Abstract
We and others have previously reported that lactotransferrin (LF), acting both as an iron-binding protein and inflammatory modulator, is greatly up-regulated in the brain of patients with Alzheimer’s disease (AD). However, it remains unknown which type of cells express LF in the brain of AD. In this study, therefore, we investigated the expression and localization of LF messenger RNA (mRNA) in the cerebral cortex of AD and control cases using real-time polymerase chain reaction (PCR) and in situ hybridization histochemistry. Real-time PCR demonstrated that LF mRNA expression in the cortex of AD cases was significantly greater than that in control cases. LF mRNA-positive granules were observed in the cortex by in situ hybridization histochemistry, and the number of positive granules was increased in AD cases compared to controls. The double staining technique of LF mRNA in situ hybridization and D-related human leukocyte antigen (HLA-DR) immunohistochemistry revealed that positive granules were localized in a subpopulation of HLA-DR-positive reactive microglia. In addition, LF mRNA-positive granules were observed in some cells that were negative for HLA-DR. These cells were also negative for CD4 and CD8 but positive for leukocyte common antigen (CD45RB), suggesting they were monocytes/macrophages. These results indicate that reactive microglia in the cerebral cortex and monocytes/macrophages infiltrating from the circulation might be responsible for synthesizing LF in AD brain.
Keywords: Alzheimer’s disease, Iron, Lactotransferrin, Microglia, Oxidative stress
Lactotransferrin (lactoferrin; LF) is an 80 kDa iron-binding glycoprotein considered to have a role in the binding, transport and storage of iron [1]. LF was first isolated from milk and is found in various secretions, such as tears, saliva, nasal secretions and intestinal mucus, and in plasma [7,11,20]. It is also stored in specific granules of neutrophilic leukocytes and released during the inflammatory process [24]. LF can bind iron tightly and in this way acts as a powerful iron chelator with antioxidant properties [5,6,33]. In addition, LF may serve as a regulator of the inflammatory-immune response [3,28], and reactive oxygen metabolism [15,19].
In the brain, excessive iron deposits occur in normal aging and in various neurodegenerative diseases [8,32,34]. LF is also associated with aging and particularly with neurodegenerative disorders including Alzheimer’s disease (AD) [16,21,22,31]. In particular, LF has been detected in senile plaques and neurofibrillary tangles [16,22]. However, little information is available about LF messenger RNA (mRNA) in AD brain. In this study, therefore, we investigated the level of LF mRNA in the cortex of neurologically normal controls and AD patients using real-time polymerase chain reaction (PCR) and demonstrated its expression by in situ hybridization. In addition, we used in situ hybridization and immunohistochemistry to further examine the localization of LF mRNA.
Human brain tissues were obtained from the Brain Donation Program at the Sun Health Research Institute [4]. The Brain Donation Program has been approved by the Institutional Review Board of the Sun Health Corporation. Total RNA was purified from the temporal cortex of six sporadic AD cases (mean age ± S.D., 86.2 ± 6.6 years) and five control cases without neurological disease (mean age ± S.D., 80.0 ± 6.3 years). The average postmortem delay for the AD and control cases was 2.23 and 2.48 h, respectively.
LF mRNA was detected by real-time PCR using a LightCycler System (Roche Applied Science, Mannheim, Germany). The sense primer was 5′–AGGCCACAAAATGCTTCCAATGGCAAA–3′, and the antisense primer was 5′–GGCTGTCTTTCGGTCCCGTAGACTTCC–3′. Real-time PCR analysis for β-actin mRNA was also employed to assess the variability of mRNA content. In order to correct for the variability of mRNA, the amount of LF mRNA from each sample was divided by the amount of β-actin mRNA. The relative value was calculated by the following formula: (LF mRNA)/(β-actin mRNA) × 105. A one-sample Kolmogorov-Smirnov test showed that the distribution of data was exponential. Thus, the original data were converted to logarithmic form (Log 2) to show a Gaussian distribution. The difference in LF mRNA between two groups was analyzed by Student t test for independent samples. Two-tailed p values of less than 0.05 were considered statistically significant.
