Abstract
Background
Given the established links between young age at first intercourse (AFI), number of sex partners, high-risk human papillomavirus infection, and squamous cell cervical cancer (SCC), we hypothesized that women diagnosed with SCC at younger ages would be more likely to report young AFI than women diagnosed later in life.
Methods
We performed a population-based investigation among invasive SCC cases who were diagnosed between 1986 and 2004, were 22 to 53 years old, and lived in the metropolitan Seattle-Puget Sound region (n=333). Using multivariate linear regression, we estimated coefficients and 95% confidence intervals (CI) to assess the association between age at SCC diagnosis and AFI (<15, 15–18, ≥19) and number of sex partners before age 20 (0, 1, 2–4, 5–14, 15+), accounting for birth year and other factors. Interactions were assessed using the likelihood ratio test.
Results
The interval between AFI and SCC diagnosis ranged from 4 to 35 years. In a multivariate model, compared to SCC cases reporting AFI≥19, the mean age of diagnosis was 3.1 years younger for SCC cases reporting AFI<15 (CI: −5.8, −0.5) and 2.6 years younger for SCC cases reporting AFI 15–18 years (CI: −4.6, −0.6). Although number of sex partners before age 20 was associated with age at SCC diagnosis in a crude analysis, the association was not independent of AFI. However, in the AFI≥19 and AFI<15 groups, differences in effect were seen by number of sex partners before age 20 (p for interaction=0.08), with the association remaining strong and significant only in the AFI<15 group that had 2 or more partners before age 20 (coefficient: −4.2, CI: −6.3, −2.1).
Conclusion
Among younger and middle-aged women with SCC, early age of diagnosis was associated with early AFI, though the effect appeared to be modified by number of sex partners before age 20.
Keywords: Cervical Carcinoma, Sexual Initiation, Age
INTRODUCTION
Cancer of the uterine cervix is the second most common cancer in women worldwide (1). In the developing world, it is thought to be responsible for the most years of life lost due to cancer, in part because it affects relatively young women (2). The majority of women with invasive squamous cell carcinoma of the cervix (SCC) are diagnosed in their mid-forties or fifties, though some women are diagnosed much earlier (3–5). In the US, the annual incidence among women 15 to 24 years of age is less than 1 per 100,000, and among women 25 to 34 years of age, it is approximately 7 per 100,000 (6). Although SCC incidence has decreased considerably among women over 34 years of age during the last 30 years in the US due to implementation of Papanicolaou smear (Pap) screening programs, the rate among younger women has remained relatively unchanged (6). In addition to the direct impact SCC can have on mortality and morbidity for cases of any age, a significant consequence especially relevant to cases who are of child-bearing age is infertility resulting from standard treatment (7, 8).
That SCC occurs in young women is perplexing given the current estimates for sojourn time from the necessary high-risk human papillomavirus (HR-HPV) infection of the cervix to invasive cancer diagnosis (3–5). The modal time between HR-HPV infection in the late teens or early twenties and diagnosis of the high-grade intraepithelial lesion that precedes invasive SCC (cervical intraepithelial neoplasia grade 3, CIN3) has been estimated as 7–15 years with diagnoses peaking at 27–30 years of age (3, 5), though there is some indication that it may be shorter (9). The transit time from CIN3 to invasive cervical cancer has been harder to estimate, because women diagnosed with CIN3 are treated by ablation or excision of the lesion. The range has been estimated as 1–20 years (4). However, if one uses the difference between median age of CIN3 diagnosis (27–30 years old) and that of invasive cervical cancer (~48 years old) to estimate median sojourn time, it appears to be at the longer end of the estimated range (3, 5). As sexual contact is thought to be the mode of transmission for virtually all anogential HPV infections (5), these estimates suggest that women diagnosed with SCC in their twenties or younger could have a very young age at first intercourse (AFI), perhaps as early as 10 or 12 years old. Early-onset SCC may also occur due to particularly rapid disease progression (10, 11), a controversial phenomenon (12).
Although early sexual debut has been identified as a risk factor for HPV infection (13, 14) and invasive SCC (15–17), we know of no study in which the association between AFI and age at invasive SCC diagnosis has been assessed. Exploring this relationship could contribute to the known natural history of SCC, and help to inform the continuing refinement of recommendations for both the age at HPV vaccination and age at initiation of routine Pap testing. Thus, we conducted a population-based, case-only study to test the hypothesis that early AFI is associated with early-onset SCC independent of other SCC risk factors.
METHODS
Study population
SCC cases in this investigation were a subset of participants from a population-based epidemiologic study in Western Washington of invasive cervical cancer, for which methods have been previously described (15). Briefly, a woman was eligible for the original study if she was diagnosed with first primary invasive squamous cell cancer of the cervix (ICD-O morphology 8010, 8070–8081 and topography codes 1800–1809) from January 1986 through June 1998 or January 2000 through December 2004, a resident of King, Pierce or Snohomish County at the time of diagnosis, and able to communicate in English. The cases were originally identified by the Cancer Surveillance System (CSS), a population-based cancer registry that is part of the Surveillance, Epidemiology and End-Results (SEER) program of the National Cancer Institute. Of the 1189 cases eligible for the original study, 62.6% (n=744) were interviewed, 10.4% died prior to contact, 21.8% refused to participate or were lost to follow up prior to interview and for 5.2% the physician approached refused contact for the participant. For statistical analysis purposes (see “Statistical Methods” below), only women diagnosed with invasive SCC born between 1951 and 1967 were included (n=333), which limited the age range at diagnosis to 22–53 years old.
