Abstract
Little is known about the molecular mechanism of recycling of intracellular receptors and lipid raft-associated proteins. Here, we have investigated the recycling pathway and internalization mechanism of a transmembrane, lipid raft-associated intracellular prohormone sorting receptor, carboxypeptidase E (CPE). CPE is found in the trans-Golgi network (TGN) and secretory granules of (neuro)endocrine cells. An extracellular domain of the IL2 receptor α-subunit (Tac) fused to the transmembrane domain and cytoplasmic tail of CPE (Tac-CPE25) was used as a marker to track recycling of CPE. We show in (neuro)endocrine cells, that upon stimulated secretory granule exocytosis, raft-associated Tac-CPE25 was rapidly internalized from the plasma membrane in a clathrin-independent manner into early endosomes and then transported through the endocytic recycling compartment to the TGN. A yeast two-hybrid screen and in vitro binding assay identified the CPE cytoplasmic tail sequence S472ETLNF477 as an interactor with active small GTPase ADP-ribosylation factor (ARF) 6, but not ARF1. Expression of a dominant negative, inactive ARF6 mutant blocked this recycling. Mutation of residues S472 or E473 to A in the cytoplasmic tail of CPE obliterated its binding to ARF6, and internalization from the plasma membrane of Tac-CPE25 mutated at S472 or E473 was significantly reduced. Thus, CPE recycles back to the TGN by a novel mechanism requiring ARF6 interaction and activity.
INTRODUCTION
Carboxypeptidase E (CPE) is a lipid raft-associated prohormone sorting receptor. Unlike other cell surface raft-associated proteins and receptors studied, CPE resides and functions at the trans-Golgi network (TGN) and within secretory granules (SGs) of the regulated secretory pathway (RSP). CPE, found in (neuro)endocrine cells, exists in two forms in the SGs of the RSP (Fricker et al., 1990). The soluble form functions as a processing enzyme and the membrane form, present at the TGN, acts as a sorting receptor to target prohormones to the SGs of the RSP (Cool et al., 1997; Shen and Loh, 1997). Membrane CPE has a C-terminal domain of ∼25 amino acids, 18 of which span the TGN and SG membrane, leaving the remaining six residues as a cytoplasmic tail (Dhanvantari et al., 2002). A significant portion of CPE is raft-associated in the TGN and remains in membrane rafts after packaging into SGs (Dhanvantari and Loh, 2000). This led us to hypothesize that membrane CPE may be recycled back to the TGN from the PM after SG exocytosis and the cytoplasmic tail may be important for this process. An initial yeast two-hybrid screen designed to identify proteins that interact with a small GTPase ADP-ribosylation factor (ARF) 6, and not ARF1, revealed a carboxyl terminal fragment of CPE. This raised the possibility that ARF6 may be involved in the recycling of CPE, because it has been implicated in the recycling and endocytosis of other proteins (Radhakrishna and Donaldson, 1997; Claing et al., 2001).
Thus far, the recycling pathway of only two types of raft-associated proteins have been studied: lipid-binding proteins, such as cholera toxin B subunit (CTxB) and shiga toxin B subunit that bind to the glycosphingolipids GM1 and Gb3, respectively (Orlandi and Fishman, 1998; Katagiri et al., 1999); and GPI-anchored proteins. A major portion of CTxB is delivered to the Golgi by a clathrin-independent pathway and is internalized through caveoli (Orlandi and Fishman, 1998) or caveolin-1-positive endosomes (Nichols et al., 2001; Nichols, 2002). Shiga toxin B subunit is endocytosed by a clathrin-dependent pathway (Sandvig et al., 1989). GPI-anchored proteins recycle between the plasma membrane (PM) and endosomes, and to varying extents to the Golgi complex, and they seem to be internalized by a nonclathrin pathway (Bamezai et al., 1992; Mayor et al., 1998; Skretting et al., 1999; Ricci et al., 2000; Nichols et al., 2001; Sabharanjak et al., 2002). However, the mechanism of internalization of these proteins is not characterized. Neither has a role of ARF6 in the internalization of raft-associated proteins been reported.
In this study, we investigated the recycling of a raft-associated, transmembrane intracellular sorting receptor CPE and the role of ARF6 in its internalization. We used a fusion protein, Tac-CPE25, to show that CPE was rapidly internalized from the PM after stimulated secretory granule exocytosis in Neuro2A cells, a neuroendocrine cell line. CPE was then trafficked to the TGN through early endosomes and the endocytic recycling compartment (ERC). Using a yeast two-hybrid screen and in vitro binding assay, we have shown that ARF6 directly interacts with the cytoplasmic tail of CPE. Site-directed mutagenesis experiments demonstrated requirements of ARF6 interaction with the CPE tail, as well as ARF6 activity, for internalization of Tac-CPE25 in vivo. These data indicate that CPE is internalized by a novel ARF6-dependent mechanism.
MATERIALS AND METHODS
Cell Transfection, DNA Constructs, and Fusion Proteins
Neuro2A and AtT20 cells obtained from American Type Culture Collection (Manassas, VA) were cultured in compete DMEM containing 10% fetal bovine serum and penicillin/streptomycin. For transient transfection, cells were grown in antibiotic-free media. The Tac-CPE25 construct, containing the extracellular domain of Tac (amino acids 1–219) fused to the C-terminal 25 amino acids of CPE (amino acids 453–477, numbering begins with M), was made as described previously (Zhang et al., 2003). Tac-CPES472A and Tac-CPEE473A point mutations were made using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The sequences of the plasmid cDNAs were verified by sequence analysis (Midland Certified Reagent, Midland, TX). Plasmids, expressing wtARF6, ARF6-T27N, ARF6-Q67L, and ARF1-Q71L were generated as described previously (Radhakrishna and Donaldson, 1997). Plasmid, expressing Eps15 mutant (GFP-EΔ95/295) was from Dr. A. Benmerah (Institut Pasteur, Paris, France) (Benmerah et al., 1999) and AP180-C construct was from Dr. H. McMahon (University College London, London, United Kingdom) (Ford et al., 2001). Dynamin-2-GFP (wild-type and K44A) constructs were provided by Dr. M. McNiven (Mayo Clinic, Rochester, MN). Transient transfections were performed using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols.
