Abstract
SEC-23 is a component of coat protein complex II (COPII)-coated vesicles involved in the endoplasmic reticulum-to-Golgi transport pathway of eukaryotes. During postembryonic life, Caenorhabditis elegans is surrounded by a collagenous exoskeleton termed the cuticle. From a screen for mutants defective in cuticle secretion, we identified and characterized a sec-23 mutant of C. elegans. By sequence homology, C. elegans has only the single sec-23 gene described herein. In addition to the cuticle secretion defect, mutants fail to complete embryonic morphogenesis. However, they progress through the earlier stages of embryogenesis, including gastrulation, and achieve substantial morphogenesis before death. We demonstrated a maternal component of SEC-23 function sufficient for progression through the earlier stages of embryogenesis and explaining the limited phenotype of the zygotic mutant. By RNA-mediated interference, we investigated the effects of perturbing COPII function during various postembryonic stages. During larval stages, major defects in cuticle synthesis and molting were observed. In the adult hermaphrodite, reduction of SEC-23 function by RNA-mediated interference caused a rapid onset of sterility, with defects in oogenesis including early maturation of the germline nuclei, probably a result of the observed loss of the GLP-1 receptor from the membrane surfaces adjacent to the developing germline nuclei.
INTRODUCTION
The cuticle of the nematode Caenorhabditis elegans is a collagenous extracellular matrix (ECM) synthesized by a specialized underlying ectodermal cell layer, termed the hypodermis, that surrounds the body of the animal. During its life cycle, C. elegans synthesizes this ECM on five occasions, once in the embryo before hatching and then at the end of each larval stage before molting. During each synthesis, the components of the cuticle are secreted apically from the hypodermal cells and then polymerize on the outside of the apical surface. The cuticle consists predominantly of small collagens encoded by a multigene family. The nematode cuticle collagens share a common overall structure, the procollagen chains being ∼30 kDa and having short, interrupted blocks of Gly-X-Y collagen sequence and clusters of conserved cysteine residues (Kramer, 1997; Johnstone, 2000). They are most similar to the nonfibrillar FACIT collagens of vertebrates (Shaw and Olsen, 1991).
For the fibrillar collagens of vertebrates, trimerization of monomers occurs within the endoplasmic reticulum (ER). Hydroxylation of a proportion of the proline residues within the repeat domains is required to stabilize the collagen structures (Kivirikko and Pihlajaniemi, 1998). This reaction is catalyzed by prolyl 4-hydroxylase, an ER enzyme that also acts as a molecular chaperone by retaining unfolded collagen chains in the ER, releasing them for transport to the Golgi apparatus for further processing only when they have folded correctly (Walmsley et al., 1999). Although less is known about the assembly and secretion of the nematode cuticular collagens, like the vertebrate fibrillar collagens they are subject to hydroxylation of proline residues by the ER enzyme prolyl 4-hydroxylase, which plays an essential role in their biosynthesis (Winter and Page, 2000). It is probable that many other aspects of collagen biosynthesis are shared between C. elegans and vertebrates.
The established route for secretory proteins to pass from the ER to the Golgi is via COPII-coated vesicles (Bednarek et al., 1996; Schekman and Orci, 1996), which in the yeast Saccharomyces cerevisiae are known to consist of two protein complexes, Sec23p-Sec24p and Sec13p-Sec31p, and a low molecular weight GTP-binding protein, Sar1p (Barlowe et al., 1994; Kirchhausen, 2000). Much of the current understanding of the eukaryotic secretory pathway is derived in combination from investigations of secretory mutants of yeast and studies in cultured mammalian cells.
C. elegans is a small animal whose cells are organized into a variety of tissues and organs, but like yeast it is genetically tractable. During the later stages of C. elegans embryogenesis, the developing embryo changes shape radically from an ellipsoidal ball of cells to the worm shape of the L1 larva. This morphogenetic event is driven largely by a circumferential contraction and concurrent increase in length of the single layer of hypodermal cells surrounding the animal (Priess and Hirsh, 1986). Once elongation is complete, the hypodermal cells secrete the components of the L1 cuticle, which polymerizes and assumes an exoskeletal role of maintaining the shape of the animal. The various collagens that comprise the bulk of the protein content of the cuticle are synthesized in a distinct sequential series over a 4-h period before cuticle assembly and hatching. This process is reiterated at the end of each larval stage (Johnstone and Barry, 1996; Johnstone, 2000). The cuticle represents a significant fraction of the total protein content of the animal, and its synthesis is a substantial biosynthetic effort for the hypodermal cells.
In a genetic screen for mutants defective in cuticle synthesis, we isolated a sec-23 mutant of C. elegans. Because a functional secretory pathway must be required for many processes of early development, it was surprising to identify such a mutant with a relatively specific phenotype affecting the final stages of embryonic development. However, zygotic expression of a sec-23::GFP reporter transgene was first detected in the hypodermal cells that secrete the cuticle and at the time of first cuticle collagen synthesis, a time at which the quantity of protein transported is disproportionate to anything in the earlier developmental stages. Thus, the specificity of the sec-23 mutant phenotype is consistent with the timing and tissue specificity of the earliest detected zygotic sec-23 expression. In this study, we used a sec-23 mutant and sec-23 RNAi to investigate the effect of perturbing COPII function at various stages of development.
MATERIALS AND METHODS
C. elegans Strains
C. elegans culture was at 20°C by using standard methods (Sulston and Hodgkin, 1988). Strains used were the wild-type strains N2 and RW7000 (used for sequence tagged site [STS] mapping); DR96 unc-76(e911); BE148 rol-9(sc148); BE150 unc-51(e369) rol-9(sc148); JK1521 fog-2(q71) pha-4(q490)/stu-3(q265) rol-9(sc148); AZ212 unc-119(ed3) ruIs32[unc-119(+) pie-1::GFP::H2B]; BS13 unc-51(e369) fog-2(q71)/fog-2(q71) rol-9(sc148); DH1033 bIs1[vit-2::GFP rol-6(su1006)]/sqt-1(sc103); IA260 unc-51(e369) rol-9(sc148)/sec-23(ij13); IA261 fog-2(q71) pha-4(q490)/sec-23(ij13) rol-9(sc148); IA384 unc-51(e369) fog-2(q71)/sec-23(ij13) rol-9(sc148); IA25 egl-23(n601); ijIs8 [col-12::lacZGFP rol-6(su1006)].
IA260 was used to generate a sec-23(ij13) rol-9(sc148) chromosome by recombination. This was used in the construction of the balanced strain IA261. BS13 is a male/female strain and was a gift from Tim Schedl (Department of Genetics, Washington University School of Medicine, St. Louis, MO). Strain DH1033 was a gift from Barth Grant (Department of Molecular Biology and Biochemistry, The State University of New Jersey, Rutgers, NJ). Some strains were obtained from the C. elegans Genetics Stock Center, which is funded by the National Institutes of Health National Center for Research Resources.
Genetics
Standard genetic methods were used (Sulston and Hodgkin, 1988). The ij13 allele was isolated from a screen of 16,000 chromosomes after ethylmethanesulfonate mutagenesis (Anderson, 1995) of the col-12::lacZGFP integrated strain IA25. ij13 was outcrossed three times and mapped to the extreme right hand end of chromosome V by STS mapping (Williams et al., 1992; Williams, 1995). Three multifactor mapping experiments were performed to refine the ij13 map position. From the strain IA260, rol-9-non-unc-51 recombinants were selected, of which seven carried ij13 and six did not, placing ij13 between unc-51 and rol-9. From the strain IA261, rol-9-non-ij13 recombinants were selected. Of these, one carried fog-2 and 4 did not, placing ij13 to the left of fog-2. From the strain IA384, both unc-51-non-fog-2 and rol-9-non-ij13 recombinant classes were selected. Of the unc-51-non-fog-2 class, three recombinants carried ij13. Of the rol-9-non-ij13 class, 1 carried both unc-51 and fog-2, whereas three carried only unc-51. These data are consistent with ij13 being positioned between unc-51 and fog-2.
Microscopy and Immunofluorescence
Time-lapse microscopy of embryos was performed using Axioplan Normaski optics (Carl Zeiss, Jena, Germany). Images were captured with an Orca camera (Hamamatsu, Bridgewater, NJ) attached to an Axioskop 2 microscope by use of Openlab 2.0.2 software (Improvision, Coventry, United Kingdom). Images were further processed with Adobe Photoshop 7.0.
Immunofluorescence microscopy was performed by standard methods (Miller and Shakes, 1995). A 1:50 dilution of monoclonal antibody (mAb) DPY7-5a (McMahon et al., 2003) was used to detect the presence of the DPY-7 collagen protein present within or secreted on the apical surface of the hypodermal cells. Other antibodies used were MH3 (Francis and Waterston, 1991), 3NB12 (Okamoto and Thomson, 1985), and anti-RME-2 (a gift from Barth Grant). All primary antibodies were detected with either an anti-mouse IgG or anti-rabbit IgG fluorescein isothiocyanate-labeled secondary antibody from Sigma-Aldrich (St. Louis, MO).
