Abstract
Mitogen-activated protein kinase cascades regulate various cellular functions, including growth, cell differentiation, development, and stress responses. We have identified a new Dictyostelium kinase (stress-activated protein kinase [SAPK]α), which is related to members of the mixed lineage kinase class of mitogen-activated protein kinase kinases. SAPKα is activated by osmotic stress, heat shock, and detachment from the substratum and by a membrane-permeable cGMP analog, a known regulator of stress responses in Dictyostelium. SAPKα is important for cellular resistance to stresses, because SAPKα null cells exhibit reduced viability in response to osmotic stress. We found that SAPKα mutants affect cellular processes requiring proper regulation of the actin cytoskeleton, including cell motility, morphogenesis, cytokinesis, and cell adhesion. Overexpression of SAPKα results in highly elevated basal and chemoattractant-stimulated F-actin levels and strong aggregation and developmental defects, including a failure to polarize and chemotax, and abnormal morphogenesis. These phenotypes require a kinase-active SAPKα. SAPKα null cells exhibit reduced chemoattractant-stimulated F-actin levels, cytokinesis, developmental and adhesion defects, and a motility defect that is less severe than that exhibited by SAPKα-overexpressing cells. SAPKα colocalizes with F-actin in F-actin–enriched structures, including membrane ruffles and pseudopodia during chemotaxis. Although SAPKα is required for these F-actin–mediated processes, it is not detectably activated in response to chemoattractant stimulation.
INTRODUCTION
The actin cytoskeleton plays a vital role in numerous cellular processes, such as cell locomotion, phagocytosis, cytokinesis, cell adhesion, cell shape changes, and stress responses. During these processes, the actin cytoskeleton is dynamically changed, a process that involves F-actin polymerization and depolymerization and the reorganization of existing filament networks. Mutations of signaling molecules or structural elements involved in these changes affect cellular processes to various extents.
Eukaryotic cells are constantly exposed to stress conditions. To survive, cells adopt various mechanisms to adapt to environmental changes. Mitogen-activated protein kinase (MAPK) pathways regulate cellular responses to external stress in various organisms, including yeast, plants, and mammals (Canman and Kastan, 1996; Jonak et al., 1996; Gustin et al., 1998; Hirt, 2000; Kyriakis and Avruch, 2001; O'Rourke et al., 2002). In Saccharomyces cerevisiae, the well-characterized HOG1 MAPK pathway is activated by osmotic stress and leads to gene expression and glycerol production, which is critical for yeast cells to withstand osmotic stress (Gustin et al., 1998; O'Rourke et al., 2002). In mammalian cells, the c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK1) pathway and the p38/SAPK2 MAP kinase pathway are both activated by diverse stimuli including UV, osmotic, and thermal stresses (Canman and Kastan, 1996; Chang and Karin, 2001; Kyriakis and Avruch, 2001). The activation of the JNK/SAPK1 pathway results in the phosphorylation and activation of transcription factors, which in turn regulate gene expression to resist stress conditions. The p38/SAPK2 MAPK pathway, in addition to its role in regulating gene expression by phosphorylation of transcription factors, has been linked to F-actin reorganization, possibly through the actin polymerization modifier HSP27 (Landry and Huot, 1995, 1999). In addition to their roles in stress responses, the JNK/SAPK1 and p38/SAPK2 pathways mediate other cellular responses. Both pathways are activated by cytokines such as tumor necrosis factor and interleukin-1 and are thereby implicated in cell differentiation and apoptosis (Ichijo, 1999; Rincon et al., 2000). However, these two pathways also exhibit specificity toward different stimuli and participate in different cellular processes. For example, in endothelial cells, the p38/SAPK2 pathway, but not the JNK/SAPK1 pathway, is activated by vascular endothelial growth factor and mediates actin reorganization and cell migration (Rousseau et al., 1997).
The activation of MAPK pathways is mediated by a family of protein kinases, MAP kinase kinase kinases (MAPKKKs) or MEK kinases (MEKKs). Identified mammalian MEK kinases include the Raf family, the MEK kinase (MEKK) family, and the mixed lineage kinase (MLK) family. The Raf family kinases selectively activate the extracellular signal-regulated kinase (ERK) pathway, which regulates growth, cell differentiation, and developmental processes (Nishida and Gotoh, 1993; Treisman, 1996). Members of the MEKK family activate both the ERK and JNK/SAPK pathways (Schlesinger et al., 1998). The recently identified MLK family of MEKKs possesses a kinase domain exhibiting characteristics of both Ser/Thr and Tyr protein kinases (Gallo and Johnson, 2002). Several members of this kinase family activate JNK/SAPK stress signaling pathways. Recently, MLK family kinases (MLTK/ZAK) were reported to activate the JNK/SAPK and p38 pathways (Gotoh et al., 2001; Gallo and Johnson, 2002). MLTKs are activated by osmotic shock, and overexpression of MLTKα, but not MLTKβ, induces the disruption of actin stress fibers and dramatic changes in cell morphology mediated through the p38 MAP kinase pathway (Gotoh et al., 2001). Dictyostelium grows as single-celled amoebae, which, upon starvation, aggregate to form a multicellular organism (Aubry and Firtel, 1999; Chen et al., 1996). Both aggregation and subsequent morphogenesis involve chemotaxis to one or more secreted factors. Dissection of the chemotaxis pathways to cAMP, which mediates aggregation, has led to important new insights into this evolutionarily highly conserved process. Chemotaxis, or directional movement toward a small molecule ligand, involves the spatially regulated polymerization of F-actin at the leading edge resulting in the forward protrusion of a pseudopod. This is followed by a myosin II/F-actin–mediated contraction of the posterior of the cell or uropod (Parent and Devreotes, 1999; Firtel and Chung, 2000; Chung et al., 2001; Iijima et al., 2002). In response to hyperosmotic stress, Dictyostelium cells withstand the extensive cell shrinkage caused by osmolarity change, largely by remodeling of the cortical cytoskeleton (Insall, 1996; Kuwayama et al., 1996; Zischka et al., 1999). This remodeling includes the rearrangement of F-actin and myosin II fibers at the cell cortex to form the core of a rigid network resembling a shell-like structure, thereby providing the physical strength to resist the osmotic shock (Insall, 1996). Other known cytoskeletal changes activated by osmotic shock in Dictyostelium include tyrosine phosphorylation of actin, threonine phosphorylation of myosin II, and an increase in the levels of RMLC (regulatory myosin light chain) and cofilin and a decrease in DdLIM in the cortex (Howard et al., 1993; Jungbluth et al., in 1994, 1995; Aizawa et al., 1999; Gamper et al., 1999; Zischka et al., 1999). In addition, the F-actin cross-linking protein α-actinin and the 120-kDa gelation factor are important for protection of Dictyostelium cells from osmotic stress and support the pivotal role the cytoskeleton plays in the osmotic response (Rivero et al., 1996). Osmotic shock results in an ∼1.6-fold increase in F-actin content in Dictyostelium, as it does in neutrophils (∼twofold) (Hallows et al., 1996; Zischka et al., 1999; Rizoli et al., 2000). In contrast to the signaling pathways controlling F-actin reorganization in response to chemoattractant stimulation, little is known about the signaling events mediating cytoskeleton reorganization in response to stress. The response of Dictyostelium cells to osmotic stress is mediated, at least in part, by cGMP and cAMP (Kuwayama et al., 1996; Oyama, 1996; Kuwayama and Van Haastert, 1998; Ott et al., 2000; Rizoli et al., 2000), which are also important for controlling Dictyostelium chemotaxis and development. Hyperosmotic treatment of cells induces both cAMP and cGMP production, with a peak at 2 and 10 min, respectively (Oyama, 1996; Ott et al., 2000). Biochemical and cell biological data indicate that cAMP accumulation in response to osmotic shock depends on DokA, which controls the intracellular cAMP phosphodiesterase RegA (Ott et al., 2000). Although there are two guanylyl cyclases in Dictyostelium, cGMP accumulation is primarily due to the activation of the soluble guanylyl cyclase both during osmotic shock and chemoattractant stimulation (Roelofs and Van Haastert, 2002). Whereas the immediate downstream target of cAMP during osmotic shock is unknown, cGMP production is required for myosin II heavy chain phosphorylation, resulting in the disassembly of myosin II filaments, a prerequisite for their reassembly at the cell cortex (Egelhoff et al., 1993; Kuwayama et al., 1996).
Dictyostelium has three partially characterized MAP kinase pathways. The pathway containing the MAPK ERK2 regulates the activity and stability of the intracellular cAMP-specific phosphodiesterase RegA, whose proper regulation is essential for aggregation, morphogenesis, patterning, and cell-type differentiation (Shaulsky et al., 1998; Thomason et al., 1999; Mohanty et al., 2001). The pathway containing MEK1 and ERK1 is essential for proper chemotaxis and aggregation (Ma et al., 1997; Sobko et al., 2002). One MEKK, MEKKα, has been characterized (Chung et al., 1998). The phenotypes of mekkα null and overexpression strains suggest that MEKKα is required for proper morphogenesis and cell-type patterning. MEKKα does not couple downstream with either the ERK1 or ERK2 pathways.
Here, we describe a putative Dictyostelium MEKK-like kinase, SAPKα, which we demonstrate is involved in stress responses and is also required other cellular processes. Dictyostelium SAPKα is activated by hyperosmotic and heat shock. SAPKα (spkA) null cells are more sensitive to hyperosmotic stress but also exhibit an altered F-actin cytoskeleton, resulting in cytokinesis and chemotaxis defects. spkA null cells aggregate, but form very small fruiting bodies when cells are plated at lower densities. In addition, spkA null cells seem to have reduced adhesion to the substratum and SAPKα is transiently activated when cells are detached from the substratum. Overexpression of SAPKα results in an elevated level of F-actin in resting cells. These cells do not polarize and move poorly toward the chemoattractant cAMP. Our results identify a novel MEKK-like kinase that is important for stress responses and other cellular processes, possibly through a direct or indirect involvement in remodeling the actin cytoskeleton.
