Abstract
Neurocutaneous melanosis (NCM) is a rare congenital disease that is characterized by the presence of large or multiple congenital melanocytic nevi and melanotic lesions of the central nervous system. We report here on the CT and MR imaging findings of an unusual case of NCM that was associated with intraventricular dermoid and Dandy-Walker malformation.
Keywords: Melanoma; Brain, growth and development
Neurocutaneous melanosis (NCM) is a rare congenital nonheritable neurocutaneous syndrome that is characterized by large or multiple pigmented nevi along with leptomeningeal melanosis or melanoma; there is usually no evidence of malignant melanoma outside the central nervous system (1). The most commonly described MR findings are enhancement of the thickened leptomeninges surrounding the brain and the spinal cord, ventricular dilatation, cerebral parenchyma involvement (especially in the temporal lobe), and inferior vermian hypoplasia (2-4).
We report here on the CT and MR imaging findings in a case of NCM that was associated with intraventricular dermoid and Dandy-Walker variant.
CASE REPORT
A 27-year-old man was admitted to our hospital with a 10-day history of headache and vomiting. He was born with multiple pigmented areas on his skin; specifically, there were multiple confluent hairy nevi on his extremities, back and most of the anterior trunk. His psychomotor development was retarded. No significant family history was elicited on the interview. The neurologic examination and laboratory findings were unremarkable.
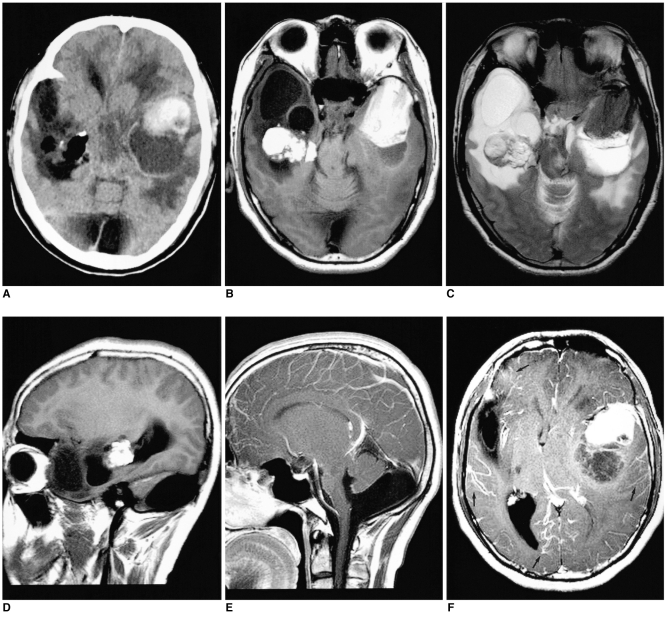
The nonenhanced CT scanning showed multiple intracranial lesions; these included a hyperattenuated mass with an adjacent cyst in the left temporal lobe, an irregular fatty mass containing marginal calcific foci within the temporal horn of the right lateral ventricle and a midline posterior fossa cyst with hypogenesis of the cerebellar vermis (Fig. 1A). The unenhanced MR scans showed a left temporal lobe mass with high signal intensity on the T1-weighted images and mixed low signal intensity on the T2-weighted images. In addition, there was a right intraventricular mass with bright signal intensity on the T1-weighted images and heterogeneous signal intensity on the T2-weighted images, relative to the cerebral cortex (Figs. 1B-D). Further, there was hypoplasia of the inferior cerebellar vermis, dilatation of the inferior fourth ventricle and an enlarged posterior fossa, which confirmed the presence of a Dandy-Walker variant (Fig. 1E). The Gd-DTPA enhanced T1-weighted images showed minimal marginal enhancement of the cystic lesion of the left temporal lobe mass with associated diffuse leptomeningeal enhancement (Fig. 1F). No apparent enhancement was noted in the right intraventricular mass. An excisional biopsy of the proximal hairy melanocytic nevus of the left ankle was done; the diagnosis proved to be a melanocytic congenital nevus. Left craniectomy was performed for the subtotal removal of the tumor. Areas of black pigmentation were seen in the brain cortex. The cyst adjacent to the left temporal lobe mass contained xanthochromic fluid. The histologic sections of the temporal lobe mass revealed a melanocytoma, and the cyst wall showed a reactive gliosis. Microscopic examination also demonstrated a diffuse melanocytosis of the leptomeninges.
Fig. 1.
Neurocutaneous melanosis in a 27-year-old man.
