Abstract
Although decreased or lack of expression of Bcl-2 has been correlated with advanced tumor stage and shortened patient survival in colorectal cancer (CRC), its value in predicting the recurrence has not been well explored. Therefore, we assessed the usefulness of phenotypic expression of Bcl-2 in non-Hispanic Caucasian patients with CRCs in identifying risk of recurrence. Archival tissues of 92 Stage II and 66 Stage III primary CRCs were evaluated for immunohistochemical expression of Bcl-2. None of these patients received either pre- or post-surgical adjuvant therapies. Kaplan-Meier and Cox proportional hazards methods were used to estimate the rates of recurrence and survival according to Bcl-2 expression. Decreased expression of Bcl-2 was associated with an increased rate of recurrence in patients with Stage II CRCs (5-year log-rank test P = 0.0015; Hazard-Ratio (HR) = 3.90, 95%C.I.: 1.55–9.77) but not with Stage III CRCs (5-year log-rank test P =0.6058; HR =1.07, 95%C.I.: 0.47–2.45) after adjusting for other demographic and clinicopathological features. Furthermore, decreased expression of Bcl-2 was an indicator of short survival in patients with Stage II CRCs but not with Stage III CRCs. Thus, decreased or lack of Bcl-2 expression in primary CRCs may serve as a molecular biomarker of high risk of recurrence for Caucasian patients with Stage II CRCs. These findings might be useful in identifying biologically aggressive phenotypes of Stage II CRCs, and may aid the oncologist in designing maximally appropriate therapeutic regimens.
Keywords: Colorectal adenocarcinoma, Bcl-2 expression, tumor stage, Caucasians, recurrence, prognosis
1. Introduction
Colorectal adenocarcinoma (CRC) is the third leading cause of cancer associated deaths in both men and women in the United States [1]. One third of CRC patients without histological evidence of lymph node involvement die within five years of surgery from distant metastasis or local recurrence. Regional lymph node metastasis is one of the most powerful indicators of aggressiveness of CRCs and aids in designing therapeutic treatments and in predicting the clinical outcome. However, nodal status alone may not predict the clinical course of CRC adequately, as groups of patients with tumors of identical stage have different treatment responses and clinical outcomes.
Traditional clinicopathological features together with molecular markers could potentially be helpful in identifying aggressive phenotypes within Stage II and III tumors and facilitate better selection of high risk patients to maximize the benefits of adjuvant therapy. Moreover, identification of such novel markers is particularly relevant in Stage II and III tumors because these stages together represent approximately 70% of CRC patients, and about 20–40% of patients with Stage II tumors will develop recurrent disease and die due to CRC [2]. However, the role of adjuvant chemotherapy in this setting is still unclear [3–5].
Multiple molecular abnormalities, including abnormal expression of the anti-apoptotic protein Bcl-2, have been implicated in human carcinogenesis. Bcl-2 onco-protein also plays a vital role in tumor progression [6,7]. Thus, the clinical implications of abnormal expression of Bcl-2 have been extensively studied in several human malignancies including CRC [8–11]; and been reported to have different biological consequences based on tumor stage [8,9,12]. Moreover, recent studies of prognostic biomarker of CRC have recommended that tumor location, tumor stage, and patient ethnicity or race be considered in the evaluation of the predictive value of molecular makers [9,10,13–17]. However, the role of Bcl-2 expression in predicting the risk of recurrence has not been studied extensively in relation to tumor stage particularly in United States.
Medical oncologists are increasingly interested in identifying and utilizing reliable factors which determine disease recurrence and patient survival to identify subsets of patients with aggressive tumors and to optimize their therapies. Therefore, in this study, we evaluated the usefulness of phenotypic expression of Bcl-2 in Stage II and III primary CRCs collected from non-Hispanic Caucasian patients in predicting their disease recurrence and survival.
2. Materials and methods
2.1. Patients
The institutional review boards of the University of Alabama at Birmingham (UAB) and its affiliated Birmingham Veterans Affairs (VA) Hospital approved this study. We identified a total of 620 non-Hispanic Caucasian patients from the UAB and VA Hospital tumor registries who had undergone surgical resection for first primary CRC from 1981 through 1993. We obtained the medical records including surgical pathology reports of these patients which were reviewed by two of the authors (CC, UM) to ascertain the key information. During our initial selection process, those patients who died within a week of their surgery, those who were shown to have Stage I or IV tumors at the time of surgery, those patients whose archival tissues were not available, those patients with surgical margin-involvement, or unspecified tumor location, or multiple primaries within the colorectum, or multiple malignancies, or those patients with family or personal history of CRC were all excluded from our study population. However, based on the information in patient charts, it will be difficult to identify the familial vs. sporadic nature of the tumors, therefore, our patient populations can be described as ‘consecutive’ populations of patients with Stages II, and III CRCs. To control for treatment bias, we included only those patients who underwent surgery as a therapeutic intervention and excluded patients who received any pre - or post surgical therapies. Since the use of adjuvant chemotherapy was not widespread during this study’s time frame (1981–1993), we could obtain such a large number of Stage III patients who have not received adjuvant therapy. The final sample size (n = 158) used for the current analysis consisted 92 Stage II & 66 Stage III non-Hispanic Caucasian patients. The patient cohort from both the hospitals was under the care of a uniform group of physicians. All the authors were blinded for the diagnosis as well as patient follow-up information.
In our study, two pathologists (NJ, WEG) reviewed hematoxylin and eosin stained slides of all cases for the degree of histologic differentiation and graded as well, moderate, poor or undifferentiated. Subsequently, we pooled well and moderately differentiated tumors into a low grade group and poor and undifferentiated tumors into a high grade group [18]. The pathologic staging was performed according to the criteria of the American Joint Commission on Cancer [19]. The International Classification of Diseases for Oncology (ICD-O) codes was used to specify anatomic location of the tumor [20]. The anatomic sub-sites were grouped into the colon and the rectum.
