-121, Jackson ImmunoResearch, Baltimore Pike, PA, USA) for 30 min at RT. The sections were then incubated in a humid chamber for 24 h at 4 °C with each of primary antibodies shown in Table 1.
Table 1.
Antisera employed in this study. lm, light microscopy, fm, fluorescence microscopy
| Antisera | Source | Host | Characteristics | Dilution lm | Dilution fm |
|---|---|---|---|---|---|
| GDNF (D-20) | sc-328 Santa Cruz Biotechnology | Rabbit | C-terminus of GDNF human origin | 1 : 400 | |
| GFR alpha 1 (H-70) | sc-10716 Santa Cruz Biotechnology | Rabbit | C-terminus of GFRalpha1human origin | 1 : 300 | 1 : 30 |
| RET (C-19) | sc-167 Santa Cruz Biotechnology | Rabbit | C-terminus of RET isoform C (RET 9) of human origin | 1 : 200 | 1 : 20 |
| RET (T-20) | sc-1291 Santa Cruz Biotechnology | Goat | C-terminus of RET isoform A (RET 51) of human origin | 1 : 200 | |
| Insulin | GHC 7303 Peninsula Lab | Guinea pig | Human antigen | 1 : 50 | |
| Glucagon | GHC 7165 Peninsula Lab | Guinea pig | Human antigen | 1 : 50 | |
| Somatostatin 14 | 20067 Incstar Lab | Rabbit | Human antigen | 1 : 50 | |
| Chromogranin | 642 DiaSorin | Rabbit | Bovine antigen | 1 : 100 | |
| PGP 9.5 | E3344 Spring Bioscience | Rabbit | Human antigen | 1 : 50 |
After incubation, the sections were washed in PBS, incubated with antiserum raised in goat against rabbit IgG (GAR, 1 : 50; 111-035-003 Jackson ImmunoResearch) or with antiserum raised in rabbit against goat IgG (RAG 1 : 50; 305-005-003 Jackson ImmunoResearch), for 30 min at RT, then washed in PBS, and incubated with rabbit PAP complex (1 : 100; 323-005-025 Jackson ImmunoResearch) or with goat PAP complex (1 : 100; 123-005-025) for 30 min at RT. The sections were rinsed again, and the immunoreactive sites were visualized using a fresh solution of 10 µg of 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma Chemical Co.) in 15 mL of a 0.5 m Tris buffer, pH 7.6, containing 1.5 mL of 0.03% H2O2.
Sections were lightly counterstained with Mayer's hematoxylin to ascertain structural details.
Double immunocytochemical staining
Double immunohistochemical staining using primary antisera raised in different species was performed as follows: dewaxed and rehydrated consecutive sections were rinsed in PBS and incubated for 48 h at RT with the primary antibodies diluted with normal donkey serum 1 : 5. After rinsing in PBS, the sections were incubated for 2 h at RT with a mixture of affinity-purified FITC-conjugated donkey anti-rabbit IgG (Jackson, West Grove, PA, USA, 711-095-152, diluted 1 : 30) and affinity-purified TRITC-conjugated donkey anti-guinea pig IgG (Jackson, 711-095-148, diluted 1 : 50).
Double immunohistochemical staining using two primary antisera raised in the same species, i.e. rabbit, was performed according to Wessel & McClay (1986), who used fluorochrome-conjugated Fab fragments to avoid cross-talk of subsequent antisera. For this case, dewaxed, rehydrated, blocked sections were rinsed in 0.01 m PBS (pH 7.4) containing 0.2% Triton X-100 and 0.1% BSA. Sections were then incubated with primary antiserum diluted with normal donkey serum 1 : 5 for 48 h at RT. Following this, the sections were washed in PBS and incubated with GAR-Fab fragment conjugated to FITC fluorochrome, diluted 1 : 30; (111-097-007, Jackson), for 2 h at RT. Thereafter the sections were rinsed in PBS and incubated with the other primary antiserum diluted with normal donkey serum 1 : 5 for 48 h at RT. After rinsing in PBS, the sections were treated with affinity-pure donkey anti-rabbit IgG conjugated to TRITC fluorochrome (711-025-152, Jackson), diluted 1 : 50 for 2 h at RT. Finally the sections were washed with PBS, mounted with glycerin diluted with PBS 1 : 1.
