Abstract
It has been generally assumed that the outflow tract of the chondrichthyan heart consists of the conus arteriosus, characterized by cardiac muscle in its walls. However, classical observations, neglected for many years, indicated that the distal component of the cardiac outflow tract of several elasmobranch species was composed of tissue resembling that of the ventral aorta. The present study was outlined to test the hypothesis that this intrapericardial, non-myocardial component might be homologous to the actinopterygian bulbus arteriosus. The material consisted of Atlantic catshark adults and embryos, which were examined by means of histochemical and immunohistochemical techniques for light and fluorescence microscopy. In this species, the distal component of the outflow tract differs histomorphologically from both the ventral aorta and the conus arteriosus; it is devoid of myocardium, is covered by epicardium and is crossed by the coronary arterial trunks. In the embryonic hearts examined, this distal component showed positive reactivity for 4,5-diaminofluorescein 2-diacetate (DAF-2DA), a fluorescent nitric oxide indicator. These findings, together with other observations in holocephals and several elasmobranch species, confirm that chondrichthyans possess a bulbus arteriosus interposed between the conus arteriosus and the ventral aorta. Therefore, the primitive heart of gnathostomates consists of five intrapericardial components, sinus venosus, atrium, ventricle, conus arteriosus and bulbus arteriosus, indicating that the bulbus arteriosus can no longer be regarded as an actinopterygian apomorphy. The DAF-2DA-positive reactivity of the chondrichthyan embryonic bulbus suggests that this structure is homologous to the base of the great arterial trunks of birds and mammals, which derives from the embryonic secondary heart field.
Keywords: bulbus arteriosus, chondrichthyans, conus arteriosus, evolution, heart, morphology
Introduction
It is widely reported that the outflow tract of the chondrichthyan heart consists of the conus arteriosus, characterized by myocardial muscle in its walls and by a variable number of valves at its luminal side (Gegenbaur, 1866; Satchell & Jones, 1967; Santer, 1985; Zummo & Farina, 1989). The conus is reported to connect the ventricle with the ventral aorta. A well-developed conus arteriosus has also been found in the heart of phylogenetically basal actinopterygians such as the polypteriformes and lepisosteiformes (Parsons, 1930). In the acipenseriformes, amiiformes, and in some species of the phylogenetically basal teleost genera Albula, Pterothrissus, Megalops and Tarpon, an intrapericardial, non-myocardial component, the bulbus arteriosus, is interposed between the conus and the ventral aorta (Stannius, 1846; Boas, 1880; Parsons, 1930; Senior, 1970a,b,c; Satchell, 1991; Icardo et al. 2002a,b).
It has been generally assumed that in most teleosts, the conus arteriosus is vestigial or even absent, a fact that has been considered to be concomitant with the remarkable development of the bulbus arteriosus in this zoological group (Smith, 1918; Santer, 1985; Satchell, 1991; Farrell & Jones, 1992). In contrast to this traditional viewpoint, recent studies indicate that the conus, although reduced in size, exists almost certainly in the majority or even in all teleost species, being actively implicated in the performance of the conal valves (Schib et al. 2002; Icardo et al. 2003; Icardo, 2006; Grimes et al. 2006).
There is general agreement that the conus arteriosus is an anatomical component of the primitive vertebrate heart. By contrast, the evolutionary origin of the actinopterygian bulbus arteriosus has been an area under discussion for more than a century. Several authors have proposed that the bulbus develops from the proximal part of the ventral aorta, which extends backwards into the pericardial cavity (Boas, 1901; Bridge, 1904; Krause, 1923; Grodzinski, 1938; Bertin, 1958; Parker & Haswell, 1962; Weichert & Presch, 1975; Lawson, 1979). Other authors consider that the bulbus forms as a modification of the anterior part of the embryonic conus arteriosus (Parsons, 1930; Licht & Harris, 1973; Priede, 1976; Yamauchi, 1980; Farrell & Jones, 1992; Hu et al. 2000; Guerrero et al. 2004). Grimes et al. (2006) have recently shown that the teleost bulbus arteriosus becomes specifically labelled with a nitric oxide indicator that also marks the smooth muscle component of the arterial pole of the chick heart, i.e. the base of both the aorta and the pulmonary artery. Taking into account that in the chick these regions are not a part of any cardiac chamber, the authors concluded that the bulbus arteriosus, which through ontogeny is never invested by myocardium, cannot be regarded as a genuine chamber of the teleost heart.
In 1930, Parsons reported that in several elasmobranch species, the myocardial portion of the conus arteriosus of the adult heart did not as a rule extend as far as the distal pericardial boundary. In such cases, the distal portion of the conus consisted of a tissue resembling that of the ventral aorta, which lies beyond the pericardial cavity. This observation, neglected for many years, was re-investigated by Guerrero et al. (2004), who suggested that the distal, non-myocardial portion of the conus might be morphologically equivalent to the actinopterygian bulbus arteriosus.
The present study was outlined to test this hypothesis. The main goals were (1) to gain new insight into the anatomy and histology of the components of the chondrichthyan cardiac outflow tract, (2) to decide whether chondrichthyans possess an outflow tract component morphologically equivalent to the bulbus arteriosus of the actinopterygians, and (3) to elucidate the possible homologies between the outflow tract components of the chondrichthyan heart and those of phylogenetically apical vertebrate taxa, in an attempt to improve our understanding of vertebrate heart evolution. The study was carried out in Atlantic catsharks, Galeus atlanticus (Carcharhiniformes; Scyliorhinidae), due to the availability of both adult and embryonic hearts of this species.
Materials and methods
The hearts examined belonged to 12 adults (five male, seven female) and 15 embryos. Adult specimens were collected in the western Mediterranean by scientific vessels belonging to the Spanish Institute of Oceanography's fleet. The total length (TL) of the animals, measured from the anterior end of the head to the tip of the tail, ranged between 33.4 and 41.0 cm.
Fertilized eggs were obtained from adult females and maintained in an indoor tank of well-aerated seawater, kept clean by means of an external filter device. The temperature of the water ranged between 15 and 18 °C. The egg capsules were opened and the embryos were removed. Their TL was between 32.0 and 65.0 mm.
