Abstract
Leishmania promastigotes synthesize an abundance of phosphoglycans, either attached to the cell surface through phosphatidylinositol anchors (lipophosphoglycan, LPG) or secreted as protein-containing glycoconjugates. These phosphoglycans are thought to promote the survival of the parasite within both its vertebrate and invertebrate hosts. The relative contributions of different phosphoglycan-containing molecules in Leishmania–sand fly interactions were tested by using mutants specifically deficient in either total phosphoglycans or LPG alone. Leishmania donovani promastigotes deficient in both LPG and protein-linked phosphoglycans because of loss of LPG2 (encoding the Golgi GDP-Man transporter) failed to survive the hydrolytic environment within the early blood-fed midgut. In contrast, L. donovani and Leishmania major mutants deficient solely in LPG expression because of loss of LPG1 (involved in biosynthesis of the core oligosaccharide LPG domain) had only a slight reduction in the survival and growth of promastigotes within the early blood-fed midgut. The ability of the LPG1-deficient promastigotes to persist in the midgut after blood meal excretion was completely lost, and this defect was correlated with their inability to bind to midgut epithelial cells in vitro. For both mutants, when phosphoglycan expression was restored to wild-type levels by reintroduction of LPG1 or LPG2 (as appropriate), then the wild-type phenotype was also restored. We conclude, first, that LPG is not essential for survival in the early blood-fed midgut but, along with other secreted phosphoglycan-containing glycoconjugates, can protect promastigotes from the digestive enzymes in the gut and, second, that LPG is required to mediate midgut attachment and to maintain infection in the fly during excretion of the digested blood meal.
Leishmania are trypanosomatids that are the etiological agents for leishmaniasis. The forms of disease produced in humans can be quite variable, depending mainly on the species of Leishmania, ranging from self-healing cutaneous lesions (e.g., Leishmania major, Leishmania tropica, and Leishmania mexicana) to progressive and fatal systemic involvement (e.g., Leishmania donovani). Leishmania have a dimorphic life cycle consisting of extracellular promastigotes that multiply and develop within the midgut of the sand fly vector and intracellular amastigotes that reside and multiply within the phagolysosomal vacuoles of host macrophages. The identification of molecules that enable Leishmania to survive within these harsh, diverse environments continues to be the main objective of much of the work devoted to these organisms. Attention has been focused on one major surface molecule in particular, the lipophosphoglycan, referred to as LPG. LPG has been identified on promastigotes of all Leishmania species that have been studied to date as the major surface glycoconjugate of these cells (reviewed in refs. 1 and 2). It is expressed on the entire surface, including the flagellum, and is organized as a densely packed filamentous glycocalyx. LPG is a tripartite molecule, consisting of a phosphoglycan (PG) domain linked via a hexasaccharide glycan core to a 1-O-alkyl-2-lyso-phosphatidylinositol lipid anchor (Fig. 1). The PG moieties of all LPGs studied to date share a common backbone consisting of repeating disaccharide units of PO4-6Gal(β1–4)Manα1, where the 3-position of the Gal residue can be unsubstituted, as in L. donovani (3), partially substituted, as in L. mexicana (4), or almost completely substituted with a variety of sugars, as in L. major (5) and L. tropica (6). The nonreducing terminus of the PG chain is capped with one of a number of different neutral oligosaccharides. The intracellular amastigote stage of some Leishmania species expresses little, if any, LPG (7), and of those that do, the molecules are expressed in much lower copy number, and they do not seem to form a densely packed surface structure (8, 9). The LPG coat, therefore, is a specialized structure designed for extracellular stages of the parasite, and any consideration of LPG function must bear in mind the usual habitat of these stages, which is the alimentary tract of the sand fly vector.
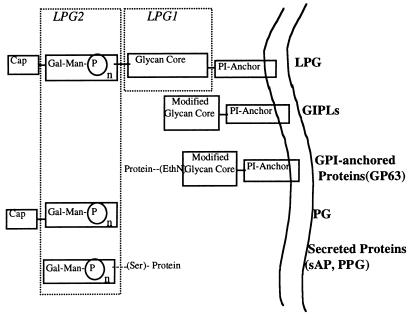
Figure 1.
Structure of PG-containing glycoconjugates from wild-type and LPG1- and LPG2-deficient mutants. Structures enclosed within the dashed boxes designate those domains specifically affected by the respective mutations in either LPG1 or LPG2. PI, phosphatidylinositol; GIPL, glycoinositol phospholipid; GPI, glycophosphatidylinositol; sAP, secreted acid phosphatase; PPG, proteophosphoglycan.
In addition to LPG, the PG repeat units are incorporated into other molecules. Leishmania promastigotes secrete the polymerized repeat units as a hydrophilic structure (PG), and they synthesize and secrete protein-bound PGs, such as PPG and sAP (reviewed in refs. 10 and 11; Fig. 1). Accordingly, straightforward biochemical approaches to assign precise functions are blurred by the sharing of the PG (and perhaps other) domains among these various lipid- and protein-linked glycoconjugates.