Microscopical photographs
Immunocytochemical stainings were photographed using a Leica microscope DM RA2 (Leica Camera AG, Solms, Germany) attached to a Leica DC 300 F camera for light microscopy or to a Leica DC300 camera for fluorescence and stored in a Leica IM1000 archive. Photomicrograph processing and lettering was carried out with the CorelDraw software (version 12; Corel Corporation, Ottawa, Canada). Color balance, contrast, and brightness of the images were adjusted to a variable extent and most of the color images were converted to gray-scale.
Controls
The specificity of the single immunocytochemical staining was tested by successively substituting the primary or secondary antisera, or the PAP complex with PBS or normal serum, in repeated trials. Adsorption controls were performed by using antigens shown in Table 2.
Table 2.
Antigens used for adsorption controls. The antisera were adsorbed at highest working dilution employed for light microscopy. Homologous antigen (antigens which induced antiserum formation) was added to antiserum up to 25 µg mL−1, heterologous antigen (antigen which can react with an antiserum but is not the one that induced its formation) was added to antiserum up to 50 µg mL−1
| Antisera adsorbed | Homologous antigen | Heterologous antigen |
|---|---|---|
| GDNF (D-20) | GDNF (sc-328 P Santa Cruz Biotechnology) | Neurturin (sc-6362 P Santa Cruz Biotechnology), persephin (sc-8684 P Santa Cruz Biotechnology), artemin (sc-9330 P Santa Cruz Biotechnology) |
| GFR alpha 1 (H-70) | GFR alpha1 (sc-6157 P Santa Cruz Biotechnology) | GFRalpha 2 (sc-7414 P Santa Cruz Biotechnology); GFRalpha 3 (sc-9340 P Santa Cruz Biotechnology); GFRalpha 4 (sc-46987 Santa Cruz Biotechnology) |
| RET (C-19) | RET (sc-167 P Santa Cruz Biotechnology) | Fibroblast growth factor receptor 1 (sc-121 P Santa Cruz Biotechnology) |
The specificity of the immunoreactivity in double labelling with Fab fragments was tested by omitting the primary antibody in the second staining, and by the two assays described below and according to Negoescu et al. (1994).
Test 1
To prove the monovalence of the Fab fragment, after the first staining, sections were incubated for 48 h at RT with a PAP rabbit complex (A 200/V, diluted 1 : 100, UCB, Braine-l’Alleud, Belgium), which serves as the second-staining primary antibody.
Test 2
To prove the complete Fab saturation of rabbit IgG epitopes, after the first staining, sections were incubated for 24 h at 4 °C with a peroxidase-coupled donkey anti-rabbit Fab fragment (711-036-132, diluted 1 : 30, Jackson), which serves as the second-staining secondary antibody.
Results
The antisera to GDNF, GFRalpha1 and RET9 (RET C-19) worked well in sections of all staged pancreas, whereas the antiserum to the long isoform of RET51 (RET T-20) did not stain any pancreatic sections. Thus, for the sake of simplicity, in the text we refer to the IR of the short isoform RET9 IR as RET IR.
In the embryos at stage 11 the pancreatic primordium was composed of small epithelial cell clusters in the mesenchyme surrounding the duodenum (Fig. 1A). These pancreatic clusters showed some faintly stained GDNF, GFRalpha1 (Fig. 1B) and RET (Fig. 1C) immunoreactive cells.