Five (TL = 35–65 mm) of the 15 embryos were used for labelling in vivo with 4,5-diaminofluorescein 2-diacetate (DAF-2DA) (Sigma Chemical Co., Poole, UK), a fluorescent nitric oxide (NO) indicator which has been proposed to be an early marker of prospective smooth muscle (Grimes et al. 2006).
All embryos were anaesthetized by immersion in seawater with 0.04% tricaine methane sulphonate (MS-222, Sigma Chemical Co.).
Both the adult and the embryonic hearts were examined by means of histochemical and immunohistochemical techniques for light microscopy. The embryonic hearts incubated in DAF-2DAF were also examined by epifluorescence.
Histochemical techniques for light microscopy
Adult hearts were perfused in situ with elasmobranch buffer (16.38 g L−1 NaCl, 0.89 g L−1 KCl, 1.11 g L−1 CaCl2, 0.38 g L−1 NaHCO3, 0.06 g L−1 NaH2PO4, 21.6 g L−1 urea, pH 7.2) and removed. Embryos were dissected under a stereomicroscope to obtain the heart together with the ventral aorta and afferent branchial arteries. Adult and embryonic hearts were fixed in 4% paraformaldehyde in elasmobranch buffer, or in MAW (methanol/acetone/water 2:2:1), dehydrated in graded series of ethanol and embedded in Histosec (Merck KGaA, Darmstadt, Germany). Serial sections of the heart, transversely, longitudinally or sagittally cut at 10 μm, were stained with haematoxylin-eosin for general assessment of the tissue structure or with Masson–Goldner's trichrome stain for connective tissue. The periodic acid–Schiff (PAS) reaction and the picrosirius-polarization staining method (Junqueira et al. 1979) were used for the detection of mucosubstances and collagen, respectively. The orcein-HCl and resorcin methods were employed for the specific detection of elastin.
Immunohistochemical techniques for light microscopy
Sections of the heart, obtained following the protocol described above, were immunolabelled with the monoclonal antibody MF20 (Developmental Studies Hybridoma Bank, University of Iowa, USA) against the myosin heavy chains of the striated and cardiac muscle, or with the monoclonal anti-smooth muscle α-actin antibody (clone 1A4, Sigma Chemical Co.).
The sections were dewaxed in xylene, hydrated in an ethanol series and washed in Tris-phosphate-buffered saline (TPBS, pH 7.8). Endogenous peroxidase activity was quenched by incubation with 3% hydrogen peroxide in TPBS for 30 min. After washing with TPBS, non-specific binding sites were saturated for 1 h with 10% sheep serum and 1% bovine serum albumin in TPBS plus 0.5% Triton X-100 (SBT). Endogenous biotin was blocked with the avidin-biotin blocking kit (Vector, Burlingame, CA, USA). Sections were washed with TPBS and then incubated overnight in the primary antibody diluted in SBT. Omission of the first antibody was used as a negative control.
After incubation with the first antibody, the sections were washed in TPBS, incubated for 1.5 h in biotin-conjugated anti-mouse IgG (Sigma) diluted 1:100 in SBT, washed again, and incubated for 1 h in ExtrAvidin® conjugate (Sigma) diluted 1:150 in SBT. Peroxidase activity was developed with Sigma Fast 3, 3′-diaminobenzidine tablets according to the instructions of the supplier.
Incubation of embryos in an NO indicator
Five live embryos were transferred into a 10 μm solution of DAF-2DA in seawater and incubated for 48 h in the dark at 17 °C. Embryonic hearts were obtained and processed as described above.
Nomenclature
Here the terms ‘proximal’ (or ‘posterior’) and ‘distal’ (or ‘anterior’) are used to describe the location of the anatomical components of the cardiac outflow tract with regard to the ventricle.
Results
Macroscopic findings in adult specimens
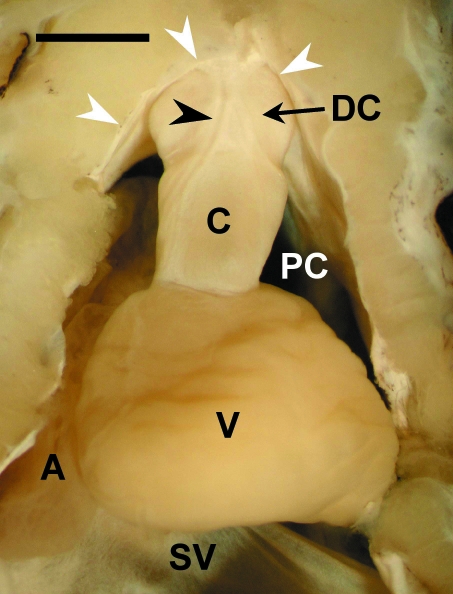
In the adult hearts, a tubular outflow tract segment extended, in a slightly ventrodorsal orientation, from the ventricle to the distal boundary of the pericardial cavity, where it connected with the ventral aorta. Macroscopically, the segment showed two components, proximal and distal, of similar length (Fig. 1). In fresh material, the proximal component was reddish and markedly harder than the distal component, which displayed a whitish coloration.
Fig. 1.
Ventral view of the heart of an adult Galeus atlanticus. A, atrium; C, proximal component (= conus arteriosus); DC, distal component of the outflow tract; PC, pericardial cavity; SV, sinus venosus; V, ventricle. The arrowhead points to a coronary artery. The white arrows indicate the pericardium. Scale bar = 0.2 cm.
The luminal surface of the proximal component was furnished with two rows, anterior and posterior, of pocket valves, indicating unequivocally that it was the conus arteriosus. Each row consisted of three valves of similar size located in the dorsal, right-ventral and left-ventral positions. Each valve was composed of the leaflet and its supporting structure, the sinus. The distal component (DC) of the tubular segment displayed a smooth luminal surface devoid of valves.