The location of expression of LF mRNA was examined by in situ hybridization using samples of temporal cortex from three AD cases and three control cases obtained from the Brain Donation Program at the Sun Health Research Institute [4]. Digoxigenin-labeled riboprobes were used. Frozen, fixed sections were mounted on RNase-free silane-coated glass slides (Dako Japan Co. Ltd., Tokyo, Japan) and air-dried. The sections were treated for 10 min at room temperature with 10 μg/ml proteinase K in 10 mM Tris-HCl buffer (pH 8.0) containing 150 mM NaCl. For pre-hybridization, the sections were reacted for 1 h at 37° C in hybridization buffer containing 50% formamide, 5 × Denhardt’s solution, 0.5 × saline/sodium citrate (SSC; 1 × SSC = 150 mM NaCl and 15 mM sodium citrate), 0.5 mg/ml yeast tRNA (Invitrogen) and 0.5 mg/ml heat-denatured salmon sperm DNA (Wako Pure Chemicals, Co., Osaka, Japan). The probes were diluted in hybridization buffer to a final concentration of 3 μg/ml, and hybridization was performed overnight at 60° C.
After hybridization, the sections were washed for 2 h in pre-warmed 0.1 × SSC buffer at 60° C, followed by a 5 min rinse in 0.1 M Tris HCl (pH 7.5) containing 150 mM NaCl (NT buffer) at room temperature. Subsequently, the sections were treated for 60 min with 1% skim milk in NT buffer to block nonspecific protein binding, and then reacted overnight at 4° C with alkaline phosphatase-labeled anti-digoxigenin antibody (1:200 dilution; Roche Diagnostics, Mannheim, Germany) in NT buffer containing 1% skim milk. After washing with NT buffer, positive signals were detected by incubating in 0.1 M Tris HCl buffer (pH 9.5) containing 100 mM NaCl, 50 mM MgCl2, 500 μg/ml nitroblue tetrazolium chloride and 187 μg/ml 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt.
After in situ hybridization, some sections from both AD and control cases were double-stained by immunohistochemistry. The sections were incubated with 0.5% hydrogen peroxide in 0.1 M PBS (pH 7.4) containing 0.3% triton-X100 (PBST) for 30 min at room temperature to eliminate endogenous peroxidase. After washing with PBST, the sections were incubated for 30 min with PBST containing 1% bovine serum albumin to block nonspecific protein binding. Then, sections were incubated overnight at 4° C with mouse monoclonal antibody directed against D-related human leukocyte antigen (HLA-DR, clone TAL.1B5; 1:100 dilution; Dako, Glostrup, Denmark), CD4 (Nichirei Co., Tokyo, Japan), CD8 leukocyte common antigen (LCA, clone PD7/26 and 2B11; 1:100 dilution; Dako). After washing with PBST, the sections were incubated for 1 h with biotinylated anti-mouse IgG (1:1000 dilution; Vector Laboratories, Burlingame, CA) and for 1 h with avidin-biotinylated peroxidase complex (1:2000 dilution; Vector Laboratories), both at room temperature. Sections were then incubated with 0.02% 3,3′-diaminobenzidine in 50 mM Tris–HCl buffer (pH 7.6) to precipitate brown chromogen.
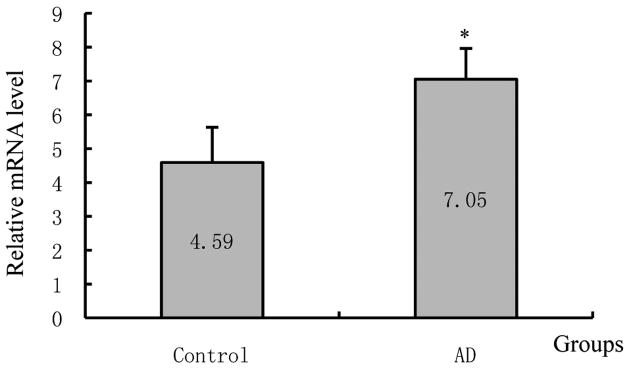
As shown in Figure 1, LF mRNA expression, determined by real-time PCR, in the normal human cortex was very low, but expression in the cortex of AD patients was significantly increased compared to that of control cases (AD/Control = 5.50, p = 0.02).
Figure 1.
Lactotransferrin (LF) mRNA expression levels in the cerebral cortex of control and Alzheimer’s disease (AD) patients. Asterisk indicates significantly different to control (p < 0.05).