A. Noncontrast CT scan demonstrates a hyperdense mass with an adjacent cyst in the left temporal lobe. The CT scan also shows an irregular fatty mass (-105 HU) with marginal calcifications within the temporal horn of the right lateral ventricle.
B, C. The axial T1-weighted (B) and T2-weighted (C) MR images show a left temporal lobe mass that is hyperintense on T1-weighted images and it is hypointense on T2-weighted images. There is a peritumoral cyst posterior to the main mass. The MR images also showed a mass in the right lateral ventricle, which appears homogeneously hyperintense on the T1-weighted images and heterogeneously hyperintense on the T2-weighted images; this is consistent with a dermoid cyst. The cystic encephalomalacia in the right temporal lobe is probably related to an early childhood insult.
D. The right parasagittal T1-weighted MR image confirms the location of the right side mass within the temporal horn of the lateral ventricle.
E. The midline sagittal contrast-enhanced T1-weighted MR image reveals hypoplasia of the inferior vermis and dilatation of the inferior fourth ventricle that communicates to the enlarged posterior fossa.
F. The axial contrast-enhanced T1-weighted MR image shows mild enhancements of the wall of the peritumoral cyst in the left temporal lobe. Also noted is mild diffuse enhancement of the leptomeninges (arrows).
DISCUSSION
Neurocutaneous melanosis has been classified as a neuroectodermal dysplasia (1). Our patient had congenital giant hairy melanotic nevi of the skin as well as CNS melanocytoma, and these findings fulfilled the diagnostic criteria of NCM as suggested by Kadonaga and Frieden (5) The pathogenesis of NCM is not yet sufficiently clear; it is thought to come about by an error that occurs in the embryonic neuroectoderm during morphogenesis, and particularly in the neural crest (5). Melanocytes that originate from the neural crest are normally found within the basal layer of the epidermis, the pia mater, the reticular formation of the medulla and the substantia nigra. In NCM, there is a marked increase of the concentration of melanotic cells in their normal location with concomitant cell infiltration into the perivascular space. The melanocytes within the pia mater are responsible for the development of the leptomeningeal melanosis (4). Parenchymal melanosis is less commonly seen than leptomeningeal melanosis, and the former is thought to be caused by either the primary migration of the melanotic cells early in development or it is caused by their subsequent secondary spread via the Virchow-Robin spaces (1). The anterior temporal lobes, and particularly the amygdala, seem to be the most frequent locations for the parenchymal melanocytic accumulation (3, 4), as was seen in the present case. The clue for the diagnosis of leptomeningeal melanosis or parenchymal melanin deposits is T1 shortening on the MR imaging. The cause of this effect is still controversial; it might be the result of the presence of stable free radicals in the melanin in which the unpaired electrons interact with the water protons via an electron dipole-dipole interaction, and this causes the subsequent shortening of both the T1 and T2 relaxation times (6). In the present case, there was a large parenchymal melanocytoma with T1 shortening. Minimal diffuse enhancement of the leptomeninges without evidence of T1 shortening also indicated the presence of leptomeningeal melanosis (2). According to Byrd et al. (2), the hyperintensity on the T1-weighted MR images depends upon the number and maturity of the melanocytes. The overall incidence of malignancy within the involved meninges is estimated to be on the order of 50% (1). It is not possible to distinguish benign accumulations of melanocytes from malignant ones just based on the MR images. Barkovich et al. (3) suggested that only necrotic or hemorrhagic intracranial masses or the masses eliciting vasogenic edema in the patients with NCM can confidently be identified as malignant melanomas; those masses without hemorrhage, edema or necrosis cannot be classified unless they show growth on the subsequent scans. In our case, a peritumoral cyst having a wall of reactive gliosis was evident. Peritumoral cyst has been described in a case of intracranial metastatic melanoma reported by Ogawa et al. (7) The cyst might have been formed either directly by the tumor itself or by pooling of the cerebrospinal fluid (CSF) that was caused by blockage of CSF flow by the tumor.