Comparisons of Leishmania–sand fly interactions in permissive and nonpermissive vector species have revealed a number of barriers to parasite growth and development that may have provided the evolutionary drive for expression of surface and secreted PG containing structures. These barriers include the lethal effects of digestive enzymes in the early blood-fed midgut and the loss of midgut infections as a consequence of excretion of the digested blood meal. LPG has been suggested to promote promastigote survival in the midgut by inhibiting the release of midgut proteases (12), by protecting the parasite surface from proteolytic attack (13), and by mediating the attachment of promastigotes to the gut wall so as to prevent loss of the parasite during passage of the digested blood meal (14). A role for LPG in midgut attachment seems especially convincing based on a number of findings: (i) purified LPG binds to midguts in vitro (15); (ii) LPG inhibits the binding of promastigotes to the gut in in vitro attachment assays (15, 16); and (iii) species-specific polymorphisms in LPG structure predict the species differences in the ability of promastigotes to persist in gut after blood meal excretion (17). The role of secreted protein-bound PGs in parasite–sand fly interactions has not been investigated.
The use of specific mutants is a powerful approach to defining the function of molecules or genes. Mutant organisms defective in LPG biosynthesis have been generated after mutagenesis and selection against binding to lectins or antibodies (18–21). Preliminary studies indicate that the LPG mutants fail to develop normally in an appropriate sand fly (17, 20). However, as these mutants were obtained after heavy mutagenesis and extended in vitro culture, their phenotype could not be ascribed definitely to the LPG defect. With the advent of functional genetic methods for identifying LPG biosynthetic genes (reviewed in ref. 22), it is now possible to generate “clean” LPG mutants by gene targeting and to confirm the role of LPG genes through specific rescue of any mutant defects by reintroduction of the relevant LPG gene (23, 24). In the present report, we used a genetic approach to distinguish the roles of LPG and protein-linked PGs in midgut survival in vivo and in midgut attachment in vitro. This approach was accomplished by comparing these Leishmania–sand fly interactions by using mutants defective in either LPG1 (R2D2 or lpg1− knockout L. major) or LPG2 (C3PO or lpg2− knockout L. donovani) expression with the same mutants in which LPG1 and LPG2 expression had been restored. LPG1-deficient L. donovani and L. major are defective in the step involving addition of a galactofuranose to the glycan core region of LPG but still assemble and secrete other protein-linked PGs (refs. 23 and 25; G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work). In contrast, LPG2-deficient mutants possess a defective transporter system for the uptake of GDP-Man in the Golgi apparatus (24, 26) and are deficient in the synthesis of all PGs. Our results show conclusively that PG synthesis is required for survival of the parasite within the digesting blood meal, whereas LPG alone is required for the maintenance of infection in the midgut after blood meal excretion.
Materials and Methods
Parasites.
Promastigotes were grown at 25°C in medium 199 supplemented with 20% (vol/vol) heat-inactivated FCS, 100 units/ml penicillin, 50 μg/ml streptomycin, and 12.5 mM l-glutamine (all from Applied Biosystems), as well as 40 mM Hepes (pH 7.4), 0.1 mM adenine, and 0.0005% hemin (M199/S). The generation of the LPG1-deficient mutant R2D2 and LPG2-deficient C3PO from the wild-type L. donovani strain 1S (MHOM/SD/00/1S-2D) has been described (18, 19). Restoration of LPG expression was achieved for R2D2 and C3PO by transfection with pX63HYG-LPG1 and pX63HYG-LPG2, respectively (23, 24), and transfectants were maintained in M199/S plus 50 μg/ml hygromycin B. The generation of L. donovani homozygous lpg2− null mutants by homologous gene replacement has also been described (24). These were maintained in hygromycin B (50 μg/ml), and a corresponding add-back line generated by stable transfection of the episomal vector pX63NEO-LPG2 (strain B3094) was maintained in 50 μg/ml G418. The generation of an lpg1− homozygous null mutant and corresponding add-back line for the LPG1 gene in L. major strain LV39 clone 5 (Rho/SU/59/P) has been described (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work); this mutant was maintained in medium supplemented with 16 μg/ml hygromycin B and 20 μM puromycin. This lpg1− null mutant was reconstituted for LPG expression by transfection with pLPG1-NEO (strain 3340) and was maintained in medium supplemented with 20 μg/ml G418.
In this work, we will refer to the lines as follows: R2D2 as LdR2D2, R2D2 transfected with pX63NEO-LPG1 as LdR2D2+LPG1, the homozygous LPG1 null mutant L. major as Lmajlpg1−, the homozygous LPG1 null mutant transfected with pLPG1-NEO as Lmajlpg1−+LPG1, C3PO as LdC3P0, C3PO transfected with pX63NEO-LPG2 as LdC3PO+LPG2, the homozygous LPG2 null mutant L. donovani as Ldlpg2−, and the homozygous LPG2 null mutant L. donovani transfected with pX63NEO-LPG2 as Ldlpg2−+LPG2.
Sand Fly Infection and Dissection.
