Abstract
Aim
Vasopeptidase inhibitors are drugs that inhibit angiotensin-converting enzyme and neutral endopeptidase (NEP). The latter is a protease that degrades vasoactive peptides and is increased in diabetes. We have previously shown that treating streptozotocin-induced diabetic rats, an animal model of type 1 diabetes, with AVE7688, a vasopeptidase inhibitor, improves neurovascular and neural function. In this study, we determined the effect of treating Zucker diabetic fatty (ZDF) rats, an animal model of type 2 diabetes, with AVE7688 on vascular and neural function.
Methods
ZDF rats at 12 weeks of age were treated for 12 weeks with AVE7688 (500 mg/kg diet). Afterwards, vascular reactivity of epineurial arterioles of the sciatic nerve and nerve conduction velocity and blood flow was determined.
Results
Vascular and neural function was significantly impaired in ZDF rats compared with age-matched lean (control) rats. Treating ZDF rats with AVE7688 improved vascular relaxation to acetylcholine and calcitonin gene-related peptide in epineurial arterioles. Motor and sensory nerve conduction velocity, endoneurial blood flow and thermal nociception end-points were also improved by treatment compared with untreated ZDF rats. Superoxide and expression of NEP were increased in epineurial arterioles from ZDF rats and attenuated by treatment with AVE7688.
Conclusions
AVE7688 is an effective treatment for microvascular and neural disease in ZDF rats. Thus, vasopeptidase inhibitors may be an effective treatment for diabetic microvascular and neural complication in type 2 diabetes.
Keywords: diabetic neuropathy, neutral endopeptidase, oxidative stress, type 2 diabetes, vascular reactivity
Introduction
Zucker diabetic fatty (ZDF) rats are an animal model for type 2 diabetes. In this model, all fatty males become hyperglycaemic by 8 weeks of age and glucose remains elevated through their lifespan [1]. Initially, ZDF rats are hyperinsulinaemic. However, by 22–42 weeks of age, serum insulin levels decline to below the levels of insulin in age-matched lean control rats [1]. A similar characteristic is seen in human type 2 diabetes, which is thought to be caused by pancreas/β-cell exhaustion. Serum free fatty acids, triglycerides and cholesterol levels are significantly higher in ZDF rats throughout their lifespan compared with lean littermate controls [1].
Previously we have demonstrated that development of microvascular and nerve dysfunction in ZDF rats is progressive with impairment of acetylcholine-mediated vascular relaxation occurring prior to nerve blood flow and conduction deficits [2,3]. At 6 weeks of age, ZDF rats were hyperinsulinaemic but had a normal blood glucose level. However, by 8–10 weeks of age, the rats became hyperglycaemic and relaxation by epineurial arterioles to acetylcholine was impaired [3]. Motor nerve conduction velocity (MNCV) was decreased at 12–14 weeks of age, endoneurial blood flow (EBF) impaired at 24 weeks of age and relaxation to calcitonin gene-related peptide (CGRP) decreased at 28 weeks of age [3].
We have also performed studies to determine whether microvascular dysfunction and slowing of nerve conduction velocity could be improved by treatment of ZDF rats with optimal doses of enalapril, α-lipoic acid, rosuvastatin or rosiglitazone [4]. Treatment was started after 16 weeks of age. The key findings of this study were that treatment of ZDF rats with enalapril or α-lipoic acid was moderately successful in decreasing nerve dysfunction. These two drugs along with rosiglitazone attenuated the impairment of vascular relaxation to acetylcholine and nerve blood flow. All four treatments improved thermal nociception and reduced superoxide levels. However, the overall success of these treatments was only modest, suggesting that more aggressive combination therapies may be required to successfully treat vascular and neural complications in type 2 diabetes.
Thus, we performed experiments with ZDF rats treated with AVE7688. AVE7688 is a vasopeptidase inhibitor, which is a new class of drug that simultaneously inhibits neutral endopeptidase (NEP) and angiotensin-converting enzyme (ACE) activity [5]. NEP is found in many tissues including vascular tissue, and its activity is increased by fatty acids and glucose in human microvascular cells [6–10]. Interestingly, NEP activity has been shown to be activated by protein kinase C, which is increased in vascular tissues by diabetes [11,12]. Our studies have demonstrated that NEP expression is increased in epineurial arterioles of streptozotocin-treated rats [13]; however, it is unknown what happens to the expression of NEP in type 2 diabetes. NEP degrades natriuretic peptides, CGRP, adrenomedullin, bradykinin and endothelin [14]. Because C-type natriuretic peptide and CGRP are expressed in epineurial arterioles, cause vascular relaxation and activity impaired by diabetes, we propose that vasopeptidase inhibitors may be an effective treatment for vascular and neural dysfunction in diabetes. In this regard, vascular conductance in the femoral artery of streptozotocin-induced diabetic rats was improved by a vasopeptidase inhibitor [15]. In streptozotocin-induced diabetic rats, vasopeptidase inhibitor treatment improved vascular relaxation in epineurial arterioles and nerve activity [13]. Treating ZDF rats with vasopeptidase inhibitors prevents nephropathy and has also been reported to decrease matrix metalloproteinases, advanced glycation endproducts (AGE) accumulation/formation and improve wound healing [16–22]. Therefore, there is great potential for treatment of diabetic vascular and neural disease in type 2 diabetes with vasopeptidase inhibitors; however, no information is available.
Methods
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical (St Louis, MO, USA).
Animal Model
Male ZDF and lean (control) rats 6 weeks of age were obtained from Charles River Laboratories, Wilmington, MA, USA. The animals were housed in a certified animal care facility and food [Harlan Teklad, #7001 (for lean rats) and #7013 (for ZDF rats), Madison, WI, USA], and water were provided ad libitum. ZDF rats when fed a high-fat diet spontaneously become hyperglycaemic at 8–10 weeks of age [1,23]. All institutional (approval animal care and use review forms ACURF #0290608) and National Institutes of Health (NIH) guidelines for the use of animals were followed. At 12 weeks of age, ZDF rats were divided into two groups. One group was fed the unsupplemented #7013 (Harlan Teklad) diet and the second group the #7013 diet containing 500 mg/kg AVE7688 [3,13]. The average amount of chow consumed by ZDF rats was 50 g/day/kg rat. Thus, these rats received approximately 25 mg/kg rat/day of AVE7688. The supplemented diet was prepared from the meal form of the diet. The AVE7688 was thoroughly mixed into the meal by stirring for 1 h. Afterwards, the diet was pelleted and dried in a vacuum oven set at 40 °C overnight. The unsupplemented diets for the lean (#7001) and untreated ZDF rats (#7013) were also prepared from meal. The treatment period lasted for 12 weeks. After 24 weeks of age, rats were used for studies described below.