Patients were followed by the UAB and VA tumor registries until their death or the date of the last documented contact within the study time frame. The tumor registries ascertain outcome (mortality) information directly from patients (or living relatives) and from the physicians of the patients through telephone and mail contacts. This information is further validated against State Death Lists. The tumor registries update follow-up information every six months and follow-up of our cohort ended in March 2004. The median follow-up period of the complete study population of 158 patients was 7.31 years (range < 1 – > 20 years).
2.2. Immunohistochemical staining
Formalin-fixed paraffin-embedded tissue blocks representative of normal mucosa and invasive adenocarcinoma were sectioned at a 5-μm thickness and mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Two tissue sections (one with and one without antigen retrieval treatment) were incubated with pre-immune rabbit serum as a negative control to test for non-specific staining and reactivity of the secondary detection system with the tissue. The tissues sections were subjected to citrate buffer-microwave antigen retrieval and were probed with anti-human Bcl-2 monoclonal antibodies (clone 124, 1:80 dilution, Cambridge Laboratories, Cambridge, UK) and were incubated for 1 hr at room temperature. BioGenex Super Sensitive Biotin-Streptavidin Horseradish Peroxidase Detection Kit was used as the secondary detection system. Sections were incubated with biotinylated goat anti-mouse and streptavidin peroxidase for 20 min each with TBS washes between steps. For visualization of the antibody-antigen complex, sections were incubated with the diaminobenzidine tetrachloride (DAB) Super Sensitive Substrate Kit (BioGenex) for 7 min. Sections were counter-stained with methyl green stain (0.5% methyl green in 0.1 M sodium acetate, pH 4.0). Staining in lymphocytes in uninvolved colonic epithelium or within the invasive lesion served as internal positive controls for the expression of Bcl-2. For negative controls, the primary antibody was omitted [8].
After being deparaffinized and rehydrated, tissue sections were placed in a Coplin jar of microwave compatible plastic that was filled with 0.01 M citric acid antigen retrieval solution (0.01 M citric acid, 1 N NaOH, pH 6.0). The Coplin jars were then placed in a microwave oven (R-3A75, 850 W; Sharp Electronics, Mahwah, NJ) in the middle of a large container of preheated water (90°C) to ensure consistent heating and heated for 5 min at high power. The antigen retrieval fluid level was checked, the evaporated buffer replenished and the slides subjected to an additional heating cycle of 5 min. Slides were allowed to cool, rinsed with de-ionized water and placed in TBS. Tissue sections were then immunostained as described above. We have previously reported that Bcl-2 antigen is stable in the archival tissues used in our study [21].
2.3. Assessment of Bcl-2 staining
The staining assessment was performed by three authors (NJ, WEG, UM) jointly to minimize the bias and disagreement. The proportion of Bcl-2 expressing cells varied from 0 to 100% and the intensities of staining were null (0), weak(+1), mild (+2), moderate (+3) or strong (+4). Therefore, a semi-quantitative immunostaining (IS) score for Bcl-2 was obtained as described previously [8,9]. In brief, the percent of cells at each intensity was multiplied by the corresponding intensity value (0 to 4) to obtain an IS score that ranged from 0 to 4.0 by each examiner. The IS scores of each examiner were averaged to obtain an overall mean IS score for each case. If there was substantial disagreement in assessment, this was resolved by re-review before combining the individual scores. An IS score of ⩾ 0.5 was chosen as cut-off value to categorize tumors into either decreased or increased expressers of Bcl-2. This cut-off value was the median value of Bcl-2 expression in the benign colonic epithelium (average of away from and adjacent to invasive lesion).
2.4. Statistical Analysis
We used the χ2-test to compare baseline characteristics [22]. Recurrence of CRC (local recurrence or distant metastases) and deaths due to CRC were the outcomes (events) of interest. The predictive and prognostic significance of Bcl-2 was analyzed using Kaplan-Meier [23] and Cox proportional hazards regression analysis [24]. Demographic variables included in the analysis were age (< 65 & ⩾ 65 years) and gender. Pathological variables were included as pT (depth of tumor invasion), pN (nodal involvement), tumor differentiation (low or high grade), tumor size (≤ 5 cm and > 5 cm) in maximal dimension, and tumor location (colon or rectum). For recurrence analyses, the time at risk was measured by calculating the number of months from date of surgery to time of recurrence or date of last contact. Patients who had recurrence of their CRC were classified as “events”, while the remaining patients without recurrence, 1) who died due to their CRC or 2) died due to causes other than CRC or 3) who were alive at the end of the follow-up period were “right censored”. For survival analyses, the risk of CRC-specific death was measured by calculating the number of months from the date of surgery to death or date of last contact. Patients who died of a cause other than CRC or who were alive at the end of the follow-up period were “right censored”. The log-rank test was used to compare Kaplan-Meier curves based on the status of Bcl-2 expression. The Kaplan-Meier estimates were used to obtain recurrence or survival status at 5 and 10 year post surgery periods. Separate multivariate Cox regression models were built for patients based on tumor stage. We controlled for demographic and clinicopathological variables in all multivariate analyses. All analyses were performed with SAS statistical software version 9.0 [25,26]. Two-sided P values were calculated and significance was analyzed at an alpha level of 0.05.
3. Results
3.1. Demographic and clinicopathologic characteristics of the patient population
The patient characteristics and Bcl-2 expression status are reported in Table 1. Mean age at surgery of the study population was 63.95 years. Median survival was 78 months and median time to relapse after surgery was 60 months. There was a slight predominance of males in our study because the majority of patients treated at the Birmingham VA medical center were men.
Table 1.
