Abstract
Extracellular signal-regulated kinase (ERK) participates in numerous cellular functions including circadian-related activities. In the retina, the activity of ERK is under circadian control. However, it is not clear whether acute illumination changes or the circadian clocks in the retina have a larger impact on ERK activity, and the cellular distribution of activated ERK (pERK) as a function of circadian time in cone photoreceptors is not known. Chick embryos were exposed to the light or dark for various lengths of time after 12:12 h light–dark (LD) cycles, or on the second day of constant darkness after LD entrainment. Retinas were excised after various exposure times and relative ERK activity was determined by western immunoblotting. We also performed immunohistochemical and immunocytochemical stainings on circadian entrained retina sections and dissociated retina cells. There is about a fourfold difference in ERK activity between retinas harvested at circadian time (CT) 4 and CT 16, and the internal circadian control of ERK activity in the retina overcomes external light exposure. Also, during the subjective night, pERK was more apparent in the outer segment of cones, while pERK distribution was more uniform throughout the photoreceptors during the subjective day. Our results imply that the activity of retinal ERK is influenced more by circadian oscillators than acute illumination changes. Hence, the circadian oscillators in retina photoreceptors play a major role in the regulation of photoreceptor physiology, which leads to the circadian control of light sensitivity in photoreceptors.
Keywords: Circadian, Photoreceptor, Signal transduction, Adaptation
Signaling through extracellular signal-regulated kinase (ERK) participates in numerous cellular functions ranging from cell division [20] to apoptosis [14]. In the retina, the ERK pathway is critical in postnatal development and visual system maturation [33] as well as responses to environmental stimuli. Inhibition of MAPK decreases neurite elongation in cultured retinal explants [40], reduces ultra violet light-induced apoptosis [38], and diminishes blue light-induced increases of rhodopsin expression in zebrafish photoreceptors [46].
The ERK pathway also partakes in many circadian activities. Visual systems anticipate daily changes in ambient illumination that can be over 10–12 orders of magnitude, and circadian oscillators in the retina provide a mechanism for visual systems to initiate more sustained adaptive changes throughout the course of the day [10,16]. The circadian oscillators in photoreceptors are endogenous, and a vast array of photoreceptor physiology and functions are under the control of these oscillators, including retinomotor movement [7,31], outer segment disc shedding and membrane renewal [5,29], ribbon synapse changes [3], photopigment gene expression [18,26,34], and the activities of ion channels [23–25]. Importantly, photoreceptors are more sensitive to intense light damage at night than during the day [41]. The activity of ERK is high during the subjective day and low during the subjective night in the suprachiasmatic nuclei (SCN) of nocturnal rodents [32,35], but is reversed in the pineal glands and retinas of diurnal species [19,23,39]. Light exposure during the subjective night induces ERK phosphorylation in rodents, and pharmacological inhibition or constitutive activation of the ERK pathway attenuates photic-induced phase-shifting without affecting the core oscillators in the SCN in these animals [8,9,13,15,17,32,35]. Genetically altered ERK signaling does not influence core oscillator genes but produces abnormalities of circadian rhythms in Drosophila locomotor activity [44]. These reports reveal the complexity of ERK signaling in the involvement of both circadian input and output pathways.
Previously, we established that there is a circadian regulation of ERK activity in chick retinas [23] with ERK activity high during the subjective night and low during the subjective day. The signaling through Ras-ERK regulates the affinity rhythm of cGMP-gated ion channels and expression of L-type voltage-gated calcium channels in cone photoreceptors [23–25]. In the present study, we found that the activity of ERK was influenced more by the circadian oscillators than acute changes in illumination. Phosphorylated ERK (pERK; activated ERK) was at least fourfold greater during the subjective night compared to the subjective day. In constant darkness after circadian entrainment, acute light exposure at either CT 4 or CT 16 did not affect pERK expression. However, when there was an acute illumination change in embryos that were never exposed to light, the pERK levels roughly doubled over 2 h. The distribution of pERK in cone photoreceptors was also under circadian control. During the subjective night, pERK was more pronounced in the outer segment than the inner segment, while pERK distribution became more uniform throughout the photoreceptors during the subjective day. Thus, the circadian rhythm of pERK may underlie the daily regulation of retina photoreceptor physiology.