Thermal Nociceptive Response
The day before the terminal studies thermal nociceptive response in the hindpaw was measured using the Hargreaves method with instrumentation provided by IITC Life Science, Woodland Hills, CA, USA (model 390G). The rat was placed in the observation chamber on top of the thermal testing apparatus and allowed to acclimate to the warmed glass surface (30 °C) and surroundings for a period of 15 min. The mobile heat source was manoeuvred so that it was under the heal of the hindpaw and then activated, a process that activates a timer and locally warms the glass surface, when the rat withdrew its paw, the timer and the heat source were turned off [24]. Following an initial recording, which was discarded, four measurements were made for each hindpaw, with a rest period of 5 min between each set of measurements. The mean of the measurements, reported in seconds, were used as a measure of the thermal nociceptive response.
On the day of the experiment, rats were anaesthetized with Nembutal i.p. (50 mg/kg, i.p.; Abbott Laboratories, North Chicago, IL, USA) and non-fasting blood glucose levels were determined with the use of glucose oxidase reagent strips (Lifescan, Milpitas, CA, USA). Blood samples were collected for determination of serum free fatty acid, triglyceride and free cholesterol using commercial kits from Roche Diagnostics, Mannheim, Germany; Sigma Chemical and Bio Vision, Mountain View, CA, USA, respectively. For these analyses, the manufacturer’s instructions were followed. Afterwards, MNCV and sensory nerve conduction velocity (SNCV) and EBF in the sciatic nerve were determined and tissue containing the epineurial arterioles were collected.
Motor and Sensory Nerve Conduction Velocities
MNCV was determined as previously described using a non-invasive procedure in the sciatic-posterior tibial conducting system [25–28]. SNCV was determined using the digital nerve to the second toe as described by Obrosova et al. [29]. The MNCV and SNCV were reported in metres per second.
Endoneurial Blood Flow
Sciatic nerve EBF was determined as previously described using the hydrogen clearance method [24–27]. The hydrogen clearance data were fitted to a mono-exponential or biexponential curve using commercial software (Prism; GraphPad, San Diego, CA, USA). Nutritive blood flow (ml/min/100 g) was calculated using the equation described by Young [14], and vascular conductance (ml/min/100 g/mmHg) was determined by dividing nutritive blood flow by the average mean arterial blood pressure.
Vascular Reactivity
Videomicroscopy was used to investigate in vitro vaso-dilatory responsiveness of arterioles vascularizing the region of the sciatic nerve (branches of the superior gluteal and internal pudendal arteries) as previously described [25–28]. The vessels used for these studies were generally oriented longitudinally in relation to the sciatic nerve; however, on occasion, radially oriented vessels were also used. The arterioles used in this study should be regarded as epineurial rather than perineurial vessels. To isolate these vessels, the common iliac was exposed and, the branch points of the internal pudendal and superior gluteal arteries were identified. The vessels were then clamped, and tissue containing these vessels and its branches dissected en bloc. The block of tissue was immediately submerged in a cooled (4 °C), oxygenated (20% O2, 5% CO2 and 75% N2) Krebs–Henseleit physiological saline solution (PSS) of the following composition (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 20, Na2EDTA 0.026 and 5.5 glucose. Branches of the superior gluteal and internal pudendal arteries (50–150 μm internal diameter and 2 mm in length) were carefully dissected and trimmed of fat and connective tissue. Both ends of the isolated vessel segment were cannulated with glass micropipettes filled with PSS (4 °C) and secured with 10–0 nylon Ethilon monofilament sutures (Ethicon, Cornelia, GA, USA). The pipettes were attached to a single pressure reservoir (initially set at 0 mmHg) under condition of no flow. The organ chamber containing the cannulated vessels was then transferred to the stage of an inverted microscope (CK2; Olympus, Lake Success, NY, USA). Attached to the microscope were a CCTV camera (WV-BL200; Panasonic, Secaucus, NJ, USA), a video monitor (Panasonic) and a video calliper (VIA-100K; Boeckeler Instruments, Tucson, AZ, USA). The organ chamber was connected to a rotary pump (Masterflex; Cole-Parmer Instrument, Vernon Hills, IL), which continuously circulated 37 °C oxygenated PSS at 30 ml/min. The pressure within the vessel was then slowly increased to 40 mmHg. At this pressure, we found that KCl gave the maximal constrictor response. Therefore, all the studies were conducted at 40 mmHg. Internal vessel diameter (resolution of 2 μm) was measured by manually adjusting the video micrometre. After 30-min equilibration, KCl was added to the bath to test vessel viability. Vessels, which failed to constrict more than 30%, were discarded. After washing with PSS, vessels were incubated for 30 min in PSS and then constricted with U46619 (10−8–10−7 M) (Cayman Chemical, Ann Arbor, MI, USA) to 30–50% of passive diameter. There was no significant difference in the amount of U46619 required to induce constriction in control and diabetic vessels. Afterwards, cumulative concentration-response relationships were evaluated for acetylcholine (10−8–10−4 M) and CGRP (10−11–10−8 M) using vessels from each group of rats. At the end of each dose–response determination, a maximal dose of sodium nitroprusside (10−4 M) was added. Afterwards, papaverine (10−5 M) was added to determine maximal vasodilation, which was consistently the same as the vascular tone of the resting vessel at 40 mmHg.
Detection of Superoxide
Hydroethidine (Molecular Probes, Eugene, OR, USA), an oxidative fluorescent dye, was used to evaluate in situ levels of superoxide (O2−) in epineurial vessels as described previously [25–27]. This method provides sensitive detection of O2−. Vessel segments from 24-week-old lean rats and untreated and treated ZDF rats were processed and imaged in parallel. The labelled vessels derived from these studies were visualized with a Zeiss LSM 510 laser scanning confocal microscope using 40× objectives. Laser settings were identical for acquisition of all images. Superoxide levels using the aorta were also measured by lucigenin-enhanced chemiluminescence as described previously [30]. Relative light units (RLUs) were measured using a Zylux FB12 luminometre. Background activity was determined and subtracted, and RLU was normalized to surface area.