Histochemical and immunohistochemical findings in adult specimens
The wall of the conus arteriosus consisted of three layers: the inner endocardial, the middle myocardial and the outer epicardial layers (Figs 2 and 3a). The endocardial layer was composed of the endocardium and the subendocardial space. This space, occupied by fibrous tissue, was thicker along the sinus wall of the anterior valves than along the sinus wall of the posterior valves. The subendocardial space of the anterior valvular sinuses was rich in elastin (Fig. 2) and collagen (Fig. 3a); in that of the posterior valve sinuses elastin was scarce. The middle myocardial layer was the thickest layer of the conus wall. It consisted of well-irrigated, compact myocardium, with the myocardial cells arranged in a circumferential orientation. The outer epicardial layer had a flattened surface, lined by epicardial cells, and a subepicardial space of fibrous tissue, very rich in collagen. Two main coronary artery trunks ran along this subepicardial fibrous tissue (Fig. 3a).
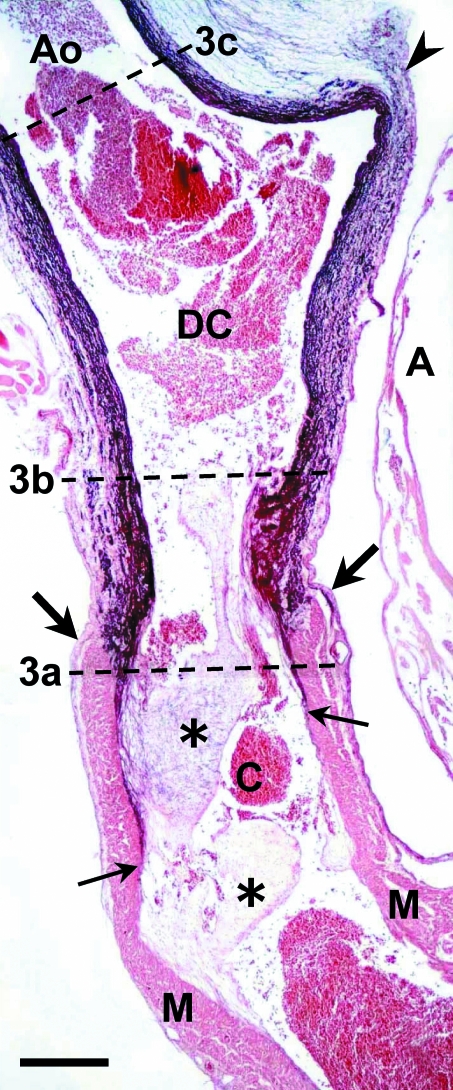
Fig. 2.
Sagittal section of the ventral aorta (Ao) and cardiac outflow tract of an adult Galeus atlanticus. In the cardiac outflow tract two components can be recognized: proximal (down) and distal (up). The boundary between them is marked with large arrows. The proximal component is the conus arteriosus (C), which contains myocardium (M) in its wall and is furnished with two rows of valves (asterisks). The distal component (DC) is devoid of myocardium and rich in elastin (dark purple staining). Note the presence of a thin layer of elastin in the subendocardial space at the level of the anterior row, and not at the level of the posterior row of conal valves. The small arrows indicate the proximal level of elastin staining. A, atrium. Arrowhead, distal boundary of the pericardial cavity. The dotted lines (3a–c) indicate the levels of the sections shown in Fig. 3. Orcein-HCl staining. Scale bar = 500 μm.
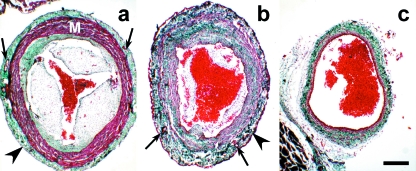
Fig. 3.
Transverse sections (see also Fig. 2) of the conus arteriosus (a), distal component of the cardiac outflow tract (b) and ventral aorta (c) of an adult Galeus atlanticus. The thickness of the distal component wall is similar to that of the conus arteriosus; the aorta has a thinner wall. The walls of both the conus and the distal component of the outflow tract are covered by epicardium (arrowheads) and crossed by coronary arteries (arrows). M, myocardium. Collagen is stained green. Masson-Goldner's trichrome. Scale bar = 300 μm.
The wall of the DC was similar in thickness to that of the conus arteriosus (Fig. 2; compare with Fig. 3a,b). Its luminal side was lined by the endothelium, surrounded by a very thick layer of smooth musculature, elastin and collagen. The smooth musculature appeared as concentric sheets (Fig. 4a), alternating with equidistant, thin layers of elastic fibres (Fig. 4b). The collagen bundles followed a more or less concentric pattern (Fig. 4c). Glycoconjugates were abundant close to the luminal part of the DC wall. They displayed an arrangement similar to that of the fibrous elements separating the smooth muscle sheets (Fig. 4d). The most external portion of the DC wall was composed of connective tissue very rich in collagen and almost devoid of elastin; it was covered by the epicardium (Figs 3b and 4a,b,d). The main coronary arterial trunks, which entered the DC at the level of the distal pericardial boundary, ran along this external fibrous layer (Figs 3b and 4a,c,d), then entering the subepicardial space of the conus arteriosus (Fig. 3a).
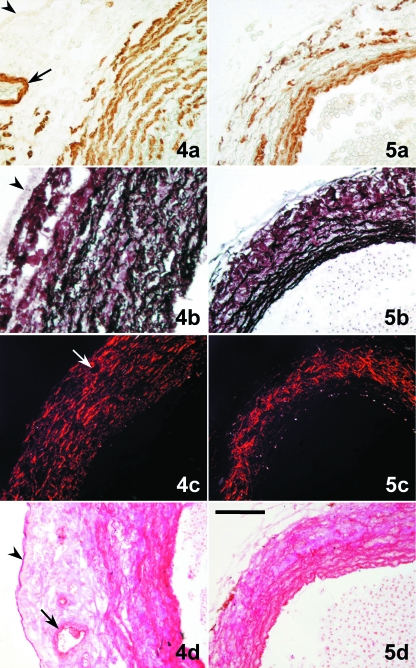
Figs 4 and 5.