MATERIALS AND METHODS
Materials
The anti-FLAG monoclonal antibody (mAb), fluorescein isothiocyanate (FITC)-phalloidin, tetramethylrhodamine B isothiocyanate (TRITC)-phalloidin, 8-bromo-cGMP (8-br-cGMP), latrunculin B, sodium orthovanadate, β-glycerophosphate, aprotinin, and leupeptin were purchased from Sigma-Aldrich (St. Louis, MO). Myelin basic protein (MBP) was purchased from Roche Diagnostics (Indianapolis, IN). [32P]γ-ATP was from ICN Pharmaceuticals (Costa Mesa, CA). Protein G plus agarose was from Santa Cruz Biotechnology (Santa Cruz, CA). Nitrocellulose filters (13 mm, 0.025 μm, catalog no. VSWP01300) used for the adhesion assay were from Millipore (Bedford, MA).
Cell Culture and Development of Dictyostelium Cells
pdeD- cells in the Ax2 background were a gift from Marcel Meima and Pauline Schaap (School of Life Sciences, University of Dundee, Dundee, Scotland). Wild-type Dictyostelium strains KAx3 and Thy minus JH10 were used to generate various mutant strains as indicated. Cells were grown in nutrient medium (Watts and Ashworth, 1970) at 23°C either in shaking culture or on a Petri dish. For the developmental study, cells were washed twice in 12 mM Na/K phosphate buffer and resuspended at 7 × 107 cells/ml and plated at various densities on agar plates.
Generation of spkA Null Cells and Cells Expressing Wild-Type and Mutant SAPKα
The spkA null mutant was obtained by a gene replacement technique based on that of Manstein et al. (1989). Because there are two copies of SPKA genes, spkA null cells were generated in JH10 cells by using two targeting constructs containing a Thy cassette and a Bsr cassette, respectively.
To make wild-type SAPKα expression constructs, SAPKα was tagged at the C terminus with either the FLAG epitope or GFP. FLAG-tagged SAPKα was produced by polymerase chain reaction (PCR) of the coding region of SAPKα with primers 5′-GTTTTTACTAGTAAAAAAAT GTCATCAACTCAACAACAACAACATC-3′ and 5′-GTTTTTCTCGAGTTATTTGTC ATCGTCATCTTTATAATCTTCTTGATTCTTTGGAAGTGG-3′. The PCR fragment was digested with SpeI and XhoI and cloned directly into the Exp4 (+) expression vector. For making SAPKα-GFP or N-terminal SAPKα-GFP constructs, GFP and SAPKα or N-terminal SAPKα were amplified by PCR and cloned simultaneously into an Exp4(+) vector through a three-piece ligation approach. For making the SAPKα K378A mutation, site-directed mutagenesis was done by a two-step PCR approach by using overlapping primers containing the desired mutation (Li and Wilkinson, 1997). The overlapping primers used were 5′-GAAGTTGCAATTGCAGTATTAAAATCAATGACTG-3′ and 5′-GATTT TAATACTGCAATTGCAACTTCTTTACCTC-3′. The DNA fragments containing the mutation were used to replace the corresponding fragments in the wild-type SAPKα constructs. All constructs were sequenced and transformed JH10 or KAx-3 or spkA null cells.
All transformations were done as described previously (Nellen et al., 1987). Transformants were selected with 10 or 20 μg/ml G418 and clones were isolated by plating cells on DM agar (12 mM Na/K phosphate buffer pH 6.1, 1% Bacto peptone, 0.2% d-glucose, 1.5% agar) containing 40 μg/ml G418. In the case of making spkA null cells, cells were selected either with 7.5 μg/ml blasticidin for the Bsr cassette or HL5 media without thymidine supplement for the Thy cassette.
Chemotaxis Assay
Aggregation-competent cells were made by pulsing cells with cAMP as described previously (Meili et al., 1999), except that cells were pulsed at 6-min intervals. Pulsed cells of 4 × 105 cells/ml were plated onto a small dish with a hole covered by a 0.17-mm glass coverslip at 40 μl/dish and allowed to adhere for 15 min. A micropipette filled with 150 μM cAMP was positioned close to cells by using a micromanipulator (Eppendorf Patchman), and the response of cells was recorded at 6-s intervals by using a time-lapse video recorder and NIH Image software. Cell movement was analyzed using the DIAS program (Wessels et al., 1998).
Immunofluorescence Microscopy
Cells expressing green fluorescent protein (GFP) fusion or FLAG-tagged SAPKα were fixed with 3.7% formaldehyde in Na/K phosphate buffer for 5 min, washed with Na/K phosphate buffer three times, and permeabilized with 0.1% Triton X-100 for 1 min. Cells were washed and incubated with phosphate-buffered saline containing 1% bovine serum albumin and 0.05% Tween 20 for 1 h. For cells expressing FLAG-tagged SAPKα, cells were first incubated with anti-FLAG (1:200) for 1 h. Cells were stained with FITC-conjugated anti-mouse IgG (1:200) and TRITC-labeled phalloidin for 1 h, washed, and mounted for observation with a 60× oil immersion lens on a Nikon Microphot-FX microscope. For cells expressing GFP fusion SAPKα proteins, cells were directly stained with TRITC-labeled phalloidin for 1 h, washed, and mounted for observation. For nuclei staining, after fixation and permeabilization, cells were mounted with mounting medium containing 0.0001% Hoechst dye.
In Vivo Actin Polymerization Assay
Cellular F-actin was quantified using a method originally developed by Howard and Oresajo (1985), except that TRITC-phalloidin was used. Cells were pulsed with 30 nM cAMP at 6-min intervals for 5 h and were then collected by centrifugation and resuspended in Na/K phosphate buffer to 1 × 107 cells/ml and shaken at 200 rpm with 2.5 mM caffeine for 20 min. Cells were spun down, resuspended again with phosphate buffer containing 2.5 mM caffeine, and stimulated with 100 μM cAMP. Aliquots of cells (100 μl) were taken at 0, 5, 10, 15, and 30 s and mixed with 1 ml of actin buffer (20 mM KH2PO4/10 mM PIPES pH 6.8/5 mM EGTA/2 mM MgCl2) containing 3.7% formaldehyde, 0.1% Triton X-100, and 0.4 μM TRITC-phalloidin. Cells were fixed and stained for 1 h and spun down at 10,000 × g for 5 min in the microcentrifuge. The pellet was extracted with 1 ml of 100% methanol and fluorescence was measured (525 excitation/565 emission).
Kinase Assay
For cAMP stimulation, kinase assays were performed as described previously (Meili et al., 1999). For responses to various stress conditions, cells were washed twice with 12 mM Na/K phosphate buffer, pH 6.1, and resuspended at 3 × 107 cells/ml in the same buffer. Cells were then shaken at 150 rpm for 1 h and treated by various stressors as indicated. For kinase assay after cell detachment, cells were first attached to plates for 30 min, starved for 1 h, and quickly resuspended in Na/K phosphate buffer by pipetting. In each case, an aliquot of 500 μl of cells was taken at the indicated time point and lysed immediately by mixing with an equal volume of 2× lysis buffer containing 50 mM Tris, pH 7.6, 200 mM NaCl, 20 mM NaF, 2 mM vanadate, 50 mM β-glycerophosphate, 6 mM sodium pyrophosphate, 2 mM EDTA, 2 mM EGTA, 4 μg/ml leupeptin, 4 μg/ml aprotinin, 2% NP-40, 20% glycerol, 2 mM dithiothreitol. The lysate was precleared by centrifugation. One microliter of anti-FLAG antibody (4 μg/μl) was added and incubated with agitation in a cold room for 1 h. The formed immune complexes were collected with 40 μl of a 1:1 slurry of protein G beads by incubation under agitation for 1 h at 4°C. The beads were washed twice with lysis buffer and twice with kinase buffer (25 mM MOPS, pH 7.4, at room temperature, 25 mM glycerophosphate, 20 mM magnesium chloride, 1 mM dithiothreitol). The kinase activity was measured using MBP as a substrate in a reaction in 60 μl of kinase buffer containing 5 μCi of [32P]γ-ATP, 5 μM cold ATP, and 5 μg of MBP. The reactions were stopped by adding 20 μl of 4× SDS buffer and boiling for 5 min. Samples were resolved by SDS-PAGE (12.5%), blotted onto polyvinylidene difluoride membrane (Millipore), and exposed to film.
Cell Viability Assay
Measurement of cell survival was performed as follows. Briefly, cells were resuspended at 3 × 107 cells/ml in Na/K phosphate buffer and stimulated with 400 mM glucose. Cells were diluted 1000 times at each time point and 200 cells were mixed with Escherichia coli B/r and plated on 1/3 SM plates. The number of surviving cells was counted several days later. The cell viability after osmotic stress is presented as percentage of cells that survived after treatment compared with the survival rate of untreated cells.
Cell Adhesion Assay
The cell-substrate adhesion assay was performed with cells attached to nitrocellulose filters. Cells were washed twice with Na/K phosphate buffer and resuspended at 2 × 107 cells/ml. The amount of 4 × 105 cells in 200 μl were plated onto 13-mm circular nitrocellulose filters (Millipore). After 30 min, unattached cells were removed by dipping filters into Na/K phosphate buffer. Filters were transferred to microcentrifuge tubes filled with 1 ml of Na/K phosphate buffer with the side of the filter containing the cells facing away from the wall of the tube. All of the tubes were placed simultaneously onto an Eppendorf 5432 mixer and vortexed for 10 s. Cells left in the buffer were counted and regarded as detached. Filters were transferred to fresh tubes each containing 1 ml buffer with 10 mM EDTA. Cells were completely detached from filters by vortexing for 1 min and were counted. These cells were regarded as attached. Cell adhesion is presented as percentage of cells attached compared with those detached. This experiment was repeated three times, each time with three filters for each strain.