Data Collection
Each woman was interviewed in-person using a structured questionnaire administered by trained female study staff. The participant was asked to refer to the time before her diagnosis when answering questions on a range of topics including sexual, reproductive, screening and cigarette smoking histories, as well as other questions relevant to health history and demographic characteristics. To address AFI, each woman was asked, “How old were you when you first had sexual intercourse with a male?” Questions regarding the number of sex partners by decade of life and over a woman’s lifetime were asked categorically (0 partners, 1, 2–4, 5–14, 15–29, 30–49, 50+), using show cards. Age at diagnosis was calculated by subtracting date of birth from date of SCC diagnosis and rounding down. Both date of birth and diagnosis were obtained from the CSS and the former was verified during the interview.
At the time of interview each participant was asked to provide a serum sample and, from consenting cases, we attempted to retrieve tumor blocks from the diagnosing pathology laboratory. The serologic HPV antibody response and PCR testing methods used have been described in detail elsewhere (15, 18, 19). Serologic antibody testing for HPV-16, HPV-18, C. trachomatis and herpes simplex virus type 2 (HSV-2) was only performed for participants with diagnosis dates between 1986 and 1998, which in this investigation meant that younger cases tended to be more likely to have test results. In the original study, blood samples were obtained from 86.5% of cases and tissue blocks were retrieved for 80.0%. The Institutional Review Boards of the Fred Hutchinson Cancer Research Center and University of Washington approved all research protocols.
Statistical Methods
Linear regression was used to estimate the relationship between AFI and age at SCC diagnosis in years. For each independent variable, the corresponding regression coefficient estimated the difference in mean age at SCC diagnosis per one unit change in that independent variable.
We modeled AFI both as a continuous variable and as a categorical variable with three categories (years old: <15, 15–18 or ≥19 (referent)). The range of the middle category of this variable was chosen based on AFI’s interquartile range. With AFI as a continuous variable we calculated univariate linear regression coefficients. We present the Spearman correlation and a scatterplot showing the data points for all cases, which are jittered to avoid overlap, as well as fitted regression lines and loess smoothes, which graphically represent weighted regressions. We used fractional polynomial regression to estimate the best-fitting fractional polynomial powers of AFI and compared it to the linear model, to explore whether the linear model was a good fit for the continuous data. We used the categorical variable for the remainder of our investigation, reporting the unadjusted coefficients, and those that were adjusted using multivariate modeling.
We hypothesized a priori that the number of male sex partners a woman had before age 20 could also be associated with age at SCC diagnosis, as another measure of early sexual experience, and could confound or modify the association between AFI and age at SCC diagnosis. Thus, we calculated unadjusted coefficients for the association between age at SCC diagnosis and number of partners before age 20, modeled categorically (0 partners, 2–4, 5–14, 15+), as well as those adjusted for AFI and other measures. Finally, we also explored stratifying AFI results by number of partners before age 20 (0–1 vs. ≥2), using a variable that combined AFI and number of male sex partners before age 20 in the following six mutually exclusive categories: 1) AFI≥19 years old and 0–1 sex partner before age 20 (referent), 2) AFI≥19 and 2 or more sex partners before age 20, 3) AFI 15–18 and 0–1 sex partner before age 20, 4) AFI 15–18 and 2 or more sex partners before age 20, 5) AFI<15 and 0–1 sex partners before age 20, and 6) AFI<15 and 2 or more sex partners before age 20.
We also hypothesized a priori that estimated associations would be confounded by birth cohort because 1) there have been changes in sexual and reproductive behavior during the last century (20), and 2) the study design linked birth year to age at SCC diagnosis, which were observed in our own preliminary examination of the data from the full case-control study (data not shown). The latter was a result of SCC cases being drawn from a study that only included women diagnosed between 1986 and 2004, which restricted the age range for each birth year with a 19-year maximum age range and smaller ranges for earlier and later birth years. In anticipation of adjustment for birth year, we restricted the study population to SCC cases born between 1951 and 1967. We choose these years from among the full range of birth years for SCC cases (1913–1981), because each year of the restricted range had at least one case that was in a designated “younger case group” (22–35 years old) and at least one in an “older age group” (36–53 years old) among the original study participants. This selection reduced the likelihood of attenuation of coefficients that can occur from restricting the range of the dependent variable in a regression. Within the years selected, birth year was associated with AFI (p-value for linear regression with AFI continuous=0.01) and age of SCC diagnosis (p-value for linear regression <0.01). Therefore, we adjusted for year of birth in multivariate models.
