Abstract
Background
Patients with Parkinson disease (PD) often have manifestations of autonomic failure. About 40% have neurogenic orthostatic hypotension (NOH), and among PD+NOH patients virtually all have evidence of cardiac sympathetic denervation; however, whether PD+NOH entails extra-cardiac noradrenergic denervation has been less clear.
Methods
Microdialysate concentrations of the main neuronal metabolite of norepinephrine (NE), dihydroxyphenylglycol (DHPG), were measured in skeletal muscle, and plasma concentrations of norepinephrine and DHPG were measured in response to i.v. tyramine, yohimbine, and isoproterenol, in patients with PD+NOH, patients with pure autonomic failure (PAF), which is characterized by generalized catecholaminergic denervation, and control subjects.
Results
Microdialysate DHPG concentrations were similarly low in PD+NOH and PAF, compared to control subjects (163±25, 153±27, and 304±27 pg/ml, p<0.01 each vs. control). The two groups also had similarly small plasma DHPG responses to tyramine (71±58 and 82±105 vs. 313±94 pg/ml; p<0.01 each vs. control) and NE responses to yohimbine (223±37 and 61±15 vs. 672±130 pg/ml, p<0.01 each vs. control) and virtually absent NE responses to isoproterenol (20±34 and 14±15 vs. 336±78 pg/ml, p<0.01 each vs. control). PD+NOH patients had normal bradycardia responses to edrophonium and normal epinephrine responses to glucagon
Conclusions
The results support the concept of generalized noradrenergic denervation in PD+NOH, with similar severity to that seen in PAF. In contrast, the parasympathetic cholinergic and adrenomedullary hormonal components of the autonomic nervous system seem intact in PD+NOH.
Keywords: Dysautonomia, Parkinson's disease, Multiple System Atrophy, Noradrenergic
The movement disorder in Parkinson disease (PD) is well known to result from loss of nigrostriatal dopaminergic neurons; however, there is increasing recognition that PD also entails several non-motor symptoms. About 90% of PD patients complain of symptoms of autonomic nervous system dysfunction,1 and about 40% have orthostatic hypotension.2 Among PD patients in whom orthostatic hypotension is neurogenic (neurogenic orthostatic hypotension, NOH),3 virtually all have a loss of sympathetic noradrenergic nerves in the heart.4, 5 Post-mortem histological studies have confirmed these observations and have consistently demonstrated a profound loss of tyrosine hydroxylase immunoreactive cardiac innervation in patients with PD6, 7 as well as involvement of autonomic ganglia.8, 9
Whether PD+NOH involves generalized noradrenergic denervation has been less clear, and reports on this topic have been inconsistent. PD+NOH patients have statistically decreased 6-[18F]fluorodopamine derived radioactivity in the thyroid gland and the renal cortex,5, 10 partial loss of noradrenergic innervation at these extra-cardiac sites. PD+NOH is also associated with low plasma levels of dihydroxyphenylglycol (DHPG), the main neuronal metabolite of norepinephrine (NE),11 and with blunted blood pressure responses to ganglion blockade with trimethaphan and to alpha-2 adrenoceptor blockade with yohimbine,12 supporting the notion of extra-cardiac noradrenergic denervation. In contrast, the rate of entry of NE into arterial plasma is not decreased in PD,5 and sympathetic denervation, as assessed by 123I-metaiodobenzylguanidine scanning, has been reported to be heart-selective.13
Therefore, in the present study we conducted five separate clinical laboratory tests, to determine whether results by different approaches point consistently to generalized noradrenergic denervation in PD+NOH. Obtaining this information, in conjunction with previous neuroimaging, neurochemical, and neuropharmacologic literature, should enhance understanding of the pathophysiology of autonomic dysfunction in PD+NOH. We hypothesized that PD+NOH and pure autonomic failure (PAF), which is known to involve generalized loss of sympathetic noradrenergic neurons, would involve similarly low values for all five tests (described in detail in the Methods), compared to control values.
Methods
The study protocol was approved by the Intramural Research Board of the National Institute of Neurological Disorders and Stroke. Each subject gave written informed consent. All testing was done in a patient observation room at the National Institutes of Health Clinical Center.
Subjects
We compared PD+NOH patients with two other groups—PAF14 and control. The control group consisted of normal volunteers and referred patients who did not have evidence of NOH or central neurodegeneration. Some patients also participated in previous neuroimaging studies that led to the present protocol.