RESULTS
Identification of the Novel Protein Kinase SAPKα
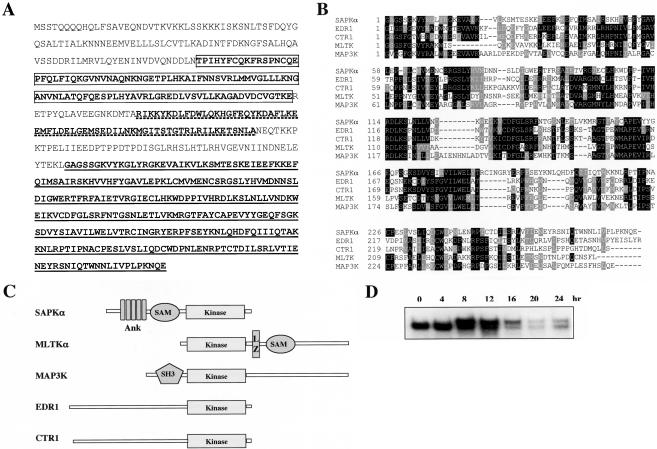
spkA, was identified by PCR with degenerate primers based on the conserved sequences within the kinase domain of MAP family members (Ma et al., 1997). The full-length cDNA was subsequently obtained by screening a λ ZAP cDNA library (see MATERIALS AND METHODS). The full-length cDNA contains an open reading frame encoding a protein of 638 amino acids and is named SAPKα. SMART analysis predicts five ankyrin motifs at the N terminus followed by a SAM domain (sterile α-motif) and a dual-specificity kinase domain at the C terminus of the protein (Figure 1A). SAM domains are involved in protein–protein interactions and dimer formation of several signaling molecules and transcription regulators (Ponting, 1995). A BLAST search against current databases failed to identify any orthologs in other organisms. However, the kinase domain showed the highest homology to MEKK family members in mammals and plants (Figure 1B). Among these are EDR1 (36% identity, 51% similar), which negatively regulates defense responses in plants (Frye et al., 2001); mammalian MLTKα (also known as ZAK, 34% identity, 54% similar), an MEKK that can activate the ERK, JNK/SAPK, p38, and ERK5 pathways (Gotoh et al., 2001); CTR1 (36% identity, 51% similar), a RAF-like kinase and a negative regulator of ethylene response in plants (Kieber et al., 1993); and MAP3K10 (also known as MST/MLK2, 34% identity, 50% similar), a member of the MLK family which directly phosphorylates and activates SEK1, an activator of JNK/SAPK (Hirai et al., 1997). MLTKα also has a SAM domain; however, unlike SAPKα, the MLTKα SAM domain is C-terminal to the kinase domain (Figure 1C).
Figure 1.
Sequence analysis of Dictyostelium SAPKα. (A) Deduced amino acid sequence of SAPKα from the 2075-base pair cDNA clone. The boxed region at the N terminus is the ankyrin repeat domain. The kinase domain at the C terminus is underlined. The SAM domain in the middle is also underlined but with dashed lines. (B) Alignment of the kinase domain of SAPKα with that of other kinases including tomato CTR1 (AF110519), barley EDR1 (AF305912), human MAP3K (NM_002446), and mouse MLTKα (AB049731). (C) Schematic diagram of the domain structures of SAPKα and other kinases with close homology in the kinase domain. Ank, ankyrin repeat motifs. (D) Northern blot showing the developmental time course of SAPKα expression. Total RNA of 8 μg per sample was resolved on a 1.0% denaturing agarose gel, blotted and probed as described previously (Datta and Firtel, 1987). The 0-h time point is for vegetative cells.
To examine the timing of SAPKα gene expression during the Dictyostelium developmental cycle, we performed RNA blot analysis. As depicted in Figure 1D, there are two SAPKα transcripts. The smaller transcript is high in vegetative cells and during early development and rapidly decreases after 12 h, the time of tipped aggregate formation. A larger transcript occurs at 8 h of development, remains high through the 12-h time point, and then decreases, like the smaller transcript. Neither transcript is detected in the spkA null strain (our unpublished data). In strains in which one of the two copies of the SpkA gene was disrupted, both transcripts are still observed. These data indicate that both transcripts derive from the same gene. As is the case for other genes in Dictyostelium expressing two transcripts, we expect this is due to two promoters rather than differential splicing within the open reading frame (Faure et al., 1990; Mann and Firtel, 1991; Saxe et al., 1991; Mann et al., 1992; Rogers et al., 1997).
spkA Null Cells Are Defective in Cytokinesis
To investigate the function of SAPKα, we generated spkA null strains by gene disruption via homologous recombination as described in the MATERIALS AND METHODS and confirmed by Southern and Northern blot analyses (our unpublished data). Our analyses indicate that there are two identical copies of SpkA in the genome, probably due to a duplication of a region of the genome. We therefore used two sequential gene disruptions with two selectable markers to obtain spkA null strains (see MATERIALS AND METHODS; our unpublished data). We identified three independent spkA null strains (both copies disrupted). All exhibited the same growth and developmental defects (our unpublished data). One was used for all of the experiments described.
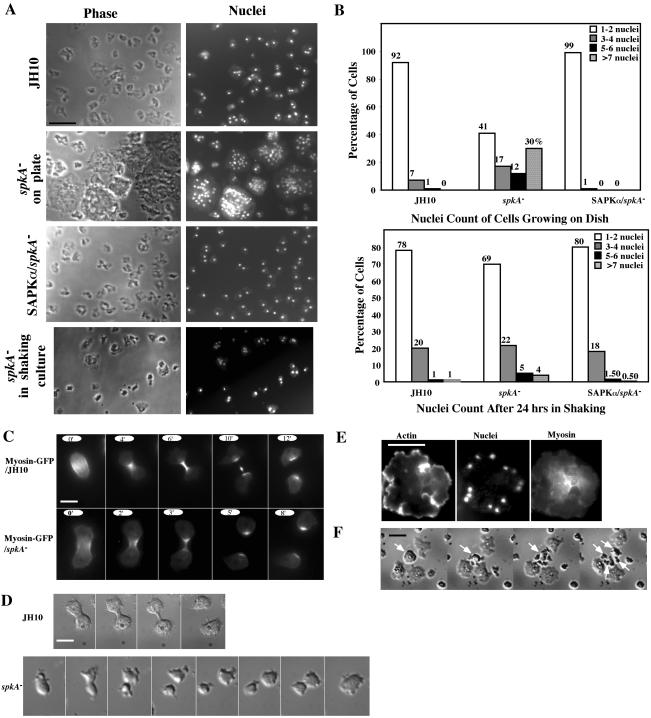
spkA null cells were very large when grown on Petri dishes, suggesting the cells have a cytokinesis defect (Figure 2A). These giant cells often rounded up and detached from plates. spkA null cells also show a very distinct cell morphology. Cells are often round or irregular with essentially no filopodia. Nuclear staining with 4,6-diamidino-2-phenylindole indicated that these giant spkA null cells could have as many as 80 nuclei/cell. Approximately 92% of the cells of the parental strain JH10 have one or two nuclei compared with ∼41% for spkA null cells (Figure 2B). Furthermore, ∼30% of spkA null cells have at least seven nuclei per cell. We examined whether spkA null cells are multinucleate when grown in suspension (shaking) culture. Twenty-four hours after transferring the cells from a Petri dish to shaking culture, spkA null cells with one or two nuclei/cell increased to ∼69% compared with ∼78% for JH10 cells. The decrease in the number of multinucleate cells when grown in suspension culture is the opposite of observations for mutants in the myosin II pathway (e.g., myosin II, PAKa, RLC null strains; Fukui et al., 1990; Zang et al., 1997; Chaudoir et al., 1999; Chung and Firtel, 1999). These strains produce large, multinucleate cells in suspension but are often mono- or dinucleate when grown on Petri plates, as the cells are able to pull themselves apart by traction-mediated cytofission (Fukui et al., 1990). Interestingly, the F-actin binding protein coronin is also required for proper cytokinesis. Like spkA null cells, coronin null cells exhibit a stronger cytokinesis defect when grown on Petri dishes than when grown in suspension culture (de Hostos et al., 1993). Expression of exogenous SAPKα under the Actin 15 promoter in spkA null cells restored normal cytokinesis (Figure 2, A and B).
Figure 2.
Cytokinesis defect of spkA null cells. (A) Images of spkA null cells growing either on plates or in shaking culture. Cells were fixed with 3.7% formaldehyde on coverslips and stained with Hoechst dye. spkA null cells grow on plates as large cells with multiple nuclei, but they divide much better in shaking culture. Control JH10 cells and spkA null cells expressing exogenous SAPKα divide normally. Bar, 30 μm. (B) Quantitative analysis of the cytokinesis defect of spkA null cells. Cells either grown on plates or 24 h after being transferred from plates to shaking culture were allowed to adhere on coverslips, fixed, and stained with Hoechst dye. The nuclei from as many as 300 cells were counted for each strain. (C) Myosin localization does not seem to be affected in spkA null cells. JH10 and spkA null cells expressing myosin-GFP were plated on coverslips and cell divisions were recorded using the GFP channel. For JH10 cells, frames were taken every 30 s. For spkA null cells, frames were taken at 1-min intervals due to increased sensitivity of cells to UV light. Bar, 10 μm. (D) Time-lapse recording of division of JH10 and spkA null cells. Although JH10 cells divide normally, spkA null cells often fail to divide due to incomplete separation and subsequent fusion of the two daughter cells. Bar, 10 μm. (E) Image showing a large dividing spkA null cell stained for actin, myosin, and nuclei. Bar, 30 μm. (F) Time-lapse recording of division of a large spkA null cell. One large spkA null cell eventually divided into 4 daughter cells, as indicated by arrows. Bar, 50 μm.
To better study cytokinesis, we examined GFP-myosin II localization during cytokinesis. As shown in Figure 2C, the localization of GFP-myosin II was similar in spkA null cell and the parental strain JH10. These results are consistent with the spkA null cytokinesis defect not being associated with a defect in the myosin II pathway.
