Abstract
Leptin has emerged over the past decade as a key hormone in not only the regulation of food intake and energy expenditure but also in the regulation of neuroendocrine and immune function as well as the modulation of glucose and fat metabolism as shown by numerous observational and interventional studies in humans with (complete) congenital or relative leptin deficiency. These results have led to proof-of-concept studies that have investigated the effect of leptin administration in subjects with complete (congenital) leptin deficiency caused by mutations in the leptin gene as well as in humans with relative leptin deficiency, including states of lipoatrophy or negative energy balance and neuroendocrine dysfunction, as for instance seen with hypothalamic amenorrhea in states of exercise-induced weight loss. In those conditions, most neuroendocrine, metabolic, or immune disturbances can be restored by leptin administration. Leptin replacement therapy is thus a promising approach in several disease states, including congenital complete leptin deficiency, states of energy deprivation, including anorexia nervosa or milder forms of hypothalamic amenorrhea, as well as syndromes of insulin resistance seen in conditions such as congenital or acquired lipodystrophy. In contrast, states of energy excess such as garden-variety obesity are associated with hyperleptinemia that reflects either leptin tolerance or leptin resistance. For those conditions, development of leptin sensitizers is currently a focus of pharmaceutical research. This article summarizes our current understanding of leptin's role in human physiology and its potential role as a novel therapeutic option in human disease states associated with a new hormone deficiency, ie, leptin deficiency.
INTRODUCTION
The discovery of leptin, the prototype adipocyte-secreted hormone or cytokine (adipokine) by positional cloning of the ob gene in 1994, has revolutionized our understanding of hormonal regulation of energy homeostasis and has changed substantially our view of adipose tissue from that of a depot storage organ to that of an active endocrine organ producing several bioactive peptides (adipokines) and inflammatory as well as antiinflammatory molecules (1, 2). This review summarizes leptin's role in the physiology and pathophysiology of several biological systems as shown by recent studies. It also discusses the potential role of leptin replacement therapy in states of absolute (congenital) or relative leptin deficiency and presents the concept of leptin tolerance or resistance in leptin excess states such as garden-variety obesity.
LEPTIN: THE PROTOTYPE ADIPOKINE
Leptin, a 167–amino acid peptide, is primarily expressed in adipose tissue, but it is also found in many other tissues, including the placenta, mammary gland, testes, ovary, endometrium, stomach, hypothalamus, pituitary, and others. Leptin exerts pleiotropic effects by binding and activating specific leptin receptors in the hypothalamus and other organs, has direct and indirect effects in metabolically active tissues, and regulates several neuroendocrine axes (2–4).
Leptin circulates in a free form (the biologically active form) and also binds to leptin-binding proteins. The hormone is secreted in a pulsatile fashion with significant diurnal-nocturnal variation. Leptin's pulsatility characteristics are similar in lean and obese subjects with the only exception being pulse amplitude, which is higher in obese subjects (5–7) (Figure 1).
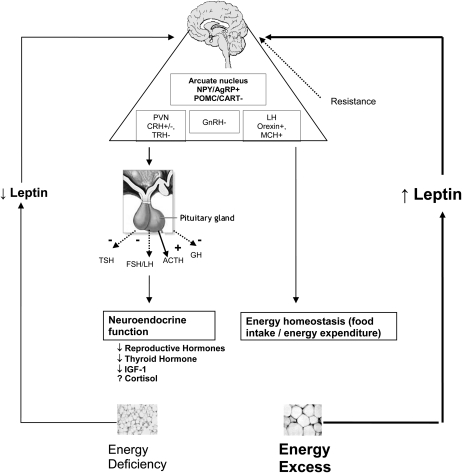
FIGURE 1.
Energy deficiency is associated with hypoleptinemia, whereas energy excess leads to hyperleptinemia. Effects of circulating leptin on neuroendocrine axes and energy homeostasis are depicted herein. Effects of leptin on immune function as well as insulin resistance are discussed in the text. NPY/AgRP, neuropeptide Y/Agouti-related peptide; POMC, proopiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; PVN, paraventricular nucleus; CRH, corticotropin-releasing hormone; TRH, thyroid-releasing hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; MCH, melanin-concentrating hormone; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; ACTH, adrenocorticotropic hormone; GH, growth hormone; IGF-I, insulin-like growth factor I. Adapted from reference 9.