Orthostatic hypotension was defined as a decrease in systolic BP of at least 20 mm Hg and diastolic BP of at least 10 mm Hg after 5 minutes of upright posture. In our study population, orthostatic hypotension was considered to be neurogenic if the subject were well hydrated and had both a progressive fall in beat-to-beat systolic blood pressure during Phase II of the Valsalva maneuver and absence of an overshoot of blood pressure during Phase IV of the maneuver.3 Virtually all such patients also had subnormal orthostatic increments in plasma norepinephrine levels.
Drugs known to affect sympathetic neuroeffector function, such as tricyclic antidepressants and sympatholytic agents, were discontinued before the testing. All subjects were examined by a board certified neurologist and the referral diagnosis of PD confirmed. PD was diagnosed if the patient had the triad of bradykinesia, resting “pill-roll” tremor, and cogwheel rigidity, developing slowly but progressively, without identified cause; or had two of the triad, developing slowly but progressively over years without identified cause, and reliable improvement in movement by levodopa treatment.
PAF was diagnosed based on a history of persistent, consistent, symptomatic orthostatic hypotension, a low plasma norepinephrine level during supine rest, and no symptoms or signs of central neurodegeneration. NOH was confirmed in all PAF patients, from abnormal blood pressure responses to the Valsalva maneuver.
Different numbers of patients and control subjects underwent neuropharmacologic testing or skeletal muscle microdialysis, as described below.
Autonomic evaluation
Beat-to-beat blood pressure and heart rate were measured noninvasively (Finometer, TNO Biomedical Instrumentation, Amsterdam, The Netherlands). Baroreflex-cardiovagal gain was measured from the slope of the cardiac interbeat interval (with one beat delay) to systolic blood pressure during Phase II of the Valsalva maneuver.15 Supine blood pressure was taken and blood was drawn through an indwelling arm venous catheter, after the patients were recumbent for at least 20 minutes and after 5 minutes of upright posture.
Drug tests
Neuropharmacologic tests assessed hemodynamic and neurochemical responses to tyramine, edrophonium, glucagon, and isoproterenol. The rationale for using each drug is presented below. For all drug tests blood pressure and heart rate were recorded continuously. With the subject supine for at least 15 minutes, blood was sampled, via an indwelling arm i.v. catheter, at baseline and after i.v. drug administration.
Tyramine
Systemic administration of the indirectly acting sympathomimetic amine, tyramine is used as a pharmacological tool to assess sympathetic nervous system function.16-20 Sympathetic nerves take up tyramine into the catecholaminergic vesicles, where tyramine displaces NE. NE that is displaced into the cytoplasm is deaminated enzymatically to form DHPG, which readily crosses the cell membrane and enters the plasma. Plasma DHPG responses to tyramine therefore provide a measure of NE stores.
Tyramine was infused i.v. at 60 cc/hour (1.0 mg/min) for 10 minutes. Blood pressure and plasma DHPG responses to tyramine were measured in a total of 8 patients with PD+NOH, 6 with PAF, and 11 control subjects.
Isoproterenol
Infusion of isoproterenol, a non-selective beta-adrenoceptor agonist, evokes release of NE and increases plasma NE levels,21 via reflexive increases in sympathetic nerve traffic in response to systemic vasodilation and stimulation of beta-2 adrenoceptors on sympathetic nerves.22 The plasma NE response to isoproterenol therefore provides a measure of the ability to release NE by exocytosis from sympathetic nerves.
Isoproterenol was infused i.v. at increasing doses (3.5, 7.0, 14.0, and 35 ng/kg/min), until a stable heart rate increase of about 25 bpm was attained. Hemodynamic and NE responses to isoproterenol were measured for a total of 6 patients with PD+NOH, 4 with PAF, and 12 control subjects.
Yohimbine
Infusion of yohimbine, an alpha-2-adrenoceptor antagonist, increases plasma NE levels via centrally evoked increases in sympathetic nerve traffic and inhibition of alpha-2-adrenoceptors on sympathetic nerves.23 Plasma NE response to yohimbine therefore also provide a measure of the ability to release stored NE by exocytosis. We previously reported attenuated pressor responses to yohimbine in PD+NOH12 but did not report whether PD+NOH patients have subnormal plasma NE responses.
Yohimbine was infused i.v. at 0.065 mg/kg/min over 3 minutes followed by 1.0 microgram/kg/min for 12 minutes. Blood pressure and NE responses to yohimbine were measured in a total of 14 patients with PD+NOH, 11 with PAF, and 17 control subjects.
Edrophonium
Edrophonium (Tensilon™) is a rapidly acting inhibitor of acetylcholinesterase. Systemic injection of edrophonium increases delivery of acetylcholine to its receptors in the heart and thereby decreases heart rate, without importantly affecting other aspects of cardiac function.24 Cardiac parasympathetic denervation would be expected to be associated with absence of a bradycardia response to edrophonium.