We performed a more detailed time-lapse video analysis of cells undergoing cytokinesis to further understand the spkA null cells' cytokinesis defects (Figure 2D). Because of Dictyostelium cells' sensitivity to the light required for fluorescence imaging in our system, these more detailed studies were not practical with GFP fusion proteins as markers. We therefore examined cells by differential interference contrast imaging. In spkA null cells, dividing cells form a cleavage furrow normally and the two daughter cells migrate away from each other. However, in many cases, the two daughter cells fail to separate and are connected by a thin “bridge” and fuse as one cell eventually. We also noticed that, whereas JH10 cells remain fully attached to the surface of the coverslip during division, the dividing spkA null cells do not remain well attached. As a result, the two spkA null daughter cells are sometimes unable to effectively pull themselves apart when trying to separate (our unpublished data). This observation might explain why spkA null cells divide better in shaking culture. We expect that the shear forces in shaking culture might break the bridge between the two cells, allowing the daughter cells to separate.
The profile of the number of nuclei per cell (some cells have a small number of nuclei; Figure 2B) indicated that cells are periodically able to undergo cytokinesis. We followed the division of larger cells labeled with GFP-myosin II. Figure 2E shows one representative large spkA null cell in the process of dividing into smaller multinucleate daughter cells. In this cell type, myosin II localized to the place where daughter cells were going to separate and nuclei were distributed along the periphery of the cell and eventually separated into a few daughter cells. Figure 2F shows a time-lapse recording of the division of one representative large spkA null cell. From these observations, we conclude that the cytokinesis defect of spkA null cells is probably not due to a defect in the myosin II pathway. The high frequency of reversion of cytokinesis during the final separation of daughter cells suggests that SAPKα function might be required, directly or indirectly, in the severing of the midbody. The cytokinesis defect could be due to an altered actin cytoskeleton and/or a strong adhesion defect (see result below). We do not know whether SAPKα is directly involved in cytokinesis or the cytokinesis defect is due to a pleiotrophic defect in a yet-to-be-defined pathway affecting the cytoskeleton.
spkA Null and SAPKαOE Cells Exhibit Developmental Defects
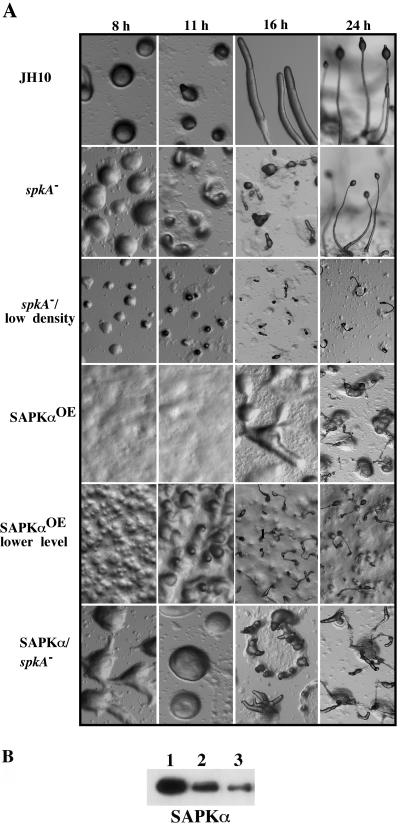
Figure 3 illustrates the development of spkA null cells, cells overexpressing SAPKα(SAPKαOE cells), and parental JH10 cells. When plated at a low density, spkA null cells formed much smaller mounds and fruiting bodies compared with the parental strain JH10 cells, which formed nearly normal-sized mounds when plated at the same density (our unpublished data). However, the developmental timing was not affected and both spkA null cells and JH10 cells were able to produce viable spores. To create cells overexpressing SAPKαOE, wild-type cells were transformed with an expression vector expressing SAPKα with a C-terminal FLAG tag. Clones were screened for SAPKα expression by Western blot analysis and clones with three different expression levels of SAPKα were examined for developmental phenotypes. Cells expressing the highest level of SAPKα did not aggregate within 24 h (our unpublished data). Cells expressing a medium level of SAPKα exhibited very delayed aggregation and formed large, amorphous aggregates (Figure 3). Small, abnormal fruiting body-like structures emerged from the mounds. No detergent-resistant spores were formed at 24 h of development. Cells exhibiting the lowest level of overexpression formed mounds with normal developmental timing, but the aggregates were very small. Some of these mounds eventually developed into very small fruiting bodies, although many remained at the mound stage through 24 h of development. Expression of a putative kinase dead SAPKα (mutation of the putative Lys in the ATP binding site to Ala, SAPKαK378A) in wild-type cells produced developmental phenotypes that were similar to but less severe than those of spkA null cells (our unpublished data).
Figure 3.
Developmental phenotypes of spkA null cells and cells expressing wild-type SAPKα. (A) Cells grown in axenic medium were washed twice with Na/K phosphate buffer and plated on nonnutrient agar plates. Photographs were taken at various developmental stages at the same magnification. For spkA null cells, developmental phenotypes at both lower and higher density are shown. (B) Western blot showing levels of expression of SAPKα in three individual clones: 1, highest expression level (developmental data not shown); 2, medium level (shown as SAPKαOE in A); and 3, lowest level (shown as SAPKαOE lower in A).
Overexpression of SAPKα in spkA null cells produced phenotypes similar to those of overexpression of SAPKα in wild-type cells, although not as severe. Cells aggregated with normal timing and formed larger mounds. Many of these broke up into smaller aggregates, some of which produced multiple abnormal-looking structures that were similar to those produced by overexpression of SAPKα in wild-type cells. We view the phenotypes as being due predominantly to overexpression of SAPKα, but we cannot exclude the possibility that some of the defects result from misexpression of SAPKα from the Actin 15 promoter.
spkA Null and SAPKαOE Cells Exhibit Motility Defects
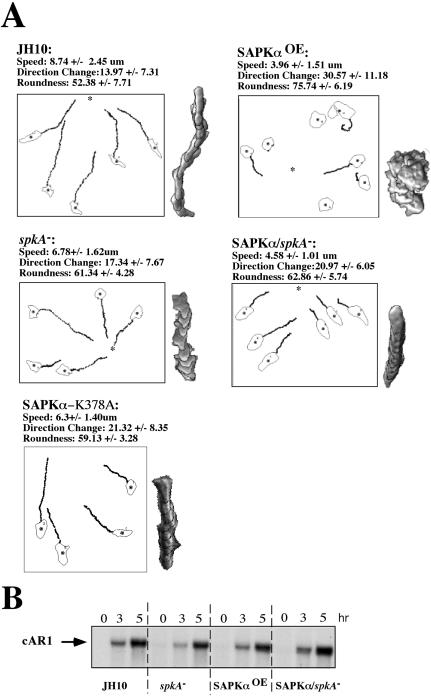
The aggregation and morphogenesis defects suggest that spkA null and SAPKαOE cells may exhibit motility and/or chemotaxis defects. Chemotaxis was examined by assaying the ability of aggregation-competent cells to migrate toward a micropipette emitting the chemoattractant cAMP. Figure 4A shows that spkA null cells are less polarized compared with JH10 cells and move more slowly. spkA null cells exhibit directionality similar to that of the parental strain. SAPKαOE cells exhibit very strong chemotaxis defects. These cells are very poorly polarized and lack a defined pseudopod. In addition, the cells undergo considerably more directional change than JH10 cells, and the speed of movement is reduced significantly. Expression of SAPKα in spkA null cells resulted in an intermediate phenotype (Figure 4A). Overexpression of a putative dominant-negative mutant of SAPKα in a wild-type background resulted in phenotypes similar to those of spkA null cells. To eliminate the possibility that the chemotaxis defects observed were due to the cells not being properly developed and thus unable to generally respond to chemoattractant signals, we performed a Northern blot analysis to check the expression of chemoattractant receptor cAR1 at different time points after pulsing the cells with cAMP, which is known to induce cAR1 expression. As shown in Figure 4B, cAR1 mRNA levels were very similar in the four cell types examined: parent strain (JH10), spkA null cells, SAPKαOE cells, and SAPKα/spkA null cells.
Figure 4.
Chemotaxis analysis of spkA null cells and cells expressing wild-type SAPKα. (A) Chemotaxis assay. Cells were washed and pulsed every 6 min for 5 h with 30 nM cAMP (see MATERIALS AND METHODS). Cells were plated on a Petri dish specifically made for observing cells by using an inverted microscope and placed close to a micropipette filled with 150 μM cAMP. Cell movements were recorded with NIH Image software and analyzed using the DIAS program. Comparison of cell shape, direction change, and speed of the movement of different cells is shown. A representative cell from each type of cells is shown. The recordings are made at 10 frames/min and 15–20 randomly chosen cells were analyzed for each strain. The image was obtained by superimposing nine frames at 1-min intervals between frames. (B) cAR1 Northern blot. Cells were pulsed and 2 × 107 cells were sampled at the indicated time points. Total RNA was prepared and 6 μg was resolved on a 1% formaldehyde denaturing agarose gel, transferred to nylon membrane, and hybridized to a cAR1 probe.
To examine whether these chemotaxis phenotypes may be due to general defects in cell motility, we examined random cell movement of these strains. For each strain, 15–20 cells were randomly chosen and the speed of movement was measured using DIAS software (Wessels et al., 1998). JH10 cells moved faster than spkA null cells (5.81 ± 0.49 versus 4.52 ± 0.51 μm/min, respectively). For the SAPKα overexpression cells, we measured clones expressing different levels of SAPKα (Figure 3B). Cells expressing the highest level of SAPKα showed a significantly reduced speed of random movement (3.55 ± 0.57 μm/min), whereas cells with the lowest level of expression exhibited a speed (5.10 ± 0.62 μm/min) similar to that of the parental strain. These data indicate that SAPKα affects the speed of random motility. Whether the chemotaxis defects are the result of general defects in motility or whether SAPKα also directly affects chemotaxis is not known.
