Abstract
Syntrophins are scaffolding proteins that link signaling molecules to dystrophin and the cytoskeleton. We previously reported that syntrophins interact with diacylglycerol kinase-ζ (DGK-ζ), which phosphorylates diacylglycerol to yield phosphatidic acid. Here, we show syntrophins and DGK-ζ form a complex in skeletal muscle whose translocation from the cytosol to the plasma membrane is regulated by protein kinase C-dependent phosphorylation of the DGK-ζ MARCKS domain. DGK-ζ mutants that do not bind syntrophins were mislocalized, and an activated mutant of this sort induced atypical changes in the actin cytoskeleton, indicating syntrophins are important for localizing DGK-ζ and regulating its activity. Consistent with a role in actin organization, DGK-ζ and syntrophins were colocalized with filamentous (F)-actin and Rac in lamellipodia and ruffles. Moreover, extracellular signal-related kinase-dependent phosphorylation of DGK-ζ regulated its association with the cytoskeleton. In adult muscle, DGK-ζ was colocalized with syntrophins on the sarcolemma and was concentrated at neuromuscular junctions (NMJs), whereas in type IIB fibers it was found exclusively at NMJs. DGK-ζ was reduced at the sarcolemma of dystrophin-deficient mdx mouse myofibers but was specifically retained at NMJs, indicating that dystrophin is important for the sarcolemmal but not synaptic localization of DGK-ζ. Together, our findings suggest syntrophins localize DGK-ζ signaling complexes at specialized domains of muscle cells, which may be critical for the proper control of lipid-signaling pathways regulating actin organization. In dystrophic muscle, mislocalized DGK-ζ may cause abnormal cytoskeletal changes that contribute to disease pathogenesis.
INTRODUCTION
Scaffold proteins are thought to enhance the efficiency and specificity of signal transduction by organizing proteins involved in the same signaling pathway into macromolecular assemblies and localizing them to specific intracellular domains (Burack and Shaw, 2000). Syntrophins are scaffold proteins that link signaling molecules to the dystrophin family of cytoskeletal proteins (Albrecht and Froehner, 2002). Dystrophin is the product of the gene mutated or missing in patients with Duchenne muscular dystrophy, a fatal, inherited disorder causing progressive weakness and wasting of skeletal muscles (Rando, 2001). Dystrophin lies on the inner face of the plasma membrane of skeletal muscle fibers, where it links cortical actin with a multisubunit complex (the dystrophin-glycoprotein complex) that spans the plasma membrane and connects to the surrounding extracellular matrix (Durbeej and Campbell, 2002). Mutations in dystrophin are thought to disrupt this linkage, causing sarcolemmal instability and contraction-induced lesions in the membrane (Petrof et al., 1993). In the absence of dystrophin, syntrophins fail to localize at the sarcolemma (Adams et al., 2001).
The syntrophin family comprises five isoforms (α1, β1, β2, γ1, and γ2) encoded by separate genes, but with the same modular domain organization. Each isoform has two tandem pleckstrin homology (PH) domains, a PSD-95, discslarge, ZO-1 (PDZ) domain, and a C-terminal syntrophin-unique region (SU) (Adams et al., 1995; Ahn et al., 1996; Piluso et al., 2000). The latter half of syntrophin, which includes the PH2 and SU domains, mediates the interaction with members of the dystrophin family of proteins, including dystrophin, utrophin, and dystrobrevin (Albrecht and Froehner, 2002). This leaves the N-terminal half, which includes the PDZ domain, available to interact with other proteins. Thus, syntrophins provide a link between signaling proteins and the actin cytoskeleton via dystrophin.
We previously identified an interaction between the PDZ domain of γ1-syntrophin and the C terminus of diacylglycerol kinase-ζ (DGK-ζ) (Hogan et al., 2001), an enzyme that phosphorylates diacylglycerol (DAG) to yield phosphatidic acid (PA). DAG is a lipid second messenger that transiently accumulates in cells stimulated by growth factors and other agonists (Hodgkin et al., 1998). It exerts its effects primarily by activating proteins that contain DAG-responsive C1 domains such as protein kinase C (PKC) isoforms, Ras-guanyl nucleotide-releasing protein, Unc-13, and chimaerins (Topham and Prescott, 1999). These proteins are key regulators of a variety of cellular functions; therefore, DAG signaling must be strictly controlled for an appropriate response. By metabolizing DAG, DGKs attenuate the activity of DAG-activated proteins. Moreover, because PA is mitogenic and modulates the activity of enzymes such as phosphatidylinositol-4-phosphate 5-kinase, Raf-1 kinase, atypical PKCs and others (Topham and Prescott, 1999; van Blitterswijk and Houssa, 2000), DGKs also have positive regulatory roles in signal transduction.
DGK-ζ is one of nine mammalian isozymes that differ remarkably in their structure, modes of tissue expression, and enzymatic properties (Topham and Prescott, 1999; van Blitterswijk and Houssa, 2000). Their structural diversity and different cellular localizations suggest different isoforms modify distinct DAG signaling events and are regulated by separate molecular mechanisms. However, translocation from the cytosol to various membrane compartments seems to be a general mechanism for activation of DGK isoforms.
Recently, Santos et al. (2002) showed that DGK-ζ translocates to the plasma membrane of T cells in response to activation of an exogenously expressed muscarinic type I receptor. DGK-ζ also translocates to the nucleus, where it regulates the amount of nuclear DAG (Topham et al., 1998). Both events seem to be dynamically regulated by PKC-mediated phosphorylation of the MARCKS domain, a cluster of basic and serine (Ser) residues homologous to the phosphorylation site domain of the MARCKS protein (McLaughlin and Aderem, 1995). Additionally, we have shown that the nuclear accumulation of DGK-ζ is regulated by its interaction with γ1-syntrophin (Hogan et al., 2001). DGK-ζ mutants with a nonfunctional C-terminal PDZ-binding motif accumulated in the nucleus, suggesting syntrophins are important for the stable retention of DGK-ζ in the cytoplasm. Because both syntrophins and DGK-ζ are expressed in multiple tissues and cell types, we hypothesized that their interaction may be a common mechanism for targeting the enzyme to specialized subcellular domains.
Here, we investigated the molecular mechanisms regulating the localization of DGK-ζ in skeletal muscle. We show that syntrophins are important for the stable association of DGK-ζ with the sarcolemma, that the two proteins form a complex whose subcellular localization is dynamically regulated by phosphorylation, and that they are involved in the regulation of the actin cytoskeleton.
MATERIALS AND METHODS
Reagents
To make polyclonal antisera to human DGK-ζ,, a peptide (CSERDAGPEPDKAPRRLNK) was synthesized, conjugated to KLH, and injected into rabbits. Antibodies were affinity purified from serum on immobilized peptide. The specificity of this antibody was verified as in Bunting et al. (1996) and by preabsorption of antibodies with the immunizing peptide. The BF-F3 monoclonal antibody (mAb) against type IIB myosin heavy chain isoform was from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). A mAb to Rac1 was from BD Biosciences (San Jose, CA), and rabbit polyclonal anti-hemagglutinin (HA) was from Zymed Laboratories (South San Francisco, CA). Texas Red-conjugated α-bungarotoxin (α-BgTx) was from Molecular Probes (Eugene, OR), fluorescein isothiocyanate (FITC)-conjugated phalloidin was from Sigma-Aldrich (St. Louis, MO), and Texas Red-, FITC-, and peroxidase-conjugated secondary antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA).
Plasmids
To visualize DGK-ζ in transfected cells, we used a construct with three tandem N-terminal HA epitope tags as described in Topham et al. (1998). A DGK-ζ construct with a C-terminal FLAG epitope tag and a mutant with all four Ser residues in the MARCKS domain changed to aspartate (Asp) (DGK-ζM1) have been described previously (Topham et al., 1998; Hogan et al., 2001). To construct DGK-ζM1-FLAG, the mutant MARCKS domain was cut from DGK-ζM1 with SmaI and HindIII enzymes and shuttled into DGK-ζFLAG in pcDNA3.1 digested with the same enzymes.