Tranverse sections of the distal component (DC) of the cardiac outflow tract (4) and ventral aorta (5). (4a) The DC wall has a thick layer of smooth muscle cells (brown labelling) forming concentric sheets. (5a) The aortic media shows several densely arranged smooth muscle layers. (4b) In the DC, elastin (black staining) forms equidistant concentric sheets that alternate with the smooth musculature. Elastin is very scarce in the subepicardial space. (5b) Elastin is abundant in the aortic media and scattered in the adventitia. (4c) Staining plus polarization microscopy enhance the natural birefringence of the collagen bundles, which follow a more or less concentric pattern through the whole wall of the DC wall and are more abundant in the subepicardial space. (5c) Collagen bundles are orientated longitudinally in the aortic media; in the adventitia they are more abundant forming a meshwork. (4d) Glycoconjugates (magenta) follow the pattern of distribution of elastin in the DC wall. (5d) Glycoconjugates follow the pattern of distribution of elastin in the aorta. (a) α-actin immunolabelling; (b) Orcein-HCl staining; (c) Picrosirius staining plus polarization microscopy; (d) PAS. The arrowhead marks the epicardium. The arrow points to a coronary artery located in the subepicardial space. Scale bars = a, b, d, 100 μm; c, 200 μm.
Beyond the distal limit of the pericardial cavity, the ventral aorta, which was narrower than the DC (Fig. 1; see Figs 3b,c, 4 and 5 for a comparison), exhibited the typical histological structure of an elastic artery; it consisted of intima, media and adventitia. The media was composed of several densely arranged smooth muscle layers (Fig. 5a), separated by thin sheets of connective tissue containing elastin (Fig. 5b), glycoconjugates (Fig. 5d) and, to a lesser extent, collagen bundles orientated longitudinally. The adventitia was well developed; it was rich in collagen bundles forming a meshwork (Fig. 5c), and contained scattered elastic fibres (Fig. 5b).
Fig. 6.
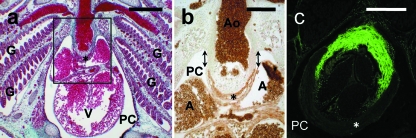
Galeus atlanticus embryos. (a) Longitudinal section of the heart and gills (G) of a 32-mm TL embryo. V, ventricle. Masson-Goldner's trichrome. (b) Detail of a section consecutive to that shown in (a), immunolabelled with MF20 (brown labelling). The arrows indicate the distal, non-myocardial (MF20-negative) component of the cardiac outflow tract. A, atrium; Ao, ventral aorta. (c) Oblique section of the conus–distal component junction of a 58-mm TL embryo labelled in vivo with DAF-2DA. Fluorescence is observed in the distal component and extends from the deepest part of the developing valvular sinuses to the distal boundary of the pericardial cavity. In a, b and c, the asterisk marks the myocardium of the developing conus arteriosus. PC, pericardial cavity. Scale bars = a, 400 μm; b, c = 200 μm.
The structural switch between the DC and the ventral aorta could be better recognized at the dorsal than at the ventral side, where the histological transition between both anatomical elements was gradual.
Embryological findings
In the embryos examined, the transverse septum was completely developed, and the heart showed an anatomical arrangement that basically corresponded to that of an adult Atlantic catshark. The cardiac tube was S-shaped, with the sinus venosus and the atrium positioned dorsally. The ventricle connected with the ventral aorta through a tubular, intrapericardial outflow tract, in which two components, proximal and distal, could be recognized (Fig. 6a). The proximal component was MF20-positive, indicating that it displayed a myocardial phenotype (Fig. 6b). The DC was MF20-negative. In embryos of 50 mm TL or longer, the DC showed positive immunoreactivity against α-actin, a smooth muscle marker. In all embryos examined, a strong DAF-2DA-positive reactivity was observed from the deepest part of the developing valvular sinuses to the distal boundary of the pericardial cavity (Fig. 6c).
Discussion
Morphological and functional aspects
The present findings show that the outflow tract of the Atlantic catshark heart consists of two anatomical components, proximal and distal, of similar length. The proximal component is the conus arteriosus; it contains myocardial muscle and is furnished with semilunar valves. The DC, which connects the conus arteriosus with the ventral aorta, is devoid of myocardium. Histologically, it exhibits an arterial-like structure. However, it differs from the ventral aorta because: (1) it displays a thicker wall, covered by epicardium; (2) it shows a different arrangement of histological elements; and (3) its walls are longitudinally crossed by the main coronary arterial trunks, which enter the DC at the boundary of the pericardial cavity and which are not mere vasa vasorum. Nonetheless, there is no striking structural discontinuity between the DC and the ventral aorta; there is a gradual, histological transition between them, especially at the ventral side.
In the sturgeon Acipenser naccarii, a species belonging to the phylogenetically basal actinopterygian group of acipenseriformes, the conus arteriosus and the ventral aorta are similarly connected by an intrapericardial, non-myocardial, tubular segment that displays an arterial-like structure, but diverges histologically from the ventral aorta (Icardo et al. 2002a,b). Moreover, it is covered by the epicardium and is crossed by the main coronary artery trunks. Guerrero et al. (2004) studied the formation of this segment, concluding that it is homologous to the teleost bulbus arteriosus. The present observations show that the outflow tract of the adult Atlantic catshark heart is similar to that of the sturgeon; it also includes a distinct non-myocardial, tubular segment, the DC, located between the conus arteriosus and the ventral aorta. This alone suggests that the DC of the Atlantic catshark and the actinopterigyan bulbus arteriosus are homologous structures. Additional support for this notion comes from embryological findings in chondrichthyans and teleosts. The embryos examined in the present study displayed a DAF-2DA-positive developing DC. This is also the case with the bulbus arteriosus of the zebrafish, Danio rerio, which is DAF-2DA-positive throughout development, from approximately 48 h post-fertilization (Grimes et al. 2006). On this basis, we conclude that the Atlantic catshark possesses a bulbus arteriosus at the arterial pole of the heart. In this context, it should be mentioned here that we have found a non-myocardial DC, i.e. a bulbus arteriosus, in all of a considerable number of chondrichthyans belonging to the chimaeriforms, hexanchiforms, squaliforms, carcharhiniforms, lamniforms, torpediniforms, rajiforms and myliobatiforms (Durán et al. 2008). This indicates that the present findings can be extended to other cartilaginous fish, thereby confirming the hypothesis of Guerrero et al. (2004) that, in the chondrichthyans, a bulbus arteriosus is interposed between the conus arteriosus and the ventral aorta.