Phlebotomus papatasi and Phlebotomus argentipes sand flies were reared and maintained in the Department of Entomology at Walter Reed Army Institute of Research. Female 3- to 5-day-old sand flies were fed through a chick skin membrane on a mixture of heparin-treated mouse blood containing 2 × 106 to 10 × 106 logarithmic phase promastigotes per ml (27). The red blood cells were washed twice in 0.86% saline and added back to the plasma, which was heat inactivated at 56°C for 45 min. Blood-engorged sand flies were separated and maintained at 28°C with 30% (vol/vol) sucrose solution. At various times after feeding, the flies were anesthetized with CO2; their midguts were dissected; and the number of midgut promastigotes was determined by placing individual midguts into a microcentrifuge tube containing 30 μl of PBS (pH 7.4). Each gut was homogenized by using a Teflon-coated microtissue grinder, and released promastigotes were counted in a hemacytometer.
In Vitro Assay for Promastigote Binding to Sand Fly Midguts.
Binding of promastigotes to sand fly midguts was quantitated by a modification of an in vitro technique previously described (17). Female 3- to 5-day-old non-blood-fed sand flies, maintained on 30% (vol/vol) sucrose solution, were dissected in PBS. Heads, crops, hindguts, and malpighian tubules were removed, and the isolated midguts were opened along the length of the abdominal segment with a fine needle. Midguts were placed in the concave wells of a microscope chamber slide. Promastigotes (2.5 × 107 per ml) in a total volume of 50 μl were added to the guts and incubated for 45 min at room temperature for P. argentipes midguts and for 10 min at room temperature for P. papatasi midguts. The guts were then individually washed by placing them in successive drops of PBS. Guts were homogenized, and released promastigotes were counted as described above. Statistics (P values) were obtained from Student's t test for paired samples.
Lectin- and Antibody-Mediated Agglutination Profiles of LPG Mutants.
Agglutination assays with peanut agglutinin (PNA)- and LPG-specific mAbs were performed in 96-well, flat-bottomed microtiter plates that contained 2 × 107 promastigotes per ml. The anti-LPG mAbs CA7AE, which recognizes the PO4-6Galβ1–4Manα1 repeat unit (28), and WIC79.3, which recognizes the galactose containing side-chains of L. major LPG (29), have each been described. Equal volumes of the parasite suspension and the lectins or antibodies in Hanks' balanced salt solution containing 1% BSA were mixed and incubated at room temperature for 30 min. After gentle mixing, the number of single, unagglutinated promastigotes was determined in a hemacytometer.
Results
The PG-Containing Structures of Leishmania Promastigotes and Mutants.
A representation of the cell surface and secreted PG-containing structures of Leishmania promastigotes is shown in Fig. 1. These molecules include the GPI-anchored LPG, the secreted PG, and secreted proteins, including sAP and PPG. The figure also depicts surface molecules that share other LPG-related structural domains, including the GIPLs and GPI-anchored proteins (e.g., gp63), which express modified core-anchor domains. Also shown is the manner in which the biosynthesis of each of these molecules is affected by defective expression of either LPG1 or LPG2.
Two LPG1-deficient lines were studied: L. donovani R2D2, obtained after mutagenesis and selection and containing an inactive mutated LPG1 gene (R. Zufferey and S.M.B., unpublished data), and L. major lpg1−, generated by homologous gene replacement (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work). The L. major lpg1− null mutant is specifically deficient in LPG synthesis and synthesizes normal levels of PPG and GIPLs, whereas R2D2 is known to secrete normal levels of protein-linked PGs (25). Two lines deficient in LPG2 expression were studied: L. donovani C3PO, generated by mutagenesis and selection and containing a mutated region of DNA that includes LPG2, and L. donovani lpg2−, generated by homologous gene replacement (24). These lines are defective in the synthesis of all PGs (24). For each mutant, add-back lines were generated by reintroduction of LPG1 or LPG2 expression constructs. Biochemical examination of the LPG1-transfected mutants revealed nearly complete quantitative restoration of full-sized LPG for L. major (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work), whereas LPG synthesis was restored to only 10% of wild-type levels in LdR2D2 (23). Similar studies of the LPG2-transfected C3P0 mutants showed nearly quantitative restoration of PG synthesis and full sized LPG (24). Finally, LPG expression was restored to 35–40% of wild-type levels in Ldlpg2−+LPG2, as determined by the recovery of biosynthetically labeled LPG (data not shown).
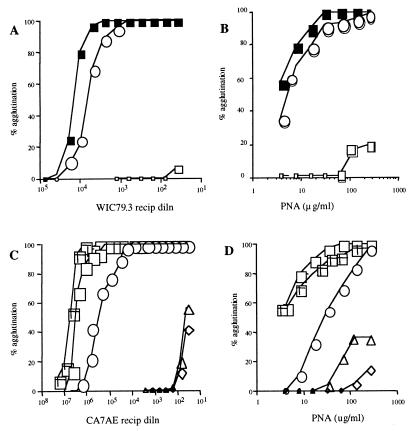
LPG deficiency and restoration were confirmed in lectin- and antibody-mediated agglutination assays with live cells. These assays provide an especially meaningful comparison of mutants for studies of promastigote–sand fly interactions, for which an abundance of surface LPG and expression of specific terminally exposed sugars have been suggested to control different aspects of midgut survival. Lmajlpg1− was not agglutinated by high concentrations of mAb WIC79.3 (Fig. 2A), specific for the galactose-containing side-chain oligosaccharides expressed by L. major LPG or by the lectin PNA (Fig. 2B), which binds with high avidity to these same sugars. The lectin- and antibody-mediated agglutination of the Lmajlpg1−+LPG1 promastigotes was restored to levels only slightly less than those in the wild-type cells. In contrast, a 20-fold higher concentration of anti-LPG antibody was required to achieve 50% agglutination of LdR2D2+LPG1 compared with that in the wild-type cells (Fig. 2C). The lectin agglutination profiles with PNA again reflect the partial restoration of surface LPG expression on the R2D2 transfectants (Fig. 2D). Agglutination profiles with either anti-LPG antibodies or PNA confirmed that the amount of surface LPG expressed on LdC3PO+LPG2 was almost fully restored to wild-type levels.