Although anthropometric and other factors (sex, fat mass and fat distribution, hormones, and cytokines; Table 1) may influence the secretion pattern of leptin, the crucial factor in regulating serum leptin concentrations seems to be short-term caloric intake and the amount of energy stored in adipocytes. Leptin concentrations are positively correlated with the amount of body fat (4–10). However, although obese subjects are hyperleptinemic compared with lean persons (11), they appear either to be tolerant or resistant to the central hypothalamic effects of leptin. The reduced sensitivity toward exogenous (administered) and endogenous leptin is commonly referred to as leptin resistance (6, 9). Leptin acts by activating specific leptin receptors. Several isoforms of the leptin receptor, resulting from alternative splicing, convey biological activity and are involved in mediating leptin's actions in the brain and peripheral organs. Alterations in the signaling of the long isoform of the leptin receptor, especially in the hypothalamic arcuate nucleus, seem to play a crucial role in leptin resistance. Additional mechanisms that were proposed to induce resistance toward the effects of leptin include alterations in the transport of leptin across the blood-brain barrier (12, 13). Moreover, increased circulating leptin concentrations (as a result of increased body fat mass associated with obesity) as well as centrally infused leptin are associated with altered expression of several molecules that impair leptin signaling, including decreased leptin-induced p-signal transducers and activators of transcription 3 (p-STAT) as well as increased suppressor of cytokine signaling 3 (SOCS-3) expression, all of which suppress leptin signaling (12). Another potential mechanism that was proposed to interfere with leptin signaling by inhibiting signaling of the long isoform of the leptin receptor is the protein tyrosine phosphatase 1B (12, 13). Other mechanisms are under investigation.
TABLE 1.
Factors that regulate circulating leptin concentrations in addition to body fat mass1
| Factors promoting leptin secretion |
| Overfeeding |
| Glucose |
| Amino acids |
| Insulin |
| Glucocorticoids |
| Estrogens |
| Factors inhibiting leptin secretion |
| Fasting |
| Free fatty acids and other lipid metabolites |
| Androgens |
| Thyroid hormones |
| Catecholamines |
| Inflammatory cytokines |
| Agonists of PPARγ (in humans the final net effect is null because PPARγ agonists also increase fat mass which negates the direct negative effect of PPARγ agonists on leptin secretion) |
Adapted from reference 6. PPARγ, peroxisome proliferative-activated receptor γ.
Deficient leptin signaling because of hyperleptinemia (as described above) or because of hypo- or aleptinemia caused by mutations of either leptin or the leptin receptor genes results in hyperphagia and decreased energy expenditure in rodents and humans (13). The result is not only an increasing degree of obesity associated with increased lipid storage in muscle, liver, and other tissues but also dysfunction of several neuroendocrine axes, including the reproductive, thyroid, and adrenal axes, as well as abnormal function of the immune and autonomic system (ie, thermoregulation, energy expenditure, and others) (5, 13, 14). These findings are consistent with physiology studies, which have proven that leptin is a crucial hormonal factor for regulating several physiologic processes, including inflammation, angiogenesis, hematopoiesis, immune function, and reproduction. According to our current understanding, leptin's main function is to inform several organs of the organism that there is enough energy to sustain life. However, the major physiologic role of leptin is to signal inadequate rather than excess energy stores. Leptin's key functions include regulating food intake and energy expenditure, regulating neuroendocrine and immune function, and modulating glucose and fat metabolism by improving insulin sensitivity and reducing intracellular lipids (3–8, 10).
LEPTIN'S ROLE IN REGULATING NEUROENDOCRINE FUNCTION
Leptin and the reproductive axis
Leptin plays a crucial role in regulating reproduction and the hypothalamic-pituitary-gonadal (HPG) axis. The percentage of leptin-bearing cells within the pituitary varies in different reproductive states and depends on sex and menstrual cycle (15). In addition, gonadotropin-releasing hormone (GnRH)–secreting neurons in the hypothalamus express leptin receptors, and the GnRH pulsatility in arcuate hypothalamic neurons regulating the release of gonadotropins is stimulated by leptin (5, 15).