Immunohistochemistry for NEP
We analysed for NEP by immunohistochemical staining 10-μm sections of epineurial arterioles. We generally followed the methods described previously [13]. Epineurial arterioles were collected as described above with minimal preparation, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA) and sectioned. These sections were then incubated with the primary antibody 40 μg/ml [anti-CD-10 rabbit polyclonal immunoglobulin G (IgG) (Santa Cruz Biotechnology, Santa Cruz, CA, USA)] for 16 h in 0.01-M phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% triton-X 100. The sections were washed and then incubated with the secondary antibody Alexa Fluor-546-conjugated IgG in buffer for 2 h (Molecular Probes). Following this incubation, vessels were washed with 0.01 M phosphate-buffered saline, water and mounted with VectorShield. The sections were then visualized using an Olympus IX71 inverted research microscope. For a negative control, the incubation step with the primary antibody was omitted. Optimal settings for the microscope and exposure was determined and left constant for recording of all the samples. Pixel intensity for NEP immunostaining was determined for each vessel segment and averaged for each condition.
Additional Biological Parameters
ACE activity in the serum was quantitated using a colorimetric assay kit from ALPCO diagnostics and the data presented as mU/ml serum [27]. One unit of ACE activity is defined as the amount of enzyme required to release 1 μmol of hippuric acid per minute and per litre of serum at 37 °C (Windham, NH, USA). Serum thiobarbituric acid reactive substance (TBARS) was determined as previously described [25–27]. Conjugated diene levels in liver were determined by measuring the absorbance at 233 nm with extraction blanks used as references [3]. An extinction coefficient of 2.52 × 104 M was used to determine the amount of conjugated diene present. The data was reported as μmol/mg wet weight.
Data Analysis
The results are presented as mean ± s.e.m. Comparisons between the groups for body weight, blood pressure, blood glucose, MNCV, SNCV, EBF, thermal nociception, serum free fatty acid, triglyceride, cholesterol, TBARS, insulin and ACE activity and aorta and epineurial arteriole superoxide levels were conducted using a one-way ANOVA and Newman–Keuls test for multiple comparisons and the Bonferroni–Dunn test (Prism software; GraphPad). Concentration response curves for acetylcholine- and CGRP-induced relaxation were compared using a two-way repeated measures ANOVA with autoregressive covariance structure using proc mixed programme of SAS [25–28]. Whenever significant interactions were noted, specific treatment–dose effects were analysed using a Bonferroni–Dunn test. A p value of <0.05 was considered significant.
Results
Effect of Diabetes and Treatment with AVE7688 on Vital Parameters in ZDF Lean and Diabetic Rats
Data in table 1 presents weight gain, blood glucose, blood pressure, serum insulin, lipid and TBARS levels, liver conjugated diene and serum ACE activity for the rats used in this study. At 6 weeks of age, ZDF rats (202 ± 3 g, p < 0.05) weighed significantly more than lean rats (175 ± 5 g). However, during the following 18 weeks of the study, lean rats gained significantly more weight than the untreated or treated ZDF rats. At the end of the study, there was no significant difference in weight between lean rats (418 ± 6 g) and treated (383 ± 11 g) and untreated (396 ± 8 g) ZDF rats. This was not surprising because we previously reported that at 16 weeks of age, lean and ZDF rats weigh about the same and that as insulin levels begin to decline at 20–24 weeks of age, ZDF rats stop gaining weight and may even decline [3]. At 24 weeks of age, blood glucose level was significantly increased in untreated and treated ZDF rats compared with that in lean rats. Serum insulin levels were significantly decreased in untreated ZDF rats compared with those in lean rats and moderately improved with AVE7688 treatment. There was no significant difference in insulin levels between treated ZDF rats and lean rats. Treatment with AVE7688 significantly lowered mean arterial blood pressure, serum cholesterol and ACE activity compared with untreated ZDF rats. In contrast, treating ZDF rats with AVE7688 did not lower serum triglyceride or free fatty acid levels and these remained significantly elevated in treated and untreated ZDF rats compared with lean rats. Results from treating ZDF rats with AVE7688 on markers of oxidative stress were mixed. Serum TBARS and liver conjugated diene levels were significantly increased in ZDF rats and not improved with treatment. However, treating ZDF rats with AVE7688 significantly reduced superoxide levels in the aorta (table 1) and epineurial arterioles (figure 1). Superoxide levels in the aorta and epineurial arterioles were not significantly different in treated ZDF rats compared with lean rats.
Table 1.
Effect of treatment of ZDF rats with AVE7688 on body weight, blood pressure, blood glucose, serum insulin, cholesterol, triglycerides, free fatty acids, TBARS and ACE, liver conjugated diene, superoxide levels in the aorta and thermal nociception
| Determination | Lean (14) | ZDF untreated (15) | ZDF AVE7688 (15) |
|---|---|---|---|
| Weight gain (g) | 237 ± 8 | 192 ± 9* | 189 ± 10* |
| Mean arterial blood pressure (mmHg) | 125 ± 7 | 130 ± 6 | 103 ± 5*+ |
| Blood glucose (mg/dl) | 103 ± 9 | 289 ± 21* | 354 ± 34* |
| Insulin (ng/ml) | 158 ± 34 | 56 ± 7* | 109 ± 42 |
| Cholesterol (mg/ml) | 3.1 ± 0.4 | 6.2 ± 0.4* | 4.7 ± 0.4*+ |
| Triglycerides (mg/dl) | 77.9 ± 8.2 | 235.9 ± 26.2* | 215.4 ± 17.9* |
| Free fatty acids (mmol/l) | 0.053 ± 0.014 | 0.389 ± 0.089* | 0.343 ± 0.062* |
| TBARS (μg/ml) | 0.72 ± 0.07 | 1.13 ± 0.12* | 1.07 ± 0.06* |
| Conjugated diene (μmol/mg) | 0.45 ± 0.02 | 0.66 ± 0.04* | 0.70 ± 0.04* |
| ACE activity (mU/ml) | 24.4 ± 4.2 | 32.0 ± 2.9 | 5.3 ± 2.0*+ |
| Aorta superoxide (RLU) | 2.19 ± 0.15 | 2.90 ± 0.18* | 1.75 ± 0.20+ |
| Thermal nociception (s) | 10.5 ± 0.4 | 17.5 ± 0.6* | 10.3 ± 0.5+ |
ACE, angiotensin-converting enzyme; RLU, relative light unit; TBARS, thiobarbituric acid reactive substances; ZDF, Zucker diabetic fatty. Data are presented as the mean ± s.e.m.
p < 0.05 compared with lean,
p < 0.05 compared with ZDF untreated. The number of experimental animals is presented in parentheses.
