Abstract
RNA interference (RNAi) has become a popular tool for analyzing gene function in cancer research. The feasibility of using RNAi in cellular and animal models as an alternative to conventional gene knock out approaches has been demonstrated. Although these studies show that RNAi can recapitulate phenotypes seen in knock out animals and their derived cell lines, a systematic study rigorously comparing downstream effector genes between RNAi and gene knock out has not been performed. Here we present data contrasting the phenotypic and genotypic changes that occur with either stable knock down via RNAi of the cyclin dependent kinase inhibitor p21 versus its somatic cell knock out counterpart in the human mammary epithelial cell line MCF-10A. Our results demonstrate that p21 knock down clones display a growth proliferative response upon exposure to Transforming Growth Factor-Beta Type 1 (TGFβ) similar to p21 knock out clones. However, gene expression profiles were significantly different in p21 knock down cells versus p21 knock out clones. Importantly p21 knock down clones did not display increased gene expression of interleukin-1α (IL-1α), a critical effector of this growth response previously validated in p21 knock out cells. We conclude that gene knock out can yield additional vital information that may be missed with gene knock down strategies.
Keywords: RNAi, gene knock down, gene knock out, p21, TGFβ
INTRODUCTION
The study of gene function in cancer research has classically relied upon two opposing but complementary methods: exogenous gene overexpression versus reducing or silencing expression of a given gene. Techniques for the latter method include antisense RNA, gene knock out and most recently RNAi (reviewed in refs. 1 and 2). In the few years since its introduction, RNAi has become a widespread tool to specifically reduce gene function in cultured cancer cells and to create mouse models of cancer.3,4 However, as more data has emerged, it has also become apparent that caveats to this technology exist including the inability to completely silence the targeted gene, the potential for eliciting cellular anti-viral responses and off target effects of a given RNAi molecule.5–8 Newer methods to overcome these limitations have emerged including the use of long double stranded RNA (dsRNA) that are thought to mitigate off target effects due to the natural cellular processing of these molecules. Despite this, recent reports from independent laboratories have also shown the presence of off target effects using long dsRNA technologies.9,10 Thus, precautionary strategies have evolved to minimize and control for these undesirable effects.7,11
Despite the limitations of RNAi, its ease of use and ability to specifically silence gene expression has led to its widespread adoption among cancer researchers. Many recent studies have demonstrated similar or identical phenotypes between RNAi mediated silencing versus knock out of cancer genes in mouse models and cell lines derived from these systems (reviewed in ref. 12). Our laboratory has a strong interest in somatic cell gene knock out as well as RNAi technology to study gene function as related to human breast carcinogenesis. We have recently presented the results of a global gene expression analysis using the genetically stable MCF-10A human breast epithelial cell line versus its somatically targeted p21 knock out counterpart and their response to TGFβ.13 p21 is a well characterized cyclin dependent kinase (CDK) inhibitor, originally cloned as a downstream effector of the tumor suppressor p53.14 Parental MCF-10A cells are normally growth inhibited by TGFβ, whereas somatic cell gene knock out of p21 results in a growth proliferative phenotype upon exposure to this cytokine. In addition, p21 knock out cells demonstrate an epithelial to mesenchymal transition after prolonged exposure to TGFβ. Gene expression analysis and functional studies revealed that the growth response to TGFβ is mediated in part by the pro-inflammatory cytokine, IL-1α. This system presented a unique opportunity to evaluate a direct phenotypic and genotypic comparison between p21 knock out cells and p21 knock down cells in a genetically stable cell line using a panel of 52 genes previously validated in their response to TGFβ.
MATERIALS AND METHODS
Cell lines and culture conditions
MCF-10A cells were purchased from ATCC (Manassas, VA). The p21−/− MCF-10A cells have been previously described.15 Cells were grown in DMEM:F12 without phenol red with 5% charcoal stripped dextran treated fetal bovine serum (Hyclone, Logan, UT), supplemented with cholera toxin at 0.1 μg/ml (Sigma, St. Louis, MO), hydrocortisone at 0.5 μg/ml (Sigma, St. Louis, MO), epidermal growth factor (EGF) at 0.02 μg/ml (Sigma, St. Louis, MO) and human insulin at 10 μg/ml (Sigma, St. Louis, MO). Cells were maintained in a 37°C incubator with 5% CO2.