His-S-CPE100, His-S-CPE100Δ6, His-S-CPES472A, His-S-CPEE473A, His-SCPET474A, His-S-CPEN476A, and His-S-CPEF477A fusion proteins were used for in vitro binding assay. C-Terminal fragments of CPE (amino acids 377–477 or 377–471, respectively) were subcloned into pET30a vector (Novagen, Madison, WI) on EcoRI/XhoI sites. The point mutations were done using QuikChange site-directed mutagenesis kit (Stratagene). The sequences of the plasmid cDNAs were verified by sequence analysis (Midland Certified Reagent). After transformation, BL21DE3 bacteria was grown to OD 0.4 and induced with 0.03 mM isopropyl β-d-thiogalactoside for 6 h at 30°C. After purification on Ni2+-NTA column proteins were dialyzed against buffer A (40 mM phosphate, pH 7.2, 2 mM dithiothreitol [DTT], 5% glycerol, 50 mM NaCl) and purified on Mono Q column using linear gradient of NaCl (50–600 mM).
Plasmids, expressing glutathione S-transferase (GST)-ARF6-T27N, GSTARF6-Q67L, and GST-ARF1-Q71L, were generated by fusing the amino terminal end of the ARFs in frame with GST in pGEX vector (Amersham Biosciences, Piscataway, NJ). After transformation BL21DE3 bacteria was grown to OD 0.4 and induced with 0.1 mM isopropyl β-d-thiogalactoside overnight at room temperature. Proteins were purified on GST-Sepharose and eluted with 20 mM glutathione. Nucleotide loading was performed by standard procedure. Briefly, proteins were incubated in the presence of proper nucleotide (GTP or GDT) in buffer containing 10 mM EDTA, 20 mM Tris-HCl, pH 8.0, on ice for 20 min followed by addition of MgCl2 up to 20 mM. Resulting complexes were separated on Mono Q column in phosphate buffer, containing 1 mM MgCl2, 1 mM DTT, and 0.2% Triton X-100 by using linear 20–500 mM NaCl gradient. Fractions eluted between 150 and 250 mM salt were pooled and used for binding studies.
GST protein was expressed and purified by using pGEX4T1 vector (Amersham Biosciences) according to manufacturer's protocol.
Antibodies and Fluorescent Ligands
Monoclonal antibodies (IgG1) against the extracellular domain of Tac were derived from two hybridoma cell lines: 2A3A1H and 7G7B6 (American Type Culture Collection). Antibodies from the 2A3A1H cell line were purified using protein G affinity chromatography. Other antibodies used were: goat anti-early endosome antigen 1 (EEA1) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-TGN38 (a gift from Sharon L. Milgram, University of North Carolina at Chapel Hill, Chapel Hill, NC), rabbit anti-CPE against the C terminus (Cool et al., 1997), mouse anti-GST (Santa Cruz Biotechnology), mouse anti-transferrin (Tf) receptor (Zymed Laboratories, South San Francisco, CA), and rabbit anti-ARF6 (Radhakrishna and Donaldson, 1997). Fluorescently conjugated secondary antibodies, Cholera toxin B subunit (CTxB)Alexa 594, and Tf-Alexa 594 were from Molecular Probes (Eugene, OR). S-protein-horseradish peroxidase (HRP) conjugate was from Novagen (Madison, WI).
Anti-Tac Antibody, Tf, and CTxB Uptake Studies
Cells expressing Tac-CPE25 were incubated in DMEM containing 50 mM KCl for 15 min at 37°C to stimulate secretion. The cells were then rinsed with ice-cold phosphate-buffered saline (PBS) and incubated for 30 min at 4°C in serum-free DMEM containing 50 μg/ml anti-Tac antibodies and/or 25 μg/ml Tf-Alexa 594 or 0.5 μg/ml CTxB-Alexa 594. After this incubation period, the cells were rinsed three times with ice-cold DMEM and then incubated in antibody- and ligand-free DMEM at 37°C for the indicated times to allow internalization. To remove membrane-bound anti-Tac antibodies, cells were placed on ice and washed three times in buffer containing 0.5 M NaCl, 0.5% acetic acid, pH 3.0 (low pH buffer) after the chase period (Radhakrishna and Donaldson, 1997).
Immunofluorescence Microscopy
Sixteen to 24 h after transfection, cells were treated as described above, rinsed with PBS, fixed in 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in PBS. Primary and secondary antibodies were diluted in PBS containing 1% bovine serum albumin. All fluorescent images were taken using an MRC-1000 confocal microscope (Bio-Rad, Hercules, CA) and processed using Laser Sharp software and Adobe Photoshop 5.0. Triton X-100 extraction was done as described previously (Mayor and Maxfield, 1995; Nichols et al., 2001). Neuro2A cells expressing Tac-CPE25 were incubated in DMEM containing 50 mM KCl for 15 min at 37°C to stimulate secretion. Then cells were rinsed in ice-cold PBS and incubated for 30 min on ice in serum-free DMEM containing 50 μg/ml anti-Tac antibodies or 0.5 μg/ml CTxB-Alexa 594. Cells were washed with ice-cold PBS and incubated for 20 min on ice in PBS containing 1% Triton X-100 followed by fixation in 4% paraformaldehyde.
Yeast Two-Hybrid System
A yeast two-hybrid screen using Matchmaker II (BD Biosciences Clontech, Palo Alto, CA) was performed. Fusion proteins of the Gal4 DNA binding domain fused at its C terminus with ARF6-Q67L, ARF6-T27N, and ARF1-Q71L were cloned into pAS2–1. A library of the activation domain fused to human fetal brain cDNAs was in pACT2. Yeast strain cg-1945 was cotransformed with pAS2–1 containing ARF6-Q67L and pACT2 library, and clones that showed growth on plates lacking histidine were identified.
An In Vitro Binding Assay
Purified fusion proteins in equal molar concentrations were incubated for 30 min on ice in binding buffer (PBS, pH 7.2, 1 mM MgCl2, 1 mM DTT, 0.2% Triton X-100, 0.1% Tween 20) followed by incubation with S-agarose beads for 30 min at 4°C. Beads were washed with binding buffer and resulting protein complexes were analyzed by SDS-PAGE and Western blotting. Nitrocellulose membrane was incubated with anti-GST antibodies followed by detection with the Super-Signal chemiluminescence system (Pierce Chemical, Rockford, IL). Some gels were stained with Owl Silver stain kit (Owl Separation Systems, Portsmouth, NH).