Patient characteristics based on tumor stage
| Variable | Total sample size (n = 158) |
|
|---|---|---|
| Stage II (n = 92) | Stage III (n = 66) | |
| Age Group (years) | ||
| <65 | 43 (47%) | 30 (45%) |
| ⩾65 | 49 (53%) | 36 (55%) |
| Gender | ||
| Male | 62 (67%) | 40 (61%) |
| Female | 30 (33%) | 26 (39%) |
| pT component of stage (bowel wall invasion) | ||
| pT1 | – | 1 (2%) |
| pT2 | – | 12 (18%) |
| pT3 | 67 (73%) | 34 (51%) |
| pT4 | 25 (27%) | 19 (29%) |
| pN component of stage (nodal involvement) | ||
| N0 | 92 (100%) | – |
| N1 | – | 41 (62%) |
| N2–3 | – | 25 (38%) |
| Tumor location | ||
| Colon | 70 (76%) | 51 (77%) |
| Rectum | 22 (24%) | 15 (23%) |
| Tumor size (cms) | ||
| ≤5 | 47 (51%) | 46 (70%) |
| >5 | 45 (49%) | 20 (30%) |
| Tumor differentiation | ||
| Low grade | 82 (89%) | 50 (76%) |
| High grade | 10 (11%) | 16 (24%) |
| Bcl-2 expression | ||
| Low (ISS* < 0.5) | 35 (38%) | 34 (52%) |
| High (ISS ⩾ 0.5) | 57 (62%) | 32 (48%) |
| Vital status (at follow-up) | ||
| Alive | 37 (40%) | 18 (27%) |
| Colon Cancer** | 19 (21%) | 31 (47%) |
| Other*** | 36 (39%) | 17 (26%) |
ISS = Immuno staining score.
Dead due to colorectal cancer.
Death due to causes other than colorectal cancer or unknown.
3.2. Bcl-2 expression
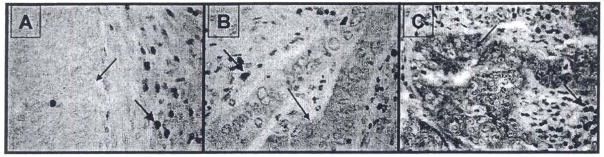
As shown in Fig. 1, varying intensities of Bcl-2 staining was observed in CRC. A strong Bcl-2 staining was observed in lymphocytes which served as internal positive controls. A higher proportion of Stage II CRCs (62%) were positive for Bcl-2 expression than Stage III CRCs (48%) (Table 1). Further analyses of tumor positivity based on other ISS cut-off values (in addition to 0.5 ISS) have suggested that 36% of Stage II and 22% of Stage III CRCs exhibited increased Bcl-2 expression at ⩾ 1.0 ISS; in contrary, only 13% of Stage II CRCs were completely negative for Bcl-2 expression (ISS = 0.0) as compared to 22% of Stage in CRCs (data not shown). These analyses suggested that there was no significant difference in the incidence of Bcl-2 positivity between Stage II and Stage III CRCs using different cut-off values. However, there was an inverse association between Bcl-2 expression and regional lymph node metastasis.
Fig. 1.
Bcl-2 expression in primary colorectal adenocarcinomas. The staining intensity of Bcl-2 expression was from very weak (A), low (B) to strong (C). Bcl-2 expression was primarily in the cytoplasm (arrows with small heads) of the malignant cells. A strong Bcl-2 expression pattern was observed in lymphocytes (arrows with large heads).
3.3. CRC recurrence based on Bcl-2 expression and clinicopathologic parameters
CRC recurrence based on clinicopathological indicators, demographic features, and Bcl-2 expression status was analyzed according to tumor stage (Table 2). In Stage II CRC patients with decreased Bcl-2 expression were more likely to have recurrence than patients with increased Bcl-2 expression, where as there was no disparity in rate of recurrence in Stage III CRC patients. The incidence of recurrence in Stage II CRC patients increased significantly as the pT component of stage increased (Table 2).
Table 2.
Associations between clinicopathologic and molecular characteristics and CRC recurrence based on tumor stage
| Stage II |
Stage III |
|||||
|---|---|---|---|---|---|---|
| Variable | Number of Patients (%) |
χ2 P-value | Number of Patients (%) |
χ2 P-value | ||
| Without recurrence 68 (74%) | With recurrence 24 (26%) | Without recurrence 30 (45%) | With recurrence 36 (55%) | |||
| Age Group (years) | ||||||
| < 65 | 29 (43) | 14 (58) | 0.1854 | 15 (50) | 15 (42) | 0.4984 |
| ⩾ 65 | 39 (57) | 10 (42) | 15 (50) | 21 (58) | ||
| Gender | ||||||
| Male | 45 (66) | 17 (71) | 0.6757 | 21 (70) | 19 (53) | 0.1539 |
| Female | 23 (34) | 7 (29) | 9 (30) | 17 (47) | ||
| pT component of stage (bowel wall invasion) | ||||||
| pT1 | – | – | 0.0168 | 0 (0) | 1 (3) | 0.2893 |
| pT2 | – | – | 8 (27) | 4 (11) | ||
| pT3 | 54 (79) | 13 (54) | 13 (43) | 21 (58) | ||
| pT4 | 14 (21) | 11 (46) | 9 (30) | 10 (28) | ||
| pN component of stage (nodal involvement) | ||||||
| N0 | 68 (74%) | 24 (26%) | – | – | 0.7457 | |
| N1 | – | – | 18 (60) | 23 (64) | ||
| N2–3 | – | – | 12 (40) | 13 (36) | ||
| Tumor location | ||||||
| Colon | 51 (75) | 19 (79) | 0.6808 | 25 (83) | 26 (72) | 0.2835 |
| Rectum | 17 (25) | 5 (21) | 5 (17) | 10 (28) | ||
| Tumor size (cms) | ||||||
| ≤ 5 | 34 (50) | 13 (54) | 0.7255 | 21 (70) | 25 (69) | 0.9610 |
| > 5 | 34 (50) | 11 (46) | 9 (30) | 11 (31) | ||
| Tumor differentiation | ||||||
| Low grade | 59 (87) | 23 (96) | 0.2198 | 23 (77) | 27 (75) | 0.8750 |
| High grade | 9 (13) | 1 (4) | 7 (23) | 9 (25) | ||
| Bcl-2 expression | ||||||
| Low (ISS* < 0.5) | 19 (28) | 16 (67) | 0.0008 | 15 (50) | 19 (53) | 0.8221 |
| High (ISS ⩾ 0.5) | 49 (72) | 8 (33) | 15 (50) | 17 (47) | ||
| Vital status (at follow-up) | ||||||
| Alive | 33 (49) | 4 (17) | < 0.0001 | 15 (50) | 3 (8) | < 0.0001 |
| Colon Cancer** | 2 (2) | 17 (71) | 2 (7) | 29 (81) | ||
| Other*** | 33 (49) | 3 (12) | 13 (43) | 4 (11) | ||
ISS = Immuno staining score.