A number of other potential characteristics were considered for inclusion in the multivariate model with the aim of estimating the relationship between AFI and age at SCC diagnosis over and above any factors that may also be associated with earlier diagnosis of SCC. As there have been no studies that examine the risk factors for younger versus older age at SCC diagnosis, the factors considered included established risk factors for invasive SCC that might also be associated with early AFI. Risk factors for HR-HPV infection and persistence were also examined, since risk of cervical carcinoma cannot be considered independent from them (21). Sexual history characteristics considered included lifetime number of sex partners (3) and serologic evidence of HSV-2 (15, 22) or C. trachomatis (19, 23, 24) infection. Reproductive history variables examined included age of first menses, combined oral contraceptive (OC) use (3, 25), and multiparity (15, 25, 26). Parity was considered both as number of full term pregnancies, and as a rate constructed as number of full term pregnancies divided by the number of reproductive years to standardize the exposure. Screening history was examined as frequency of Pap in the most recent decade and interval since last screening Pap smear in months (described in (27)). Other behavioral and socioeconomic characteristics examined included cigarette smoking history (3, 15, 28), race (29), income level, years of education, and marital status (15). All characteristics were considered in the categories presented in Table 1.
Table 1.
Selected characteristics of SCC cases by age at diagnosis*
| 22–35 years old n=165 |
36–53 years old n=168 |
|||
|---|---|---|---|---|
| Characteristic | Frequency† | % | Frequency | % |
| Age (years) | ||||
| Mean (standard deviation) | 31.0 (3.2) | 41.1 (4.0) | ||
| Median | 32 | 40 | ||
| Birth year | ||||
| 1951–1959 | 73 | 44 | 120 | 71 |
| 1960–1967 | 92 | 56 | 48 | 29 |
| Race | ||||
| White | 152 | 92 | 143 | 85 |
| Non-White | 13 | 8 | 25 | 15 |
| Education | ||||
| High school or less | 74 | 45 | 62 | 37 |
| More than high school | 91 | 55 | 106 | 63 |
| Income | ||||
| Less than $30,000 per year | 93 | 57 | 64 | 39 |
| $30,000 per year or more | 71 | 43 | 100 | 61 |
| Unknown | 1 | 4 | ||
| Marital status | ||||
| Never married | 27 | 16 | 21 | 13 |
| Ever married ‡ | 138 | 84 | 147 | 88 |
| Cigarette smoking | ||||
| Never | 49 | 30 | 74 | 44 |
| Former | 48 | 29 | 33 | 20 |
| Current | 68 | 41 | 61 | 36 |
| Age at menses (years) | ||||
| Less than 12 | 32 | 19 | 34 | 20 |
| 12 to 13 | 80 | 48 | 94 | 56 |
| 14 or older | 53 | 32 | 40 | 24 |
| Combined OC use | ||||
| Never to <6 months | 29 | 18 | 52 | 31 |
| 6 months to < 5 years | 57 | 35 | 59 | 35 |
| 5 or more years | 79 | 48 | 57 | 34 |
| Number of full term pregnancies | ||||
| 0 | 46 | 28 | 30 | 18 |
| 1 | 41 | 25 | 30 | 18 |
| 2 or more | 78 | 47 | 108 | 64 |
| Full term pregnancy rate | ||||
| None | 18 | 11 | 15 | 9 |
| < 1 per 10 years | 29 | 18 | 57 | 34 |
| 1 per 10 to <2 per 10 years | 55 | 34 | 61 | 37 |
| 2 per 10 years or greater | 62 | 38 | 34 | 20 |
| Pap frequency in most recent decade | ||||
| None | 7 | 4 | 2 | 1 |
| 1 to 5 per decade | 67 | 41 | 102 | 63 |
| 6 to 8 per decade | 10 | 6 | 15 | 9 |
| 1 per year | 61 | 37 | 34 | 21 |
| Greater than 1 per year | 19 | 12 | 10 | 6 |
| Unknown | 1 | 5 | ||
| Lifetime number of sex partners | ||||
| 1 | 5 | 3 | 11 | 7 |
| 2 to 4 | 46 | 28 | 52 | 31 |
| 5 to 14 | 74 | 45 | 69 | 41 |
| 15 or more | 40 | 24 | 35 | 21 |
| Unknown | 0 | 1 | ||
| Chlamydia trachomatis serology§ | ||||
| Negative | 45 | 52 | 26 | 58 |
| Positive | 41 | 48 | 19 | 42 |
| Test not performed or unknown | 79 | 123 | ||
| HSV-2 serology § | ||||
| Negative | 79 | 56 | 34 | 47 |
| Positive | 61 | 44 | 39 | 53 |
| Test not performed or unknown | 25 | 95 | ||
| FIGO Stage | ||||
| Stage 1 | 151 | 92 | 131 | 81 |
| Stage 2–4 | 13 | 8 | 30 | 19 |
| Unknown | 1 | 7 | ||
| HPV DNA results | ||||
| HPV negative | 14 | 11 | 9 | 9 |
| HPV-16 positive | 88 | 71 | 60 | 62 |
| HPV-18 positive | 8 | 6 | 10 | 10 |
| HPV-16 and -18 positive | 6 | 5 | 9 | 9 |
| HPV positive for other types** | 8 | 6 | 9 | 9 |
| HPV positive, type unknown | 3 | 0 | ||
| Insufficient sample | 8 | 3 | ||
| Test not performed or unknown | 30 | 68 | ||
| HPV 16/18 serology | ||||
| HPV-16 and -18 negative | 80 | 56 | 30 | 42 |
| Only HPV-16 positive | 29 | 20 | 14 | 19 |
| Only HPV-18 positive | 13 | 9 | 16 | 22 |
| HPV-16 and -18 positive | 20 | 14 | 12 | 17 |
