SUMMARY
Normal cellular development and function requires tight spatiotemporal control of actin assembly. Formins are potent actin assembly factors that protect the growing ends of filaments from capping proteins. However, it is unresolved how the duration of formin-mediated actin assembly events is controlled, whether formins are actively displaced from growing ends, and how filament length is regulated in vivo. Here, we identify Bud14 as a high affinity inhibitor of the yeast formin Bnr1 that rapidly displaces the Bnr1 FH2 domain from growing barbed ends. Consistent with these activities, bud14Δ cells display fewer actin cables, which are aberrantly long, bent, and latrunculinA-resistant, leading to defects in secretory vesicle movement. Moreover, bud14Δ suppressed mutations that cause abnormally numerous and shortened cables, restoring wild type actin architecture. From these results, we propose that formin displacement factors regulate filament length and are required in vivo to maintain proper actin network architecture and function.
Keywords: Actin, formin, Rho, polarity, yeast
INTRODUCTION
Formins are a conserved family of actin assembly-promoting factors that employ a novel mechanism for building actin networks that drive cell movement, morphogenesis, and division (Faix and Grosse, 2006; Goode and Eck, 2007; Kovar, 2006). The conserved FH2 domain of formins catalyzes de novo actin polymerization, and processively associates with growing barbed ends of nascent filaments, protecting them from capping proteins. The adjacent FH1 domain accelerates filament growth by recruiting profilin-actin. These activities of the FH1 and FH2 are employed in vivo to assemble diverse actin-based structures, including polarized cables, stress fibers, filopodia, cytokinetic actin rings, and lammelipodia.
How formin activities are regulated spatially and temporally in vivo remains only partially understood. A large class of formins are autoinhibited via intramolecular interactions between their N-terminal DID and C-terminal DAD domains, and released/activated by Rho GTPase binding. However, it has been uncertain whether additional, non-Rho factors contribute to regulating formin transitions between their active and inactive states. It is also unclear how the length of actin filaments produced by formins is controlled. This is particularly important given that many actin networks assembled by formins are comprised of relatively short filaments 0.3–13 μm in length (e.g. cytokinetic rings, stress fibers, and cables) (Cramer et al., 1997; Kamasaki et al., 2005). The length of these filaments suggests that the duration of a formin activity cycle must be very rapid (< 5 sec). However, purified formins persist on barbed ends much longer than could account for the short filament lengths produced in vivo, even when challenged with capping proteins in vitro (Kovar and Pollard, 2004; L. Blanchoin, personal communication). Thus, additional cellular factors may be required to catalyze formin displacement from barbed ends to restrict filament length.
In this study, we have investigated formin temporal regulation in S. cerevisiae. Yeast contain two prominent F-actin structures visible throughout the cell cycle, cortical patches assembled by Arp2/3 complex, which mediate endocytosis, and cables assembled by formins, which direct polarized secretion and cell morphogenesis (Moseley et al., 2006). S. cerevisiae expresses two formins, Bni1 and Bnr1, which have distinct localization patterns and functions. Bni1 particles are transiently recruited from the cytoplasm to the bud cortex (Buttery et al., 2007), where they assemble short actin filaments organized into cables (Pruyne et al., 2004), then are released and/or incorporated into the cables. In contrast, Bnr1 remains stably anchored to the neck region, and assembles filaments incorporated into cables that fill the mother cell. These two complementary sets of cables serve as polarized tracks for myosin V-dependent transport of secretory vesicles and other cargoes required for growth of the daughter cell. Genetic evidence has suggested that Bni1 and Bnr1 are autoinhibited, then activated by Rho GTPases (Evangelista et al., 2003). Further, Bud6 has been shown to function as a co-factor for Bni1-mediated actin assembly (Moseley et al., 2004). Little else is known about how Bni1 and Bnr1 activities are regulated in vivo, but the stable localization of Bnr1 suggests that it must be regulated through rapid and tightly controlled cycles of activation/inactivation.
Here, we identify the S. cerevisiae cell polarity factor Bud14 as a novel Bnr1 regulator. Previous studies showed that Bud14 interacts with Glc7 (protein phosphatase type 1) to control dynein-dependent microtubule sliding at the bud cortex and facilitate nuclear migration (Knaus et al., 2005). In addition, deletion of BUD14 caused a poorly understood hyper-elongated bud phenotype (Cullen and Sprague, 2002). The observation that Bud14 overexpression was conditionally lethal and exacerbated by bni1Δ hinted that it may have additional roles in actin-based cell morphogenesis events (Cullen and Sprague, 2002). We show that Bud14 directly controls formin activity to regulate actin cable architecture and function.
RESULTS
Loss of BUD14 causes altered actin cable architecture
To identify novel regulators of Bnr1-mediated actin cable assembly, we visually screened yeast strains carrying deletions of non-essential genes that encode bud neck resident proteins for actin cable abnormalities in the mother cell compartment. Of the 28 strains examined, one (bud14Δ) showed clear defects in cable organization. A large percentage of bud14Δ cells contained at least one cable that was exaggerated in length and kinked or buckled where it reached the cell cortex (Fig. 1A, arrows in lower panels; quantified in Fig. 1B). Such hyper-extended cables were rarely observed in wild type cells, and cable ‘turns’ at the cortex in wild type cells were not as sharp as in bud14Δ cells (Fig. 1A, arrows in upper panels). In addition, bud14Δ cells showed a significant decrease (~50%) in number of visible cables compared to wild type cells (Fig. 1C and Fig. S1).
Figure 1. Actin cable defects in bud14Δ cells.
(A) Representative architectural defects in actin cables found in bud14Δ cells. Whereas cables in wild type cells contoured the cell cortex (large arrow, upper panels), cables in bud14Δ cells were “kinked” or “buckled” (small arrow, lower panels). Cables that changed direction with > 90 degree turn at the cortex were scored as “bent”. (B) Data quantified from two experiments. (C) Total number of actin cables visible in bud14Δ versus wild type cells. Data averaged from two experiments. (D) Representative images from experiments in which wild type and bud14Δ cells were treated with 20 μM LatA for 60 seconds, fixed, and stained with Alexa-488-phalloidin to score percentage cells with visible cables. “Enhanced” panels are the same images (LatA-treated) contrast-enhanced to highlight the remaining cables. (E) Cells were treated as above with LatA for different lengths of time before fixation and imaging (0–120 sec). Data were quantified from at least two independent experiments (**=p<.001, *= p<.05). (F) Yeast strains were serially diluted and compared for growth at 25 C and 34 C on YPD plates. (G) GFP-Sec4 localization in strains grown at 25°C. Percentage of cells with a bright spot of GFP-Sec4 at the bud tip was scored (n>100), and is listed in each panel. (H) DIC microscopy of strains in F.