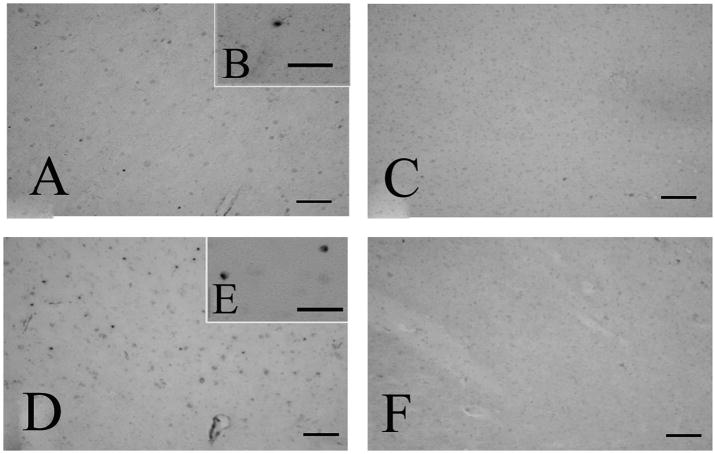
In the cerebral cortex, granular signals of LF mRNA were detected in both control (Fig. 2A and B) and AD (Fig. 2D and E) by in situ hybridization using the antisense probe. The number of LF-positive granules was increased in AD cases compared to controls (Fig. 2). The sense probe did not show any signal (Fig. 2C and F).
Figure 2.
In situ hybridization histochemistry of the cerebral cortex of control (A, B, C) and Alzheimer’s disease (AD) cases (D, E, F) using antisense (A, B, D, E) and sense (C, F) probes. Granules imply the localization of lactotransferrin mRNA. The number of positive granules is increased in AD cases (D) compared to controls (A). Using sense probes (C and F) as the control for the antisense probe, no granule of blue color is detected in the cortex. Bars = 100 μm in A, C, D, F, and 50 μm in B, E.
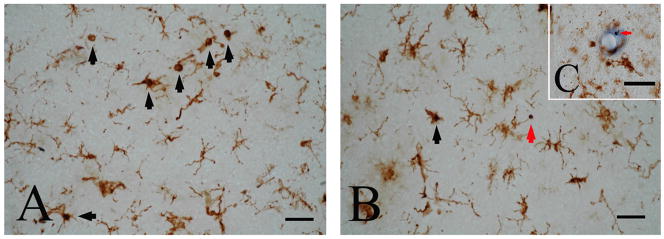
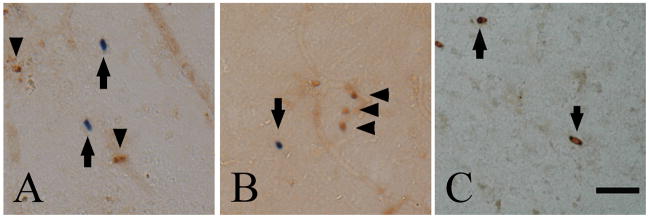
Using the double staining technique of LF mRNA in situ hybridization and HLA-DR immunohistochemistry, LF mRNA-positive granules were localized in cell bodies of a subpopulation of HLA-DR-positive reactive microglia in both AD (black arrows in Fig. 3A and B) and control cases (data not shown). Approximately 4 % and 8% of HLA-DR-positive microglia showed LF-positive staining, in control and AD cases, respectively. In AD cases, some LF mRNA-positive granules were negative for HLA-DR (red arrows in Fig. 3B and C), and these granules were often seen in the vicinity of microvessels (red arrow in Fig. 3C). These cells were negative for CD4 (Fig. 4A) and CD8 (Fig. 4B) but positive for LCA (Fig. 4C), suggesting they were monocytes/macrophages.
Figure 3.
Double staining of lactotransferrin (LF) mRNA in situ hybridization and D-related human leukocyte antigen (HLA-DR) immunohistochemistry in the cerebral cortex of an Alzheimer’s disease case. LF mRNA is expressed in a subpopulation of HLA-DR-positive reactive microglia (black arrows in A and B). Some LF mRNA-positive cells which are negative for HLA-DR are seen in the parenchyma of the cerebral cortex (red arrow in B) and in the vicinity of microvessels (red arrow in C). Bars = 50 μm.
Figure 4.
Double staining of lactotransferrin (LF) mRNA in situ hybridization and CD4 (A), CD8 (B) or leukocyte common antigen (LCA) immunohistochemistry (C) in the cerebral cortex of an Alzheimer’s disease case. LF-positive signals (arrows in A and B) were negative for CD4 (arrowheads in A) and CD8 (arrowheads in B). Granular signals for LF mRNA are seen in the cytoplasm of LCA-positive cells (arrows in C). Bar = 50 μm.