Subjects with congenital leptin deficiency or loss of leptin function because of leptin mutations or leptin receptor mutations have clinically evident hypogonadotropic hypogonadism and present with low concentrations of follicle-stimulating hormone and luteinizing hormone and complete loss of luteinizing hormone pulsatility, lack of pubertal growth spurt, reduced expression of secondary sexual characteristics, and primary or secondary amenorrhea (16). The clinical features of hypothalamic hypogonadism and associated disturbances can be restored by leptin administration in replacement doses (17). However, not only decreased circulating leptin concentrations but also elevated concentrations of the hormone because of increased body fat mass associated with (morbid) obesity may have an inhibitory effect on the HPG axis (18). These results underline a pivotal role of leptin in regulating reproductive function and strengthen the hypothesis that leptin is one of the factors mediating reproductive abnormalities in several disease states. We have shown that leptin may serve as a signal to convey information to the reproductive system that the amount of energy stored in the body as fat is adequate not only for the survival of the person but also for carrying a pregnancy to term. Because a certain threshold of energy and body fat mass, which may vary from person to person, seems to be necessary for the onset of puberty and normal fertility, leptin has been proposed to be a permissive signal that can activate the reproductive axis and maintain normal reproductive function by conveying needed information on available energy reserves in the adipose tissue (5, 6, 17). Moreover, in states of secondary failure of the HPG axis associated with loss of fat mass, such as in exercise-induced amenorrhea or anorexia nervosa, exogenously administered leptin may fully normalize function of this axis (5, 7, 9, 17).
Leptin and the adrenal axis
The role of leptin in the regulation of the adrenal axis in humans remains controversial. In contrast to findings in rodents, subjects with mutations of the leptin gene or the leptin receptor gene present with normal adrenal function (16, 18). Interventional studies in healthy, normal-weight subjects failed to show an effect of leptin on adrenal steroids (19).
Leptin and the hypothalamic-pituitary-growth hormone–insulin-like growth factor I axis
A direct effect and an indirect effect of leptin on the growth hormone (GH)–insulin-like growth factor I (IGF-I)–insulin-like growth factor binding protein (IGF-BP) axis have also been suggested. Subjects with congenital leptin deficiency because of a mutation in the leptin gene or the leptin receptor gene have been described to have a significant growth delay in early childhood because of decreased GH secretion and low IGF-I and IGF-BP3 concentrations (16, 18). Administering leptin in replacement doses to healthy, normal-weight men after a period of acute fasting that resulted in low IGF-I concentrations partially prevented the decrease in IGF-I and IGF-BP3 but had no (short-term) effect on circulating concentrations of GH (19). We have observed similar effects of leptin to up-regulate IGFs and to alter concentrations of IGF-PB3 after long-term leptin administration (20). However, the exact long-term effect of leptin on the hypothalamic-pituitary-GH-IGF axis remains to be fully elucidated.
Leptin and the hypothalamic-pituitary-thyroid axis
A significant role of leptin in the regulation of the hypothalamic-pituitary-thyroid axis has been well established in humans (6–8). Subjects with leptin receptor mutations present with hypothalamic hypothyroidism, low circulating thyroxine concentrations, normal circulating basal thyroid-stimulating hormone (TSH), and a sustained response of TSH to a thyroid-releasing hormone challenge (18). Healthy, normal-weight subjects exhibit a similar leptin and TSH 24-h secretion pattern that is impaired in subjects with congenital leptin deficiency (21). Leptin administration in replacement doses to healthy lean men during a 3-d starvation period significantly blunted the fasting-induced decrease in TSH pulsatility and increased free thyroxine to concentrations within the normal range (19). In contrast, in leptin-replete states, leptin seems to lack the ability to regulate the hypothalamic-pituitary-thyroid axis (7, 21). These data indicate that leptin may regulate TSH, influencing secondarily circulating thyroxine concentrations in leptin-deplete states. The exact mechanisms underlying these effects are still not completely understood and require further investigation.