Dead due to colorectal cancer.
Death due to causes other than colorectal cancer or unknown.
3.4. Kaplan-Meier analyses for disease recurrence
Kaplan-Meier analyses demonstrated that the rate of recurrence was higher especially within 5 years after surgery in patients with Stage III CRCs compared to patients with Stage II CRCs (Fig. 2A). We analyzed the time to recurrence according to Bcl-2 expression in Stage II and Stage III patients separately. The association between the status of Bcl-2 expression and the rate of recurrence was significant only in patients with Stage II tumors (Fig. 2B), but not in patients with Stage III tumors (Fig. 2C). It was interesting to note that the low Bcl-2 expressers of Stage II CRCs exhibited a similar rate of recurrence as those of Stage III CRCs.
Fig. 2.
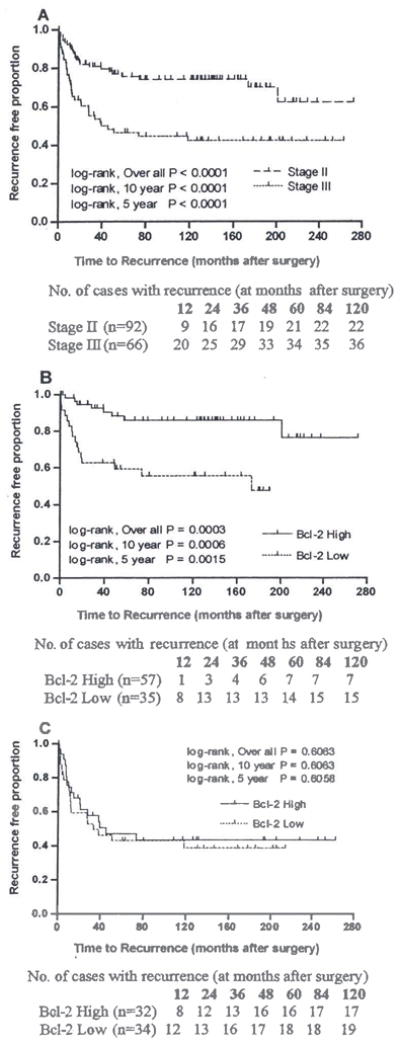
Kaplan-Meier curves and log-rank tests to assess the risk of recurrence based on the Bcl-2 expression status and tumor stage in Caucasians with CRCs: Significant difference in rate of recurrence was observed between stage II and stage III CRC cases (A). Deceased or lack of Bcl-2 expression was significantly associated with an increased rate of recurrence only in patients with stage II (B) but not in Stage III (C) CRCs.
3.5. Cox proportional hazard analyses for recurrence
Individual multivariate Cox regression models based on tumor stage have demonstrated, as observed in univariate analyses, that patients with II tumors with decreased Bcl-2 expression were 3.90 times more likely to have recurrence within 5 years after surgery when compared to the group of Stage II patients with tumors exhibiting increased expression of Bcl-2 (HR: 3.90, 95% CI: 1.55–9.77) after adjusting for other demographic and clinicopathological variables in study (Table 3). Patients with Stage II disease who suffered perforation into the visceral peritoneum (pT4) had a recurrence rate 2.84 times higher that of patients with Stage II tumors with pT3 stage (HR: 2.84, 95% CI: 1.15–7.00) within 5 years of surgery (Table 3). Patient age, gender, tumor size, and tumor location were not independent predictors of recurrence in patients with Stage II CRCs. In Stage III CRC patients none of the demographic, clinicopathological variables were independent predictors of recurrence along with Bcl-2 expression and depth of tumor invasion.
Table 3.
