Abstract
The burgeoning obesity epidemic has fueled the drive to describe, mechanistically, metabolic homeostasis. From the early theories implicating glucose as a principal modulator grew an understanding of a complex array of metabolic signals, sensed by peripheral organs along with specific locations within the central nervous system (CNS). The discovery that leptin, an adipose-derived hormone, acts within the mediobasal hypothalamus to control food intake and energy expenditure ushered in a decade of research that went on to describe not only the specific nuclei and cell type, such as proopiomelanocortin neurons of the arcuate nucleus, that respond to leptin but also the signaling cascades that mediated its effects. This review thus highlights the sites and mechanisms of action of leptin, both in the hypothalamus and in extrahypothalamic sites within the CNS, and shows our current knowledge and direction of future research aimed at understanding the multifunctional role of leptin in maintaining metabolic homeostasis.
INTRODUCTION
The incidence of obesity has risen sharply over the past half-century. Currently, more than one-half of adults within the United States are classified as overweight or obese (1, 2). The prevalence of obesity, along with its associated diseases, such as type 2 diabetes, poses a serious threat to public health. Because of the widespread lack of clinical success with long-term weight-loss maintenance, new therapeutic approaches are needed to promote effective weight reduction (3). Despite this recognized need, a basic lack of knowledge exists about how the body compensates for weight loss and permits weight gain. This review attempts to summarize our current understanding of the mechanism of action of leptin, a key peptide hormone in the regulation of metabolic homeostasis, in the mediobasal hypothalamus.
LIFE BEFORE LEPTIN: GLUCOSE AS THE PRINCIPAL REGULATOR OF METABOLIC HOMEOSTASIS
It could be argued that the journey to understanding obesity and its comorbidities started >150 y ago when Claude Bernard made a series of simple yet monumental descriptions of the role of glucose in the periphery and the brain, although it was not until nearly a century later that Bernard's observations could begin to be understood within the framework of hypotheses proposed by such scientists as Mayer, Kennedy, Coleman, and others. Mayer first described the glucostatic theory, which suggested that eating maintains glucostasis and that hunger as well as spontaneous meal initiation are stimulated in response to lowered circulating glucose concentrations (4, 5). In addition, he proposed that the brain may alter acute feeding behavior by directly sensing changing glucose concentrations, and a similar adipostatic mechanism could account for the longer-term effects on body weight and energy balance (6). After the dissemination of the glucostatic theory, several studies proceeded to establish the role of glucose concentrations in the acute effects on hunger and food intake. For example, plasma glucose concentrations were slightly reduced before the onset of meals in rats, whereas after a meal plasma glucose concentrations as well as insulin rose (7, 8). In addition, insulin, which reduces plasma glucose concentrations by facilitating cellular glucose utilization, and 2-deoxy-d-glucose, a competitive inhibitory of glucose metabolism, initiated food intake (9–12). However, several reports were inconsistent with the glucostatic hypothesis, which together with the addition of the “dual center” model for the regulation of feeding and the later description of peripheral factors (eg, insulin, free fatty acids, glucagon, cholecystokinin, gastrin, catecholamines, and leptin) capable of modifying feeding behavior, forced the abandonment of the glucostatic hypothesis.
FAT TALKING TO THE BRAIN: ADIPOCYTE-DERIVED LEPTIN IS REQUIRED FOR METABOLIC HOMEOSTASIS
Kennedy (13) first proposed in 1953 that inhibitory signals released into the circulation in proportion to body adipose stores act directly in the brain to reduce food intake. Since the formulation of the adipostatic hypothesis many researchers have attempted to identify the factors involved. Coleman (14, 15), while working at Jackson Laboratories, pioneered a series of seminal experiments that studied C57Bl6 mice that had 2 spontaneous autosomal recessive mutations that resulted in obesity and diabetes (ob/ob and db/db). The ob/ob phenotype exhibits similar characteristics to mild type 2 diabetes, insulin resistance, glucose intolerance, and mild hyperglycemia. These mice also displayed hyperphagia and decreased energy expenditure, resulting in excessive lipid accumulation and visually detectable obesity at 3–5 wk of age. Similar to the ob/ob mice, the db/db phenotype also displays hyperphagia and low energy expenditure, resulting in obesity. However, unlike the ob/ob mutant, the db/db mice develop rapid, early-onset, severe hyperglycemia and type 2 diabetes.