Neurocutaneous melanosis may be associated with other neurocutaneous syndromes such as Sturge-Weber or von Recklinghausen's disease. Associations were also reported with Dandy-Walker complex, spinal lipoma and arachnoid cyst (8, 9). However, to the best of our knowledge, we are not aware of any previous description of the concurrence of intraventricular dermoid in a patient with NCM. A review of the relevant embryological data is helpful for considering the pathogenesis of such concurrent lesions in relation to the neural crest. Intracranial dermoids originate from ectodermal inclusions of the primitive pleuripotential cells, and this is due to defects of the neural tube closure at around 3-5 weeks of gestation (10). They are often located at the cranial midline within the posterior cranial fossa, the suprasellar cistern and the subfrontal areas. Intraventricular dermoid tumors are most frequently located in the fourth ventricle. Dermoids have characteristic CT and MRI appearances; they appear round or lobulated on CT with attenuation values from -150 to 0 HU, and they usually show a slight mass effect and foci of calcification without evidence of enhancement or surrounding edema after contrast. They have high signal intensity on T1-weighted MR images due to their lipid content, with a heterogeneous signal on the T2-weighted MR images due to the mixed composition of the tumor. Fat-suppression techniques can be used to definitely demonstrate the presence of lipid within these lesions (10). Although pathologic correlation of the intraventricular dermoid in this case was not available since the patient's family refused further surgical intervention, we feel quite confident of the diagnosis of dermoid because of the characteristic CT and MR imaging findings.
The Dandy-Walker malformation is a rare developmental abnormality of the CNS; it is characterized by hypoplasia or aplasia of the cerebellar vermis, cystic dilation of the posterior fossa and a cystic dilatation of the fourth ventricle that communicates with the broad posterior fossa. The Dandy-Walker variant is a less severe form of the Dandy-Walker complex in which there is a better development of the vermis and the fourth ventricle posterior fossa cyst is smaller. Kadonaga et al. (8) have proposed that the concurrent development of the Dandy-Walker malformation and the NCM, as seen in our patient, is not an incidental finding. Dandy-Walker complex may result from an insult to the development of both the cerebellar hemisphere and the fourth ventricle. Any failure of incorporation between the choroid plexus and the roof of the fourth ventricle or the delayed opening of the foramen Magendie may form the fourth ventricle-cisterna magna cyst. The meningeal cells play a role in cerebellar development. In NCM, the melanin-containing abnormal leptomeninges may disrupt the development of both the cerebellum and the fourth ventricle (8).
In summary, we report here on a case of NCM that manifested as a temporal lobe melanocytoma and leptomeningeal melanosis with coexistent intraventricular dermoid cyst and Dandy-Walker malformation. This type of unusual presentation should be added to the spectrum of imaging abnormalities of NCM.
References
- 1.Fox H. Neurocutaneous melanosis. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Amsterdam: North Holland; 1972. pp. 414–428. [Google Scholar]
- 2.Byrd SE, Darling CF, Tomita T, Chou P, de Leon GA, Radkowski MA. MR imaging of symptomatic neurocutaneous melanosis in children. Pediatr Radiol. 1997;27:39–44. doi: 10.1007/s002470050060. [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ, Frieden IJ, Williams ML. MR of neurocutaneous melanosis. AJNR Am J Neuroradiol. 1994;15:859–867. [PMC free article] [PubMed] [Google Scholar]
- 4.Demirci A, Kawamura Y, Sze G, Duncan C. MR of parenchymal neurocutaneous melanosis. AJNR Am J Neuroradiol. 1995;16:603–606. [PMC free article] [PubMed] [Google Scholar]
- 5.Kadonaga JN, Frieden IJ. Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol. 1991;24:747–755. doi: 10.1016/0190-9622(91)70115-i. [DOI] [PubMed] [Google Scholar]
- 6.Gomori JM, Grossman RI, Shields JA, Augsburger JJ, Joseph PM, DeSimeone D. Choroidal melanomas: correlation of NMR spectroscopy and MR imaging. Radiology. 1986;158:443–445. doi: 10.1148/radiology.158.2.3941871. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa R, Aoki R, Hyakusoku H. A rare case of intracranial metastatic amelanotic melanoma with cyst. J Clin Pathol. 2003;56:548–551. doi: 10.1136/jcp.56.7.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadonaga JN, Barkovich AJ, Edwards MS, Frieden IJ. Neurocutaneous melanosis in association with the Dandy-Walker complex. Pediatr Dermatol. 1992;9:37–43. doi: 10.1111/j.1525-1470.1992.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 9.Kasantikul V, Shuangshoti S, Pattanaruenglai A, Kaoroptham S. Intraspinal melanotic arachnoid cyst and lipoma in neurocutaneous melanosis. Surg Neurol. 1989;31:138–141. doi: 10.1016/0090-3019(89)90328-5. [DOI] [PubMed] [Google Scholar]
- 10.Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437–1455. doi: 10.1148/radiographics.15.6.8577967. [DOI] [PubMed] [Google Scholar]