| Test not performed or unknown | 23 | 96 | ||
SCC= squamous cell cervical cancer, HSV-2=Herpes simplex virus type 2, FIGO=International Federation of Gynecology, and Obstetrics (FIGO), HPV=Human papillomavirus
Unless otherwise indicated
Includes currently or formerly married or lived with a partner for greater than 6 months
Chlamydia trachomatis, HSV-2, HPV-16 and HPV-18 serology only available on cases enrolled prior to 1999, which excludes 94 cases (88 diagnosed at 36–53 years of age and 8 diagnosed at 22–36 years of age)
Includes HPV types 6, 31, 33, 45, 53, 58, and/or 73
The process of choosing characteristics to include in the multivariate model was done in steps. Each of the potential covariates was added sequentially to a model that included AFI, number of sex partners before age 20, and birth year. If, with the addition of the potential covariate to the model, any of the AFI coefficients changed by greater than or equal to 10%, then the potential covariate was left in the model. Once those covariates were chosen, LR tests were performed to determine whether each characteristic could be modeled with fewer categories. Of those characteristics still in the model, if its removal did not change any of the levels of the combined variable’s terms by at least 10%, then the characteristic was removed from the multivariate model.
Once the multivariate model was established, we explored stratification of AFI by number of partners before age 20, as discussed above, and also the association between AFI and age at diagnosis in among women with International Federation of Gynecology and Obstetrics (FIGO) Stage 1a cancer. This restriction was performed to explore the relationship in a potentially more homogenous group in terms of timely diagnosis and/or disease aggressiveness, and the factors that are associated with these conditions. Stratification by stage and HPV type was not possible given the small number of cases of later stage cancer. The effect of stratifying AFI by number of partners before age 20 was tested using LR tests comparing a model with the interaction of these two factors to one without.
Statistical significance by level of exposure, including p-values and 95% confidence intervals (CI), was estimated by individual Wald tests, whereas significance of characteristics at any level, interactions, and sufficiency of modeling covariates with fewer categories were judged by LR tests at a two-sided significance level of 5%. Two participants missing data on the AFI and number of sex partners prior to 20 years were excluded from the analysis. The final multivariate model and the models to which it was compared included only those with information for every covariate incorporated. Covariates with missing data are detailed in Table 1. All statistical procedures were performed using Intercooled Stata 9.0 for Windows.
RESULTS
The distributions of many characteristics differed when comparing SCC cases who were 22–35 years old at diagnosis (“younger cases”) to those who were 36–53 years old (“older cases”) (Table 1). Younger cases were more likely to be white, to have had less education, to have a lower annual income at diagnosis, to have ever smoked cigarettes, and to have never been married. They were also more likely to have had one Pap per year in the most recent decade, and twice as likely to have had multiple Paps per year. Younger cases were more likely to have had an older age at first menses, and to use combined OCs for longer than 6 months, though less likely to have had more than one full pregnancy. Younger cases were less likely to have had only one sex partner in their lifetime. Of those cases with serologic test results, younger cases were less likely to have HSV-2 antibodies, and slightly more likely to have positive antibody results for C. trachomatis. The percentage with anti-HPV-16 antibodies was similar for the younger cases (34%) and the older cases (36%), though there was a higher proportion of older cases that were HPV-18 positive with no evidence of HPV-16 antibodies.
Most younger and older cases were diagnosed at FIGO Stage 1, with 57% and 42% diagnosed at Stage 1a and 1b, respectively. Among those with HPV DNA results, most were HPV-16 or −18 positive. The younger cases’ mean AFI was approximately 1 year earlier than older cases and they were more likely to have a greater number of sex partners before age 20 (Table 2). The number of years between AFI and age at SCC diagnosis ranged between 4 and 35 years.
Table 2.