ROLE OF LEPTIN IN GLUCOSE AND FAT METABOLISM OR DEVELOPMENT OF INSULIN RESISTANCE IN STATES OF LEPTIN DEFICIENCY
States of congenital leptin deficiency because of mutations of the leptin gene have been associated with severe obesity, glucose intolerance, and insulin resistance in humans. These disturbances can be completely reversed by leptin administration (6). Lipodystrophic syndromes, ie, conditions of complete or partial absence of subcutaneous fat, have also been associated with low leptin concentrations. Long-term effects of leptin in such conditions have been studied in the context of controlled clinical trials in patients with partial lipoatrophy induced by administration of highly active antiretrovirals in HIV-positive patients who have milder insulin resistance and a milder metabolic syndrome. It has been shown that leptin administration in replacement doses significantly improved glycemia, dyslipidemia, and hepatic steatosis in lipoatrophic, hypoleptinemic patients with severe insulin resistance. It also improved lipidemia and insulin resistance in HIV-positive patients with partial lipoatrophy (10, 22–26).
Lipodystrophic subjects accumulate adipose tissue intrahepatically and intramyocellularly, which is probably the mechanism by which they become insulin resistant. The resolution of insulin resistance in states of congenital leptin deficiency may be either the primary result of leptin or a secondary result because of a loss or redistribution of body fat. This remains to be clarified by future studies. In general, data derived from initial proof-of-concept studies indicate that leptin deficiency, because of a lack of subcutaneous fat (congenital or acquired), might be responsible for the metabolic abnormalities seen in these subjects and that leptin administration in replacement doses could restore metabolic function (6, 10, 23–26) (Table 2).
TABLE 2.
Leptin deficiency and leptin resistance states1
| Disease state | Estimated prevalence | Associated features |
| Leptin deficiency | ||
| Hypothalamic amenorrhea | 3–8.5% in women aged 13–44 y | Strenuous exercise, stress, energy deficit, neuroendocrine dysfunction |
| Lipoatrophy (congenital) | Rare | Insulin resistance, impaired glucose tolerance, dyslipidemia |
| Anorexia nervosa | 1–3% of college-age subjects | Disturbed body image, severe restriction of food intake, loss of body weight, neuroendocrine disturbances |
| HIV-lipodystrophy | ≤50% of antiretroviral-treated patients | Insulin resistance, impaired glucose tolerance or type 2 diabetes, dyslipidemia, increased risk of cardiovascular disease |
| Obesity as a manifestation of leptin deficiency | ||
| Complete congenital leptin deficiency | Rare | Hypogonadotrophic hypogonadism, hyperphagia, advanced bone age, hyperinsulinemia, immune dysfunction in the context of early onset morbid obesity |
| Heterozygous leptin deficiency | ≤5–6% of the obese | Garden-variety obesity with low leptin concentrations relative to fat mass |
| Obesity as a manifestation of leptin resistance (involving leptin and molecular pathways downstream of the leptin receptor) | ||
| Leptin receptor gene mutations | Rare | Hypogonadotrophic hypogonadism, abnormal growth hormone, and TSH secretion |
| POMC mutations | Rare | ACTH deficiency, red hair, pale skin |
| Prohormone convertase deficiency | Rare | Hypogonadotrophic hypogonadism, hypocortisolemia, postprandial hypoglycemia |
| MC4R mutations | 5–8% of childhood obesity | Increased fat and lean body mass, increased linear growth and bone density |
| Mutations of other molecules downstream of leptin receptor | Rare | Obesity with onset in childhood |
| Mechanism to be discovered | >90% of obese subjects | Garden-variety obesity |
ROLE OF LEPTIN IN STATES OF LEPTIN EXCESS
Obesity is associated with hyperleptinemia, which may reflect either leptin tolerance or leptin resistance. It has been proposed that elevated circulating leptin concentrations may directly induce a state of inflammation that might be underlying the development of features of the metabolic syndrome, such as insulin resistance, type 2 diabetes, and atherosclerosis. We have not found direct supporting evidence of this hypothesis in our interventional studies (22). Because of an overlap between signal-transducing pathways of leptin and insulin, a common pathogenesis of leptin and insulin resistance has also been suggested and would offer a variety of new approaches for novel therapies. This area remains a significant area of research (6, 27).