Multivariate Cox regression analyses to assess the value of Bcl-2 expression in predicting risk of recurrence in Caucasian patients with colorectal cancer
| Recurrence within 5 Years |
Recurrence within 10 Years |
|||||
|---|---|---|---|---|---|---|
| Variable | Crude HR | Adjusted* HR (95% CI) | P | Crude HR | Adjusted* HR (95% CI) | P |
| Stage II (n = 92) | ||||||
| Bcl-2 expression | ||||||
| Low vs. High | 3.91 | 3.90 (1.55, 9.77) | 0.0037 | 4.21 | 4.32 (1.74, 10.71) | 0.0016 |
| pT component of stage(bowel wall invasion) | ||||||
| pT4 vs. pT3 | 3.01 | 2.84(1.15, 7.00) | 0.0234 | 2.82 | 2.80 (1.15, 6.84) | 0.0237 |
| Age group | ||||||
| ⩾ 65 years vs. < 65 years | 0.67 | 0.64(0.26, 1.55) | 0.3203 | 0.74 | 0.70 (0.29, 1.65) | 0.4112 |
| Gender | ||||||
| Male vs. Female | 1.74 | 1.21(0.43, 3.43) | 0.7209 | 1.46 | 1.01 (0.38, 2.68) | 0.9903 |
| Tumor location | ||||||
| Rectum vs. Colon | 1.06 | 0.79 (0.27, 2.34) | 0.6755 | 1.01 | 0.81 (0.28, 2.38) | 0.7080 |
| Tumor differentiation | ||||||
| High grade vs. Low grade | 2.23 | 2.75 (0.72, 3.94) | 0.2876 | 2.14 | 2.32 (0.64, 3.34) | 0.3213 |
| Tumor size (cms) | ||||||
| > 5 vs. ≤ 5 | 0.73 | 0.70 (0.28, 1.76) | 0.4541 | 0.82 | 0.80 (0.33, 1.95) | 0.6263 |
| Stage III (n = 66) | ||||||
| Bcl-2 expression | ||||||
| Low vs. High | 1.19 | 1.07(0.47, 2.45) | 0.8704 | 1.19 | 1.04 (0.47, 2.30) | 0.9240 |
| pN component of stage (nodal involvement) | ||||||
| N2–3 vs. N1 | 1.53 | 1.77(0.76, 4.13) | 0.1833 | 1.38 | 1.66 (0.73, 3.77) | 0.2266 |
| pT component of stage (bowel wall invasion) | ||||||
| pT4 vs. pT3 | 1.16 | 0.75 (0.29, 1.96) | 0.5606 | 1.04 | 0.75 (0.30, 1.91) | 0.5528 |
| Age group | ||||||
| ⩾65 years vs. < 65 years | 1.41 | 1.30 (0.57, 2.95) | 0.5270 | 1.44 | 1.31 (0.59, 2.89) | 0.5031 |
| Gender | ||||||
| Male vs. Female | 0.56 | 0.46 (0.20, 1.08) | 0.0747 | 0.62 | 0.55 (0.24, 1.25) | 0.1521 |
| Tumor location | ||||||
| Rectum vs. Colon | 1.15 | 1.18 (0.41, 3.41) | 0.7570 | 1.25 | 1.29 (0.47, 3.50) | 0.6222 |
| Tumor differentiation | ||||||
| High grade vs. Low grade | 1.41 | 1.52 (0.61, 3.78) | 0.3711 | 1.33 | 1.36 (0.56, 3.32) | 0.5001 |
| Tumor size (cms) | ||||||
| > 5 vs. ≤ 5 | 1.53 | 1.36 (0.55, 3.34) | 0.5049 | 1.39 | 1.22 (0.51, 2.95) | 0.6574 |
Hazard ratios adjusted for Bcl-2 expression, depth of the tumor invasion into the bowel wall, number of lymph nodes involved, tumor differentiation, tumor size, tumor location, patient age, and gender.
3.6. Survival analyses
We also analyzed the association between CRC-specific mortality, expression of Bcl-2 and other confounding features based on tumor stage.
3.6.1. Univariate survival analyses
In the study population, 21 % (19 of 92) of Stage II, and 47% (31 of 66) of Stage III patients died due to CRC. In Kaplan-Meier survival analysis, Stage II patients had better survival than Stage III patients during every time point of the follow-up period (log rank, overall, 10 yr and 5 yr, P < 0.0001) (data not shown). We observed that decreased Bcl-2 expression was associated with shortened survival in patients with Stage II tumors (log rank, over-all P = 0.0012; 10-year P = 0.0012; 5-year P < 0.0001) but not in patients with Stage III tumors (data not shown).
3.6.2. Multivariate survival analyses
Proportional hazards models were built separately according to tumor stage (Table 4). Bcl-2 expression was an independent prognostic factor for patients with Stage II CRCs (HR = 8.48, 95% CI: 2.29, 31.45) but not with Stage III CRCs (HR = 1.52, 95% CI: 0.60, 3.88) after adjusting for other demographic and clinicopathological variables in study. Patients with Stage II CRC who suffered perforation into the visceral peritoneum (pT4) were 6.37 times more likely to die due to CRC within 5 years after surgery compared to Stage II CRC patients with pT3 stage (HR: 6.37, 95% CI: 1.97–20.60) within 5 years of surgery (Table 4). Patient age, gender, tumor size, and tumor location were not independent predictors of survival in patients with Stage II CRCs. In Stage III CRC patients none of the demographic, clinicopathological variables, other than nodel invasion, were independent predictors of mortality due to CRC along with Bcl-2 expression and depth of tumor invasion (Table 4).
Table 4.