RNA-mediated Interference (RNAi)
RNAi by microinjection of the adult hermaphrodite gonad (Fire et al., 1998) was performed on the 12 C. elegans predicted genes: Y113G7A.1, Y113G7A.3, Y113G7A.4, Y113G7A.5, Y113G7A.6, Y113G7A.8, Y113G7A.9, Y113G7A.10, Y113G7A.11, Y113G7A.12, Y113G7A.13, and Y113G7A.14. Details of sequences used for RNAi of these genes can be obtained from the authors. Postembryonic RNAi of sec-23 was performed by the bacterial feeding method; polymerase chain reaction (PCR)-generated fragments of the genes were subcloned into the RNAi vector L4440 and transformed into Escherichia coli strain HT115 (Timmons et al., 2001). Feeding RNAi was performed at 20°C. Details of sequences used can be obtained from the authors.
Reporter Gene Constructs, sec-23 Mini-Gene, and Other Gene Fusions
Green fluorescent protein (GFP) reporter gene fusions were constructed for the two cuticle collagen genes dpy-7 and col-12 by using the GFP vector pPD 95.67 (http://www.ciwemb.edu; Fire et al., 1990), generating the dpy-7::GFP reporter gene fusion pMW002 and the col-12::GFP reporter gene fusion pMW003. pMW002 contains ∼450 base pairs of upstream dpy-7 gene sequence; pMW003 contains ∼850 base pairs of upstream col-12 gene sequence. sec-23 reporter gene fusions were constructed using the lacZ::GFP reporter vector pPD96.04 (http://www.ciwemb.edu; Fire et al., 1990). Two reporter constructs were generated: pABR004, containing ∼1 kb of sec-23 upstream sequence, and pABR012, containing ∼2.5 kb of sec-23 upstream sequence. The upstream fragments containing the putative promoter region of sec-23 were generated by PCR by using primers y113prom52S (5′TCGGTCGACCTCCTGACGGTATTTTG 3′), y1135promA (5′GTGGGATCCATTTTTACTGGAATAAACTG 3′), and y113prom5S3 (5′TCGGTCGACTCATCTCCAGTCAAATCCCG 3′), and were subcloned into the vector pPD96.04.
A sec-23 mini-gene was constructed from three PCR-generated fragments. A 2.5-kb cDNA fragment including exons 4–12 was generated by PCR amplification from cDNA generated from mixed stage RNA from the wild-type strain N2, by using primers y113exon-4S (5′-ATTGCCGAGGATAACCGC-3′) and y113exon-12A2 (5′-GTTGAAGAGCTGGAGGAGAG-3′), and subcloned into PCRscript (Stratagene, La Jolla, CA) to produce plasmid clone pABR009. A 350-base pair genomic 3′ fragment containing the predicted polyadenylation site was generated by PCR of N2 wild-type genomic DNA, by using primers y113fragFS (5′-CTATTAATTTATTGATTTATTG-3′) and y113-fragEA (5′-GTTAGGCGGTCCAATGGGCT-3′), and subcloned into NotI-SacII–digested plasmid clone pABR009 to produce pABR010. A 5′ genomic fragment containing ∼1 kb of upstream genomic sequence and exons and introns 1–3 was generated by PCR amplification of N2 wild-type genomic DNA, by using primers y113prom52S (5′-TCGGTCGACCTCCTCCTGACGGTATTTTG-3′) and y113promA2 (5′-GCTCCTCTGCAGTCATACAC-3′), and subcloned as a SalI fragment into pABR010 to form plasmid pABR014. The integrity of this construct was confirmed by sequencing.
A dpy-7::sec-23 fusion construct was obtained by generating a 700-base pair dpy-7 promoter fragment by PCR with primers DPY7S (5′-GGAGTCGACAAAGTTTGGAGAAGTGATGATTG-3′) and DPY7A (5′-TCTGGTACCGGAACAAAATGTAAGAATATTC-3′). This fragment was then cloned into the sec-23 mini-gene construct, replacing the native sec-23 promoter sequences with those of dpy-7, to produce clone pABR040.
A SEC-23::GFP fusion protein transgene was generated by subcloning the intact functional sec-23 mini-gene contained in plasmid pABR014 into the GFP vector pPD95.75 (http://www.ciwemb.edu; Fire et al., 1990). This was done by PCR amplification of the mini-gene sequences from pABR014 by using primers: Prom-XbaGFP (5′-AGTTCTAGACTCCTCCTGACGGTATTTTG-3′) and Prom-SmaGFP (5′-CATCCCGGGATGTTGAAGAGCTGGAGGAG-3′). The fusion between SEC-23 and GFP is at the carboxy terminal end of SEC-23. Two independent clones termed pABR020a and pABR020b were generated from two independent PCR amplification reactions.
C. elegans Transgenesis
Transgenic phenotypic rescue of sec-23(ij13) was performed using strain IA261. Simple free arrays were generated by microinjection into adult hermaphrodite gonads (Mello and Fire, 1995) of the sec-23 mini-gene plasmid ABR014 at concentrations of between 2 and 10 ng/μl and, as a marker for transgenesis, the dpy-7::GFP plasmid pMW002 at 10 ng/μl. As carrier DNA, pTAg cloning vector (R & D Systems, Minneapolis, MN) was included at 120 ng/μl. Complex free arrays (Kelly et al., 1997) were generated by microinjection of linearized plasmids ABR014 and pMW002 at 2 ng/μl. In this instance, carrier DNA was PvuII-digested genomic C. elegans DNA at 100 ng/μl.
Transgenic lines carrying the SEC-23::GFP fusion protein vectors pABR020a and pABR020b were generated in strain IA261. pABR020a or pABR020b was microinjected at 2 ng/μl with pTAg carrier at 120 ng/μl. GFP expression from pABR020a or pABR020b was used as the only marker for transgenesis in this case.
Transgenic lines carrying the dpy-7::sec-23 promoter::mini-gene fusion pABR040 were generated in strain IA260. pABR040 was microinjected at 1 ng/μl, with coinjected col-12::GFP plasmid pMW003 at 10 ng/μl and pTAG as carrier at 120 ng/μl. Transgenic lines were established in the heterozygous IA260 strain, and the effect of the dpy-7::sec-23 transgene was observed in the homozygous sec-23(ij13) segregants.
Transgenic lines carrying the sec-23::lacZ::GFP reporter gene fusions pABR004 and pABR012 were generated in strain DR76, by using rescue of the Unc-76 phenotype as a marker for transgenesis. pABR004 or pABR012 was injected at 20 ng/μl, and the unc-76 rescuing plasmid p76–16B (Bloom and Horvitz, 1997) was coinjected at 120 ng/μl.
Sequencing
All sequencing was performed on the ABI stretch automated sequencer (Applied Biosystems, Foster City, CA). DNA from sec-23(ij13) embryos was isolated by the same method used for STS mapping (Williams, 1995) and used for PCR amplification. The PCR-generated products were cloned into pGEM-T and sequenced using primers M13 Forward and M13 Reverse (Promega, Madison, WI).
Reverse Transcription-PCR and 5′ Rapid Amplification of cDNA Ends
Intron/exon boundaries were determined by sequencing cDNA. RNA was prepared as described previously (Johnstone and Barry, 1996). First-strand cDNA was generated using the ProSTAR first-strand reverse transcription-PCR kit (Stratagene) as per the manufacturer's instructions. Full-length gene-specific cDNA molecules were produced by PCR with primers y113cDNA-S (5′-GAAGAATATTTGGGTGCTCAACA-3′) and y113cDNA-A (5′-TTCGGATCGTCTTTCGAGTAT-3′). The cDNA was subcloned into PCRscript (Stratagene) and sequenced as described above.
5′ RACE was performed using the 5′ RACE system kit (Invitrogen, Carlsbad, CA) for rapid amplification of cDNA ends kit. The sec-23 gene-specific primers used were RACEX6A (5′-GCCGGCCATTACATCCTTGATTTG-3′), y113exon5A2 (5′-CGTAGCTTCTCGAGATTCCC-3′), and RACEX3A (5′-CTCGGCAATTGCCGCATAATGAG-3′). PCR products were subcloned into PCRscript (Stratagene) and were sequenced.
RESULTS
Identification of C. elegans Mutants Defective in Cuticle Synthesis
We performed a clonal zygotic embryonic lethal mutant screen with C. elegans strain IA25, which carries a chromosomally integrated col-12::GFP reporter transgene causing expression of GFP within most or all hypodermal cells. col-12 is a C. elegans cuticular collagen gene that is expressed specifically in hypodermal cells late within each cuticle synthetic phase, concurrent with secretion of each new cuticle (Johnstone and Barry, 1996). During C. elegans embryogenesis it is expressed at the late threefold stage toward the end of embryonic morphogenesis and just before secretion of the L1 larval cuticle. The resulting GFP expression or lack of it was used to facilitate phenotypic characterization and the identification of mutants defective in cuticle synthesis.