It is well known that the teleost bulbus arteriosus smoothes cardiac output, i.e. it (1) reduces both the pulsality of the blood flow to the gill vasculature and haemodynamic afterload of the ventricular muscle by expanding during ventricular systole, and (2) provides constant perfusion of the gills through the ventral aorta by rebounding during ventricular diastole (Icardo et al. 1999a,b; Braun et al. 2003; Evans et al. 2003). The question is whether the function of the chondrichthyan bulbus arteriosus is similar to that of the teleost bulbus.
The gill vasculature of the chondrichthyans is an intricate system, containing several elastic elements, the cavernous bodies, which have been adduced to serve as elastic blood reservoirs (De Vries & De Jager, 1984). With this in mind, one is tempted to assume that the bulbus arteriosus and the cavernous bodies may be the elements which protect the delicate capillary network of the gill vasculature of the chondrichthyans from exposure to high-pressure pulses of blood. However, further studies testing the effects of vasoactive substances are needed to verify this notion. Characterization of such effects on the teleost bulbus indicates that it is under neurohormonal control (Evans et al. 2003), a fact which denotes that the bulbus is much more than a ‘Windkessel’ (Von Skramlick, 1935), as classically assumed. In this context, it should be emphasized that NO, which is commonly accepted as the major endothelium-derived relaxing factor in vertebrates (Evans & Gunderson, 1998), has been shown to occur in the developing bulbus arteriosus of both the zebrafish (Grimes et al. 2006) and the Atlantic catshark (present results). In terrestrial vertebrates, NO derived from the vascular endothelium is proposed to be a ubiquitous vasodilator. However, studies in both teleosts and elasmobranchs suggest that the paraphyletic group of fishes relies upon other vasoregulatory mechanisms. These data, mainly derived from ex vivo isolated blood vessels, are often confusing and contradictory (Olson & Villa, 1991; McGeer & Eddy, 1996; Eddy & Tibbs, 2003), but it does appear that NO produces significant dilation of the teleost bulbus arteriosus (Evans et al. 2003). Similar experiments with isolated blood vessels have shown that there may be a mild vasoconstrictive response to NO in the vascular smooth musculature of chondrichthyans (Evans & Gunderson, 1998; Evans, 2001; Donald et al. 2004; Pellegrino et al. 2005). However, in vivo, under normoxic conditions, a vasodilator role for a neuronally derived NO has been proposed and this response is greatly enhanced by hypoxia, at which point endothelially derived NO may also be implicated (Renshaw & Dyson, 1999; Swenson et al. 2005). Moreover, as the chondrichthyan bulbus arteriosus has apparently been overlooked, there are no data available regarding a specific role for NO in this structure. The possibility of a paradoxical response to NO in chondrichthyans and actinopterygians versus terrestrial vertebrates raises interesting questions about the evolution of blood pressure regulatory mechanisms and undoubtedly increases the imperative for further studies of the effects of different cell-signalling agents, including NO, in the chondrichthyan bulbus arteriosus.
Embryological aspects
Classical work suggested that all segments of the vertebrate heart develop from the primary straight tube derived from the bilateral primary heart fields. It was assumed that the components of the straight tube represent all of the myocardial and endocardial progenitors. Currently, it is well known that the heart forms in a sequential manner by continued addition of cells to the anterior and posterior limits of the primitive cardiac tube (Mjaatvedt et al. 2001; Waldo et al. 2001, 2005; Abu-Issa et al. 2004; Abu-Issa & Kirby, 2007). Information about this embryological process has been gained from experimental studies carried out in birds and, to a lesser extent, in mammals.
A recent, essential finding concerning heart formation in tetrapods is that the components of the outflow tract are recruited from a field of splanchnic mesoderm, termed anterior (Kelly et al. 2001; Mjaatvedt et al. 2001) or secondary (Waldo et al. 2001) heart field. Another crucial finding is that, at least in birds, the secondary heart field contributes not only myocardium but also smooth muscle to the arterial pole of the developing heart, forming the proximal walls of the aorta and pulmonary trunk (Waldo et al. 2005). Interestingly, from early developmental stages, DAF-2DA labels regions of the arterial pole of the chick heart that ultimately adopt a smooth muscle phenotype (Grimes et al. 2006). As mentioned above, the bulbus arteriosus of the zebrafish is also labelled by DAF-2DA during development (Grimes et al. 2006), suggesting that the teleost bulbus is akin to the intrapericardial base of the great arterial trunks of birds. On this basis, Grimes et al. (2006) hypothesized that a secondary heart field, giving rise to both the conal myocardium and the bulbar smooth musculature, might exist in teleosts. The DAF-2DA-positive reactivity of the embryonic bulbus arteriosus of the Atlantic catshark suggests that the chondrichthyan bulbus is also homologous to the base of the great arterial trunks of birds and mammals. This leads to the idea that a secondary heart field might be present in the phylogenetically basal, jawed vertebrates (gnathostomates), i.e. the chondrichthyans.