Fig. 1.
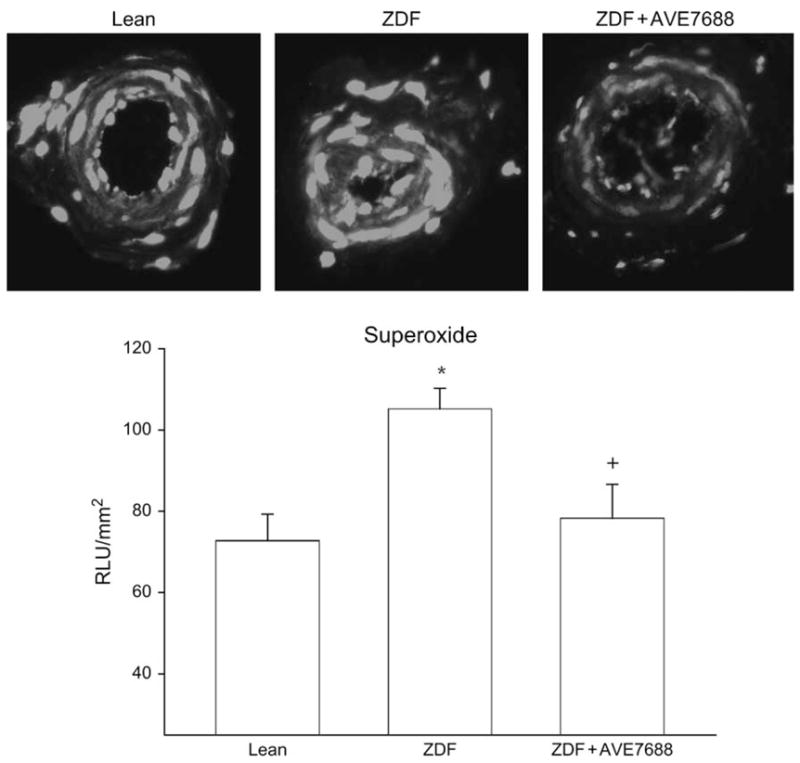
Detection of superoxide in epineurial arterioles of the sciatic nerve from lean (control), untreated Zucker diabetic fatty (ZDF) and ZDF rats treated with AVE7688. Presented are representative fluorescent photomicrographs of confocal microscopic sections of epineurial arterioles of the sciatic nerve for superoxide immunostaining (top). Analysis of these images using SimplePCI imaging software is presented below the images. The data are presented as relative light units (RLUs) and normalized to surface area. *p < 0.05 compared with lean rats. +p < 0.05 compared with untreated ZDF rats. These values were obtained from three different rats, and five vessel segments were analysed for each individual rat.
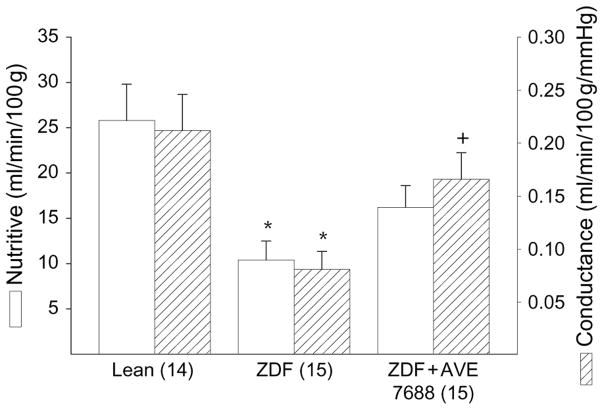
Effect of AVE7688 Treatment on EBF in the Sciatic Nerve
Data in figure 2 demonstrate that treating ZDF rats with AVE7688 significantly improved EBF. EBF was not significantly different in untreated ZDF rats compared with lean rats.
Fig. 2.
Determination of the effect of treatment of Zucker diabetic fatty (ZDF) rats with AVE7688 for 12 weeks starting at 12 weeks of age on endoneurial blood flow. Data are presented as the mean ± s.e.m. for nutritive flow (ml/min/100 g) and conductance (ml/min/100 g/mmHg). The number of experimental determinations is presented in parentheses. *p < 0.05 compared with lean rats, +p < 0.05 compared with untreated ZDF rats.
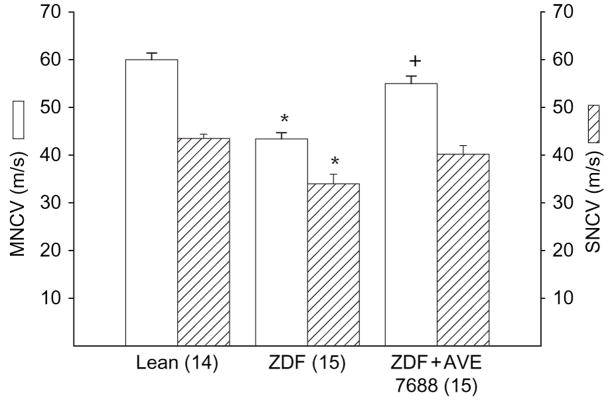
Effect of AVE7688 Treatment of ZDF Rats on MNCV and SNCV and Thermal Nociception
Treating ZDF rats with AVE7688 improved MNCV and SNCV (figure 3). MNCV and SNCV were not significantly different between treated ZDF rats and lean rats. AVE7688 treatment also prevented the development of thermal hypoalgesia, as determined by measuring thermal nociception in the hindpaw (table 1). Responsiveness to thermal stimulation was similar for treated ZDF rats and lean rats.