One model to explain these observations is that bud14Δ cells contain fewer cables comprised of abnormally long individual filaments. We tested this possibility using two independent methods. First, we compared wild type and bud14Δ cells for retention of visible cables after treatment with the actin monomer sequestering drug latrunculin A (LatA) (Fig. 1D). A single cable is comprised of many short, bundled actin filaments and is highly dynamic. Cables are continuously synthesized and rapidly turned over. As a result, visible cable staining in wild type cells is completely lost after less than one min of exposure to LatA (Okada et al., 2006; Yang et al., 2001). Therefore, a cable that is comprised of abnormally short filaments should be hyper-sensitive to LatA treatment, whereas a cable that is comprised of abnormally long filaments should be resistant to LatA. Wild type and bud14Δ cells were treated for different times with a low concentration of LatA (20 μM) and scored for percentage of cells with visible actin cables (Fig. 1E). Further, from a plot of these data we derived the time required for half of the cells to lose visible cables (see Methods): wild type, T1/2 = 27 sec; bud14Δ, T1/2 = 53 sec. These data indicate that bud14Δ cells contain LatA-resistant cables. Further, these cables appeared to be the same hyper-extended and/or kinked cables described above, suggesting a correlation between abnormal architecture and increased LatA resistance. These observations support the model that the altered cables in bud14Δ cells are comprised of abnormally long filaments.
An alternative model is that bud14Δ changes the decoration of cables by stabilizers and/or destabilizers to reduce cable turnover. However, this model seems less likely given that Bud14 does not directly bind F-actin, does not affect F-actin dynamics in vitro, and does not decorate F-actin in vivo (see below).
As a second test of the cable architecture model, we performed genetic crosses to examine bud14Δ interactions with two other mutations that affect cable architecture, tpm1Δ and aip1Δ. Loss of the filament stabilizer Tpm1 results in greatly diminished actin cable staining and loss of polarity, resulting in enlarged cell morphology and temperature sensitive growth (Liu and Bretscher, 1992). In contrast, loss of the filament destabilizer Aip1 results in thickened, hyper-stabilized cables without obvious effects on cell morphology (Okada et al., 2006; Rodal et al., 1999). Notably, bud14Δ strongly suppressed the temperature sensitive cell growth defects and partially rescued the cell morphology and actin defects of tpm1Δ (Fig. 1F and 1H; also Fig. S2). This result supports our model, since bud14Δ cells have exaggerated cables and tpm1Δ cells have diminished shortened cables. To further assess rescue, we transformed each strain with a low copy plasmid expressing GFP-Sec4, a marker for secretory vesicles (Schott et al., 2002). Delivery of secretory vesicles to the bud tip requires type V myosin (Myo2) transport on actin cables, which makes polarized accumulation of GFP-Sec4 a sensitive in vivo assay for cable function. Each strain was scored for percentage of cells with a bright spot of GFP-Sec4 at the bud tip (Fig. 1G): wild type (97%), bud14Δ (93%), tpm1Δ (3%), and bud14Δtpm1Δ (49%).
In contrast to the rescue of tpm1Δ by bud14Δ, bud14Δaip1Δ double mutants showed compounded defects in cell morphology, with approximately 34% of double mutant cells being grossly elongated and misshapen (Fig. 1H). Thus, bud14Δ has opposite genetic interactions with tpm1Δ and aip1Δ. Taken together with the observation that bud14Δ cells contain LatA-resistant cables, these data support the model that loss of Bud14 results in elongated and bent cables comprised of abnormally long actin filaments.
Bud14 functions upstream of Bnr1 to control actin cable assembly and polarized cell growth
We next tested the genetic relationship between Bud14 and the formins Bni1 and Bnr1. Since BNI1 and BNR1 function in genetically redundant pathways for cable assembly, mutation of a gene that regulates Bnr1 is predicted to have synthetic interactions with bni1Δ. Indeed, bni1Δbud14Δ double mutants showed synthetic defects in cell growth at 37°C, whereas bnr1Δbud14Δ double mutants displayed no synthetic defects in cell growth (Fig. 2A). Synthetic defects in the bni1Δbud14Δ strain were supported further by an examination of GFP-Sec4 localization (Fig. S3). Together, these data reveal that in cells lacking Bni1, the further loss of Bud14 impairs polarized cell growth, and suggest that BUD14 functions in the same pathway as BNR1, in parallel to BNI1.
Figure 2. BUD14functions upstream ofBNR1and in parallel toBNI1.
(A) Yeast strains were serially diluted and compared for growth at 25 C and 37 C on YPD plates. (B) DIC microscopy of strains grown at 25°C. (C) Percentage of cells with elongated buds was scored for each strain (n > 200) in two independent experiments.
We also compared the cell morphologies of the single and double mutants (Fig. 2B). bud14Δ cells showed a high penetrance elongated bud phenotype, as previously reported (Cullen and Sprague, 2002), which we quantified (Fig. 2C). The elongated bud phenotype can arise from a delay in the switch from anisotropic to isotropic bud growth at G2/M, a transition controlled by the SWE1 checkpoint (Lew and Reed, 1993). Accordingly, we found that deletion of SWE1 suppressed the elongated bud phenotype of bud14Δ (Fig. 2C). Interestingly, bnr1Δ also suppressed the elongated bud phenotype of bud14Δ (Fig. 2C), further supporting the view that BNR1 functions downstream of BUD14.
Our data also may have implications for checkpoint control. It has been suggested that Swe1 responds to actin cytoskeletal stress to delay the switch from apical to isotropic bud growth (Keaton and Lew, 2006). However, the nature of the stress being monitored has remained elusive. Our data suggest that Swe1 may detect abnormalities in cable architecture and function resulting from Bnr1 mis-regulation in the absence of Bud14. This could impair delivery to the neck of factors that influence Swe1 kinase activity and/or degradation.