RESULTS
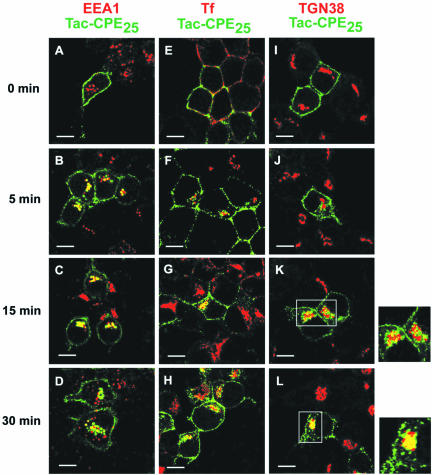
To examine the hypothesis that after secretory granule exocytosis, CPE recycles back to the TGN from the PM, we designed a Tac-CPE25 construct, where the extracellular domain of Tac was fused with the membrane binding domain and cytoplasmic tail of CPE (C-terminal 25 amino acids of CPE). Neuro2A cells that were transiently transfected with Tac-CPE25 were stimulated to exocytose by KCl. Tac-CPE25 exposed at the PM of these cells were labeled with 50 μg/ml mouse monoclonal anti-Tac antibody (ab) for 30 min at 4°C (Figure 1). Cells were then chased for 0, 5, 15, and 30 min at 37°C in ab-free DMEM, fixed, permeabilized, and probed with fluorescently conjugated anti-mouse secondary ab to detect internalized Tac-CPE25. The cells were also stained with anti-TGN38 ab to label the TGN (Figure 1, I–L). The endocytosed Tac-CPE25 was extensively colocalized with TGN38 after 30 min of internalization (Figure 1L). This observation indicates that Tac-CPE25 recycled from the PM to the TGN.
Figure 1.
Tac-CPE25 traffics from the PM through the endocytic recycling compartment to the TGN. Neuro2A cells expressing Tac-CPE25 were labeled for 30 min at 4°C with anti-Tac monoclonal antibodies alone (A–D and I–L) or with both anti-Tac antibodies and fluorescent-labeled Tf (red) (E–H). Cells were chased at 37°C for the indicated times. The cells were then fixed in 4% paraformaldehyde, permeabilized, and incubated with anti-EEA1 (A–D) or anti-TGN38 (I–L) antibodies followed by appropriate fluorescent-labeled secondary antibodies (both red). To follow anti-Tac antibody uptake, cells were probed with Alexa 488-conjugated goat anti-mouse (E–H and I–L) or rabbit anti-mouse (A–D) secondary antibodies (green). Single confocal microscope sections are shown. Colocalization on the merged images looks yellow. High resolution analysis of the TGN area (boxed K and L) are shown to the right of the panel confirming colocalization of Tac-CPE25 with TGN38 at 30 min. Bars, 10 μM. Note that Tac-CPE25 was found in early endosomes after 5 min, in the endocytic recycling compartment, labeled with Tf, after 15 min, and in TGN after 30 min of internalizaition.
To determine whether the Tac-CPE25 from the PM was internalized by a clathrin-dependent mechanism, we used fluorescent-conjugated Tf as a marker for the clathrin-mediated recycling pathway. After stimulation of exocytosis, Neuro2A cells expressing Tac-CPE25 were coincubated at 4°C for 30 min with Tf and anti-Tac ab, chased for 0, 5, 15, and 30 min at 37°C in Tf- and ab-free media, followed by fixation (Figure 1, E–H). Tac-CPE25 was partially colocalized with Tf only after 15 min of internalization (Figure 1G), but not at the early (5 min; Figure 1F) or late (30 min; Figure 1H) steps of endocytosis. This suggests that Tac-CPE25 was internalized by a clathrin-independent mechanism into early endosomes and subsequently moved into the Tf-positive endocytic recycling compartment, a common compartment for both clathrin-dependent, as well as -independent pathways (Chatterjee et al., 2001). The extensive colocalization of internalized Tac-CPE25 with the early endosomal marker EEA1 at 5 (Figure 1B) and 15 min (Figure 1C) of endocytosis further indicates that Tac-CPE25 trafficked to the TGN through the recycling compartment, but not through late endosomes. After a 90-min chase, some Tac-CPE25 was detected in secretory granules (our unpublished data). The same results were also obtained in the AtT20 cell line (our unpublished data), indicating that recycling of Tac-CPE25 through an EEA1-positive, Tf-negative early endosomal compartment, and the ERC, to the TGN, is not cell-type specific.
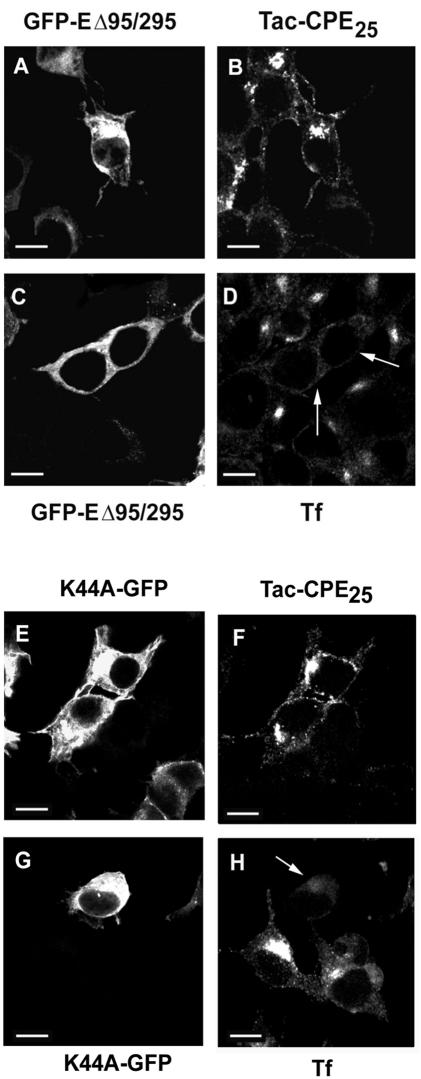
To further confirm that CPE was internalized from the PM by a clathrin-independent pathway, we used the Eps15 mutant GFP-EΔ95/295, which was reported to inhibit the assembly of clathrin-coated pits and therefore blocks clathrin-dependent endocytosis (Benmerah et al., 1999). Neuro2A cells were cotransfected overnight with Tac-CPE25 and Eps15 mutant. Then cells were incubated for 30 min at 4°C with anti-Tac ab (Figure 2, A and B) or with Tf-Alexa 594 (Figure 2, C and D) and then for 15 min at 37°C to allow endocytosis. As shown in Figure 2, Eps15 mutant effectively blocked internalization of Tf (Figure 2D), but did not inhibit endocytosis of Tac-CPE25 (Figure 2B). Furthermore, coexpression of another mutant protein, AP180-C, which completely inhibits the clathrin-mediated pathway (Ford et al., 2001), also had no effect on the internalization of Tac-CPE25 (our unpublished data). To determine whether internalization of Tac-CPE25 was dependent on dynamin-2, we cotransfected cells with Tac-CPE25 and wild-type dynamin-2 (our unpublished data) or a mutant dynamin-2-K44A (Figure 2, E–H), which has been reported to block endocytosis from clathrin-coated pits (Schmid et al., 1998). We found that neither wild type (our unpublished data) nor K44A mutant of dynamin-2 had any effect on Tac-CPE25 internalization (Figure 2, E and F), but K44A mutant inhibited internalization of Tf (Figure 2, G and H). Together, these data strongly indicate that CPE is internalized by a clathrin and dynamin-2-independent pathway.