SAPKα Is Involved in Modulating the Actin Cytoskeleton
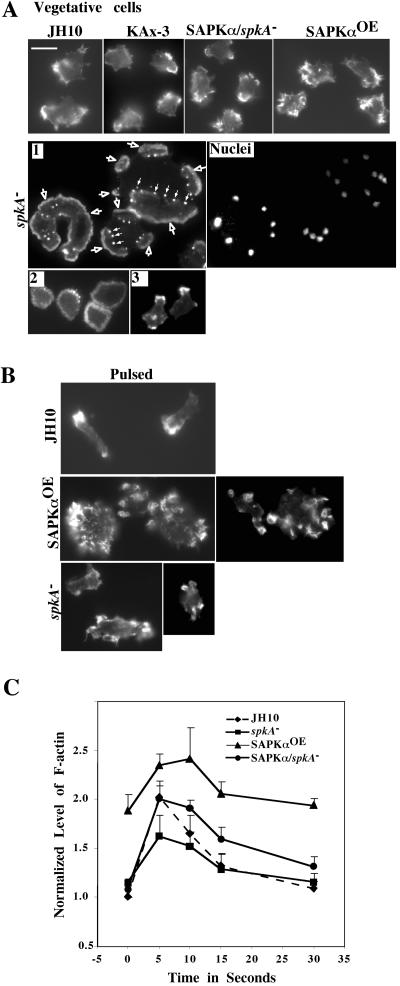
Because spkA null cells exhibit altered aggregation and morphogenesis, we examined the possibility that the SAPKα mutants might affect the actin cytoskeleton. To test this, we examined the distribution of F-actin by using FITC-phalloidin staining of F-actin in the SAPKα mutant strains under various physiological conditions (Figure 5, A and B). In wild-type cells, F-actin localized predominantly to the cortical areas at the sites of cell protrusions. SAPKαOE cells seem to have an increase in the level of F-actin staining and an increase in the number of visible membrane ruffles. spkA null cells exhibited actin staining strikingly distinct from that of wild-type cells. As shown in Figure 5A, we observed three types of staining patterns. In large multinucleated cells, F-actin is localized in flattened lamellipodia (indicated by white arrow with a black center) and eupodia (indicated by white arrow), cortical structures proposed to be responsible for cell-substratum anchorage during amoeboid movement, and occur transiently in multiple units at the base of certain pseudopodia (Fukui and Inoue, 1997). Although these structures are present in a large proportion of spkA null cells, they are only seen occasionally in wild-type cells. Some spkA null cells exhibit a broad ring-like actin staining in the cortex region, which is not normally seen in wild-type cells (Figure 5A, 2). A small fraction of spkA null cells (<10%) look similar to wild-type cells (Figure 5A, 3). In SAPKα reconstituted spkA null cells, the normal, wild-type-cell distribution of F-actin is restored (Figure 5A).
Figure 5.
Abnormal actin organization in spkA null cells and SAPKαOE cells. Cells in the vegetative growth state (A) or after pulsing (B) were plated on cover slips and fixed and stained with phalloidin as described in MATERIALS AND METHODS. For vegetative spkA null cells, representative cells from three different populations were shown (1–3). Population 1 (1) are mainly large, multinucleate cells which were also stained for nuclei. Population 2 (2) are cells with one or two nuclei and show ring-like staining. Population 3 (3) are cells with one or two nuclei and have a staining pattern more like that of parental JH10 cells. White arrows indicate flattened lamellipodia of different sizes typically seen in these cells. Multiple eupodia were also present in theses cells (indicated by white arrows with black centers). Bar, 10 μm. (C) Actin polymerization assay. F-actin levels before and after cAMP stimulation were measured and normalized against the amount of protein.
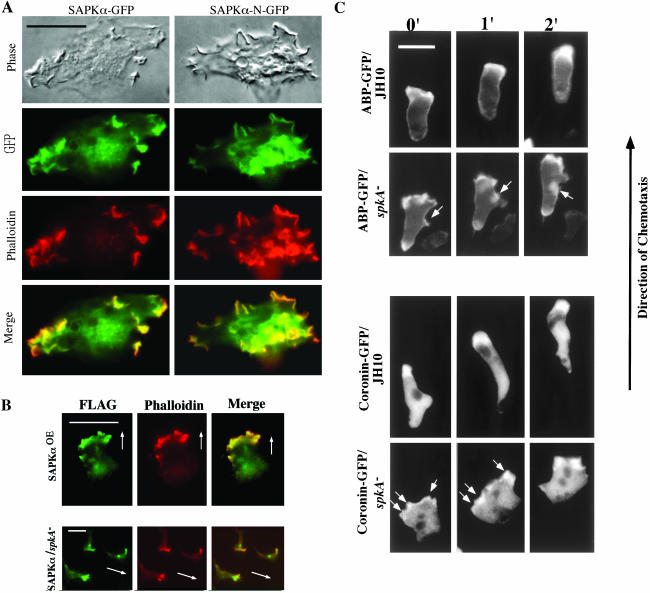
In cells starved for 6 h or pulsed for 6 h with 30 nM cAMP every 6 min to make cells maximally express components of the signaling pathway required for chemotaxis to cAMP (Insall et al., 1994), SAPKαOE cells do not polarize and F-actin localizes to either multiple crown-like structures or ruffles (Figure 5B). Starved spkA null cells do not polarize, and we observed actin staining along the cell cortex. Pulsed spkA null cells elongate, but F-actin localizes along the entire cell cortex in small protrusions along the edge of the cells. These observations suggest that SAPKα may be important for a normal actin cytoskeleton. To further study F-actin levels in SAPKα mutant strains, we examined F-actin levels in pulsed cells before and after cAMP stimulation. Figure 5C shows that SAPKαOE cells have a significantly elevated basal level of F-actin compared with the parental strain as determined by our assay for F-actin levels (see MATERIALS AND METHODS). On chemoattractant stimulation, SAPKαOE exhibited an ∼30% increase, compared with the approximate doubling of F-actin levels in wild-type cells. The level of F-actin after stimulation in SAPKαOE cells was still higher than that in stimulated wild-type cells. In spkA null cells, the basal level of F-actin was similar to that of wild-type cells, but there was only an ∼60% increase after stimulation by cAMP. Because our results suggest that SAPKα is involved in modulating the actin cytoskeleton, we tested whether SAPKα colocalizes with F-actin. Cells expressing either SAPKα-GFP or SAPKα-N-GFP were fixed and stained for actin with TRITC-phalloidin (Figure 6A). The result showed that both full-length SAPKα and the N terminus of SAPKα were enriched in cell surface actin-rich structures, inferring the N-terminal domain mediates the targeting of SAPKα. We also examined the localization of SAPKα-FLAG in chemotaxing cells. SAPKα-FLAG seems to localize in the leading edge of chemotaxing cells. However, as SAPKαOE cells polarized poorly and many cells did not show directional movement up the chemoattractant gradient, we only examined cells that exhibited some polarization. In these cells, SAPKα localized to cell surface protrusions during cell movement (Figure 6B, top). To confirm this result, we examined SAPKα localization during chemotaxis in spkA null cells expressing SAPKα-FLAG. These cells chemotax significantly better than SAPKαOE cells and we still observed localization of SAPKα-FLAG in the leading edge of the cells (Figure 6B, bottom). We used the F-actin–binding proteins ABP120 and coronin fused with GFP to examine F-actin dynamics in spkA null cell chemotaxis (Figure 6C). In JH10 cells, both a GFP fusion of the F-actin–binding domain of ABP120 (ABP-GFP) and coronin-GFP localized to a well-defined single pseudopod at the leading edge of chemotaxing cells, as described previously (Gerisch et al., 1995; Pang et al., 1998). In spkA null cells, ABP-GFP and coronin-GFP had a significantly broader leading edge and we observed labeling at sites along the lateral edge and posterior of cells. These combined results suggest that SAPKα is involved in cortical F-actin organization.
Figure 6.
Localization of SAPKα. (A) Wild-type cells expressing full-length SAPKα-GFP or N-terminal SAPKα-GFP were pulsed with cAMP, fixed, and stained with TRITC-phalloidin as described in MATERIALS AND METHODS. Phase contrast and fluorescence images indicate that SAPKα was enriched in multiple actin-rich structures on the cell surface. Bar, 5 μm. (B) Localization of SAPKα in chemotaxing cells. Wild-type cells (top) or spkA null cells (bottom) expressing full-length SAPKα-FLAG were pulsed with cAMP and cells were plated onto cover slips and allowed to aggregate. Cells were then fixed and stained with TRITC-phalloidin and anti-FLAG mAb as described in MATERIALS AND METHODS. White arrows indicate the directions of movement. Bar, 10 μm. (C) Chemotaxis of spkA null cells was examined using ABP-GFP and coronin-GFP as markers. In JH10 cells, both ABP-GFP and coronin-GFP localized to a well-defined leading edge pseudopodium. spkA null cells, however, had a broader leading edge and both markers localized to the lateral cortex (as indicated by arrows).
SAPKα Is Activated by Stress and Required for Full Osmoprotection
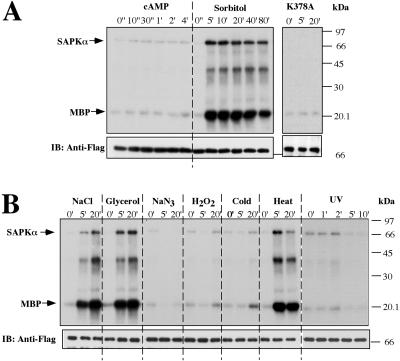
In response to cAMP stimulation during chemotaxis or to osmotic shock, the actin cytoskeleton undergoes a major reorganization. We therefore examined whether SAPKα kinase activity increases in response to these stimuli (see MATERIALS AND METHODS). As depicted in Figure 7A, despite the strong developmental and motility defects exhibited by SAPKαOE cells and spkA null cells, SAPKα kinase was not detectably activated by cAMP stimulation in wild-type cells. In contrast, hyperosmotically shocking cells by treatment with 0.4 M sorbitol resulted in a very significant activation of SAPKα kinase activity in 5 min, as observed by the phosphorylation of the heterologous substrate MBP. There is a subsequent gradual decline in kinase activity. The kinetics of increase in kinase activity are similar to those of other responses stimulated by osmotic shock in Dictyostelium and much slower than responses stimulated by chemoattractants, which normally peak between 5 and 40 s, depending on the response (Kuwayama et al., 1996; Oyama, 1996). The putative kinase dead SAPKα, SAPKαK378A, did not have any kinase activity. In addition to the phosphorylation of MBP, we observed the phosphorylation of a band that comigrates with tagged SAPKα. We suggest that this is autophosphorylated SAPKα rather than being due to another protein that is phosphorylated by and coimmunoprecipitates and comigrates with SAPKα. This labeled band is not observed when kinase dead SAPKα is assayed, indicating this band is probably not due to another kinase. In addition, we observed a third phosphorylated band with a mobility corresponding to ∼40 kDa, which is not detected in assays by using kinase dead SAPKα. We suggest that this protein coimmunoprecipitates with and is phosphorylated by SAPKα.