Short hairpin RNA specific for p21 (shp21) or GFP (shGFP) vector construction and generation of stable cell clones
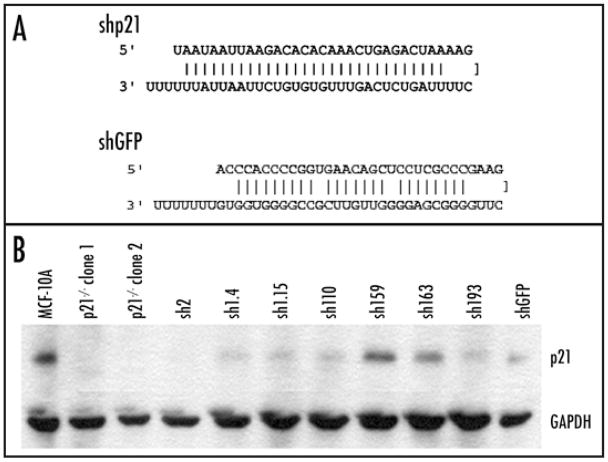
Short hairpins were designed using open access on line software (www.katahdin.cshl.org:9331/homepage/siRNA/RNAi.cgi?type=shRNA) to identify shRNA sequences specifically targeting human p21 and green fluorescent protein (GFP) mRNA (Fig. 1A). These sequences were synthesized as DNA oligos and then cloned into a plasmid vector containing a U6 promoter and a neomycin resistance gene for selection. All constructs were verified via automated sequencing. Plasmid DNA containing shp21 or shGFP was transfected into MCF-10A cells using Fugene 6 (Roche, Indianapolis, IN) as per the manufacturer’s directions and selected in G418 containing media (120 μg/ml) for three weeks. Single cell clones were evaluated for reduced p21 protein levels using Western blot as described below.
Figure 1.
Stable transfection of short hairpin RNA sequences against p21 in MCF-10A cells leads to clones with variable degrees of gene knock down. (A) Short hairpin RNA sequence (shp21) used for stable gene knock down. Short hairpin RNA sequence for GFP (shGFP) used as a control is also shown. (B) Western blot analysis of protein levels in p21 knock out and knock down clones. Results are representative of multiple blots.
Western blot analysis
Whole cell lysates were prepared from cells grown in culture and resuspended in Laemmli buffer. Equivalent amounts of samples were subjected to gel electrophoresis. Western blotting was performed using NuPage gels (Invitrogen, Carlsbad, CA) as per the manufacturer’s recommendations. Primary antibodies for human p21 (clone AB-1, Oncogene Research Products, Boston, MA) and GAPDH (Abcam, Cambridge, England) and secondary antibody for mouse IgG (Pierce Endogen, Rockford, IL) were used at the manufacturer’s recommended dilutions. Blots were exposed to Kodak XAR film using chemiluminescence for detection (Perkin Elmer, Boston, MA).
TGFβ cell proliferation assay
Proliferation assays have been previously described.15 In brief, MCF-10A cells and their derivatives (knock out and knock down clones) were cultured in assay media, which is identical to growth media without EGF, and then supplemented with TGFβ at 2 ng/ml (R&D systems, Minneapolis, MN). Cells were seeded at equal density in 24 well plates and then cultured for six days. Media was replaced on a daily basis to prevent arrested cells from dying, thus ensuring that cell counts reflected only cell proliferation and not increased survival. Cells were counted using a Coulter Cell Counter (Beckman Coulter, Fullerton, CA) as per the manufacturer’s directions.
Quantitative real time RT-PCR analysis
Total RNA was isolated using the RNeasy system (Qiagen, Valencia, CA). Cells were seeded as above for proliferation assays, and harvested on day one, thirty minutes after the addition of TGFβ corresponding with the kinetics of IL-1α gene induction as previously reported.13 Single stranded cDNA was generated using First Strand cDNA Synthesis Kit (Amersham Biosciences, UK) following the manufacturer’s directions. Control template reactions were prepared in parallel without the addition of reverse transcriptase. Real time quantitative RT-PCR was then performed as previously described.15 Primers used for real time RT-PCR are listed in Supplementary Table 1.
Multidimensional scaling analysis
Values from real time PCR experiments were used to generate a multidimensional scaling diagram based upon linear discriminate analysis, representing the similarities between samples as Euclidean distance, as previously described.16 Relationships between samples were plotted in three- dimensional space. This analysis was performed using the in-house software available from The National Human Genome Research Institute’s array analysis website https://arrayanalysis.nih.gov.