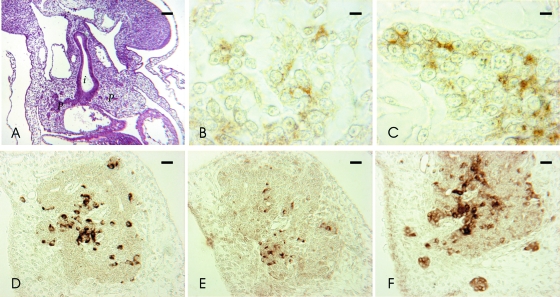
Fig. 1.
Hematoxylin-eosin and immunocytochemical stainings in early cat embryo pancreas. (A–C) Stage 11. Hematoxylin-eosin of transversal section (A), GFRalpha1 (B) and RET (C) IR in the pancreas primordium. (D–F) Stage 14. GDNF (D), GFRalpha1 (E) and RET (F) IR in embryonic pancreas. I, intestine; p, pancreas. Scale bars: (A) 40 µm; (E) 10 µm; (D,F) 20 µm; (B,C) 5 µm.
In the embryos at stage 14 GDNF, GFRalpha1 and RET immunoreactivity (IR) appeared in closely intermingled cells of the pancreas parenchyma or in small clusters scattered in the surrounding mesenchyme (Fig. 1D–F). Generally, GFRalpha1 immunoreactive cells were less numerous than GDNF and RET immunoreactive cells.
In the embryos at stage 16 GDNF and GFRalpha IR (Fig. 2A,B) were localized in both the dorsal and the ventral pancreas. In particular, the IR was seen in clustered cells, often forming ring-like cords, and in single scattered cells in the exocrine parenchyma. RET IR (Fig. 2C,D) was detected in small clusters of neurons in the mesenchyme surrounding the pancreas.
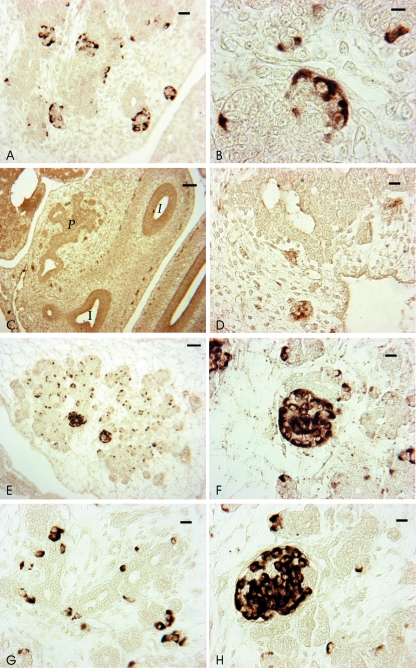
Fig. 2.
GDNF, GFRalpha1 and RET IR in cat embryos and fetuses. (A–D) Stage 16. GFRalpha1 IR (A,B) is localized at the periphery of islets and RET IR (C,D) in grouped neurons at the periphery of pancreas and intestine. (E,F) Stage 19. GDNF IR in cells scattered in the exocrine portion (E) and in islets (F). (G,H) Stage 22. GFRalpha1 IR in cells scattered in the exocrine portion (G) and in islets (H). For abbreviations, see Fig 1. Scale bars: (C) 50 µm; (E) 20 µm; (A,D,F–H) 10 µm; (B) 5 µm.
In the fetuses at stage 19 and 22, GDNF and GFRalpha IR (Fig. 2E–H) were seen in morphologically well-distinguishable islets and in the cells of the exocrine portion of dorsal and ventral pancreas. In the islets, GDNF and GFR alpha immunoreactive cells formed closely folded cords or rings at the periphery. The IR was diffused throughout the cytoplasm, mainly localized in the basal region of the cell. In the exocrine portion, single cells or two to three cells lined up were seen. In a few neurons, RET IR was detected occasionally.
By employing double immunohistochemical staining, in embryos at stage 14 GFRalpha1 IR (Fig. 3A) was localized in some GDNF and RET (Fig. 3B) immunoreactive cells, whereas in embryos and fetuses at stage 16, 19 (Fig. 3C,D) and 22 (Fig. 3E–H) GFRalpha1 IR was localized with GDNF IR in the majority of islets and exocrine pancreatic cells. Furthermore, GDNF IR and RET IR were never co-localized in any pancreatic cells of any stage studied (data not shown).