From 8000 F1 progeny of mutagenized parents, ∼2000 zygotic embryonic lethal mutants were obtained, and we identified three that achieved a relatively normal “comma” stage of embryonic development and proceeded to elongate considerably, but failed to express the col-12::GFP transgene. That these mutants initiate the process of embryonic elongation in an apparently normal way indicates that the hypodermal tissue is present and is initiating this major feature of hypodermal terminal developmental fate. The failure to induce the col-12::GFP reporter could either be a direct effect, if the mutated gene was necessary for the transcriptional activation of the col-12 promoter, or indirect, if it was required for the hypodermis to reach the developmental stage at which col-12 is normally expressed. The three mutations of this class identified are not allelic, mapping to different chromosomal locations and differing in the extent of elongation achieved before collapse and death. One of these mutations, ij13 is discussed herein.
Identification of ij13 as a sec-23 Mutation
To facilitate cloning of the gene identified by ij13, we used STS (Williams, 1995) and standard multifactor mapping (Sulston and Hodgkin, 1988). We positioned ij13 between unc-51 and fog-2 on the extreme right-hand end of chromosome V (Figure 1A; see MATERIALS AND METHODS). Both of these genes have previously been cloned: unc-51 corresponds to the predicted gene Y60A3A.1, and fog-2 corresponds to the predicted gene Y113G7B.5 (Ogura et al., 1994; Clifford et al., 2000). We performed RNAi on the predicted genes within the sequence Y113G7A, looking for a phenocopy of the ij13 phenotype. RNAi was carried out by the microinjection method (Fire et al., 1998). A detectable RNAi effect was obtained with only one of the 12 genes tested, Y113G7A.3. The RNAi effect we obtained for this gene followed a very reproducible progression. The first few embryos laid after injection were not affected. Y113G7A.3 RNAi-treated hermaphrodites then produced some embryos that died with phenocopies ranging from the same extent of elongation as that seen for the zygotic ij13 mutant to a much earlier developmental arrest at a precomma stage of embryogenesis. After producing a small brood of 10–30 embryos, the treated mothers became sterile. We suggest that the spectrum of effects seen with Y113G7A.3 RNAi can be explained by a gradual reduction in function as the RNAi takes effect (discussed below). As a percentage of the F1 progeny of treated mothers mimicked the ij13 zygotic mutant phenotype, we concluded that Y113G7A.3 was a strong candidate for the gene identified by the ij13 lesion.
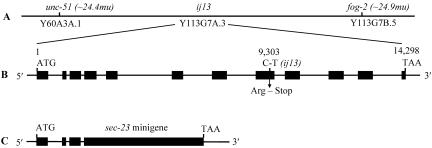
Figure 1.
ij13 genomic location, sec-23 gene structure, and sec-23 mini-gene. (A) Extreme right-hand end of chromosome V showing the position of the ij13 mutation. (B) Genomic organization of sec-23 (predicted gene Y113G7A.3). The black boxes represent exons. The position of the base change in ij13 is indicated. (C) Schematic representation of the sec-23 mini-gene (see MATERIALS AND METHODS for details).
Y113G7A.3 encodes the C. elegans homolog of the S. cerevisiae gene sec23 (Figure 2), so we termed it sec-23. To ascertain whether sec-23 contained a mutation in the ij13 mutant background, we sequenced its exons from ij13 homozygotic mutant animals. Because the sec-23 gene spans ∼14.3 kb of genomic sequence (Figure 1B), we determined exon sequence only. We identified a CGA to TGA substitution (Figure 1B) in the genomic sequence at base 9303 relative to the ATG in sec-23(ij13) exon 8, consistent in DNA derived from five homozygotic mutant embryos. This amber mutation affects Arg486 of the predicted protein and would result in the production of a truncated protein of 485 residues, which would lack a region of strong similarity to other Sec23 proteins (Figure 2). The mutation also causes a restriction site polymorphism that can be used to distinguish sec-23(ij13) gene sequence from that of the wild-type.
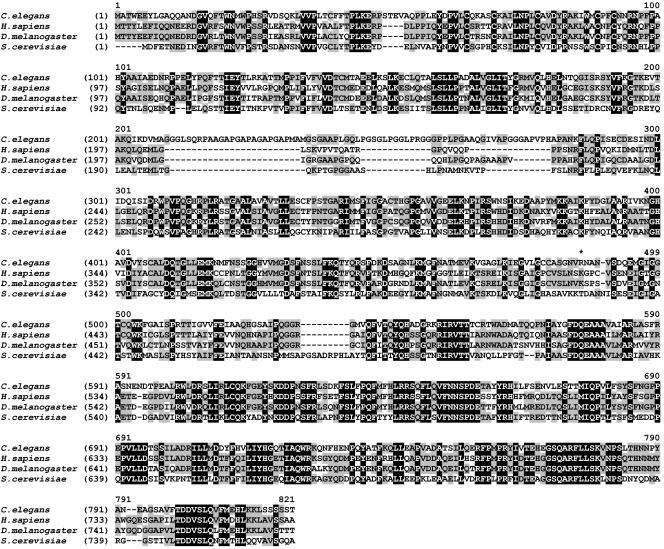
Figure 2.
Sequence comparison of SEC23 proteins. Predicted protein sequences were aligned using the AlignX program of VectorNTI. The sequences were obtained from databases at the European Bioinformatics Institute (http://www.ebi.ac.uk/), with the following accession numbers: C. elegans, Q9U2Z1; D. melanogaster, Q9VNF8; human (SEC23A), Q15436; and S. cerevisiae, P15303. Residues shared among all four sequences are shown as white on black; residues shared between two or three of the sequences are shown as black on gray. The residue affected by the ij13 mutation is marked with *.
We sequenced the cDNA of sec-23 to confirm the structure of the gene predicted for Y113G7A.3 by Genefinder (accession no. AL132858). The intron/exon structure is as currently predicted (Figure 1B). We mapped the 5′ end of the sec-23 transcript by using 5′ RACE and determined that the gene is trans-spliced to the SL1 splice leader at position -7 relative to the predicted ATG start codon. The 3′ end of the mature transcript is confirmed by the end point of several cDNA clones sequenced by the genome project.
Because of the length of the sec-23 genomic sequence, we constructed a mini-gene assembled from wild-type genomic and cDNA sequence (Figure 1C) before attempting phenotypic rescue by using the balanced strain IA261. Transgenic rescue would result in survival of the sec-23(ij13) rol-9(sc148) homozygotic segregants of this strain. Initial attempts by using circular plasmid mini-gene and plasmid carrier DNA resulted in the appearance of significant numbers of larvae of this genotype; however, these larvae developed into sterile adults. We confirmed chromosomal homozygosity for sec-23(ij13) by PCR amplification of the genomic sec-23 copies, by using primers that do not amplify the sec-23 mini-gene, and by testing for the restriction site polymorphism of the ij13 lesion. Thus, those animals were phenotypically rescued for the somatic function of sec-23, but their sterility suggested a germline function that was not rescued by the transgenesis. Circular plasmid DNA injected into the syncytial gonad forms heritable extrachromosomal arrays composed of multiple copies of the injected plasmid(s) (Mello and Fire, 1995). Transgenes on such repetitive arrays are very poorly expressed in the C. elegans germline, a problem that can be overcome by using complex arrays generated by injecting linearized transgene DNA at low concentration plus linearized genomic C. elegans DNA as a complex DNA carrier (Kelly et al., 1997). We obtained phenotypic rescue of both the somatic and germline functions of sec-23 by this method; however, the germline rescue was partial as transgenic animals gave relatively low brood sizes (see below).
The phenotypic rescue of the ij13 mutant with the sec-23 mini-gene and the presence of an amber mutation in the sec-23 gene in the ij13 mutant background confirm that ij13 is an allele of sec-23.
C. elegans Has a Single sec-23 Gene and Two Apparent sec-24 Genes
Searches against all predicted C. elegans proteins and against translations of the complete genomic sequence indicated that C. elegans has only one SEC23 homolog, as described above. C. elegans SEC-23 shares ∼43% sequence identify with S. cerevisiae Sec23p, ∼56% identity with human SEC23A and SEC23B, and ∼59% identity with Drosophila melanogaster Sec23 (Figure 2). In addition, C. elegans ZC518.2 and F12F6.6 (accession nos. Q23368 and Q19371), share ∼26 and ∼22% identity with S. cerevisiae Sec24p. Sec23 and Sec24 proteins form a heterodimeric complex, and the S. cerevisiae proteins have weak similarity (∼12% sequence identity) over their entire lengths. Similarly, C. elegans SEC-23 shares ∼12 and ∼13% sequence identity with the predicted proteins ZC518.2 and F12F6.6.
Relative to other Sec23 proteins, C. elegans SEC-23 contains an apparent insertion at residues ∼210 to ∼280 (Figure 2). There seems to be weak similarity between this region and the much shorter sequence of this region in D. melanogaster Sec23. Except for this, the D. melanogaster protein is more closely related (∼75% identity) to the human proteins.
