90% of all cervical cancers diagnosed every year are the result of an infection with the human papilloma virus (HPV).1,2 HPV is a small (8000 bp) double stranded DNA virus which primarily infects basal epithelial cells of the mucosa and skin.3 40 of the more than 130 identified human papilloma viruses identified to date are known to infect the genital mucosa.1 HPV viruses can be divided into low- and high risk types, depending upon their oncogenic potential. Importantly, infection with either the low- or high-risk phenotype results in proliferative disease, but only the high risk phenotype causes malignant transformation. Of the high risk HPV sub-types, HPV-16 accounts for 50–70%, and HPV-18 for 10–20%, of all cervical cancers diagnosed.1,2 In contrast, infection with the low risk HPV sub-types, such as HPV-6 or HPV-11, causes formation of genital warts.3
HPV infects basal keratinocytes, but full viral DNA replication occurs only in the fully differentiated keratinocyte of the upper layers of the stratum spinosum and granulosum of squamous epithelia during the later stages of the HPV life cycle.4 Since viral replication in the keratinocyte is highly dependent on the host DNA synthesis machinery, and DNA polymerases and replication factors are produced only in actively proliferating cells, HPV induces the expression of the E6 and E7 viral proteins from its own genome to override the host differentiation program and to activate DNA synthesis and prevent apoptosis of the host cell in response to the aberrant proliferative stimulation. Importantly, expression and cooperative action of both the E6 and E7 viral proteins is known to be necessary and sufficient for induction and maintenance of transformation of keratinocytes infected with HPV.5,6
Both the E6 and E7 viral proteins of transforming HPV viruses interact with and inactivate the action of intracellular proteins that are key to regulating cell cycle progression and apoptosis. For example, the E7 protein binds and inhibits the action of the retinoblastoma (Rb) tumor suppressor protein family.1,3 In contrast the main target of the E6 viral protein is p53.1–3 p53 is a transcription factor and tumor suppressor protein. In response to DNA damage, genotoxic stress, oncogenic alterations, and aberrant mitogenic stimulation p53 induces either growth arrest or apoptosis, depending upon cell type and/or external stimuli.7 p53 exerts its tumor suppressor functions by at least two mechanisms. As a transcription factor, p53 up- or down regulates the expression of genes necessary for promoting or inhibiting cell cycle arrest and apoptosis, respectively.7 In addition to its transcription-dependent functions, p53 regulates apoptosis induction by a transcription-independent mechanism which involves translocation of p53 to the mitochondria.8 At the mitochondria, p53 activates the main apoptotic effector proteins BAK and BAX, inducing their oligomerization and thereby initiating the apoptotic program.9–11 Activation of BAK and BAX by p53 involves a conformational change in these proteins and formation of homo-oligomers on the outer mitochondrial membrane. These oligomers are thought to function as channels through which inner mitochondrial pro-apoptotic factors necessary for the initiation of apoptosis are released.11 In addition, at the mitochondria p53 can bind and neutralize the anti-apoptotic function of the BCL-2 and BCL-XL proteins.12,13 Hence, induction of cell cycle arrest or apoptosis by p53 in response to E7 overexpression and subsequent reprogramming of the differentiation status of an HPV infected cell is countered by E6 overexpression, which induces p53 degradation.1–3
While the mechanisms of malignant transformation in response to infection with high risk HPV have been extensively studied and well characterized, little is known about why low-risk HPV fails to cause malignant disease. In this issue of Cancer Biology and Therapy14, Sun and colleagues present data that explores the reasons why low risk HPVs cause non-invasive lesions of infected primary epithelia. Since interaction with and degradation of p53 by the high risk HPV-E6 proteins is key to cellular transformation induced in response to infection with high risk HPV, Sun and coworkers hypothesized that the E6 proteins from low risk HPV might interact differently with p53, and exert different regulatory influences on this tumor suppressor protein. This hypothesis is supported by early studies that examined the binding and degradation of p53 by E6 proteins from low and high risk HPV. In particular, while high risk E6 proteins have been shown to be able to induce degradation of p53, low risk HPV E6 proteins are able to interact with p53 but are unable to induce degradation.15 Interestingly, low and high risk HPV E6 proteins bind to different regions within p53.16 The C-terminal domain of p53 (amino acid 376–384) interacts with both low and high risk E6 proteins, while the p53 core domain (amino acid 66–326) is contacted only by high risk E6 proteins; the latter interaction is believed to mediate interaction with E6-AP and target p53 for degradation.