Figure 2.
Eps15 mutant and dynamin-2 mutant K44A do not block internalization of Tac-CPE25. Neuro2A cells cotransfected with GFP-EΔ95/295 and Tac-CPE25 (A–D) or with K44A and Tac-CPE25 (E–H) were incubated with anti-Tac ab (A, B, E, and F) or with Tf-Alexa 594 (C, D, G, and H) for 30 min at 4°C. Cells were then chased for 15 min at 37°C in Tf- or ab-free media, fixed, permeabilized, and probed with Alexa 568-conjugated anti-mouse antibody to follow internalization of Tac-CPE25. Single confocal microscope sections are shown. Arrows point to the cells where mutants were expressed and Tf was not internalized. Of 100 cells transfected with Eps15 mutant (GFP-EΔ95/295) or dominant negative mutant of Dynamin 2 (K44A-GFP) counted, >85% of them showed internalization of Tac-CPE25 but not transferrin. Bars, 10 μM.
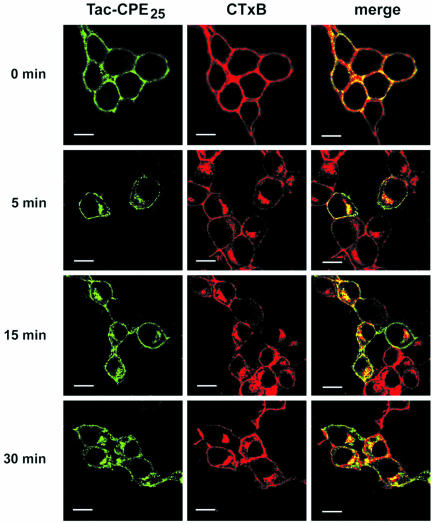
We next examined the distribution of Tac-CPE25 and CTxB, a lipid raft marker, which traffics from the PM to the Golgi complex by a clathrin-independent pathway (Orlandi and Fishman, 1998). Neuro2A cells, expressing Tac-CPE25 were stimulated, coincubated with CTxB and anti-Tac ab for 30 min at 4°C, and then chased in serum-free DMEM for 0, 5, 15, and 60 min at 37°C (Figure 3). As shown in Figure 3, a major portion of internalized Tac-CPE25 was found in CTxB-positive endocytic compartments during recycling from the PM to the TGN. In addition, CTxB was found in EEA1-positive, transferrin-negative early endosomes (our unpublished data). The same results were obtained in AtT20 cells (our unpublished data). These findings indicate that Tac-CPE25 is delivered from the PM to the TGN through the ERC by a clathrin-independent pathway, similar to the lipid raft marker CTxB.
Figure 3.
Tac-CPE25 and the lipid raft marker, cholera toxin B subunit, internalize and reach the TGN by the same endocytic pathway. Neuro2A cells expressing Tac-CPE25 were labeled for 30 min at 4°C with anti-Tac monoclonal ab and CTxB-Alexa 594 (red), and then chased for the indicated times at 37°C. Cells were fixed, permeabilized, and labeled with goat anti-mouse secondary antibody-Alexa 488 (green) to visualize anti-Tac antibody uptake. Single confocal microscope sections are shown. Colocalization on the merged images looks yellow. Bars, 10 μM.
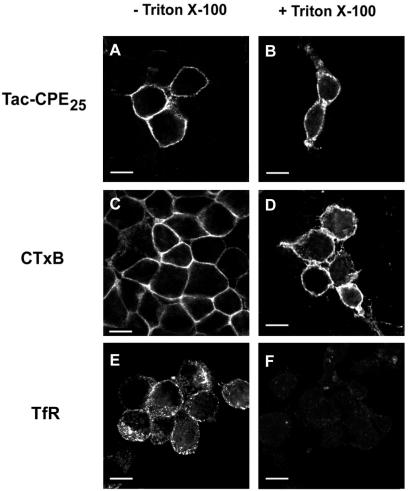
We have previously shown that CPE is associated with lipid rafts in secretory granules and in TGN membranes (Dhanvantari and Loh, 2000). Here, we examined the hypothesis that Tac-CPE25 remains associated with rafts in the PM after exocytosis. We used Neuro2A cells expressing Tac-CPE25 that were stimulated to exocytose. To label raft and nonraft components at the PM, cells were incubated at 4°C with anti-Tac ab or CTxB. Before fixation, cells were treated with 1% Triton X-100 to remove nonraft proteins (Figure 4). We found that Tac-CPE25 and GM1 (as a component of lipid rafts) were resistant to Triton X-100 extraction (Figure 4, B and D, respectively), whereas transferrin receptor (TfR) (a nonraft protein) was completely removed from the PM (Figure 4F). These data show that Tac-CPE25 remains associated with rafts after delivery to the PM.
Figure 4.
Tac-CPE25 remains in lipid rafts on the cell surface after exocytosis. Neuro2A cells expressing Tac-CPE25 were stimulated to exocytose and then were incubated at 4°C with anti-Tac ab (A and B) or CTxB-Alexa Fluor 594 (C and D). Before fixation, cells were treated for 30 min at 4°C with 1% Triton X-100. After fixation cells were stained with anti-TfR ab (E and F). Bars, 10 μM.