Fertilized eggs (Gallus gallus) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX). Chick embryos from embryonic day 11 (E11) were entrained to 12:12 h light–dark (LD) cycles at 39 °C in ovo, and Zeitgeber time zero (ZT 0) was designated as when the lights come on, and ZT 12 was the time when the lights turn off. After in ovo LD entrainment for 6 days, eggs were kept in constant darkness (DD) for another day. On the second day of DD, retinas were dissected out at various circadian times (CT) of the day. For some experiments, chick retinas were dissociated at E11 and cultured on glass coverslips for 6–7 days as described previously [22–25]. Cultures prepared in this way in the presence of ciliary neurotrophic factor (R&D Systems, Minneapolis, MN) yield a highly enriched population of cone photoreceptors [1,2,4]. Cell culture incubators (maintained at 39 °C and 5% CO2) were equipped with lights and timers, which allowed for the entrainment of retinal circadian oscillators to LD cycles in vitro for 5 days, after which the cultures were kept in DD for another day. On the second or third day of DD, cultured photoreceptors were processed for immunocytochemistry.
To study acute light or dark adaptation, chick embryos were either exposed to light (at ZT 16) or dark (at ZT 4) for 0, 5, 15, 30, 45, 60, and 120 min after several days of LD entrainment. In other experiments, chick embryos were exposed to the light for various lengths on the second day of DD at CT 4 (subjective day) or CT 16 (subjective night). The retinas were excised at different exposure times, washed in ice-cold PBS, and lysed in RIPA buffer [23]. The samples were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. The primary antibodies used in this study were a monoclonal antibody specific for di-phospho-extracellular signal-related kinase (pERK; Sigma, St. Louis, MO) and a polyclonal antibody insensitive to the phosphorylation state of ERK (total ERK, used for loading control, Santa Cruz Biochemicals, Santa Cruz, CA). Blots were visualized using anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology, Danvers, MA) and an ECL detection system (Pierce, Rockford, IL). The ratio of pERK to total ERK in each sample was determined by densitometry using Scion Image (NIH, Bethesda, MD). All measurements were repeated six to eight times.
For immunohistochemical stainings, on the second day of DD at CT 4 and CT 16, the eyes were dissected and fixed with Zamboni’s fixative (Newcomer Supply, Middleton, WI) in phosphate buffer (PB; 0.1 M, pH 7.4) overnight at 4 °C followed by submersion in series of gradient sucrose-PBS for cryo-protection. One whole eye section (15 μm) from CT 4 and CT 16 were mounted on the same glass slide. Whole eye sections were incubated with anti-pERK (1:200, Sigma) antibody in 1% normal goat serum at 4 °C overnight. Sections were then washed in PBS several times and incubated with a fluorescence conjugated secondary antibody (Alexa 488 nm goat anti-rabbit, 1:1500 dilution; Molecular Probes, Carlsbad, CA) for 2 h in the dark. After several washes, coverslips were mounted and stored at 4 °C for later observation on a Zeiss microscope with epi-fluorescence to determine the distribution of pERK. The images taken from CT 4 and CT 16 were under identical conditions including illumination intensities, exposure time, and magnification.