To visualize α1-syn in transfected cells, we made a construct encoding the full-length protein with two tandem N-terminal myc epitope tags. This was done by isolating a 1.3-kb restriction fragment by digestion of the α1-syn clone BC1012 [in pBluescript SK ([minus)] with EcoR I and BamH I restriction enzymes. A fragment encoding the N terminus of α1-syn was amplified by the polymerase chain reaction with specific primers then digested with EcoR I and ApaI. Complementary oligonucleotides containing 5′ NheI and 3′ ApaI sites and encoding two myc epitope tags were synthesized, annealed, and ligated along with the other fragments into pQBI25-fc1 (Quantum Biotechnologies, Montreal, PQ) cut with NheI and BamH I.
Mutations near the ankyrin repeats of DGK-ζ were introduced using site-directed mutagenesis. An Mxa I restriction site (2429T→C) was engineered into the DGK-ζ cDNA and then oligonucleotides encoding the desired mutations were ligated into the Bsa BI + XmaI restriction sites as described in Topham et al. (1998). The Raf-ER and MKP3 expression plasmids were a gift from Dr. Andrew Thorburn (Wake Forest University, Winston-Salem, NC).
Muscle Homogenates
Skeletal muscles were harvested from adult male C57BL/6 mice, flash-frozen in liquid nitrogen, and stored at -80°C. One to 2 g of frozen muscle was added to 10 volumes of homogenization buffer (25 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, and 100 mM NaCl) plus protease inhibitors [10 μg/ml each of leupeptin, antipain, 4-(2-aminoethyl)-benzenesulfonyl-fluoride HCl, pepstatin A, and benzamidine HCl]. The mixture was homogenized in a Waring blender and then with a PT-3100 Polytron (Kinematica, Luzern, Switzerland). Nuclei were pelleted for 10 min at 1,000 × g. The supernatant was centrifuged at 48,000 × g for 20 m, removed, and assayed for protein content using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). A fraction of the sample was mixed with 5× SDS-PAGE sample buffer and heated for 5 min at 95°C.
Cell Culture and Transfection
C2C12 myoblasts were grown on dishes coated with Matrigel (Collaborative Research, Bedford, MA) in DMEM high-glucose with 10% fetal bovine serum, 100 U/ml penicillin-streptomycin, and 2 mM l-glutamine to 80-100% confluence. To induce differentiation, cells were switched to fusion medium containing 5% horse serum.
C2 myoblasts were transfected 18-24 h after plating at 70-80% confluence by using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. For transfections of myoblasts plated on glass coverslips, 1 μg of purified DNA was added to 3 μl of FuGENE 6 (3:1 ratio) diluted in serum-free DMEM to a final volume of 100 μl. The mixture was incubated for 20 min at room temperature (RT) and added to 3 ml of growth medium in 35-mm dishes containing the coverslips. For large-scale transfections, 5.3 μg of DNA was added to 8 μl of FuGENE 6 (3:2 ratio) diluted in serum-free DMEM to a final volume of 100 μl. Transfections proceeded for 18-24 h.
For Raf activation experiments, plasmids were transfected into COS-7 cells as described in Topham et al. (1998). Twenty-four hours later, cells were treated with 2 μM estrogen for 30 min and then harvested. Equivalent amounts of protein were used for SDS-PAGE followed by Western blotting with anti-DGK-ζ antibodies.
Subcellular Fractionation
Cultures of C2 myoblasts in 100-mm dishes were washed three times with cold phosphate-buffered saline (PBS) and then scraped into 1 ml of PBS and centrifuged. All subsequent steps were performed on ice or at 4°C. The cells were resuspended in 200 μl of lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, and protease inhibitors) and lysed by 10-s sonication with a Branson sonifier equipped with a 5-mm tip (power output 1, at 50% duty cycle; Branson, Ultrasonics, Danbury, CT). An aliquot (total fraction) was removed and boiled in SDS-PAGE loading buffer. The lysates were centrifuged at 14,000 × g for 10 min to remove nuclei and unbroken cells. Then the supernatant was collected and centrifuged at 100,000 × g for 1 h at 4°C. This supernatant (cytosol fraction) was removed and the pellet was resuspended by vortexing in buffer containing 1% Triton X-100 (TX-100) and then centrifuged at 100,000 × g for 1 h at 4°C. The final supernatant (membrane fraction) was collected and the pellet (cytoskeleton fraction) was resuspended and boiled in SDS-PAGE loading buffer.
Immunofluorescence
C57BL/6 and C57BL/10ScSn-Dmdmdx/J (mdx) mice were sacrificed by CO2 overdose. The tibialis anterior, soleus, and plantaris muscles were immediately removed, placed in Histo Prep (Fisher Scientific Co., Fair Lawn, NJ), frozen in liquid nitrogen-cooled isopentane, and stored at -80°C. Transverse sections (8 μm thick) were cut on a cryostat and stored at -80°C. The sections were thawed, ringed with a hydrophobic boundary, and rinsed with buffer A (0.5% bovine serum albumin and 0.15% glycine in PBS, pH 7.4). Then they were fixed with 4% paraformaldehyde, washed, and blocked in buffer A + 5% normal goat serum. They were then incubated with affinity-purified antibody diluted in buffer A + 0.3% TX-100 in a closed, damp chamber for 1 h at RT or overnight at 4°C. The sections were then rinsed in buffer A and incubated with a 1:300 dilution of FITC- or Texas Red dye-conjugated secondary antibody in buffer A + 0.3% TX-100 for 1 h at RT.The sections were rinsed in PBS for 3 × 15 min and cover-slipped with Fluoromount G (EMS, Fort Washington, PA). Images were captured on an Axioskop microscope (Carl Zeiss, Thornwood, NY) equipped with an Axiocam digital camera by using Axiovision 3.0 software. Images were processed using Adobe Photoshop 5.5.
Quantification of Membrane Localization
C2 myoblasts were fixed and stained as in Hogan et al. (2001). Transfected cells were scored as having cytoplasmic, partially membrane-associated, or completely membrane-associated protein according to the following criteria. A cytoplasmic localization was defined as protein diffusely and equally distributed throughout the cell. Complete membrane localization was defined as protein highly concentrated at the plasma membrane around the entire perimeter of the cell. Cells with partial membrane localization had protein concentrated at the plasma membrane in at least one part of the cell. In double-labeled cells, each fluorophore was scored separately. The results are the average of three separate experiments with a minimum of 200 cells/condition.
Immunofluorescence intensity profile plots were made using Scion Image software (Scion, Frederick, MD), and data were exported and graphed using SigmaPlot 8.0 (SPSS Science, Chicago, IL). Scanned regions were selected by drawing a 50-μm reference line, 8 pixels wide, near the middle of the cell so as to include the plasma membrane, cytoplasm and nucleus. Values for the profile plots were derived by averaging the pixel intensity values across the width of the line.
Immunoprecipitation
All steps were carried out at 4°C or on ice. Five-d-old C2 myotube cultures were washed twice with 10 ml of ice-cold PBS, pH 7.4, and then lysed with 0.5 ml of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% TX-100, and protease inhibitors)/100-mm dish. Cells were lysed on ice for 30 min, then scraped and centrifuged for 10 min at 4°C. The supernatant was collected and an aliquot of this starting material (input) was boiled in reducing SDS-PAGE sample buffer. Samples were precleared for 1-2 h by using 50 μl of a 50% slurry of washed Protein A/G Plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). One to 5 μg of antibody was added to 0.8 ml of precleared supernatant and incubated at 4°C with mixing for 2 h. Then 50 μl of Protein A/G Plus agarose beads was added and incubated an additional 2-3 h at 4°C with mixing. The immune complexes were collected by centrifugation and washed 3 × 10 min with lysis buffer. Proteins were eluted from the beads by boiling in SDS-PAGE sample buffer. The samples were then centrifuged at 21,000 × g for 1-2 min and subjected to SDS-PAGE and Western blot analysis.
Pull-Down Assays
Bacterial fusion proteins were expressed and purified as in Gee et al. (2000). Glutathione S-transferase (GST) (2.5 mg) or a fusion protein consisting of GST and amino acids 787-928 of human DGK-ζ (GST-DGK-ζ) (Hogan et al., 2001) were incubated with 500 μl of a 50% slurry of glutathione-Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ) for 20 min at 4°C, followed by washing in PBS, pH 7.2. Then an equal volume of beads (100 μl) was incubated with the soluble fraction of mouse skeletal muscle homogenates for 2 h at 4°C with constant mixing. A fraction of the starting material (input) was boiled in SDS-PAGE loading buffer. The beads were washed twice with ice-cold PBS, pH 7.2, and then once with PBS + 1% TX-100. The bound proteins were eluted in an equal volume of loading buffer and boiled for 5 min.