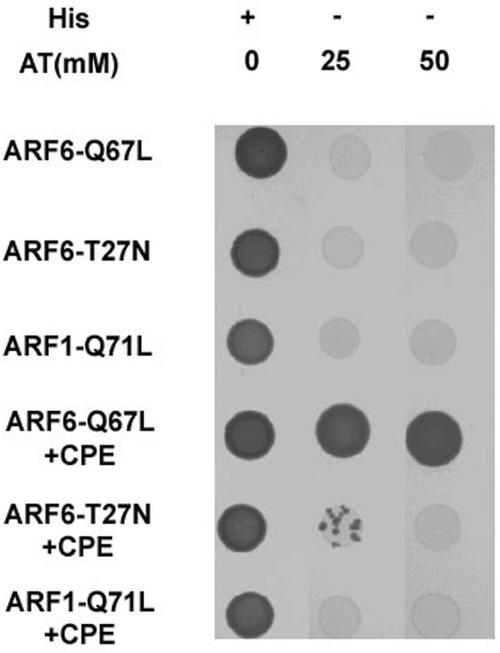
A yeast two-hybrid screen of a human fetal brain library by using ARF6-Q67L as bait identified a clone containing the last 90 amino acids of human CPE. This clone was isolated several times. This interaction was tested against ARF6-Q67L (ARF6-GTP form), ARF6-T27N (dominant negative mutant of ARF6), and ARF1-Q71L (ARF1-GTP) in a growth assay. In the absence of histidine, strong growth occurred only in yeast expressing ARF6-Q67L (Figure 5). Some growth occurred with ARF6-T27N at low stringency, but significantly, no growth occurred with expression of ARF1-Q71L. This ARF specificity is particularly striking given the similarity in the effector, switch I and II, regions in both ARF1 and ARF6.
Figure 5.
A yeast two-hybrid screen using ARF6-Q67L as bait and a fetal brain library identified a clone containing the last 90 amino acids of CPE. ARF6-Q67L, ARF6-T27N, and ARF1-Q71L were cloned into bait vector pAS2–1. Yeast strain CG-1945 was cotransformed with prey plasmid pACT2 alone (top three rows) or the pACT2 library clone containing the C-terminal 90 amino acids of CPE (bottom three rows). Transformants were spotted onto plates lacking leucine and tryptophan with (+) or without (-) histidine (His) in the presence of 25 or 50 mM 3-amino-1,2,4-triazole (3-AT). Vigorous growth was observed for cells coexpressing the ARF6-Q67L bait plasmid and the CPE clone, even at the highest concentration of 3-AT tested (fourth row). Slight growth was observed for cells coexpressing ARF6-T27N and CPE, but only at the lower concentration of 25 mM 3-AT (fifth row). ARF1-Q71L showed no interaction with CPE. No growth was observed at either concentration of 3-AT for cells expressing ARF6-Q67L and the pACT2 vector alone (top row).
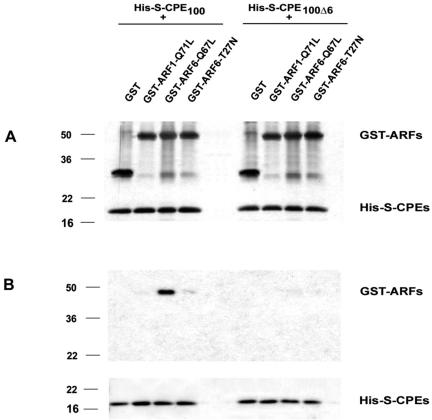
A direct interaction between CPE and ARF6 was further confirmed by an in vitro binding assay using purified fusion proteins (Figure 6). Fusion proteins containing the C terminus of CPE with (His-S-CPE100) or without the cytoplasmic tail (His-S-CPE100Δ6) were incubated with different forms of ARF6 followed by incubation with S-protein agarose beads for 30 min at 4°C. Proteins that remain bound to the beads after extensive washes were analyzed by Western blot. As shown in Figure 6B, ARF6-Q67L bound to His-S-CPE100, but not to His-S-CPE100Δ6, indicating that only the cytoplasmic tail of CPE was involved in this interaction. We also found that ARF1-GTP did not bind with the cytoplasmic tail of CPE. The dominant negative mutant of ARF6, ARF6-T27N, showed very low interaction with His-S-CPE100. Thus, the results of the in vitro binding assay and yeast two-hybrid data show specificity of binding between the cytoplasmic tail of CPE and the active form of ARF6.
Figure 6.
The cytoplasmic tail of CPE interacts with ARF6. In vitro binding assay of fusion proteins shows interaction of six amino acids of CPE C-terminus with active form of ARF6. (A) Input (10%) of fusion proteins was analyzed by SDS-PAGE followed by staining with Silver Stain kit. (B) Fusion proteins, bound to S-agarose beads were analyzed by Western blot. Nitrocellulose membrane was probed with anti-GST ab (top) or with Ponceau S (bottom).
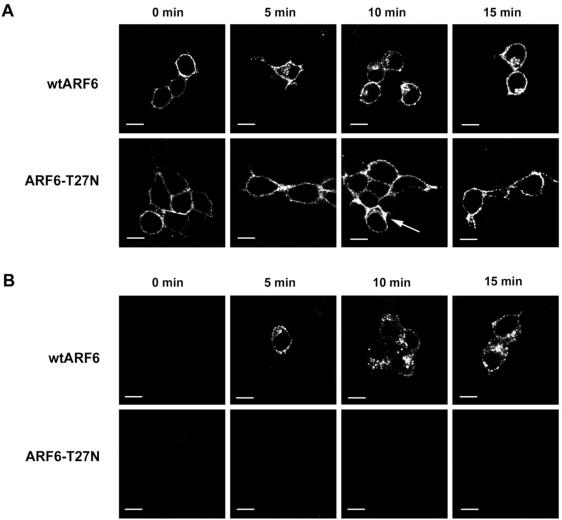
To determine whether ARF6 function is required for the recycling of Tac-CPE25, we examined whether expression of a dominant negative mutant of ARF6, T27N, would block recycling of Tac-CPE back to the TGN. Neuro2A cells were cotransfected with Tac-CPE25 and either the wtARF6 (Figure 7, A and B, top) or ARF6-T27N (Figure 7, A and B, bottom) constructs. Sixteen to 24 h after transfection, cells were stimulated to secrete. Cells were then labeled with anti-Tac ab for 30 min at 4°C and chased for 5, 10, and 15 min at 37°C in ab-free media. Cells expressing Tac-CPE25 and wtARF6 showed extensive internalization of anti-Tac ab (Figure 7A). However, in cells expressing the negative mutant of ARF6 (ARF6-T27N), anti-Tac ab bound with Tac-CPE25 remained on the PM even after 15-min chase (Figure 7B). These results indicate that expression of ARF6-T27N blocks internalization of Tac-CPE25. The same experiments have been done with uptake of Tf-Alexa 594. We found that in Neuro2A cells the dominant negative mutant of ARF6 did not inhibit internalization of Tf. As shown on Table 1, after 10 min of endocytosis only 24.6% ± 4.5 of cells coexpressing ARF6-T27N and Tac-CPE25 demonstrated internalization of anti-Tac ab. However, 88% ± 2.96 of these cotransfected cells showed internalization of Tf.