For immunocytochemical stainings, cultured cells on glass coverslips were fixed with Zamboni’s fixative in PB for 30 min at room temperature followed by three washes in PB containing 0.1% Triton-X, a blocking step with 0.1% triton-X/PB containing 3% normal goat serum for an hour, and then the cells were incubated with two primary antibodies, anti-L-type voltage gated calcium channel α1D (VGCCα1D, 1:100; Alomone Labs, Jerusalem, Israel) and anti-pERK (1:200) overnight. The cells were washed with PB and incubated with fluorescent conjugated secondary antibodies (Alexa 488 nm goat anti-rabbit and Alexa 594 nm goat anti-mouse) in PB containing 1.5% normal goat serum for 1 h in the dark. After washing in PB, coverslips were prepared as above for later observation on a Zeiss microscope with epi-fluorescence to determine the localization of VGCCα1D and pERK. A pair of coverslips from CT 4 and CT 16 along with controls (omitted primary antibodies substituted with the appropriate antiserums) was processed concurrently for each experiment. A single fluorescence image from CT 4, CT 16, or control was taken under identical settings, including exposure time and magnification. The averaged fluorescence intensities per pixel (F) of the outlined structures were analyzed without any modification using the luminosity channel of the histogram function in Adobe Photoshop 6.0 software, and the green or red fluorescence intensity was measured on a scale of 0–255. Three cells (F1, F2, and F3) from each experiment were measured. The background fluorescence intensity was acquired from an adjacent area without any cells (B). The relative fluorescence intensity from one single image of a particular time point (T) was converted as: T = [(F1 − B) + (F2 − B) + (F3 − B)]/3. All of the data are presented as mean ± S.E. (standard error). Student’s t-test or one-way ANOVA followed by Tukey’s post hoc test for unbalanced n were used for statistical analysis. Throughout, *p < 0.05 was regarded as significant.
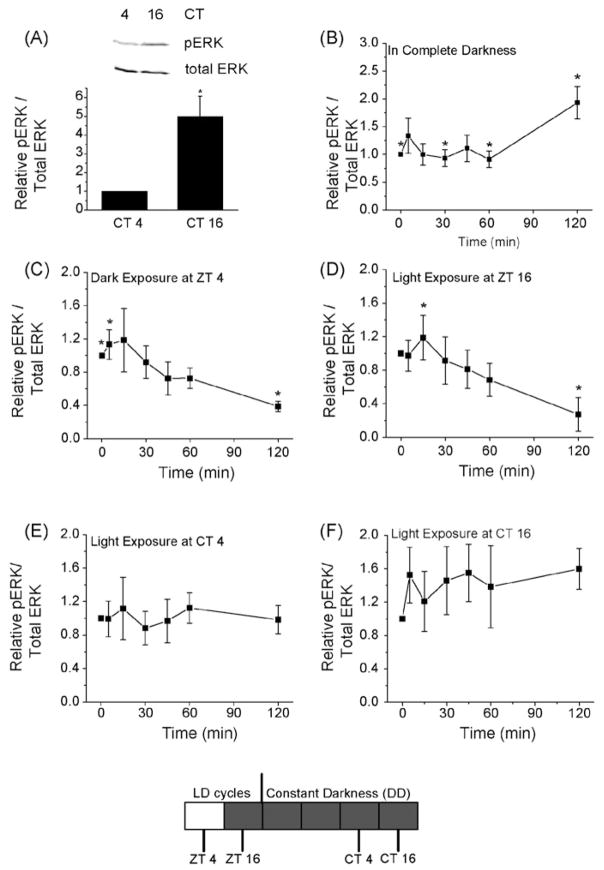
In chick embryos that were entrained to LD cycles and kept in DD for 2 days (in free-running rhythm), we found that the relative expression of pERK in retinas harvested at CT 16 was at least four times higher than CT 4 retinas (Fig. 1A), which was consistent with our previous reports in younger embryos [23,24]. In chick embryos that were never exposed to light before, brief exposure to light for 2 h increased pERK expression (Fig. 1B), which was similar to other reports where exposure to light for 12 h causes an up-regulation of pERK in rat retinas [30], and exposure to light for 4 h causes an up-regulation of pERK in cultured photoreceptors [45].
Fig. 1.