RESULTS
DGK-ζ and Syntrophins Associate in Skeletal Muscle
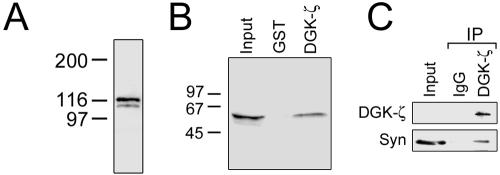
Western blotting indicated that both DGK-ζ and syntrophins are detectable in extracts of adult skeletal muscle (Figure 1, A and B). To verify the biochemical association of DGK-ζ and syntrophins, we performed pull-down experiments from muscle extracts by using a GST fusion protein of DGK-ζ. The bound proteins were analyzed by immunoblotting for syntrophins. GST-DGK-ζ, but not GST alone captured a significant fraction of syntrophins in the extract (Figure 1B). For coimmunoprecipitations, C2 myotubes were used because more DGK-ζ could be solubilized from cells than from muscle tissue. DGK-ζ was efficiently precipitated by its specific antibody, but not by control rabbit IgG (Figure 1C). Although syntrophins were specifically coimmunoprecipitated by the DGK-ζ antibody, only a fraction of the total bound DGK-ζ. This result may be explained by the fact that syntrophins have additional binding partners in skeletal muscle (Brenman et al., 1996; Gee et al., 1998). Nevertheless, these results suggest DGK-ζ and syntrophins form a stable complex in muscle cells.
Figure 1.
DGK-ζ and syntrophins associate in skeletal muscle. (A) DGK-ζ migrates as a doublet of ∼116 kDa in immunoblots of mouse skeletal muscle homogenates. (B) Beads charged with GST alone or with a GST-DGK-ζ fusion protein were incubated with skeletal muscle extracts. GST-DGK-ζ, but not GST, captured a significant portion of syntrophin in the offered extract. Input, 5% of starting material. (C) DGK-ζ and syntrophins are coimmunoprecipitated from muscle cell extracts. Lysates of C2 myotubes were immunoprecipitated with an antibody to DGK-ζ or with control IgG. The immunoprecipitates were analyzed by immunoblotting for DGK-ζ and syntrophins. With longer exposures, DGK-ζ was detectable in the input lane. Input, 5% of starting material.
DGK-ζ and Syntrophins Colocalize in Skeletal Muscle
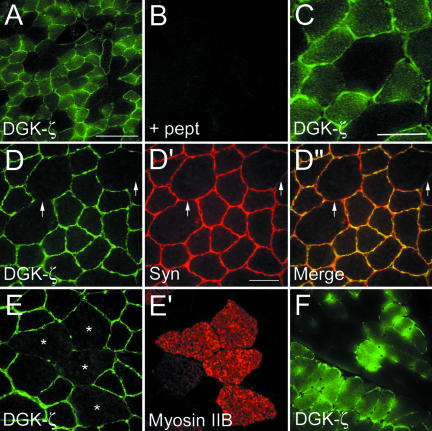
Indirect immunofluorescence labeling of transverse sections of mouse tibialis anterior (TA) muscle revealed a fiber type-specific pattern of DGK-ζ expression, which was blocked by preincubation of the antibody with its immunogenic peptide (Figure 2, A and B). Higher magnification revealed strong staining of the sarcolemma and punctate intracellular staining (Figure 2C). In double-labeled sections, DGK-ζ and syntrophins were colocalized at the sarcolemma (Figure 2, D-D″). Combined with the biochemical data mentioned above, these results suggest DGK-ζ and syntrophins form a complex at the sarcolemma of skeletal muscle fibers.
Figure 2.
Immunofluorescence localization of DGK-ζ in skeletal muscle and colocalization with syntrophins. (A-C) Transverse sections of mouse TA muscle were fixed and stained with affinity-purified antibodies to DGK-ζ and FITC-conjugated secondary antibodies. At low magnification (A) DGK-ζ immunoreactivity was observed in a subset of muscle fibers. (B) Specificity of the DGK-ζ immunolabeling was confirmed by preincubation of the antibody with its immunogenic peptide, which completely eliminated the signal. (C) Higher magnification revealed diffuse cytoplasmic and strong sarcolemmal labeling. (D-D″) Mouse TA muscle was double labeled for DGK-ζ (D) and syntrophins (D′). Syntrophins were visualized with mAb 2101 and Texas Red-conjugated secondary antibodies. (D″) Merged image shows syntrophins and DGK-ζ colocalize at the sarcolemma. The arrows indicate regions where DGK-ζ staining is absent and a corresponding reduction in syntrophin staining. (E and E′) In mouse TA muscle, DGK-ζ is absent or greatly reduced in type IIB fibers. (F) DGK-ζ is expressed in all fibers of the soleus muscle (bottom left), but not in the adjacent plantaris muscle (top right). The asterisks indicate DGK-ζ-negative fibers stained for myosin IIB. Bars, 100 μm (A) and 25 μm (C-F).
The mouse TA muscle contains both oxidative (types IIA and IIX) and glycolytic (type IIB) fibers. In the latter, DGK-ζ staining was undetectable (Figure 2, E and E′). In plantaris muscle, which also contains a mix of fiber types, a mosaic pattern of DGK-ζ labeling was observed (Figure 2F, top right). However, all fibers were labeled in soleus muscle, which contains only type I and IIA fibers (Figure 2F, bottom left). These results suggest DGK-ζ is found on the sarcolemma of all fiber types except IIB.
DGK-ζ Is Mislocalized in mdx Muscle
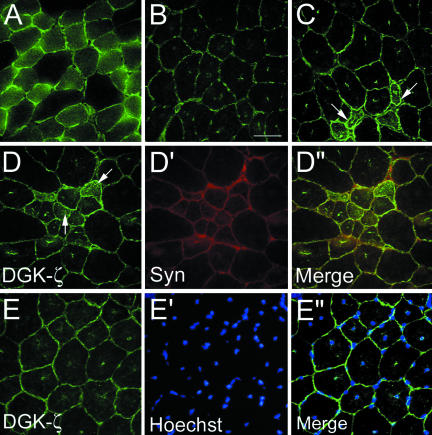
In mdx mouse muscle, the absence of dystrophin results in a dramatic reduction of sarcolemmal syntrophins (Froehner et al., 1987). Compared with control muscle fibers (Figure 3A), mdx fibers had substantially lower levels of DGK-ζ, although detectable sarcolemmal staining remained (Figure 3B). Interspersed among these were groups of small caliber regenerating fibers where DGK-ζ and syntrophin staining was noticeably increased (Figure 3, C and D-D″). Central patches of DGK-ζ immunoreactivity were observed in mdx (Figure 3E), but not control fibers (Figure 3A). Hoechst staining revealed they correspond to central nuclei and the merged image shows that virtually every nucleus was associated with DGK-ζ immunoreactivity (Figure 3, E-E″). In normal muscle, there was no obvious enrichment of DGK-ζ in large punctate structures at the sarcolemma, suggesting the enzyme is not normally associated with peripheral myofiber or satellite cell nuclei. Thus, these results suggest DGK-ζ accumulates in the nucleus of muscle fibers in the absence of sarcolemmal syntrophin and dystrophin.
Figure 3.
DGK-ζ is reduced on the sarcolemma of mdx myofibers and is associated with central nuclei. Sections of TA muscle from normal (A) and mdx (B) mice were stained for DGK-ζ and photographed with identical exposure times. (C) In mdx muscle, occasionally groups of small caliber fibers (arrows) with increased DGK-ζ expression were observed. (D-D″) The increased DGK-ζ immunoreactivity in small caliber fibers coincided with increased syntrophin expression. (E-E″) In mdx TA muscle fibers, DGK-ζ was associated with central nuclei revealed by Hoechst stain (E′ and E′′). Bar, 25 μm.