Early sexual experience characteristics of SCC cases by age at diagnosis*
| 22–35 years old n=165 |
36–53 years old n=168 |
|||
|---|---|---|---|---|
| Characteristic | Frequency2 | % | Frequency | % |
| Age at first intercourse (years) | ||||
| Mean (standard deviation) | 16.0 (2.7) | 17.3 (2.7) | ||
| Median | 16 | 17 | ||
| 19 or older | 16 | 10 | 52 | 31 |
| 15–18 | 118 | 72 | 96 | 57 |
| Less than 15 | 30 | 18 | 19 | 11 |
| Unknown | 1 | 1 | ||
| Difference between age at first intercourse and age at SCC diagnosis (years) | ||||
| 4–10 | 20 | 12 | 1 | 1 |
| 11–20 | 137 | 83 | 39 | 23 |
| 21–30 | 7 | 4 | 114 | 68 |
| 31–35 | 0 | 0 | 13 | 8 |
| Number of sex partners before age 20 | ||||
| None | 9 | 5 | 26 | 15 |
| 1 | 43 | 26 | 56 | 33 |
| 2 to 4 | 70 | 42 | 69 | 41 |
| 5 to 14 | 34 | 21 | 14 | 8 |
| 15 or more | 9 | 5 | 3 | 2 |
SCC= squamous cell cervical cancer
Unless otherwise indicated
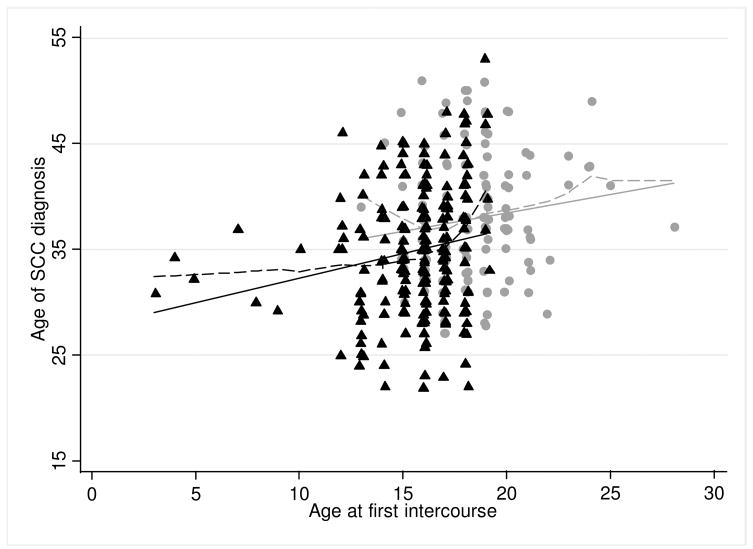
The adjusted coefficient for AFI measured continuously estimated that each one year change in AFI resulted in a 0.6-year change in age at diagnosis (95% CI: 0.4 – 0.8, p-value<0.01); the Spearman correlation between AFI and age at diagnosis was 0.26. The relationship among those with 2 or more sex partners before age 20 (unadjusted coefficient= 0.5, 95% CI: 0.1, 0.8, p-value=0.01) was similar to those with zero or one sex partner before age 20 (unadjusted coefficient= 0.4, 95% CI: −0.1, 0.8, p-value=0.13), though the latter was not statistically significant. These results assume a linear relationship between AFI and age at SCC diagnosis, though the loess curve results suggested that the relationship may not be linear, particularly around the minimum and maximum AFIs (Figure 1).
Figure 1. Age of SCC diagnosis by age at first intercourse*,†.
 0-1 sex partner before age 20
0-1 sex partner before age 20
 Linear regression-based line
Linear regression-based line
 Loess curve
Loess curve
▲2+ sex partner before age 20
—Linear regression-based line
—Loess curve
*SCC= squamous cell cervical cancer; †Scatter plot points are jittered
In both the univariate and multivariate models, an association was seen between age at SCC diagnosis and AFI in three categories (Table 3). In the univariate analysis, compared to SCC cases whose AFI was 19 years old or older (referent group), the mean age of diagnosis was 3.5 years younger for an AFI between 15 and 18, and 5.8 years younger for an AFI less than 15. The AFI coefficients from the multivariate model were decreased, but remained statistically significant, with the mean age of SCC diagnosis 2.6 years younger for an AFI between 15 and 18, compared to the referent group, and 3.1 years younger for an AFI less than 15. The multivariate model was adjusted for number of sex partners before age 20, birth year, age at first menses and annual income at diagnosis. All other characteristics examined did not appreciably affect the AFI coefficients.
Table 3.
Linear regression coefficients, CIs and p-values for association between age at SCC diagnosis and age at first intercourse and number of sex partners before age 20*
| Univariate Model† |
Adjusted for each other |
Adjusted for each other and birth year |
Multivariate Model‡ |
||
|---|---|---|---|---|---|
| Characteristic | n | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) |
| Age at first intercourse (years) | |||||
| 19 or older | 66 | Referent | Referent | Referent | Referent |
| 15–18 | 121 | −3.5 (−5.2, −1.9) | −3.0 (−5.3, −0.7) | −3.1 (−5.0, −1.1) | −2.6 (−4.6, −0.6) |
| Less than 15 | 48 | −5.8 (−8.0, −3.5) | −4.0 (−7.0, −1.0) | −4.1 (−6.7, −1.4) | −3.1 (−5.8, −0.5) |
| Number of sex partners before age 20 | |||||
| None | 32 | Referent | Referent | Referent | Referent |
| 1 | 98 | −1.1 (−3.5,1.3) | 1.1 (−1.8,3.9) | 1.2 (−1.3,3.7) | 0.6 (−1.9, 3.1) |
| 2 to 4 | 136 | −3.0 (−5.3, −0.7) | 0.01 (−3.2,3.2) | 1.0 (−1.8, 3.7) | 0.6 (−2.2, 3.3) |
| 5 to 14 | 48 | −5.6 (−8.3, −2.9) | −2.2 (−5.8, 1.3) | −0.1 (−3.2, 3.1) | −0.5 (−3.6, 2.6) |
| 15 or more | 12 | −7.2 (−11.2, −3.2) | −3.4 (−8.2,1.5) | −0.6 (−4.8,3.6) | −1.0 (−5.2, 3.2) |
SCC= squamous cell cervical cancer, AFI= Age at first intercourse, CI=95% confidence interval
All models presented include only cases with information on all characteristics included in the multivariate model
Adjusted for each other birth year, age at first menses (<14 years old/≥14), annual income at diagnosis (<$30,000/≥$30,000)
Although number of sex partners before age 20 is significantly associated with age at SCC diagnosis in the univariate model, this relationship was not independent of AFI (Table 3). To address our a priori hypothesis that the relationship between AFI and age at SCC diagnosis might be modified by number of sex partners before age 20, we stratified AFI by a binary measure of number partners before age 20 (Table 4). In the multivariate analysis, number of partners before age 20 appeared to affect the coefficients for AFI of 19 or greater and AFI less than 15, although the number of participants in these categories were small. There was little difference between the coefficients for the two groups of AFI between 15 and 18. The interaction between AFI and number of partners before age 20 was statistically significant in a model with no other covariates (LR test for interaction p-value=0.02), and of borderline statistical significance in the model adjusted for birth year, age at first menses, and annual income at diagnosis (LR test for interaction p-value=0.08).