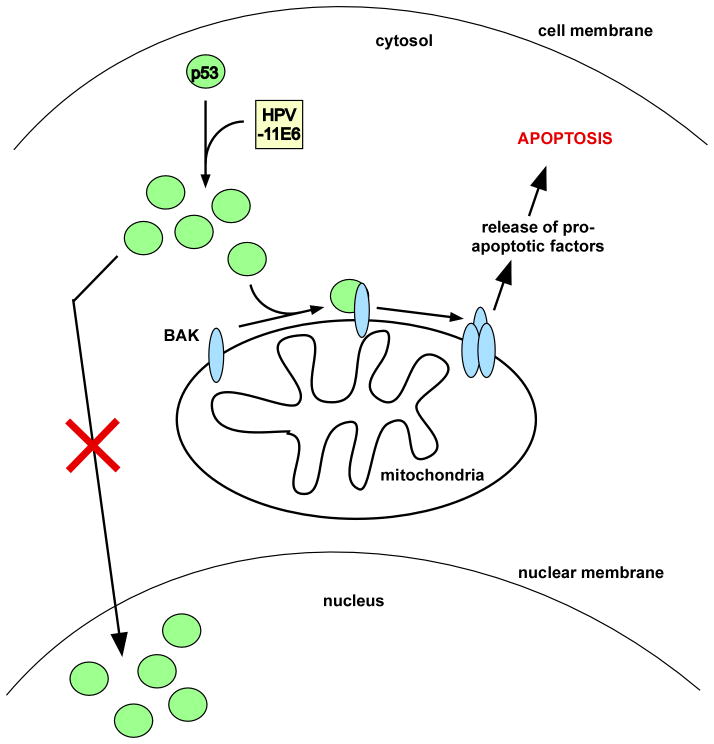
High risk HPV E6 appears to localize primarily to the nucleus.17,18 In the present study, Sun et al.14 expressed either green fluorescent protein-tagged or polyhistidine-tagged low risk HPV-11E6 fusion proteins in cell lines containing wild type p53. The authors found that both tagged versions of HPV-11E6 are primarily expressed in the cytoplasm and further that overexpression of HPV-11E6 results in cytoplasmic translocation of p53, binding of p53 to BAK, and induction of apoptosis (See Figure 1). Importantly, the authors find that induction of apoptosis in response to HPV-11E6 overexpression is dependent upon p53, thereby supporting the mitochondrial, transcription-independent role of p53 in apoptosis induction. Interestingly, in contrast to the observation by Sun et al.14, high risk HPV-E6 protein can induce the degradation not only of p53, but also of BAK; the ability of high risk E6 proteins to induce degradation of both p53 and BAK has been correlated with a global deficiency of high risk E6-infected cells to undergo apoptosis in response to a stress stimulus.15,19,20
Figure 1. HPV-11E6 traps p53 in the cytoplasm and induces apoptosis.
While high risk HPV E6 proteins are primarily localized to the nucleus, low risk HPV-11E6 localizes to the cytosol, and traps and stabilizes p53 in the cytosol. A fraction of the cytosolic p53 pool interacts with the pro-apoptotic mitochondrial protein BAK. As a result of interaction with p53, BAK undergoes a conformational change that allows BAK to oligomerize, allowing pro-apoptotic factors to be released into the cytosol and induce apoptosis.