Multivariate Cox regression analyses to assess the value of Bcl-2 expression in predicting the risk of mortality due to colorectal cancer in Caucasian patients
| Variable | 5 Year Survival |
10 Year Survival |
||||
|---|---|---|---|---|---|---|
| Crude HR | Adjusted* HR (95% CI) | P | Crude HR | Adjusted* HR (95% CI) | P | |
| Stage II (n = 92) | ||||||
| Bcl-2 expression | ||||||
| Low vs. High | 7.68 | 8.48 (2.29, 31.45) | 0.0014 | 4.33 | 5.77 (2.04, 16.33) | 0.0009 |
| pT component of stage (bowel wall invasion) | ||||||
| pT4 vs. pT3 | 5.58 | 6.37 (1.97, 20.60) | 0.0020 | 5.65 | 7.96 (2.75, 23.01) | 0.0001 |
| Age group | ||||||
| ⩾65 years vs. < 65 years | 1.04 | 1.08 (0.36, 3.22) | 0.8834 | 1.26 | 1.25 (0.47, 3.30) | 0.6553 |
| Gender | ||||||
| Male vs. Female | 7.72 | 4.62 (0.58, 36.67) | 0.1473 | 2.15 | 1.30 (0.40, 4.20) | 0.6606 |
| Tumor location | ||||||
| Rectum vs. Colon | 0.86 | 0.47 (0.12, 1.87) | 0.2847 | 0.67 | 0.47 (0.12, 1.83) | 0.2756 |
| Tumor differentiation | ||||||
| High grade vs. Low grade | 3.25 | 3.48 (0.35, 5.21) | 0.4715 | 3.78 | 3.92 (0.42, 4.92) | 0.5187 |
| Tumor size (cms) | ||||||
| > 5 vs. ≤ 5 | 0.68 | 0.52 (0.17, 1.60) | 0.2542 | 0.92 | 0.76 (0.29, 1.99) | 0.5722 |
| Stage III (n = 66) | ||||||
| Bcl-2 expression | ||||||
| Low vs. High | 1.62 | 1.52 (0.60, 3.88) | 0.3810 | 1.50 | 1.35 (0.55, 3.32) | 0.5115 |
| pN component of stage (nodal involvement) | ||||||
| N2–3 vs. N1 | 2.14 | 3.42 (1.37, 8.51) | 0.0083 | 1.99 | 2.92 (1.21, 7.07) | 0.0176 |
| pT component of stage (bowel wall invasion) | ||||||
| pT4 vs. pT3 | 0.94 | 0.56 (0.19, 1.60) | 0.2783 | 1.05 | 0.63 (0.23, 1.76) | 0.3836 |
| Age group | ||||||
| ⩾65 years vs. < 65 years | 1.52 | 1.74 (0.70, 4.34) | 0.2369 | 1.46 | 1.51 (0.62, 3.66) | 0.3628 |
| Gender | ||||||
| Male vs. Female | 0.65 | 0.52 (0.20, 1.33) | 0.1718 | 0.61 | 0.51 (0.20, 1.27) | 0.1466 |
| Tumor location | ||||||
| Rectum vs. Colon | 1.21 | 1.19 (0.37, 3.89) | 0.7692 | 1.18 | 1.16 (0.36, 3.73) | 0.8050 |
| Tumor differentiation | ||||||
| High grade vs. Low grade | 1.36 | 1.29 (0.50, 3.33) | 0.5994 | 1.32 | 1.25 (0.49, 3.23) | 0.6390 |
| Tumor size (cms) | ||||||
| > 5 vs. ≤ 5 | 2.18 | 1.78 (0.68, 4.62) | 0.2366 | 2.04 | 1.63 (0.64, 4.17) | 0.3070 |
Hazard ratios adjusted for Bcl-2 expression, depth of the tumor invasion into the bowel wall, number of lymph nodes involved, tumor differentiation, tumor size, tumor location, patient age, and gender.
4. Discussion
In this study, we investigated the utility of Bcl-2 expression in predicting the disease recurrence and overall survival in patients with CRC who have undergone surgery as their only therapeutic regimen. Lack of or decreased expression of Bcl-2 was an independent predictor of increased risk of recurrence in Caucasians with Stage II CRCs. It was also observed that the low Bcl-2 expressers with Stage II CRCs have similar trends of recurrence compared to high Bcl-2 expressers with Stage III disease. This also supports the concept that not all Stage II CRCs are indolent and there are some Stage II CRC cases which behave as if they are Stage III CRCs in terms of risk of recurrence. These aggressive Stage II CRCs can be identified by using Bcl-2 expression as a biomarker and should be treated aggressively (equivalent to Stage III CRCs).
The prognostic significance of Bcl-2 expression in CRCs has been reported in several studies [8,27–30]; however, its value in predicting disease recurrence has not been studied extensively [30–33]. A study of Stage II CRCs from the US has demonstrated a positive association with increased expression of Bcl-2 and better relapse free survival; however, its role in predicting the recurrence of the disease has not been studied [30]. A recent study from Italy did not find any association between Bcl-2 expression and recurrence of CRCs in univariate analysis in patients of Stage II and III CRCs combined [34]. The lack of association between Bcl-2 expression and recurrence found in the above study might be due to pooling stage II and III CRCs together in the analysis. Moreover, their conclusion is based on a univariate analysis rather than multivariate estimation adjusted for other known variables. A study from the UK by Ilyas et al. [31] demonstrated, similar to our study, that in patients with moderately differentiated Stage II CRCs (n = 66) with low Bcl-2 expression was associated with increased incidence of recurrence when adjusted for tumor stage and other clinicopathologic factors. Schwandner et al. [32] from Germany also reported similar findings in rectal carcinomas (Stages I to III analyzed together). These results reinforce our current findings that decreased or lack of Bcl-2 expression is an important biomarker in the prediction of recurrence in Caucasians with Stage II CRCs. This finding may be evidence supporting the premise that there is a possible relation between decreased Bcl-2 expression and an increased incidence of micro-metastasis to lymph nodes (unobservable metastasis to lymph nodes); contributing to increased rate of recurrence in patients with Stage II. The association between nodal involvement and Bcl-2 expression particularly needs further comprehensive investigation.
Our results are consistent with previous literature with respect to poor patient survival [8,27–29] and to an increased rate of disease recurrence among patients with tumors which lack or exhibit decreased levels of Bcl-2 expression [31,32]. In addition to CRC, in several other human malignancies including those of gastric [35], cervical [36], esophageal [37], and endometrial origin [38], it was demonstrated that a less aggressive malignant phenotype exists with increased expression of Bcl-2. However, these findings are paradoxical because increased expression of Bcl-2 together with other anti-apoptotic proteins should block the apoptotic pathway and thus provide a growth advantage to the tumor. Previous studies demonstrated that Bcl-2 protein appears to be involved in cellular processes other than apoptosis which influence tumor progression. Indeed, experimental studies on animals provided evidence for this paradoxical anti-tumor progression function of Bcl-2 based on its negative effect on cell proliferation [39,40]. It has also been suggested that Bcl-2 mediated inhibition of apoptosis is not the primary pathway by which CRC cells avoid programmed cell death. Nevertheless, immunohistochemical evidence in colorectal neoplasia indicates that it may be an early step by which dysplastic cells of the colonic mucosa can accumulate genetic alterations and thereby escape apoptosis [41]. It has also been suggested that adenomatous growth and tumor invasion are two different phases of tumor progression, and it may be during the later phase of tumor invasion, when the apoptotic stresses are different, that selection of a different means of inhibition of apoptosis (such as p53 dependency) occurs [31,32,41]. Furthermore, Gurova and Gudkov [42] recently reviewed studies related to the paradoxical role of apoptosis in tumor progression and concluded that inhibition of apoptosis does not lead to loss of genomic stability but rather creates a tumor environment that no longer supports further tumor progression. Therefore, inhibitors of apoptosis can be considered as factors suppressing tumor progression.