Effects of acute changes in illumination on ERK phosphorylation depend on previous light-experience and circadian oscillators in the retinas. (A) The pERK levels of CT 16 retinas was at least four times than CT 4 retinas; chick embryos were entrained in LD cycles and kept in DD for 2 days; n = 4 for each group. *p < 0.05, Student’s t-test. (B) Chick E18 embryos that were continuously kept in compete darkness were exposed to light for 0, 5, 15, 30, 45, 60, and 120 min (min). Brief exposure to light for 120 min significantly increased the pERK expression compared with other exposure time periods 0, 30, or 60 min. *p < 0.05; n = 6–9 for each group. (C and D) E18 embryos were under LD cycles for 7 days. At ZT 4 (4 h after lights on) or ZT 16 (4 h after lights off), embryos were exposed to the dark or light briefly for 0, 5, 15, 30, 45, 60, and 120 min. (C) Exposure to the dark for 120 min at ZT 4 caused a significant decrease of pERK compared with 0 and 5 min dark exposure. *p < 0.05; n = 6–8 for each group. (D) Exposure to light for 120 min at ZT 16 caused a significant decrease of pERK compared with 15 min light exposure. *p < 0.05; n = 6–7 for each group. (E and F) Chick embryos were entrained in LD cycles and kept in DD for 2 days. On the second day of DD at CT 4 and CT 16, embryos were exposed to light for 0, 5, 15, 30, 45, 60, and 120 min. At both CT 4 (E) and CT 16 (F), brief light exposure did not have any effect on pERK. n = 6–7 for each group. The bottom panel shows the different experimental paradigms from (C) to (F).
Interestingly, in LD-entrained E18 embryos that were not transferred to constant darkness (DD), exposure to complete darkness at ZT 4 (4 h after lights on) for 2 h caused a significant decrease in pERK (Fig. 1C), while exposure to light at ZT 16 (4 h after lights off) for 2 h also caused a down-regulation of pERK (Fig. 1D). Brief light exposure in embryos under free-running rhythm during the subjective day (CT 4) or the subjective night (CT 16) did not have any significant effect on the phosphorylation of ERK (Fig. 1E and F), while pERK was significantly higher at CT 16 than CT 4 without light exposure as presented earlier (Fig. 1A).
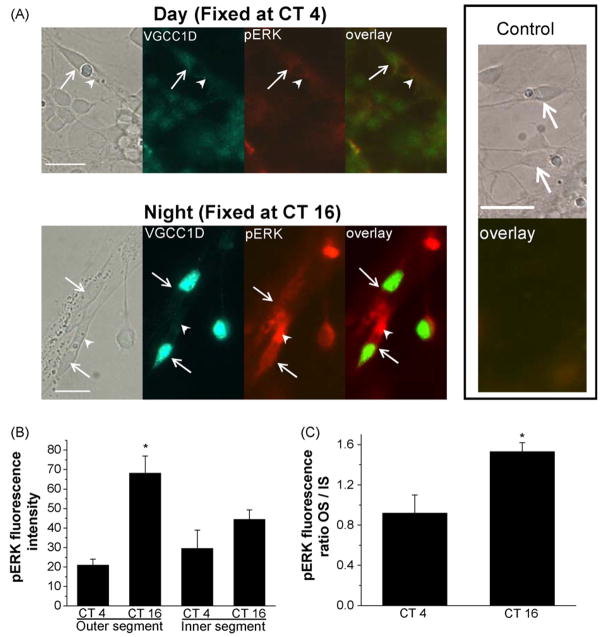
The pERK immuno-staining intensity was higher in retina tissue fixed at CT 16 than tissue fixed at CT 4 (Fig. 2). In our double immunostaining with anti-VGCCα1D and anti-di-phorphorylated ERK (pERK) antibodies of cultured retinal cells, we found that pERK was present in all cells (including glia cells, neurons, and fibroblasts, Fig. 3A). In chick retina cones, oil droplets are present at the base of the outer segment, thereby providing a convenient marker to differentiate outer segments from inner segments and cell bodies. As we reported previously, VGCCα1D is primarily present in neurons and in the cell bodies and inner segments of photoreceptors but not glia cells ([25]; Fig. 3A). Here, we show the immunostaining of VGCCα1D as a reference for photoreceptor cell bodies. In photoreceptors, pERK was distributed in outer and inner segments and cell bodies. However, the fluorescence intensity was higher in cells fixed at CT 16. Interestingly, there was more pERK fluorescence intensity present in the outer segments (OS) than inner segments-cell bodies (IS) of the photoreceptors fixed at CT 16, while pERK appeared to be less intense and distributed more evenly in photoreceptors fixed at CT 4 (Fig. 3A). Further analysis of fluorescence intensities of pERK in the inner and outer segments revealed that pERK intensity was significantly stronger in cells fixed at CT 16 than at CT 4 in photoreceptor OS, while comparisons between pERK intensity of IS from cells fixed at CT 16 and CT 4 were not significantly different (Fig. 3B). The immunofluorescence intensities for VGCCα1D was also much higher in photoreceptors fixed at CT 16 than at CT 4 (Fig. 3A), which was similar to our previous observation [25], and we showed that pERK serves as part of the circadian output to regulate L-type VGCC channels, and inhibition of the ERK pathway dampens the protein expression rhythm of VGCCα1D [25]. The CT 4 pERK pictures shown in Fig. 3A were photo-enhanced with prolonged exposure for viewing purpose and not used for statistical comparisons (Fig. 3B and C), since under normal exposure time, the pERK fluorescence intensity in CT 4 cells was very faint.
