Abstract
Involvement of rabphilin-3A-like (RPH3AL), or Noc2, the potential effector of Ras-associated binding proteins Rab3A and Rab27A in the regulation of exocytotic processes in the endocrine pancreas has been demonstrated in experimental models. Noc2 expression together with other regulatory molecules of the exocytotic machinery in human tissues, however, has not been studied. We evaluated immunohistochemical expression of the key molecules of the exocytotic machinery, Noc2, Rab3A, Rab27A, and RIM2, together with the characteristic islet cell hormones, insulin and glucagon in normal and endocrine tumor tissues of human pancreas. Normal pancreatic islets were stained for all of these proteins and showed strong cytoplasmic localization. A similar pattern of strong cytoplasmic expression of these proteins was observed in the majority of endocrine tumors. By contrast, the exocrine portions of normal appearing pancreas completely lacked Rab27A staining and showed decreased expression of the proteins, Noc2, Rab3A, and RIM2. The staining pattern of Noc2 and Rab27A was similar to the staining pattern of glucagon-producing cells within the islets. The concomitant expression of Noc2 with these molecules suggests that Noc2 may serve as an effector for Rab3A and Rab27A and that it is involved in the regulation of exocytosis of the endocrine pancreas in humans.
Keywords: endocrine tumor, endocrine pancreas, Noc2, Rab3A, Rab27A, RIM2
Exocytosis is important for the primary function of several tissues including exocrine and endocrine glands. It involves a two-step process: docking of secretory granules at specific sites on the plasma membrane, and subsequent fusion of the granules with the plasma membrane and extracellular release of their contents. GTP-binding proteins are involved in the exocytotic process. A number of proteins that modulate GTP-binding proteins during exocytosis have been identified (Watson 1999).
Rab (Ras super family) proteins regulate defined steps of intracellular membrane trafficking including endocytosis and exocytosis (Zerial and Stenmark 1993). The correct targeting of vesicles to the appropriate acceptor membrane necessitates interaction with specific effectors, such as modulation of the GTP-bound form of Rab3A (ras-associated binding protein 3A) by rabphilin-3 isoforms (Bourne 1988).
Rabphilin-3A-like (RPH3AL), or Noc2, a 38 kDa protein, was identified by Kotake et al. (1997) in a mouse pancreatic beta-cell line, MIN6. It consists of 302 amino acids and has 40.7% amino acid identity and 77.9% similarity to the N-terminal Rab3A-binding region (amino acids 1–304) of rabphilin-3A, but lacks the two carboxy domains; thus, RPH3AL is also referred to as Noc2 (no 2 carboxy domains). The Noc2 protein is expressed abundantly not only in pancreatic β cells, but also in other endocrine cells (Kotake et al. 1997). In addition, it has been reported that alterations of the expression levels of Noc2 profoundly impairs β cell exocytosis, which suggests its involvement in the regulation of insulin exocytosis (Cheviet et al. 2004, Abderrahmani et al. 2006). In addition, Noc2 interacts preferentially with Rab27A rather than with Rab3A in vitro (Cheviet et al. 2004, Fukuda et al. 2004). Rab3A is expressed in neuroendocrine and neuronal cells and in insulin secreting cell lines, and is present in high levels in pancreatic β cells (Lankat-Buttgereit et al. 1992, Regazzi et al. 1992, Kowluru et al. 1994, Lankat-Buttgereit et al. 1994, Regazzi et al. 1996). Despite the presence of large amounts of Rab3A in pancreatic β cells, its effectors, rabphillin-3 isoforms, cannot be detected in endocrine cells (Regazzi et al. 1996). Thus, the role of Rab3A in insulin secretion is uncertain because direct evidence for the involvement of Rab3A in the control of insulin release is lacking.
Rab27A, which is highly and specifically expressed in pancreatic islets and pituitary tissues, is localized on the membranes of insulin granules (Yi et al. 2002). Rab27A overexpression causes enhancement of high K+-induced insulin secretion (Yi et al. 2002). Rab3-interacting molecule (RIM), another putative effector of Rab3A, has been detected in brain and pancreatic β cells as well as in the pancreas-derived cell lines, INS-1E and HIT-T15 (Wang et al. 1997, Hata et al. 1998). Transfection of INS-1E cells with the Rab3-binding domain of RIM enhanced glucose–and Ca2+-stimulated exocytosis. Co-expression of Rab3A reversed the effect of RIM, indicating the importance of RIM in the control of insulin secretion (Hata et al. 1998).