Figure 2.
Agglutination profiles of L. major wild-type (LV39) and LPG1-targeted null mutants with mAb WIC79.3 (A) and PNA lectin (B). LmLV39, closed squares; Lmajlpg1−+LPG1, open circles; Lmajlpg1−, open squares. Agglutination profiles of L. donovani wild-type (1S) and mutant promastigotes with mAb CA7AE (C) and PNA (D). Ld1S, open squares; LdR2D2, open diamonds; LdR2D2+LPG1, open circles; LdC3PO, open triangles; LdC3PO+LPG2, cross-hatched squares.
Survival of L. major LPG1 Mutant in P. papatasi.
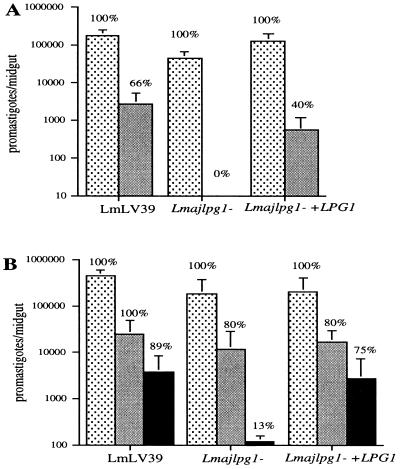
When P. papatasi sand flies, which are the natural vectors of L. major transmission throughout most endemic regions, were infected with wild-type L. major promastigotes, Lmajlpg1−, or Lmajlpg1−+LPG1, the day-2 midgut infections in each group were not significantly different (Fig. 3A). For each of the L. major strains, 100% of the flies were positive, and the number of promastigotes per midgut was high and reduced only slightly in the flies infected with Lmajlpg1−. Thus, the absence of LPG on L. major promastigotes does not seem to affect adversely their early survival and growth in the abdominal midgut of P. papatasi. A consequence of the LPG deficiency was clear, however, by day 7, at which time the digested blood meal had been passed, and no flies remained infected with Lmajlpg1−. In contrast, 40% of the flies infected with Lmajlpg1−+LPG1 remained infected on day 7, compared with 66% of the flies infected with wild-type promastigotes. To compensate for the relatively high loss of parasites observed with even the wild-type promastigotes of this particular strain, flies were infected with a 5-fold higher concentration of promastigotes (Fig. 3B). Again, Lmajlpg1− survived and grew extremely well in the early blood-fed midgut, up until day 3. When the infected flies were examined on day 5, it was possible at this same time to compare flies that either had recently passed or still retained their blood meals. Even at this relatively late time after parasite delivery, 80% of the flies infected with Lmajlpg1− that still contained blood also continued to harbor parasites in numbers that were not significantly different than those observed in the flies infected with wild-type or Lmajlpg1−+LPG1 promastigotes. In contrast, the Lmajlpg1− promastigotes were almost completely lost from day 5 flies that had passed their blood meals, with the few remaining infected flies containing less than 300 promastigotes per gut. With the higher-dose inoculum, a higher percentage of flies infected with either wild-type (89%) or Lmajlpg1−+LPG1 transfectants (75%) sustained infection after blood meal excretion, and the number of promastigotes per midgut was also comparable, indicating that Lmajlpg1−+LPG1 had their ability to persist in the gut almost completely restored.
Figure 3.
Infection of P. papatasi sand flies with L. major LPG1-targeted null mutants. (A) Flies were fed on mouse blood containing 2 × 106 promastigotes per ml and were dissected on day 2 (stippled bars) or day 7 (solid bars). (B) Flies were fed on mouse blood containing 107 promastigotes per ml, and midguts were dissected on day 3 (stippled bars) and on day 5, at which time midguts were grouped according to those that still retained blood (light solid bars) and those that had no detectable blood (dark solid bars). Graphs show the means ± SEM of promastigotes per midgut from a single experiment (10–12 flies per group). The percentages of flies positive for parasites in each group are shown.
The ability of Leishmania promastigotes to maintain midgut infections is thought to be associated with their ability to bind and to remain anchored to the gut wall during excretion of the digested blood meal. Midgut binding can be assayed directly in vitro with dissected whole midguts. The in vivo outcomes were clearly reflected in the in vitro binding assays with P. papatasi midguts, in which the mean number of wild-type and Lmajlpg1−+LPG1 promastigotes that bound were, respectively, 12,200 ± 6,200 and 9,800 ± 6,900, compared with only 1,100 ± 900 for the Lmajlpg1− promastigotes.