ROLE OF LEPTIN IN THE REGULATION OF IMMUNE FUNCTION
Leptin has been shown to have several effects on the innate immune system in leptin-deficient states: It exerts direct effects on macrophages by up-regulating the phagocytic activity, stimulates the secretion of proinflammatory cytokines, and stimulates chemotaxis in polymorphonuclear cells (6). As shown by animal studies as well as observational studies in humans with congential leptin deficiency or obesity-related hyperleptinemia, both leptin excess and states of energy deprivation associated with leptin deficiency may increase the individual susceptibility to infection: Children with congenital leptin deficiency are prone to early death as a result of severe infections during childhood because of defects in T cell number and function (28). However, immune function could be markedly improved with leptin replacement (29, 30).
Further insight into the role of leptin in immune function could be gained from 3 interventional studies involving administration of recombinant methionyl human leptin (r-metHuLeptin) to lean, otherwise healthy obese, and obese diabetic subjects to investigate whether increasing circulating leptin concentrations over a wide spectrum of values (from low physiologic to high pharmacologic) would alter serum concentrations of inflammatory markers and other cytokines important in the T helper cell response. r-metHuLeptin administered in replacement doses to healthy subjects (after starvation for 72 h) prevented the starvation-induced reduction of CD4+ CD45RA+ peripheral mononuclear blood cells (31).
Thus, leptin may increase circulating cytokine concentrations and regulate the T helper type 1 and 2 cell immune balance in states of congenital leptin deficiency and immune dysfunction, supporting a role for leptin in regulating immune function in these conditions (29, 30). Similar to congenital leptin deficiency, relative leptin deficiency because of long-term energy deprivation is associated with defects in immunologic variables that may be corrected with exogenous r-metHuLeptin administration: Leptin replacement therapy alters circulating cytokine concentrations in women with hypothalamic amenorrhea who present with chronic relative leptin deficiency resulting from a chronic energy deficit (6, 31).
Although obesity is also associated with disturbed immune function, the role of leptin in regulating immune function in obesity has been suggested to be a permissive one. Interventional studies investigating the effect of leptin administration to persons with leptin deficiency compared with leptin sufficiency or leptin excess could clearly show a role for leptin in regulating the immune system in states of leptin deficiency, but not leptin excess, as seen in obesity (22, 27, 31). However, data derived from those interventional studies show that congenital complete leptin deficiency (29) or induction of acute hypoleptinemia to leptin concentrations of <1 ng/mL in humans induces changes in reproductive, thyroid, and IGF axes (20) and immune function (31). Increasing serum leptin concentrations to >2–3 ng/mL with r-metHuLeptin administration corrects, fully or in part, these abnormalities (20, 29, 31). However, in leptin-sufficient obese subjects with type 2 diabetes, the administration of r-metHuLeptin for 4 or 16 wk resulted in high pharmacologic leptin concentrations but did not activate the tumor necrosis factor α system or increase cytokines or inflammatory markers above the normal range. These data support a permissive role for leptin in the regulation of the immune system but do not support an etiopathogenic role for leptin in proinflammatory states associated with leptin excess such as obesity (32).
All these findings from observational and interventional studies taken together indicate that congenital leptin deficiency as well as negative energy balance may alter the nature and vigor of the immune response by leptin-dependent mechanisms. The role of leptin in regulating immune function appears to be a permissive one (33), because there might exist a critical leptin threshold above which leptin has no major additional physiologic effect on neuroendocrine or immune function (7).
CLINICAL APPLICATIONS
Leptin replacement therapy in complete leptin deficiency
Very rare genetic forms of obesity result from congenital leptin deficiency as a result of mutations in the leptin genes, which are frequently associated with consanguineous marriage. These leptin-deficient subjects respond in a dramatic fashion to leptin administered in replacement doses, which induces markedly decreased appetite and food intake and which in turn result in a significant reduction in body weight (28–30). In addition, leptin treatment in children with congenital leptin deficiency results in normalization of several neuroendocrine axes, including the reproductive and thyroid axis, as well as immune function. These children, when given leptin, have an appropriately timed pubertal development (28, 29). To answer the question whether leptin replacement therapy has long-term beneficial effects in subjects with congenital leptin deficiency, we need more long-term interventional studies.