We investigated the immunohistochemical (IHC) expression patterns of Noc2, Rab3A, Rab27A, and RIM2 proteins in normal and endocrine tumor tissues of human pancreas. The primary objective of our preliminary study was to assess whether these proteins correspond to the characteristic pancreatic islet cell hormones, insulin and glucagon. A secondary aim of this report is to make it known that the anti-human Noc2 antibody we developed is available for use in studies directed toward understanding the pathobiology of different pancreatic diseases.
Materials and methods
Tissues
We randomly picked formalin fixed paraffin embedded archival tissue blocks of seven pancreatic endocrine tumors from the files of the UAB-Surgical Pathology Section that also had adjacent normal appearing pancreatic tissue. All of these tumors were incidentalomas (clinically asymptomatic pancreatic endocrine tumors detected incidentally upon routine investigation). Hematoxylin and eosin stained slides of all cases were reviewed by three pathologists (CKS, NCJ, WEG) to select the representative blocks to be used for IHC. This study was approved by UAB-Institutional Review Board.
Rabphilin-3A-like (Noc2/RPH3AL) antibody development
Polyclonal antisera were raised by immunizing rabbits with Noc2 specific peptide 1 (TPGRAD DPHFRPLPC) and peptide 2 (DRLPSTGVRDRK GDC) (GenScript Corp., Piscataway, NJ). Noc2 polyclonal antibody was affinity purified using protein-A-agarose and specificity of the antibody was determined by enzyme-linked immunosorbent assay (ELISA) and dot blot analysis.
Enzyme-linked immunosorbent assay (ELISA)
One hundred microliters of diluted antigens (peptide 1, peptide 2), 4 μg/ml, was added to each microplate well and allowed to stand overnight at 4°C. After removing coating solution, plates were washed three times with phosphate buffered saline with 0.05% Tween 20 (PBST) and 200 μl of blocking solution was added to the wells and incubated at 37°C for 30 min. The blocking solution was removed and plates were washed three times with PBST. To the antigen coated wells, 100 μl of each dilution of purified antibody in diluting buffer (1% bovine serum albumin (BSA) in PBST) was added. The plate was covered with adhesive plastic and incubated for 1 h at 37°C. After washing three times with PBST, 100 μl of diluted secondary antibody (goat anti-rabbit IgG, horseradish peroxidase (HRP) conjugation (Abcam Inc, Cambridge, MA) was added to each well and incubated for 30 min at 37°C. The plates were washed five times with PBST and developed using tetramethyl benzidine (TMB) substrate solution (GenScript Corp.) by incubating for 30 min at room temperature. To stop the reaction, 100 μl of 2 N HCl was added to the wells. Optical density was measured at 405 nm in a multiwell plate reader (BioTek Synergy2, Winooski, VT).
Dot blot analysis
Synthetic peptides 1 and 2 specific for Noc2 and purified Noc2 from stably transfected LoVo cells with Noc2 were spotted onto an Optitran nitrocellulose membrane (Bio-Rad Labs, Hercules, CA) and dried at 50°C for 20 min. After blocking in blocking buffer (3% BSA and 0.05% Tween 20 in PBS) for 1 h at room temperature with gentle agitation, the blot was incubated with anti-human Noc2 polyclonal antibody raised in rabbit (1:10,000) and washed three times with PBS buffer. The blot was incubated with HRP-conjugated anti-rabbit IgG antibody (1:10,000) (Abcam Inc.) for 1 h and washed three times with PBS buffer. Detection was carried out using the enhanced chemiluminescence (ECL™) detection kit (Amersham Biosciences, Piscataway, NJ).