RESULTS
Variability of gene knock down using a short hairpin RNA targeting the p21 gene
To begin our analyses, we chose to use DNA based short hairpin RNA (shRNA) to knock down p21 gene expression. For these studies, commercially available short interfering RNA (siRNA) molecules could not be used for two reasons. First, although siRNA molecules have a high transfection efficiency in MCF-10A cells, we could only achieve 50% gene knock down with these reagents, though in other cell lines such as MCF-7 and HEK293, p21 mRNA and protein were greatly reduced (data not shown). Others have also shown that reduction of gene expression via RNAi can have varying effects that are cell type dependent.17,18 Furthermore, growth assays used to assess TGFβ responses require six days in culture necessitating the creation of stable knock down clones, as gene knock down using transient transfection of siRNA or shRNA is generally extinguished after two to three days in culture. We therefore constructed a series of shRNA vectors designed to target the p21 gene. Using online software (see Materials and Methods), we designed eight different shRNA sequences. As before, with transient transfection none of these sequences were capable of reducing p21 mRNA levels by more than 30% in the MCF-10A cell line, though one demonstrated marked reduction of gene expression in HEK293 cells (data not shown). However, the transfection efficiency of MCF-10A cells with plasmid DNA is greatly reduced compared to siRNA molecules approaching 50% under optimal conditions. We therefore chose the shRNA vector that yielded the best gene knock down with transient transfection (Fig. 1A) and stably transfected this construct into MCF-10A cells with the rationale that stable integration of shRNA constructs in a clonal population may be able to confer a high degree of gene knock down. Using Western blotting, we analyzed p21 protein levels in over five hundred individual stably selected clones to assess the degree of p21 reduction. As shown in (Fig. 1B), the level of p21 protein varied, with clone sh2 demonstrating the highest amount of gene knock down and clone sh159 having almost no knock down relative to the MCF-10A parental control. As a negative control, we used a shRNA vector against green fluorescent protein (GFP) termed shGFP (Fig. 1A). Unexpectedly, three stable clones derived from this construct also demonstrated a significant reduction of p21 protein, though this was not seen with transient transfections (representative clone shown in Fig. 1B). We presume that this particular shRNA sequence has off target effects resulting in p21 knock down that only manifested after stable integration and expression, and was not seen transiently due to poor transfection efficiency.
Stable shRNA p21 knock down clones are growth stimulated by TGFβ
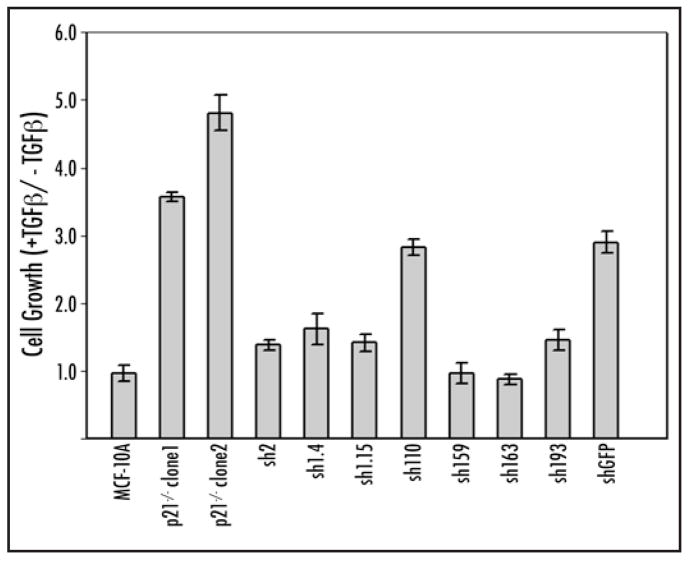
We have previously shown that gene knock out of p21 in MCF-10A cells reverses the cellular response to TGFα from growth inhibitory to growth stimulatory, and that a component of this effect is mediated by an increase in IL-1α gene expression.13,15 Therefore, we assessed whether knock down of p21 induced a similar TGFβ growth phenotype by performing a cell proliferation assay as previously described.15 To ensure accurate comparisons, earlier passages of p21 knock out clones were used so that these studies were performed on knock out and knock down clones of equivalent passage number. MCF-10A cells require external growth factors such as epidermal growth factor (EGF) for normal growth. Removal of arrest facilitating our ability to this cytokine induces a complete G1 assess even small increases in cell proliferation mediated by TGFβ.15 As seen in Figure 2, TGFβ gave no growth promotion phenotype to parental MCF-10A cells while both MCF-10A p21−/− knock out clones (clone1 and clone2) showed a strong growth proliferative effect as expected. The majority of p21 knock down clones also demonstrated a growth proliferative phenotype upon TGFβ exposure. However, knock down clones showed varying degrees of cell proliferation relative to plating efficiencies, and this effect was not entirely consistent with the amount of p21 protein levels. For example, the most severe knock down clone, sh2, exhibited only a slight growth enhancement in response to TGFβ. In addition, all other clones demonstrating appreciable levels of p21 knock down (sh1.4, sh1.15, sh110, sh193) also displayed a growth proliferative phenotype though again, the magnitude of this response varied. Clones sh159 and sh163 had minimal to no p21 knock down and accordingly did not show a TGFβ growth proliferative phenotype identical to parental MCF-10A cells. As described above, the control clone shGFP, which is a stably transfected shRNA construct against GFP, also had a significant decrease in p21 protein. Similar to p21 knock down clones, this clone had a growth proliferative response upon TGFβ exposure.
Figure 2.
TGFβ growth response in p21 knock out and stable p21 knock down clones. Cells were seeded at equal density and grown under conditions described in Materials and Methods. Cell counts for each sample were taken on day one and day six with each bar depicting the relative change in cell number with and without TGFα (+TGFβ/-TGFβ). Results are expressed as the average of triplicate measurements and are representative of three independent experiments. Error bars represent standard error of the mean.