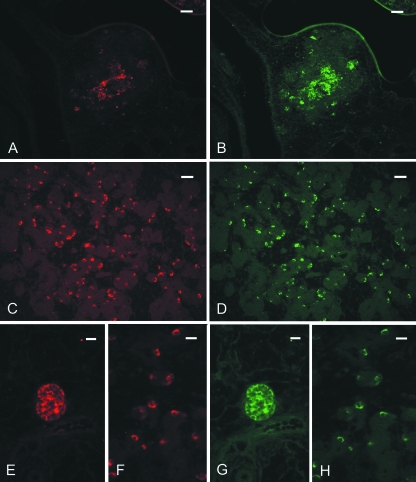
Fig. 3.
Double immunofluorescent staining in cat pancreas. (A,B) Stage 14. GFRalpha1 (A) is partially co-localized with RET (B) IR; (C,D) Stage 19. GDNF IR (C) and GFRalpha1(D) IR are completely co-localized in cells scattered in the exocrine portion. (E–H) Stage 22. GDNF (E,F) and GFRalpha1 (G,H) IR are co-localized in an islet (E,G) and in the exocrine portion (F,H). Scale bars: (A–D) 20 µm; (E–H) 10 µm.
To characterize the cell populations showing IR to GDNF and its receptor complex, antisera to chromogranin and anti-protein gene product (PGP) 9.5, an endocrine and neuronal marker, respectively, were employed. By double immunofluorescence staining, embryos at stage 14 showed GDNF IR co-localized with chromogranin IR (data not shown) and RET IR co-localized with PGP 9.5 IR (Fig. 4A,B). Some GFRalpha1 immunoreactive cells were co-localized with chromogranin and with PGP 9.5 IR (Fig. 4C,D). Fetuses at later stages showed both GDNF and GFRalpha1 IR co-localized with chromogranin IR and RET IR co-localized with PGP 9.5 IR (data not shown).
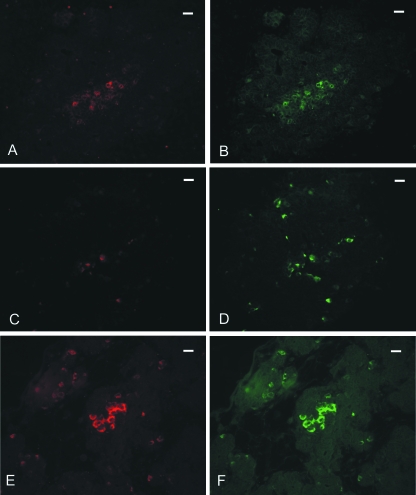
Fig. 4.
Double immunofluorescent staining in cat pancreas. (A,B) Stage 14. RET (A) is co-localized with PGP 9.5 (B) IR. (C,D) Stage 14. GFRalpha (C) is partially co-localized with PGP 9.5 (D) IR. (E,F) Stage 19. GDNF IR (E) is completely co-localized with glucagon (F) IR. Scale bars: (A–F) 10 µm.
To further define the GDNF and GFRalpha1 cell source in the endocrine islets of elderly fetuses, antisera to insulin, glucagon and somatostatin were employed. Using double immunocytochemical staining, both GDNF and GFRalpha1 IR were shown co-localized with glucagon IR (Fig. 4E,F).
Controls
Controls obtained by substituting the primary antibodies with PBS, normal serum or antibody adsorbed by their homologous antigens showed the lack of reaction. In contrast, substitution of primary antibodies with antibodies adsorbed by heterologous antigens did not modify the reaction. Moreover, test 1 and test 2 controls were negative.