Morphogenetic Defects in sec-23(ij13) Embryos
sec-23(ij13) homozygous animals die late in embryogenesis. Affected embryos develop in an apparently normal manner through gastrulation, and start elongation as in wild type (Figure 3, A and E). At around the two-fold stage of elongation, the sec-23 embryos have a morphology similar to wild type, indicating that hypodermal morphogenesis proceeds relatively normally at least until this stage (Figure 3, B and F). Elongation continues variably to ∼60% of the length of the wild-type, at which point elongation prematurely terminates. After some time at this stage (typically <1 h), material is seen rupturing from the hypodermal surface of the mutant (Figure 3G), and the body of the affected animals starts to collapse (Figure 3H). At the terminal phenotype, the mutant embryos have lost much of their organized shape and have protrusions visible over the surface (Figure 3I). The sec-23 mutant embryos do not produce a functional cuticle and hypodermal cells frequently dissociate from the body of the animal.
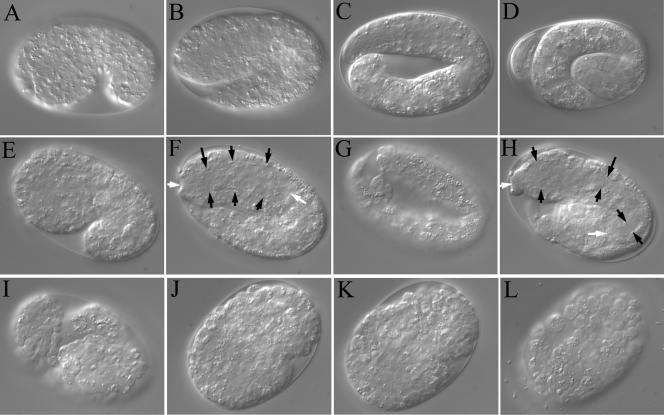
Figure 3.
Morphogenesis in sec-23(ij13) embryos. (A–D) Elongation of a wild-type embryo: comma stage (A), two-fold stage (B), three fold stage (C), and pretzel stage (D) after secretion of the cuticle. (E–I) Elongation and collapse of a sec-23(ij13) embryo. (E–H) Images taken at 30-min intervals. (I) Image taken 90 min after H. White arrows, anterior and posterior ends of the pharynx (anterior to the left); black arrows, the width of the pharynx at various points. (J–L) Images taken at 150-min intervals of a homozygotic sec-23(ij13) mutant embryo derived from a chromosomally sec-23(ij13) homozygous mutant mother, transgenic for sec-23. In J, the animal has just initiated hypodermal morphogenesis.
Approximately concurrent with the morphogenetic changes in the hypodermis that drive much of the elongation of the body, changes in shape occur in the cells of the pharynx, an organ that pumps food into the animal's intestine, resulting in the generation of an elongate bilobed tube (Albertson and Thomson, 1976; Portereiko and Mango, 2001). The pharynx also fails to complete morphogenesis in the sec-23(ij13) mutant, but its elongation continues after the onset of collapse in the hypodermis (Figure 3, F and H). Subsequently, the pharynx also collapses, as indicated by staining pharyngeal muscle cells with the 3NB12 antibody (Figure 4, A and B), and there is a general loss of structural integrity in the animal (Figure 3I). Cell boundaries become more visible, and many cells detach from their neighbors, possibly as a result of a loss of cell adhesion.
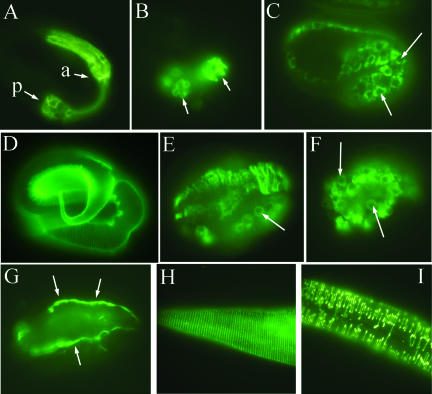
Figure 4.
Effect of sec-23 mutation on pharyngeal development and on the secretion of collagen and a basement membrane marker. (A and B) A pretzel-stage wild-type embryo (A) and a sec-23(ij13) mutant (B) were stained with antibody 3NB12, which labels pharyngeal muscle cells (Priess and Thomson, 1987). The anterior (a) and posterior (p) pharyngeal bulbs are indicated in A; the two bulbs are also indicated in B, but due to the severity of the morphological defects, it is not possible to distinguish anterior and posterior. (C–F) Immunolocalization of the DPY-7 collagen with the DPY7–5a mAb. (C) A wild-type late comma-stage embryo showing perinuclear localization of DPY-7 in hypodermal cells before secretion. Two nuclei are indicated by white arrows. (D) A wild-type pretzel-stage embryo with DPY-7 in the cuticle. (E) A sec-23(ij13) mutant embryo at terminal zygotic phenotype, with some DPY-7 retained in a perinuclear location (arrow) and most in aberrant strands or clumps. (F) Perinuclear DPY-7 (arrows) in a sec-23(ij13) mutant embryo derived from a chromosomally sec-23(ij13) homozygous mutant mother transgenic for sec-23. (G) Nonperinuclear localization of the basement membrane component perlecan with MH3 antiserum in a mutant embryo like that in F. (H and I) Localization of DPY-7 to circumferential bands in the cuticle of a wild-type larva (H) and to discontinuous bands in a sec-23 RNAi larva.
The intestine is present in sec-23 embryos, and there is muscle function as evidenced by movement of the affected embryos. Although there may also be defects in the morphogenesis of these tissues, this is hard to determine due to the general collapse as a result of the hypodermal defects.
Cuticle Collagen Gene Expression in sec-23(ij13) Embryos
sec-23(ij13) mutants do not express the col-12::GFP transgene. This is unlikely to reflect a direct effect on transcriptional activation and presumably is an indirect result of disruption in the function of the hypodermis before the late-class collagen genes are expressed. This interpretation is consistent with the failure of the mutants to reach the extent of elongation at which col-12 is expressed in the wild type. To test whether sec-23(ij13) affects the expression of early-expressed cuticle collagen genes, the mutation was crossed into a strain carrying a dpy-7::GFP reporter construct. The dpy-7 cuticle collagen gene is expressed 4-h before secretion of the cuticle, at approximately the comma stage of elongation (Johnstone and Barry, 1996). The expression of this transgene was normal in the sec-23(ij13) homozygous embryos (our unpublished data), supporting the conclusion from morphological observations that the hypodermis in sec-23(ij13) mutants behaves normally through the early stages of development.
Maternal Supply of sec-23 Function
As discussed above, we obtained phenotypic rescue of both the somatic and germline functions of sec-23 with a mini-gene carried on a complex free array. In C. elegans, such arrays are transmitted in a non-Mendelian manner, being lost from a proportion of the progeny of any transgenic parent. To facilitate distinction between animals with the free array and those that had lost it, we included a dpy-7::GFP reporter as a cotransformed marker. For the rescued lines tested, 100% of embryos that had lost the array died, because they were homozygous mutant sec-23(ij13). Ninety-five percent of embryos that carried the free array hatched. Of these, ∼20% died at some stage during larval development, whereas the remainder reached adulthood. Only 13% of these adults were fertile, and those fertile adults had reduced brood sizes of ∼60% that of wild type. Thus, the sec-23 transgene contained within a complex array was more efficient at rescuing the zygotic than germline function.
The embryos that died after loss of the free array did so at a considerably earlier stage of development (Figure 3, J–L) than did homozygous mutant embryos derived from sec-23(ij13)/+ heterozygous mothers (Figure 3, E–I). This difference in phenotype must result from differences in maternal contribution of sec-23 function. The transgenic rescued mothers have only the sec-23 transgene, which is relatively inefficient at rescuing the germline sec-23 function, whereas the sec-23(ij13)/+ heterozygous mothers have one wild-type chromosomal copy of sec-23, which is adequate for normal function as 100% of such mothers are fertile. This establishes that there is significant maternal contribution of sec-23 function in wild-type C. elegans and that this function is sufficient for the earlier stages of embryonic development.
Secretion Defects in sec-23(ij13) Embryos
The herniations and protrusions from the surface of the affected embryos suggest that the sec-23(ij13) mutant does not secrete a functional cuticle. To explore this further, we used an anti-DPY-7 mAb to test the localization of the DPY-7 cuticle collagen in sec-23 mutant embryos. In wild-type embryos at the comma stage, DPY-7 is detected within hypodermal cells in a perinuclear location (Figure 4C), consistent with location in the ER (McMahon et al., 2003). After elongation is complete, DPY-7 is detected within the newly secreted cuticle (Figure 4D). In sec-23(ij13) mutant embryos derived from heterozygous mothers, some DPY-7 was retained in the perinuclear location and some was located in disorganized threads or band-like structures (Figure 4E). In mutant embryos derived from transgenic rescued mothers (see above), all of the detectible DPY-7 collagen was retained in a perinuclear location (Figure 4F). Thus, SEC-23 is required for secretion of DPY-7, and maternally supplied sec-23 function is sufficient to permit the transport of some DPY-7 collagen from the ER.
We also examined the localization of the basement membrane component perlecan (Francis and Waterston, 1991). We did not see perinuclear retention of perlecan in either class of sec-23(ij13) homozygotic mutant embryos (Figure 4G). In all cases, the detected perlecan was peripheral to the expressing cells.