In the present study, using real-time PCR, we found LF mRNA expression was up-regulated in the cortex of AD patients. These results are in agreement with previous immunohistochemical studies [16,22] showing up-regulation of LF at the protein level. We also demonstrated that LF mRNA was detected in granular structures in the cortex of both control and AD cases using in situ hybridization. Histochemical double staining for LF mRNA and HLA-DR confirmed that LF mRNA was expressed by a subpopulation of HLA-DR-positive microglia. These results are supported by a previous report showing that LF is produced by activated microglia in the substantia nigra in Parkinson’s disease [14]. In neurodegenerative disorders such as AD, a subtle and chronic inflammation reaction takes place leading to a marked activation of glia indicated by HLA-DR-positive staining [25,26,27].
We also observed LF mRNA in some cells that were negative for HLA-DR and which were often observed in the vicinity of microvessels. Since T cells and monocytes/macrophages are known to infiltrate the brain parenchyma in AD, we employed double staining techniques for the T cell markers, CD4 and CD8, as well as LCA (CD45RB). These cells were negative for the T cell markers, CD4 and CD8, but positive for LCA (CD45RB), suggesting they were monocytes or macrophages. Thus, LF protein is synthesized in the brain not only by reactive microglia, but also by infiltrating monocytes/macrophages. However, it cannot be ruled out that some LF protein is transported from the blood into the brain [12,13].
The precise role of LF in AD still remains unknown, however this and previous studies raise several possible explanations. LF markedly inhibits classical C3 convertase [17,18] and thus suppresses the complement classical pathway that occurs in the brain of AD [10]. LF also suppresses the production of inflammatory cytokines such as interleukin 1 and tumor necrosis factor [9,23]. Therefore, it is possible that the up-regulation of LF in areas affected by AD may be a defensive response of the AD brain. Another possibility is that LF may have a role in anti-oxidative stress [5,6,33], because oxidative stress is involved in the pathology of AD [29, see 35 for review] and increased levels of oxidative damage occur prior to the onset of amyloid beta deposition [30]. Redox-active iron, a source of redox-generated free radicals which contribute to the oxidative damage, is associated with the senile plaques and neurofibrillary tangles in AD [32,34]. It has been reported that transferrin and ferritin are responsible for iron regulation and play an important role in the disruption of brain iron homeostasis in AD [8]. Like transferrin, LF consists of two lobes, each possessing an iron-binding site capable of reversibly binding one ferric ion and LF binds iron more tightly than transferring [2]. Therefore, LF acts as a powerful iron chelator with antioxidant properties [2,5,6,33]. Both inflammatory processes and oxidative stress contribute to an increased production of LF by microglia, or to an increased number of activated cells leading to enhanced LF production which might explain its accumulation. So it is likely that LF may prevent cell injury and tissue damage, and therefore protect brain integrity, through both its anti-inflammatory and antioxidant functions.
In conclusion, this present study has shown that the expression of LF mRNA is up-regulated in the cortex of AD patients. We have also demonstrated that LF mRNA is expressed by activated microglial cells or leukocytes that have infiltrated the cortex of the AD brain.