Fig. 3.
Determination of the effect of treatment of Zucker diabetic fatty (ZDF) rats with AVE7688 for 12 weeks starting at 12 weeks of age on motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV). Data are presented as the mean ± s.e.m. in metres/second. The number of experimental determinations is presented in parentheses. *p < 0.05 compared with lean rats, +p < 0.05 compared with untreated ZDF rats.
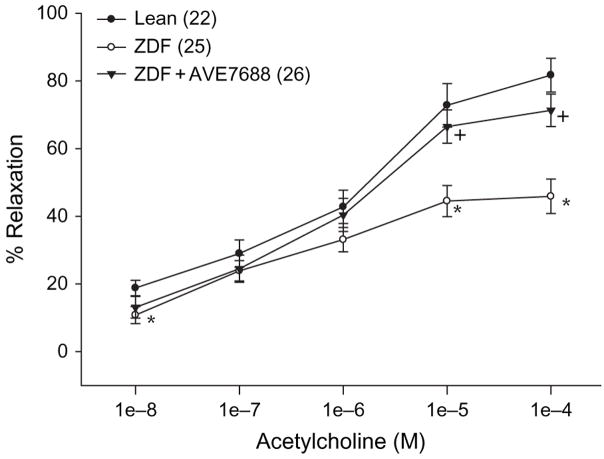
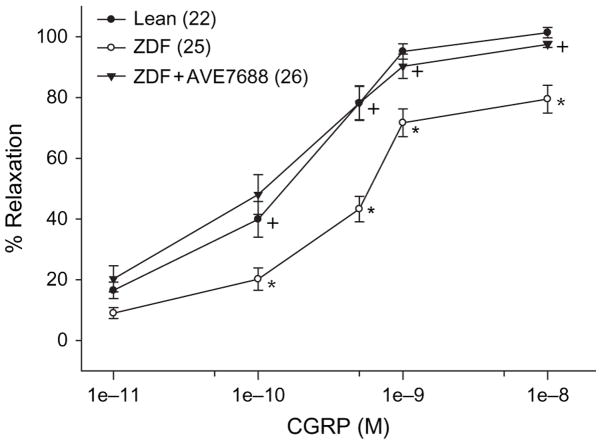
Effect of AVE7688 Treatment of ZDF Rats on Vascular Relaxation in Response to Acetylcholine Or CGRP
Epineurial arterioles of the sciatic nerve are innervated by sensory nerves that contain CGRP [31]. We have previously reported that vascular relaxation in response to acetylcholine or CGRP was impaired in ZDF rats [2,3]. Data in figures 4 and 5 demonstrate that treating ZDF rats with AVE7688 significantly improved vascular relaxation to acetylcholine and CGRP in epineurial arterioles compared with untreated ZDF rats. There was no significant difference in acetylcholine- and CGRP-mediated vascular relaxation between treated ZDF rats and lean rats.
Fig. 4.
Determination of the effect of treatment of Zucker diabetic fatty (ZDF) rats with AVE7688 for 12 weeks starting at 12 weeks of age on acetylcholine-mediated vascular relaxation of epineurial arterioles of the sciatic nerve. Pressurized arterioles (40 mmHg) were constricted with U46619 (30–50%), and incremental doses of acetylcholine were added to the bathing solution while recording steady-state vessel diameter. Data are presented as the mean of per cent relaxation ± s.e.m. The number of experimental determinations is presented in parentheses. *p < 0.05 compared with lean rats, +p < 0.05 compared with untreated ZDF rats.
Fig. 5.
Determination of the effect of treatment of Zucker diabetic fatty (ZDF) rats with AVE7688 for 12 weeks starting at 12 weeks of age on calcitonin gene-related peptide (CGRP)-mediated vascular relaxation of epineurial arterioles of the sciatic nerve. Pressurized arterioles (40 mmHg) were constricted with U46619 (30–50%), and incremental doses of CGRP were added to the bathing solution while recording steady-state vessel diameter. Data are presented as the mean of % relaxation ± s.e.m. The number of experimental determinations is presented in parentheses. *p < 0.05 compared with lean rats, +p < 0.05 compared with untreated ZDF rats.
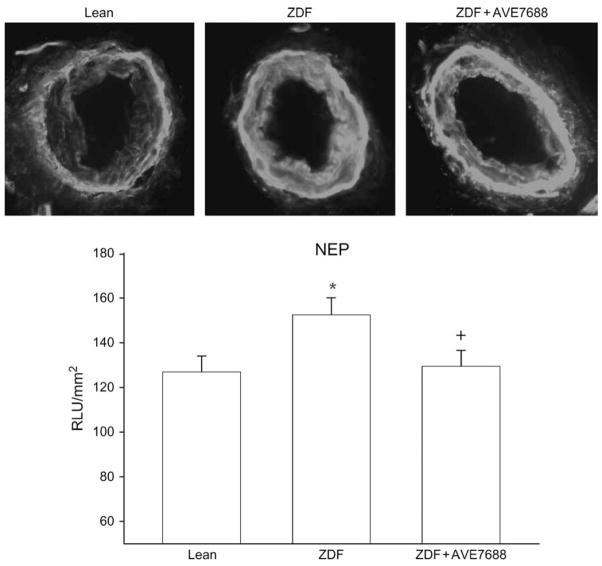
Changes of Expression of NEP in Epineurial Arterioles from ZDF Rats Treated with Or without AVE7688
Previously we demonstrated that streptozotocin-induced diabetes caused a significant increase in NEP expression in epineurial arterioles [13]. In untreated ZDF rats, expression of NEP in epineurial arterioles is modestly increased (25%) compared with lean rats (figure 6). Treating ZDF rats with AVE7688 significantly decreased NEP expression in epineurial arterioles. NEP expression in epineurial arterioles in treated ZDF rats was similar to NEP expression lean rats.
Fig. 6.
Detection of neutral endopeptidase (NEP) in epineurial arterioles of the sciatic nerve from lean (control), untreated Zucker diabetic fatty (ZDF) and ZDF rats treated with AVE7688. Presented are representative fluorescent photomicrographs of confocal microscopic sections of epineurial arterioles of the sciatic nerve for NEP immunostaining (top). Analysis of these images using SimplePCI imaging software is presented below the images. The data are presented as relative light units (RLUs) and normalized to surface area. *p < 0.05 compared with lean rats. +p < 0.05 compared with untreated ZDF rats. These values were obtained from three different rats and five vessel segments were analysed for each individual rat.