Fig. 2.
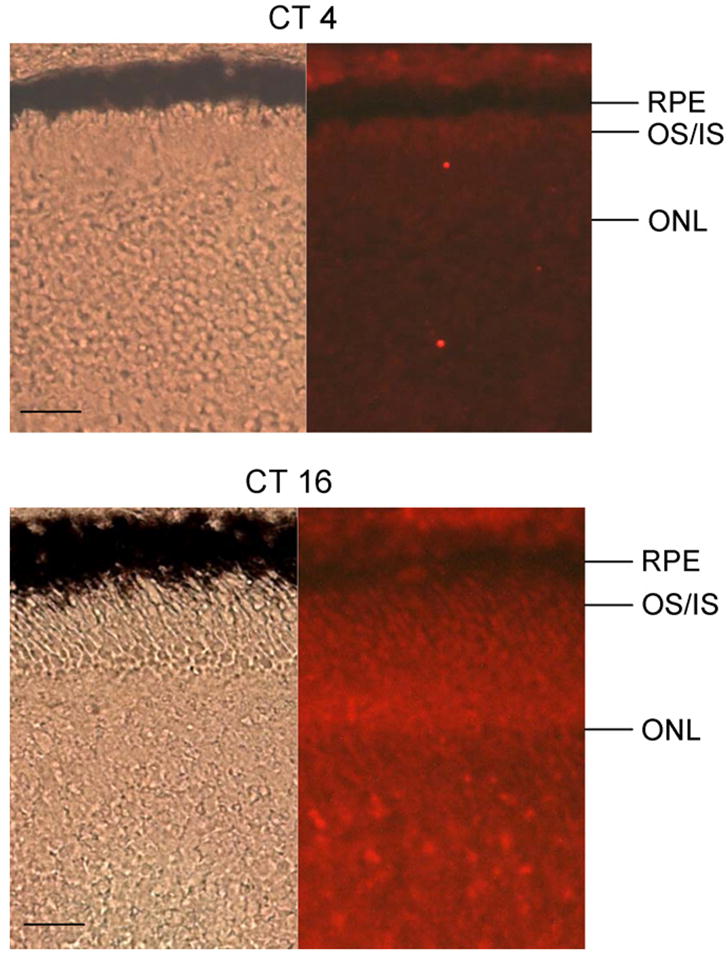
There is a circadian regulation of pERK fluorescence intensity in the retina tissue sections. The immunofluorescence intensity of pERK is higher in the retina tissue fixed at CT 16 than at CT 4. RPE: retina pigment epithelium; OS/IS: outer segment/inner segment; ONL: outer nuclear layer; bar = 20 μm.
Fig. 3.