Acknowledgments
The Brain Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011 and 05–901 to the Arizona Parkinson’s Disease Consortium) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aisen P, Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 2.Baker EN, Anderson BF, Baker HM, Day CL, Haridas M, Norris GE, Rumball SV, Smith CA, Thomas DH. Three-dimensional structure of lactoferrin in various functional states. Adv Exp Med Biol. 1994;357:1–12. doi: 10.1007/978-1-4615-2548-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: A multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 4.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britigan BE, Hasset DJ, Rosen GM, Hamill DR, Cohen MS. Neutrophil degranulation inhibits potential hydroxy-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxy-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem J. 1989;264:447–455. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Mao J, Rasmussen T, Serody JS, Britigan BE. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis. 1992;166:1375–1378. doi: 10.1093/infdis/166.6.1375. [DOI] [PubMed] [Google Scholar]
- 7.Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr. 2001;20:389S–395S. doi: 10.1080/07315724.2001.10719173. [DOI] [PubMed] [Google Scholar]
- 8.Connor JR, Menzies SL, St Martin SM, Mufson E. A histochemical study of iron, transferrin and ferritin in Alzheimer’s disease brains. J Neurosci Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 9.Crouch SP, Slater KJ, Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992;80:235–240. [PubMed] [Google Scholar]
- 10.Emmerling MR, Spiegel K, Watson MD. Inhibiting the formation of classical C3-convertase on the Alzheimer’s beta-amyloid peptide. Immunopharmacology. 1997;38:101–109. doi: 10.1016/s0162-3109(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 11.Farnaud S, Evans RW. Lactoferrin--a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 12.Fillebeen C, Dehouck B, Benaïssa M, Dhennin-Duthille I, Cecchelli R, Pierce A. TNF-α increases lactoferrin transcytosis through the blood–brain barrier. J Neurochem. 1999;73:2491–2500. doi: 10.1046/j.1471-4159.1999.0732491.x. [DOI] [PubMed] [Google Scholar]
- 13.Fillebeen C, Descamps L, Dehouck MP, Fenart L, Benaïssa M, Spik G, Cecchelli R, Pierce A. Receptor mediated-transcytosis of lactoferrin through the blood–brain barrier. J Biol Chem. 1999;274:7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 14.Fillebeen C, Ruchoux MM, Mitchell V, Vincent S, Benaïssa M, Pierce A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor α or 1-methyl-4-phenylpyridinium treatment. Brain Res Mol Brain Res. 2001;96:103–113. doi: 10.1016/s0169-328x(01)00216-9. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge JM, Paterson SK, Segal AW, Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981;199:259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamata T, Tooyama I, Yamada T, Walker DG, McGeer PL. Lactotransferrin immunocytochemistry in Alzheimer and normal human brain. Am J Pathol. 1993;142:1574–1585. [PMC free article] [PubMed] [Google Scholar]
- 17.Kievits F, Kijlstra A. Inhibition of C3 deposition on solid-phase bound immune complexes by lactoferrin. Immunology. 1985;54:449–456. [PMC free article] [PubMed] [Google Scholar]
- 18.Kijlstra A, Jeurissen SH. Modulation of classical C3 convertase of complement by tear lactoferrin. Immunology. 1982;47:263–270. [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanoff SJ, Waltersdorph AM. Peroxidant activity of transferrin and lactoferrin. J Exp Med. 1990;172:1293–1303. doi: 10.1084/jem.172.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 21.Leveugle B, Faucheux B, Bouras C, Nillesse N, Spik G, Hirsch EC, Agid Y, Hof PR. Immunohistochemical analysis of the iron-binding protein lactotransferrin in Parkinson’s disease. Acta Neuropathol. 1996;91:566–572. doi: 10.1007/s004010050468. [DOI] [PubMed] [Google Scholar]
- 22.Leveugle B, Spik G, Perl DP, Bouras C, Fillit HM, Hof PR. The iron-binding protein lactotransferrin is present in pathologic lesions in a variety of neurodegenerative disorders: a comparative immunohistochemical analysis. Brain Res. 1994;650:20–31. doi: 10.1016/0006-8993(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 23.Machnicki M, Zimecki M, Zagulski T. Lactoferrin regulates the release of tumour necrosis factor-α and interleukin 6 in vivo. Int J Exp Pathol. 1993;74:433–439. [PMC free article] [PubMed] [Google Scholar]
- 24.Masson PL, Heremans JF, Schönne E. Lactoferrin: an iron binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 26.McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76:550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 27.McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 28.Nuijens JH, van Berkel PHC, Schanbacher FL. Structure and biological actions of lactoferrin. J Mamm Gland Biol Neoplas. 1996;1:285–295. doi: 10.1007/BF02018081. [DOI] [PubMed] [Google Scholar]
- 29.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 31.Osmand AP, Switzer RC., III . Differential distribution of lactoferrin and Alz-50 immunoreactivities in neuritic plaques and neurofibrillary tangles in Alzheimer’s disease. In: Iqbal K, McLachlan DRC, Winblad B, Wisniewski HM, editors. Alzheimer’s Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies. Wiley; New York: 1991. pp. 219–228. [Google Scholar]
- 32.Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 33.Shinmoto H, Dosako S, Nakajima I. Anti-oxidant activity of bovine lactoferrin on iron/ascorbate induced lipid peroxidation. Biosci Biotechnol Biochem. 1992;56:2079–2080. [Google Scholar]
- 34.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MA, Sayre LM, Monnier VM, Perry G. Radical AGEing in Alzheimer’s disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]