Table 4.
Linear regression coefficients, CIs and p-values for association between age at SCC diagnosis and the combination of age at first intercourse and number of sex partners before age 20*
| Characteristic |
Univariate Model† |
Multivariate Model‡ |
||||
|---|---|---|---|---|---|---|
| AFI | Number of sex partners before age 20 | n | Coefficient (CI) | p-value | Coefficient (CI) | p-value |
| AFI=19 | 0–1 partners | 60 | Referent | Referent | ||
| AFI=19 | 2 or more partners | 6 | 4.2 (−0.8, 9.1) | 0.10 | 2.2 (−2.1, 6.4) | 0.32 |
| AFI 15–18 | 1 partner | 68 | −1.9 (−3.9, 0.2) | 0.08 | −2.3 (−4.1, −0.6) | 0.01 |
| AFI 15–18 | 2 or more partners | 144 | −3.7 (−5.5, −2.0) | <0.01 | −2.5 (−4.0, −0.9) | <0.01 |
| AFI<15 | 1 partner | 2 | 3.2 (−5.2, 11.5) | 0.46 | 3.2 (−4.1, −10.4) | 0.39 |
| AFI<15 | 2 or more partners | 46 | −5.8 (−8.1, −3.5) | <0.01 | −4.2 (−6.3, −2.1) | <0.01 |
SCC= squamous cell cervical cancer, AFI= Age at first intercourse, CI=95% confidence interval
Univariate model includes only cases with information on all characteristics included in the multivariate model
Adjusted for each other birth year, age at first menses (<14 years old/≥14), annual income at diagnosis (<$30,000/≥$30,000)
In analyses restricted to Stage 1a cases the coefficients were similar to those presented in Tables 3 and 4.
DISCUSSION
In this investigation of younger and middle-aged women with invasive SCC, sexual experience at younger ages was associated with younger age of diagnosis. The association between AFI and age at SCC diagnosis was independent of a number of sexual, reproductive, heath behavior, and demographic factors, including birth cohort. Although number of sex partners before age 20 was associated with age at SCC diagnosis in the univariate analysis, this relationship was not independent AFI. However, it appeared that that the effect of AFI may be modified by number of partners before age 20, though the number of participants in some categories was small.
One of the main goals of this investigation was to help address questions regarding sojourn time between HPV infection and SCC diagnosis in young women. Our primary results suggest young AFI, especially in combination with having multiple sex partners prior to age 20, contributes to an earlier age at SCC diagnosis. A young AFI could be a risk factor for younger SCC diagnosis simply because of the time needed for disease progression or, possibly, the adolescent cervix, compared to the adult cervix, is more susceptible to persistent HR-HPV infection (30). The possible effect of number of sex partners before age 20 suggests that factors that increase the probability of HPV infection at a young age also may increase the probability of young SCC diagnosis. Two other observations from this investigation may provide some clues about SCC sojourn time. One was the time interval between AFI and age at SCC diagnosis. For 21 cases the interval was 10 years or less, with a minimum of 4 years, providing evidence for the previously described phenomenon of rapid onset cervical cancer (23, 31). The other observation of interest was that the youngest ages of AFI are not inevitably associated with the youngest ages of SCC diagnosis. These observations, and the low Spearman correlation between AFI and age at diagnosis, appear to indicate that young AFI is related to earlier diagnosis of SCC diagnosis, but not strongly.
To our knowledge, the relationship between AFI and age at SCC diagnosis has not been reported previously. Our ability to examine this question was aided by the fact that the original study from which data for this investigation was obtained had a wide range of ages at diagnosis of invasive SCC, and a high number of younger cases. Another strength is the population-based design of the original study, with active recruitment of all cases of invasive SCC residing in the Puget Sound area. However, the case participation rate (63%) of the original study may have limited how representative these cases are compared to all SCC cases, especially if the association between AFI and age at diagnosis non-participants differed from participants (15). For example, if rapidly progressing young cases (i.e., with older AFIs) or older cases with young AFIs who did not want to answer questions with regard to sexual history were less likely to participate, then our results could be biased towards finding an association between younger AFI and earlier age at diagnosis.