Discussion
Our previous studies with ZDF rats demonstrated that vascular and neural complications are progressive [3]. Vascular dysfunction appeared within 8 weeks of age followed by decreased MNCV (12–14 weeks of age) and EBF (24 weeks of age) [3]. Because many patients are not diagnosed with type 2 diabetes until they develop symptoms of complications, we decided to initiate 12 weeks of treatment in ZDF rats at 12 weeks of age. At 12 weeks of age, ZDF rats have been hyperglycaemic for 4 weeks and vascular and neural complications have or are developing [3].
The key finding of this study was that treatment of ZDF rats with AVE7688 was successful in preventing/reversing nerve and vascular dysfunction in ZDF rats. In a previous study, we found treatment with enalapril, α-lipoic acid, rosuvastatin or rosiglitazone to be modestly effective in improving vascular and neural dysfunction in ZDF rats using an intervention protocol [4]. Based on that study, we concluded that a more aggressive combination therapy would be required to successfully treat vascular and neural complications in type 2 diabetes. Hence, we examined the effect of AVE7688 treatment of ZDF rats. As discussed above, AVE7688 is a vasopeptidase inhibitor, which is a drug that has both ACE and NEP inhibitor properties [5].
In several different rat models of hypertension, treatment with a vasopeptidase inhibitor was found to be an effective antihypertensive agent and provide renal protection [14,32,33]. Moreover, in Munich-Wistar rats subjected to 5/6 nephrectomy, treatment with omapatrilat, a vasopeptidase inhibitor, offered greater renal protection than enalapril [33]. Although we examined different end-points in a rat model of type 2 diabetes, we obtained similar results. We found that treating ZDF rats with AVE7688 was more efficacious in preventing/reversing vascular defects than enalapril although both drugs provided about the same amount of inhibition of serum ACE activity and improvement in nerve conduction velocity and blood flow [4]. This was also true in studies using streptozotocin-induced diabetic rats [13,28]. In streptozotocin-induced diabetic rats, treatment with enalapril for 12 weeks after 4 weeks of untreated diabetes partially improved MNCV, EBF and acetylcholine-mediated vascular relaxation in epineurial arterioles [28]. Using the same experimental protocol treatment of streptozotocin-induced diabetic rats with AVE7688 completely restored MNCV, EBF and acetylcholine-mediated vascular relaxation in epineurial arterioles [13]. In comparing the efficacy of AVE7688 treatment of streptozotocin-induced diabetic rats and ZDF rats, we found that AVE7688 was generally more efficacious in the type 1 streptozotocin diabetic rat model than in the type 2 (ZDF) diabetic rats [13]. In both studies, AVE7688 was provided through the diet at the same dose and the 12-week treatment was started after 4 weeks of untreated hyperglycaemia [13]. This suggests that improving vascular and neural dysfunction in type 2 diabetes may be more difficult and require earlier treatment and perhaps additional therapeutic drugs. It should be noted that treatment of ZDF rats with AVE7688 did not reduce the elevated serum triglyceride or free fatty acid levels, and serum markers of oxidative stress were not improved. Thus, the improvement that occurred by treating ZDF rats with AVE7688 did not require correction of all the lipid abnormalities and may not be necessary for improving vascular and neural function.
In our studies, treatment of ZDF rats with AVE7688 was found to reverse vascular dysfunction as determined by examining vascular responsiveness to acetylcholine and CGRP. In ZDF rats, we previously reported that acetylcholine-mediated vascular relaxation in epineurial arterioles of the sciatic nerve is decreased by 8 weeks of age. In streptozotocin-induced diabetic rats, acetylcholine-mediated vascular relaxation is impaired after 2 weeks of hyperglycaemia [34]. We found that treatment with AVE7688 of ZDF rats after 12 weeks of age or streptozotocin-induced diabetic rats after 4 weeks of hyperglycaemia completely corrected acetylcholine-mediated vascular relaxation [13].
We attribute the improvement of vascular responsiveness to acetylcholine to two mechanisms. First, in epineurial arterioles, vascular relaxation to acetylcholine is mediated by two mechanisms, the generation of nitric oxide by endothelial nitric oxide synthase and the formation of endothelium-derived hyperpolarizing factor (EDHF) [34,35]. The biological activity of nitric oxide is inhibited by the generation of superoxide. Superoxide can rapidly bind with nitric oxide and form peroxynitrite. We have evidence that this occurs in epineurial arterioles. Nitrotyrosine staining, a biological marker for peroxynitrite formation, is increased in epineurial arterioles from streptozotocin-induced diabetic rats and ZDF rats [4,13]. Furthermore, we have shown that formation of superoxide and increased nitrotyrosine staining can be decreased by treating diabetic rats with enalapril, AVE7688 or α-lipoic acid. Each of these treatments also improved acetylcholine-mediated vascular relaxation in epineurial arterioles [4,13,27]. Thus, it seems that preventing superoxide formation and/or quenching superoxide protects the biological activity of nitric oxide.
Decreasing oxidative stress may have a role in reducing the expression of NEP in diabetes. Muangman et al. [10] demonstrated in vitro that antioxidant treatment of microvascular endothelial cells reduced the increase in NEP expression caused by high glucose or fatty acid levels. In our studies, treatment of ZDF rats with AVE7688 reduced expression of NEP as well as superoxide levels in epineurial arterioles.
The biological compound(s) responsible for EDHF activity is unknown. However, we have demonstrated that C-type natriuretic peptide has biological properties consistent with EDHF; epineurial arterioles express C-type natriuretic peptide, and in epineurial arterioles from streptozotocin-induced diabetic rats, relaxation mediated by exogenous C-type natriuretic peptide is decreased [13]. Studies conducted with human pineal and rat mesenteric arteries also suggest that the biological properties of C-type natriuretic peptide are consistent with EDHF [36–38]. In epineurial arterioles from streptozotocin-induced diabetic rats and ZDF rats, the expression of NEP is increased [13]. NEP regulates the biological activity of natriuretic peptides through degradation prior to binding at active sites [14,39]. Therefore, we propose that the increased expression of NEP reduces acetylcholine-mediated vascular relaxation in part by reducing the biological activity of C-type natriuretic peptide [13]. This is supported by our studies with ZDF rats demonstrating that vascular relaxation mediated by exogenous CGRP is decreased compared with lean rats [3]. NEP degrades and mediates the biological activity of CGRP [39,40]. Therefore, we believe that reduced vascular relaxation in response to CGRP in epineurial arterioles in ZDF rats is because of degradation of CGRP and that treatment of ZDF rats with AVE7688 protects CGRP bioactivity by inhibiting and reducing the expression of NEP.