Survival of L. donovani LPG1 Mutants in P. argentipes.
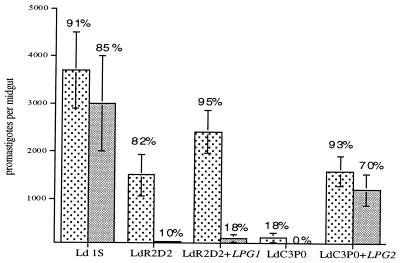
P. argentipes sand flies, which are the natural vectors of L. donovani transmission in India, were fed on blood containing 106 promastigotes per ml of each mutant strain, and midgut infections were evaluated on day 2 and on day 5, by which time blood meals had been passed in every fly examined (Fig. 4). Wild-type L. donovani promastigotes produced infections in 91% of the flies on day 2, and as we have reported previously, the growth of L. donovani promastigotes in this laboratory colony of P. argentipes is significantly less compared with that of L. major in P. papatasi (17). Although the mean number of day-2 parasites in flies infected with LdR2D2 was reduced compared with those in wild-type controls, the percentage of infected flies remained high (82%), and there was an average of 1,500 viable promastigotes per midgut. LdR2D2+LPG1 showed a moderate, though statistically insignificant, effect on LdR2D2 survival. The ability of LdR2D2 to sustain infection in the midgut after excretion of the blood meal (day 5) was dramatically impaired, and this ability was not restored in LdR2D2+LPG1, which expressed only 10% of the wild-type level of LPG. These in vivo outcomes were again completely consistent with the results of in vitro binding assays with P. argentipes midguts; an average of 2,800 ± 1,200 wild-type promastigotes bound per gut, whereas both R2D2 and LdR2D2+LPG1 bound poorly, with less than 240 ± 40 and 400 ± 380 promastigotes, respectively, bound per gut.
Figure 4.
Infection of P. argentipes sand flies with L. donovani R2D2 and C3P0 mutants. Flies were fed on mouse blood containing 2 × 106 promastigotes per ml, and midguts were dissected on day 2 (stippled bars) and day 5 (solid bars) after feeding. Graphs show the means ± SEM of promastigotes per midgut, from a pool of three separate experiments (22–30 individuals flies) for day 2 and two separate experiments (14–19 total flies) for day 5. The percentages of flies positive for parasites in each group are shown.
Survival of L. donovani LPG2 Mutants in P. argentipes.
In contrast to the results with LdR2D2, the early survival and growth of promastigotes in flies infected with LdC3P0 were sharply reduced; infections were totally absent in 82% of the flies, and few viable promastigotes were found in the positive flies (Fig. 4). LdC3PO+LPG2, which expressed close to wild-type levels of LPG, showed substantial increases in both the percentage and intensity of day 2 midgut infections; 93% of the flies were infected, and the mean number of promastigotes increased from less than 100 to 1,650. LPG2 also restored the capacity of the C3P0 mutant to persist in the midgut after passage of the digested blood meal; 70% of the flies maintained infection, with parasite loads reduced only slightly from the levels present on day 2. No positive flies were found in the C3P0 infected group at this time point. The binding of C3P0 to P. argentipes midguts in vitro was reduced by over 90% compared with wild-type promastigotes (240 ± 90 vs. 2,800 ± 1,200), and in contrast to the results with the LdR2D2+LPG1, LdC3PO+LPG2 had its binding capacity restored (1,930 ± 820). The data suggest that a threshold level of surface LPG is required to mediate stable attachment.
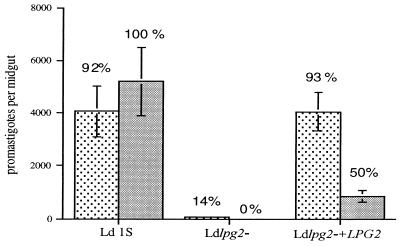
LPG2-deficient L. donovani mutants have also been generated by targeted gene replacement (24). LPG synthesis was partially restored (35–40%) in the LPG2 add-back lines. Approximately 90% of the P. argentipes sand flies infected with either wild-type or Ldlpg2−+LPG2 had promastigotes present in their abdominal midguts on day 2, and the mean intensity of infection in these two groups was comparable (4,200 promastigotes/midgut; Fig. 5). In contrast, in the flies infected with Ldlpg2−, only 14% of the flies were still infected on day 2, and the few flies that were infected had less than 450 promastigotes per midgut. Thus Ldlpg2− was again unable to survive the conditions present in the early blood-fed midgut. When the midguts were examined 5 days after infection, after blood meal excretion, 100% of flies infected with wild-type L. donovani promastigotes sustained infection with a mean intensity slightly greater than that observed on day 2, whereas in the flies infected with the Ldlpg2−+LPG2, the proportion that remained infected had declined to 50%, and the number of promastigotes in each infected fly also declined significantly (P < 0.02). Thus, Ldlpg2−+LPG2 was not completely rescued with respect to their ability to persist in the midgut after passage of the digested blood meal. There were no infected flies remaining in the group infected with Ldlpg2−.