Coleman used a technique called parabiosis, the process of making artificial conjoined twins such that each animal share, in part, a common circulation, to determine whether a circulating factor could be shared from the lean littermates to correct the obesity or diabetes in either mutant or vice versa. Coleman performed 3 parabiotic pairings: 1) the ob/ob mutant paired to the lean littermate, 2) the db/db mutant to the lean littermate, and 3) the ob/ob mutant paired to the db/db mutant. In the first pairing, the ob/ob mouse responded with a reduction in food intake and weight gain and the lean littermate remained unchanged. The results of the second pairing were opposite to those of the first in that the db/db mutant was unaffected and the lean littermate became severely hypophagic and lost significant weight, so much so that the lean mouse eventually died. On postmortem investigation, the lean mouse was found without any food in its stomach, suggestive of starvation. Coleman's findings supported the adipostatic hypothesis in that the lean animal made and responded to some circulating factor that regulated its energy balance. Coleman proposed that the ob/ob mutant did not produce the circulating factor, whereas the db/db mouse made this factor in excess but was “resistant” to its effects because of either the lack of the receptor or the signaling necessary to respond. The final pairing provided confirmation of this hypothesis in that the db/db mutant was unaffected, whereas the ob/ob mouse normalized food intake and body weight. Ultimately, it was determined that the ob/ob mutant lacked the circulating factor (OB), and the db/db mutant had a defect in the OB receptor (OBr). However, it was not until the early 1990s when Jeffrey Friedman's laboratory at Rockefeller University identified the ob gene expressed in adipose (fat) cells through positional cloning (16). The cognate secreted factor was also identified and subsequently named leptin after the Greek word leptos, meaning “thin.” The effects of leptin injected into the obese mouse mutant mirrored the parabiotic data, with leptin reducing the degree of obesity. As predicted, leptin injection had no effect in db/db mice that were deficient in the receptor for leptin (17). Further experiments showed that the main target of the secreted hormone leptin was the central nervous system (CNS) (18, 19). Leptin given by intraperitoneal or intracerebroventricular injection was shown to reduce food intake and weight gain, whereas deletion of leptin receptor expression solely in the CNS recapitulated the db/db phenotype. These data finally provided significant support for the role of an identified adipose tissue secreted factor in energy balance regulation acting primarily through modulation of neuronal activity.
LEPTIN SIGNALING AND MODULATION OF NEURONAL ACTIVITY
Substantial evidence shows that the leptin pathway plays a significant role in body weight control by communicating the status of energy stores to the brain (Figure 1A). Leptin has been implicated in activating, primarily through the long-form leptin receptor, a variety of intracellular signaling cascades [reviewed by Myers et al (24)]. Briefly, the leptin receptor is a member of the interleukin-6 receptor family of type I cytokine receptors and lacks an endogenous kinase activity (25, 26). The extracellular leptin signal is instead transduced by Janus activated kinases (JAKs) that are soluble tyrosine kinase receptors that are noncovalently associated with the leptin receptor. On ligand binding, the JAKs autophosphorylate and subsequently phosphorylate tyrosine residues on the intracellular domain of the leptin receptor. Specifically, leptin has been predominately linked to the activation of JAK-2 (27), although JAK-1 activation has also been observed (28). Phosphorylation of the leptin receptor tyrosine residues are required to recruit and activate signal transduction and activators of transcription (STATs) that dimerize and translocate to the nucleus to facilitate transcription (29, 30). Although the leptin receptor has been associated with the activation of STAT-1, -3, -5, and -6 (31–33), the hypothalamus and possibly the brainstem appear exclusively linked to the activation of STAT-3 (34–36). Moreover, the cytoplasmic tyrosine residue (Tyr1198) was shown to be necessary for proper STAT-3 signaling in the hypothalamus (37). Along with eliciting changes in transcription, leptin receptor phosphorylation has been shown to affect neuronal activity, through activation of phosphoinositide 3-kinase (PI3K) and other poorly described mechanisms.