The distribution of di-phosphorylated ERK (pERK) is under circadian control in cone photoreceptors. On the second day of DD, photoreceptors were fixed at CT 4 and CT 16, followed by immunocytochemistry dual-staining with antibodies against pERK and L-VGCCα1D (VGCC1D) subunit. (A) The distribution of VGCCα1D (green fluorescence) is mainly confined to the somata (inner segment and cell bodies) of the cone photoreceptors, and the fluorescence is higher in cells fixed at CT 16 (lower panel) than at CT 4 (upper panel). The arrows indicate the photoreceptors. The overall pERK fluorescence intensity (red fluorescence) is also higher in cells fixed at CT 16 (lower panel) than in cells fixed at CT 4 (upper panel). The distribution of pERK in photoreceptors is also under circadian control. In photoreceptors fixed at CT 16 (lower panel), the pERK fluorescence intensity is much stronger in the outer segments (OS, arrow heads) than in the inner segments-cell bodies (IS). The control shows no fluorescence activity when the specific primary antibodies were omitted and substituted with serums. (B) The pERK fluorescence intensity is significantly higher in the OS of photoreceptors fixed at CT 16 than the other three groups (*p < 0.05, ANOVA, Tukey post hoc test). (C) The pERK fluorescence intensity ratio (outer segment/inner segment-cell body; OS/IS) is significantly higher in photoreceptors fixed at CT 16 than at CT 4 (*p < 0.05, Student’s t-test). The fluorescence intensity data are taken from nine photoreceptors in three different experiments of each time point (CT4 or CT16). Bar = 10 μm.
Our studies indicate that the internal circadian control of retina ERK activity overcomes external light exposure (Fig. 1A, E, and F), since there was about fourfold more ERK activity in retinas harvested during the subjective night (CT 16) than during the subjective day (CT 4), but light exposure for various lengths of time did not affect pERK levels (Fig. 1E and F) at these time points when the retinas were under free-running conditions. The fourfold oscillatory difference between the peak and nadir of pERK levels does not reflect the total extent to which ERK activity can be extended [21]. Cyclic AMP or forskolin (adenylate cyclase activator) can further activate ERK beyond levels observed during the subjective night when the circadian ERK activity is at peak, while PKA or adenylate cyclase inhibitors can further decrease pERK levels when the ERK activity is at its nadir [21]. However, when retinas were kept in a cycling light environment, retinas were able to respond to acute illumination changes through modifications in ERK activity (Fig. 1B, C, and D). Previously, Warren and Molday [43] showed that exposure to bright light for 20 min during the subjective night causes a decrease in tyrosine phosphorylation of ERK in chick pineal glands, while others showed that exposure to light causes an up-regulation of pERK in rat retinas [30] as well as in cultured photoreceptors that were not exposed to light before [45]. Since the visual systems have to adapt to long-term changes in ambient illumination throughout the day [10,16] in addition to short-term light/dark adaptation [6,7,28], it is possible that pERK participates in the circadian regulation of photoreceptor physiology as well as acute light/dark adaptation.
While light did not affect ERK activity in circadian entrained photoreceptors that were free-running in constant darkness, illumination changes did affect pERK levels when embryos were in LD cycles or were never exposed to light before. Interestingly, photoreceptors are more sensitive to intense light damage at night than during the day even in animals kept in constant darkness after LD entrainment [41]. It is possible that in these animals, on top of higher ERK activity in the subjective night, intense light might trigger activation of other signaling pathways (e.g. the p38 stress-kinase pathway) that lead to light-induced retina damage. The elevated levels of pERK seen during the night may be a protective/adaptive measure to sudden, drastic illumination changes. Active ERK can phosphorylate and activate hypoxia-induced factor (HIF), which has been shown to protect photoreceptors from light-induced degeneration in the mouse retina [36]. The decrease in ERK activity seen in photoreceptors at ZT 4 (dark exposure) and ZT 16 (light exposure) after 2 h may be one factor that leads to light-induced damage in the retina. Hence, the circadian oscillators in retina photoreceptors play a major role in regulation of photoreceptor physiology, which leads to the circadian control of light sensitivity of photoreceptors.