Figure 5.
Infection of P. argentipes sand flies with L. donovani LPG2-targeted null mutants. Flies were fed on mouse blood containing 2 × 106 promastigotes per ml, and midguts were dissected on day 2 (stippled bars) and day 5 (solid bars) after feeding. Graphs show the means ± SEM of promastigotes per midgut from a single experiment (13–19 flies per group). The percentages of flies positive for parasites in each group are shown.
Discussion
The PG-containing molecules of Leishmania, bearing the repeat unit structure Gal(β1, 4)Man(α1)-PO4, are the major surface and secreted molecules of these parasites. To date, there have been four PG family members that have been characterized: LPG, PG, sAP, and PPG. Of these molecules, most attention has been focused on the structure and function of LPG. LPG is the major glycoconjugate on the surface of Leishmania promastigotes. It has been implicated in a variety of virulence-related activities in both the vertebrate and invertebrate host environments, including inhibition of midgut proteases (12), attachment of promastigotes to the midgut wall (15), resistance to complement mediated lysis in the blood (30), attachment to and entry into host macrophages (31), and inhibition of macrophage signal transduction pathways (32). The evidence that LPG is a virulence determinant is based primarily on studies in which purified LPG has been shown to mediate these various activities in vitro. Additional support is based on the altered behavior of LPG-deficient mutants in vitro and in vivo. These have been generated in L. donovani and L. major by mutagenesis followed by negative selection with LPG binding lectins and antibodies. In each case, the LPG-deficient promastigotes had diminished capacity to survive in macrophages (33–36) or to maintain infection within the sand fly midgut (17, 20). Although informative, the studies involving these mutants remain inconclusive with respect to the role of LPG, for one or more of the following reasons: (i) the mutants were generated by heavy mutagenesis, and they may possess other molecular defects; (ii) they may be compromised by culture-associated loss of virulence, which occurs spontaneously and with high frequency during subpassage of promastigotes in vitro (37, 38); and (iii) they were selected not for loss of LPG per se but for loss of PG determinants—thus, they may be deficient in other PG-containing structures. The proof that LPG is an essential virulence molecule rests on the demonstration that, on specific restoration of gene function in mutant lines with a defect that is confined to LPG, virulence behavior can also be restored (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work). In the present studies, these conditions have largely been fulfilled, and the data provide the strongest evidence to date that LPG is required for survival of Leishmania within the sand fly vector. The data confine the essential role of LPG to midgut attachment and the maintenance of infection after blood meal excretion and reveal a role along with other secreted PG-containing proteins in the survival of promastigotes within the early blood-fed midgut.
Mutants defective in expression of two distinct classes of biosynthetic LPG genes have been explored in these studies. LPG1 was identified by transfection of the L. donovani R2D2 mutant (23) and is involved in addition of galactofuranose to the glycan core of LPG (25). As such, the defective expression of PG-containing molecules in R2D2 or in mutants generated by targeted deletion of the LPG1 gene are restricted to LPG (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work). In addition, the expression of GIPLs, which contain LPG related core oligosaccharide structures, seemed normal in these cells (G.S., L.E., S. Singer, H. Avila, S.J.T., and S.M.B., unpublished work). Study of the interaction of the L. major LPG1-deficient mutant with its natural vector, P. papatasi, revealed that promastigote growth was reduced only slightly within the early blood-fed midgut. The mutant promastigotes were lost completely, however, after excretion of the digested blood meal. This point is forcefully made by the comparison of midgut infections in flies that either had retained or had passed their blood meals on day 5; the former remained heavily infected, whereas the latter were virtually devoid of parasites. The inability of Lmajlpg1− promastigotes to persist in the midgut during blood meal excretion was correlated with their loss of binding to P. papatasi midguts in vitro. The finding that the LPG1 transfectants that were fully restored for LPG expression were also almost fully restored for in vitro midgut attachment and for day 5 in vivo midgut persistence is conclusive evidence that LPG controls these interactions. That there might be a threshold level of surface LPG required to mediate attachment is suggested by the inability of LdR2D2+LPG1 to rescue LdR2D2 for in vitro attachment and in vivo persistence, presumably because the line had less than 10% of wild-type levels of LPG restored. This conclusion is supported by the finding that the Ldlpg2−+LPG2, which showed efficient restoration of surface LPG expression, was successfully rescued in its ability to maintain infection in the P. argentipes midgut and to attach to these midguts in vitro.