Gene expression analysis after TGFβ exposure yields distinct groups between knock out and knock down clones
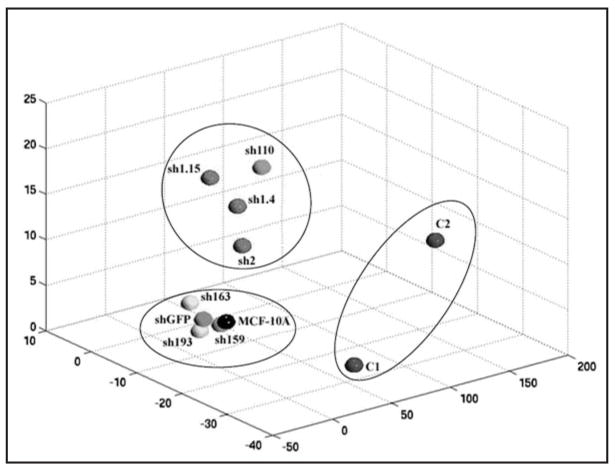
As seen in Table 1, the expression of 52 genes was analyzed by quantitative real time RT-PCR following stimulation of the cells with TGFβ. The majority of the genes in this subset had previously been validated in our study using p21 knock out clones.13 For the current study, we repeated all assays with knock out and knock down clones and harvested the cells for RNA at an earlier time point based upon our prior work demonstrating the upregulation of IL-1α in the p21 knock out cell lines.13 As predicted, IL-1α gene expression increased by almost three-fold in p21 knock out clones consistent with our previous report demonstrating this cytokine’s role in mediating the growth proliferative effects of TGFβ.13 Somewhat unexpectedly, increased IL-1α gene expression was not seen in any of the knock down clones that demonstrated a growth proliferative response to TGFβ. Clone sh159 did show a two-fold increase in IL-1α gene expression upon TGFβ exposure, but curiously this clone has relatively little to no gene knock down and no proliferative response to TGFβ. In order to visualize the global relationships of the samples, the data were subjected to a multidimensional scaling analysis, which measures the magnitude of difference between the sum gene expression profiles of each sample,16 thus providing an overview of their similarity to each other (Fig. 3). This analysis yielded three distinct subsets, with knock out clones being vastly different than their knock down counterparts. Notably, knock down clones clustered in one of two groups, but the availability of only two knock out clones raises the possibility that additional knock out clones could also show varied patterns of gene expression.
Table 1.
TGFβ induced gene expression in wild type, p21−/− knock out and p21 stable knock down MCF-10A cell lines
| +TGFβ/−TGFβ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | McF10A | p21−/− c1 | p21−/− c2 | sh2 | sh1.4 | sh1.15 | sh110 | sh159 | sh163 | sh193 | shGFP |
| EZRIN | 2.2 | 2.0 | 3.7 | 0.7 | 2.3 | 0.4 | 0.3 | 0.6 | 1.1 | 1.0 | 0.7 |
| RAN | 1.4 | 1.1 | 1.2 | 1.0 | 2.1 | 0.3 | 1.7 | 0.5 | 1.6 | 2.5 | 1.9 |
| CD63 | 2.3 | 1.5 | 1.2 | 2.2 | 3.9 | 0.8 | 1.5 | 1.1 | 2.1 | 0.8 | 1.7 |
| CDC23 | 5.5 | 17.4 | 12.5 | 4.6 | 2.6 | 0.5 | 5.2 | 4.6 | 0.6 | 0.7 | 1.4 |
| CDC20 | 3.1 | 6.1 | 7.8 | 0.4 | 2.8 | 1.0 | 5.5 | 5.6 | 0.3 | 11.5 | 0.4 |
| FAP | 3.6 | 1.0 | 1.2 | 1.2 | 0.9 | 0.4 | 2.2 | 0.7 | 2.1 | 0.3 | 1.1 |
| CDC28 | 1.3 | 1.1 | 2.0 | 3.2 | 8.0 | 18.0 | 9.1 | 0.1 | 1.1 | 0.1 | 0.2 |