Figure 7.
Inactive ARF6 blocks internalization of Tac-CPE25. (A) Neuro2A cells cotransfected with wtARF6 and Tac-CPE25 (wtARF6) or with ARF6-T27N and Tac-CPE25 (ARF6-T27N) constructs after stimulation were labeled with anti-Tac antibody for 30 min at 4°C and chased for 5, 10, and 15 min at 37°C in antibody-free media. Cells were fixed, permeabilized, and incubated with rabbit anti-ARF6 antiserum followed by Alexa 568 goat anti-rabbit and Alexa 488 goat anti-mouse secondary antibodies to detect expressed ARF6 and anti-Tac antibody uptake, respectively. Images were obtained from cells, expressing both Tac-CPE25 and wtARF6 (or ARF6-T27N) proteins. Single confocal microscopy sections show the Tac-CPE25 immunostaining only. Arrow points to a cell, where ARF6-T27N was not expressed and therefore internalization of Tac-CPE25 was not inhibited. Note that cells expressing wtARF6 demonstrate PM-bound and internalized anti-Tac antibody staining, whereas cells expressing negative mutant of ARF (ARF6-T27N) show only PM-bound anti-Tac antibodies. Bars, 10 μM. (B) Neuro2A cells cotransfected with wtARF6 and Tac-CPE25 (wtARF6) or with ARF6-T27N and Tac-CPE25 (ARF6-T27N) constructs were labeled with anti-Tac antibody for 30 min at 4°C and chased for 5, 10, and 15 min at 37°C in antibody-free media, as in A. To remove anti-Tac antibodies remaining at the PM, the cells were washed with Na-acetate buffer (pH 3.0) at 4°C before fixation. To detect ARF6 and internalized anti-Tac antibodies, fixed cells were labeled with anti-ARF6 rabbit antiserum followed by incubation with appropriate fluorescent-conjugated secondary antibodies. Images were taken from cells, expressing both Tac-CPE25 and wtARF6 (or ARF6-T27N) proteins. Single confocal microscopy sections show the Tac-CPE25 immunostaining only. Bars, 10 μM.
Table 1.
Quantification of Tac-CPE25 and Tf internalization in the presence of wild-type or dominant negative mutant of ARF6
| wtARF6 | ARF6-T27N | |
|---|---|---|
| Tac-CPE25 | 91.15 ± 1.84% | 24.61 ± 4.5%* |
| Tf | 89.75 ± 0.85% | 88.00 ± 3.0% |
Neuro2A cells were cotransfected with Tac-CPE25 and either the wtARF6 or ARF6-T27N constructs. Anti-Tac ab and Tf uptake experiments were performed as described in MATERIALS AND METHODS. Numbers represent mean % ± SEM of total cells counted that showed internalized Tac-CPE25 or Tf after 10 min of endocytosis. Cells (100) were counted for each condition from each of four independent experiments. Difference between wild-type and mutant ARF6 is significant to P < 0.001 (*) (analysis by Student's t test).
To distinguish whether Tac-CPE25 coexpressed with ARF6-T27N was actually trapped on the PM or in early endosomes just beneath the PM, cells were washed after anti-Tac ab uptake with low pH buffer to remove all ab remaining on the PM after internalization (Figure 7B). Internalized Tac was observed only in the cells coexpressing Tac-CPE25 and wild-type ARF6, and not in the cells coexpressing Tac-CPE25 and ARF6-T27N (Figure 7B). Expression of a dominant negative mutant of ARF6 such as T27N in addition to being in the inactive form, blocks the activation of the endogenous, wild-type protein. This result indicates that ARF6 activity is necessary for internalization of Tac-CPE25.
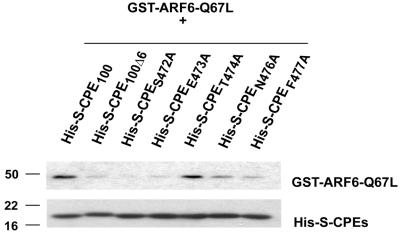
To further investigate whether direct interaction of the CPE cytoplasmic tail with active form of ARF6 is necessary for internalization of CPE, we mutated each amino acid on the cytoplasmic tail of CPE singly and performed in vitro binding assays (Figure 8). Purified fusion proteins containing the mutated C terminus of CPE (His-S-CPES472A, His-SCPEE473A, His-S-CPET474A, His-S-CPEN476A, and His-SCPEF477A) were incubated for 30 min at 4°C with GST-ARF6-Q67L followed by incubation with S-protein agarose beads for 30 min at 4°C. Proteins bound to the beads were analyzed by Western blot by using anti-ARF6 ab (Figure 8, top) and S-protein-HRP conjugate (Figure 8, bottom). As shown on Figure 8, two mutants (His-S-CPES472A and His-SCPEE473A) were unable to interact with active form of ARF6 (top, lanes 3 and 4, respectively). The other mutants showed various degrees of interaction with ARF6.
Figure 8.
Mutation of the CPE cytoplasmic tail prevents its interaction with active form of ARF6. In vitro binding assay of fusion proteins shows two mutations that prevents interaction of CPE C terminus with ARF6-Q67L. Fusion proteins, bound to S-agarose beads were analyzed by Western blot. Nitrocellulose membrane was probed with anti-ARF6 ab (top) or with S-protein-HRP conjugate (bottom).
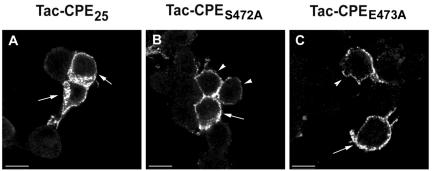
We made the same two mutations on the Tac-CPE25 construct (Tac-CPES472A and Tac-CPEE473A) and transfected these constructs into Neuro2A cells. After overnight transfection, cells were stimulated to exocytose by KCl and then incubated at 4°C for 30 min with anti-Tac ab. Cells were then chased for 0, 5, 10, and 15 min at 37°C in ab-free media, fixed, permeabilized, and probed with fluorescently conjugated anti-mouse secondary ab to detect internalized Tac-CPES472A, Tac-CPEE473A, or Tac-CPE25 (Figure 9). Even after 15 min of endocytosis both mutants stayed on the PM, whereas Tac-CPE25 was effectively internalized (Figure 9). Only 36.4 ± 4.7% of cells expressing Tac-CPES472A and 25.15% ± 5.9 of cells expressing Tac-CPEE473A had internalized anti-Tac ab (Table 2). These data show that mutants unable to bind to ARF6-Q67L were poorly internalized. Therefore, direct interaction with active form of ARF6 is necessary for internalization of CPE.