Immunohistochemical (IHC) analysis
Serial sections 5 μm thick were cut from the representative paraffin blocks and mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Tissue sections were cut 1–2 days prior to immunostaining to avoid potential problems with antigen recognition due to storage degradation of cut tissue sections on glass slides (Jacobs et al. 1996). Immunostaining was performed as described in our earlier studies of antigen expression in different tissues (Myers et al. 1994, Manne et al. 1997). Briefly, the sections were melted at 60°C for 1.5 h, then incubated overnight at 37°C prior to staining. The tissue sections were deparaffinized in xylene and subsequently rehydrated through graded alcohols. The sections were then transferred to a Tris-buffer bath (0.05 M Tris base, 0.15 M NaCl and 0.01% Triton X-100, pH 7.6). Based on preliminary evaluations, heat-induced antigen retrieval (AR) was performed on the sections using a pressure cooker for 10 min with EDTA buffer 0.01 M at pH 9 (2.1 g EDTA, 1000 ml H2O, adjusting pH with NaOH) for Noc2 and Rab27A, and with citrate buffer (0.01 M at pH 6, 2.1 g citric acid monohydrate, 1000 ml H2O, adjusting pH with NaOH) for glucagon. AR was not performed for tissue sections stained for Rab3A, RIM2, and insulin, however, because in our preliminary analyses, AR produced no difference. Each section was treated with an aqueous solution of 3% H2O2 for 5 min to quench endogenous peroxidase activity. Each section then was incubated with 3% goat serum at room temperature for 1 h to reduce non-specific immunostaining. Tissue sections were then incubated with anti-human Noc2/RPH3AL (rabbit polyclonal; dilution 5 μg/ml, developed by our laboratory), anti-human Rab3A mouse monoclonal (clone 4H7; dilution 1 μg/ml; Novus Biologicals, Littleton, CO), anti-human Rab27A mouse monoclonal (clone 1G7; dilution 1 μg/ml; Novus Biologicals), anti-human RIM2 mouse monoclonal (clone 3C12; dilution 1 μg/ml; Novus Biologicals), anti-human glucagon rabbit polyclonal (clone Ab-1; dilution 1:100; NeoMarkers, Fremont, CA), and anti-human insulin mouse monoclonal (clone Ab-6; dilution 1:800; NeoMarkers) primary antibodies. Sections on which the primary antibody was not applied served as negative controls.
Secondary detection was accomplished using a multi-species detection system (Signet Lab Inc., Dedham, MA). Sections were incubated in biotinylated multi-species antibodies including anti-mouse antibodies for 20 min, then incubated with peroxidase-labeled streptavidin for 20 min (Signet Lab Inc.). A diaminobenzidine tetrachloride super sensitive substrate kit (BioGenex, San Ramon, CA) was used to visualize the antibody–antigen complex. Each section was then counterstained using hematoxylin, dehydrated through graded alcohols, and soaked in xylene before coverslipping.
Immunostaining evaluation
The stained slides were systematically evaluated by three pathologists (CKS, NCJ, WEG) together for the expression status as well as the cellular localization patterns of each protein and their correlations. The extent of staining was graded on a scale of 0–3+. Weak, moderate, and strong staining levels were recorded as 1+, 2+ and 3+, respectively. Lack of staining was scored 0. All negative control slides (deletes) were negative for staining.