Discussion
This study reports the presence of GDNF, GFRalpha1 and RET IR in the cat pancreas during development. All the results obtained from the positive and negative controls confirmed the specificity of the reactions. Regarding RET IR, the results in cat pancreas showed only the presence of RET9. This finding is in accordance with previous data that RET9 and RET51 do not associate with each other (Tsui-Pirchala et al. 2002; Degl’Innocenti et al. 2004; Scott et al. 2005). Moreover, RET9 and RET51 show different associated signaling complexes; mice lacking RET51 seem to be normal, whereas mice lacking RET9 have kidney abnormalities and enteric aganglionosis (for a review see Sariola & Saarma, 2003). Bearing in mind these different characteristics regarding RET9 and RET51, it is plausible that in developing cat pancreas only one isoform was detected.
GDNF and RET immunoreactive cells always expressed the endocrine marker chromogranin and the neuronal marker PGP 9.5, respectively. Thus, from the early stages onward the endocrine nature of GDNF immunoreactive cells and the neuronal nature of RET immunoreactive cells appear to be established. Conversely, GFRalpha1 was seen first in some GDNF/chromogranin and RET/PGP 9.5 immunoreactive cells and then in the majority of GDNF/chromogranin/glucagon immunoreactive cells.
In early embryos, the presence of GDNF IR in chromogranin immunoreactive cells and the presence of GFRalpha1/RET complex IR in PGP 9.5 immunoreactive cells, which are close to each other in the pancreas at stage 14, seems to suggest a paracrine action of GDNF. Thus, neuronal cell precursors expressing GFRalpha1/RET receptor complex could be controlled by GDNF released by endocrine cells. No previous findings regarding the influence of GDNF on the development of pancreatic innervation are available in the literature. However, similarly to our findings, enteric neuronal precursors also express RET/GFRalpha1 (for a review see Young et al. 2001, 2004; Burns et al. 2004). In our study, RET-positive neurons were seen in the intestine of cat embryos and fetuses (unpublished data, Fig. 2C). Consistently, pancreatic ganglia derive from a subset of vagal neural crest precursors that, after entering and migrating proximodistally into the gut and perpendicularly toward the mucosa to form myenteric ganglia (Gershon et al. 1993; Young et al. 1998), migrates in the opposite direction out of the gut to form pancreatic ganglia (for a review see Jiang et al. 2003). All these findings suggest a close relationship during pancreatic development between the autonomic nervous system and the islets. A recent study reported that neural crest cells regulate the size of the beta-cell populations and the pancreatic epithelium regulates the neural crest cell expression after migration into the pancreas (Nekrep et al. 2008), presumably by means of various signaling molecules, one of which could be GDNF.
The presence of the co-receptor GFRalpha1 IR in GDNF immunoreactive cells without the co-presence of the receptor RET, could be explained by the biology of GDNF signaling, which is much more complex than previously assumed. A number of studies have demonstrated that GDNF/GFRalpha1 can signal independently of RET through GFRalpha1. GDNF-GFRalpha1 dimer can promote phosphorylation of Met, whose activation is indirect and is mediated by Src-type kinases (Trupp et al. 1999), or can bind to NCAM and activate Fyn (Paratcha et al. 2003). Moreover, differentiation and tangential migration of cortical neurons suggest the existence of another unknown alternative transmembrane signaling mediator or mediators for GDNF-GFRalpha complex (Pozas & Ibañez, 2005). However, studies conducted in mice in which RET-independent GFRalpha1 was eliminated, showed that GFRalpha1 in cells lacking RET was dispensable for organogenesis and regeneration (Enomoto et al. 2004). In any case, as previously reported, the expression of GFRalpha1 and RET does not coincide in many tissues (Trupp et al. 1997; Yu et al. 1998; Golden et al. 1999) and it was also observed in developing cat pancreas. Thus, the co-presence of GDNF and GFRalpha1 could lead to the hypothesis that GDNF can act in an autocrine manner by means of unidentified mediators. On the other hand, an autocrine mode of action has been reported in rat endocrine pancreas for other neurotrophic factors, such as the nerve growth factor and its specific receptor TrkA (Rosenbaum et al. 1998).