| CDH2 | 0.9 | 3.8 | 4.0 | 0.6 | 0.2 | 0.2 | 3.0 | 1.9 | 0.9 | 2.3 | 0.8 |
| FN1 | 16.1 | 49.1 | 189.7 | 44.2 | 43.7 | 64.0 | 7.2 | 17.6 | 19.3 | 19.8 | 4.4 |
| SYS6 | 0.6 | 0.5 | 0.6 | 1.1 | 0.8 | 0.4 | 2.1 | 0.8 | 2.0 | 1.6 | 1.3 |
| LOX | 7.2 | 8.4 | 2.8 | 12.3 | 13.0 | 2.6 | 19.9 | 4.6 | 2.8 | 2.9 | 7.2 |
| IL-1α | 0.3 | 2.7 | 2.8 | 1.1 | 0.7 | 0.5 | 1.0 | 2.0 | 0.3 | 0.9 | 0.3 |
| CDC25A | 1.5 | 1.9 | 2.1 | 2.0 | 1.7 | 1.4 | 1.3 | 0.4 | 1.1 | 0.9 | 1.5 |
| MAPK13 | 2.0 | 1.9 | 1.6 | 2.4 | 1.8 | 1.4 | 0.9 | 1.9 | 1.0 | 1.0 | 4.6 |
| IL-15 | 2.0 | 2.4 | 1.3 | 1.3 | 1.0 | 0.6 | 1.0 | 0.4 | 1.2 | 1.1 | 0.5 |
| CNB1 | 3.0 | 6.1 | 30.0 | 6.3 | 2.5 | 1.4 | 10.1 | 2.6 | 3.5 | 3.3 | 2.4 |
| SUR | 1.5 | 12.2 | 8.0 | 2.3 | 2.0 | 1.4 | 13.9 | 4.4 | 1.3 | 1.0 | 2.8 |
| IGFBP7 | 1.2 | 3.1 | 1.2 | 1.6 | 2.9 | 2.9 | 4.6 | 2.5 | 4.6 | 3.0 | 1.9 |
| KU-antigen | 1.3 | 1.1 | 1.5 | 0.2 | 1.1 | 0.6 | 2.2 | 0.6 | 1.1 | 1.6 | 0.5 |
| PLK | 1.1 | 2.1 | 1.5 | 1.4 | 2.3 | 1.1 | 1.2 | 0.9 | 1.4 | 2.5 | 0.5 |
| GIOT1 | 1.1 | 2.1 | 1.6 | 3.4 | 2.1 | 1.4 | 2.0 | 2.0 | 0.6 | 0.9 | 1.4 |
| IL-8 | 0.7 | 4.4 | 3.7 | 2.3 | 1.2 | 0.7 | 4.1 | 1.4 | 1.1 | 0.9 | 1.5 |
| KIF3C | 3.1 | 1.2 | 1.4 | 3.4 | 3.4 | 2.6 | 6.2 | 3.7 | 1.8 | 1.4 | 1.0 |
| Met2A | 7.4 | 19.6 | 5.0 | 2.0 | 7.1 | 1.1 | 5.1 | 3.0 | 1.3 | 1.1 | 3.0 |
| Met1E | 1.6 | 0.3 | 0.9 | 1.4 | 1.4 | 0.9 | 2.1 | 0.6 | 1.1 | 0.3 | 1.5 |
| HSP90 | 1.9 | 2.1 | 0.3 | 0.4 | 2.0 | 0.5 | 0.5 | 2.1 | 1.3 | 1.4 | 1.0 |
| cMyc | 1.4 | 1.5 | 0.5 | 2.7 | 3.3 | 1.1 | 1.5 | 0.9 | 1.2 | 1.5 | 1.3 |
| cyclinD1 | 1.3 | 1.1 | 1.0 | 1.0 | 0.9 | 0.7 | 1.1 | 1.2 | 0.9 | 0.5 | 1.3 |
| CATD | 1.4 | 1.5 | 0.5 | 0.9 | 1.0 | 0.7 | 1.1 | 1.1 | 0.9 | 0.8 | 1.9 |
| HSP70 | 1.6 | 0.3 | 0.7 | 1.1 | 1.8 | 0.9 | 2.0 | 1.3 | 1.8 | 2.9 | 0.9 |
| AQP9 | 9.8 | 0.7 | 0.9 | 0.1 | 5.1 | 0.4 | 0.4 | 0.2 | 0.5 | 3.6 | 1.2 |
| MAPK3 | 0.7 | 1.5 | 1.2 | 0.7 | 2.0 | 0.7 | 0.7 | 2.2 | 1.2 | 0.6 | 0.9 |
| ARCH | 1.8 | 1.4 | 1.1 | 1.2 | 1.3 | 0.9 | 1.6 | 1.6 | 1.2 | 0.6 | 1.8 |
| TP53 | 3.9 | 10.6 | 0.3 | 2.9 | 1.8 | 1.0 | 1.0 | 1.3 | 3.1 | 3.3 | 2.6 |
| ITGA5 | 1.6 | 1.7 | 1.7 | 1.8 | 1.7 | 1.3 | 2.3 | 2.4 | 1.2 | 0.9 | 2.2 |
| MMP2 | 5.3 | 5.0 | 12.0 | 0.9 | 1.4 | 5.6 | 4.4 | 1.1 | 0.7 | 0.5 | 0.4 |
| SRPN1 | 7.1 | 12.7 | 11.0 | 6.1 | 4.8 | 3.3 | 9.5 | 8.7 | 2.8 | 4.2 | 11.8 |
| NSEP1 | 2.0 | 1.1 | 1.0 | 1.3 | 1.0 | 0.8 | 0.6 | 1.0 | 1.3 | 0.4 | 1.1 |
| CREBL1 | 1.7 | 1.9 | 1.7 | 0.8 | 0.8 | 0.8 | 0.9 | 0.9 | 0.6 | 1.7 | 0.6 |
| E48 | 1.5 | 1.0 | 2.0 | 1.3 | 1.0 | 0.7 | 0.4 | 0.4 | 1.7 | 0.2 | 1.2 |
| Met1X | 0.6 | 1.7 | 0.5 | 0.4 | 3.3 | 0.5 | 0.6 | 0.7 | 0.8 | 0.8 | 0.9 |
| RAB3D | 2.6 | 0.2 | 0.4 | 1.2 | 4.3 | 0.9 | 0.8 | 0.8 | 0.8 | 1.4 | 1.8 |