GFP-Bud14 localizes with Bnr1 at the bud neck
Next, we tested whether Bud14 localization in cells overlaps with that of Bnr1. Previously, it was shown that a YFP-Bud14 fusion protein overexpressed from the ADH1 promoter localized to the bud cortex during most stages of bud emergence and growth, and accumulated at the neck post-anaphase (Knaus et al., 2005). Here, we examined localization using a low copy plasmid expressing GFP-Bud14 under the control of its own promoter. Importantly, this construct complements BUD14 function (Fig. S4). We observed faint GFP-Bud14 staining at the neck in small and medium budded cells, and prominent staining at the bud cortex (Fig. 3A). By the large budded stage, neck staining was intensified and cortex staining was diminished. Given the stable localization of Bnr1 at the neck (Buttery et al., 2007), these data suggest that Bud14 and Bnr1 at least partially co-localize throughout daughter cell growth. One interpretation of this localization pattern is that Bud14 takes on a greater role in regulating Bnr1 function at later stages of bud growth. However, an equally likely interpretation is that Bud14 strongly regulates Bnr1 function throughout early and late stages of bud development without accumulating at the neck in earlier stages, reflecting more transient interactions with Bnr1 and/or other neck factors.
Figure 3. Requirements for GFP-Bud14 localization.
(A) Localization of GFP-Bud14. (B) GFP-Bud14 localization after treatment of cells with 50 μM LatA. (C) GFP-Bud14 localization after genetic disruption of actin cables or Myo2 function. Each of the indicated strains carrying GFP-Bud14 was imaged after growth at 25 C or a 10 min shift to the non-permissive temperature (37 C). (D) GFP-Bud14 localization in bnr1Δ cells. For all strains, GFP-Bud14 localization patterns were uniform in the populations.
To better understand how Bud14 is recruited to the neck and characterize the dynamics of its association there, we examined Bud14 localization after treating cells with LatA. One previous report showed that YFP-Bud14 localization is sensitive to a 30 min treatment of cells with LatA (Knaus et al., 2005). Similarly, we found that GFP-Bud14 localization after treating cells with LatA was diminished after 10 min and minimal by 20 min (Fig. 3B), confirming that GFP-Bud14 localization is dynamic and requires filamentous actin structures. These properties differ from those of Bnr1-GFP, which stably localizes to the neck even after 30 min treatment with LatA (Buttery et al., 2007).
We next examined GFP-Bud14 staining in a tpm2Δtpm1-ts strain to address whether localization requires actin cables. Localization was lost rapidly after shifting cells to the non-permissive temperature (Fig. 3C). Further, we examined GFP-Bud14 staining in a myo2-ts strain, and found that localization was lost within 10 min upon shift to the non-permissive temperature (Fig. 3C). Together, these observations suggest that Myo2 may transport Bud14 on actin cables to the bud neck. This also indicates that distinct mechanisms regulate localization of Bud14 and Bnr1 to the neck. Consistent with this view, bnr1Δ did not significantly affect GFP-Bud14 localization (Fig. 3D), and bud14Δ did not significantly affect Bnr1-GFP localization (Fig. S5). We also quantified Bnr1-GFP signal intensity at the neck of wild type and bud14Δ medium to large budded cells (n=25) and found there to be no appreciable difference (30.7 +/− 7.0 and 31.1 +/−9.5 fluorescence arbitrary units, respectively). On the other hand, we did observe that Bnr1-GFP localization to the incipient bud neck was modestly decreased in unbudded bud14Δ compared to wild type cells (Fig. S5). The significance of this observation remains unclear, since the decreased localization could result from a short delay in G1 polarization in bud14Δ cells.
These observations also show that the exaggerated cable phenotype in bud14Δ cells does not result from Bnr1 mislocalization, and suggest instead that it results from misregulation of Bnr1 activity in the absence of Bud14.
Bud14 directly inhibits the Bnr1 FH2 domain
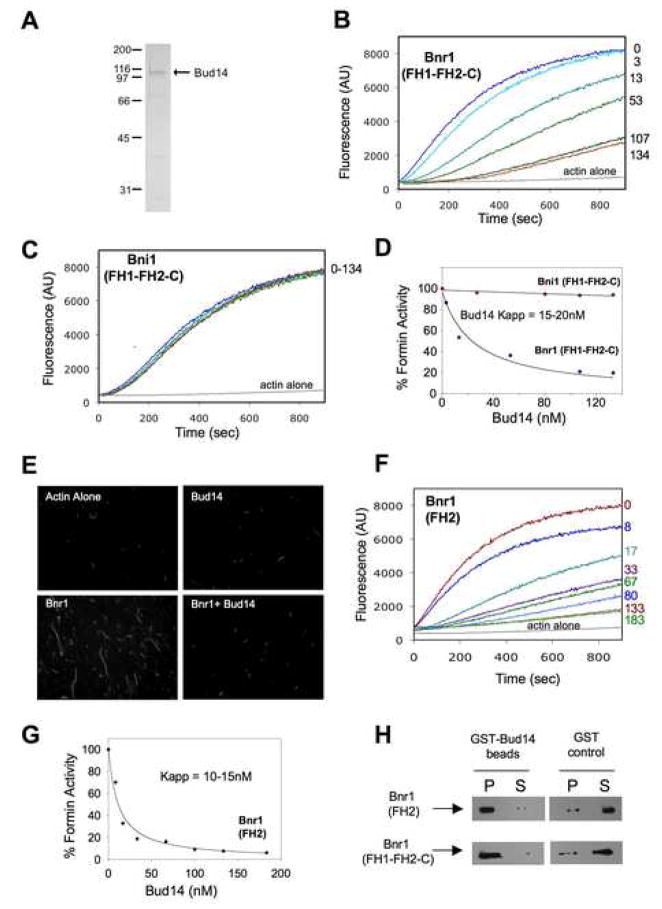
To better understand the mechanistic basis of the genetic observations above, we purified full-length Bud14 from E. coli (Fig. 4A) and tested its in vitro effects on formin-mediated actin assembly. Bud14 had no effect on Bni1(FH1-FH2-C) activity, but potently inhibited Bnr1(FH1-FH2-C) activity in a concentration-dependent manner (Fig. 4B–D) with half-maximal inhibition at ~25nM Bud14 (Fig. 4D). Samples of the assembly reactions were removed at 10 min, and filaments were stabilized with Alexa-488 phalloidin and imaged (Fig. 4E), providing visual demonstration of Bud14 inhibitory effects. Bud14 alone showed no effects on actin assembly in the absence of Bnr1; it also failed to bind F-actin in co-sedimentation assays (Fig. S6). These observations suggest that Bud14 interacts with Bnr1 rather than actin to inhibit actin assembly.
Figure 4. Purified Bud14 directly inhibits Bnr1.