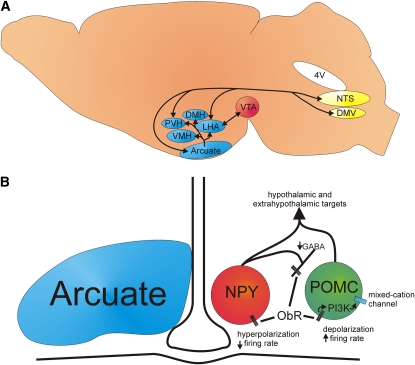
FIGURE 1.
Targets of leptin action in the brain. (A) Illustration shows hypothalamic (blue) and extrahypothalamic (yellow and red) sites of leptin action that are or may be involved in the control of energy and glucose homeostasis. (B) Illustration shows our current understanding of the pro-opiomelanocortin (POMC) and neuropeptide Y–Agouti-related peptide (NPY-AgRP) circuit in the arcuate nucleus. POMC cell: Acute stimulation of the long-form leptin receptor results in activation of various associated kinases that in turn activates phosphoinositide 3-kinase (PI3K). PI3K subsequently activates a putative mixed-cation channel, which leads to a depolarization and increased firing frequency responsible for POMC-mediated acute leptin actions (20). NPY-AgRP cell: acute stimulation of the leptin receptor results in a membrane hyperpolarization, and a suppression of γ-aminobutyric acid (GABA) release from NPY terminals (21–23). This results in a disinhibition of POMC neurons and enhancement of melanocortin activity. ObR, ob receptor; DMH, dorsomedial hypothalamic nucleus; PVH, paraventricular hypothalamic nucleus; LHA, lateral hypothalamic area; VMH, ventromedial hypothalamic nucleus; NTS, nucleus tractus solitarii; DMV, dorsal motor nucleus of the vagus; 4V, fourth ventricle; VTA, ventral tegmental area.
Over the past 10 y, leptin was shown to regulate local circuit activity within the mediobasal hypothalamus. Several studies have shown heterogeneous cellular responses to leptin in different CNS nuclei (21, 22, 38). In particular, leptin was shown to induce a membrane hyperpolarization by activating ATP-sensitive K+ channels in cell lines (39, 40) and in hypothalamic neurons (21, 38), both by a PI3K-dependent mechanism. In contrast, leptin was shown to directly depolarize pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (22) and steroidogenic factor 1 (SF-1) neurons in the ventromedial hypothalamus (VMH) (39). Specifically, POMC neurons displayed an increase in action potential firing, concomitant with a dose-dependent depolarization and a decrease in inhibitory synaptic inputs in response to 0.1–100 nmol/L leptin (22). In contrast, the resting membrane potential of non–green fluorescent protein-labeled arcuate neurons was variably altered, being hyperpolarized, depolarized, or unaffected by leptin. In addition, a subpopulation of neurons in a slice preparation containing the ventromedial and arcuate nuclei experienced a K+-dependent hyperpolarization and a decrease in action potential firing subsequent to 4–50 nmol/L leptin administration (214). Thus, leptin heterogeneously modulates activity in various brain regions because leptin can directly activate or inhibit neurons in the CNS.
EXAMINING THE SITES OF LEPTIN ACTION IN THE CNS
Although direct microinjection of leptin into the third ventricle, the mediobasal hypothalamus, or both, similar to lesion studies, results in a reduction in food intake and weight gain, it is difficult to delineate the sites of action in intact animals. Moreover, examining cellular activity in electrophysiologic studies may isolate leptin effects at the nucleus or cell-specific level; however, conclusions are limited because it remains unclear whether the alteration in cellular activity observed directly correlates with the behavior in question. This means that much of the data examining energy homeostasis have been based on indirect, circumstantial (often convincing) evidence. Therefore, researchers have begun to use Cre-loxP (cyclization recombination-locus of X over P1) technology to 1) perturb circuits guided by neuroanatomic information coupled with the power of mouse genetics and 2) assess effects on energy homeostasis in awake, unrestrained mice (20, 41–46).