Our study also demonstrated that the pT component of stage (bowel wall invasion) is an independent predictor of recurrence in patients with Stage II CRCs, confirming the existing knowledge that tumor invasion is an important predictor of risk of recurrence. When analyzed for the predictive importance of Bcl-2 expression together with the depth of tumor invasion (pT component of stage) in patients with Stage II CRCs, it was demonstrated that the risk of disease recurrence for a patient with a tumor that expresses low levels of Bcl-2 (< 0.5 ISS) and has invaded the visceral peritoneum (pT4) versus a patient with a tumor that expresses increased levels of Bcl-2 (⩾ 0.5 ISS) and without visceral peritoneal involvement (pT3) is 11.08 times.
In conclusion, we have shown that decreased expression of Bcl-2 is an independent indicator of early recurrence and shortened patient survival after adjusting for other clinical, pathologic and demographic features in non-Hispanic Caucasians with Stage II CRCs. These findings also demonstrate that the low Bcl-2 expressers of Stage II CRCs behave similar to Stage III CRCs in predicting recurrence; thus, suggesting that Stage II CRCs should be evaluated for Bcl-2 expression before categorizing them into cases with good prognosis. This study also suggests that the association between the lack of Bcl-2 expression in primary CRCs and the nodal metastasis should be further explored. These findings suggest that by estimating Bcl-2 expression in CRCs may aid in assessing the risk of recurrence and help in designing the appropriate treatment strategies.
Acknowledgments
We thank Prof. G. P. Siegal for his critical review of the manuscript. Also, we thank Venkatramakrishna Neelagiri M.D. and Vanita Jain M.D., for their assistance in the collection of patient information. This work is supported in part by grants from the National Cancer Institute (RO1-CA98932-01 & RO3-CA097542-01), National Institute of Health.
References
- 1.American Cancer Society. Cancer facts and figures. Atlanta, GA: 2003. pp. 1–52. [Google Scholar]
- 2.G Nodal staging of colorectal carcinomas and sentinel nodes. J Clin Pathol. 2003;56(5):327–335. doi: 10.1136/jcp.56.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol. 1999;17(5):1356–1363. [PubMed] [Google Scholar]
- 4.Mamounas, Wieand, Wolmark, Bear, Atkins, Song, Jones, Rockette Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17(5):1349–1355. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 5.McDonald Adjuvant therapy of colorectal cancer. CA Cancer J Clin. 1999;49:202–219. doi: 10.3322/canjclin.49.4.202. [DOI] [PubMed] [Google Scholar]
- 6.Bedi, Pasricha, Akhtar, Barber, Bedi, Giardiello, Zehnbauer, Hamilton, Jones Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55(9):1811–1816. [PubMed] [Google Scholar]
- 7.Reed Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124(1–2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manne, Myers, Moron, Poczatek, Dillard, Weiss, Brown Srivastava and Grizzle, Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. International Journal of Cancer. 1997;74(3):346–358. doi: 10.1002/(sici)1097-0215(19970620)74:3<346::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Manne, Weiss, Grizzle Bcl-2 expression is associated with improved prognosis in patients with distal colorectal adenocarcinomas. Int J Cancer. 2000;89(5):423–430. doi: 10.1002/1097-0215(20000920)89:5<423::aid-ijc5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Manne, Weiss, Myers, Danner, Moron, Srivastava, Grizzle Nuclear accumulation of p53 in colorectal adenocarcinoma: prognostic importance differs with race and location of the tumor. Cancer. 1998;83:2456–2467. doi: 10.1002/(sici)1097-0142(19981215)83:12<2456::aid-cncr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Grizzle, Manne, Weiss, Jhala, Talley Molecular staging of colorectal cancer in African-American and Caucasian patients using phenotypic expression of p53, Bcl-2, MUC-1 AND p27(kip-1) Int J Cancer. 2002;97(4):4030–409. doi: 10.1002/ijc.1617. [DOI] [PubMed] [Google Scholar]
- 12.Hoos, Nissan, Stojadinovic, Shia, Hedvat, Leung, Paty, Klimstra, Cordon-Cardo, Wong Tissue microarray molecular profiling of early, node-negative adenocarcinoma of the rectum: a comprehensive analysis. Clin Cancer Res. 2002;8(12):3841–3849. [PubMed] [Google Scholar]
- 13.Takanishi, Hart, Covarelli, Chappell, Michelassi Ploidy as a prognostic feature in colonic adenocarcinoma. Arch Surg. 1996;131(6):587–592. doi: 10.1001/archsurg.1996.01430180013002. [DOI] [PubMed] [Google Scholar]
- 14.Manne, Weiss, Grizzle Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clinical Cancer Research. 2000;6:4017–4025. [PubMed] [Google Scholar]
- 15.Ashktorab, Smoot, Carethers, Rahmanian, Kittles, Vosganian, Doura, Nidhiry, Naab, Momen, Shakhani, Giardiello High incidence of microsatellite instability in colorectal cancer from African Americans. Clin Cancer Res. 2003;9(3):1112–1117. [PubMed] [Google Scholar]
- 16.Manne, Jhala, Jones, Weiss, Chatla, Meleth, Suarez-Cuervo, Grizzle Prognostic significance of p27(kip-1) expression in colorectal adenocarcinomas is associated with tumor stage. Clin Cancer Res. 2004;10(5):1743–1752. doi: 10.1158/1078-0432.ccr-03-0037. [DOI] [PubMed] [Google Scholar]