(A) Coommassie stained gel of purified full-length Bud14. (B,C) Monomeric actin (2 μM, 5% pyrene labeled) was polymerized in the presence of (B) 5nM Bnr1(FH1-FH2-C) or (C) 30nM Bni1(FH1-FH2-C) and the indicated concentrations of Bud14. (D) Concentration-dependent effects of Bud14 on Bnr1 or Bni1 activity. Percent activity was determined by dividing the slope of the actin polymerization curve in the presence of a given concentration of Bud14 by the slope of the curve in the absence of Bud14 (considered 100% activity). (E) Visualization of filaments assembled by Bnr1 in the presence and absence of Bud14. Reaction samples were removed (same conditions as B) 10 min after initiation; filaments were stabilized with Alexa-488-phalloidin and imaged. (F) Bud14 inhibits Bnr1(FH2). Reaction conditions as in B, except using 20nM Bnr1(FH2). (G) Concentration-dependent effects of Bud14 on Bnr1(FH2) (determined as in D). (H) Association of 500nM 6His-Bnr1(FH1-FH2-C) or 6His-Bnr1(FH2) with GST-Bud14 or GST (control) immobilized on glutathione agarose. Levels of 6His-Bnr1 in the pellet (P) and supernatant (S) fractions compared by immunoblotting with 6His antibodies.
To determine whether Bud14 targets the conserved FH2 domain of Bnr1 (the catalytic core of the actin-assembly apparatus) or instead the sequences flanking the FH2, we tested the effects of Bud14 on purified Bnr1(FH2). Bud14 strongly inhibited Bnr1(FH2) activity, with half-maximal inhibition at ~15 nM Bud14 (Fig. 4F and 4G). Further, Bud14 had no effect on purified Bni1(FH2) (not shown). We also observed direct binding of Bnr1(FH2) and Bnr1(FH1-FH2-C) to Bud14 immobilized on beads (Fig. 4H). Together, these data suggest that Bud14 inhibits Bnr1 by directly associating with the FH2 domain.
Bud14 displaces Bnr1 from growing barbed ends
One plausible model to explain how Bud14 inhibits Bnr1 is that a Bud14-Bnr1 complex tightly associates with the barbed end as a static cap, blocking FH2 processive movement and inhibiting growth. An alternative model is that Bud14 binding to the FH2 blocks its ability to interact with actin, which would displace the FH2 from barbed ends. To distinguish between these models, we employed a filament elongation assay that monitors barbed end growth on F-actin “seeds”. Freshly sheared filaments are added to a mixture of formins, capping proteins, and/or other proteins, then mixed with pyrene-actin monomers to initiate assembly. As expected, Bnr1 protected barbed end growth both in the presence and absence of capping protein (Fig. 5A and 5B). However, further addition of Bud14 greatly reduced filament growth specifically in the presence of capping protein. These observations are inconsistent with Bud14-Bnr1 forming a stationary cap, which would inhibit elongation in the absence of capping protein. Instead, they support the model that Bud14 disrupts FH2-actin interactions, displacing the FH2 from barbed ends.
Figure 5. Bud14 displaces Bnr1 from growing barbed ends.
(A) Effects of Bud14 on Bnr1-capped filament barbed end growth in the presence and absence of capping protein (CP). At time zero, monomeric actin (0.5μM) was added to mechanically sheared F-actin seeds in the presence of Bnr1(FH1-FH2-C), CP, and/or Bud14 (concentrations indicated in B). (B) Rates of elongation averaged from multiple experiments. (C) Elongation assays performed as in A, except instead of premixing Bnr1 with Bud14, Bud14 or control buffer was added to the reactions 65 seconds after initiation. (D) Rates of elongation averaged from multiple experiments.
We next changed the order of addition in these assays to address whether Bud14 actively disrupts (rather than simply prevents) FH2-actin interactions. Approximately 1 min after reactions containing formins and capping protein were initiated, Bud14 or control buffer was added (Fig. 5C and 5D). Almost immediately, assembly decreased, indicating that Bud14 rapidly displaced Bnr1 from barbed ends. Quantification of these data (Fig. 5D) showed that the inhibitory effects were equally potent whether Bud14 was premixed with Bnr1 (as in Fig. 5A) or added post-initiation of assembly (as in Fig. 5C).
Deletion of BUD14 suppresses the actin cable architecture defects of a hyper-activated bnr1 allele
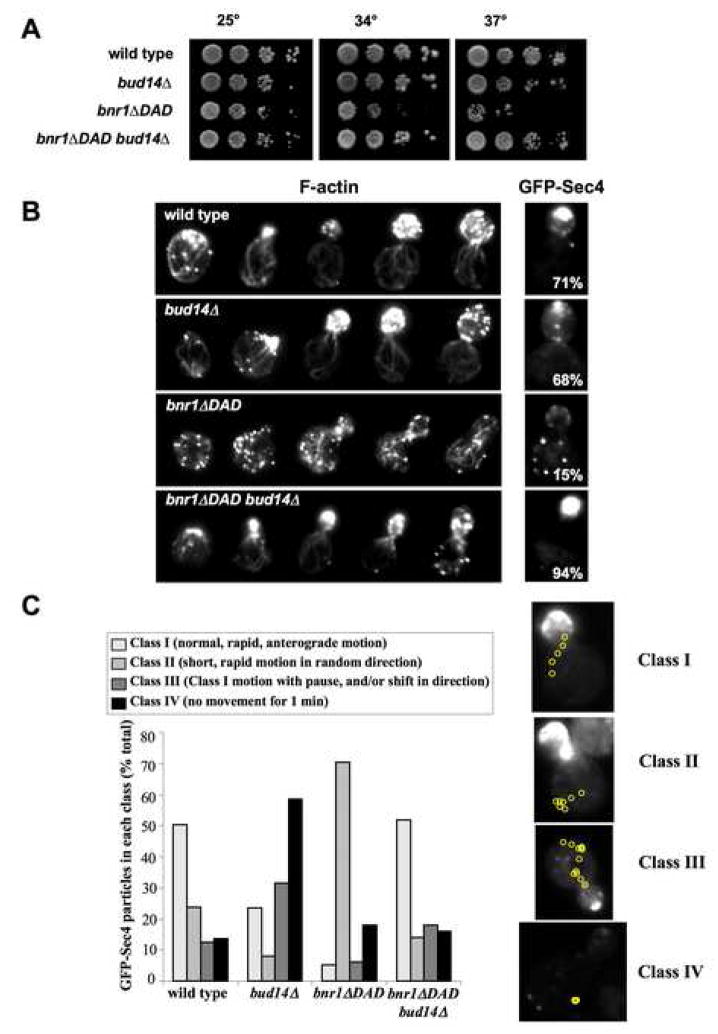
To better understand how these activities of Bud14 contribute in vivo to regulating Bnr1, we generated a hyper-activated allele of bnr1 (bnr1ΔDAD) and examined its genetic relationship with bud14Δ. Deletion of the DAD domain is predicted to constitutively activate Bnr1 and increase the frequency of Bnr1-mediated actin assembly events. This would produce an abundance of short cables, very different from the effects of bud14Δ, which produces fewer, more exaggerated cables. This is because in the absence of Bud14, Bnr1-mediated actin assembly events would be too long in duration rather than too frequent.