Evolutionary aspects
The presence of a bulbus arteriosus in the phylogenetically basal gnatostomates leads us to conclude that this anatomical structure can no longer be regarded as an actynopterigian apomorphy. However, the evolutionary origin of the bulbus remains an open question. More studies are needed to decide whether it appeared in the chondrichthyans or in an earlier period of the craniata (vertebrate) story, i.e. in the agnates. Hagfish hearts have generally been described to be composed of sinus venosus, atrium and ventricle, while many authors consider that in lampreys, a conus arteriosus is present at the most distal cardiac portion (for reviews, see Moorman & Christoffels, 2003; Guerrero et al. 2004; Simoes-Costa et al. 2005). However, although it may be a simple case of confused nomenclature, other authors state that the most distal segment of the lamprey's heart consists of a bulbus arteriosus (Fontaine, 1958; Yamauchi, 1980; Santer, 1985), containing elastin and smooth muscle cells (Yamauchi, 1980). Whatever it might be, the existence of a bulbus arteriosus in chondrichthyans is of evolutionary relevance, because, among vertebrates, chondrichthyans constitute the living group that symbolizes the primitive gnathostomate condition. The living actinopterygians belong to a phylogenetically more advanced lineage, which diverged from the stem of tetrapods very early in evolution. Therefore, the chondrichthyan and not the actinopterygian heart should be regarded as that which reflects faithfully the primitive status of the gnathostomate heart.
On this basis, we can affirm that the classical concept of the primitive heart of the gnathostomates, where the conus arteriosus connects the ventricle with the ventral aorta, is incorrect. A distinct bulbus arteriosus is located between them. Therefore, the primitive heart of the gnathostomates consists of five intrapericardial components: sinus venosus, atrium, ventricle, conus arteriosus and bulbus arteriosus. In this regard it should be noted that in the chondrichthyan heart, a distinct atrioventricular segment, characterized by a low content of myosin heavy chain, is interposed between the atrium and ventricle (Franco et al. 2002). However, it remains unclear whether this atrioventricular segment has to be regarded as a sixth component of the primitive gnathostomate heart.
In basal actinopterygians, the bulbus arteriosus persists as a tubular component, coexisting with a well-developed conus arteriosus. Within teleosts, the bulbus has developed as a prominent structure, splitting into a wide range of structural variants (Icardo et al. 1999a,b, 2000), while the conus has been subjected to a considerable reduction in size (Icardo, 2006).
In the African lungfish, Protopterus dolloi, a representative of the sarcopterigyan lineage, the cardiac outflow tract consists of a proximal portion, the conus arteriosus, which extends one-third the length of the outflow and contains only cardiac muscle. The remaining two-thirds of the outflow tract has been described as being arterial-like, but, in fact, possesses a myocardial coat over its entire length (Icardo et al. 2005). Thus, although the lungfish may not possess an entirely smooth muscle bulbus arteriosus like that of chondrichthyans and actinopterygians, both smooth muscle and myocardial components are present, but are morphologically difficult to distinguish.
In the avian and mammalian embryo, the common outflow connects the developing right ventricular component of the looped heart with the aortic sac, and is the embryonic cardiac segment that gives rise to the mesenchymal cushions or ridges from which the outflow tract valves form. At the onset, the junction between the distal end of the common outflow with the aortic sac is found at the cranial boundary of the pericardial cavity (Webb et al. 2003). Later in development, the distal part of the outflow tract consists of the intrapericardial portions of the aortic and pulmonary trunks. The mechanism causing the apparent disappearance or retraction of the distal myocardial wall of the truncus has for a long time been one of the most intriguing aspects of the formation of the tetrapod heart (Webb et al. 2003; Restivo et al. 2006). The finding that the secondary heart field contributes smooth muscle to the arterial pole of the heart (Waldo et al. 2005) is crucial for a better understanding of this morphogenetic change.
The mechanism by which the cardiac outflow tract components form in the basal gnathostomates is similar to that occurring in amniotes. In sturgeons, which share many morphological features with chondrichthyans, the whole embryonic outflow tract shows a myocardial phenotype at the onset of heart development (Guerrero et al. 2004). Later in development, the distal portion of the outflow tract is seen to consist of the non-myocardial bulbus arteriosus (Guerrero et al. 2004). It is likely that this smooth muscle component is added to the arterial pole, rather than any phenotypic transition that has been previously proposed (Guerrero et al. 2004). These data, together with the results obtained by using DAF-2DA in Atlantic catshark (present results), zebrafish and chick (Grimes et al. 2006) embryos indicate that the evolutionarily derived condition of the basal gnathostomate bulbus arteriosus in amniotes is the intrapericardial base of the aortic and pulmonary trunks. With this in mind, it seems reasonable to assume, in accordance with Icardo (2006), that the derived condition of the primitive gnathostomate conus arteriosus in amniotes is the conotruncus. The amniote conotruncus (common outflow) gives rise to the endocardial ridges from which the outflow tract valves form. In its primitive condition, as is the case with chondrichthyans, the embryonic conus arteriosus appears as a straight tube furnished by mesenchymal ridges, which give rise to a variable number of conal valves (for a review, see Sans-Coma et al. 1995).
In summary, from the evolutionary viewpoint, the present findings, together with data from the literature, indicate that: (1) the outflow tract of the heart of basal gnathostomates consists of two distinct components, namely the myocardial conus arteriosus and the non-myocardial bulbus arteriosus; (2) in adult forms, these anatomical elements persist through evolution and are distinct components in actinopterygians but less so in sarcopterygians; (3) the embryonic conus arteriosus of chondrichthyans is morphologically equivalent to the embryonic conotruncus of amniotes; and (4) in amniotes, the non-myocardial component of the outflow tract, which is homologous to the bulbus arteriosus of chondrichthyans, has been remodelled and septated, becoming the intrapericardial, basal portions of the great arterial trunks.
Acknowledgments
This study was supported by grant CGL2006-00693 from the Ministerio de Educación y Ciencia (Spain). C.R. is the recipient of fellowship BES-2007-17632. We thank Dr Luis Gil de Sola and Dr David Macías, from the Spanish Institute of Oceanography, Fuengirola, Málaga, for their contribution in obtaining the specimens studied, and to Luis Vida, Málaga, for his technical assistance.