HYPOTHALAMIC LEPTIN ACTION IS CRITICAL FOR MAINTAINING METABOLIC HOMEOSTASIS
POMC neurons of the arcuate nucleus were proposed as the primary cell type for mediating the anorexigenic effect of leptin. In a test of this hypothesis, the Cre-loxP system was used to delete OBr from POMC neurons in mice to first determine whether OBrs on POMC neurons are critical mediators of leptin's effects. Selective deletion of leptin receptors from POMC neurons results in obesity (41, 47). However, the degree of obesity is only 15–20% of that observed with systemic leptin deficiency. Deletion of OBrs in Agouti-related peptide–neuropeptide Y neurons produces a similar level of obesity with respect to mice that lack OBrs in POMC cells (48). This may not be surprising because leptin receptors are expressed widely throughout several hypothalamic sites outside the arcuate nucleus, such as in the VMH. In fact, deletion of leptin receptor from a population of VMH neurons with the use of mice expressing Cre recombinase from the promoter of the VMH selective transcription factor SF-1 resulted in modest obesity (41, 42). However, mice lacking leptin receptor in both POMC and SF-1 cells were observed to have an additive effect of leptin deletion on body weight. These data suggest that the role of POMC and Agouti-related peptide–neuropeptide Y neurons in responding to leptin to regulate food intake and body weight is significant but perhaps less pronounced than predicted. Additional studies will be required to determine which brain targets are required or sufficient for leptin regulation of energy balance.
Along with regulation of body weight and energy expenditure, leptin has been implicated in the maintenance of glucose homeostasis. Recent data suggest that leptin acts in the hypothalamus, specifically in the POMC neuron that impinges on hypothalamic melanocortin neuron activity, to maintain blood glucose homeostasis. Initially, glucose concentrations were unaffected when leptin receptors were selectively deleted in POMC neurons from animals fed a chow diet (43). However, overexpression of the POMC gene lowered blood glucose concentrations in mice, and the selective deletion of suppressor of cytokine signaling 3 in POMC cells resulted in improved glucose homeostasis on chow diet and attenuated weight gain and glucose homeostasis on a high-fat diet (49). Moreover, unilateral reactivation of arcuate leptin receptor in mice homozygous for a flipase recombination enzyme–reactivatable, leptin receptor null allele (Obrneo/neo mice), resulted in normalized blood glucose concentrations (46). Similar results were found in a rat model with leptin receptor mutations (50). In addition to the melanocortin-mediated effects on energy and glucose homeostasis, it appears that subgroups of neurons can detect changes and alter intrinsic cellular activity in response to glucose concentrations, including the POMC neurons of the arcuate nucleus. It is unclear whether this response is purely a neuroprotective adaptation or whether the glucose-induced change of activity correlates directly with a modification in energy balance. Recent evidence suggests that melanocortin neurons are activated in response to elevated glucose concentrations (51, 52), which together suggests that the role of the arcuate POMC neurons to regulate energy and glucose homeostasis may have been underestimated. Future studies should clearly delineate the relative contribution of glucose to overall energy regulation.
With respect to the time course of leptin action, both pre- and postnatal leptin signaling may be important in the regulation of metabolic homeostasis. Although the viral reactivation experiments have shown the ability of leptin to act in the adult to affect glucose homeostasis, leptin was also shown to affect the neuronal development of arcuate neurons. In the leptin-deficient mouse, arcuate projections to the paraventricular hypothalamic nucleus are greatly reduced (53). In addition, in diet-induced obese rats, leptin administration is unable to rescue the impaired neurite outgrowth of the arcuate nucleus (54), suggesting that leptin acts as a crucial developmental factor in determining the ability of the arcuate to respond to metabolic cues.