A test dose of 0.2 mL (2 mg), then a main dose of 0.8 mL (8 mg), was given as an i.v. bolus, with flushes each time of 5 mL of normal saline i.v. Heart rate recordings were obtained in a total of 7 patients with PD+NOH, 4 with PAF, and 7 control subjects.
Glucagon
Glucagon injection increases plasma epinephrine levels.25 Since adrenomedullary chromaffin cells do not possess glucagon receptors,26 plasma epinephrine response to glucagon result from increased sympathetic preganglionic outflow to the adrenal medulla.
Subjects received 1 mg of glucagon as a bolus i.v. Blood was sampled at baseline and at 1, 2, 3, and 5 minutes after glucagon injection. Plasma levels of epinephrine to glucagon were measured in a total of 6 patients with PD+NOH, 6 with PAF, and 13 control subjects.
Microdialysis
For microdialysis a commercially available probe (CMA 60, CMA Microdialysis AB, Solna, Sweden) was inserted percutaneously into quadriceps skeletal muscle after local anesthesia of the overlying skin, as described previously.27 Normal saline was administered via the probe at a rate of 0.18 ml per hour (3 microliters per minute) in and microdialysate collected in 30-minute aliquots. Microdialysate DHPG concentrations provided a measure of noradrenergic innervation of skeletal muscle. Data for the first collection period were excluded and DHPG levels in subsequent periods averaged.
Data were obtained for a total of 6 patients with PD+NOH, 6 with PAF, and 16 control subjects.
Neurochemical Assays
Plasma catechols were assayed by high-pressure liquid chromatography with electrochemical detection after batch alumina extraction, as was validated previously in our laboratory.28 Since isoproterenol is a catechol, plasma levels of isoproterenol were quantified in the same samples as used for measuring levels of endogenous catechols.21
Statistical Analysis
Comparisons among the subject groups were made using factorial analyses of variance with Fisher's probable least-squares difference post hoc test. For categorical variables, we calculated χ2. For relationships within subjects between numeric variables, we used simple linear regression (StatView 5.01, SAS Institute). Mean values were expressed ± SEM. A P value less than 0.05 defined statistical significance; non-significant results were designated as “ns.”
Results
Baseline
The PD+NOH group was older than the other groups (Table 1). Dysautonomic symptoms were common among the patient groups and included orthostatic intolerance, constipation, urinary difficulties, and sexual dysfunction. Supine blood pressure was higher in the autonomic failure groups than in the control group, with larger falls in blood pressure during the Valsalva maneuver. All patients had low baroreflex-cardiovagal gain. Orthostatic hypotension was not associated with an increase in forearm vascular resistance. Baseline plasma levels of DHPG and NE were lower in PAF than in PD+NOH patients or control subjects.
Table 1. Clinical characteristics, hemodynamics, and plasma catechols at baseline.
| PD+NOH | PAF | Control | |
|---|---|---|---|
| Age years | 71±1* | 62±3 | 60±2 |
| M/F | 25/14 | 11/10 | 34/16 |
| Motor symptoms duration years | 9±1 | - | - |
| SBP mm Hg | 163±5 | 166±9 | 136±6** |
| DBP mm Hg | 85±2.5 | 86±3 | 79±4 |
| HR bpm | 68±2 | 67±3 | 67±3 |
| Orthostasis | |||
| Fall in SBP mm Hg | -55±4 | -73±7 | -2±2** |
| Change in FAVR units | -14±8 | -1±7 | 44±36** |
| Valsalva | |||
| Fall in SBP mm Hg | 90±15 | 58±8 | 34±3** |
| Increase in HR bpm | 11±3 | 6±2 | 14±28 |
| BRS gain mec/mm Hg | 1.1 ±0.3 | 1.5±0.4 | 4.5±0.6** |
| BL NE pg/mL | 261±37 | 91±16† | 302±12 |
| BL DHPG pg/mL | 878±39 | 433±23† | 936±24 |
Notes: Data was recorded while the patients were supine and after 20 minutes of resting recumbent. Abbreviations: PD+NOH=Parkinson disease with neurogenic orthostatic hypotension; PAF=pure autonomic failure; Control =control subjects; BRS = baroreflex gain (msec/mm Hg); SBP = systolic blood pressure (mm Hg); DBP = diastolic blood pressure (mm Hg); HR = heart rate (bpm); FAVR = forearm vascular resistance; NE = norepinephrine (pg/ml); DHPG = dihydroxyphenylglycol (pg/ml).
P<0.05 PD+NOH vs. all other;
P<0.05 Control vs. all other;
P<0.05 PAF vs. all other.