Consistent with this model, bnr1ΔDAD cells grew poorly at elevated temperatures (Fig. 6A) and contained disorganized arrays of short cables (Fig. 6B). Moreover, the cables did not support efficient polarized secretion (Fig. 6B). We also tracked real-time movements of GFP-Sec4 particles in these strains (Fig. 6C). In the wild type strain, about 50% of GFP-Sec4 particles exhibited rapid, anterograde movements toward the bud (“Class I” movements) (Movie S1). Similar dynamics were described previously for wild type cells, and arise from Myo2-dependent transport of secretory vesicles on cables (Pruyne et al., 1998). The remaining 50% of GFP-Sec4 particles in wild type cells exhibited one of three distinct categories of movement: rapid bursts in random directions (“Class II”) (Movie S2), long pauses and/or changes of direction (“Class III”) (Movie S3), and no detectable movement for at least 1 min (“Class VI”) (Movie S4). The four classes of GFP-Sec4 movement were scored (Fig. 6C), which showed that bud14Δcaused a marked increase in Class IV (~60% of particles exhibited no movement), whereas bnr1ΔDAD caused an increase in Class II (~70% of particles exhibited short, directionless movement).
Figure 6. bud14Δ suppresses the actin cable architecture and secretory transport defects of abnr1ΔDADstrain.
(A) Yeast strains were serially diluted and compared for growth at 25°C, 34°C and 37°C on YPD plates. (B) Cells were fixed and stained with Alexa-488-phalloidin, or transformed with a GFP-Sec4 plasmid to localize secretory vesicles. Images of representative cells shown. GFP-Sec4 panels include the percentage of cells having >50% GFP-Sec4 fluorescence in the bud (n>100). (C) Time-lapse movement of GFP-Sec4 particles in live cells. The four categories of movement observed (Class I, II, III and IV) were scored in each strain (n>100 cells). Shown to the right of the bar graph are representative cells depicting each category of movement, with yellow circles tracing the path of movement recorded at 300 millisecond intervals. See supplementary materials for movies: wild type (Movie S1), bud14Δ (Movie S2), bnr1ΔDAD (Movie S3), and bud14Δbnr1ΔDAD (Movie S4).
Collectively, these observations show that the cable architecture defects in bud14Δ and bnr1ΔDAD cells are highly distinct, and that they differentially impair polarized secretion. This led us to test suppression between the two alleles. Indeed, in the bnr1ΔDADbud14Δ strain, we observed strong and mutual suppression of the defects in cell growth, actin cable organization, and GFP-Sec4 movement (Fig. 6A, B, and C). These data suggest that deletion of Bud14 restores normal cable architecture by rebalancing actin filament length control.
Separation of Bud14 in vivo functions
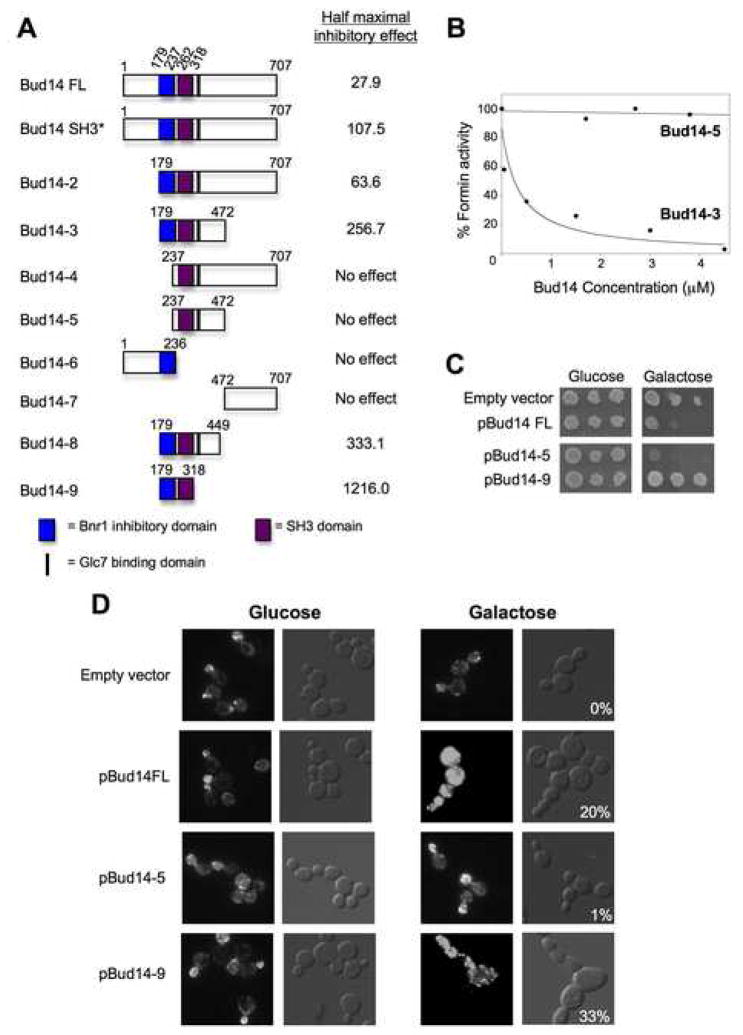
To better understand the relationship between the role we describe here for Bud14 in controlling Bnr1-mediated actin assembly and its previously described role in regulating Glc7 to promote dynein-dependent steps in nuclear migration, we dissected Bud14 function (Fig. 7A). Bud14 has a predicted molecular weight of 79 kDa and contains only one recognizable structural element, an SH3 domain (residues 262–318). We purified a set of truncated Bud14 polypeptides and full length Bud14 bearing a point mutation (W296A) that disrupts canonical SH3 binding to target sequences (Fazi et al., 2002). These Bud14 polypeptides were compared over a range of concentrations for their inhibitory effects on Bnr1-mediated actin assembly (Fig. 7A). Activity was expressed as the concentration of Bud14 polypeptide required for half-maximal inhibition. Representative titrations are shown for Bud14-3 and Bud14-5 (Fig. 7B). Four mutants (Bud14-4, Bud14-5, Bud14-6, and Bud14-7) failed to inhibit Bnr1 even at high concentrations, suggesting that they lack appreciable activity. Three mutants (Bud14-3, Bud14-8, and Bud14-9) showed pronounced decreases in activity, ranging from a 9-fold to 43-fold reduction compared to wild type Bud14. The last two mutants (Bud14 SH3* and Bud14-2) exhibited more modest reductions in activity, approximately 4-fold and 2-fold respectively.