Expression of sec-23 and Rescue of Its Hypodermal Function
Although sec-23(ij13) mutants do not secrete a cuticle, they die before the normal developmental stage at which cuticle collagens are secreted, as indicated by their failure to express the col-12::GFP reporter. Because SEC-23 is likely to be required in all cells, we wanted to determine whether the hypodermal-elongation and cuticle-secretion defects resulted specifically from the absence of SEC-23 in the hypodermal cells or just from the general sickness of the mutant animals. To determine when and where zygotic expression of sec-23 begins, we constructed a sec-23::GFP reporter trans-gene. The earliest expression that we detected with this reporter, when transmitted from the maternal germline on a free array, was specifically in hypodermal cells just after the comma stage of embryogenesis (Figure 5, A and B). This GFP expression was only observed in those embryos to which a free array was transmitted, and hence must be zygotic expression. This time of expression is slightly before the time at which elongation terminates in the mutant. The number of cell types in which sec-23::GFP expression was detected increased toward the end of embryogenesis; during larval and adult stages, it was evident in most cell types including hypodermis, body wall muscle, pharynx, intestine, and nerve cells (our unpublished data).
Figure 5.
sec-23::GFP reporter gene expression and restoration of sec-23 function in the hypodermis. Nomarski images (left column) are of the same embryos shown in the fluorescence images. (A–D) Expression of sec-23::GFP (A and B) and dpy-7::GFP (C and D) reporter genes in the hypodermal cells of wild-type embryos at the one-and-a-half-fold (A and B) or comma (C and D) stage. (E–G) Rescue of morphogenesis and of late-stage cuticle collagen col-12::GFP reporter gene expression by a dpy-7::sec-23 gene fusion. (E and F) A homozygous sec-23(ij13) mutant embryo containing only the col-12::GFP reporter gene. (G and H) A mutant embryo containing also the dpy-7::sec-23 gene fusion.
The early hypodermal expression of sec-23::GFP was very similar to that seen for the dpy-7 cuticle collagen gene, which is expressed in all hypodermal cells and only in them (Figure 5, C and D) (Gilleard et al., 1997). To express sec-23 exclusively in hypodermal cells, we constructed a dpy-7::sec-23 gene fusion that contained the dpy-7 promoter and the coding sequence of sec-23, derived from the sec-23 mini-gene described above. Using strain IA260, we generated transgenic lines in which the dpy-7::sec-23 fusion was cotransformed with a col-12::GFP reporter construct. Transgenic lines with only the col-12::GFP reporter were generated as a control. Because IA260 is heterozygous for sec-23, 25% of its progeny should be sec-23(ij13) homozygotes. Such segregants that carried only the col-12::GFP reporter displayed the standard elongation and collapse phenotypes and failed to express the col-12::GFP reporter (Figure 5, E and F). However, sec-23(ij13) homozygotes that carried also the dpy-7::sec-23 gene fusion elongated completely, produced a functional cuticle, and expressed the col-12::GFP reporter (Figure 5, G and H). Thus, restoration of zygotic sec-23 function only in the hypodermis was sufficient to rescue the embryonic elongation and cuticle synthesis defects of the sec-23(ij13) mutant. These animals wriggled inside the egg shell for a day or more, but most did not hatch and eventually died. A few did hatch and lived for 1 or 2 days. These observations suggest that secretion of some product from a tissue other than the hypodermis is essential for hatching. When hatching was assisted by mechanical means, the L1 larvae could move for a while but did not feed and quickly became paralyzed and limp, and then died. Consistent with the hypothesis that a nonhypodermal source of a secreted product is required for hatching, it has previously been shown that hatching requires pharyngeal pumping (Hall and Hedgecock, 1991). Thus, possible sources of this product could be the pharynx itself or another tissue affected by pharyngeal pumping, such as the intestine.
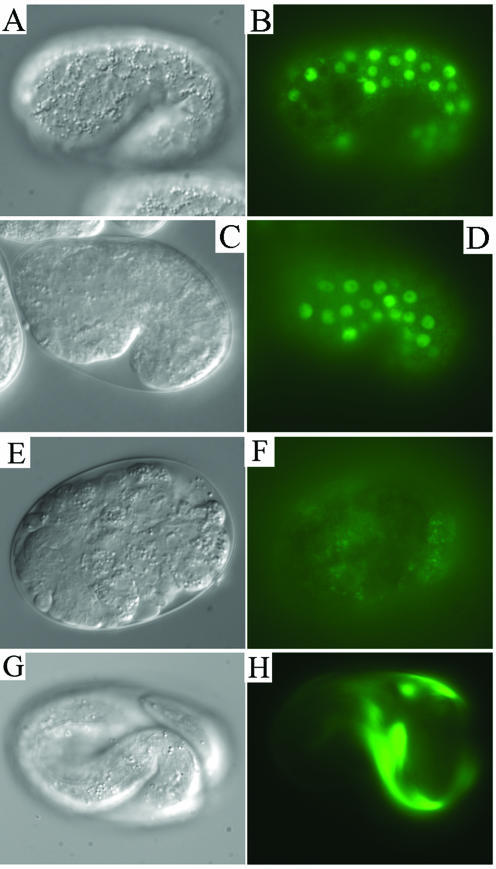
Subcellular Localization of SEC-23
As noted above, Sec23 and Sec24 proteins form a heterodimeric complex. A SEC24::GFP protein fusion has previously been used to investigate the localization and dynamics of this complex in cultured mammalian cells (Stephens et al., 2000). In that work, the function of the SEC24::GFP protein could not be assessed because endogenous wild-type SEC24 was also present; cell lines mutant for SEC24 do not exist. We constructed a C. elegans transgene in which GFP sequences were fused to the 3′ end of the functional sec-23 mini-gene (see MATERIALS AND METHODS) and generated transgenic lines with the balanced sec-23(ij13) mutant strain IA261. The transgene did not rescue the sec-23(ij13) mutant phenotype, indicating that the SEC-23::GFP fusion protein is not fully functional. However, like the mammalian SEC24::GFP fusion reported previously, SEC-23::GFP localized to discrete spots or foci surrounding the ER (Figure 6A). In the C. elegans hypodermis at the time of first cuticle synthesis, an extensive ER network surrounds the hypodermal nuclei and can be observed by immunofluorescence detection of the ER resident enzyme protein disulfide isomerase (PDI-2) (Figure 6B) (Myllyharju et al., 2002). The SEC-23::GFP foci were positioned in a nonrandom manner around the ER network, with the majority of foci on the apical side of the ER networks, closest to the apical surface of the cells (Figure 6, C and D). In deeper focal planes toward the basal side of these cells, there were relatively few foci (Figure 6, E and F). The greater concentration of the SEC-23::GFP foci on the side of the ER that faces the surface from which the cuticle collagens are secreted, at the time of first cuticle synthesis, is unlikely to be coincidental, and it suggests that SEC-23::GFP is incorporated into COPII structures even though it is not fully functional.
Figure 6.
Subcellular localization of a SEC-23::GFP fusion protein. All images are of the same embryo. (A and B) Images in the same focal plane showing SEC-23::GFP with anti-GFP antibody (A) and the ER resident enzyme PDI-2 (B). PDI-2 is detected as a halo surrounding the nuclei, which look like non-stained almost spherical structures. The arrows indicate the position of four hypodermal cells. (C–F) Higher magnification, composite images of the cells indicated in A and B, taken in four different focal planes. (C) Surface of the ER closest to the apical surface of the cells. (D–F) Sequentially deeper focal planes. The nuclei are clearly visualized in D and E but are almost out of vision in the focal plane of F (close to the basal side of the cells).
Postembryonic Reduction of SEC-23 by RNAi
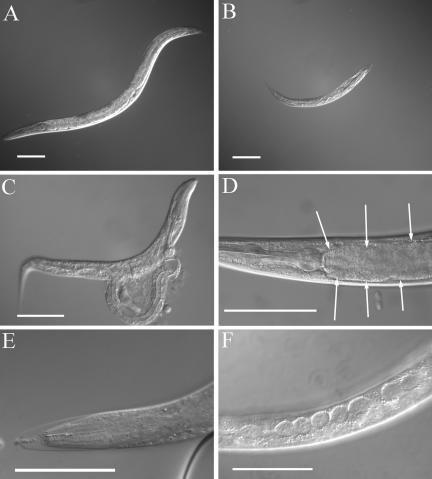
Because sec-23(ij13) has an embryonic-lethal phenotype, the effect of loss or reduction of sec-23 function during postembryonic stages cannot be observed in the mutant. To investigate this, we used sec-23 RNAi by the bacterial feeding method (Timmons et al., 2001). To determine whether sec-23 is required for postembryonic cuticle production, we placed wild-type embryos or mixed stage larvae onto sec-23 RNAi bacterial lawns. These animals went through one or two larval stages before dying as the sec-23 RNAi took effect. They grew very slowly (Figure 7, A and B) and became extremely fragile, frequently rupturing during transfer for microscopy (Figure 7C). They also became constipated, with undigested bacteria in the lumen of the intestine (Figure 7D). Many animals died during cuticle synthesis and displayed molting defects (Figure 7E), and the specialized hypodermal cells termed seam cells became swollen (Figure 7F). The DPY-7 collagen was disorganized in the cuticles of animals that went through a molt (Figure 4, H and I). We conclude that sec-23 is required, among other things, for postembryonic cuticle synthesis and for digestion of food. It is probable that the molting and cuticle-synthesis defects mask other problems. These defects will contribute to the general flaccidity and lethargic behavior that these animals demonstrate before death; however, defects in muscle and neuronal function may contribute also.