Orthostasis
By definition, systolic blood pressure fell markedly in the PD+NOH and PAF groups during orthostasis. The orthostatic increment in plasma NE levels was attenuated similarly in the two groups (Figure 1).
Figure 1.
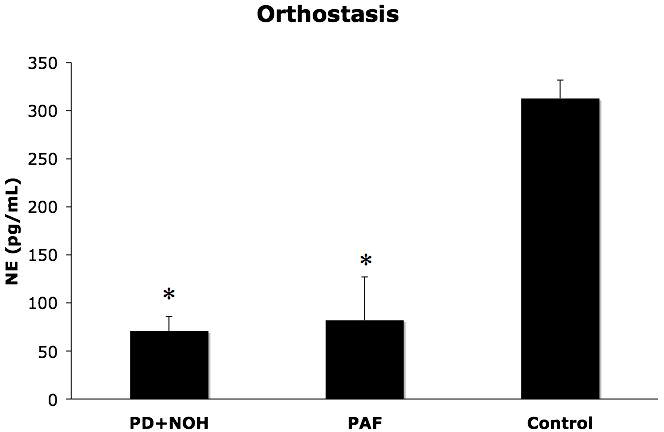
Responses of plasma norepinephrine (NE) levels (means ± SEM) to orthostasis in patients with pure autonomic failure (PAF, N=6), Parkinson disease with neurogenic orthostatic hypotension (PD+NOH, N=8), and control subjects (Control, N=11). Both patient groups had subnormal norepinephrine responses to orthostasis. (*) indicates P<0.05 between PD+NOH vs. the control group.
Tyramine
The PAF group tended to have smaller pressor responses to tyramine than did the control group (p=0.06), whereas the PD+NOH group did not differ from the control group (Table 2). Plasma DHPG responses to tyramine were significantly smaller in the PD+NOH and PAF groups than in the control group (p<0.01 for control vs. both patient groups; Figure 2).
Table 2. Systolic blood pressure responses (mm Hg) to tyramine and isoproterenol.
| PD+NOH | PAF | Control | |
|---|---|---|---|
| Tyramine | 31±6 | 49±8 | 20±6 |
| Isoproterenol | -16±6 | -18±5 | 13±5* |
Notes: Abbreviations as in Table 1.
P<0.05, Control vs. all other. 6 patients with PD+NOH, 4 with PAF, and 12 control subjects performed the Isoproterenol test and 8 patients with PD+NOH, 6 with PAF, and 11 control subjects performed the Tyramine test.
Figure 2.
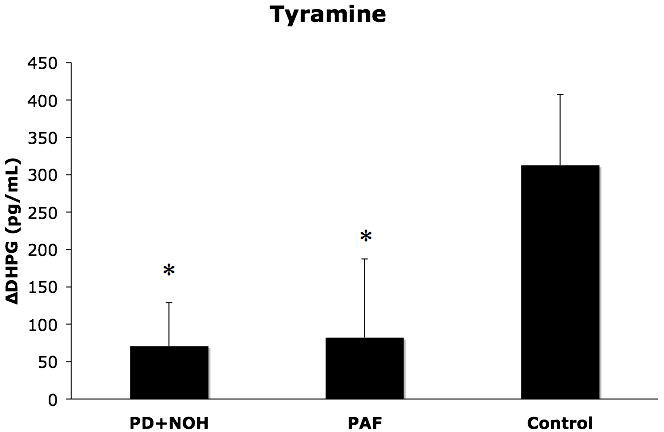
Plasma dihydroxyphenylglycol glycol (DHPG) responses (means ± SEM) to tyramine infusion in patients with pure autonomic failure (PAF, N=6), Parkinson disease with neurogenic orthostatic hypotension (PD+NOH, N=8), and control subjects (Control, N=11). The PAF and PD+NOH group had similarly subnormal DHPG responses to tyramine. (*) indicates P<0.05 between vs. the control group.
Isoproterenol
Isoproterenol infusion increased heart rate in all subjects. Systolic blood pressure increased in the control group but not in the PD+NOH or PAF group (Table 2). The magnitude of increase in plasma NE was less in the PD+NOH (p=0.004) and PAF (p=0.009) groups than in the control group (Figure 3).
Figure 3.