LEPTIN AND INSULIN MODULATION OF HYPOTHALAMIC NEURON ACTIVITY: THE ROLE OF PI3K
Pharmacologic experiments have established that PI3K signaling is required for leptin's effects on feeding (55, 56); however, the contribution of the PI3K pathway in specific hypothalamic neurons to long-term energy regulation by leptin has been controversial. Recently, the role of PI3K in leptin effects observed by POMC neurons of the hypothalamus was described (20). The Cre-loxP system was used to delete the regulatory subunits of PI3K from POMC neurons. The results suggested that PI3K signaling is required for leptin to depolarize POMC neurons and to acutely suppress food intake after central leptin injection (20) (Figure 1B). Aside from the effects of leptin on acute cellular activity and food intake, the effect of leptin was markedly different in this model than was previously described in the POMC Obr null, STAT-3 mutant and the POMC STAT-3 mutant (37, 41, 43, 57). Specifically, all variables examined, including long-term food intake and body weight maintenance, remained unchanged in mice lacking PI3K in POMC cells. These data stress the importance of multiple signal cascades in the leptin-induced regulation of energy balance.
The β cell–derived metabolic factor insulin activates signaling pathways that mirror those activated by leptin in the CNS to regulate energy balance. The insulin receptor is a tyrosine kinase that undergoes rapid autophosphorylation and subsequently activates various molecular proteins, including insulin receptor substrates. Insulin receptor substrates in turn associate with PI3K and result in the modification of cellular activity within various CNS nuclei. Interestingly, the acute effects of insulin were also shown to require PI3K in POMC cells. These data suggest that insulin and leptin differentially modify cellular activity of POMC cells by an identical pathway. Overall, our findings suggest that the PI3K pathway, through its control of POMC neuronal excitation, plays a major role in the neuroendocrine response to acute alterations in circulating leptin concentrations and reconcile findings that show a role for both PI3K-dependent and -independent pathways in the actions of leptin. However, these data only add to the conundrum that insulin and leptin can disparately affect the same population of cells by an indistinguishable intracellular cascade. One model suggests responses to these peptides are highly compartmentalized within the cell which results in sequestering of PI3K signals, such as phosphoinositol triphosphate, to areas that have insulin receptors in close proximity to Katp (ATP-sensitive K+) channels, whereas other parts of the cell contain leptin receptors in close apposition to the putative mixed-cation channel. Insulin receptor binding and subsequent increase in PI3K activity then only affects the nearby Katp channels producing the observed hyperpolarization, whereas the leptin-induced activation of PI3K only modifies the mixed-cation channel activity because of their close proximity. However, these data are difficult to reconcile, and any ideas or models are purely conjecture at this point. In any case, these findings extend previous observations and undoubtedly show the power of mouse genetics in determining effects within intact animals. It will be of great interest to see how leptin and insulin cross talk is regulated and synchronized within specific cells which ultimately results in a coordinated control over energy and glucose homeostasis.
EXTRAHYPOTHALAMIC SITES OF LEPTIN ACTION IN THE MIDBRAIN AND HINDBRAIN
Extrahypothalamic effects of leptin on central autonomic regions such as the brainstem await thorough investigation (58–60). Recent studies that used in situ hybridization histochemistry and mice expressing green fluorescent protein under the control of the leptin receptor have reported leptin receptor expression in the vagal complex (58) (J Lachey and JK Elmquist, unpublished observations, 2008). Consistent with the neuroanatomical evidence, leptin induces c-fos expression in the nucleus tractus solitarius (NTS) (61, 62). In addition, fourth ventricle administration of leptin reduces food ingestion and weight gain within 24 h, and these effects are mimicked by microinjection of the peptide into either the fourth ventricle or the NTS (58). Within the vagal complex, leptin hyperpolarizes most of the NTS and the dorsal motor nucleus of the vagus nerve neurons by activation of an ATP-sensitive K+ conductance at the level of the obex, including those involved in the control of the stomach (63, 64). Leptin also results in a suppression of the overall glutamatergic (excitatory) tone in the vagal complex (63, 64). However, it is unclear as of yet whether the effects of leptin to reduce food intake and weight gain by the vagal complex correlates with the acute leptin-induced suppression of excitatory activity of neurons in this location. Future studies will probably attempt to delineate what role leptin-responsive neurons of the vagal complex plays in the leptin-induced regulation of energy balance.