Figure 7. Bud14 domain analysis.
(A) Schematic of purified, truncated Bud14 polypeptides. Each Bud14 polypeptide was compared at a range of concentrations for its effects on Bnr1(FH1-FH2-C)-mediated pyrene-actin assembly. Concentrations of Bud14 required for half-maximal Bnr1 inhibition are shown. (B) Dose-dependent effects of two constructs, Bud14-5 and Bud14-3, on Bnr1(FH1-FH2-C) activity. (C) Effects on cell growth after Gal-driven overexpression of full length Bud14, Bud14-5, and Bud14-9. Cells were grown in selective media, serially diluted on selective plates containing glucose or galactose and grown at 25°C. (D) Cell morphology and actin organization defects caused by overexpression of Bud14 constructs. After 24 hr growth in galactose-containing medium, cells were fixed and stained with Alexa-488 phalloidin. DIC panels for Gal-induced cultures include percentage of cells with an obvious ‘chained’ phenotype and/or with grossly elongated or misshapen buds.
These data show that a short, N-terminal region of Bud14 (179–236) is required but not sufficient for Bnr1 inhibition (Fig. 7A). Inhibition by this fragment required the addition of C-terminal flanking sequences. Inhibitory activity increased with the inclusion of progressively longer stretches of the C-terminus. The SH3 domain lies within these sequences, but cannot account for the full effects, as a W296A mutation in Bud14 (Bud14 SH3*) only reduced inhibition by 3–4 fold. We note that Bud14 SH3* showed a similar 3–4 fold reduction in inhibitory activity on Bnr1 FH2 and FH1-FH2-C (not shown), suggesting that its target is the FH2 rather than the FH1.
We used the mapping information above to compare the in vivo contributions of the Bnr1 inhibitory and Glc7 regulatory domains of Bud14 to the Bud14 overexpression phenotype. Overexpression of full-length Bud14 is lethal, and lethality can be suppressed by mutation of the Glc7-binding site (372–382) (Cullen and Sprague, 2002; Knaus et al., 2005). Therefore, we compared the phenotypes of cells overexpressing full-length Bud14, Bud14-5 (which includes the Glc7-binding site but lacks Bnr1 inhibitory activity), and Bud14-9 (which contains Bnr1 inhibitory activity but lacks the Glc7-binding site). Consistent with the studies above, we found that overexpression of full-length Bud14 was lethal (Fig. 7C) and caused gross abnormalities in cell morphology, including ~20% of cells with elongated and misformed buds in “chains” (Fig. 7D). Overexpression of Bud14-5 was lethal, similar to full-length Bud14, but caused no visible defects in cell morphology or actin staining. In contrast, Bud14-9 overexpression was not lethal, but caused severe defects in cell morphology. Further, these cells showed a severe loss of visible actin cables and depolarization of actin patches (Fig. 7D), consistent with the predicted effects of overexpressing a formin displacement factor. These data demonstrate that the lethality and cell morphology defects can be genetically uncoupled, and map to separate domains/activities of Bud14.
DISCUSSION
We have identified Bud14 as a factor that directly removes formins from growing barbed ends, and shown that this activity is required in vivo for proper actin cable architecture and function. Bud14 is the third biochemical inhibitor of formins to be identified, the other two being DIP/WISH and the Spire KIND domain (Eisenmann et al. 2007; Quinlin et al. 2007). However, Bud14 is the first to be shown to displace formins from growing barbed ends, and this difference alludes to a unique role for Bud14 in vivo. The in vivo role of formin inhibition by the Spire KIND domain is unknown, but all available genetic data for DIP/WISH indicate that it down-regulates mDia2-dependent filopodial formation (Eisenmann et al. 2007), and thus acts as a classic inhibitor, suppressing formin activity. In contrast, all of our genetic data indicate that Bud14 does not suppress Bnr1 activity, but instead controls the duration of Bnr1-mediated actin assembly events, regulating the architecture of the actin arrays produced by Bnr1.
In this study, we have addressed how the duration of formin-mediated actin assembly events and filament length control are regulated. If formins are not inhibited/displaced from barbed ends to limit the growth of individual actin filaments, the architecture of actin networks will be altered. In addition, many actin arrays assembled by formins require rapid and on-going synthesis, suggesting that formins must be cycled between their active and inactive states to enable each new round of assembly. We propose that by removing Bnr1 from growing barbed ends, Bud14 controls filament length to produce proper actin cable architecture and function. Bud14 also may promote Bnr1 cycling between its active and inactive states to promote actin assembly. A parallel can be drawn to GAPs, which serve as Rho inhibitors yet positively influence Rho cellular functions by promoting cycling of Rho proteins between their activated and inactivated states.
Taken together, our findings reveal an unexpected function for formin inhibitors, and suggest that different inhibitors may serve mechanistically distinct roles in vivo, some functioning as classic suppressors of formin activity, others promoting formin recycling and restricting filament length.
Temporal regulation of formins and the control of filament length
Many autoinhibited formins are released/activated by Rho GTPases (Li and Higgs, 2003; Wallar and Alberts, 2003). While this has offered a compelling mechanism to explain the initial release/activation of formins, it has left uncertain the question of how formins are subsequently inactivated and/or displaced from growing filament ends (i.e. temporally regulated), permitting termination of growth by capping proteins to restrict length. We dissected regulation of the yeast formin Bnr1, because it is spatially confined to the bud neck (Buttery et al., 2007), which allowed us to focus on temporal control of its activity.