The main limitation in interpretation of our results is that the timing of the HPV infection that led to an invasive lesion is unknown. Although AFI has been suggested as a crude method to estimate first HPV exposure (32), we acknowledge that estimation of sojourn time was not possible because infection may have occurred later. Other limitations of this investigation and its interpretation are mainly consequences of its retrospective study design, though given the current state of medical treatment for cervical pre-neoplasia a prospective study would not have been possible. Although differential recall based on case-control status is not an issue in this analysis, it is possible that younger cases remembered sexual activity events from adolescence more clearly because they were more recently experienced. If the difference between younger and older SCC cases was systematic in this regard the effect on our results would depend on whether the older cases estimated their AFI as younger or older than their actual AFI, and if it was the latter then our results would be biased towards an association. It is also possible that answers to questions about AFI and number of sex partners could have been influenced by the social undesirability for reporting young sexual experience, especially experience related to abuse. Misreporting of AFI and especially failing to report very early sexual experience could influence our results if it was also related to age at SCC diagnosis. Additionally, though we consider the ability to adjust for birth year a strength, the inclusion of birth year in the model necessitated limiting our analysis to women aged 22 to 53.
Taking these limitations into account, our results regarding the relationship between AFI and age at invasive SCC diagnosis appear to support the recommendations for HPV vaccination during early adolescence or younger. The Advisory Committee on Immunization Practices at the Centers for Disease Control and Prevention (CDC) recommends the routine HPV vaccination for 11–12 year-old girls, though it can be administered as young as 9 (33). According to the most recent estimates from the CDC, 3.7% of girls in the US have had sex before 13 years old, and 13% before 15 (34, 35). Girls with young AFIs are at higher risk for invasive SCC (15–17) and, as our results indicate, among SCC cases they may also be at higher risk for a younger age of diagnosis. Even though cases of young SCC are rare (6), its impact on fertility in young women (7, 8) may influence those parents, clinicians and policy-makers who have been resistant to vaccinating pre-teen girls (36–38).
Another clinical implication of the association found between AFI and age at SCC diagnosis, as well as the minimum time interval between the two (4 years), is that they support current recommendations for initiation of Pap smears no earlier than 21 years old or 3 years following AFI (39). Lastly, although changes in screening practices have certainly been the main driver of the declining age of invasive SCC diagnosis over the last 50 years in the US (6), this investigation suggests that the increasingly higher percentages of women having sex in their teens during the 1970s and 80s (20, 40), combined with higher numbers of sex partners, could have also contributed to the lack of a decline in the incidence in early-onset SCC.
In summary, our results suggest that among younger and middle-aged women with invasive SCC there is a positive relationship between AFI and age at diagnosis. Although AFI is probably one of many factors that determine the age at invasive SCC diagnosis, this association should be considered in the refinement and implementation of recommendations for age at HPV vaccination and age at Pap screening initiation.
Acknowledgments
Financial support: National Institutes of Health (P01CA042792, N01-PC-35142, 5T32CA009168) and institutional support from the Fred Hutchinson Cancer Research Center
The authors would like to express great appreciation to the study participants, without whom this investigation would not be possible, and to the study staff who were responsible for collecting the data.
References
- 1.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 21;24(Suppl 3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 2.Yang BH, Bray FI, Parkin DM, Sellors JW, Zhang ZF. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. Int J Cancer. 2004;109:418–24. doi: 10.1002/ijc.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24:S42–S51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol. 2000;43:352–62. doi: 10.1097/00003081-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Kjaer SK. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003;31:14–9. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 6.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100:1035–44. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 7.Maltaris T, Seufert R, Fischl F, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007;130:148–55. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Seli E, Tangir J. Fertility preservation options for female patients with malignancies. Curr Opin Obstet Gynecol. 2005;17:299–308. doi: 10.1097/01.gco.0000169108.15623.34. [DOI] [PubMed] [Google Scholar]
- 9.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 10.Hildesheim A, Hadjimichael O, Schwartz PE, et al. Risk factors for rapid-onset cervical cancer. Am J Obstet Gynecol. 1999;180:571–7. doi: 10.1016/s0002-9378(99)70256-5. [DOI] [PubMed] [Google Scholar]
- 11.Bain RW, Crocker DW. Rapid onset of cervical cancer in an upper socioeconomic group. Am J Obstet Gynecol. 1983;146:366–71. doi: 10.1016/0002-9378(83)90814-1. [DOI] [PubMed] [Google Scholar]