Figure 9.
CPE mutants unable to bind active ARF6 are not internalized. Neuro2A cells expressing (A), Tac-CPES472A (B), and Tac-CPEE473A (C) were stimulated to exocytose, incubated at 4°C with anti-Tac ab and then chased for 15 min at 37°C. Cells were fixed, permeabilized, and labeled with goat anti-mouse secondary antibody-Alexa 488 (green) to visualize anti-Tac antibody uptake. Single confocal microscope sections are shown. Arrows point to cells that were counted as having internalized anti-Tac ab (Table 2), albeit the internalization of mutants (B and C) was much less than in wild-type Tac-CPE25 (A). Arrowheads show cells that were counted as having no internalized ab (Table 2). Bars, 10 mM.
Table 2.
Quantification of internalization of Tac-CPE25 mutants
| Mean ± SEM of cells with internalized anti-Tac ab (%) | |
|---|---|
| Tac-CPE25 | 71.25 ± 7 |
| Tac-CPES472A | 36.4 ± 4.7* |
| Tac-CPEE473A | 25.15 ± 5.9** |
Neuro2A cells were transfected with Tac-CPE25, Tac-CPES472A, and Tac-CPEE473A constructs. Anti-Tac ab uptake experiments were performed as described in MATERIALS AND METHODS. More than 100 cells were counted for each condition from each of four independent experiments. Difference between wild-type and mutants is significant to P < 0.05 (*) for Tac-CPES472A and P < 0.01 (**) for Tac-CPEE473A (analysis by Student's t test).
DISCUSSION
In this study, we show that the raft-associated transmembrane domain together with the cytoplasmic tail of CPE is sufficient for directing the internalization and transport of a foreign protein Tac, from the PM to the TGN in neuroendocrine and endocrine cells (Neuro2A and AtT20, respectively). Lipid raft-associated Tac-CPE25 was first internalized from the PM into early endosomes, separate from Tf. Subsequently, it was transported to the ERC as evidenced by its colocalization with Tf after 15 min of endocytosis. Tac-CPE25 was then transported to the TGN and later was observed in the secretory granules. Furthermore, appending the C-terminal 25 amino acids onto Tac clearly alters the trafficking of this protein because Tac alone is not a raft-associated protein and is not sorted to the TGN after endocytosis (Naslavsky et al., 2003). The presence of raft-associated CPE on the PM, and in EEA1-positive compartments of AtT20 cells suggests that endogenous CPE is also recycled in a similar manner (Arnaoutova, unpublished data).
The present study indicates for the first time that transmembrane CPE deposited on the PM after exocytosis is rapidly internalized and recycled back to the TGN. Interestingly, it seems to be resident in the TGN for >1 h before being packaged into SGs, consistent with the role of membrane CPE as a prohormone sorting receptor at the TGN. Lack of inhibition of Tac-CPE25 internalization when Neuro2A cells were cotransfected with Eps15 or AP180 mutants indicated that Tac-CPE25 was internalized by a clathrin-independent pathway. Indeed, raft-associated GPI-anchored proteins are also internalized by clathrin-independent pathways (Bamezai et al., 1992; Mayor et al., 1998; Skretting et al., 1999; Ricci et al., 2000; Nichols et al., 2001). CPE recycles through the ERC en route to the TGN, unlike other proteins such as furin (Mallet and Maxfield, 1999) and P-selectin (Straley and Green, 2000) that move from the PM to the TGN, passing through the early endosomes and late endosomes, but without entering the ERC.
Here, we also describe for the first time a novel mechanism of internalization of a raft-associated protein, CPE. We demonstrated that the binding of ARF6 with the cytoplasmic tail of CPE is required for its internalization. No internalization of Tac-CPE25 was observed upon coexpression with T27N, a GTP-binding defective mutant of ARF6 that inhibits endogenous ARF6 function (Radhakrishna et al., 1996; Radhakrishna and Donaldson, 1997; Song et al., 1998). In contrast, internalization seemed to be normal for Tac-CPE25, coexpressed with wtARF6 or ARF6-Q67L. Yeast two-hybrid and in vitro binding experiments and in vivo site-directed mutagenesis studies showed a direct physical interaction of active ARF6 with the cytoplasmic tail (S472ETLNF477) of CPE. Remarkably, the activated form of ARF1 does not interact with CPE attesting to the specificity of this interaction. Together, the results indicate that SETLNF might be an ARF6 binding motif.
ARF6 is localized at the PM in the GTP-bound form and its activity is required for multiple cellular functions, including remodeling of F-actin (Radhakrishna et al., 1996; Song et al., 1998), Fcγ receptor-mediated phagocytosis (Zhang et al., 1998), and various PM/endosomal recycling events (Radhakrishna and Donaldson, 1997; Bose et al., 2001; Lawrence and Birnbaum, 2001). ARF6 is also associated with endosomal membranes derived from clathrin-independent endocytosis, through which full-length Tac enters and recycles. Although ARF6 activities can modulate the flow of membrane through this system, it is not specifically required for internalization into this pathway (Brown et al., 2001; Naslavsky et al., 2003). However, ARF6 activity was recently shown to be involved in the internalization of ligand-activated β2-adrenergic and choriogonadotropic receptors from the PM via clathrin-mediated endocytosis (Claing et al., 2001; Salvador et al., 2001). Furthermore, in our present study we have demonstrated a requirement not only for ARF6 activity but also physical interaction with ARF6 for endocytosis of CPE via clathrin-independent, raft-mediated internalization. ARF6 activates phospholipase D and phosphatidylinositol 4-phosphate 5-kinase, enzymes involved in membrane lipid remodeling. The interaction of ARF6 with the SETLNF cytoplasmic tail of CPE may induce changes in the membrane lipid environment, perhaps involving other protein interactions as well, to allow CPE anchored to lipid rafts at the PM to be internalized.