Our model, that Bud14 displaces Bnr1 from growing barbed ends to ensure proper actin architecture and function, is supported by genetic and biochemical evidence. First, purified Bud14 directly associated with the FH2 domain of Bnr1 with high affinity (Kapp = 20 nM) (Fig. 4) and rapidly displaced Bnr1 from growing barbed ends, allowing capping protein to terminate elongation (Fig. 5). Second, loss of Bud14 in vivo resulted in abnormal mother cable architecture (long, bent or buckled cables resistant to LatA) (Fig. 1), leading to defects in polarized secretion revealed by tracking GFP-Sec4 particle movements (Fig. 6). Third, the hyper-elongated bud phenotype of bud14Δ cells was suppressed by bnr1Δ, suggesting that Bud14 functions upstream of Bnr1 (Fig. 2C). Fourth, bud14Δ (which produces abnormally long cables) rescued the temperature sensitive growth defects and cell morphology defects of mutants with abnormally short cables, tpm1Δ (Fig. 1) and bnr1ΔDAD (Fig. 6). All of these data are consistent with Bud14 regulating Bnr1 activity to control filament length, and in turn, actin cable organization and function.
Our data emphasize that inactivation, not just activation/release from autoinhibition, represents a critical control point in governing the formin regulatory cycle. The need for strict temporal regulation of formins is demonstrated by the severe dominant cable defects in bnr1ΔDAD cells (disorganized networks of short cables that impair polarized secretion). These defects are far more detrimental to cell growth and morphology than the partial loss of cables caused by a deletion of BNR1. The cable length defects in bnr1ΔDAD are rescued by bud14Δ, which restores normal cable architecture, and thereby rescues polarized secretion, cell growth and cell morphology (Fig. 6). Why do bud14Δ and bnr1ΔDAD mutations cause opposite defects in cable architecture to begin with? We propose that in the absence of Bud14, Bnr1 assembles fewer but longer filaments, which causes cables to become hyper-extended, buckled, and latA-resistant (Fig 1). bnr1ΔDAD has the opposite effect (more numerous and shorter cables) because Bnr1 is constitutively active, which elevates the frequency of actin assembly events. Due to a limited pool of actin monomers, the filaments in bnr1ΔDAD cells would be predicted to grow at a slower rate, producing shorter cables. The phenotype of bud14Δ is completely distinct, because it functions at the end of the Bnr1 cycle, to retrieve and recycle Bnr1 for new rounds of assembly. Thus, in the absence of Bud14, actin assembly events are fewer but of abnormally long duration. The mutual suppression of bud14Δ and bnr1ΔDAD lends strong support for this model.
Given the potent biochemical activities of Bud14, it was somewhat surprising that the cable phenotypes of bud14Δ cells were not more severe. This suggests that Bud14 is not the only cellular factor that attenuates Bnr1 activity to influence actin filament length. Indeed, capping protein, which directly competes for barbed end association, can promote formin displacement biochemically (L. Blanchoin, personal communication). To test this model genetically, we crossed bud14Δ to cap2Δ (which fully abolishes capping protein function). We found that bud14 cap2Δ cells have synthetic growth defects at elevated temperatures and compounded defects in GFP-Sec4 polarization (Fig. S7). These data are consistent with Bud14 and capping protein making complementary contributions to formin displacement in vivo. We note however, that capping protein likely has additional roles in cable assembly, unrelated to formin displacement, that complicate the analysis of its role in cable formation, such as stabilization of filaments in cables due to its association with barbed ends. This may explain why cap2Δ cells show diminished cable staining, rather than an exaggerated cable phenotype like bud14Δ cells. Collectively, these observations suggest that capping protein and Bud14 provide distinct but complementary mechanisms for displacing formins from barbed ends, with capping protein competing for barbed end association and Bud14 directly targeting the FH2 domain to remove formins.
Our observations raise new questions to be addressed, including how Bud14-Bnr1 interactions are regulated. Since Bud14 potently inhibits Bnr1 activity, the timing and duration of the Bud14-Bnr1 association must be regulated in vivo. Our data suggest that dynamics of Bud14 localization may factor into this mechanism. While Bnr1 is stably anchored at the neck, Bud14 neck localization is dynamic and requires on-going Myo2-dependent transport on cables (Fig 3). This suggests that Myo2 may deliver Bud14 to Bnr1-attached filament ends, promoting displacement of Bnr1 and release of nascent filaments. Another important question is how Bud14-Bnr1 interactions are disrupted to make Bnr1 available for new rounds of filament assembly, which could involve posttranslational modifications and/or other ligands.
Finally, how might the functions of Bud14 be related to those of its closest homologue by sequence, S. pombe cell polarity factor tea4p? tea4p directly associates with the formin for3p and is required for actin cable assembly and polarized growth (Martin et al., 2005). However, no biochemical activities have yet been reported for tea4p or for3p, leaving the specific role of their association unclear. Thus, it is difficult to make close comparisons of tea4p and Bud14 phenotypes to assess their relatedness. There are also key differences in the two experimental systems (S. pombe and S. cerevisiae) that complicate interpretations. Whereas Bnr1 and Bud14 localize to the bud neck independently of one another, for3p localization to the new end of the cell depends on tea4p. Thus, deletion of tea4 results in loss of actin cables, making it difficult to know whether this phenotype arises from misregulation of for3p activity or rather mislocalization of for3p. In contrast, Bnr1 localization to the neck is stable and does not depend on Bud14, which has allowed us to correlate bud14Δ cable defects with the biochemical effects of Bud14 on Bnr1.
In summary, our data reveal an important mode of formin regulation that influences filament length and is critical for normal morphology of actin arrays. We have also defined a new cellular function for Bud14 in actin assembly. Given the previous described role of Bud14 in regulating microtubule-dependent events to promote nuclear migration (Knaus et al., 2005), this points to Bud14 being a multi-faceted cytoskeletal regulator that coordinates microtubule- and actin-based functions. While these two interactions/activities of Bud14 mapped to separate domains (Fig. 7), future studies will be needed to understand if and how these functions are integrated in vivo.