One thing that should be noted is that susceptibility to the development of HPV induced cancers may not be dependent solely on negative regulatory influences of the high risk HPV E6 protein on p53, and that other factors may play a role. For example, it has been reported that a polymorphism at codon 72 of p53 (encoding proline or arginine, p53-P72 or p53-R72) influences the ability of high risk E6 to interact with p53.20 Additionally, high risk HPV-E6 has been found to induce the degradation of p53-R72 to a greater extent than p53-P72.21 Importantly, the p53-R72 variant is reported to have increased apoptotic potential, relative to the p53-P72 variant, due to increased localization of R72 to the mitochondria as well as enhanced interaction with BAK.22 The influence of the codon 72 polymorphism of p53 on interaction and apoptotic activity of p53 in response to infection of cells with low risk HPV E6 is an interesting issue that remains to be determined.
Ultimately, the study presented by Sun et al. suggests two interesting premises. First, the observation that trapping of p53 in the cytosol by HPV-11E6 results in apoptosis represents a novel mechanism to explain why low risk HPV infection does not result in malignant transformation; this explanation remains tentative at present, and awaits further testing. Additionally, however, these data provide further evidence for the significance and function of mitochondrially-localized p53 in mediating tumor suppression. Although not formally tested by cell fractionation or immunofluoresecence studies, data presented by Sun et al. strongly suggest that in response to HPV-11E6 a portion of the p53 protein trapped in the cytosol localizes to the mitochondria and interacts with the outer mitochondrial membrane protein BAK. This study therefore supports and strengthens prior reports that have demonstrated that targeting of p53 exclusively to the mitochondria by fusion of the mitochondrial leader peptide of ornithine transcarbamylase to the N-terminus of p53 results in induction of apoptosis in mammalian cells grown in culture which translates to repression of tumor formation in vivo.22,23 Clearly then there exist scenarios where p53 exclusively utilizes its mitochondrial functions in order to suppress cellular transformation.
References
- 1.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–11. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanley MA, Pett MR, Coleman N. HPV: from infection to cancer. Biochem Soc Trans. 2007;35:1456–60. doi: 10.1042/BST0351456. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32 (Suppl 1):S7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 8.Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–6. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–50. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 10.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 11.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 12.Tomita Y, Marchenko N, Erster S, Nemajerova A, Dehner A, Klein C, et al. WT p53, but not tumor-derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization. J Biol Chem. 2006;281:8600–6. doi: 10.1074/jbc.M507611200. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–90. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Zhang G, Lei T, Huang C, Song T, Si L. Two different HPV-11E6 fusion proteins trap p53 in the cytoplasm and induce apoptosis. Cancer Biol Ther. 2008;X:XXX. doi: 10.4161/cbt.7.12.6941. [DOI] [PubMed] [Google Scholar]
- 15.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70:4509–16. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao M, Kruhlak M, Xia S, Androphy E, Zheng ZM. Signals that dictate nuclear localization of human papillomavirus type 16 oncoprotein E6 in living cells. J Virol. 2003;77:13232–47. doi: 10.1128/JVI.77.24.13232-13247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman L, Schlegel R. Serum- and calcium-induced differentiation of human keratinocytes is inhibited by the E6 oncoprotein of human papillomavirus type 16. J Virol. 1996;70:3269–79. doi: 10.1128/jvi.70.5.3269-3279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–73. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leverrier S, Bergamaschi D, Ghali L, Ola A, Warnes G, Akgül B, et al. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis. 2007;12:549–60. doi: 10.1007/s10495-006-0004-1. [DOI] [PubMed] [Google Scholar]
- 21.Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–34. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 22.Dumont P, Leu JI, Della Pietra AC, 3rd, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–65. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 23.Talos F, Petrenko O, Mena P, Moll UM. Mitochondrially targeted p53 has tumor suppressor activities in vivo. Cancer Res. 2005;65:9971–81. doi: 10.1158/0008-5472.CAN-05-1084. [DOI] [PubMed] [Google Scholar]