Figure 7.
Effects of sec-23 RNAi on larvae. (A) An untreated wild-type L4 larva. (B–F) Larvae subjected to sec-23 RNAi by the bacterial feeding method. (B) An animal of age similar to that in A, showing a lack of growth. (C) An animal that ruptured upon handling, demonstrating a weak cuticle. (D) An accumulation of undigested bacteria (arrows) in the intestine. (E) A molting defect; the head of an animal is stuck inside an old cuticle. (F) Bloated seam cells. Bars, 0.1 mm.
sec-23 Is Required for Oocyte Formation and Function
When sec-23 RNAi was administered to young adult hermaphrodites, they produced a small brood of embryos and then become sterile. Because the gonad was already mature at the time of RNAi, the cause of this sterility is subsequent to gonad development and the generation of spermatocytes. We saw a progression of effects in the mature hermaphrodite gonad as sec-23 RNAi took effect.
The adult C. elegans hermaphrodite gonad is a bilobed organ. The distal region of each lobe, or arm, is a syncytium of germline nuclei, which proliferate mitotically and then enter meiotic prophase. As these nuclei proceed around the turn of the gonad and into the proximal region, they cellularize to produce oocytes, which increase in size as they mature (Figure 8A) (Hirsh et al., 1976; McCarter et al., 1999). Fertilization occurs as the oocytes pass through the spermatheca. During oocyte maturation, yolk (lipids plus lipid-binding proteins) is transported into the oocytes. The yolk is synthesized in the intestine and secreted into the pseudocoelomic space. From there, it passes through pores in the gonadal sheath cells before being taken up into vesicles in the developing oocytes by receptor-mediated endocytosis involving receptors of the LDL (low density lipoprotein) superfamily (Kimble and Sharrock, 1983; Hall et al., 1999).
Figure 8.
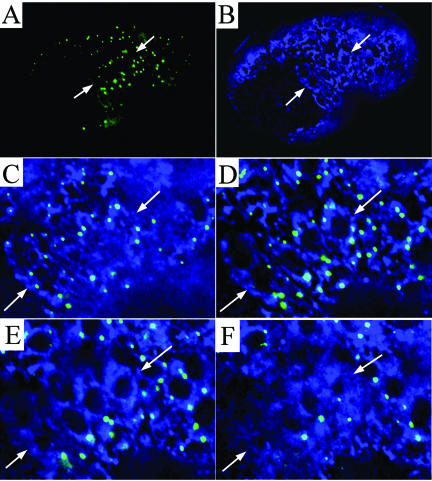
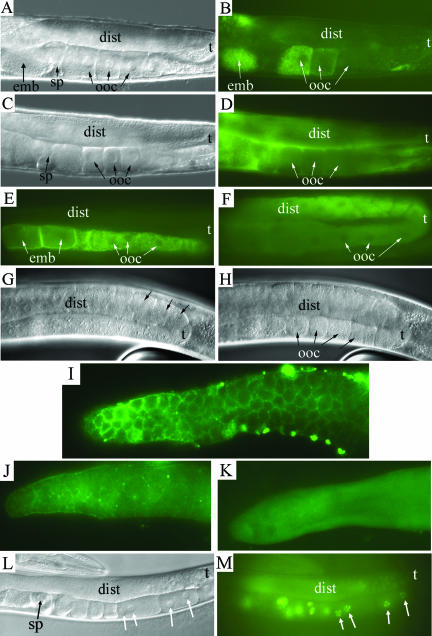
Effect of sec-23 RNAi on the germ-line. Young adult hermaphrodites grown under normal culture conditions were transferred onto sec-23 RNAi bacterial lawns and allowed to feed for 18–48 h. The distal region (dist), turn (t), oocytes (ooc), spermatheca (sp), and in utero embryo (emb) are indicated. (A and B) Nomarski (A) and fluorescence (B) images of an adult hermaphrodite gonad expressing YP170::GFP. (C and D) Like A and B, but of an RNAi treated animal. (E and F) Fluorescence images of untreated (E) and RNAi treated (F) animals showing immunolocalization of the yolk receptor RME-2. (G and H) Nomarski images in two focal planes of an animal treated with RNAi for a few hours longer than those in C–F. (I–K) Fluorescence images of distal gonad arms stained with anti-GLP-1 antibody. Gonads are from an untreated animal (I) or from animals treated during adulthood for 18 h (J) or 36 h (K) with sec-23 bacterial-feeding RNAi. (L and M) Nomarski (L) and fluorescence (M) images of an animal after an RNAi treatment like that of G and H. A histone::GFP protein fusion expressed in the germline from a pie-1::GFP::H2B gene fusion permits visualization of chromatin. Arrows, two binucleate oocytes.
To test whether yolk uptake was affected by sec-23 RNAi, we followed the uptake of a yolk::GFP protein fusion, YP170::GFP (Grant and Hirsh, 1999), in live animals. In the untreated wild-type, accumulation of YP170::GFP was seen in maturing oocytes and in formed embryos (Figure 8, A and B). In RNAi-treated animals, the first effect detected, after ∼18 h, was a failure of oocyte uptake of YP170::GFP and its accompanying accumulation in the pseudocoelom and uterus (Figure 8, C and D). Given the known function of Sec23 in secretory transport between the ER and Golgi, the defect is probably an indirect rather than a direct effect of SEC-23 depletion upon endocytosis.
We next examined the localization of the yolk receptor RME-2 (Grant and Hirsh, 1999) in RNAi-treated animals. In untreated animals, RME-2 was detected only in the proximal region of the gonad in oocytes and young embryos (Figure 8E). As the oocytes mature, the RME-2 receptor becomes concentrated on the oocyte cell membrane. In treated animals, concurrent with the failure of oocyte yolk uptake, we observed two distinct defects in localization of RME-2. First, it did not seem to become concentrated on the oocyte cell membrane but instead remained distributed within the cell body (Figure 8F). It is probable that localization of RME-2 to the cell membrane requires its transport through the secretory apparatus in a SEC-23–dependent manner and that the failure to localize RME-2 properly causes the yolk-uptake failure in RNAi-treated animals. Second, we detected RME-2 throughout both the proximal and distal arms of the gonad in the treated animals (Figure 8F). The presence of RME-2 in the distal gonad suggests premature maturation of the germline nuclei. Consistent with this hypothesis, shortly after the time at which these earliest defects were detected, cellularization of germline nuclei within the distal region of sec-23 RNAi treated animals became evident (Figure 8, G and H).
The switch from the mitotic proliferation of germline nuclei to entry into meiosis and subsequent oocyte maturation is controlled by GLP-1, a NOTCH receptor family member (Crittenden et al., 1994). GLP-1 is normally localized to membranes that partially surround the germline nuclei within the syncytial region of the gonad (Figure 8I). We tested the localization of GLP-1 at various times after administration of sec-23 RNAi. After 18 h, we observed a substantial reduction in the amount of GLP-1 detected in the membranes in the distal arm of the gonads (Figure 8J). After 36 h, no membrane-localized GLP-1 was detected (Figure 8K). Thus, SEC-23 seems to be necessary for the delivery of GLP-1 to the membranes.
The oocytes that formed prematurely as a result of sec-23 RNAi were defective. When they entered into the proximal region of the gonad, they failed to increase in size as they approached the spermatheca, presumably because they failed to take up yolk. They degraded to various extents as they passed through the spermatheca. Also, after prolonged (36–48 h) sec-23 RNAi, we observed the production of some binucleate oocytes (Figure 8, L and M) and of some with no chromatin (our unpublished data). We saw no evidence for endoreduplication as reported previously for a mutant of emo-1, a C. elegans homolog of the Sec61p γ subunit, believed to be involved in protein translocation into the ER (Iwasaki et al., 1996).
DISCUSSION
ECM plays a ubiquitous role in the organization of cells into tissues and organs in all animals. Biosynthesis of the C. elegans cuticle shares many features with that of other animal ECMs. The cells that synthesize the cuticle are an epithelial layer, and the ECM components are sorted and transported in a directional manner, through the apical surface of the cells. The collagen components of this matrix require molecular chaperones for assembly that are known to be involved also in vertebrate collagen folding (Winter and Page, 2000).