DGK-ζ Is Concentrated at NMJs
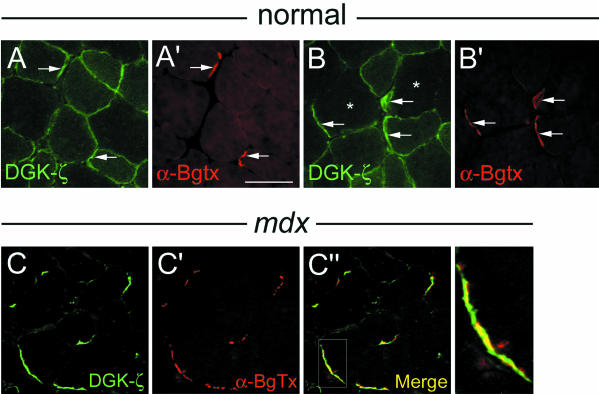
Three syntrophin isoforms are enriched at NMJs in normal muscle: β2-syn is restricted to the postsynaptic membrane, whereas α1- and β1-syn are additionally present on the extrajunctional sarcolemma (Peters et al., 1994; Peters et al., 1997). In fibers from control mice, sarcolemmal DGK-ζ labeling was noticeably increased at NMJs revealed by staining with α-BgTx, which specifically labels nicotinic acetylcholine receptors (AChRs) (Figure 4, A and A′, arrows). However, in type IIB fibers (Figure 4B, asterisks), DGK-ζ immunoreactivity was localized exclusively at NMJs (Figure 4, B and B′, arrows).
Figure 4.
DGK-ζ is specifically targeted to NMJs in type IIB fibers and retained at NMJs in mdx muscle. TA muscles were labeled with an antibody to DGK-ζ and with α-BgTx, which labels AChRs. (A and A′) In most fibers, DGK-ζ was present on the extrajunctional sarcolemma and was concentrated at NMJs (arrows). (B and B′) In type IIB fibers (indicated by asterisks), DGK-ζ was restricted to junctional regions (arrows). (C-C″) In mdx myofibers, DGK-ζ is absent from extrajunctional regions but remains concentrated at synaptic sites. Higher magnification of the boxed region in C″ (bottom right) shows DGK-ζ extends beyond the AChR-rich regions. Bar, 25 μm.
Although DGK-ζ staining was dramatically reduced on the extrajunctional sarcolemma of mdx fibers, it remained concentrated at synaptic sites (Figure 4, C and C′), likely due to its association with syntrophins, which are specifically retained at NMJs (Peters et al., 1994, 1997; Yang et al., 1995). Notably, DGK-ζ immunoreactivity extended beyond the AChR-rich regions and into the surrounding perisynaptic membrane (Figure 4C″, boxed region and panel at right).
Reciprocal Interactions Regulate the Localization of DGK-ζ and Syntrophin in Muscle Cells
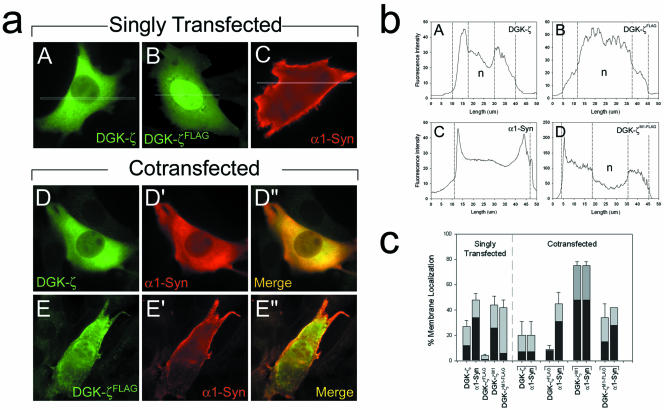
To gain insight into the molecular mechanisms regulating the membrane localization of DGK-ζ in muscle cells, we transiently transfected C2 myoblasts with a plasmid encoding wild-type DGK-ζ with three N-terminal HA epitope tags. Wild-type DGK-ζ was associated with the plasma membrane in 27 ± 5% of the cells examined, with only 12% demonstrating complete membrane localization (Figure 5c). In cells with a cytoplasmic distribution of DGK-ζ, the protein was diffusely distributed throughout and was largely excluded from the nucleus (Figure 5a, A).
Figure 5.
Reciprocal interactions regulate the localization of DGK-ζ and α1-syn in muscle cells. (a) C2 myoblasts were singly (A-C) or doubly transfected (D-E″) with cDNAs encoding the indicated epitope-tagged proteins. The cells were fixed and labeled with antibodies specific for each epitope tag. (b) Representative profile plots of the fluorescence intensity of transfected C2 myoblasts labeled for the indicated constructs showing cytoplasmic (A), nuclear (B), complete membrane localization (C), and partial membrane localization (D). The profile plot in D is taken from the cell shown in Figure 6a, B. The vertical lines indicate transition points in the fluorescence intensity between cellular compartments. (c) The graph shows the percentage of cells with membrane localized protein in C2 myoblasts expressing the indicated constructs. Membrane localization was quantified as described in MATERIALS AND METHODS. Gray bars indicate the percentage of cells with any membrane localization and black bars indicate complete membrane localization. The data are the average of three independent experiments. Error bars indicate SEM. n, nucleus.
We have previously shown that a C-terminal FLAG epitope tag blocks the binding of the DGK-ζ C terminus to the PDZ domain of syntrophin (Hogan et al., 2001). In C2 cells transfected with DGK-ζFLAG, only 4 ± 1% of the cells had any discernible membrane localization, and only 1% of the cells showed complete membrane localization (Figure 5c). DGK-ζFLAG also accumulated in the nucleus (Figure 5a, B), as previously observed in HeLa cells (Hogan et al., 2001). Thus, in the absence of syntrophin interaction, DGK-ζ is decreased at the plasma membrane and accumulates in the nucleus.
PDZ-containing proteins act as scaffolds for the assembly of protein complexes at the plasma membrane (Sheng and Sala, 2001). Thus, we reasoned that α1-syntrophin, the predominant isoform in skeletal muscle (Adams et al., 1993), might recruit DGK-ζ to the plasma membrane. By itself, myc-tagged α1-syntrophin (α1-Syn) was localized at the plasma membrane (Figure 5a, C) in 48 ± 5% (34% complete) of transfected C2 cells (Figure 5c). Surprisingly, DGK-ζ and α1-syn were colocalized in the cytoplasm in the majority of cotransfected cells (Figure 5a, D-D″). Only 20 ± 11% (7% complete) of the cells showed any membrane localization, which is comparable with cells expressing DGK-ζ alone and substantially lower than for α1-syn alone (Figure 5c).
In contrast, DGK-ζFLAG failed to redistribute α1-Syn from the plasma membrane, even though it was expressed at similar levels to DGK-ζ (Figure 5a, E-E″). In 45 ± 9% (31% complete) of the doubly transfected cells, α1-syn was localized at the membrane, whereas in the same cells only 9 ± 3% (8% complete) had observable DGK-ζ at the membrane (Figure 5c). Moreover, as noted above, DGK-ζFLAG amassed in the nucleus (Figure 5a, E). These results suggest that the redistribution of α1-Syn from the plasma membrane occurs by a specific interaction with the DGK-ζ C terminus and argue against the idea that it is an artifact of overexpression.
Phosphorylation Targets DGK-ζ and Syntrophin to the Plasma Membrane
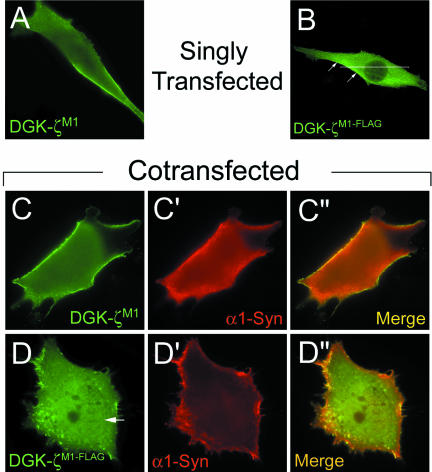
To determine whether phosphorylation of the MARCKS domain regulates the association of DGK-ζ with the plasma membrane, we generated a mutant (DGK-ζM1) mimicking phosphorylation by substituting Asp for Ser. DGK-ζM1 was membrane associated (Figure 6A) in 44 ± 7% (26% complete) of the transfected cells (Figure 5c), approximately a twofold increase compared with cells expressing wild-type DGK-ζ. These results suggest phosphorylation of the MARCKS domain targets DGK-ζ to the plasma membrane of muscle cells.
Figure 6.