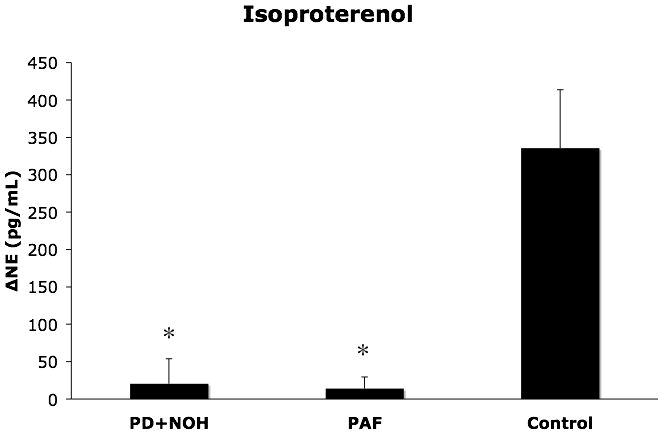
Plasma norepinephrine (NE) responses (means ± SEM) to isoproterenol infusion in patients with pure autonomic failure (PAF, N=4), Parkinson disease with neurogenic orthostatic hypotension (PD+NOH, N=6), and control subjects (Control, N=12). The PAF and PD+NOH groups had similarly subnormal norepinephrine responses to isoproterenol. (*) indicates P<0.05 vs. the control group.
Yohimbine
The PAF and PD+NOH groups had attenuated plasma NE responses to yohimbine, compared to responses in the control group (p=0.0001, p=0.003; Figure 4). The groups did not differ in systolic pressure responses to yohimbine.
Figure 4.
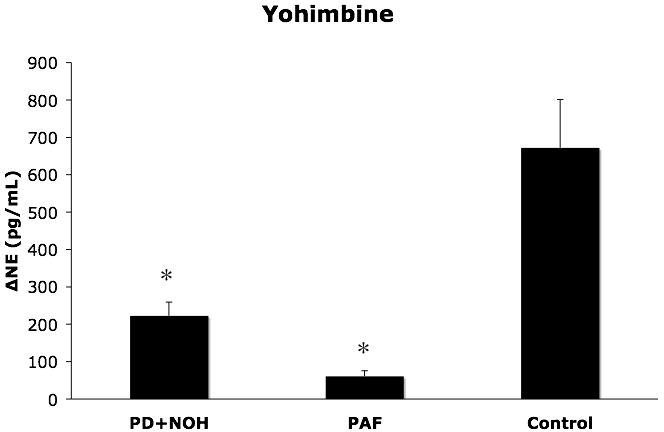
Plasma norepinephrine (NE) responses (means ± SEM) to yohimbine infusion in patients with pure autonomic failure (PAF, N=11), Parkinson disease with neurogenic orthostatic hypotension (PD+NOH, N=14), and control subjects (Control, N=17). The PAF and PD+NOH groups had similarly subnormal norepinephrine responses to yohimbine. (*) indicates P<0.05 vs. the control group.
Microdialysate Catechols
PD+NOH patients had skeletal muscle microdialysate concentrations of DHPG that were similar to those in PAF patients and lower than in control subjects (p<0.01 for control vs. both patient groups; Figure 5)
Figure 5.
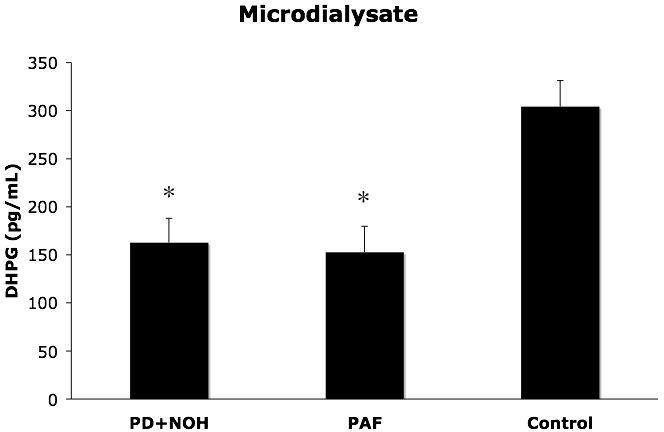
Skeletal muscle microdialysate concentrations (means ± SEM) of dihydroxyphenylglycol glycol (DHPG) in patients with pure autonomic failure (PAF, N=6), Parkinson disease with neurogenic orthostatic hypotension (PD+NOH, N=6), and control subjects (Control, N=12). The PAF and PD+NOH groups had similarly subnormal microdialysate DHPG levels. (*) indicates P<0.05 vs. the control group
Edrophonium
Maximum bradycardia responses to edrophonium were similar in the PD+NOH, PAF, and control groups (8±2 bpm, 2±1 bpm and 7±1 bpm, P=ns).
Glucagon
Glucagon injection increased plasma epinephrine levels similarly in the PD+NOH (35±16 pg/ml), PAF (71±59 pg/ml), and control (38±19 pg/ml) groups.