References
- Abu-Issa R, Waldo K, Kirby ML. Heart fields: one, two or more? Dev Biol. 2004;272:281–285. doi: 10.1016/j.ydbio.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Bertin L. Appareil circulatoire. In: Grassé PP, editor. Traité de Zoologie. XII/II. Paris: Masson; 1958. pp. 1399–1458. [Google Scholar]
- Boas JEV. Über den Conus arteriosus bei Butirinusund bei anderen Knochenfischen. Morphol Jahrb. 1880;6:527–534. [Google Scholar]
- Boas JEV. Lehrbuch der Zoologie. Jena: Gustav Fischer Verlag; 1901. [Google Scholar]
- Braun MH, Brill RW, Gosline JM, Jones DR. Form and function of the bulbus arteriosus in yellowfin tuna (Thunnus albacares), bigeye tuna (Thunus obesus) and blue marlin (Makaira nigricans): static properties. J Exp Biol. 2003;206:3311–3326. doi: 10.1242/jeb.00575. [DOI] [PubMed] [Google Scholar]
- Bridge TW. Fishes (exclusive of the systematic account of teleostei) In: Harmer SF, Shipley AE, editors. The Cambridge Natural History. Vol. 7. London: McMillan; 1904. pp. 141–537. [Google Scholar]
- De Vries R, De Jager S. The gill in the spiny dogfish, Squalus acanthias: respiratory and nonrespiratory function. Am J Anat. 1984;169:1–29. doi: 10.1002/aja.1001690102. [DOI] [PubMed] [Google Scholar]
- Donald JA, Broughton BRS, Bennett MB. Vasodilator mechanisms in the dorsal aorta of the giant shovelnose ray, Rhinobatus typus(Rajiformes; Rhinobatidae) Comp Bioch Physiol A. 2004;137:21–31. doi: 10.1016/s1095-6433(03)00260-5. [DOI] [PubMed] [Google Scholar]
- Durán AC, Rodríguez C, Fernández B, et al. Chondrichthyans have a bulbus arteriosus at the arterial pole of the heart. EB2008 Meeting. FASEB J. 2008;22:586, 1. doi: 10.1111/j.1469-7580.2008.00973.x. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy FB, Tibbs P. Effects of nitric oxide synthase inhibitors and a substrate, L-arginine, on the cardiac function of juvenile salmonid fish. Comp Bioch Physiol C. 2003;135:137–144. doi: 10.1016/s1532-0456(03)00079-6. [DOI] [PubMed] [Google Scholar]
- Evans DH, Gunderson MP. A prostaglandin, not NO, mediates endothelium-dependent dilation in ventral aorta of shark (Squalus acanthias. Am J Physiol. 1998;274:R1050–R1057. doi: 10.1152/ajpregu.1998.274.4.R1050. [DOI] [PubMed] [Google Scholar]
- Evans DH. Vasoactive receptors in abdominal blood vessels of the dogfish shark, Squalus acanthias. Physiol Biochem Zool. 2001;74:120–126. doi: 10.1086/319308. [DOI] [PubMed] [Google Scholar]
- Evans DH, Harrie AC, Kozlowski MS. Characterization of the effects of vasoactive substances on the bulbus arteriosus of the eel, Anguilla rostrata. J Exp Zool. 2003;297A:45–51. doi: 10.1002/jez.a.10238. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Jones DR. The heart. In: Hoar WS, Randall D, Farrel AP, editors. Fish Physiology. XII. San Diego: Academic Press; 1992. pp. 1–87. The Cardiovascular System, Part A. [Google Scholar]
- Fontaine M. Classe des Cyclostomes. Formes actuelles. Super-ordres des Petromyzonoidea et des Myxinoidea. In: Grassé PP, editor. Traité de Zoologie. XIII/I. Paris: Masson; 1958. pp. 13–172. [Google Scholar]
- Franco D, Gallego A, Habets PEMH, Sans-Coma V, Moorman AFM. Species-specific differences in myosin content in the developing cardiac chambers of fish, birds, and mammals. Anat Rec. 2002;268:27–37. doi: 10.1002/ar.10126. [DOI] [PubMed] [Google Scholar]
- Gegenbaur C. Zur vergleichenden Anatomie des Herzens. Jenaisch Z Naturw. 1866;2:365–383. [Google Scholar]
- Grimes AC, Stadt HA, Shepherd IT, Kirby ML. Solving an enigma: arterial pole development in the zebrafish heart. Dev Biol. 2006;290:265–276. doi: 10.1016/j.ydbio.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Grodzinski Z. Das Blutgefäβsystem der Fishe. In: Bronn HG, editor. Klassen und Ordnungen des Tierreichs. Vol. 6. Leipzig: CF Winter; 1938. pp. 1–77. [Google Scholar]
- Guerrero A, Icardo JM, Durán AC, et al. Differentiation of the cardiac outflow tract components in alevins of the sturgeon Acipenser naccarii(Osteichthyes, Acipenseriformes): implications for heart evolution. J Morphol. 2004;260:172–183. doi: 10.1002/jmor.10200. [DOI] [PubMed] [Google Scholar]
- Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. Anat Rec. 2000;260:148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Bulbus arteriosus of Antarctic teleosts. I. The white-blooded Chiondraco hamatus. Anat Rec. 1999a;254:396–407. doi: 10.1002/(SICI)1097-0185(19990301)254:3<396::AID-AR11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Bulbus arteriosus of Antarctic teleosts. II. The red-blooded Trematomus bernachii. Anat Rec. 1999b;256:116–126. doi: 10.1002/(SICI)1097-0185(19991001)256:2<116::AID-AR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Light and electron microscopy of the bulbus arteriosus. J Fish Biol. 2000;57(Suppl A):121–135. doi: 10.1159/000016781. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart. I. The conus valves and the subendocardium. Anat Rec. 2002a;267:17–27. doi: 10.1002/ar.10080. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart. II. The myocardium, the subepicardium and the conus-aorta transistion. Anat Rec. 2002b;268:388–398. doi: 10.1002/ar.10170. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Schib JL, Ojeda JL, et al. The conus valves of the adult gilthead seabream. J Anat. 2003;202:537–550. doi: 10.1046/j.1469-7580.2003.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardo JM, Brunelli E, Perrotta I, Colvée E, Wong WP, Ip YK. Ventricle and outflow tract of the African lungfish Protopterus dolloi. J Morphol. 2005;265:43–51. doi: 10.1002/jmor.10340. [DOI] [PubMed] [Google Scholar]