MARCKS domain-phosphorylation regulates DGK-ζ localization. C2 myoblasts expressing the indicated constructs were fixed and visualized as described in Figure 5. The arrow in D indicates nuclear DGK-ζ staining.
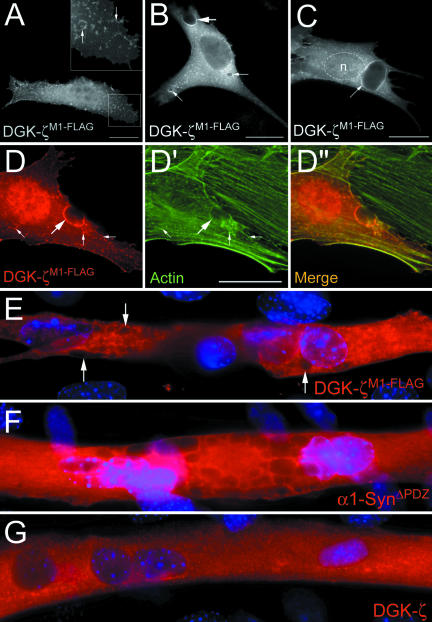
DGK-ζM1 with a C-terminal FLAG tag (DGK-ζM1-FLAG) was membrane associated (Figure 6B) in 42 ± 6% of the cells, similar to DGK-ζM1; however, only 6% of the cells showed complete membrane localization (Figure 5c). A plot of the immunofluorescence intensity is shown in Figure 5b, D. These results suggest syntrophin is not absolutely required for the plasma membrane localization of DGK-ζ, but may stabilize (phosphorylated) DGK-ζ at the plasma membrane.
Syntrophins Increase the Membrane Localization of Phosphorylated DGK-ζ
To test this idea, we coexpressed DGK-ζM1 with α1-syn in C2 cells. Both proteins were membrane associated in 75 ± 3% of the cotransfected cells, a substantial increase compared with cells expressing either protein alone (Figure 5c). In 48% of the cells, the two proteins were concentrated at the plasma membrane (Figure 6, C-C″). These results suggest that phosphorylation of the MARCKS domain signals the complex of DGK-ζ and syntrophin to move to the plasma membrane.
As a control, we coexpressed DGK-ζM1-FLAG with α1-syn. As expected, α1-syn did not increase the membrane localization of DGK-ζM1-FLAG and the percentage of cells with membrane localization was similar to DGK-ζM1-FLAG expressed alone (Figure 5c). Moreover, the two proteins were not exactly colocalized (Figure 6, D-D″). Collectively, our results suggest syntrophins promote the stable association of phosphorylated DGK-ζ with the plasma membrane through PDZ interactions, which may be critical for the proper targeting of DGK-ζ to specialized membrane domains in muscle cells.
Endogenous DGK-ζ and Syntrophin Are Concentrated in Regions of the Plasma Membrane Undergoing Actin Reorganization
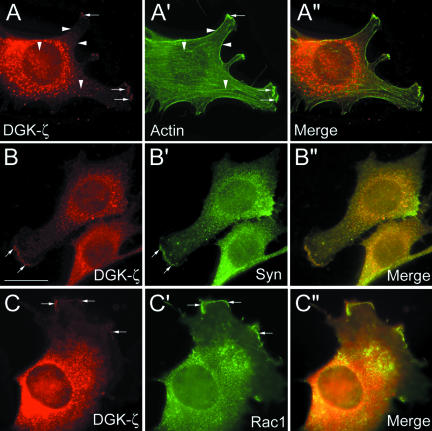
Because overexpressed proteins may not reflect the location of their native counterparts, we determined the subcellular distribution of endogenous DGK-ζ and syntrophins in C2 myoblasts. Punctate DGK-ζ labeling was found throughout most of the cytoplasm and was largely excluded from the nucleus, similar to the overexpressed HA-tagged protein (Figure 7A). DGK-ζ was also concentrated in specific regions of the cell periphery, including membrane ruffles and the leading edge of lamellipodia (Figure 7, A-C, small arrows). At these sites, DGK-ζ immunoreactivity partially overlapped with F-actin (Figure 7A′, small arrows), but it did not colocalize with stress fibers or with cortical F-actin (arrow-heads). It also colocalized with syntrophins in these regions (Figure 7, B-B″, small arrows) and with Rac1, a Rho-family GTPase that mediates actin reorganization underlying membrane ruffling and lamellipodia formation (Figure 7, C-C″, arrows) (Etienne-Manneville and Hall, 2002). Together, these results suggest DGK-ζ and syntrophins participate in the assembly or organization of the actin cytoskeleton.
Figure 7.
DGK-ζ colocalizes with actin, syntrophin, and Rac1 in membrane ruffles and at the leading edge of lamellipodia. C2 myoblasts were stained with an antibody to DGK-ζ and with either phalloidin to detect F-actin (A-A″), or with antibodies to syntrophin (B-B″) or Rac1 (C-C″). (A-A″) Small arrows indicate membrane ruffles and lamellipodia leading edges, vertical arrowheads actin stress fibers and horizontal arrowheads cortical actin. Bar, 20 μm.
Ras/Extracellular Signal-regulated Kinase (ERK) Mitogen-activated Protein (MAP) Kinase Phosphorylation Regulates DGK-ζ Association with the Cytoskeleton
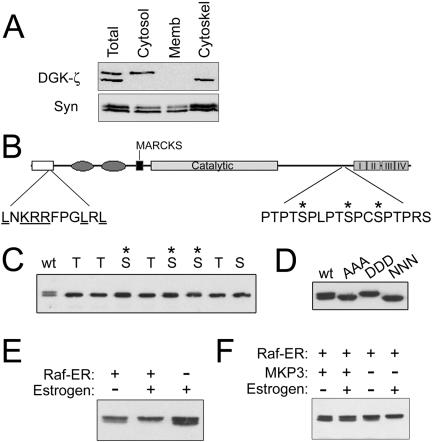
Subcellular fractionation revealed that the two DGK-ζ species detected in immunoblots of muscle extracts have different localizations (Figure 8A). The upper band was present in the cytosolic fraction, whereas the lower band was recovered in the detergent-insoluble cytoskeleton fraction (Figure 8A). Neither band was detected in the membrane fraction. In contrast, syntrophins were detected in every fraction.
Figure 8.
ERK phosphorylation regulates the association of DGK-ζ with the cytoskeleton. (A) C2 myoblast lysates were fractionated as described in MATERIALS AND METHODS, and equal amounts of protein from each fraction were analyzed by SDS-PAGE and immunoblotting for DGK-ζ and syntrophin. (B) Schematic of DGK-ζ showing the domain organization, the sequence of the proline-rich region near the ankyrin repeats (I-IV) and the ERK docking-site consensus sequence. The N-terminal (open box), C1 (ellipses), MARCKS, and catalytic domains are indicated. (C) COS-7 cells were transfected with DGK-ζ constructs in which all the Ser and Thr residues in the proline-rich sequence were mutated to Ala except the one shown above the corresponding lane of the immunoblot. The presence of a slightly higher shifted band in the lanes marked by an asterisk indicates that the residue is phosphorylated. (D) COS-7 cells were transfected with cDNAs encoding wild-type DGK-ζ (wt), a mutant in which all the Ser/Thr residues shown in B were changed to alanines (AAA), or mutants in which the three Ser residues indicated by asterisks were changed to Asp (DDD) or Asn (NNN). The lysates were analyzed by SDS-PAGE and immunoblotting for DGK-ζ. (E) COS-7 cells were transfected with cDNAs encoding DGK-ζ and either Raf-ER or a control vector. 24 h later the cells were treated for 10 h with 1 μM estrogen or control vehicle. (F) COS-7 cells expressing DGK-ζ, Raf-ER and either the ERK phosphatase MKP3 or a control vector were treated with estrogen or vehicle as described above and then analyzed for DGK-ζ.
To investigate the basis for the difference in the size and subcellular localization of the two DGK-ζ species, we analyzed potential phosphorylation sites in a proline-rich sequence near the C terminus (Figure 8B). Every Ser and Thr residue in the sequence was changed to Ala except for the one shown at the top of the corresponding lane of the immunoblot in Figure 8C. Mutation of the three Ser residues indicated by asterisks gave rise to a second, more slowly migrating species, indicating that they can be phosphorylated. The three Ser residues were changed to Asp to mimic phosphorylation or to Asn, which is structurally similar but uncharged at physiological pH. Immunoblotting revealed that the Asp mutant (DDD) comigrates with the upper band, whereas the Asn mutant (NNN) comigrates with the lower band (Figure 8D). An additional mutant, in which all the Ser and Thr residues were changed to Ala (AAA) also comigrated with the lower band, suggesting there are no additional phosphorylation sites within the proline-rich sequence.