Results
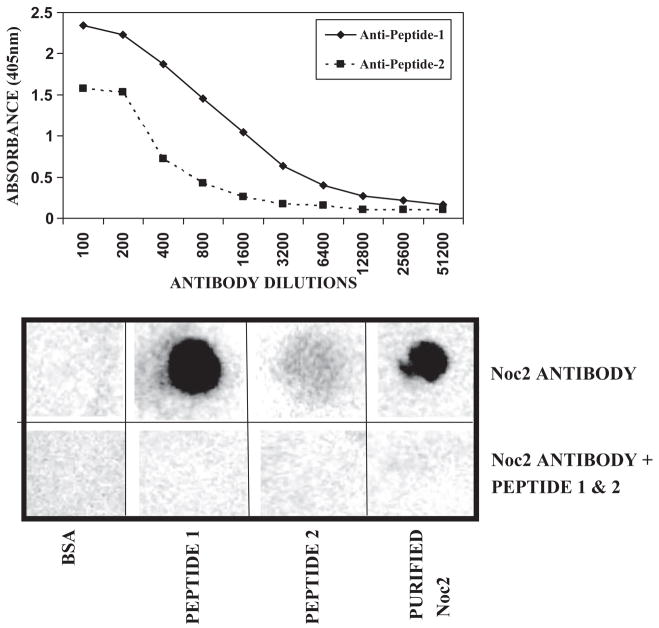
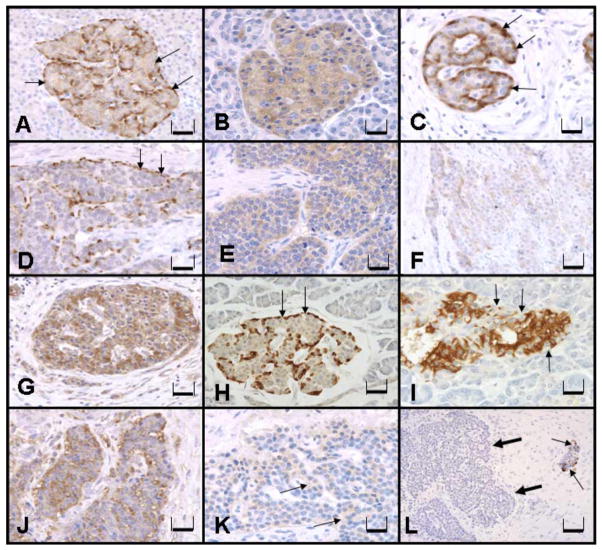
The specificity of the anti-human Noc2 (RPH3AL) antibody was confirmed by ELISA and dot blot techniques (Fig. 1A,B). All normal appearing pancreatic islets were stained at a moderate level (2+) for Noc2, Rab3A, Rab27A, and RIM2 with cytoplasmic localization (Fig. 2). Weak immunostaining for Noc2, Rab3A, and RIM2 was also observed in the exocrine portion of the pancreas (ducts and acini), but more prominent staining was consistently present in the islets compared to exocrine portions of normal appearing pancreas (Fig. 2A,B,G). Rab27A staining was observed in the islets of normal pancreas, but not in the exocrine portions (Fig. 2C). Five of seven endocrine tumors stained for all of these proteins; two did not stain for any (Table 1). Staining for glucagon was observed in only one of seven endocrine tumors (Case 6, Table 1; Fig. 2K). None of these tumors showed staining for insulin (Fig. 2L).
Fig. 1.
Specificity assay for anti-human Noc2 polyclonal antibody, ELISA (A) and dot blot (B) techniques; Noc2 antibody+Peptide-1 & Peptide-2 = Noc2 antibody pre-incubated with Peptide-1 and Peptide-2 at 37°C for 30 min. Peptide-1 is more immunogenic than peptide-2.
Fig. 2.
Immunohistochemical expression of Noc2, Rab3A, Rab27A, and RIM2 in human normal endocrine pancreas and endocrine tumors. (A) Normal islet exhibiting weak to moderate cytoplasmic Noc2 staining with peripheral accentuation (arrows), Bar = 400 μm. (B) Normal islet exhibiting Rab3A (diffuse cytoplasmic) staining, Bar = 400 μm. (C) Normal islet exhibiting weak to moderate cytoplasmic Rab27A staining, Bar = 200 μm. Peripheral islet cells (arrows) exhibit strong immunostaining similar to Noc2 staining in (A). (D) Endocrine tumor demonstrating Noc2 diffuse cytoplasmic staining with peripheral accentuation (arrows) similar to that seen in the normal islet in (A), Bar = 400 μm. (E) Endocrine tumor with diffuse cytoplasmic Rab3A expression, Bar = 400 μm. (F) Endocrine tumor with diffuse cytoplasmic Rab27A expression, Bar = 200 μm. (G) Normal islet exhibiting moderate RIM2 staining in the cytoplasm, Bar = 400 μm. (H) Normal islet exhibiting strong cytoplasmic staining for glucagon in the peripheral cells (arrows), Bar = 400 μm. (I) Normal islet exhibiting strong cytoplasmic staining for insulin within cells in the central area of the islet. The peripheral islet cells are devoid of stain (arrows), Bar = 400 μm. (J) Endocrine tumor exhibiting weak to moderate cytoplasmic RIM2 expression, Bar = 400 μm. (K) Endocrine tumor of the same case as in (D) exhibiting weak, but diffuse, cytoplasmic staining for glucagon (arrow), Bar = 400 μm. (L) Endocrine tumor of the same case as in (D) and (K) exhibiting no staining for insulin (thick arrows). The adjacent normal islet cells stain weakly to moderately for insulin (thin arrow), Bar = 200 μm.
Table 1.