Another understudied site of leptin action is in the ventral tegmental area (VTA) of the midbrain. Dopaminergic neurons within the VTA have classically been implicated in the valuing and salience of drug reward, through projections to the nucleus accumbens, prefrontal cortex, hippocampus, and amygdala (65, 66). Recent data suggest abnormalities in dopaminergic neurotransmission with the development of obesity. In cocaine-addicted humans, a reduction in nucleus accumbens dopamine D2-3 receptor ligand binding is observed, whereas reduced D2 receptor expression is also seen in the striatum of obese persons (67, 68). In the rodent, there is considerable evidence linking leptin signaling with the VTA dopamine system. For example, Leptin-receptor-null mice require dopamine neurotransmission to elicit leptin-dependent hyperphagia (69). In VTA-dopamine–dependent tests of reward, leptin administration in rats was shown to block the development of a conditioned place preference for high-fat diet (which was reversed with the use of a dopamine receptor antagonist), suggesting leptin may act through this VTA circuit to regulate the rewarding value of nutrient-dense foods (70, 71). Electrophysiologic experiments support this contention, because peripheral administration of leptin was subsequently shown to reduce the frequency of dopamine neuron firing in anesthetized rats, whereas a reduction in frequency of dopamine neuron firing was also seen in acutely isolated VTA tissue sections (72). Unlike in the NTS, the conductance modulated by leptin was not described. Furthermore, as predicted from the previously described data, injection of leptin directly into the VTA of rats acutely decreases food intake. Finally, reducing leptin receptor expression selectively in the VTA with the use of RNA interference increases daily food consumption along with an increase in preference for sucrose and a high-fat diet (72). In summary, the assembled data point to a direct action of leptin in the VTA on feeding. However, an effect on acute metabolic state and long-term metabolic homeostasis awaits investigation. Similar to the extrahypothalamic action of leptin in the NTS, the magnitude and significance of the VTA-leptin interaction remains to be fully described. Interestingly, unlike the action of leptin in the NTS, systemic leptin fails to increase phosphorylation of STAT-3 in the VTA (MM Scott and JK Elmquist, unpublished observations, 2008), suggesting the mechanism of leptin action differs significantly between these 2 nuclei. Collectively, these results suggest that the body weight and food intake effects of leptin are mediated by a distributed network of leptin-responsive cells that includes additional hypothalamic (ie, dorsal hypothalamus) and likely extrahypothalamic (eg, brainstem and VTA) sites of action that warrant further study (73).
FUTURE DIRECTIONS: THE “MODERN ERA” OF GLUCOSE AND ENERGY BALANCE
Within the past decade there has been a marked increase in the prevalence of obesity in the United States, along with its associated diseases, such as type 2 diabetes, which pose a serious threat to public health. With the occurrence of obesity growing to epidemic levels, the importance of understanding all facets of body weight regulation is evident. Great advances were made over the past 150 y leading to the development of many therapeutic strategies for the control of obesity and diabetes. However, as discussed here much of these advances were based on circumstantial evidence. The “modern era” of glucose and energy homeostasis will embrace current (eg, neuron-specific targeting with the use of transgenic Cre lines) and any future technologies that use multidisciplinary in vivo and in vitro approaches to challenge and extend conventional hypotheses about the role of the brain in glucose and energy balance. Importantly, these strategies will allow the sites and mechanisms of action of leptin in the CNS in the control of feeding, metabolism, and glucose homeostasis to be precisely described. Such findings will be crucial to understanding the development of obesity and other comorbid conditions that are rampant within our society. (Other articles in this supplement to the Journal include references 74–77.)