| 20B | 1.8 | 1.2 | 1.4 | 2.0 | 1.9 | 1.5 | 0.8 | 1.2 | 1.3 | 0.6 | 2.3 |
| K-RAS | 0.6 | 1.8 | 1.2 | 1.3 | 2.4 | 1.2 | 2.1 | 1.5 | 0.8 | 0.9 | 3.5 |
| NFKB1 | 1.3 | 2.3 | 0.9 | 1.2 | 1.6 | 1.1 | 0.6 | 1.0 | 0.9 | 0.6 | 1.6 |
| Vim | 0.4 | 3.3 | 1.9 | 1.4 | 2.2 | 1.0 | 0.3 | 0.2 | 5.5 | 0.5 | 1.2 |
| Ecad | 2.9 | 0.4 | 0.9 | 2.6 | 9.1 | 0.5 | 3.4 | 1.5 | 2.7 | 1.0 | 1.5 |
| ENO1 | 1.3 | 1.0 | 1.0 | 1.0 | 1.2 | 0.9 | 1.5 | 1.0 | 1.2 | 0.6 | 1.4 |
| MX1 | 1.2 | 1.6 | 1.5 | 1.8 | 1.3 | 1.4 | 1.2 | 1.2 | 1.5 | 0.6 | 1.1 |
| Cullin3 | 3.4 | 0.5 | 0.8 | 0.6 | 0.6 | 2.6 | 0.1 | 0.1 | 2.8 | 0.2 | 0.1 |
| DSC | 3.3 | 3.3 | 1.3 | 0.8 | 0.4 | 2.2 | 0.5 | 0.5 | 0.6 | 0.4 | 2.2 |
| COL4 | 2.2 | 1.1 | 1.3 | 0.6 | 4.2 | 2.1 | 8.3 | 0.9 | 3.8 | 3.3 | 0.6 |
Gene expression by quantitative real time RT-PCR using primers listed in supplementary information. Cells were cultured and harvested as described in Material and Methods. For each gene, results are expressed as the fold change difference between TGFβ exposure (+TGFβ) versus vehicle control (αTGFβ).
Figure 3.
Clustering of parental MCF-10A cells and p21 knock out/knock down clones based on gene expression profiles of 52 genes by quantitative real time RT-PCR after TGFβ exposure. Cells were seeded and harvested as described in Materials and Methods, and RNA was extracted, and used to perform quantitative real time PCR. Values from real time PCR were used to generate a multidimensional scaling diagram, providing a visualization of the global relationships between the samples. X, Y and Z represent Euclidean distance between each sample based upon the summation and comparison of the gene expression differences as described in Materials and Methods.
DISCUSSION
The discovery of RNAi has revolutionized the evaluation of gene function for cancer research. Clearly this powerful means of regulating gene expression has led to numerous insights and discoveries concerning cancer gene function. Recently, there have been various studies comparing phenotypes observed with RNAi versus the more traditional approaches of gene knock out. For example, large scale RNAi screens in Caenorhabditis elegans and Drosophila melanogaster have demonstrated that RNAi can recapitulate knock out/mutant phenotypes, though the rate of false negative in some of these screens was quite elevated.19,20 In addition, stable heritable gene knock down using RNAi has been demonstrated in murine systems and can lead to phenotypes similar to their knock out mouse counterparts.12 Despite this, to our knowledge there has not been a systematic analysis of the gene expression changes associated with stable RNAi versus knock out of a given gene.