We also found that there was a circadian regulation in the distribution of activated ERK (pERK). During the subjective night (CT 16), even though pERK was distributed throughout the photoreceptor, it was more prominent in the outer segments, while during the subjective day (CT 4), pERK was less abundant and distributed more evenly in both inner and outer segments. What are the functional roles of activated ERK in the outer segments of photoreceptors? Thus far, ERK has not been directly linked to the phototransduction process, but overexpression of the γ subunit of phosphodiesterase 6, the primary effector of phototransduction, increases ERK activity in HEK 293 cells [42]. In zebrafish retina photoreceptors, pharmacologically blocking MAPK signaling diminishes blue-light-induced increases in rhodopsin promoter expression but has no effect on white-light mediated rhodopsin promoter expression [46]. These evidences imply that MAP kinase ERK may play a modulatory role in phototransduction. Previously, we showed that MAPK signaling serves as the circadian output pathway to regulate the affinity rhythm of cGMP-gated ion channels [23,24]. The cGMP-gated ion channels (CNGCs) in photoreceptor outer segments serve as the last step in phototransduction and carry the “dark currents” [12,27,43]. Coincidently, CNGCs are also present in the cone synaptic terminals that regulate exocytosis in photoreceptors [37]. The apparent heavier distribution of pERK in photoreceptor outer segments at night indicates that the major contribution of MAPK signaling in the outer segments is the circadian regulation of CNGC affinity rhythms, in which the affinity of cGMP to CNGCs is higher at night [23–25]. The very last step of the molecular mechanism underlying the circadian regulation of cGMP affinity to CNGCs is the tyrosine phosphorylation of the auxiliary subunits of CNGCs [11]. Therefore, MAPK signaling serves as an upstream regulator to tyrosine kinase and/or phosphatase activity that leads to the circadian regulation of CNGCs. In summary, the MAPK signaling pathway participates in the circadian output regulation of photoreceptor daily adaptation to environmental illumination as well as short-term light/dark adaptation.
Acknowledgments
We thank Dr. Robert Burghardt, Director of the Image Analysis Laboratory, College of Veterinary Medicine and Biomedical Sciences at Texas A&M University for his assistance in using the imaging facility. The Image Analysis Laboratory has been supported in part by NIH grants S10RR022532, P42ES004917, and P30ES009106. This project was supported by NIHRO1EY017452 and a start-up fund from Texas A&M University to GK.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- 2.Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984;99:1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adly MA, Spiwoks-Becker I, Vollrath L. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest Ophthalmol Vis Sci. 1999;40:2165–2172. [PubMed] [Google Scholar]
- 4.Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev Biol. 1996;178:304–315. doi: 10.1006/dbio.1996.0220. [DOI] [PubMed] [Google Scholar]
- 5.Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt DA. Light adaptation and contrast in the outer retina. Prog Brain Res. 2001;131:407–418. doi: 10.1016/s0079-6123(01)31033-6. [DOI] [PubMed] [Google Scholar]
- 7.Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- 8.Butcher GQ, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- 9.Butcher GQ, Lee B, Obrietan K. Temporal regulation of light-induced extra-cellular signal-regulated kinase activation in the suprachiasmatic nucleus. J Neurophysiol. 2003;90:3854–3863. doi: 10.1152/jn.00524.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Retinal Eye Res. 1995;14:267–291. [Google Scholar]
- 11.Chae KS, Ko GY, Dryer SE. Tyrosine phosphorylation of cGMP-gated ion channels is under circadian control in chick retina photoreceptors. Invest Ophthalmol Vis Sci. 2007;48:901–906. doi: 10.1167/iovs.06-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SK, Ko GY, Dryer SE. Somatostatin peptides produce multiple effects on gating properties of native cone photoreceptor cGMP-gated channels that depend on circadian phase and previous illumination. J Neurosci. 2007;27:12168–12175. doi: 10.1523/JNEUROSCI.3541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coogan AN, Piggins HD. Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. J Neurosci. 2003;23:3085–3093. doi: 10.1523/JNEUROSCI.23-07-03085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 15.Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- 16.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 17.Hainich EC, Pizzio GA, Golombek DA. Constitutive activation of the ERK-MAPK pathway in the suprachiasmatic nuclei inhibits circadian resetting. FEBS Lett. 2006;580:6665–6668. doi: 10.1016/j.febslet.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Haque R, Chaurasia SS, Wessel JH, 3rd, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Sanada K, Fukada Y. Circadian and photic regulation of MAP kinase by Ras- and protein phosphatase-dependent pathways in the chick pineal gland. FEBS Lett. 2001;491:71–75. doi: 10.1016/s0014-5793(01)02153-6. [DOI] [PubMed] [Google Scholar]
- 20.Khavari TA, Rinn J. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle. 2007;6:2928–2931. doi: 10.4161/cc.6.23.4998. [DOI] [PubMed] [Google Scholar]
- 21.Ko GY, Ko M, Dryer SE. Circadian and cAMP-dependent modulation of retinal cone cGMP-gated channels does not require protein synthesis or calcium influx through L-type channels. Brain Res. 2004;1021:277–280. doi: 10.1016/j.brainres.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 22.Ko GY, Ko ML, Dryer SE. Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J Neurosci. 2003;23:3145–3153. doi: 10.1523/JNEUROSCI.23-08-03145.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 24.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- 27.Kramer RH, Molokanova E. Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol. 2001;204:2921–2931. doi: 10.1242/jeb.204.17.2921. [DOI] [PubMed] [Google Scholar]
- 28.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retinal Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- 30.Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menger GJ, Koke JR, Cahill GM. Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci. 2005;22:203–209. doi: 10.1017/S0952523805222083. [DOI] [PubMed] [Google Scholar]
- 32.Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira CS, Rigon AP, Leal RB, Rossi FM. The activation of ERK1/2 and p38 mitogen-activated protein kinases is dynamically regulated in the developing rat visual system. Int J Dev Neurosci. 2008;26:355–362. doi: 10.1016/j.ijdevneu.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- 35.Pizzio GA, Hainich EC, Ferreyra GA, Coso OA, Golombek DA. Circadian and photic regulation of ERK, JNK and p38 in the hamster SCN. Neuroreport. 2003;14:1417–1419. doi: 10.1097/00001756-200308060-00002. [DOI] [PubMed] [Google Scholar]
- 36.Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 37.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 38.Roduit R, Schorderet DF. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis. 2008;13:343–353. doi: 10.1007/s10495-008-0179-8. [DOI] [PubMed] [Google Scholar]
- 39.Sanada K, Hayashi Y, Harada Y, Okano T, Fukada Y. Role of circadian activation of mitogen-activated protein kinase in chick pineal clock oscillation. J Neurosci. 2000;20:986–991. doi: 10.1523/JNEUROSCI.20-03-00986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanimoto S, Kanamoto T, Mizukami M, Aoyama H, Kiuchi Y. Pigment epithelium-derived factor promotes neurite outgrowth of retinal cells. Hiroshima J Med Sci. 2006;55:109–116. [PubMed] [Google Scholar]
- 41.Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–553. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Wan KF, Sambi BS, Tate R, Waters C, Pyne NJ. The inhibitory gamma subunit of the type 6 retinal cGMP phosphodiesterase functions to link c-Src and G-protein-coupled receptor kinase 2 in a signaling unit that regulates p42/p44 mitogen-activated protein kinase by epidermal growth factor. J Biol Chem. 2003;278:18658–18663. doi: 10.1074/jbc.M212103200. [DOI] [PubMed] [Google Scholar]
- 43.Warren R, Molday RS. Regulation of the rod photoreceptor cyclic nucleotide-gated channel. Adv Exp Med Biol. 2002;514:205–223. doi: 10.1007/978-1-4615-0121-3_12. [DOI] [PubMed] [Google Scholar]
- 44.Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science. 2001;293:2251–2256. doi: 10.1126/science.1063097. [DOI] [PubMed] [Google Scholar]
- 45.Yang LP, Zhu XA, Tso MO. Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2007;48:4766–4776. doi: 10.1167/iovs.06-0871. [DOI] [PubMed] [Google Scholar]
- 46.Yu CJ, Gao Y, Willis CL, Li P, Tiano JP, Nakamura PA, Hyde DR, Li L. Mitogen-associated protein kinase- and protein kinase A-dependent regulation of rhodopsin promoter expression in zebrafish rod photoreceptor cells. J Neurosci Res. 2007;85:488–496. doi: 10.1002/jnr.21157. [DOI] [PubMed] [Google Scholar]