We have demonstrated in these studies with ZDF rats and in a previous study using streptozotocin-induced diabetic rats that treatment with AVE7688 improves or prevents diabetes-induced nerve dysfunction [13]. In both streptozotocin-induced diabetic rats and ZDF rats, we have demonstrated that vascular impairment precedes the appearance of nerve dysfunction as measured by slowing of nerve conduction velocities [3,25]. This has led us to propose that a contributing factor to diabetic neuropathy is microvascular dysfunction. These studies with ZDF rats treated with AVE7688 provide additional support for this hypothesis. Treatment with AVE7688 protects vascular tissue from the pathogenesis associated with increased superoxide production and expression of NEP. Therefore, we propose that maintaining vascular function in the presence of the metabolic derangements caused by diabetes provides protection to nervous tissue.
Treatment of ZDF rats and streptozotocin-induced diabetic rats with AVE7688 was found to prevent the development of thermal hypoalgesia. NEP expression and activity is increased in skin from patients with diabetes, and this has been linked to slow wound healing because of increased degradation of neuroactive and vasoactive peptides [16,41]. CGRP is found in sensory nerves innervating the skin and the dermis of the hindpaw of rats [42]. CGRP has multiple physiological functions and along with substance P is involved in pain modulation [43,44]. At this time, it is not certain how inhibition of NEP improves pain perception. However, if CGRP is associated with pain perception as reported then increased degradation by NEP of CGRP along with degeneration of nerve fibres could cause increased latency of paw withdrawal in tests of thermal nociception. Additional studies will be required to confirm this hypothesis.
In summary, AVE7688 was found to be an effective treatment for vascular and neural dysfunction in diabetes in ZDF rats, a model for type 2 diabetes. The beneficial effects of AVE7688 are likely because of several factors including protection of vasoactive peptides such as CGRP and reduction of oxidative stress. Overall, these studies suggest that the use of vasopeptidase inhibitors may be a valuable approach for the treatment of diabetic vascular and neural dysfunction in type 2 diabetes.
Acknowledgments
This work was supported by a Merit Review Grant from the Veterans Affairs Administration and by a National Institute of Diabetes and Digestive and Kidney Diseases Grant DK073990 from National Institutes of Health (NIH). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would also like to extend our appreciation to sanofi-aventis for supplying AVE7688 for these studies.
References
- 1.Peterson RG, Shaw WN, Neel MA, et al. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR News. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppey LJ, Gellett JS, Davidson EP, et al. Changes in epineurial blood flow motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev. 2002;18:1–9. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- 3.Oltman CL, Coppey LJ, Gellett JS, et al. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty (ZDF) and Zucker rats. Am J Physiol. 2005;289:E113–E122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- 4.Oltman CL, Davidson EP, Coppey LJ, et al. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes Metab. 2008;10:67–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 5.Weber M. Emerging treatments for hypertension: potential role for vasopeptidase inhibition. Am J Hypertens. 1999;12:139S–147S. doi: 10.1016/s0895-7061(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez W, Soleilhac JM, Fournie-Zaluski MC, et al. Characterization of neutral endopeptidase in vascular cells, modulation of vasoactive peptide levels. Eur J Pharmacol. 1998;345:323–331. doi: 10.1016/s0014-2999(98)00038-7. [DOI] [PubMed] [Google Scholar]
- 7.Vatter H, Schilling L, Schmiedek P, et al. Evidence for functional endothelin-converting enzyme activity in isolated rat basilar artery: effect of inhibitors. J Cardiovasc Pharmacol. 1998;31:S64–S67. doi: 10.1097/00005344-199800001-00021. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RM, Pullen M, Nambi P. Distribution of neutral endopeptidase activity along the rat and rabbit nephron. Pharmacology. 1999;59:45–50. doi: 10.1159/000028304. [DOI] [PubMed] [Google Scholar]
- 9.Ebihara F, Di Marco GS, Juliano MA, et al. Neutral endopeptidase expression in mesangial cells. J Renin Angiotensin Aldosterone Syst. 2003;4:228–233. doi: 10.3317/jraas.2003.037. [DOI] [PubMed] [Google Scholar]
- 10.Muangman P, Spenny ML, Tamura RN, et al. Fatty acids and glucose increase neutral endopeptidase activity in human microvascular endothelial cells. Shock. 2003;19:508–512. doi: 10.1097/01.shk.0000055815.40894.16. [DOI] [PubMed] [Google Scholar]
- 11.Suzki T, Ino K, Kikkawa F, et al. Neutral endopeptidase/CD10 expression during phorbol ester-induced differentiation of choriocarcinoma cells through the protein kinase C- and extracellular signal-regulated kinase-dependent signaling pathway. Placenta. 2002;23:475–482. doi: 10.1053/plac.2002.0820. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa F, Shibata K, Suzuki T, et al. Signal pathway involved in increased expression of neutral endopeptidase by gonadotropin releasing hormone in choriocarcinoma cells. Placenta. 2004;25:176–183. doi: 10.1016/j.placenta.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Davidson EP, Kleinschmidt TL, Oltman CL, et al. Treatment of streptozotocin-induced diabetic rats with AVE7688, a vasopeptidase inhibitor, on vascular and neural disease. Diabetes. 2007;56:355–362. doi: 10.2337/db06-1180. [DOI] [PubMed] [Google Scholar]
- 14.Pu Q, Schiffrin EL. Effect of ACE/NEP inhibition on cardiac and vascular collagen in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2001;14:1067–1072. doi: 10.1016/s0895-7061(01)02157-4. [DOI] [PubMed] [Google Scholar]