Expression of Rab3A, Noc2, RIM2 and Rab27A together with glucagon and insulin in pancreatic endocrine tumors.
| Case number | Rab3A | Noc2 | RIM2 | Rab27A | Glucagon | Insulin |
|---|---|---|---|---|---|---|
| Case 1 | 2+ | 2+ | 2+ | 2+ | 0 | 0 |
| Case 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Case 3 | 2+ | 2+ | 2+ | 2+ | 0 | 0 |
| Case 4 | 2+ | 2+ | 2+ | 2+ | 0 | 0 |
| Case 5 | 2+ | 2+ | 2+ | 2+ | 0 | 0 |
| Case 6 | 2+ | 2+ | 2+ | 2+ | 1+ | 0 |
| Case 7 | 0 | 0 | 0 | 0 | 0 | 0 |
Note: Semiquantitative staining intensities were evaluated using following scores: negative, 0; weakly positive, 1+; moderately positive, 2+; strongly positive, 3+.
Noc2 immunostaining was observed throughout the exocrine and endocrine pancreas (Fig. 2A). However, stronger diffuse cytoplasmic staining was observed in the islets. Within normal islets, the peripherally located islet cells showed strong immunostaining compared to cells in remainder of the islet, resembling the pattern of cells that produce glucagon (Fig. 2H). Insulin secreting cells were localized throughout the central portions of the islets as typically described (Fig. 2I). These cells also showed diffuse cytoplasmic staining of varying intensity with Noc2, although not as intense as the cells located in the periphery of the islet. Five of seven endocrine tumors were stained for Noc2 with a diffuse cytoplasmic localization, but strong peripheral staining (Fig. 2D).
The immunostaining of Rab3A (Fig. 2B) and RIM2 (Fig. 2G) followed the same pattern as Noc2 in both normal pancreas and endocrine tumors, but without the peripheral accentuation. Comparison with insulin staining revealed that RIM2 was expressed in all islet cells, even those negative for insulin (Fig. 2G,I).
Rab27A staining was observed in islets of normal appearing pancreas, but not in the exocrine portion. Like Noc2, Rab27A showed diffuse cytoplasmic localization with peripheral cells in the islets exhibiting stronger staining (Fig. 2C). Five of seven endocrine tumors that stained for other proteins also stained for Rab27A without peripheral accentuation (Fig. 2F).
Discussion
To our knowledge, this is the first descriptive report on the collective IHC expression of Noc2, Rab3A, Rab27A, and RIM2 proteins in human pancreatic islets and endocrine tumor tissues. We have demonstrated that in the islets of normal human pancreas, these proteins were expressed in the cytoplasm. The exocrine and endocrine pancreas both demonstrated immunostaining for all of these proteins except Rab27A, which was found only in the islets, not in the exocrine pancreas. Noc2 staining was more prominent in the islets; within the islets, the peripheral cells demonstrated stronger immunostaining, resembling the pattern of cells that secrete glucagon. The immunostaining patterns of Rab3A and Rab27A were consistent with Noc2 in normal pancreatic islets and in the islet cells of tumors. These results suggest that these proteins are interrelated and may be involved collectively in the regulation of exocytosis in human endocrine pancreas.
Noc2 interaction with Rab3A has been implicated in vesicle transport and Ca2+-dependent exocytosis, and in the secretion of neurotransmitters (Geppert et al. 1994). Although an initial study on Noc2 failed to demonstrate interaction between Noc2 and Rab3A (Kotake et al. 1997), subsequent studies with COS-1 cells demonstrated that Noc2 binds to all of the Rab3 isoforms (Rab3A–D) in a GTP-dependent manner (Matsumoto et al. 2004), and specifically to Rab3A in PC12 cells (Haynes et al. 2001). On the other hand, Noc2 interacts preferentially with Rab27A, rather than with Rab3A in vitro (Cheviet et al. 2004, Fukuda et al. 2004). These inconsistent findings concerning the relation between Noc2 and Rab proteins may indicate that each cell type possesses different sets of Rab and effector proteins. Thus, a single Rab protein may interact with multiple effector proteins resulting in different patterns of expression based on the cell type (Fukuda 2003).