- Icardo JM. Conus arteriosus of teleost heart: dismissed, but not missed. Anat Rec. 2006;288A:900–908. doi: 10.1002/ar.a.20361. [DOI] [PubMed] [Google Scholar]
- Junqueira LCV, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–435. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms FgF10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Krause R. Mikroskopische Anatomie der Wirbeltiere. IV. Teleostier, Plagiostomen, Zyklostomen und Leptokardier. Berlin: Walter de Gruyter; 1923. [Google Scholar]
- Lawson R. The comparative anatomy of the circulatory system. In: Wake MH, editor. Hayman's Comparative Anatomy. Chicago: University of Chicago Press; 1979. pp. 448–554. [Google Scholar]
- Licht JH, Harris WS. The structure, composition and elastic properties of the teleost bulbus arteriosus in the carp, Cyprinus carpio. Comp Biochem Physiol. 1973;46:699–708. doi: 10.1016/0300-9629(73)90122-9. [DOI] [PubMed] [Google Scholar]
- McGeer JC, Eddy FB. Effects of sodium nitroprusside on blood circulation, acid-base and ionic balance in rainbow trout: evidence for nitric oxide induced vasodilation. Can J Zool. 1996;74:1211–1219. [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, et al. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Moorman AFM, Christoffels VM. Cardiac chamber formation: development, genes and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Olson KR, Villa J. Evidence against nonprostanoid endothelium-derived relaxing factor(s) in trout vessels. Am J Physiol. 1991;260:R925–R933. doi: 10.1152/ajpregu.1991.260.5.R925. [DOI] [PubMed] [Google Scholar]
- Parker TJ, Haswell WA. A Textbook of Zoology. London: Macmillan; 1962. [Google Scholar]
- Parsons CW. The conus arteriosus of fishes. Q J Microsc Sci. 1930;73:145–176. [Google Scholar]
- Pellegrino D, Tota B, Randall DJ. Adenosin/nitric oxide crosstalk in the branchial circulation of Squalus acanthias and Anguilla anguilla. Comp Bioch Physiol Part A. 2005;142:198–204. doi: 10.1016/j.cbpb.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Priede IG. Functional morphology of the bulbus arteriosus of rainbow trout (Salmo gairdnieri Richardson) J Fish Biol. 1976;9:209–216. [Google Scholar]
- Renshaw GM, Dyson SE. Oxide synthase in the vasculature of the epaulette shark brain following hypoxia. Neuroreport. 1999;10:1707–1712. doi: 10.1097/00001756-199906030-00015. [DOI] [PubMed] [Google Scholar]
- Restivo A, Placentini G, Placidi S, Saffiro C, Marino B. Cardiac outflow tract: a review of some embryogenetic aspects of the conotruncal region of the heart. Anat Rec. 2006;288A:936–943. doi: 10.1002/ar.a.20367. [DOI] [PubMed] [Google Scholar]
- Sans-Coma V, Gallego A, Muñoz-Chápuli R, De Andrés AV, Durán AC, Fernández B. Anatomy and histology of the cardiac conal valves of the adult dogfish (Scyliorhinus canicula. Anat Rec. 1995;241:496–504. doi: 10.1002/ar.1092410407. [DOI] [PubMed] [Google Scholar]
- Santer RM. Morphology and innervation of the fish heart. Adv Anat Embryol Cell Biol. 1985;89:1–192. doi: 10.1007/978-3-642-70135-1. [DOI] [PubMed] [Google Scholar]
- Satchell GH. Physiology and Form of Fish Circulation. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Satchell GH, Jones MP. The function of the conus arteriosus in the Port Jackson shark, Heterodontus portusjacksoni. J Exp Biol. 1967;46:373–382. doi: 10.1242/jeb.46.2.373. [DOI] [PubMed] [Google Scholar]
- Schib JL, Icardo JM, Durán AC, et al. The conus arteriosus of the adult gilthead seabream (Sparus auratus. J Anat. 2002;201:395–404. doi: 10.1046/j.0021-8782.2002.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior HD. The conus arteriossus in Tarpon atlanticus(Cuvier & Valenciennes) Biol Bull. 1970a;12:146–151. [Google Scholar]
- Senior HD. Note on the conus of Megalops cyprinoides(Broussonet) Biol Bull. 1970b;12:378–379. [Google Scholar]
- Senior HD. Teleosts with a conus having more than one row of valves. Anat Rec. 1970c;4:83–84. [Google Scholar]
- Simoes-Costa MS, Vasconcelos M, Sampaio AC, et al. The evolutionary origin of cardiac chambers. Dev Biol. 2005;277:1–15. doi: 10.1016/j.ydbio.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Smith WC. On the process of disappearance of the conus arteriosus in teleosts. Anat Rec. 1918;15:65–71. [Google Scholar]
- Stannius H. Bemerkungen über das Verhältniss der Ganoiden zu den Clupeiden, insbesondere zu Butirinus. Rostock: 1846. [Google Scholar]
- Swenson KE, Eveland RL, Gladwin MT, Swenson ER. Nitric oxide (NO) in normal and hypoxic vascular regulation of the spiny dogfish, Squalus acanthias. J Exp Zool A: Comp Exp Biol. 2005;303:154–160. doi: 10.1002/jez.a.145. [DOI] [PubMed] [Google Scholar]
- Von Skramlick E. Über den Kreislauf bei den Fischen. Ergbn Biol. 1935;11:1–130. [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3186. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Ward CC, et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;28:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat. 2003;202:327–342. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert CK, Presch W. Elements of Chordate Anatomy. New York: McGraw-Hill; 1975. [Google Scholar]
- Yamauchi A. Fine structure of the fish heart. In: Bourne GH, editor. Hearts and Heart-like Organs. Vol. 1. New York: Academic Press; 1980. pp. 119–149. [Google Scholar]
- Zummo G, Farina F. Ultrastructure of the conus arteriosus of Scyliorhinus stellaris. J Exp Zool. 1989;2:158–164. [Google Scholar]