- 15.Arbin V, Claperon N, Fournie-Zaluski MC, et al. Effects of combined neutral endopeptidase 24–11 and angiotensin-converting enzyme inhibition on femoral vascular conductance in streptozotocin-induced diabetic rats. Br J Pharmacol. 2001;130:1297–1304. doi: 10.1038/sj.bjp.0703442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spenny ML, Muangman P, Sullivan SR, et al. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10:295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- 17.Schafer S, Linz W, Bube A, et al. Vasopeptidase inhibition prevents nephropathy in Zucker diabetic fatty rats. Cardiovasc Res. 2003;60:447–454. doi: 10.1016/s0008-6363(03)00544-3. [DOI] [PubMed] [Google Scholar]
- 18.Schafer S, Linz W, Vollert H, et al. The vasopeptidase inhibitor AVE7688 ameliorates Type 2 diabetic nephropathy. Diabetologia. 2004;47:98–103. doi: 10.1007/s00125-003-1264-8. [DOI] [PubMed] [Google Scholar]
- 19.Schafer S, Schmidts H-L, Bleich M, et al. Nephroprotection in Zucker diabetic fatty rats by vaso-peptidase inhibition is partly bradykinin B2 receptor dependent. Br J Pharmacol. 2004;143:27–32. doi: 10.1038/sj.bjp.0705884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzone D, Rossi GP, Porteri E, et al. Bradykinin and matrix metalloproteinases are involved the structural alterations of rat small resistance arteries with inhibition of ACE and NEP. J Hypertens. 2004;22:759–766. doi: 10.1097/00004872-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Cha YM, Redfield MM, Shah S, et al. Effects of omapatrilat on cardiac nerve sprouting and structural remodeling in experimental congestive heart failure. Heart Rhythm. 2005;2:984–990. doi: 10.1016/j.hrthm.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Wihler C, Schafer S, Schmid K, et al. Renal accumulation and clearance of advanced glycation end-products in type 2 diabetic nephropathy: effect of angiotensin-converting enzyme and vasopeptidase inhibition. Diabetologia. 2005;48:1645–1653. doi: 10.1007/s00125-005-1837-9. [DOI] [PubMed] [Google Scholar]
- 23.Unger RH. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. 1997;7:276–282. doi: 10.1016/s1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 24.Calcutt NA, Jorge MC, Yaksh TL, et al. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–299. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- 25.Coppey LJ, Davidson EP, Dunlap JA, et al. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that provide circulation to the sciatic nerve. Int J Exp Diabetes Res. 2000;1:131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppey LJ, Gellett JS, Davidson EP, et al. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular function of epineurial arterioles of the sciatic nerve. Br J Pharmacol. 2001;134:121–129. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppey LJ, Gellett JS, Davidson EP, et al. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 28.Coppey LJ, Davidson EP, Rinehart TW, et al. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55:341–348. doi: 10.2337/diabetes.55.02.06.db05-0885. [DOI] [PubMed] [Google Scholar]
- 29.Obrosova IG, Li F, Abatan OI, et al. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 30.Miller FJ, Gutterman DD, Rios CD, et al. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 31.Yorek MA, Coppey LJ, Gellett JS, et al. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: effect of streptozotocin-induced diabetes. Exp Diabetes Res. 2004;5:187–193. doi: 10.1080/15438600490486732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quaschning T, D’Uscio LV, Shaw S, et al. Vasopeptidase inhibition restores renovascular endothelial dysfunction in salt-induced hypertension. J Am Soc Nephrol. 2001;12:2280–2287. doi: 10.1681/ASN.V12112280. [DOI] [PubMed] [Google Scholar]
- 33.Taal MW, Nenov VD, Wong W, et al. Vasopeptidase inhibition affords greater renoprotection than angiotensin-converting enzyme inhibition alone. J Am Soc Nephrol. 2001;12:2051–2059. doi: 10.1681/ASN.V12102051. [DOI] [PubMed] [Google Scholar]
- 34.Terata K, Coppey LJ, Davidson EP, et al. Acetylcholine-induced arteriolar dilation is reduced in streptozotocin-induced diabetic rats with motor nerve dysfunction. Br J Pharmacol. 1999;128:837–843. doi: 10.1038/sj.bjp.0702856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppey LJ, Gellett JS, Yorek MA. Mediation of vascular relaxation in epineurial arterioles of the sciatic nerve: effect of diabetes in type 1 and type 2 diabetic rat models. Endothelium. 2003;10:1–6. doi: 10.1080/10623320303366. [DOI] [PubMed] [Google Scholar]
- 36.Kun A, Kiraly I, Pataricza J. C-type natriuretic peptide hyperpolarizes and relaxes human penile resistance arteries. J Sex Med. 2008;5:1114–1125. doi: 10.1111/j.1743-6109.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan SD, Hobbs AJ, Ahluwalia A. C-type natriuretic peptide: new candidate for endothelium-derived hyperpolarizing factor. Int J Biochem Cell Biol. 2004;36:1878–1881. doi: 10.1016/j.biocel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Chauhan SD, Nilsson H, Ahluwalia A, et al. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer HH, Schmidt K, Leis S, et al. Angiotensin converting enzyme has an inhibitory role in CGRP metabolism in human skin. Peptide. 2006;27:917–920. doi: 10.1016/j.peptides.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Kramer HH, Schmidt K, Leis S, et al. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp Neurol. 2005;195:179–184. doi: 10.1016/j.expneurol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Antezana MA, Sulllivan SR, Usui ML, et al. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol. 2002;119:1400–1404. doi: 10.1046/j.1523-1747.2002.19618.x. [DOI] [PubMed] [Google Scholar]
- 42.Ellington HC, Cotter MA, Cameron NE, et al. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Lundeberg T, Yu L-C. Involvement of CGRP and CGRP1 receptor in nociception in the nucleus accumbens of rats. Brain Res. 2001;901:161–166. doi: 10.1016/s0006-8993(01)02341-1. [DOI] [PubMed] [Google Scholar]
- 44.Yu L-C, Weng X-H, Wang J-W, et al. Involvement of calcitonin gene-related peptide and its receptor in antinociception in the periaqueductal grey of rats. Neurosci Lett. 2003;349:1–4. doi: 10.1016/s0304-3940(03)00273-8. [DOI] [PubMed] [Google Scholar]