The sequence identified above contains motifs conforming to the minimal MAP kinase phosphorylation consensus sequence (Ser/Thr-Pro). In addition, the N terminus of DGK-ζ contains an ERK docking-site consensus sequence (Figure 8B) (Sharrocks et al., 2000). To activate the Ras/ERK signaling pathway, we used a chimeric cDNA consisting of the hormone-binding domain of the estrogen receptor fused to an oncogenic form of Raf-1 (Samuels et al., 1993; Topham and Prescott, 2001). Activation of the chimeric protein (Raf-ER) by estrogen in COS-7 cells induced phosphorylation of DGK-ζ, indicated by the accumulation of the slowly migrating band (Figure 8E). In contrast, coexpression of stress-activated protein kinase did not alter the migration of either DGK-ζ band (our unpublished data). Coexpression of Raf-ER with MKP3, a dual Tyr-Ser/Thr phosphatase that inactivates ERK (Zhou et al., 2002), completely attenuated the band shift even in the presence of estrogen, indicating that phosphorylation of DGK-ζ occurs downstream of ERK activation (Figure 8F). Another ERK phosphatase, CL100, had a similar effect (our unpublished data). These results suggest ERK-dependent phosphorylation of DGK-ζ negatively regulates its association with the cytoskeleton.
DGK-ζM1-FLAG Induces Changes in the Actin Cytoskeleton
C2 myoblasts expressing DGK-ζM1-FLAG displayed prominent membrane ruffles enriched in both DGK-ζ (Figure 9A, inset, arrows) and F-actin (Figure 9, D-D″, small arrows). In addition, they often contained large vesicles and invaginations of the plasma membrane that were occasionally as large as the nucleus (Figure 9, B-D). DGK-ζ labeling on the perimeter of these structures was colocalized with actin (Figure 9, D-D″, arrows). Large vesicles were also observed in C2 myotubes expressing DGK-ζM1-FLAG (Figure 9E arrows) but not in myotubes expressing either wild-type DGK-ζ (Figure 9G, arrows) or DGK-ζM1 (our unpublished data), suggesting the DGK-ζ/syntrophin interaction is important for proper control of actin cytoskeleton organization. Consistent with this interpretation, overexpression of α1-syntrophin lacking the PDZ domain (α1-SynΔPDZ) (Adams et al., 2001) produced a similar phenotype (Figure 9F).
Figure 9.
Expression of DGK-ζM1-FLAG in C2 cells induces F-actin-rich membrane ruffles and large vesicles. (A-C) Representative images of C2 myoblasts expressing DGK-ζM1-FLAG. (A and inset) DGK-ζ was concentrated in membrane ruffles (arrows). (B) DGK-ζ was localized on the perimeter of large invaginations of the plasma membrane (large arrow) and vesicles ranging in size from 2 to 5 μm (small arrows). In some cells, vesicles as large as the nucleus were observed (C, arrow). (D-D″) DGK-ζM1-FLAG-induced membrane ruffles (small arrows) and invaginations (large arrow) were enriched in F-actin, which colocalized with DGK-ζ. (E-G) Representative images of C2 myotubes expressing the indicated constructs. 4,6-Diamidino-2-phenylindole-stained nuclei are shown in blue. Vesicles were present in myotubes expressing DGK-ζM1-FLAG (E, arrows), α1-SynΔPDZ (F), but not wild-type DGK-ζ (G). n, nucleus. Bars, 20 μm.
DISCUSSION
Recent studies indicate that the regulation of DGK activity depends on the correct subcellular localization of the enzyme (Topham et al., 1998; Imai et al., 2002; Santos et al., 2002). Because DGK-ζ is found in the cytoplasm, at the plasma membrane and in the nucleus, the mechanisms regulating its localization are likely to be complex. Here, we investigated the role of syntrophins in the control of DGK-ζ localization in skeletal muscle. We demonstrated that DGK-ζ and syntrophins form a stable complex in muscle cells, in agreement with our previous studies showing the DGK-ζ C terminus binds to the PDZ domains of syntrophin isoforms (α1, β1, and β2) expressed in skeletal muscle (Hogan et al., 2001). In addition, we showed DGK-ζ and syntrophin reside in part on the sarcolemma of adult muscle fibers. Syntrophins seem to promote a more stable association of DGK-ζ with the plasma membrane, which in our studies, leads to an increase in cells with membrane-associated DGK-ζ. In agreement with this idea, there was a substantial loss of sarcolemmal DGK-ζ in mdx muscles, where the absence of dystrophin results in decreased syntrophin expression and mislocalization of the remaining membrane-associated protein to the cytosol (Froehner et al., 1997). The latter finding suggests the membrane localization of DGK-ζ in skeletal muscle fibers depends on the formation of a complex containing both syntrophin and dystrophin.
Syntrophins Stabilize Active DGK-ζ at the Plasma Membrane and Anchor the Inactive Enzyme in the Cytoplasm
Recently, Santos et al. (2002) showed PKC-dependent phosphorylation of the MARCKS domain signals DGK-ζ translocation to the plasma membrane of T cells. Our results confirm and extend their findings by showing that the localization of DGK-ζ and syntrophin is coordinately regulated by phosphorylation. Immunofluorescence data demonstrated that constitutive phosphorylation of the MARCKS domain increased the association of DGK-ζ with the plasma membrane relative to the wild-type protein. Moreover, coexpression of phosphorylated DGK-ζ with α1-syn leads to an increase in syntrophin localization at the plasma membrane. An interesting possibility is that phosphorylation of DGK-ζ causes a conformational change in syntrophin that increases its affinity for dystrophin. This could serve as a mechanism for targeting syntrophin/DGK-ζ complexes to the sarcolemma.
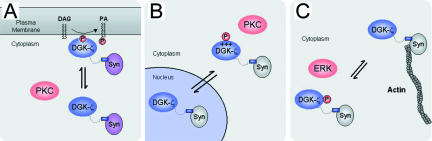
Syntrophins have been regarded as scaffold proteins that function exclusively at the plasma membrane (Froehner et al., 1997); however, substantial amounts are found in the cytosolic fraction of skeletal muscle cell and tissue lysates (Figure 8; Gee, unpublished observations), implying they have functional roles in the cytoplasm. Our data raise the possibility that cytoplasmic syntrophins sequester DGK-ζ in the cytoplasm of resting cells, away from sources of DAG. In this scheme, phosphorylation of the MARCKS domain by PKC would allow DGK-ζ and syntrophin to translocate to the plasma membrane, thereby regulating DGK-ζ activity (Figure 10A). These findings imply that syntrophin localization is dynamically regulated in muscle cells.
Figure 10.
Model depicting the regulation of DGK-ζ localization in muscle cells. (A) PKC-dependent phosphorylation of the MARCKS domain regulates the association of DGK-ζ and syntrophin with the plasma membrane. (B) Syntrophin interaction and PKC-dependent phosphorylation regulate the nuclear-cytoplasmic shuttling of DGK-ζ. (C) ERK-dependent phosphorylation of DGK-ζ negatively regulates its association with the cytoskeleton.
Regulation of Nuclear Localization
Consistent with our previous studies (Hogan et al., 2001), we showed that DGK-ζ accumulates in the nucleus of C2 muscle cells in the absence of syntrophin interaction. We also observed DGK-ζ immunoreactivity associated with central nuclei of mdx muscles fibers, raising the possibility that the absence of dystrophin and the secondary loss of syntrophins cause the inappropriate accumulation of DGK-ζ in the nucleus. Because DGK-ζ regulates the levels of DAG in the nucleus (Topham et al., 1998), which in turn regulates PKC-dependent changes in cell growth, inappropriate levels of DGK-ζ in nuclei of mdx muscles could affect the regulation of muscle cell growth. However, more detailed studies are required to verify that there are differences in nuclear DGK-ζ levels in normal versus mdx muscles. Nevertheless, these results, together with our previous studies, reinforce the idea that syntrophins are important for the stable retention of DGK-ζ in the cytoplasm and the regulation of nuclear DGK-ζ levels.