Furthermore, GDNF/GFRalpha1 IR was seen to be localized in glucagon immunoreactive cells This finding seems to suggest, in addition to an autocrine mode of action of this neurotrophic factor, a particular ‘sensitivity’ of glucagon cells to neurotrophic factors and their ability to synthesize and/or store them. The latter consideration is further confirmed by previous studies. Glucagon cells were seen to express: (1) neurotrophin 3 in man (Ohta et al. 1997); (2) TrkB, the specific BDNF receptor, in teleost fish (Lucini et al. 2001); (3) neurotrophin 3 and its specific receptor TrkC in buffalos of different ages (Lucini et al. 2003a); (4) brain-derived neurotrophic factor (BDNF) in various vertebrate species (Lucini et al. 2003b).
Later on in development, RET IR was detected in intrapancreatic ganglia, without any co-localization with GFRalpha1 and/or GDNF. Thus at later stages of pancreatic development RET receptor could be activated by other GFR alphas and ligands of GDNF family which may be present but which we did not test in this study. However, we cannot exclude the presence of GFRalpha1 and GDNF, although their level in pancreatic ganglia might be undetectable by immunocytochemistry. Furthermore, there is evidence in the literature that TrkA, the specific receptor of the nerve growth factor, and TrkB can act on RET by an intracellular mechanism which does not require GFRalpha1 or GDNF (Tsui-Pirchala et al. 2002; Esposito et al. 2008).
In conclusion, the present results represent, to our knowledge, the first evidence regarding the involvement of GDNF in pancreatic development. It could act by means of a paracrine/autocrine regulatory loop on endocrine cells and neuronal precursors. Moreover, our findings may also suggest a sequential receptorial change in GDNF transduction signal. The physiological meaning of these findings are worthy of further studies.
References
- Burns AJ, Pasricha PJ, Young HM. Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil. 2004;16:3–17. doi: 10.1111/j.1743-3150.2004.00466.x. Review. [DOI] [PubMed] [Google Scholar]
- Case RM. Is the rat pancreas an appropriate model of the human pancreas? Pancreatology. 2006;6:180–190. doi: 10.1159/000091849. [DOI] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Degl’Innocenti D, Arighi E, Popsueva A, et al. Differential requirement of Tyr1062 multidocking site by RET isoforms to promote neural cell scattering and epithelial cell branching. Oncogene. 2004;23:7297–7309. doi: 10.1038/sj.onc.1207862. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Hughes I, Golden J, et al. GFRα1 expression in cells lacking RET is dispensable for organogenesis and nerve regeneration. Neuron. 2004;44:623–636. doi: 10.1016/j.neuron.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Esposito CL, D’alessio A, de Franciscis V, Cerchia L. A cross-talk between TrkB and Ret tyrosine kinases receptors mediates neuroblastoma cells differentiation. PloS ONE. 2008;3:e1643. doi: 10.1371/journal.pone.0001643. doi: 10.1371/journal.pone.0001643. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Huleihel M, Abuelhija M, Lunenfeld E. In vitro culture of testicular germ cells: regulatory factors and limitations. Growth Factors. 2007;25:236–252. doi: 10.1080/08977190701783400. [DOI] [PubMed] [Google Scholar]
- Ito Y, Okada Y, Sato M, et al. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery. 2005;138:788–794. doi: 10.1016/j.surg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu MT, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Knospe C. Periods and stages of the prenatal development of the domestic cat. Anat Histol Embryol. 2002;31:37–51. doi: 10.1046/j.1439-0264.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- Lucini C, Maruccio L, De Girolamo P, Vega JA, Castaldo L. TrkA and TrkB neurotrophin receptor immunoreactivity in the teleost (Scorpaena porcus) endocrine pancreas. Anat Rec. 2001;263:113–117. doi: 10.1002/ar.1081. [DOI] [PubMed] [Google Scholar]