Rab3A is expressed significantly in human insulinomas and rat insulin-releasing insulinoma-derived RIN-cells, but not in normal islets of the rat (Lankat-Buttgereit et al. 1992, 1994). By contrast, our study revealed strong cytoplasmic expression of Rab3A in both human normal pancreatic islets and exocrine glands, although the intensity of staining was stronger in the normal islets compared to the exocrine pancreas. However, we did not find appreciable differential expression of Rab3A between normal pancreatic islets and endocrine tumors. Regazzi (1996) and Lankat-Buttgereit and co-workers (1994) were unable to detect expression of Rab3A or rabphilin-3A in human pancreatic cells by Western blotting and/or by the GTP-overlay technique. By contrast, we demonstrated expression of Rab3A, Rab27A, Noc2, and RIM2 in the human endocrine pancreas using immunohistochemistry.
In our study, both Noc2 and Rab27A immunostaining demonstrated moderate cytoplasmic staining intensity, with the peripheral cells of the islets staining much stronger than the other islet cells and resembling the pattern of staining exhibited by cells secreting glucagon. This finding agrees with a study of mouse tissues in which double staining with anti-Noc2 and either the anti-glucagon or anti-insulin antibody revealed intense staining of cells located at the periphery of islets corresponding to the glucagon secreting cells (Teramae et al. 2007). This finding suggests a possible role for Noc2 and Rab27A in regulation of exocytosis of pancreatic hormones, specifically glucagon. These observations are strengthened further by the finding that transfection of the Noc2 gene rescues impaired insulin secretion in cultured pancreatic islets of Noc2−/− mice, while the transfer of a mutant Noc2 gene without the capacity to bind Rab3 isoforms does not (Matsumoto et al. 2004). In addition, even at low concentration, Noc2 might be required for the final step in the release of secretory products (Teramae et al. 2007). To the contrary, our study also demonstrated Noc2 immunoreactivity in the exocrine portions, but with low intensity compared to the islets. The variability of Noc2 immunostaining within the islets may be attributed to the possibility that the content of Noc2 in cells changes during the secretory process and that the difference in expression level is associated with the secretory phase of endocrine cells.
In the pancreatic β cells of rat and INS-1E cells, RIM2 is localized to the plasma membrane (Hata et al. 1998, Iezzi et al. 2000). We observed a similar distribution for RIM2 in our study, with uniform, moderate staining of entire islets. This localization, the enhanced stimulation of exocytosis caused by transfection of INS-1E cells with the Rab3 binding domain of RIM2, and the reversal of the effect of RIM2 on exocytosis by co-expression of Rab3A indicate that RIM2 functions in insulin exocytosis as an effector for Rab3A (Hata et al. 1998, Iezzi et al. 2000). Rab27A is expressed in pancreatic islets and pituitary tissues, and is localized on the membranes of insulin granules (Yi et al. 2002). Rab27A overexpression causes enhancement of high K+-induced insulin secretion (Yi et al. 2002). Formation of the Rab27A/granuphilin complex is readily detected in the pancreatic β cell line, MIN6, suggesting that granuphilin may function as a Rab27A effector in pancreatic β cells. We were unable to stain for granuphilin expression, however, because an antibody for it was not available commercially. Cytoplasmic distribution and peripheral accentuation of Rab27A immunostaining in the islets of the normal appearing pancreas was similar to Noc2. It has been reported that mutations in Noc2 prevent the binding of Rab3A and Rab27A and alter the subcellular localization of Noc2, implying that Noc2 is recruited on the secretory granules by interacting with these proteins (Cheviet et al. 2004). Selective impairment of Rab3A binding did not affect Noc2 distribution, suggesting that Rab27A functions through Noc2 in pancreatic β cells (Cheviet et al. 2004).
Although most of the earlier studies focused on elucidating the role of Noc2 in insulin secretion, our study, based on similar staining pattern of Noc2 and Rab27A with that of glucagon-producing cells, implies that Noc2 may act as a mediator in the regulation of glucagon secretion. This finding must be confirmed by future studies.
Noc2, Rab3A, Rab27A, and RIM2 are expressed in the islets of normal human pancreas and can be detected by IHC. The concomitant expression of Noc2 with these molecules, as reported in earlier experimental in vitro and in vivo studies, may be involved in regulation of exocytosis of the endocrine pancreas in humans. Further studies must be performed to understand more completely the role of each protein and its interactions. This information would aid our understanding of the regulated exocytotic process of various hormones and also delineate the clinical implications of the expression of these proteins in various pancreatic disease states including diabetes and endocrine tumors.