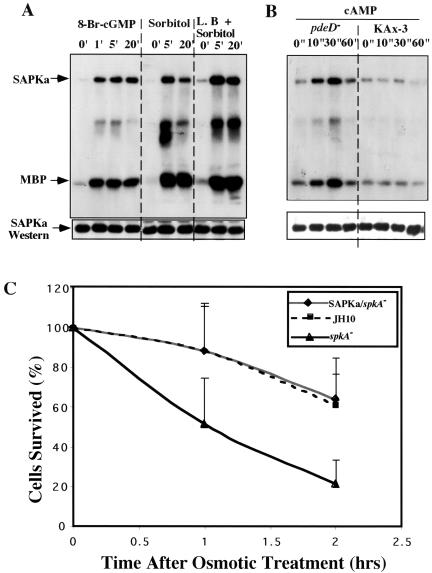
Figure 7.
Activation of SAPKα by various stimuli. (A) Activation of SAPKα by osmotic shock but not by cAMP. For cAMP stimulation, aggregation-competent cells made by pulsing (see MATERIALS AND METHODS) were stimulated with 100 μM cAMP. For osmotic shock stimulation, vegetative cells were washed with Na/K phosphate buffer, starved for 1 h, and treated with 0.4 M sorbitol. Aliquots were taken at the indicated time points. Cells were lysed, SAPKα or its mutant was immunoprecipitated with anti-FLAG mAb, and kinase activity was measured as described in MATERIALS AND METHODS. The kinase dead SAPKα K378A was used as a negative control. (B) Activation of SAPKα in other stress conditions. To confirm the osmotic response of SAPKα, 0.4 M NaCl, and 0.4 M glycerol were also tested. For heat shock treatment, cells were incubated at 30°C. For cold shock, cells were incubated at 4°C. For UV treatment, cells were placed in a dish on a shaker and exposed to 300-nm UV light. Cells were also treated with 3 mM H2O2 and 1 mM NaN3. The top band shows the autophosphorylated SAPKα. The bottom band shows phosphorylated MBP. The middle band might represent a protein coimmunoprecipitated and phosphorylated by SAPKα. To examine the immunoprecipitated SAPKα protein level, a Western blot was carried out on the same blot used for kinase assay (bottom).
To further test whether SAPKα is involved in generally responding to cellular stress, we examined the effects of other stress conditions on SAPKα kinase activity (Figure 7B). Other hyperosmotic stresses, NaCl and glycerol, produced a similar activation of SAPKα. Heat shock at 30°C for 5 min strongly activated the kinase. However, cold shock at 4°C or treatment of cells with NaN3 or H2O2 did not increase kinase activity. Although spkA null cells seemed to round up faster than JH10 cells (our unpublished data), SAPKα was not activated by UV light treatment. We think that the faster rounding-up of spkA null cells may be due to an adhesion defect as discussed below (see Figure 9C). Our results suggest that SAPKα kinase activity is selectively regulated by certain stress responses and may play a role in these responses.
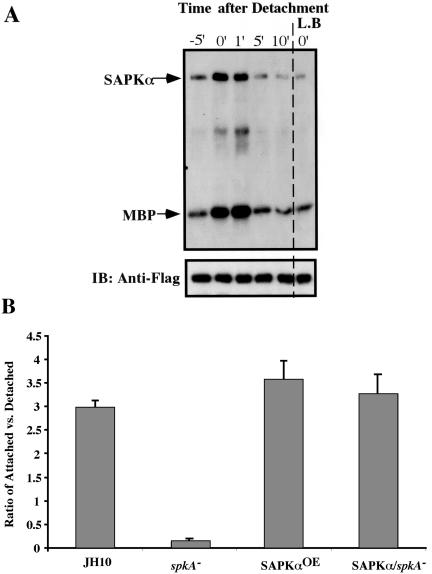
Figure 9.
SAPKα affects cell adhesion. (A) Activation of SAPKα by detachment. Cells were plated onto culture plates and allowed to attach for 30 min. Cells were then quickly resuspended by pipetting and transferred to shaking culture. At the time point indicated, cells were lysed in an equal volume of 2× lysis buffer and SAPKα kinase activity was determined accordingly. For the 0-min time point, cells were lysed immediately after detachment from the plate. For the -5-min time point, cells were lysed 5 min before the experiment directly on the plate by adding 1× lysis buffer. Kinase activation by detachment was also measured after treatment of cells with 1 μM latrunculin B. (B) Cell-substratum adhesion assay on spkA null and SAPKαOE cells. Cell adhesion assays were performed using nitrocellulose filters as described in MATERIALS AND METHODS. Adhesion was measured by the ratio of attached compared with unattached cells. Experiments were performed at least three times. Bar, 10 μm.
It was shown previously that guanylyl cyclase is activated within a few minutes after osmotic shock, resulting in large increases in cGMP (Kuwayama et al., 1996; Oyama, 1996; Roelofs and Van Haastert, 2002). Total intracellular and extracellular cGMP levels start to rise significantly after 2 min, reach a peak at ∼10 min, and slowly decrease. Intracellular cGMP levels peak at 3–5 min. To test whether SAPKα can be activated by cGMP, we assayed SAPKα kinase activity after addition of the membrane-permeable cGMP (8-Br-cGMP). Figure 8A indicates that 8-Br-cGMP addition results in a rapid increase in SAPKα activity, although the level is lower than that observed after sorbitol treatment. The fact that SAPKα was activated by several stress treatments suggests that SAPKα might be required for cells to survive osmotic stress. Figure 8B shows that spkA null cells exhibit a decreased resistance to osmotic stress. This increased sensitivity was complemented by expression of SAPKα in these cells.
Figure 8.
SAPKα provides protection against osmotic stress. (A) SAPKα activation by 8-Br-cGMP. A kinase assay was performed with the addition of 20 mM 8-Br-cGMP, a cell membrane-permeable cGMP. As a positive control, cells were treated with 0.4 M sorbitol. To examine the effects of latrunculin B, cells were treated with 1 μM latrunculin B (L.B.) for 10 min before osmotic stress stimulation. (B) SAPKα activation by cAMP in pdeD null cells. Cells were pulsed and stimulated with 150 μM cAMP for 5 h and SAPKα kinase assays were performed as usual. (C) Cell survival after osmotic stress. Cells were shaken in Na/K phosphate buffer in the presence or absence of 0.4 M sorbitol for the times indicated, diluted with buffer, and plated onto SM agar plates with Klebsiella aerogenes bacteria. Cell viability was determined as the percentage of colonies compared with wild-type controls.
Because cGMP can activate SAPKα kinase activity, we reinvestigated chemoattractant activation of SAPKα in cells lacking the cGMP phosphodiesterase (pdeD null cells) primarily responsible for degrading cGMP in response to cAMP stimulation (Meima et al., 2002). In this phosphodiesterase null mutation, cAMP-stimulated CGMP levels are highly elevated. As shown in Figure 8B, cAMP does result in a low-level activation of SAPKα kinase activity in PDE null but not wild-type cells with kinetics of activation similar to those of the kinetics of cGMP accumulation. The results suggest that chemoattractants may stimulate SAPKα kinase activity in wild-type cells but the level of activation is too low to be measured using our assay.
SAPKα Is Activated by Detachment and Might Be Involved in Cell Adhesion
Our experiments assayed sensitivity to stress in cells grown in suspension culture (Figure 7). We also performed experiments to test the effect of UV irradiation on SAPKα activity by using attached cells, because spkA null cells grown on Petri plates seemed to round up more rapidly than wild-type cells after treatment with UV light (our unpublished data). Unexpectedly, when cells grown on plates were assayed for SAPKα kinase activity, we observed elevated SAPKα activity in unstimulated cells, immediately after they were detached from the substratum (comparable with unstimulated cells grown in suspension; our unpublished data). These data suggested that the detachment of cells from the plate resulted in SAPKα kinase activation. To test this possibility, we assayed SAPKα kinase activity at different times after detachment from Petri plates and in suspension in buffer. The result showed that SAPKα was activated upon cell detachment, followed by its inactivation within 5 min (Figure 9B). We think this activation occurred after cell detachment because, in a control experiment in which cells were lysed directly on a plate before detachment, the kinase activity was significantly lower (Figure 9B, lane 1). This proposed activation by detachment probably takes place through a different mechanism than by osmotic shock, because latrunculin B treatment of cells inhibits the detachment-mediated activation of SAPKα but has no effect on the osmotic shock stimulated activation (Figures 8A and 9B). Because latrunculin B specifically disrupts the F-actin cytoskeleton, it is possible that cell detachment-mediated activation is transmitted through the actin cytoskeleton.
spkA null cells are more easily detached from Petri dishes than wild-type cells and can be readily detached by gentle pipetting. Figure 9B quantifies the attachment and demonstrates that spkA null cells exhibit a greatly reduced adhesion to the substratum compared with wild-type, SAPKαOE, and SAPKα/spkA null cells (see MATERIALS AND METHODS).