Discussion
The neurochemical findings in this study support the concept of generalized noradrenergic denervation in PD+NOH. The extent of loss of noradrenergic innervation seems similar in severity to that in PAF, since the PD+NOH and PAF groups had about the the same extent of attenuation of plasma DHPG responses to tyramine and of NE responses to isoproterenol and yohimbine and had similarly low skeletal muscle microdialysate DHPG concentrations. These neurochemical profiles differed clearly from those in the control group. The neurochemical findings fit with previous reports of low plasma concentrations of both NE29 and DHPG30 in PD+NOH and with neuroimaging evidence of reduced renal cortical 6-[18F]fluorodopamine-derived31 and thyroid 123I-meta-iodobenzylguanidine-derived32 radioactivity.
Neurochemical evidence for noradrenergic denervation in PD+NOH was not accompanied by attenuated pressor responses to tyramine. This lack of association might be explained by adaptive up-regulation of adrenoceptor-mediated processes to the loss of sympathetic nerves, as has been noted for both alpha-adrenoceptors29 and beta-adrenoceptors.33
We have previously shown that the sympathetic lesion in PD+NOH is neurotransmitter-specific,34 since PD+NOH patients have normal results of the quantitative sudomotor axon reflex test, indicating intact cutaneous sympathetic cholinergic innervation. The present finding of normal bradycardia responses to edrophonium suggests generally intact parasympathetic cholinergic innervation of the heart. The autonomic lesion in PD+NOH therefore does not seem to entail as severe loss of cardiac or extra-cardiac cholinergic as noradrenergic innervation.
PD+NOH patients had approximately normal increases in plasma epinephrine levels in response to glucagon, indicating intact adrenomedullary secretory responses. This finding is in line with a previous report describing normal plasma epinephrine and metanephrine levels in PD+NOH.30 We infer that the peripheral catecholaminergic lesion in PD+NOH is more prominent in noradrenergic than in adrenergic cells. Bases for this distinction remain unknown.
Since attenuation of plasma NE responses to orthostasis might reflect baroreflex failure, such attention does not necessarily indicate generalized noradrenergic denervation. This limitation highlights the importance of neuropharmacologic tests results to test the notion of generalized noradrenergic denervation in PD+NOH.
The similarities noted here between PD+NOH and PAF suggest that these conditions lie along a spectrum of the same pathogenetic process. Support for this concept comes from reports that both diseases feature Lewy bodies in the substantia nigra and sympathetic ganglia,8, 9, 35 decreased 6-[18F]fluorodopamine-derived radioactivity in the left ventricular myocardium and renal cortex,31 virtually absent tyrosine hydroxylase immunoreactivity in epicardial nerves,6, 36 and decreased 6-[18F]fluorodopa-derived radioactivity in the substantia nigra.35 Nevertheless, the diseases do seem to differ in other features. PAF entails decreased adrenomedullary secretion of epinephrine, and PD+NOH does not.30 PAF patients have normal striatal 6-[18F]fluorodopa-derived radioactivity, in marked contrast to decreased radioactivity in PD with or without NOH.37 So far we have not observed any downward trend over time in striatal 6-[18F]fluorodopa-derived radioactivity in PAF patients. The presence of normal striatal dopaminergic innervation in PAF suggests a pathogenetic mechanism somewthat different from that in PD; however, long-term follow-up data comparing the two conditions seem necessary.
Parkinsonism with NOH occurs in multiple system atrophy (MSA), which can mimic PD+NOH clinically. Many neurochemical, neuropharmacologic, neuroimaging, and post-mortem pathology studies have agreed, however, that most patients with MSA have normal noradrenergic innervation,7, 12, 38, 39 with occasional exceptions.40, 41
Our study has several limitations. This study did not report on the neurochemical and neuropharmacologic profile of PD patients who do not have NOH. This issue is somewhat separate from the hypothesis the study was designed to address. PD patients without NOH are heterogeneous; some have evidence for loss of cardiac and extra-cardiac noradrenergic innervation, others have evidence for cardiac denervation in the inferolateral or apical wall only, and others have no evidence for loss of noradrenergic innervation at all. Because the results in patients with PD and no NOH might be variable, we decided that to test for generalized noradrenergic denervation in PD+NOH it would be more appropriate to compare this group with a non-parkinsonian control group.
The finding of a progressive decline in blood pressure during Phase II of the Valsalva maneuver does not necessarily imply a neurogenic basis of OH, because this abnormality can be seen in other conditions, such as dehydration. The combination of abnormal blood pressure in both Phases II and IV, consistent, persistent OH, and a small orthostatic increment in plasma NE in patients who are well hydrated and off medications that could affect neurocirculatory reflexes in our view does support a diagnosis of NOH.