- 17.Alexander, Chatla, Funkhouser, Meleth, Grizzle, Manne Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma. Cancer. 2004;101(1):66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compton, Fielding, Burgart, Conley, Cooper, Hamilton, Hammond, Henson, Hutter, Nagle, Nielsen, Sargent, Taylor, Weiton, Willett Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 19.Green, Page, Fleming, Fritz, Balch, Haller, Morrow . Cancer Staging Handbook from the AJCC Cancer Staging Manual. 6. Springer; New York: 2002. American Joint Committee on Cancer; pp. 127–138. [Google Scholar]
- 20.World Health organization. International classification of diseases for oncology. 2. Geneva: World Health Organization; 1990. [Google Scholar]
- 21.Manne, Myers, Srivastava, Grizzle Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1997;89:585–586. doi: 10.1093/jnci/89.8.585. [DOI] [PubMed] [Google Scholar]
- 22.Fleiss . Statistical methods for rates and proportions. John Wiley and Sons; New York, NY: 1981. [Google Scholar]
- 23.Kaplan, Meier Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(457–481) [Google Scholar]
- 24.Cox Regression models and life tables. J Roy Stat Soc. 1972;34:187–220. [Google Scholar]
- 25.Allison . Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc; Gary, NC: 1995. [Google Scholar]
- 26.Kleinbaum . Survival Analysis: A Self Learning Text. Springer-Verlag; New York, NY: 1996. [Google Scholar]
- 27.Kaklamanis, Savage, Whitehouse, Doussis-Anagnostopoulou, Biddolph, Tsiotos, Mortensen, Gatter, Harris Bcl-2 protein expression: association with p53 and prognosis in colorectal cancer. Br J Cancer. 1998;77(11):1864–1869. doi: 10.1038/bjc.1998.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hague, Moorghen, Hicks, Chapman, Paraskeva BCL-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994;9(11):3367–3370. [PubMed] [Google Scholar]
- 29.Ofner, Riehemann, Maier, Riedmann, Nehoda, Totsch, Bocker, Jasani, Schmid Immunohistochemically detectable bcl-2 expression in colorectal carcinoma: correlation with tumour stage and patient survival. Br J Cancer. 1995;72(4):981–985. doi: 10.1038/bjc.1995.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinicrope, Hart, Michelassi, Lee Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clinical Cancer Research. 1995;1(10):1103–1110. [PubMed] [Google Scholar]
- 31.Ilyas, Hao, Wilkinson, Tomlinson, Abbasi, Forbes, Bodmer, Talbot Loss of Bcl-2 expression correlates with tumour recurrence in colorectal cancer. Gut. 1998;43(3):383–387. doi: 10.1136/gut.43.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwandner, Schiedeck, Bruch, Duchrow, Windhoevel, Broll p53 and Bcl-2 as significant predictors of recurrence and survival in rectal cancer. Eur J Cancer. 2000;36(3):348–356. doi: 10.1016/s0959-8049(99)00271-3. [DOI] [PubMed] [Google Scholar]
- 33.Giatromanolaki, Sivridis, Stathopoulos, Fountzilas, Kalofonos, Tsamandas, Vrettou, Scopa, Polychronidis, Simopoulos, Koukourakis Bax protein expression in colorectal cancer association with p53, bcl-2 and patterns of relapse. Anticancer Res. 2001;21(1A):253–259. [PubMed] [Google Scholar]
- 34.Rosati, Chiacchio, Reggiardo, De Sanctis, Manzione Thymidylate synthase expression, p53, bcl-2, Ki-67 and p27 in colorectal cancer: relationships with tumor recurrence and survival. Tumour Biol. 2004;25(5–6):258–263. doi: 10.1159/000081389. [DOI] [PubMed] [Google Scholar]
- 35.Liu, Liu Fang and Men, Expression of bcl-2 protein in gastric carcinoma and its significance. World J Gastroenterol. 1998;4(3):228–230. doi: 10.3748/wjg.v4.i3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjalma, De Cuyper, Weyler, Van Marck, De Pooler, Albertyn, van Dam Expression of bcl-2 in invasive and in situ carcinoma of the uterine cervix. Am J Obstet Gynecol. 1998;178(1 Pt 1):113–117. doi: 10.1016/s0002-9378(98)70636-2. [DOI] [PubMed] [Google Scholar]
- 37.Koide, Koike, Adachi, Amano, Usuda, Nagata Immunohistochemical expression of bcl-2 protein in squamous cell carcinoma and basaloid carcinoma of the esophagus. Surg Today. 1997;27(8):685–691. doi: 10.1007/BF02384977. [DOI] [PubMed] [Google Scholar]
- 38.Saegusa, Okayasu Bcl-2 is closely correlated with favorable prognostic factors and inversely associated with p53 protein accumulation in endometrial carcinomas: immunohistochemical and polymerase chain reaction/loss of heterozygosity findings. J Cancer Res Clin Oncol. 1997;123(8):429–434. doi: 10.1007/BF01372546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deichman, Matveeva, Kashkina, Dyakova, Uvarova, Nikiforov, Gudkov Cell transforming genes and tumor progression: in vivo unified secondary phenotypic cell changes. Int J Cancer. 1998;75(2):277–283. doi: 10.1002/(sici)1097-0215(19980119)75:2<277::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 40.Vail, Pierce, Fausto Bcl-2 delays and alters hepatic carcinogenesis induced by transforming growth factor alpha. Cancer Res. 2001;61(2):594–601. [PubMed] [Google Scholar]
- 41.Popescu, Lohri, de Kant, Thiede, Reuter, Herrmann, Rochlitz bcl-2 expression is reciprocal to p53 and c-myc expression in metastatic human colorectal cancer. Eur J Cancer. 1998;34(8):1268–1273. doi: 10.1016/s0959-8049(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 42.Gurova, Gudkov Paradoxical role of apoptosis in tumor progression. J Cell Biochem. 2003;88(1):128–137. doi: 10.1002/jcb.10382. [DOI] [PubMed] [Google Scholar]