The infection outcomes with both the L. major and L. donovani LPG1 mutants in their respective vectors indicate, surprisingly, that LPG is not required for the parasite to survive within the proteolytic environment of the blood-fed midgut. The requirement for other PG-containing molecules was indicated, however, by the outcome of early midgut infections in the LPG2-deficient L. donovani mutants. LPG2 was originally identified by transfection of the L. donovani C3P0 mutant and encodes a translocation activity involved in transport of GDP mannose into the Golgi lumen, where it is required to assemble the disaccharide-phosphate repeats of LPG and other PG-containing molecules (24, 26). Deletion of this gene, as occurred in C3P0 by chemical mutagenesis and in the L. donovani lpg2− parasites by targeted gene replacement, gives rise to promastigotes that are completely deficient in all PG-containing glycoconjugates. In contrast to the lpg1− mutants, they are unable to survive during their early exposure to the blood-fed midgut. It is important to note that proteins, such as the acid phosphatase, are still secreted and enzymatically active in these mutants but are devoid of PG chains (24). Transfection of C3P0 and Ldlpg2− with LPG2 restored their surface LPG expression and their ability to assemble other PGs and also restored their capacity to survive the conditions in the digesting blood meal. The rapid killing of promastigotes in the abdominal midgut after blood feeding has been observed previously by using inappropriate sand fly–Leishmania combinations, e.g., L. donovani infections in P. papatasi (39, 40). This killing has been attributed to digestive enzymes released into the midgut to which the inappropriate Leishmania species is especially vulnerable. Taken together, the results with the LPG1 and LPG2 mutants suggest that one or more of the other released PG containing structures, e.g., sAP or PPG, play important roles in protecting the parasite during its early exposure to digestive enzymes in the gut. These data are consistent with an earlier observation by Schlein et al. (12) that released glycoconjugates from cultured promastigotes will promote the early survival of parasites in the midgut. At the time, it was assumed that the active molecule was LPG; the glycoconjugates were not characterized; and it seems likely that sAP and/or PPG were also present in the released material. Released molecules bearing the PG epitope were detected in high abundance in L. major-infected P. papatasi midguts as early as day 2 (41), and the fibrous network of secreted PPG and sAP produced by some Leishmania species in vitro (10) has been suggested to correspond to a similar gel-like matrix observed in infected sand flies (42, 43). An abundance of these secreted PG-containing products, by virtue of their negative charge, might protect the promastigote by acting as a transient barrier against digestive enzymes in the vicinity of the parasite. Such protection would be analogous to that bestowed by gastric mucins, to which PPG shows structural similarities (44), on intestinal epithelial cells in humans. Alternatively, secreted parasite enzymes, such as sAP, might inhibit the activity of the sand fly proteases, and these enzymes might themselves be destroyed in the gut in the absence their PG domains.
Taken together, the comparison of four different mutant lines, involving two different LPG biosynthetic genes, two different Leishmania species, and their respective sand fly vectors, has revealed distinct roles for LPG and the secreted PG-containing proteins in parasite vector interactions and has provided conclusive evidence that these glycoconjugates are required to maintain the transmission cycle of Leishmania in nature.
Acknowledgments
We thank R. Zufferey for providing information concerning the LPG1 mutation in R2D2. We gratefully acknowledge support by National Institutes of Health Grant AI 31078 (to S.J.T. and S.M.B.) and a Human Frontiers Science Program fellowship (to G.S.).
Abbreviations
- LPG
lipophosphoglycan
- PPG
proteophosphoglycan
- PG
phosphoglycan
- sAP
secreted acid phosphatase
- PI
phosphatidylinositol
- GIPL
glycoinositol phospholipid
- GPI
glycophosphatidylinositol
- PNA
peanut agglutinin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Turco S J, Descoteaux A. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 2.McConville M J, Ferguson M A J. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turco S J, Hull S R, Orlandi P A, Jr, Shepherd S D, Homans S W, Dwek R A, Rademacher T W. Biochemistry. 1987;26:6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- 4.Ilg T, Etges R, Overath P, McConville M J, Thomas-Oates J, Thomas J, Homans S W, Ferguson M A. J Biol Chem. 1992;267:6834–6840. [PubMed] [Google Scholar]
- 5.McConville M J, Thomas-Oates J E, Ferguson M A, Homans S W. J Biol Chem. 1990;265:19611–19623. [PubMed] [Google Scholar]
- 6.McConville M J, Schnur L F, Jaffe C, Schneider P. Biochem J. 1995;310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConville M J, Blackwell J M. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 8.Turco S J, Sacks D L. Mol Biochem Parasitol. 1991;45:91–99. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]