Acknowledgments
The authors’ responsibilities were as follows—KWW, MMS, and JKE: contributed to the writing and preparation of this review. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723–7 [DOI] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults: the National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA 1994;272:205–11 [DOI] [PubMed] [Google Scholar]
- 3.Hensrud DD. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes. Obes Res 2001;9(suppl 4):348S–53S [DOI] [PubMed] [Google Scholar]
- 4.Mayer J. The glucostatic theory of regulation of food intake and the problem of obesity. Bull New Engl Med Cent 1952;14:43–9 [PubMed] [Google Scholar]
- 5.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med 1953;249:13–6 [DOI] [PubMed] [Google Scholar]
- 6.Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci 1955;63:15–43 [DOI] [PubMed] [Google Scholar]
- 7.Muller K, Scharrer E, Zucker H. [Suspension, by change in diet composition, of a 12-hour rhythmic blood glucose concentration fixed by the meal sequence.] Naturwissenschaften 1967;54:201–2(in German) [DOI] [PubMed] [Google Scholar]
- 8.Louis-Sylvestre J, Le Magnen J. Fall in blood glucose level precedes meal onset in free-feeding rats. Neurosci Biobehav Rev 1980;4(suppl 1):13–5 [DOI] [PubMed] [Google Scholar]
- 9.Novin D, VanderWeele DA, Rezek M. Infusion of 2-deoxy-D-glucose into the hepatic-portal system causes eating: evidence for peripheral glucoreceptors. Science 1973;181:858–60 [DOI] [PubMed] [Google Scholar]
- 10.Smith GP, Epstein AN. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol 1969;217:1083–7 [DOI] [PubMed] [Google Scholar]
- 11.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979;282:503–5 [DOI] [PubMed] [Google Scholar]
- 12.Woods SC, Stein LJ, McKay LD, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am J Physiol 1984;247:R393–401 [DOI] [PubMed] [Google Scholar]
- 13.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953;140:578–96 [DOI] [PubMed] [Google Scholar]
- 14.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 1973;9:294–8 [DOI] [PubMed] [Google Scholar]
- 15.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol 1969;217:1298–304 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32 [DOI] [PubMed] [Google Scholar]
- 17.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995;269:543–6 [DOI] [PubMed] [Google Scholar]
- 18.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–9 [DOI] [PubMed] [Google Scholar]
- 19.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 1997;94:8878–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill JW, Williams KW, Ye C, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 2008;118:1796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997;390:521–5 [DOI] [PubMed] [Google Scholar]
- 22.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001;411:480–4 [DOI] [PubMed] [Google Scholar]
- 23.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 2004;7:493–4 [DOI] [PubMed] [Google Scholar]
- 24.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537–56 [DOI] [PubMed] [Google Scholar]
- 25.Tartaglia LA. The leptin receptor. J Biol Chem 1997;272:6093–6 [DOI] [PubMed] [Google Scholar]
- 26.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263–71 [DOI] [PubMed] [Google Scholar]
- 27.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG., Jr Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem 2002;277:41547–55 [DOI] [PubMed] [Google Scholar]
- 28.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A 1998;95:6061–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darnell JE., Jr STATs and gene regulation. Science 1997;277:1630–5 [DOI] [PubMed] [Google Scholar]
- 30.Zabeau L, Lavens D, Peelman F, Eyckerman S, Vandekerckhove J, Tavernier J. The ins and outs of leptin receptor activation. FEBS Lett 2003;546:45–50 [DOI] [PubMed] [Google Scholar]
- 31.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A 1996;93:8374–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A 1996;93:6231–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenblum CI, Tota M, Cully D, et al. Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology 1996;137:5178–81 [DOI] [PubMed] [Google Scholar]
- 34.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 1996;14:95–7 [DOI] [PubMed] [Google Scholar]
- 35.McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology 1998;139:4442–7 [DOI] [PubMed] [Google Scholar]
- 36.Buyse M, Ovesjo ML, Goiot H, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci 2001;14:64–72 [DOI] [PubMed] [Google Scholar]
- 37.Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003;421:856–9 [DOI] [PubMed] [Google Scholar]