DISCUSSION
We have identified a putative novel member of the MEKK class of kinases that are most closely related to the MLK subfamily. The N terminus of SAPKα, which is required for SAPKα subcellular localization, contains five ankyrin repeats and a SAM domain, which we expect mediate protein– protein interactions. Analysis of the phenotypes of spkA null and SAPKα overexpressing cells suggests that disruption of normal SAPKα function affects various cellular processes, presumably due to the altered actin cytoskeleton observed in these cells. Both strains exhibit defects in cell motility, morphogenesis, and aggregation. spkA null cells also have a reduced adhesion to the substratum and seem to have increased number of eupodia, a cytoskeletal structure that has been linked in Dictyostelium to the substratum adhesion. Overexpression of SAPKα leads to very elevated F-actin levels in unstimulated cells that increase further upon stimulation. However, the ability of spkA null cells to increase F-actin levels in response to chemoattractant stimulation, albeit to only 50% of the level observed in stimulated wild-type cells, suggests SAPKα is not essential for this process. Furthermore, SAPKα is associated with the cytoskeletal cortex in response to chemoattractant stimulation. Our results indicate that both spkA null and SAPKα overexpressing cells exhibit general motility defects, and SAPKα is not activated by cAMP in a wild-type background. It is therefore possible that the effects of SAPKα mutants or overexpressor strain on chemotaxis are indirect. However, we cannot completely rule out a possible involvement of SAPKα in chemotaxis, because we observe a low-level stimulation of SAPKα kinase activity in cells lacking one of the cGMP PDEs, demonstrating that the chemoattractant pathway is able to activate this response. If SAPKα does play a role in chemotaxis, we expect that SAPKα is activated in wild-type cells in response to chemoattractant stimulation, but the activity may be highly localized within the cell or highly transient and thus may not be detectable in our assays.
Interestingly, SAPKα kinase is rapidly activated in response to several stress stimuli, including heat and osmotic shock. We found that SAPKα activity can be stimulated by 8-Br-cGMP, a membrane-permeant cGMP. cGMP levels rapidly increase in response to osmotic stress to a point that is significantly higher than their activation in response to chemoattractant stimulation, and guanylyl cyclase activation has been linked to stress survival. The activation of SAPKα by several stress stimuli and 8-Br-cGMP suggests that SAPKα is a major downstream effector pathway controlling the cell's response to certain stress stimuli. Consistent with this result, spkA null cells exhibit a reduced ability to survive osmotic shock treatment. At present, we do not know which pathway lies between cGMP and SAPKα activation, although recently two cGMP binding proteins that are putative downstream effectors of cGMP have been identified and may lie upstream of SAPKα.
In addition, SAPKα is stimulated by detaching cells adhered to a substratum. This stimulation does not occur when cells are pretreated with latrunculin B, suggesting that a cytoskeleton-mediated pathway may sense the disruption that leads to SAPKα stimulation. This may parallel responses of mammalian cells, which also stimulate stress-related pathways upon detachment from the substratum. In mammalian cells, cell detachment activates the p38 MAP kinase, which is required for detachment-induced apoptosis (Rosen et al., 2002). There is evidence suggesting that MAP kinase pathways may participate in modulating focal adhesion and/or cell adhesion (Schneider et al., 1998; Takino et al., 2002). Alternatively, as detachment by pipetting (the method used in this article) applies mechanical force to cells, it is possible that shear stress may also trigger signaling pathways leading to SAPKα activation. In mammalian cells, shear stress activates MAP kinases and may participate in wound healing and other cellular responses (Kippenberger et al., 2000a,b; Langille, 2001).
The level of cGMP produced in response to chemoattractant stimulation is significantly lower than that produced in stress pathways. It is possible that this lower level of cGMP production may be insufficient to induce an observable SAPKα activity if cGMP is the sole mechanism of SAPKα activation. However, it is possible that low levels of SAPKα activity may be activated by basal or chemoattractant-stimulated levels of cGMP, and these levels of kinase activity may suffice to mediate cellular responses under these physiological conditions but may be insufficient to mediate an observed increase in biochemical activity that is measurable in our assays.
In conclusion, we have identified a novel MEK kinase-like kinase that is a downstream effector of several stress-induced pathways. Results suggest that SAPKα might be involved in a number of actin-based cellular processes. The presence of an MEKK-like domain suggests that SAPKα may lie at the top of a MAP kinase cascade, although downstream components have not been identified. The N-terminal domain mediates subcellular localization, presumably through protein–protein interactions. Although SAPKα does not have a direct ortholog in mammalian cells, there are significant parallels between SAPKα function and the function of members of the MLK subfamily of MEK kinases and the role of MAP kinases in stress and detachment-mediated responses. For example, MLK family members mediate the activation of p38 as well as JNK MAPK pathways by multiple stimuli, including cellular stresses (Kyriakis and Avruch, 2001). One of these, MLTKβ, induces actin cytoskeleton reorganization through the p38 MAPK pathway and is activated by osmotic stress (Gotoh et al., 2001). Furthermore, the p38 MAPK pathway directly phosphorylates the actin polymerization modifier HSP27 and regulates actin reorganization (Landry and Huot, 1995, 1999). This finding suggests that the Dictyostelium SAPKα-regulated pathway may have direct parallels in other systems.
Acknowledgments
We thank M. Meima and P. Schaap for the PDE null mutant and members of the Firtel laboratory for helpful suggestions and comments during the course of this work. The work was supported by a U.S. Public Health Service grant to R.A.F.
References
- Aizawa, H., Katadae, M., Maruya, M., Sameshima, M., Murakami-Murofushi, K., and Yahara, I. (1999). Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells 4, 311-324. [DOI] [PubMed] [Google Scholar]
- Aubry, L., and Firtel, R. (1999). Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15, 469-517. [DOI] [PubMed] [Google Scholar]
- Canman, C.E., and Kastan, M.B. (1996). Signal transduction. Three paths to stress relief. Nature 384, 213-214. [DOI] [PubMed] [Google Scholar]
- Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410, 37-40. [DOI] [PubMed] [Google Scholar]
- Chaudoir, B.M., Kowalczyk, P.A., and Chisholm, R.L. (1999). Regulatory light chain mutations affect myosin motor function and kinetics. J. Cell Sci. 112, 1611-1620. [DOI] [PubMed] [Google Scholar]
- Chen, M.Y., Insall, R.H., and Devreotes, P.N. (1996). Signaling through chemoattractant receptors in Dictyostelium. Trends Genet. 12, 52-57. [DOI] [PubMed] [Google Scholar]
- Chung, C.Y., and Firtel, R.A. (1999). PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J. Cell Biol. 147, 559-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C.Y., Funamoto, S., and Firtel, R.A. (2001). Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem. Sci. 26, 557-566. [DOI] [PubMed] [Google Scholar]
- Chung, C.Y., Reddy, T.B., Zhou, K., and Firtel, R.A. (1998). A novel, putative MEK kinase controls developmental timing and spatial patterning in Dictyostelium and is regulated by ubiquitin-mediated protein degradation. Genes Dev. 12, 3564-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, S., and Firtel, R.A. (1987). Identification of the sequences controlling cyclic AMP regulation and cell-type specific expression of a prestalk-specific gene in Dictyostelium discoideum. Mol. Cell. Biol. 7, 149-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos, E.L., Rehfuess, C., Bradtke, B., Waddell, D.R., Albrecht, R., Murphy, J., and Gerisch, G. (1993). Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J. Cell Biol. 120, 163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff, T.T., Lee, R.J., and Spudich, J.A. (1993). Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 75, 363-371. [DOI] [PubMed] [Google Scholar]
- Faure, M., Franke, J., Hall, A.L., Podgorski, G.J., and Kessin, R.H. (1990). The cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum contains 3 promoters specific for growth, aggregation, and late development. Mol. Cell. Biol. 10, 1921-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel, R.A., and Chung, C.Y. (2000). The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. Bioessays 22, 603-615. [DOI] [PubMed] [Google Scholar]
- Frye, C.A., Tang, D., and Innes, R.W. (2001). Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA 98, 373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui, Y., De Lozanne, A., and Spudich, J.A. (1990). Structure and function of the cytoskeleton of a Dictyostelium myosin-defective mutant. J. Cell Biol. 110, 367-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui, Y., and Inoue, S. (1997). Amoeboid movement anchored by eupodia, new actin-rich knobby feet in Dictyostelium. Cell Motil. Cytoskeleton 36, 339-354. [DOI] [PubMed] [Google Scholar]
- Gallo, K.A., and Johnson, G.L. (2002). Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat. Rev. Mol. Cell. Biol. 3, 663-672. [DOI] [PubMed] [Google Scholar]
- Gamper, M., Kim, E., Howard, P.K., Ma, H., Hunter, T., and Firtel, R.A. (1999). Regulation of Dictyostelium protein-tyrosine phosphatase-3 (PTP3) through osmotic shock and stress stimulation and identification of pp130 as a PTP3 substrate. J. Biol. Chem. 274, 12129-12138. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., Albrecht, R., Heizer, C., Hodgkinson, S., and Maniak, M. (1995). Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using green fluorescent protein-coronin fusion protein. Curr. Biol. 5, 1280-1285. [DOI] [PubMed] [Google Scholar]
- Gotoh, I., Adachi, M., and Nishida, E. (2001). Identification and characterization of a novel MAP kinase kinase kinase, MLTK. J. Biol. Chem. 276, 4276-4286. [DOI] [PubMed] [Google Scholar]