Two reports have previously compared phenotypic differences between RNAi versus knock out of the DNA methyltransferase 1 (DNMT1) gene in human somatic cells.21,22 Using the same RNAi sequences and identical cell lines these groups yielded disparate results. The first of these two studies by Robert et al. suggested potential phenotypic discrepancies between DNMT1 somatic knock out cell lines versus their RNAi gene knock down counterparts when comparing promoter methylation of genes. However, this was not seen in the study by Ting et al. The conflicting results observed by these two groups could conceivably be explained by the use of the HCT-116 cell line. This cell line is known to have a “mutator” phenotype due to mutations in nucleotide mismatch repair genes,23 and preexisting mutations within this cancer cell line could potentially obscure conclusions regarding gene function as recently reported by our group.24 To avoid these potential confounding effects, we have conducted the current study using the non-tumorigenic MCF-10A human breast epithelial cell line.25 These cells are genetically stable as assessed for microsatellite instability (our unpublished observations) and chromosomal instability,26 thus making them ideal for these analyses.
There were several notable results from these experiments. First, severe (>90%) gene knock down could only be achieved in one out of five hundred clones screened in the MCF-10A cell line. This appeared to be cell line specific, as others have also reported difficulty in obtaining high levels of gene knock down in the MCF-10A cell line (Moustakis A, personal communication). Second, there was generally no consensus between the levels of p21 knock down and the ability of these clones to proliferate in response to TGFβ. Somewhat paradoxically the most severe knock down clone, sh2, did not exhibit a strong growth proliferative response upon TGFβ exposure, yet other clones with less p21 knock down (sh110 and shGFP) demonstrated a more robust response to this cytokine. The basis of this is unclear. Third, gene expression profiles using a previously validated gene set clearly demonstrated wide variability between knock out versus knock down clones. In addition, knock down clones segregated into one of two clusters that did not correlate with their growth response phenotype. Although it is possible that stable integration of the shRNA vector by itself altered the gene expression profiles of knock down clones and caused them to be vastly different than knock out cell lines, the fact that parental MCF-10A cells also clustered within one of these two groups strongly argues against this possibility. Fourth, we confirmed that experimental control RNAi molecules must be used with caution, as our results demonstrate that shRNA vectors designed to target non-human genes such as GFP, can lead to off target effects. Similarly, we have recently observed that a commercially available GFP short hairpin vector was also found to significantly reduce levels of the PTEN protein in MCF-10A cells (unpublished observations). Presumably, this could also be true for “scrambled” control RNAi molecules commonly used in many gene knock down studies. Finally, although p21 knock down correlated with a TGFβ induced growth proliferative response in the majority of stable shRNA clones, none of these clones could reproduce the magnitude of the growth proliferative response seen in p21 knock out clones. Consistent with this notion, knock down clones did not demonstrate an increase in IL-1α gene expression upon TGFβ exposure. We recently extensively characterized and validated this gene’s role in promoting the growth proliferative phenotype induced by TGFβ in our p21 knock out cell lines, including the ability of TGFβ to directly stimulate the IL-1α promoter, leading to increased gene transcription.13 This would suggest that complete absence of this cyclin dependent kinase inhibitor is required for TGFβ to increase IL-1α gene expression. These results are also consistent with our earlier report demonstrating that IL-1α mediates part but not all of the growth proliferative response to TGFβ in p21 null cell lines. Therefore, in addition to the known caveats of RNAi, our study raises an additional concern of missing important downstream genes using this technology. Although there has been widespread use of RNAi to functionally assess a gene’s importance to carcinogenesis, gene knock out strategies may still provide additional information that could serve to further elucidate molecular mechanisms of a given phenotype.
Acknowledgments
The authors thank members of the Park Lab for critical review of the manuscript. We also thank Drs. Nickolas Papadopoulos and Bert Vogelstein for thoughtful discussions of this work.
This work was supported by the Department of Defense Breast Cancer Research Program (DAMD17-03-1-0241), NIH CA109274, The Elsa Pardee Foundation, and The Summer Running Fund (www.stompcancer.org). J.G. is a recipient of a Department of Defense Breast Cancer Research Program Predoctoral Fellowship Award (W81XWH-06-1-0325), A.A. is supported on an NIH Institutional Training Grant (T32CA67751) and H.K. is a recipient of a Young Clinician Scientist Award from The Flight Attendant Medical Research Institute (FAMRI). This work was also supported in part by funds from the intramural research program of the National Institute on Aging. B.H.P. is an Avon Scholar for Breast Cancer Research and also receives generous support from The FAMRI and The V Foundation for Cancer Research via The NFL Referee’s Blow the Whistle on Cancer Campaign.