The process of eukaryotic secretion is probably best understood in the yeast S. cerevisiae, where it has been the subject of extensive and productive genetic investigation. The synthesis of the C. elegans cuticle provides a model for ECM secretion and assembly that, like yeast, is tractable to genetic analysis. Herein, we have isolated and investigated a mutant in the sec-23 gene of C. elegans, identified from the phenotype of a failure to secrete a functional cuticle. That this central component of the secretory pathway was identified from such a mutant screen was unexpected, because protein secretion is essential for many processes during earlier stages of embryogenesis, including signal transduction required for the specification of cell fate (Kaletta et al., 1997; Rocheleau et al., 1997; Rose and Kemphues, 1998) and the transport of molecules involved in processes such as cell adhesion, gastrulation (Knight and Wood, 1998; Nance and Priess, 2002), and organ morphogenesis. That C. elegans sec-23(ij13) homozygous mutants progress in a relatively normal way through the earlier stages of embryogenesis and undergo substantial morphogenesis is explained by the maternal supply of sec-23 product in the embryo. The earliest zygotic expression of sec-23 that we detected by reporter gene assay is in the cuticle secreting hypodermal cells at about the comma stage of embryogenesis. This coincides with the onset of collagen biosynthesis in the hypodermis for the production of the L1 larval cuticle (Johnstone and Barry, 1996). Also, in the sec-23(ij13) mutant, hypodermal morphogenesis arrests about 1 h after we first detect zygotic sec-23 reporter gene expression in this tissue. Thus, it is likely that the switch from maternal to zygotic sec-23 function occurs in the hypodermis between the comma and two-fold stages of C. elegans embryogenesis.
Two lines of evidence suggest that maternal sec-23 function is adequate until later in development in most other cell types. First, sec-23 reporter gene expression is restricted to the hypodermis until relatively close to hatch when it is detected in many additional cell types. Second, when we restored zygotic sec-23 function only to the hypodermis by driving its expression from a hypodermal specific promoter, these transgenic animals completed elongation, generated the L1 larval cuticle and moved within the egg very much like the wild type. Most failed to hatch, probably because of a failure to secrete enzymes required to weaken the eggshell and permit hatching. The movement of these animals indicates that the body wall muscle is functional and that the muscle is attached through the hypodermis to the cuticle; this is required for animal locomotion. It also indicates that at least those nerves required for movement are functional. When hatching is assisted by mechanical means, they live for 1 or 2 days. Their gradual paralysis and then death is presumably the result of maternal sec-23 function running out in cell types other than the hypodermis.
The quantity of protein that is secreted to synthesize the cuticle is on a greater scale from anything earlier in C. elegans embryonic development. The cuticle contains a significant proportion of the total protein content of the entire animal, yet it is synthesized by an epithelial cell layer only one cell thick. It is probable that the scale of secretion required to synthesize the cuticle explains the earlier requirement for zygotic function of sec-23 in the hypodermis compared with other C. elegans cell types.
Secretion of the cuticle components is directed toward the apical surface of the hypodermal cells. The ER network in these cells surrounds the nuclei, as indicated by the localization of the ER resident enzyme prolyl disulfide isomerase (Figure 6) (Myllyharju et al., 2002). The asymmetric distribution of foci containing the SEC-23::GFP fusion protein during cuticle synthesis in the hypodermal cells, is unlikely to be coincidental. Although the SEC-23::GFP fusion protein cannot substitute for the function of the wild-type SEC-23, it is clearly assembled into ER-associated sites. That these sites are concentrated on the side of the ER network closest to the apical surface of the cells toward which the cuticle components are secreted, may reflect an asymmetric recruitment of COPII components to this side of the hypodermal ER network. It indicates a polarity of the ER network that reflects the apical/basal polarity of these epithelial cells.
We demonstrate retention of the DPY-7 cuticle collagen in a perinuclear location when sec-23 function is removed from the embryo either by mutation or RNAi. Postembryonic RNAi performed on larvae by the feeding method results in cuticle synthesis defects and problems with molting. Animals frequently die while attempting to molt and generate physically weak cuticles as indicated by their fragility. That the apparent cause of death in these larvae is predominantly cuticle synthesis defects is not surprising. Although sec-23 function must be required in most or all cell types, the secretion of a new cuticle at the end of each larval stage presumably places an absolute requirement for a functional secretory pathway at the end of each larval stage. The effect of loss of sec-23 function from other cell types may take longer to become apparent.
Although loss of sec-23 function causes perinuclear retention of the DPY-7 collagen, we did not find a similar retention of the basement membrane protein perlecan, which is synthesized by muscle (Moerman et al., 1996). There are various isoforms of C. elegans perlecan generated by differential splicing of the gene unc-52 (Mullen et al., 1999). The predicted encoded proteins are very large, being ∼2480 residues with estimated molecular mass of ∼273 kDa. In comparison, the DPY-7 procollagen chain that we have demonstrated is secreted in a sec-23–dependent manner is small being ∼30 kDa. There has been speculation as to the mechanism of transport of molecules that are too large to fit within standard COPI- and COPII-coated vesicles. Suggestions include transport via tubules that may extend directly from the ER to Golgi (Bonfanti et al., 1998; Stephens and Pepperkok, 2002), and the possibility that COP carrier structures may have sufficient flexibility to adapt to large cargos (Schekman and Mellman, 1997). The failure to cause retention of perlecan is not restricted to the zygotic sec-23(ij13) mutant where we know maternal sec-23 function is present. In C. elegans, RNAi performed on a mother can remove or reduce both maternal and zygotic gene function from its F1 progeny (Fire et al., 1998). When sec-23 function is depleted by RNAi in this manner, it generates a spectrum of earlier embryonic death, the result of interfering both zygotic and maternal sec-23 function. In these sec-23 RNAi embryos, in all where perlecan is detected it is seen to be secreted. Our inability to cause even partial ER retention of perlecan by sec-23 RNAi, under conditions where most DPY-7 collagen is retained, may suggest perlecan is transported in a sec-23–independent manner. However, because perlecan and the DPY-7 collagen are synthesized by different cell types, we cannot exclude the possibility that there is more residual SEC-23 function in muscle cells than the hypodermis under these conditions of sec-23 RNAi. It is also possible that the absolute levels of collagen secretion during cuticle synthesis are much greater than perlecan secretion from muscle, and that lower levels of SEC-23 function are sufficient to secrete all of the perlecan produced.
Assessment of the effect of the loss of sec-23 function in a developing embryo is complicated by the presence of maternal sec-23. Among the embryos generated by sec-23 RNAi–treated mothers, where both maternal and zygotic gene function may be interfered, are ones that die substantially earlier than the zygotic mutant, some having failed to complete gastrulation indicating that as expected, sec-23 function is required at earlier stages of embryogenesis. We do not see embryos that die with only a few cells present. There are two possible explanations for this that are not mutually exclusive. Either sec-23 function is not required for early cell proliferation in the embryo, although it would presumably be required for cell fate specification; or the complete absence of sec-23 function results in the failure to generate a functional zygote. Our data indicates the latter is true and as a result, we cannot address the former. sec-23 RNAi-treated adult hermaphrodites become sterile, and we show defects in oogenesis, including mislocalization of the egg yolk uptake receptor RME-2 and a failure to uptake egg yolk. These defective oocytes degrade either before or upon entry into the spermatheca. We therefore cannot produce a zygote which we are certain has no sec-23 function.
sec-23 RNAi of adult hermaphrodites also results is a loss of GLP-1 from the membrane that partly surrounds the germline nuclei in the distal arm of the gonad. GLP-1 is a trans-membrane receptor related to NOTCH and is therefore expected to be transported to the cell membrane surface in a COPII-dependent manner. The requirement for GLP-1 function to prevent premature entry into meiosis of germline nuclei, and subsequent cellularization, is well documented (Berry et al., 1997; Seydoux and Schedl, 2001). It is probable that the various features of premature maturation of germ-line nuclei that we observe in the distal arm of the gonad with sec-23 RNAi is a direct result of the loss of GLP-1 function. It is not clear what happens to the GLP-1 protein because we detect no evidence for its retention within the secretory apparatus. It is possible that when transport from the ER to the Golgi is blocked by sec-23 RNAi, that GLP-1 contained within the ER is rapidly degraded.
Although sec-23 and COPII function is required presumably in all cell types, the effect of its reduction at specific developmental stages by RNAi, or loss by mutation cause a variety of developmental defects in C. elegans. The most evident are defects in cuticle synthesis at all stages, defects in oogenesis in the adult hermaphrodite, and failure in digestion resulting in extreme constipation at all postembryonic stages. All of these processes share the common elements of involving a substantial biosynthetic effort and secretion of large amounts of protein.
Acknowledgments
We acknowledge Tim Schedl (Department of Genetics, Washington University School of Medicine) for information on the position of fog-2 before publication. Some C. elegans strains were obtained from the Genetics Stock Center, which is funded by the National Institutes of Health National Center for Research Resources. We thank Robert H. Waterston (Department of Genetics, Washington University School of Medicine), Barth Grant (Department of Molecular Biology and Biochemistry, The State University of New Jersey) and Tim Schedl for reagents and strains. I.L.J. is a Medical Research Council Senior Fellow in Biomedical Sciences.
References
- Albertson, D.G., and Thomson, J.N. (1976). The pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci. 275, 299-325. [DOI] [PubMed] [Google Scholar]
- Anderson, P. (1995). Mutagenesis. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism, ed. H.F. Epstein and D.C. Shakes, San Diego: Academic Press, 31-58.