EXPERIMENTAL PROCEDURES
Cell Imaging
For construction of yeast strains and plasmids, see Supplementary Methods. To image F-actin, cells were grown to log phase, fixed, and stained with Alexa-488-phalloidin (Molecular Probes, Eugene, OR). To examine polarized secretion, cells were transformed with a low copy plasmid expressing GFP-Sec4 under the control of its own promoter (a gift from Ruth Collins) and grown to early log phase. For live imaging of GFP-Sec4, cells were mounted on slides and observed at room temperature. Movies of single focal planes were acquired through consecutive 300-ms exposures. All images were captured on a Zeiss Axioskop-2 mot plus microscope (Carl Zeiss, Thornwood, NY) using a Hamamatsu ORCA-ER digital CCD camera (Hamamatsu Photonics, Bridgewater, NJ) running OpenLab software (Improvision, Lexington, MA). Unless noted, all quantifications score over 200 cells.
In vivo LatA-induced actin turnover assays
Isogenic wild type and bud14Δ strains were grown in 5 ml YPD cultures to log phase, then cells were pelleted and resuspended in 1 ml YPD. 100–200 μl cells were incubated at 30°C with control buffer or buffer containing a final concentration of 20 μM Latruncullin A (LatA; a gift from Phil Crews). At 0, 30, 60, 90, and 120 sec time points cells were fixed, stained with Alexa-488-phalloidin, imaged as above, and scored for visible actin cables (n>200 cells per sample) as described (Okada et al., 2006). Data in figure 1E were plotted (as % cells with visible cables vs. time after treatment with LatA), and the curve fits were used to calculate time required to reach 50% of cells lacking visible cables.
In vitro actin assays
Purification of all proteins is described in Supplemental Methods. For actin assembly assays, monomeric RMA (final 2 μM, 5% pyrene-labeled) was converted to Mg-ATP-actin for 2 min immediately prior to use in reactions. 45 μl of Mg-ATP-actin was added to 12 μl of control buffer or proteins in the same buffer and mixed with 3 μl of 20X initiation mix (40 mM MgCl2, 10 mM ATP, 1 M KCl). Fluorescence was monitored at 25 °C at excitation 365 nm and emission 407 nm in a fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ). Rates of assembly were determined from the slopes of curves at 25–50% polymerization. For elongation assays, 5 μL of freshly sheared F-actin (10 μM) were added to a mixture of 0.5 nM Bnr1 (FH1-FH2-C), 20 nM Bud14, and/or 1 μM capping protein, then immediately mixed with 0.5 μM monomeric actin (10% pyrene-labeled). Rates of elongation were determined as above. To visualize the F-actin products from assembly assays, samples were removed after 10 min and diluted 10-fold into F-buffer plus 0.6 μM Alexa-488-phalloidin. After 5 min, filaments were diluted another 50 fold, and then 0.5 μl was applied to a nitrocellulose-coated cover slip and filaments were examined immediately by fluorescence microscopy.
In vivo over-expression of Bud14 constructs
Yeast cells transformed with plasmids expressing fragments of Bud14 were grown to log phase in selective media containing raffinose, then switched to galactose-containing media, and grown for 24 hr at 25°C. Samples were removed at time zero (at media switch) and 24 hr post-galactose induction, fixed, stained with Alexa488-phalloidin, and imaged as above.
Supplementary Material
Acknowledgments
We thank D. Lew for helpful guidance and reagents for SWE1 disruption experiments, S. Maiti and A. Potashinsky for purifying Bnr1 (FH2), R. Collins for the Sec4-GFP plasmid, and D. Pellman lab for the PY5462 strain. We also thank L. Blanchoin, K. Daugherty, A.G. DuPage, and A. Rodal for helpful comments and critical reading of the manuscript. This work was supported by grants from the NIH (GM083137 and GM063691) to B.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buttery SM, Yoshida S, Pellman D. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol Biol Cell. 2007;18:1826–1838. doi: 10.1091/mbc.E06-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J Cell Biol. 1997;136:1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Sprague GF., Jr The Glc7p-interacting protein Bud14p attenuates polarized growth, pheromone response, and filamentous growth in Saccharomyces cerevisiae. Eukaryot Cell. 2002;1:884–894. doi: 10.1128/EC.1.6.884-894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Zigmond S, Boone C. Formins: signaling effectors for assembly and polarization of actin filaments. J Cell Sci. 2003;116:2603–2611. doi: 10.1242/jcs.00611. [DOI] [PubMed] [Google Scholar]
- Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fazi B, Cope MJ, Douangamath A, Ferracuti S, Schirwitz K, Zucconi A, Drubin DG, Wilmanns M, Cesareni G, Castagnoli L. Unusual binding properties of the SH3 domain of the yeast actin-binding protein Abp1: structural and functional analysis. J Biol Chem. 2002;277:5290–5298. doi: 10.1074/jbc.M109848200. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Kamasaki T, Arai R, Osumi M, Mabuchi I. Directionality of F-actin cables changes during the fission yeast cell cycle. Nat Cell Biol. 2005;7:916–917. doi: 10.1038/ncb1295. [DOI] [PubMed] [Google Scholar]
- Keaton MA, Lew DJ. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr Opin Microbiol. 2006;9:540–546. doi: 10.1016/j.mib.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Knaus M, Cameroni E, Pedruzzi I, Tatchell K, De Virgilio C, Peter M. The Bud14p-Glc7p complex functions as a cortical regulator of dynein in budding yeast. EMBO J. 2005;24:3000–3011. doi: 10.1038/sj.emboj.7600783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR. Molecular details of formin-mediated actin assembly. Curr Opin Cell Biol. 2006;18:11–17. doi: 10.1016/j.ceb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Pollard TD. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc Natl Acad Sci U S A. 2004;101:14725–14730. doi: 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Bretscher A. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J Cell Biol. 1992;118:285–299. doi: 10.1083/jcb.118.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, McDonald WH, Yates JR, 3rd, Chang F. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev Cell. 2005;8:479–491. doi: 10.1016/j.devcel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Maiti S, Goode BL. Formin proteins: purification and measurement of effects on actin assembly. Methods Enzymol. 2006;406:215–234. doi: 10.1016/S0076-6879(06)06016-2. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ravi H, Smith EM, Goode BL. Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol Biol Cell. 2006;17:2855–2868. doi: 10.1091/mbc.E06-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Gao L, Bi E, Bretscher A. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol Biol Cell. 2004;15:4971–4989. doi: 10.1091/mbc.E04-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC. Aip1p interacts with cofilin to disassemble actin filaments. J Cell Biol. 1999;145:1251–1264. doi: 10.1083/jcb.145.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–446. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Yang HC, Simon V, Swayne TC, Pon L. Visualization of mitochondrial movement in yeast. Methods Cell Biol. 2001;65:333–351. doi: 10.1016/s0091-679x(01)65020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.