The PD+NOH group was older than either the PAF or control group, and for most of the tests employed in this study there are no normative data to correct for effects of aging. Sympathetic neuronal outflows increase with normal aging, and responses to manipulations of sympathetic neuronal outflows are if anything augmented.42, 43 Age mismatching therefore would not be expected to explain the large obtained differences between the PD+NOH and control groups. The numbers of patients studied using any particular manipulation were relatively small; however, this deficiency was offset by remarkably consistent results across a variety of testing modalities.
We conclude that PD+NOH entails a loss of extra-cardiac noradrenergic nerves that in most respects is about as severe as in PAF. In contrast, the sympathetic cholinergic, parasympathetic cholinergic, and adrenomedullary hormonal components of the autonomic nervous system seem intact. The stage is now set for biopsy studies of accessible tissues in patients with PD+NOH, to confirm directly the neuropharmacologic and neurochemical findings reported here.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NINDS. We thank Dr. Basil Eldadah for assistance in carrying out the many clinical procedures that were crucial for the present report.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Semin Neurol. 2003;23:351–363. doi: 10.1055/s-2004-817719. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson disease. Clin Auton Res. 2006;16:46–64. doi: 10.1007/s10286-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–291. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Holmes C, Cannon RO, III, et al. Sympathetic cardioneuropathy in dysautonomias. N Engl J Med. 1997;336:696–702. doi: 10.1056/NEJM199703063361004. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS, Holmes C, Li ST, et al. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 6.Orimo S, Oka T, Miura H, et al. Sympathetic cardiac denervation in Parkinson's disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orimo S, Ozawa E, Oka T, et al. Different histopathology accounting for a decrease in myocardial MIBG uptake in PD and MSA. Neurology. 2001;57:1140–1141. doi: 10.1212/wnl.57.6.1140. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson's disease. Eur Neurol. 1997;38 2:2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 9.Rajput AH, Rozdilsky B. Dysautonomia in Parkinsonism: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1976;39:1092–1100. doi: 10.1136/jnnp.39.11.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein DS, Holmes C, Dendi R, et al. Orthostatic hypotension from sympathetic denervation in Parkinson's disease. Neurology. 2002;58:1247–1255. doi: 10.1212/wnl.58.8.1247. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Eldadah BA, Holmes C, et al. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension. Independence from levodopa treatment. Hypertension. 2005;46:1–7. doi: 10.1161/01.HYP.0000188052.69549.e4. [DOI] [PubMed] [Google Scholar]
- 12.Sharabi Y, Eldadah B, Li ST, et al. Neuropharmacologic distinction of neurogenic orthostatic hypotension syndromes. Clin Neuropharmacol. 2006;29:97–105. doi: 10.1097/01.WNF.0000220822.80640.0D. [DOI] [PubMed] [Google Scholar]
- 13.Taki J, Nakajima K, Hwang EH, et al. Peripheral sympathetic dysfunction in patients with Parkinson's disease without autonomic failure is heart selective and disease specific. Eur J Nucl Med. 2000;27:566–573. doi: 10.1007/s002590050544. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama M, Hakusui S, Koike Y, et al. A scintigraphical qualitative analysis of peripheral vascular sympathetic function with meta-[123I]iodobenzylguanidine in neurological patients with autonomic failure. J Auton Nerv Syst. 1995;53:230–234. doi: 10.1016/0165-1838(95)00002-f. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66:432–439. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- 16.Bianchetti MG, Minder I, Beretta-Piccoli C, et al. Effects of tyramine on blood pressure and plasma catecholamines in normal and hypertensive subjects. Klin Wochenschr. 1982;60:465–470. doi: 10.1007/BF01720361. [DOI] [PubMed] [Google Scholar]
- 17.Jacob G, Costa F, Vincent S, et al. Neurovascular dissociation with paradoxical forearm vasodilation during systemic tyramine administration. Circulation. 2003;107:2475–2479. doi: 10.1161/01.CIR.0000065605.37863.C0. [DOI] [PubMed] [Google Scholar]
- 18.Jacob G, Gamboa A, Diedrich A, et al. Tyramine-induced vasodilation mediated by dopamine contamination: a paradox resolved. Hypertension. 2005;46:355–359. doi: 10.1161/01.HYP.0000172353.62657.8b. [DOI] [PubMed] [Google Scholar]