Modulation of the Actin Cytoskeleton
We found that DGK-ζ and syntrophins associate with actin in regions undergoing cytoskeletal rearrangements, suggesting they play a role in modulating actin dynamics. Consistent with this interpretation, DGK-ζ was colocalized with Rac1, a member of the Rho family of small GTPases that control the assembly and organization of F-actin (Etienne-Manneville and Hall, 2002). Moreover, Tolias et al. (1998) demonstrated that Rac immunoprecipitates contain DGK activity with biochemical characteristics like those of DGK-ζ. Thus, DGK-ζ and syntrophins may function in a complex with Rac1 to control actin cytoskeleton remodeling, possibly by facilitating Rac1 activation and/or its localization to the cell surface. Activation of Rac1 produces lamellipodia and membrane ruffles, which serve as sites of actin polymerization, endocytosis, receptor tyrosine kinase signaling, and protease activity (Small et al., 2002). Consistent with a role in regulating actin dynamics, overexpression of DGK-ζM1-FLAG, which does not interact with syntrophins, produced dramatic changes in cell morphology and actin organization, including the formation of membrane ruffles and large vesicles. This finding suggests that the control of DGK-ζ localization by syntrophins is important for regulating actin cytoskeleton rearrangements. For example, syntrophins might direct the assembly of Rac1/DGK-ζ complexes to the leading edges of lamellipodia where other actin regulatory proteins are assembled.
This study demonstrates that ERK-dependent phosphorylation near the C terminus of DGK-ζ negatively regulates its association with the cytoskeleton (Figure 10C). The mechanism by which this occurs is unclear, but DGK-ζ likely interacts with F-actin, because the two were colocalized in ruffles and at the leading edge of lamellipodia. The MARCKS domain might mediate this interaction directly, as demonstrated for the MARCKS protein (Hartwig et al., 1992). Alternatively, syntrophins might mediate the interaction with the cytoskeleton via dystrophin family members, which bind actin at their N terminus (Rybakova et al., 1996; Galkin et al., 2002). Moreover, at least one isoform, α1-syn, has been reported to bind directly to actin (Iwata et al., 1998). Because the phosphorylated residues in DGK-ζ lie close to the C terminus, ERK phosphorylation might affect the binding of DGK-ζ to syntrophin, thereby modulating its interaction with the cytoskeleton. Interestingly, ERK is concentrated at the leading edges of lamellipodia in migrating cells where it promotes the phosphorylation of myosin light chain kinase, leading to activation of the actin/myosin motor and cell migration (Howe et al., 2002). Therefore, DGK-ζ may play a role in the cascade of signals that regulate actin reorganization at the leading edge of migrating muscle cells.
The Role of DGK-ζ at NMJs
DGK-ζ was concentrated at NMJs in normal myofibers, and its localization at NMJs was unaffected in mdx muscle, suggesting it can associate with syntrophins bound to utrophin, a dystrophin homolog found primarily at synaptic sites. Additional observations suggest a role for utrophin in the localization of DGK-ζ. Utrophin is present at low levels on the extrasynaptic sarcolemma of slow twitch muscle fibers (Gramolini et al., 2000), which may explain the residual sarcolemmal DGK-ζ observed in mdx muscles. In type IIB fibers, DGK-ζ was present exclusively at NMJs. In these fibers, sarcolemmal dystrophin levels are normal and utrophin is restricted to synaptic sites (Gramolini et al., 2000), supporting the idea that DGK-ζ associates with utrophin at NMJs. Finally, utrophin is up-regulated in regenerating fibers, accounting for the observed increase in DGK-ζ and syntrophin expression in a subset of mdx muscle fibers.
The function of DGK-ζ at adult NMJs is not known; however, its close association with AChRs suggests it could play a role in the maintenance of postsynaptic AChR density through its effects on actin organization. At the developing NMJ, agrin secreted by motor neurons induces the clustering of AChRs and other proteins at synaptic sites (Sanes and Lichtman, 1999). This process involves extensive changes in the organization of the subsynaptic cytoskeleton, which anchors and stabilizes AChRs in the membrane. Studies using cultured myotubes have established that actin polymerization is necessary for the formation of AChR clusters (Dai et al., 2000) and that agrin activates Rac, which is required for cluster formation (Weston et al., 2000). Thus, DGK-ζ could have a role in the signaling pathways leading to the initial clustering of synaptic proteins at developing NMJs or in the maintenance of their density in the adult. In this regard, it is interesting to note that NMJs from α1-syn null mice have reduced levels and an abnormal distribution of AChRs (Adams et al., 2000).
Mislocalization of DGK-ζ in Dystrophic Muscle
Our results showing the mislocalization of DGK-ζ leads to alterations in the actin cytoskeleton suggest a basis for the widespread changes in the organization of the membrane-associated cytoskeleton observed in mdx myofibers (Williams and Bloch, 1999). These changes are thought to contribute to the fragility of the sarcolemma of dystrophic muscle and may ultimately contribute to the pathogenesis of the disease. Furthermore, DGK-ζ mislocalization could affect the regulation of DAG- or PA-activated proteins. In support of this idea, mutations in the Drosophila retinal degeneration A gene, which encodes a DGK isoform closely related to DGK-ζ, result in a severe retinal phenotype (Hardie and Raghu, 2001). The degeneration is caused by Ca2+ influx through constitutively active transient receptor potential channels, which are regulated by DAG and its metabolites (Raghu et al., 2000). Interestingly, dystrophic muscles have chronically elevated intracellular Ca2+ levels caused by a persistent influx through Ca2+-permeable channels of the transient receptor potential channel family (Franco and Lansman, 1990; Vandebrouck et al., 2002). This is thought to activate Ca2+-dependent proteases, which contribute to muscle degeneration.
CONCLUSIONS
Recent studies suggest that the association of DGK-ζ and its target proteins into organized complexes is a mechanism to spatially regulate DAG signals (Topham and Prescott, 2001; Luo et al., 2003). To function optimally, these complexes must be targeted to regions of the membrane where DAG is produced. Our findings suggest syntrophins participate in the assembly and organization of DGK-ζ signaling complexes at the plasma membrane of skeletal muscle fibers, which may be critical for the proper control of actin reorganization. In dystrophic muscle, mislocalized DGK-ζ may cause signaling and cytoskeletal changes that contribute to disease pathogenesis.
Acknowledgments
We thank Drs. Bernard Jasmin and Stephen Prescott for helpful discussions. This work was supported by the Neuromuscular Research Partnership Program, an alliance of the Amyotrophic Lateral Sclerosis Society of Canada, the Muscular Dystrophy Association of Canada, and the Canadian Institutes of Health Research. S.G. is supported by a Canadian Institutes of Health Research New Investigator Award.
Abbreviations used: AChR, acetylcholine receptor; α-BgTx, α-bungarotoxin; α1-Syn, α1-syntrophin; DAG, diacylglycerol; DGK-ζ, diacylglycerol kinase-ζ; ERK, extracellular signal-regulated kinase; NMJ, neuromuscular junction; PA, phosphatidic acid; PDZ, Postsynaptic density protein-95/Discs-large/Zona Occludens-1; PH, pleckstrin homology; SU, syntrophin unique; TX-100, Triton X-100.