Acknowledgments
We thank the Tissue Procurement Facility of UAB-Comprehensive Cancer Center for the histology services. Also, we thank Dr. Donald L. Hill, of Department of Preventive Medicine of UAB for his critical review of this manuscript. This work is supported by grants from the National Institute of Health/National Cancer Institute (RO1-CA98932-01, U54-CA118948, U24-CA086359, and the Pancreatic SPORE at UAB-P20CA101955-05) and funds from the Department of Pathology, UAB.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Abderrahmani A, Cheviet S, Ferdaoussi M, Coppola T, Waeber G, Regazzi R. ICER induced by hyperglycemia represses the expression of genes essential for insulin exocytosis. Embo J. 2006;25:977–986. doi: 10.1038/sj.emboj.7601008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR. Do GTPases direct membrane traffic in secretion? Cell. 1988;53:669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R. The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol. 2004;18:117–126. doi: 10.1210/me.2003-0300. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem. 2004;279:13065–13075. doi: 10.1074/jbc.M306812200. [DOI] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Hata Y, Nakanishi H, Takai Y. Synaptic PDZ domain-containing proteins. Neurosci Res. 1998;32:1–7. doi: 10.1016/s0168-0102(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Evans GJ, Morgan A, Burgoyne RD. A direct inhibitory role for the Rab3-specific effector, Noc2, in Ca2+-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2001;276:9726–9732. doi: 10.1074/jbc.M006959200. [DOI] [PubMed] [Google Scholar]
- Iezzi M, Regazzi R, Wollheim CB. The Rab3-interacting molecule RIM is expressed in pancreatic beta-cells and is implicated in insulin exocytosis. FEBS Lett. 2000;474:66–70. doi: 10.1016/s0014-5793(00)01572-6. [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88:1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- Kotake K, Ozaki N, Mizuta M, Sekiya S, Inagaki N, Seino S. Noc2, a putative zinc finger protein involved in exocytosis in endocrine cells. J Biol Chem. 1997;272:29407–29410. doi: 10.1074/jbc.272.47.29407. [DOI] [PubMed] [Google Scholar]
- Kowluru A, Rabaglia ME, Muse KE, Metz SA. Subcellular localization and kinetic characterization of guanine nucleotide binding proteins in normal rat and human pancreatic islets and transformed beta cells. Biochim Biophys Acta. 1994;1222:348–359. doi: 10.1016/0167-4889(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Fehmann HC, Hering BJ, Bretzel RG, Goke B. Expression of the ras-related rab3a gene in human insulinomas and normal human pancreatic islets. Pancreas. 1994;9:434–438. doi: 10.1097/00006676-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Goke R, Fehmann HC, Niess C, Goke B. Expression of the ras-related rab3a gene in insulinoma-derived cell lines. FEBS Lett. 1992;312:183–186. doi: 10.1016/0014-5793(92)80931-6. [DOI] [PubMed] [Google Scholar]
- Manne U, Myers RB, Moron C, Poczatek RB, Dillard S, Weiss H, Brown D, Srivastava S, Grizzle WE. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer. 1997;74:346–358. doi: 10.1002/(sici)1097-0215(19970620)74:3<346::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Miki T, Shibasaki T, Kawaguchi M, Shinozaki H, Nio J, Saraya A, Koseki H, Miyazaki M, Iwanaga T, Seino S. Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc Natl Acad Sci USA. 2004;101:8313–8318. doi: 10.1073/pnas.0306709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160erbB-3 and p185erbB-2 in prostatic intraepithelial neoplasia and prostatic adeno-carcinoma. J Natl Cancer Inst. 1994;86:1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Kikuchi A, Takai Y, Wollheim CB. The small GTP-binding proteins in the cytosol of insulin-secreting cells are complexed to GDP dissociation inhibitor proteins. J Biol Chem. 1992;267:17512–17519. [PubMed] [Google Scholar]
- Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y, Wollheim CB. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996;109:2265–2273. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- Teramae H, Fujimoto W, Seino S, Iwanaga T. Cellular expression of Noc2, a Rab effector protein, in endocrine and exocrine tissues in the mouse. Histochem Cell Biol. 2007;127:1–11. doi: 10.1007/s00418-006-0207-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Watson EL. GTP-binding proteins and regulated exocytosis. Crit Rev Oral Biol Med. 1999;10:284–306. doi: 10.1177/10454411990100030301. [DOI] [PubMed] [Google Scholar]
- Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22:1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]