- 38.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 2000;3:757–8 [DOI] [PubMed] [Google Scholar]
- 39.Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J Physiol 1997;504:527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin BE. Glucosensing neurons do more than just sense glucose. Int J Obes Relat Metab Disord 2001;25(suppl 5):S68–72 [DOI] [PubMed] [Google Scholar]
- 41.Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006;49:191–203 [DOI] [PubMed] [Google Scholar]
- 42.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 2008;149:2138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balthasar N, Coppari R, McMinn J, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004;42:983–91 [DOI] [PubMed] [Google Scholar]
- 44.Cui Y, Huang L, Elefteriou F, et al. Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 2004;24:258–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choudhury AI, Heffron H, Smith MA, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest 2005;115:940–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coppari R, Ichinose M, Lee CE, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 2005;1:63–72 [DOI] [PubMed] [Google Scholar]
- 47.Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005;123:493–505 [DOI] [PubMed] [Google Scholar]
- 48.van de Wall E, Leshan R, Xu AW, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 2008;149:1773–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuno TM, Kelley KA, Pasinetti GM, Roberts JL, Mobbs CV. Transgenic neuronal expression of proopiomelanocortin attenuates hyperphagic response to fasting and reverses metabolic impairments in leptin-deficient obese mice. Diabetes 2003;52:2675–83 [DOI] [PubMed] [Google Scholar]
- 50.Morton GJ, Niswender KD, Rhodes CJ, et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology 2003;144:2016–24 [DOI] [PubMed] [Google Scholar]
- 51.Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007;449:228–32 [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim N, Bosch MA, Smart JL, et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 2003;144:1331–40 [DOI] [PubMed] [Google Scholar]
- 53.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–10 [DOI] [PubMed] [Google Scholar]
- 54.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 2008;7:179–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001;413:794–5 [DOI] [PubMed] [Google Scholar]
- 56.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension 2003;41:763–7 [DOI] [PubMed] [Google Scholar]
- 57.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 2007;148:72–80 [DOI] [PubMed] [Google Scholar]
- 58.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 2002;143:239–46 [DOI] [PubMed] [Google Scholar]
- 59.Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 2002;143:3779–84 [DOI] [PubMed] [Google Scholar]
- 60.Burdakov D, Jensen LT, Alexopoulos H, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006;50:711–22 [DOI] [PubMed] [Google Scholar]
- 61.Elias CF, Kelly JF, Lee CE, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 2000;423:261–81 [PubMed] [Google Scholar]
- 62.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 1997;138:839–42 [DOI] [PubMed] [Google Scholar]
- 63.Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol 2006;573:395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 2007;148:1868–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 2000;25:515–32 [DOI] [PubMed] [Google Scholar]
- 66.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005;8:1481–9 [DOI] [PubMed] [Google Scholar]
- 67.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet 2001;357:354–7 [DOI] [PubMed] [Google Scholar]
- 68.Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007;315:1267–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet 2000;25:102–4 [DOI] [PubMed] [Google Scholar]
- 70.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci 2004;118:479–87 [DOI] [PubMed] [Google Scholar]
- 71.Spyraki C, Fibiger HC, Phillips AG. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology (Berl) 1982;77:379–82 [DOI] [PubMed] [Google Scholar]
- 72.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006;51:801–10 [DOI] [PubMed] [Google Scholar]
- 73.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 2005;493:63–71 [DOI] [PubMed] [Google Scholar]
- 74.Lebenthal E. Introduction to the symposium. Am J Clin Nutr 2009;89(suppl):971S–2S [DOI] [PubMed] [Google Scholar]
- 75.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr 2009;89(suppl):973S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr 2009;89(suppl):980S–4S [DOI] [PubMed] [Google Scholar]
- 77.Blüher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr 2009;89(suppl):991S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]