References
- Adams, M.E., Butler, M.H., Dwyer, T.M., Peters, M.F., Murnane, A.A., and Froehner, S.C. (1993). Two forms of mouse syntrophin, a 58 kD dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11, 531-540. [DOI] [PubMed] [Google Scholar]
- Adams, M.E., Dwyer, T.M., Dowler, L.L., White, R.A., and Froehner, S.C. (1995). Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J. Biol. Chem. 270, 25859-25865. [DOI] [PubMed] [Google Scholar]
- Adams, M.E., Kramarcy, N., Krall, S.P., Rossi, S.G., Rotundo, R.L., Sealock, R., and Froehner, S.C. (2000). Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J. Cell Biol. 150, 1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, M.E., Mueller, H.A., and Froehner, S.C. (2001). In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J. Cell Biol. 155, 113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, A.H., Freener, C.A., Gussoni, E., Yoshida, M., Ozawa, E., and Kunkel, L.M. (1996). The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J. Biol. Chem. 271, 2724-2730. [DOI] [PubMed] [Google Scholar]
- Albrecht, D.E., and Froehner, S.C. (2002). Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals 11, 123-129. [DOI] [PubMed] [Google Scholar]
- Brenman, J.E., et al. (1996). Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84, 757-767. [DOI] [PubMed] [Google Scholar]
- Bunting, M., Tang, W., Zimmerman, G.A., McIntyre, T.M., and Prescott, S.M. (1996). Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J. Biol. Chem. 271, 10230-10236. [PubMed] [Google Scholar]
- Burack, W.R., and Shaw, A.S. (2000). Signal transduction: hanging on a scaffold. Curr. Opin. Cell Biol. 12, 211-216. [DOI] [PubMed] [Google Scholar]
- Dai, Z., Luo, X., Xie, H., and Peng, H.B. (2000). The actin-driven movement and formation of acetylcholine receptor clusters. J. Cell. Biol. 150, 1321-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej, M., and Campbell, K.P. (2002). Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 12, 349-361. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Franco, A., Jr., and Lansman, J.B. (1990). Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344, 670-673. [DOI] [PubMed] [Google Scholar]
- Froehner, S.C., Adams, M.E., Peters, M.F., and Gee, S.H. (1997). Syntrophins: modular adapter proteins at the neuromuscular junction and the sarcolemma. Soc. Gen. Physiol. 52, 197-207. [PubMed] [Google Scholar]
- Froehner, S.C., Murnane, A.A., Tobler, M., Peng, H.B., and Sealock, R. (1987). A postsynaptic Mr 58, 000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J. Cell Biol. 104, 1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin, V.E., Orlova, A., VanLoock, M.S., Rybakova, I.N., Ervasti, J.M., and Egelman, E.H. (2002). The utrophin actin-binding domain binds F-actin in two different modes: implications for the spectrin superfamily of proteins. J. Cell Biol. 157, 243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., Madhavan, R., Levinson, S.R., Caldwell, J.H., Sealock, R., and Froehner, S.C. (1998). Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci. 18, 128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., Quenneville, S., Lombardo, C.R., and Chabot, J. (2000). Single-amino acid substitutions alter the specificity and affinity of PDZ domains for their ligands. Biochemistry 39, 14638-14646. [DOI] [PubMed] [Google Scholar]
- Gramolini, A.O., Wu, J., and Jasmin, B.J. (2000). Regulation and functional significance of utrophin expression at the mammalian neuromuscular synapse. Microsc. Res. Tech. 49, 90-100. [DOI] [PubMed] [Google Scholar]
- Hardie, R.C., and Raghu, P. (2001). Visual transduction in Drosophila. Nature 413, 186-193. [DOI] [PubMed] [Google Scholar]
- Hartwig, J.H., Thelen, M., Rosen, A., Janmey, P.A., Nairn, A.C., and Aderem, A. (1992). MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356, 618-622. [DOI] [PubMed] [Google Scholar]
- Hodgkin, M.N., Pettitt, T.R., Martin, A., Michell, R.H., Pemberton, A.J., and Wakelam, M.J. (1998). Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem. Sci. 23, 200-204. [DOI] [PubMed] [Google Scholar]
- Hogan, A., Shepherd, L., Chabot, J., Quenneville, S., Prescott, S.M., Topham, M.K., and Gee, S.H. (2001). Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J. Biol. Chem. 276, 26526-26533. [DOI] [PubMed] [Google Scholar]
- Howe, A.K., Aplin, A.E., and Juliano, R.L. (2002). Anchorage-dependent ERK signaling - mechanisms and consequences. Curr. Opin. Genet. Dev. 12, 30-35. [DOI] [PubMed] [Google Scholar]
- Imai, S., Sakane, F., and Kanoh, H. (2002). Phorbol ester-regulated oligomerization of diacylglycerol kinase delta linked to its phosphorylation and translocation. J. Biol. Chem. 277, 35323-35332. [DOI] [PubMed] [Google Scholar]
- Iwata, Y., Pan, Y., Yoshida, T., Hanada, H., and Shigekawa, M. (1998). Alpha1-syntrophin has distinct binding sites for actin and calmodulin. FEBS Lett. 423, 173-177. [DOI] [PubMed] [Google Scholar]
- Luo, B., Prescott, S.M., and Topham, M.K. (2003). Association of diacylglycerol kinase {zeta} with protein kinase C {alpha}: spatial regulation of diacylglycerol signaling. J. Cell. Biol. 160, 929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, S., and Aderem, A. (1995). The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem. Sci. 20, 272-276. [DOI] [PubMed] [Google Scholar]
- Peters, M.F., Adams, M.E., and Froehner, S.C. (1997). Differential association of syntrophin pairs with the dystrophin complex. J. Cell Biol. 138, 81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, M.F., Kramarcy, N.R., Sealock, R., and Froehner, S.C. (1994). β2-Syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuroreport 5, 1577-1580. [PubMed] [Google Scholar]
- Petrof, B.J., Shrager, J.B., Stedman, H.H., Kelly, A.M., and Sweeney, H.L. (1993). Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 90, 3710-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piluso, G., Mirabella, M., Ricci, E., Belsito, A., Abbondanza, C., Servidei, S., Puca, A.A., Tonali, P., Puca, G.A., and Nigro, V. (2000). gamma 1- and gamma 2-Syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J. Biol. Chem. 275, 15851-15860. [DOI] [PubMed] [Google Scholar]
- Raghu, P., Usher, K., Jonas, S., Chyb, S., Polyanovsky, A., and Hardie, R.C. (2000). Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26, 169-179. [DOI] [PubMed] [Google Scholar]
- Rando, T.A. (2001). The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 24, 1575-1594. [DOI] [PubMed] [Google Scholar]
- Rybakova, I.N., Amann, K.J., and Ervasti, J.M. (1996). A new model for the interaction of dystrophin with F-actin. J. Cell Biol. 135, 661-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels, M.L., Weber, M.J., Bishop, J.M., and McMahon, M. (1993). Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol. Cell Biol. 13, 6241-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes, J.R., and Lichtman, J.W. (1999). Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389-442. [DOI] [PubMed] [Google Scholar]
- Santos, T., Carrasco, S., Jones, D.R., Merida, I., and Eguinoa, A. (2002). Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J. Biol. Chem. 277, 30300-30309. [DOI] [PubMed] [Google Scholar]
- Sharrocks, A.D., Yang, S.H., and Galanis, A. (2000). Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 25, 448-453. [DOI] [PubMed] [Google Scholar]
- Sheng, M., and Sala, C. (2001). PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24, 1-29. [DOI] [PubMed] [Google Scholar]
- Small, J.V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112-120. [DOI] [PubMed] [Google Scholar]
- Tolias, K.F., Couvillon, A.D., Cantley, L.C., and Carpenter, C.L. (1998). Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol. Cell Biol. 18, 762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham, M.K., Bunting, M., Zimmerman, G.A., McIntyre, T.M., Blackshear, P.J., and Prescott, S.M. (1998). Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature 394, 697-700. [DOI] [PubMed] [Google Scholar]
- Topham, M.K., and Prescott, S.M. (1999). Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J. Biol. Chem. 274, 11447-11450. [DOI] [PubMed] [Google Scholar]
- Topham, M.K., and Prescott, S.M. (2001). Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism. J. Cell Biol. 152, 1135-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk, W.J., and Houssa, B. (2000). Properties and functions of diacylglycerol kinases. Cell Signal. 12, 595-605. [DOI] [PubMed] [Google Scholar]
- Vandebrouck, C., Martin, D., Colson-Van Schoor, M., Debaix, H., and Gailly, P. (2002). Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 158, 1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, C., Yee, B., Hod, E., and Prives, J. (2000). Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol. 150, 205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M.W., and Bloch, R.J. (1999). Extensive but coordinated reorganization of the membrane skeleton in myofibers of dystrophic (mdx) mice. J. Cell Biol. 144, 1259-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B., Jung, D., Rafael, J.A., Chamberlain, J.S., and Campbell, K.P. (1995). Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J. Biol. Chem. 270, 4975-4978. [DOI] [PubMed] [Google Scholar]
- Zhou, B., Wang, Z.X., Zhao, Y., Brautigan, D.L., and Zhang, Z.Y. (2002). The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J. Biol. Chem. 277, 31818-31825. [DOI] [PubMed] [Google Scholar]