- 9.Pimenta P F, Saraiva E M, Sacks D L. Exp Parasitol. 1991;72:191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 10.Ilg T, Stierhof Y D, Wiese M, McConville M J, Overath P. Parasitology. 1994;108:S63–S71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- 11.Mengeling B J, Turco S J. Curr Opin Struct Biol. 1998;8:572–577. doi: 10.1016/s0959-440x(98)80146-2. [DOI] [PubMed] [Google Scholar]
- 12.Schlein Y, Schnur L F, Jacobson R L. Trans R Soc Trop Med Hyg. 1990;84:353–355. doi: 10.1016/0035-9203(90)90315-6. [DOI] [PubMed] [Google Scholar]
- 13.Pimenta P F, Modi G B, Pereira S T, Shahabuddin M, Sacks D L. Parasitology. 1997;115:359–369. doi: 10.1017/s0031182097001510. [DOI] [PubMed] [Google Scholar]
- 14.Sacks D L, Saraiva E M, Rowton E, Turco S J, Pimenta P F. Parasitology. 1994;108:S55–S62. doi: 10.1017/s0031182000075727. [DOI] [PubMed] [Google Scholar]
- 15.Pimenta P F, Turco S J, McConville M J, Lawyer P G, Perkins P V, Sacks D L. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D L, Pimenta P F, McConville M J, Schneider P, Turco S J. J Exp Med. 1995;181:685–697. doi: 10.1084/jem.181.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pimenta P F, Saraiva E M, Rowton E, Modi G B, Garraway L A, Beverley S M, Turco S J, Sacks D L. Proc Natl Acad Sci USA. 1994;91:9155–9156. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King D L, Turco S J. Mol Biochem Parasitol. 1988;28:285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- 19.McNeely T B, Tolson D L, Pearson T W, Turco S J. Glycobiology. 1990;1:63–69. doi: 10.1093/glycob/1.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Butcher B A, Turco S J, Hilty B A, Pimenta P F, Panunzio M, Sacks D L. J Biol Chem. 1996;271:20573–20579. doi: 10.1074/jbc.271.34.20573. [DOI] [PubMed] [Google Scholar]
- 21.Descoteaux A, Mengeling B J, Beverley S M, Turco S J. Mol Biochem Parasitol. 1998;94:27–40. doi: 10.1016/s0166-6851(98)00047-4. [DOI] [PubMed] [Google Scholar]
- 22.Beverley S M, Turco S J. Trends Microbiol. 1998;6:35–40. doi: 10.1016/S0966-842X(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 23.Ryan K A, Garraway L A, Descoteaux A, Turco S J, Beverley S M. Proc Natl Acad Sci USA. 1993;90:8609–8613. doi: 10.1073/pnas.90.18.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Descoteaux A, Luo Y, Turco S J, Beverley S M. Science. 1995;269:1869–1872. doi: 10.1126/science.7569927. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Turco S J. J Biol Chem. 1993;268:24060–24066. [PubMed] [Google Scholar]
- 26.Ma D, Russell D G, Beverley S M, Turco S J. J Biol Chem. 1997;272:3799–3805. [PubMed] [Google Scholar]
- 27.Modi G B, Tesh R B. J Med Entomol. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 28.Tolson D L, Turco S J, Pearson T W. Infect Immun. 1990;58:3500–3507. doi: 10.1128/iai.58.11.3500-3507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelleher M, Curtis J M, Sacks D L, Handman E, Bacic A. Mol Biochem Parasitol. 1994;66:187–200. doi: 10.1016/0166-6851(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 30.Puentes S M, Sacks D L, da Silva R P, Joiner K A. J Exp Med. 1988;167:887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handman E, Goding J W. EMBO J. 1985;4:329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Descoteaux A, Matlashewski G, Turco S J. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 33.Cappai R, Morris L, Aebischer T, Bacic A, Curtis J M, Kelleher M, McLeod K S, Moody S F, Osborn A H, Handman E. Parasitology. 1994;108:397–405. doi: 10.1017/s0031182000075946. [DOI] [PubMed] [Google Scholar]
- 34.Elhay M, Kelleher M, Bacic A, McConville M J, Tolson D L, Pearson T W, Handman E. Mol Biochem Parasitol. 1990;40:255–267. doi: 10.1016/0166-6851(90)90047-p. [DOI] [PubMed] [Google Scholar]
- 35.McNeely T B, Turco S J. J Immunol. 1990;144:2745–2750. [PubMed] [Google Scholar]
- 36.Opat A, Ng K, Currie G, Handman E, Bacic A. Glycobiology. 1996;6:387–397. doi: 10.1093/glycob/6.4.387. [DOI] [PubMed] [Google Scholar]
- 37.Handman E, Hocking R E, Mitchell G F, Spithill T W. Mol Biochem Parasitol. 1983;7:111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- 38.da Silva R, Sacks D L. Infect Immun. 1987;55:2802–2806. doi: 10.1128/iai.55.11.2802-2806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlein Y, Jacobson R L. Parasitology. 1998;117:467–473. doi: 10.1017/s0031182098003321. [DOI] [PubMed] [Google Scholar]
- 40.Schlein Y, Romano H. Exp Parasitol. 1986;62:376–380. doi: 10.1016/0014-4894(86)90045-7. [DOI] [PubMed] [Google Scholar]
- 41.Davies C R, Cooper A M, Peacock C, Lane R P, Blackwell J M. Parasitology. 1990;101:337–343. doi: 10.1017/s0031182000060522. [DOI] [PubMed] [Google Scholar]
- 42.Lawyer P G, Ngumbi P M, Anjili C O, Odongo S O, Mebrahtu Y B, Githure J I, Koech D K, Roberts C R. Am J Trop Med Hyg. 1990;43:31–43. doi: 10.4269/ajtmh.1990.43.31. [DOI] [PubMed] [Google Scholar]
- 43.Walters L L, Modi G B, Tesh R B, Burrage T. Am J Trop Med Hyg. 1987;36:294–314. doi: 10.4269/ajtmh.1987.36.294. [DOI] [PubMed] [Google Scholar]
- 44.Ilg T, Stierhof Y D, Craik D, Simpson R, Handman E, Bacic A. J Biol Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]