- 12.Janerich DT, Hadjimichael O, Schwartz PE, et al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995;85:791–4. doi: 10.2105/ajph.85.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. Mediators of the association between age of first sexual intercourse and subsequent human papillomavirus infection. Pediatrics. 2002;109:E5. doi: 10.1542/peds.109.1.e5. [DOI] [PubMed] [Google Scholar]
- 14.Burchell AN, Winer RL, de SS, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24:S52–S61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Daling JR, Madeleine MM, McKnight B, et al. The relationship of human papillomavirus-related cervical tumors to cigarette smoking, oral contraceptive use, and prior herpes simplex virus type 2 infection. Cancer Epidemiol Biomarkers Prev. 1996;5:541–8. [PubMed] [Google Scholar]
- 16.Berrington de GA, Sweetland S, Green J. Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis. Br J Cancer. 2004;90:1787–91. doi: 10.1038/sj.bjc.6601764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinton LA, Herrero R, Reeves WC, de Britton RC, Gaitan E, Tenorio F. Risk Factors for Cervical Cancer by Histology. Gynecologic Oncology. 1993;51:301–6. doi: 10.1006/gyno.1993.1294. [DOI] [PubMed] [Google Scholar]
- 18.Carter JJ, Madeleine MM, Shera K, et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61:1934–40. [PubMed] [Google Scholar]
- 19.Madeleine MM, Anttila T, Schwartz SM, et al. Risk of cervical cancer associated with Chlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int J Cancer. 2007;120:650–5. doi: 10.1002/ijc.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aral SO, Holmes KK. Social and behavioral determinants of epidemiology of STDs: Industrialized and developing countries. In: Holmes KK, Mardh P-A, Sparling PF, et al., editors. Sexually transmitted diseases. 4. New York, NY: McGraw-Hill; 1999. pp. 36–76. [Google Scholar]
- 21.Wacholder S. Chapter 18: Statistical issues in the design and analysis of studies of human papillomavirus and cervical neoplasia. J Natl Cancer Inst Monogr. 2003;31:125–30. doi: 10.1093/oxfordjournals.jncimonographs.a003474. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Herrero R, Bosetti C, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604–13. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- 23.Bain RW, Crocker DW. Rapid onset of cervical cancer in an upper socioeconomic group. Am J Obstet Gynecol. 1983;146:366–71. doi: 10.1016/0002-9378(83)90814-1. [DOI] [PubMed] [Google Scholar]
- 24.Smith JS, Bosetti C, Munoz N, Herrero R, Bosch FX, Eluf-Neto J, et al. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer. 2004;111:431–9. doi: 10.1002/ijc.20257. [DOI] [PubMed] [Google Scholar]
- 25.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–8. [PubMed] [Google Scholar]
- 26.Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119:1108–24. doi: 10.1002/ijc.21953. [DOI] [PubMed] [Google Scholar]
- 27.Madeleine MM, Daling JR, Schwartz SM, et al. Human papillomavirus and long-term oral contraceptive use increase the risk of adenocarcinoma in situ of the cervix. Cancer Epidemiol Biomarkers Prev. 2001;10:171–7. [PubMed] [Google Scholar]
- 28.Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118:1481–95. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 29.Xi LF, Kiviat NB, Hildesheim A, et al. Human papillomavirus type 16 and 18 variants: race-related distribution and persistence. J Natl Cancer Inst. 2006;98:1045–52. doi: 10.1093/jnci/djj297. [DOI] [PubMed] [Google Scholar]
- 30.Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. The interval between menarche and age of first sexual intercourse as a risk factor for subsequent HPV infection in adolescent and young adult women. J Pediatr. 2002;141:718–23. doi: 10.1067/mpd.2002.128893. [DOI] [PubMed] [Google Scholar]
- 31.Hildesheim A, Hadjimichael O, Schwartz PE, et al. Risk factors for rapid-onset cervical cancer. Am J Obstet Gynecol. 1999;180:571–7. doi: 10.1016/s0002-9378(99)70256-5. [DOI] [PubMed] [Google Scholar]
- 32.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention. HPV and HPV Vaccine - Information for Healthcare Providers. Center for Disease Control and Prevention 2007. [cited 2007 Jun 28];Available from: URL: http://www.cdc.gov/std/hpv/STDFact-HPV-vaccine-hcp.htm.
- 34.Center for Disease Control and Prevention. 2005 Youth Risk Behavior Survey. Center for Disease Control and Prevention 2007 June 6. [cited 2007 Jun 30];Available from: URL: www.cdc.gov/yrbss.
- 35.Dailard C. Legislating against arousal: The growing divide between federal policy and teenage sexual behavior. Guttamacher Policy Rev. 2006;9:12–6. [Google Scholar]
- 36.Charo RA. Politics, parents, and prophylaxis--mandating HPV vaccination in the United States. N Engl J Med. 2007;356:1905–8. doi: 10.1056/NEJMp078054. [DOI] [PubMed] [Google Scholar]
- 37.Kahn JA, Zimet GD, Bernstein DI, et al. Pediatricians’ intention to administer human papillomavirus vaccine: the role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005;37:502–10. doi: 10.1016/j.jadohealth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Marlow LA, Waller J, Wardle J. Parental attitudes to pre-pubertal HPV vaccination. Vaccine. 2007;25:1945–52. doi: 10.1016/j.vaccine.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 39.Cervical Cancer. NIH Consensus Statement. April 1–3 1996. 14[1], 1–38. 1996.
- 40.Center for Disease Control and Prevention. Current Trends Premarital Sexual Experience Among Adolescent Women -- United States, 1970–1988. MMWR. 1991;39:929–32. [PubMed] [Google Scholar]