Leptin replacement therapy in relative leptin deficiency
Relative leptin deficiency is an emerging clinical syndrome seen in several clinical conditions, including congential or acquired lipodystrophy as well as exercise-induced energy deficiency and hypothalamic amenorrhea or anorexia nervosa. Interventional studies in women with hypothalamic amenorrhea and low circulating leptin concentrations have proven the concept that r-metHuLeptin in replacement doses may restore reproductive function, induce ovulation, and increase circulating concentrations of thyroxine, IGF-I, IGF-BP3, bone alkaline phosphatase, and other bone markers. In addition, r-metHuLeptin replacement restored GnRH pulsatility (17), raising thus the intriguing possibility that leptin administration in replacement, physiologic doses could also be a potential treatment of some patients with infertility. This hypothesis has been strengthened by the observation that leptin and the soluble leptin receptor are not only highly interrelated with each other but also with other intrafollicular hormones that regulate fertility (34).
r-metHuLeptin therapy in replacement doses improved insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy (10). Whether r-metHuLeptin alone or in combination with other treatments will find a place in our therapeutic armamentarium for this condition remains to be seen.
Taking these initial data together, leptin replacement therapy might prove to be a therapeutic option for patients with disorders that are associated with a new hormone deficiency state, ie, the leptin deficiency. Further studies in this area are needed.
Leptin administration in states of obesity
Despite initial hopes, placebo-controlled trials in obese persons over periods of ≤24 wk have shown a statistically significant but clinically not impressive weight loss in leptin-treated subjects compared with placebo-treated controls. Given the initial high expectations for r-metHuLeptin as a potential treatment of obesity and the overwhelming results seen in leptin-deficient patients (6), results in subjects with garden-variety obesity have thus been considered to be disappointing. More recent evidence indicates, however, that coadministration of leptin with medications that could sensitize the body to leptin's actions may provide better weight-loss outcomes. This remains an active and exciting field of research (35).
SUMMARY AND FURTHER DIRECTIONS
During the past decade, leptin has been proven to be the adipocyte-secreted hormone that communicates information to several organs about the body's energy reserves. Subsequent studies have also shown that leptin is involved in regulating several neuroendocrine axes, immune function, and glucose and lipid metabolism. Our understanding of the potential applications of leptin in states of complete or relative leptin deficiency has been substantially advanced by recent observational and interventional studies in humans, ranging from pharmacokinetic studies to leptin replacement therapy in patients with leptin deficiency syndromes such as hypothalamic amenorrhea or acquired lipodystrophy. Similar to other hormone deficiency syndromes, leptin deficiency syndromes call for leptin replacement treatment in humans.
Thus, r-metHuLeptin replacement could prove to be a new potentially useful medication to be added to our therapeutic armamentarium for disease states of absolute or relative leptin deficiency to restore neuroendocrine, metabolic, or immune function in low-leptin states such as (congenital or acquired) lipodystrophy or exercise-induced hypothalamic amenorrhea. Other applications, such as anorexia nervosa and infertility, are currently under intense investigation and hold promise as potential future therapeutic options. Finally, the development of leptin sensitizers or results of trials evaluating coadministration of leptin with other medications that could act as leptin sensitizers are anticipated with great expectations. (Other articles in this supplement to the Journal include references 36–39.)
Acknowledgments
Both authors contributed parts of the manuscript and refined the entire manuscript. The senior author's laboratory has received funds from Amgen and Amylin. No other conflicts of interest were reported.