Acknowledgments
We thank Dr. F. Maxfield (Weill Medical College of Cornell University, New York, NY) and Dr. J. Lippincott-Schwartz (National Institutes of Health, Bethesda, MD) for helpful discussions. We thank Dr. A. Arnaoutov (National Institutes of Health, Bethesda, MD) for help in the generation of the His-SCPE constructs and purification of the fusion proteins. We also thank Dr. S. Milgram (University of North Carolina at Chapel Hill) for anti-TGN38 antibody, Dr. A. Benmerah (Institut Pasteur, Paris, France) for the GFP-EΔ95/295 construct, Dr. H. McMahon (University College London) for the AP180-C construct, and Dr. M. McNiven (Mayo Clinic, Rochester, MN) for Dynamin-2-CFP (wild type and K44A) constructs.
References
- Bamezai, A., Goldmacher, V.S., and Rock, K.L. (1992). Internalization of glycosyl-phosphatidylinositol (GPI)-anchored lymphocyte proteins. II. GPI-anchored and transmembrane molecules internalize through distinct pathways. Eur. J. Immunol. 22, 15-21. [DOI] [PubMed] [Google Scholar]
- Benmerah, A., Bayrou, M., Cerf-Bensussan, N., and Dautry-Varsat, A. (1999). Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112, 1303-1311. [DOI] [PubMed] [Google Scholar]
- Bose, A., Cherniack, A.D., Langille, S.E., Nicoloro, S.M., Buxton, J.M., Park, J.G., Chawla, A., and Czech, M.P. (2001). G(alpha)11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane. Mol. Cell Biol. 21, 5262-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, F.D., Rozelle, A.L., Yin, H.L., Balla, T., and Donaldson, J.G. (2001). Phosphatidylinositol 4, 5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, S., Smith, E.R., Hanada, K., Stevens, V.L., and Mayor, S. (2001). GPI anchoring leads to sphingolipid-dependent retention of endocytosed proteins in the recycling endosomal compartment. EMBO J. 20, 1583-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing, A., Chen, W., Miller, W.E., Vitale, N., Moss, J., Premont, R.T., and Lefkowitz, R.J. (2001). beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J. Biol. Chem. 276, 42509-42513. [DOI] [PubMed] [Google Scholar]
- Cool, D.R., Normant, E., Shen, F., Chen, H.C., Pannell, L., Zhang, Y., and Loh, Y.P. (1997). Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88, 73-83. [DOI] [PubMed] [Google Scholar]
- Dhanvantari, S., Arnaoutova, I., Snell, C.R., Steinbach, P.J., Hammond, K., Caputo, G.A., London, E., and Loh, Y.P. (2002). Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry 41, 52-60. [DOI] [PubMed] [Google Scholar]
- Dhanvantari, S., and Loh, Y.P. (2000). Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J. Biol. Chem. 275, 29887-29893. [DOI] [PubMed] [Google Scholar]
- Ford, M.G., Pearse, B.M., Higgins, M.K., Vallis, Y., Owen, D.J., Gibson, A., Hopkins, C.R., Evans, P.R., and McMahon, H.T. (2001). Simultaneous binding of PtdIns(4, 5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051-1055. [DOI] [PubMed] [Google Scholar]
- Fricker, L.D., Das, B., and Angeletti, R.H. (1990). Identification of the pH-dependent membrane anchor of carboxypeptidase E (EC 3.4.17.10). J. Biol. Chem. 265, 2476-2482. [PubMed] [Google Scholar]
- Katagiri, Y.U., Mori, T., Nakajima, H., Katagiri, C., Taguchi, T., Takeda, T., Kiyokawa, N., and Fujimoto, J. (1999). Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 274, 35278-35282. [DOI] [PubMed] [Google Scholar]
- Lawrence, J.T., and Birnbaum, M.J. (2001). Adp-ribosylation factor 6 delineates separate pathways used by endothelin 1 and insulin for stimulating glucose uptake in 3T3–L1 adipocytes. Mol. Cell Biol. 21, 5276-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, W.G., and Maxfield, F.R. (1999). Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146, 345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor, S., and Maxfield, F.R. (1995). Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol. Biol. Cell 6, 929-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor, S., Sabharanjak, S., and Maxfield, F.R. (1998). Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 17, 4626-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky, N., Weigert, R., and Donaldson, J.G. (2003). Convergence of nonclathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell 14, 417-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J. (2002). A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4, 374-378. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J., Kenworthy, A.K., Polishchuk, R.S., Lodge, R., Roberts, T.H., Hirschberg, K., Phair, R.D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi, P.A., and Fishman, P.H. (1998). Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141, 905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., and Donaldson, J.G. (1997). ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna, H., Klausner, R.D., and Donaldson, J.G. (1996). Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell Biol. 134, 935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci, V., Galmiche, A., Doye, A., Necchi, V., Solcia, E., and Boquet, P. (2000). High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPI-anchored protein and is not blocked by inhibition of the clathrin-mediated pathway of endocytosis. Mol. Biol. Cell 11, 3897-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411-423. [DOI] [PubMed] [Google Scholar]
- Salvador, L.M., et al. (2001). Activation of the luteinizing hormone/choriogonadotropin hormone receptor promotes ADP ribosylation factor 6 activation in porcine ovarian follicular membranes. J. Biol. Chem. 276, 33773-33781. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., Olsnes, S., Brown, J.E., Petersen, O.W., and van Deurs, B. (1989). Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J. Cell Biol. 108, 1331-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, S.L., McNiven, M.A., and De Camilli, P. (1998). Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10, 504-512. [DOI] [PubMed] [Google Scholar]
- Shen, F.S., and Loh, Y.P. (1997). Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in cpefat mice associated with a carboxypeptidase E mutation. Proc. Natl. Acad. Sci. USA 94, 5314-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skretting, G., Torgersen, M.L., van Deurs, B., and Sandvig, K. (1999). Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J. Cell Sci. 112, 3899-3909. [DOI] [PubMed] [Google Scholar]
- Song, J., Khachikian, Z., Radhakrishna, H., and Donaldson, J.G. (1998). Localization of endogenous ARF6 to sites of cortical actin rearrangement and involvement of ARF6 in cell spreading. J. Cell Sci. 111, 2257-2267. [DOI] [PubMed] [Google Scholar]
- Straley, K.S., and Green, S.A. (2000). Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules. J. Cell Biol. 151, 107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C.F., Dhanvantari, S., Lou, H., and Loh, Y.P. (2003). Sorting of carboxypeptidase E to the regulated secretory pathway requires interaction of its transmembrane domain with lipid rafts. Biochem. J. 369, 453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q., Cox, D., Tseng, C.C., Donaldson, J.G., and Greenberg, S. (1998). A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 273, 19977-19981. [DOI] [PubMed] [Google Scholar]