- 19.Charkoudian N, Joyner MJ, Sokolnicki LA, et al. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meck JV, Martin DS, D'Aunno DS, Waters WW. Pressor response to intravenous tyramine is a marker of cardiac, but not vascular, adrenergic function. Journal of cardiovascular pharmacology. 2003;41:126–131. doi: 10.1097/00005344-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DS, Zimlichman R, Stull R, Keiser HR. Plasma catecholamine and hemodynamic responses during isoproterenol infusions in humans. Clin Pharmacol Ther. 1986;40:233–238. doi: 10.1038/clpt.1986.168. [DOI] [PubMed] [Google Scholar]
- 22.Majewski H, Hedler L, Starke K. Evidence for a physiological role of presynaptic alpha-adrenoceptors: modulation of noradrenaline release in the pithed rabbit. Naunyn Schmiedeberg's Arch Pharmacol. 1983;324:256–263. doi: 10.1007/BF00502620. [DOI] [PubMed] [Google Scholar]
- 23.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol. 1991;260:R142–R147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JA, Baratta F, Frieden J, Grossman J. Hemodynamic effects of edrophonium chloride (Tensilon) infusion. Chest. 1975;67:147–151. doi: 10.1378/chest.67.2.147. [DOI] [PubMed] [Google Scholar]
- 25.Sharabi Y, Goldstein DS, Bentho O, et al. Sympathoadrenal function in patients with paroxysmal hypertension: pseudopheochromocytoma. J Hypertens. 2007;25:2286–2295. doi: 10.1097/HJH.0b013e3282ef5fac. [DOI] [PubMed] [Google Scholar]
- 26.Sharabi Y, Zimlichman R, Alesci S, et al. Glucagon does not affect catecholamine release in primary cultures of bovine adrenal chromaffin cells. Horm Metab Res. 2005;37:205–208. doi: 10.1055/s-2005-861415. [DOI] [PubMed] [Google Scholar]
- 27.Bruce S, Tack C, Patel JN, et al. Local sympathetic function in human skeletal muscle and adipose tissue assessed by microdialysis. Clin Auton Res. 2002;12:13–19. doi: 10.1007/s102860200005. [DOI] [PubMed] [Google Scholar]
- 28.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatog B Biomed Applic. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 29.Senard JM, Valet P, Durrieu G, et al. Adrenergic supersensitivity in parkinsonians with orthostatic hypotension. European journal of clinical investigation. 1990;20:613–619. doi: 10.1111/j.1365-2362.1990.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS, Holmes C, Sharabi Y, et al. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 31.Tipre DN, Goldstein DS. Cardiac and extra-cardiac sympathetic denervation in Parkinson disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med. 2005;46:1775–1781. [PubMed] [Google Scholar]
- 32.Matsui H, Udaka F, Oda M, et al. Metaiodobenzylguanidine (MIBG) uptake in Parkinson's disease also decreases at thyroid. Ann Nucl Med. 2005;19:225–229. doi: 10.1007/BF02984609. [DOI] [PubMed] [Google Scholar]
- 33.Milon D, Allain H, Bentue-Ferrer D, et al. Cardiac beta-adrenoceptor sensitivity and Parkinson's disease. Fundam Clin Pharmacol. 1991;5:539–548. doi: 10.1111/j.1472-8206.1991.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharabi Y, Li ST, Dendi R, et al. Neurotransmitter specificity of sympathetic denervation in Parkinson's disease. Neurology. 2003;60:1036–1039. doi: 10.1212/01.wnl.0000052690.91036.ff. [DOI] [PubMed] [Google Scholar]
- 35.Hague K, Lento P, Morgello S, et al. The distribution of Lewy bodies in pure autonomic failure: autopsy findings and review of the literature. Acta Neuropathol (Berl) 1997;94:192–196. doi: 10.1007/s004010050693. [DOI] [PubMed] [Google Scholar]
- 36.Amino T, Orimo S, Takahashi A, et al. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Path. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DS, Holmes C, Sato T, et al. Central dopamine deficiency in pure autonomic failure. Clin Auton Res. 2008 doi: 10.1007/s10286-008-0457-0. in press. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein DS, Polinsky RJ, Garty M, et al. Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol. 1989;26:558–563. doi: 10.1002/ana.410260410. [DOI] [PubMed] [Google Scholar]
- 39.Polinsky RJ, Kopin IJ, Ebert MH, Weise V. Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology. 1981;31:1–7. doi: 10.1212/wnl.31.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Raffel DM, Koeppe RA, Little R, et al. PET measurement of cardiac and nigrostriatal denervation in parkinsonian syndromes. J Nucl Med. 2006;47:1769–1777. [PubMed] [Google Scholar]
- 41.Orimo S, Kanazawa T, Nakamura A, et al. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol (Berl) 2007;113:81–86. doi: 10.1007/s00401-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 42.Esler MD, Turner AG, Kaye DM, et al. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- 43.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