REFERENCES
- 1.Zhang Y, Proenca M, Maffei M, et al. Positional cloning of the obese gene and its human homologue. Nature 1994;372:425–32 [DOI] [PubMed] [Google Scholar]
- 2.Laclaustra M, Corella D, Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis 2007;17:125–39 [DOI] [PubMed] [Google Scholar]
- 3.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–65 [DOI] [PubMed] [Google Scholar]
- 4.Brennan AM, Mantzoros CS. Leptin and adiponectin: their role in diabetes. Curr Diab Rep 2007;7:1–2 [DOI] [PubMed] [Google Scholar]
- 5.Blüher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes 2007;14:1–7(Review) [DOI] [PubMed] [Google Scholar]
- 6.Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology: emerging clinical applications in leptin deficient states. Nat Clin Pract Endocrinol Metab 2006;2:318–27 [DOI] [PubMed] [Google Scholar]
- 7.Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 2006;103:8481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelesidis T, Mantzoros CS. The emerging role of leptin in humans. Pediatr Endocrinol Rev 2006;3:239–48(Review) [PubMed] [Google Scholar]
- 9.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005;366:74–85 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab 2006;91:2605–11 [DOI] [PubMed] [Google Scholar]
- 11.Kiess W, Petzold S, Töpfer M, et al. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab 2008;22:135–53 [DOI] [PubMed] [Google Scholar]
- 12.Munzberg H. Differential leptin access into the brain–a hierarchical organization of hypothalamic leptin target sites? Physiol Behav 2008;94:664–9 [DOI] [PubMed] [Google Scholar]
- 13.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 2008;70:537–56 [DOI] [PubMed] [Google Scholar]
- 14.Blüher S, Mantzoros CS. Insulin resistance in states of energy excess–pathophysiological concepts. Mantzoros CS. The metabolic syndrome Totowa, NJ: Humana Press, in press [Google Scholar]
- 15.Akhther N, Johnson BW, Crane C, et al. Anterior pituitary leptin expression changes in different reproductive states: stimulation, in vitro, by gonadotropin releasing hormone (GnRH). J Histochem Cytochem 2007;55:151–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 2007;356:237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004;351:987–97 [DOI] [PubMed] [Google Scholar]
- 18.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401 [DOI] [PubMed] [Google Scholar]
- 19.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 2003;111:1409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan JL, Williams CJ, Raciti P, et al. Leptin does not mediate short-term fasting-induced changes in GH pulsatility, but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab 2008;93:2819–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001;86:3284–91 [DOI] [PubMed] [Google Scholar]
- 22.Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab 2005;90:1618–24 [DOI] [PubMed] [Google Scholar]
- 23.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–8 [DOI] [PubMed] [Google Scholar]
- 24.Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 2005;54:1994–2002 [DOI] [PubMed] [Google Scholar]
- 25.Tsiodras S, Mantzoros C. The role of leptin and adiponectin in the HAART induced metabolic syndrome. Am J Infect Dis 2006;2:141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeney LL, Brennan AM, Mantzoros CS. The role of adipokines in relation to HIV lipodystrophy. AIDS 2007;21:895–904 [DOI] [PubMed] [Google Scholar]
- 27.Matarese G, Mantzoros C, La Cava A. Leptin and adipocytokines: bridging the gap between immunity and atherosclerosis. Curr Pharm Des 2007;13:3676–80 [DOI] [PubMed] [Google Scholar]
- 28.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 1999;84:3686–95 [DOI] [PubMed] [Google Scholar]
- 29.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002;110:1093–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooqi IS. Monogenic human obesity. Front Horm Res 2008;36:1–11 [DOI] [PubMed] [Google Scholar]
- 31.Chan JL, Moschos SJ, Bullen J, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab 2005;90:1625–31 [DOI] [PubMed] [Google Scholar]
- 32.Brennan AM, Li TY, Kelesidis I, Gavrila A, Hu FB, Mantzoros CS. Circulating leptin levels are not associated with cardiovascular morbidity and mortality in women with diabetes: a prospective cohort study. Diabetologia 2007;50:1178–85 [DOI] [PubMed] [Google Scholar]
- 33.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol 2006;176:7745–52 [DOI] [PubMed] [Google Scholar]
- 34.Welt CK, Schneyer AL, Heist K, Mantzoros CS. Leptin and soluble leptin receptor in follicular fluid. J Assist Reprod Genet 2003;20:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A 2008;105:7257–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebenthal E. Introduction to the symposium. Am J Clin Nutr 2009;89(suppl):971S–2S [DOI] [PubMed] [Google Scholar]
- 37.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr 2009;89(suppl):973S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooqi IS, O'Rahilly S. Leptin: a pivotal action of human energy homeostasis. Am J Clin Nutr 2009;89(suppl):980S–4S [DOI] [PubMed] [Google Scholar]
- 39.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: leptin regulation in the mediobasal hypothalamus. Am J Clin Nutr 2009;89(suppl):985S–90S [DOI] [PMC free article] [PubMed] [Google Scholar]