Abbreviations
- RNAi
RNA interference
- TGFβ
transforming growth factor-Beta type 1
- IL-1α
interleukin-1α
- dsRNA
double stranded RNA
- CDK
cyclin dependent kinase
Footnotes
Note
Supplementary Table 1 can be found at: www.landesbioscience.com/supplement/KarakasCBT6-7-sup.pdf
References
- 1.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–24. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 2.Lavery KS, King TH. Antisense and RNAi: Powerful tools in drug target discovery and validation. Curr Opin Drug Discov Devel. 2003;6:561–9. [PubMed] [Google Scholar]
- 3.Sandy P, Ventura A, Jacks T. Mammalian RNAi: A practical guide. Biotechniques. 2005;39:215–24. doi: 10.2144/05392RV01. [DOI] [PubMed] [Google Scholar]
- 4.Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Crelox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–5. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–4. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- 6.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–9. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 7.Cullen BR. Enhancing and confirming the specificity of RNAi experiments. Nat Methods. 2006;3:677–81. doi: 10.1038/nmeth913. [DOI] [PubMed] [Google Scholar]
- 8.Pei Y, Tuschl T. On the art of identifying effective and specific siRNAs. Nat Methods. 2006;3:670–6. doi: 10.1038/nmeth911. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni MM, Booker M, Silver SJ, Friedman A, Hong P, Perrimon N, Mathey-Prevot B. Evidence of off-target effects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat Methods. 2006;3:833–8. doi: 10.1038/nmeth935. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Creanga A, Lum L, Beachy PA. Prevalence of off-target effects in Drosophila RNA interference screens. Nature. 2006;443:359–63. doi: 10.1038/nature05179. [DOI] [PubMed] [Google Scholar]
- 11.Echeverri CJ, Beachy PA, Baum B, Boutros M, Buchholz F, Chanda SK, Downward J, Ellenberg J, Fraser AG, Hacohen N, Hahn WC, Jackson AL, Kiger A, Linsley PS, Lum L, Ma Y, Mathey-Prevot B, Root DE, Sabatini DM, Taipale J, Perrimon N, Bernards R. Minimizing the risk of reporting false positives in large-scale RNAi screens. Nat Methods. 2006;3:777–9. doi: 10.1038/nmeth1006-777. [DOI] [PubMed] [Google Scholar]
- 12.Prawitt D, Brixel L, Spangenberg C, Eshkind L, Heck R, Oesch F, Zabel B, Bockamp E. RNAi knock-down mice: An emerging technology for post-genomic functional genetics. Cytogenet Genome Res. 2004;105:412–21. doi: 10.1159/000078214. [DOI] [PubMed] [Google Scholar]
- 13.Karakas B, Weeraratna A, Abukhdeir A, Blair BG, Konishi H, Arena S, Becker K, Wood W, IIIrd, Argani P, De Marzo AM, Bachman KE, Park BH. Interleukin-1 alpha mediates the growth proliferative effects of transforming growth factor-beta in p21 null MCF-10A human mammary epithelial cells. Oncogene. 2006;25:5561–9. doi: 10.1038/sj.onc.1209540. [DOI] [PubMed] [Google Scholar]
- 14.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 15.Bachman KE, Blair BG, Brenner K, Bardelli A, Arena S, Zhou S, Hicks J, De Marzo AM, Argani P, Park BH. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3:221–5. doi: 10.4161/cbt.3.2.666. [DOI] [PubMed] [Google Scholar]
- 16.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds A, Anderson EM, Vermeulen A, Fedorov Y, Robinson K, Leake D, Karpilow J, Marshall WS, Khvorova A. Induction of the interferon response by siRNA is cell type- and duplex length-dependent. RNA. 2006;12:988–93. doi: 10.1261/rna.2340906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting AH, Jair KW, Schuebel KE, Baylin SB. Differential requirement for DNA methyl-transferase 1 in maintaining human cancer cell gene promoter hypermethylation. Cancer Res. 2006;66:729–35. doi: 10.1158/0008-5472.CAN-05-1537. [DOI] [PubMed] [Google Scholar]
- 19.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 20.Armknecht S, Boutros M, Kiger A, Nybakken K, Mathey-Prevot B, Perrimon N. High-throughput RNA interference screens in Drosophila tissue culture cells. Methods Enzymol. 2005;392:55–73. doi: 10.1016/S0076-6879(04)92004-6. [DOI] [PubMed] [Google Scholar]
- 21.Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, MacLeod AR. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet. 2003;33:61–5. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- 22.Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE. CpG island hypermethylation is maintained in human colorectal cancer cells after RNAi-mediated depletion of DNMT1. Nat Genet. 2004;36:582–4. doi: 10.1038/ng1365. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos N, Nicolaides NC, Wei YF, Ruben SM, Carter KC, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625–9. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 24.Weiss MBVMI, Baerenfaller K, Marra G, Park BH, Bachman KE. Persistent mismatch repair deficiency following targeted correction of hMLH1. Cancer Gene Ther. 2007;14:98–104. doi: 10.1038/sj.cgt.7700997. [DOI] [PubMed] [Google Scholar]
- 25.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 26.Yoon DS, Wersto RP, Zhou W, Chrest FJ, Garrett ES, Kwon TK, Gabrielson E. Variable levels of chromosomal instability and mitotic spindle checkpoint defects in breast cancer. Am J Pathol. 2002;161:391–7. doi: 10.1016/S0002-9440(10)64194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]