- Lucini C, Costagliola C, Borzacchiello G, Castaldo L. Neurotrophin 3 and its receptor TrkC immunoreactivity in glucagon cells of buffalo pancreas. Anat Histol Embryol. 2003a;32:253–256. doi: 10.1046/j.1439-0264.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Lucini C, Maruccio L, De Girolamo P, Castaldo L. Brain-derived neurotrophic factor in higher vertebrate pancreas: immunolocalization in glucagon cells. Anat Embryol. 2003b;206:311–318. doi: 10.1007/s00429-002-0304-3. [DOI] [PubMed] [Google Scholar]
- Mwangi S, Anitha M, Mallikarjun C, et al. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology. 2008;134:727–737. doi: 10.1053/j.gastro.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoescu A, Labat-Moleur F, Lorimier P, et al. F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J Histochem Cytochem. 1994;42:433–437. doi: 10.1177/42.3.7508473. [DOI] [PubMed] [Google Scholar]
- Nekrep N, Wang J, Takeshi M, German MS. Signals from the neural crest regulate beta-cell mass in the pancreas. Development. 2008;135:2151–2160. doi: 10.1242/dev.015859. [DOI] [PubMed] [Google Scholar]
- Ohta T, Numata M, Tsukioka Y, et al. Neurotrophin-3 expression in human pancreatic cancers. J Pathol. 1997;181:405–412. doi: 10.1002/(SICI)1096-9896(199704)181:4<405::AID-PATH786>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ibañez CF, Ledda F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibañez C. GDNF and GFRα1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Reynolds GM, Young FI, Young JA, Williams A, Rowlands DC. Microwave oven antigen retrieval applied to the immunostaining of cytopathology specimens. Cytopathology. 1994;5:345–358. doi: 10.1111/j.1365-2303.1994.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Vidaltamayo R, Sánchez-Soto MC, Zentella A, Hiriart M. Pancreatic beta cells synthesize and secrete nerve growth factor. Proc Natl Acad Sci USA. 1998;95:7784–7788. doi: 10.1073/pnas.95.13.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeberg-Roos P, Saarma M. Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Ann Med. 2007;39:572–580. doi: 10.1080/07853890701646256. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. Review. [DOI] [PubMed] [Google Scholar]
- Scott RP, Eketjäll S, Aineskog H, Ibañez CF. Distinct turnover of alternatively spliced isoforms of the RET kinase receptor mediated by differential recruitment of the Cbl ubiquitin ligase. J Biol Chem. 2005;280:13442–13449. doi: 10.1074/jbc.M500507200. [DOI] [PubMed] [Google Scholar]
- Sternberger. Immunocytochemistry. 3rd edn. New York: Wiley; 1986. [Google Scholar]
- Toma H, Winston JH, Micci MA, Li H, Hellmich HL, Pasricha PJ. Characterization of the neurotrophic response to acute pancreatitis. Pancreas. 2002;25:31–38. doi: 10.1097/00006676-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibañez C. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Scott R, Whittemore SR, Ibañez CF. Ret-dependent and independent mechanisms of glial cell line-derived neurotrophic factor signalling in neuronal cells. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- Tsui-Pirchala BA, Ahrens RC, Crowder RJ, Milbrandt J, Johnson EM., Jr The long and short isoforms of Ret function as independent signaling complexes. J Biol Chem. 2002;277:34618–34625. doi: 10.1074/jbc.M203580200. [DOI] [PubMed] [Google Scholar]
- Wessel GM, McClay DR. Two embryonic, tissue-specific molecules identified by a double-label immmunofluorescence technique for monoclonal antibodies. J Histochem Cytochem. 1986;34:703–706. doi: 10.1177/34.6.3084626. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Ciampoli D, Southwell BR, Brunet JF, Newgreen DF. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998;202:67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. Review. [DOI] [PubMed] [Google Scholar]
- Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R. Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci. 1998;18:4684–4696. doi: 10.1523/JNEUROSCI.18-12-04684.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]