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII a membrane coat formed by SEC proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895-907. [DOI] [PubMed] [Google Scholar]
- Bednarek, S.Y., Orci, L., and Schekman, R. (1996). Traffic COPs and the formation of vesicle coats. Trends Cell Biol. 6, 468-473. [DOI] [PubMed] [Google Scholar]
- Berry, L.W., Westlund, B., and Schedl, T. (1997). Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124, 925-936. [DOI] [PubMed] [Google Scholar]
- Bloom, L., and Horvitz, H.R. (1997). The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc. Natl. Acad. Sci. USA 94, 3414-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti, L., Mironov, A.A., Martinez-Menarguez, J.A., Martella, O., Fusella, A., Baldassarre, M., Buccione, R., Geuze, H.J., Mironov, A.A., and Luini, A. (1998). Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell 95, 993-1003. [DOI] [PubMed] [Google Scholar]
- Clifford, R., Lee, M.H., Nayak, S., Ohmachi, M., Giorgini, F., and Schedl, T. (2000). FOG-2, a novel F-box containing protein, associates with the GLD-1 RNA binding protein and directs male sex determination in the C. elegans hermaphrodite germline. Development 127, 5265-5276. [DOI] [PubMed] [Google Scholar]
- Crittenden, S.L., Troemel, E.R., Evans, T.C., and Kimble, J. (1994). GLP-1 is localized to the mitotic region of the C. elegans germ line. Development 120, 2901-2911. [DOI] [PubMed] [Google Scholar]
- Fire, A., Harrison, S.W., and Dixon, D. (1990). A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene 93, 189-198. [DOI] [PubMed] [Google Scholar]
- Fire, A., Xu, S.Q., Montgomery, M.K., Kostas, S.A., Driver, S.E., and Mello, C.C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806-811. [DOI] [PubMed] [Google Scholar]
- Francis, R., and Waterston, R.H. (1991). Muscle cell attachment in Caenorhabditis elegans. J. Cell Biol. 114, 465-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard, J.S., Barry, J.D., and Johnstone, I.L. (1997). cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17, 2301-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, B., and Hirsh, D. (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D.H., and Hedgecock, E.M. (1991). Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837-847. [DOI] [PubMed] [Google Scholar]
- Hall, D.H., Winfrey, V.P., Blaeuer, G., Hoffman, L.H., Furuta, T., Rose, K.L., Hobert, O., and Greenstein, D. (1999). Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212, 101-123. [DOI] [PubMed] [Google Scholar]
- Hirsh, D., Oppenheim, D., and Klass, M. (1976). Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49, 200-219. [DOI] [PubMed] [Google Scholar]
- Iwasaki, K., McCarter, J., Francis, R., and Schedl, T. (1996). emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134, 699-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, I.L. (2000). Cuticle collagen genes - expression in Caenorhabditis elegans. Trends Genet. 16, 21-27. [DOI] [PubMed] [Google Scholar]
- Johnstone, I.L., and Barry, J.D. (1996). Temporal reiteration of a precise gene-expression pattern during nematode development. EMBO J. 15, 3633-3639. [PMC free article] [PubMed] [Google Scholar]
- Kaletta, T., Schnabel, H., and Schnabel, R. (1997). Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature 390, 294-298. [DOI] [PubMed] [Google Scholar]
- Kelly, W.G., Xu, S.Q., Montgomery, M.K., and Fire, A. (1997). Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146, 227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble, J., and Sharrock, W.J. (1983). Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev. Biol. 96, 189-196. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell. Biol. 1, 187-198. [DOI] [PubMed] [Google Scholar]
- Kivirikko, K.I., and Pihlajaniemi, T. (1998). Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325-398. [DOI] [PubMed] [Google Scholar]
- Knight, J.K., and Wood, W.B. (1998). Gastrulation initiation in Caenorhabditis elegans requires the function of gad-1, which encodes a protein with WD repeats. Dev. Biol. 198, 253-265. [PubMed] [Google Scholar]
- Kramer, J. M. (1997). Extracellular Matrix. In: C. elegans II, ed. D.L. Riddle, T. Blumenthal, B.J. Meyer, and J.R. Priess, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 471-500. [PubMed]
- McCarter, J., Bartlett, B., Dang, T., and Schedl, T. (1999). On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205, 111-128. [DOI] [PubMed] [Google Scholar]
- McMahon, L., Muriel, J.M., Roberts, B., Quinn, M., and Johnstone, I.L. (2003). Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol. Biol. Cell 14, 1366-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C., and Fire, A. (1995). DNA transformation. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism, ed. H.F. Epstein and D.C. Shakes, San Diego: Academic Press, 451-482.
- Miller, D. M., and Shakes, D. C. (1995). Immunofluorescence microscopy. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism, ed. H.F. Epstein and D.C. Shakes, San Diego: Academic Press, 365-394.
- Moerman, D.G., Hutter, H., Mullen, G.P., and Schnabel, R. (1996). Cell autonomous expression of perlecan and plasticity of cell shape in embryonic muscle of Caenorhabditis elegans. Dev. Biol. 173, 228-242. [DOI] [PubMed] [Google Scholar]
- Mullen, G.P., Rogalski, T.M., Bush, J.A., Gorji, P.R., and Moerman, D.G. (1999). Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol. Biol. Cell 10, 3205-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju, J., Kukkola, L., Winter, A.D., and Page, A.P. (2002). The exoskeleton collagens in Caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J. Biol. Chem. 277, 29187-29196. [DOI] [PubMed] [Google Scholar]
- Nance, J., and Priess, J.R. (2002). Cell polarity and gastrulation in C. elegans. Development 129, 387-397. [DOI] [PubMed] [Google Scholar]
- Ogura, K., Wicky, C., Magnenat, L., Tobler, H., Mori, I., Muller, F., and Ohshima, Y. (1994). Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 8, 2389-2400. [DOI] [PubMed] [Google Scholar]
- Okamoto, H., and Thomson, J.N. (1985). Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous-tissue of the nematode Caenorhabditis elegans. J. Neurosci. 5, 643-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portereiko, M.F., and Mango, S.E. (2001). Early morphogenesis of the Caenorhabditis elegans pharynx. Dev. Biol. 233, 482-494. [DOI] [PubMed] [Google Scholar]
- Priess, J. R., and Hirsh, D. I. (1986). Caenorhabditis elegans morphogenesis - the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117, 156-173. [DOI] [PubMed] [Google Scholar]
- Priess, J.R., and Thomson, J.N. (1987). Cellular interactions in early C. elegans embryos. Cell 48, 241-250. [DOI] [PubMed] [Google Scholar]
- Rocheleau, C.E., Downs, W.D., Lin, R.L., Wittmann, C., Bei, Y.X., Cha, Y.H., Ali, M., Priess, J.R., and Mello, C.C. (1997). Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90, 707-716. [DOI] [PubMed] [Google Scholar]
- Rose, L.S., and Kemphues, K.J. (1998). Early patterning of the C. elegans embryo. Annu. Rev. Genet. 32, 521-545. [DOI] [PubMed] [Google Scholar]
- Schekman, R., and Mellman, I. (1997). Does COPI go both ways? Cell 90, 197-200. [DOI] [PubMed] [Google Scholar]
- Schekman, R., and Orci, L. (1996). Coat proteins and vesicle budding. Science 271, 1526-1533. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., and Schedl, T. (2001). The germline in C. elegans: origins, proliferation, and silencing. Int. Rev. Cytol. 203, 139-185. [DOI] [PubMed] [Google Scholar]
- Shaw, L.M., and Olsen, B.R. (1991). Facit collagens - diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 16, 191-194. [DOI] [PubMed] [Google Scholar]
- Stephens, D.J., Lin-Marq, N., Pagano, A., Pepperkok, R., and Paccaud, J.P. (2000). COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 113, 2177-2185. [DOI] [PubMed] [Google Scholar]
- Stephens, D.J., and Pepperkok, R. (2002). Imaging of procollagen transport reveals COPI-dependent cargo sorting during ER-to-Golgi transport in mammalian cells. J. Cell Sci. 115, 1149-1160. [DOI] [PubMed] [Google Scholar]
- Sulston, J., and Hodgkin, J. (1988). Methods. In: The Nematode Caenorhabditis elegans, ed. W.B. Wood, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 587-606.
- Timmons, L., Court, D., and Fire, A. (2001). Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103-112. [DOI] [PubMed] [Google Scholar]
- Walmsley, A.R., Batten, M.R., Lad, U., and Bulleid, N.J. (1999). Intracellular retention of procollagen within the endoplasmic reticulum is mediated by prolyl 4-hydroxylase. J. Biol. Chem. 274, 14884-14892. [DOI] [PubMed] [Google Scholar]
- Williams, B. D. (1995). Genetic mapping with polymorphic sequence-tagged sites. In: Caenorhabditis elegans: Modern Biological Analysis of an Organism, ed. H.F. Epstein and D.C. Shakes, San Diego: Academic Press, 81-96.
- Williams, B.D., Schrank, B., Huynh, C., Shownkeen, R., and Waterston, R.H. (1992). A genetic-mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131, 609-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, A.D., and Page, A.P. (2000). Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol. Cell. Biol. 20, 4084-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]