- Gustin, M.C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows, K.R., Law, F.Y., Packman, C.H., and Knauf, P.A. (1996). Changes in cytoskeletal actin content, F-actin distribution, and surface morphology during HL-60 cell volume regulation. J. Cell. Physiol. 167, 60-71. [DOI] [PubMed] [Google Scholar]
- Hirai, S., Katoh, M., Terada, M., Kyriakis, J.M., Zon, L.I., Rana, A., Avruch, J., and Ohno, S. (1997). MST/MLK2, a member of the mixed lineage kinase family, directly phosphorylates and activates SEK1, an activator of c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 272, 15167-15173. [DOI] [PubMed] [Google Scholar]
- Hirt, H. (2000). MAP kinases in plant signal transduction. Results Probl. Cell Differ. 27, 1-9. [DOI] [PubMed] [Google Scholar]
- Howard, T.H., and Oresajo, C.O. (1985). A method for quantifying F-actin in chemotactic peptide activated neutrophils: study of the effect of tBOC peptide. Cell Motil. 5, 545-557. [DOI] [PubMed] [Google Scholar]
- Howard, P.K., Sefton, B.M., and Firtel, R.A. (1993). Tyrosine phosphorylation of actin in Dictyostelium associated with cell-shape changes. Science 259, 241-244. [DOI] [PubMed] [Google Scholar]
- Ichijo, H. (1999). From receptors to stress-activated MAP kinases. Oncogene 18, 6087-6093. [DOI] [PubMed] [Google Scholar]
- Iijima, M., Huang, Y.E., and Devreotes, P. (2002). Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469-478. [DOI] [PubMed] [Google Scholar]
- Insall, R.H. (1996). Cyclic GMP and the big squeeze. Osmoregulation. Curr. Biol. 6, 516-518. [DOI] [PubMed] [Google Scholar]
- Insall, R.H., Soede, R.D.M., Schaap, P., and Devreotes, P.N. (1994). Two cAMP receptors activate common signaling pathways in Dictyostelium. Mol. Biol. Cell 5, 703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak, C., Kiegerl, S., Ligterink, W., Barker, P.J., Huskisson, N.S., and Hirt, H. (1996). Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc. Natl. Acad. Sci. USA 93, 11274-11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth, A., Eckerskorn, C., Gerisch, G., Lottspeich, F., Stocker, S., and Schweiger, A. (1995). Stress-induced tyrosine phosphorylation of actin in Dictyostelium cells and localization of the phosphorylation site to tyrosine-53 adjacent to the DNase I binding loop. FEBS Lett. 375, 87-90. [DOI] [PubMed] [Google Scholar]
- Jungbluth, A., Vonarnim, V., Biegelmann, E., Humbel, B., Schweiger, A., and Gerisch, G. (1994). Strong increase in the tyrosine phosphorylation of actin upon inhibition of oxidative phosphorylation: correlation with reversible rearrangements in the actin skeleton of Dictyostelium cells. J. Cell Sci. 107, 117-125. [DOI] [PubMed] [Google Scholar]
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.A., and Ecker, J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427-441. [DOI] [PubMed] [Google Scholar]
- Kippenberger, S., Bernd, A., Loitsch, S., Guschel, M., Muller, J., Bereiter-Hahn, J., and Kaufmann, R. (2000a). Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Investig. Dermatol. 114, 408-412. [DOI] [PubMed] [Google Scholar]
- Kippenberger, S., Loitsch, S., Muller, J., Guschel, M., Ramirez-Bosca, A., Kaufmann, R., and Bernd, A. (2000b). Melanocytes respond to mechanical stretch by activation of mitogen-activated protein kinases (MAPK). Pigment Cell Res. 13, 278-280. [DOI] [PubMed] [Google Scholar]
- Kuwayama, H., Ecke, M., Gerisch, G., and van Haastert, P.J.M. (1996). Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science 271, 207-209. [DOI] [PubMed] [Google Scholar]
- Kuwayama, H., and Van Haastert, P.J. (1998). Chemotactic and osmotic signals share a cGMP transduction pathway in Dictyostelium discoideum. FEBS Lett. 424, 248-252. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J.M., and Avruch, J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807-869. [DOI] [PubMed] [Google Scholar]
- Landry, J., and Huot, J. (1995). Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem. Cell Biol. 73, 703-707. [DOI] [PubMed] [Google Scholar]
- Landry, J., and Huot, J. (1999). Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27). Biochem. Soc. Symp. 64, 79-89. [PubMed] [Google Scholar]
- Langille, B.L. (2001). Morphologic responses of endothelium to shear stress: reorganization of the adherens junction. Microcirculation 8, 195-206. [DOI] [PubMed] [Google Scholar]
- Li, S., and Wilkinson, M.F. (1997). Site-directed mutagenesis: a two-step method using PCR and DpnI. Biotechniques 23, 588-590. [DOI] [PubMed] [Google Scholar]
- Ma, H., Gamper, M., Parent, C., and Firtel, R.A. (1997). The Dictyostelium MAP kinase kinase DdMEK1 regulates chemotaxis and is essential for chemoattractant-mediated activation of guanylyl cyclase. EMBO J. 16, 4317-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, S.K.O., and Firtel, R.A. (1991). A developmentally regulated, putative serine/threonine protein kinase is essential for development in Dictyostelium. Mech. Dev. 35, 89-101. [DOI] [PubMed] [Google Scholar]
- Mann, S.K.O., Yonemoto, W.M., Taylor, S.S., and Firtel, R.A. (1992). DdPK3, which plays essential roles during Dictyostelium development, encodes the catalytic subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89, 10701-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstein, D.J., Titus, M.A., De Lozanne, A., and Spudich, J.A. (1989). Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 8, 923-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili, R., Ellsworth, C., Lee, S., Reddy, T.B., Ma, H., and Firtel, R.A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meima, M.E., Biondi, R.M., and Schaap, P. (2002). Identification of a novel type of cGMP phosphodiesterase that is defective in chemoattractant stmF mutants. Mol. Biol. Cell. 13, 3870-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, S., Lee, S., Yadava, N., Dealy, M.J., Johnson, R.S., and Firtel, R.A. (2001). Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium. Genes Dev. 15, 1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen, W., Datta, S., Reymond, C., Sivertsen, A., Mann, S., Crowley, T., and Firtel, R.A. (1987). Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 28, 67-100. [DOI] [PubMed] [Google Scholar]
- Nishida, E., and Gotoh, Y. (1993). The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18, 128-131. [DOI] [PubMed] [Google Scholar]
- O'Rourke, S.M., Herskowitz, I., and O'Shea, E.K. (2002). Yeast go the whole HOG for the hyperosmotic response. Trends Genet. 18, 405-412. [DOI] [PubMed] [Google Scholar]
- Ott, A., Oehme, F., Keller, H., and Schuster, S.C. (2000). Osmotic stress response in Dictyostelium is mediated by cAMP. EMBO J. 19, 5782-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama, M. (1996). cGMP accumulation induced by hypertonic stress in Dictyostelium discoideum. J. Biol. Chem. 271, 5574-5579. [PubMed] [Google Scholar]
- Pang, K.M., Lee, E., and Knecht, D.A. (1998). Use of a fusion protein between GFP and an actin-binding domain to visualize transient filamentous-actin structures. Curr. Biol. 8, 405-408. [DOI] [PubMed] [Google Scholar]
- Parent, C.A., and Devreotes, P.N. (1999). A cell's sense of direction. Science 284, 765-770. [DOI] [PubMed] [Google Scholar]
- Ponting, C.P. (1995). SAM: a novel motif in yeast sterile and Drosophila polyhomeotic proteins. Protein Sci. 4, 1928-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon, M., Flavell, R.A., and Davis, R.A. (2000). The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic. Biol. Med. 28 1328-1337. [DOI] [PubMed] [Google Scholar]
- Rivero, F., Koppel, B., Peracino, B., Bozzaro, S., Siegert, F., Weijer, C.J., Schleicher, M., Albrecht, R., and Noegel, A.A. (1996). The role of the cortical cytoskeleton: F-actin crosslinking proteins protect against osmotic stress, ensure cell size, cell shape and motility, and contribute to phagocytosis and development. J. Cell Sci. 109, 2679-2691. [DOI] [PubMed] [Google Scholar]
- Rizoli, S.B., Rotstein, O.D., Parodo, J., Phillips, M.J., and Kapus, A. (2000). Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am. J. Physiol. Cell Physiol. 279, C619-C633. [DOI] [PubMed] [Google Scholar]
- Roelofs, J., and Van Haastert, P.J. (2002). Characterization of two unusual guanylyl cyclases from Dictyostelium. J. Biol. Chem. 277, 9167-9174. [DOI] [PubMed] [Google Scholar]
- Rogers, K.C., Ginsburg, G.T., Mu, X., Gollop, R., Balint-Kurti, P., Louis, J.M., and Kimmel, A.R. (1997). The cAMP receptor gene family of Dictyostelium discoideum: expression, regulation, function. In: Dictyostelium: A Model System for Cell and Developmental Biology, ed. Y. Maeda, K. Inouye, and I. Takeuchi, Tokyo: Universal Academy Press, 163-172.
- Rosen, K., Shi, W., Calabretta, B., and Filmus, J. (2002). Cell detachment triggers p38 mitogen-activated protein kinase-dependent overexpression of Fas ligand. J. Biol. Chem. 277, 46123-46130. [DOI] [PubMed] [Google Scholar]
- Rousseau, S., Houle, F., Landry, J., and Huot, J. (1997). p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15, 2169-2177. [DOI] [PubMed] [Google Scholar]
- Saxe III, C. L., Johnson, R.L., Devreotes, P.N., and Kimmel, A.R. (1991). Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Dev. 5, 1-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger, T.K., Fanger, G.R., Yujiri, T., and Johnson, G.L. (1998). The TAO of MEKK. Front. Biosci. 3, D1181-D1186. [DOI] [PubMed] [Google Scholar]
- Schneider, G.B., Hamano, H., and Cooper, L.F. (1998). In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J. Cell Physiol. 177, 575-584. [DOI] [PubMed] [Google Scholar]
- Shaulsky, G., Fuller, D., and Loomis, W.F. (1998). A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development 125, 691-699. [DOI] [PubMed] [Google Scholar]
- Sobko, A., Ma, H., and Firtel, R.A. (2002). Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev. Cell 2, 745-756. [DOI] [PubMed] [Google Scholar]
- Takino, T., Yoshioka, K., Miyamori, H., Yamada, K.M., and Sato, H. (2002). A scaffold protein in the c-Jun N-terminal kinase signaling pathway is associated with focal adhesion kinase and tyrosine-phosphorylated. Oncogene 21, 6488-6497. [DOI] [PubMed] [Google Scholar]
- Thomason, P.A., Traynor, D., Stock, J.B., and Kay, R.R. (1999). The RdeA-RegA system, a eukaryotic phospho-relay controlling cAMP breakdown. J. Biol. Chem. 274, 27379-27384. [DOI] [PubMed] [Google Scholar]
- Treisman, R. (1996). Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8, 205-215. [DOI] [PubMed] [Google Scholar]
- Watts, D.J., and Ashworth, J.M. (1970). Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J. 119, 171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, D., Voss, E., Von Bergen, N., Burns, R., Stites, J., and Soll, D.R. (1998). A computer-assisted system for reconstructing and interpreting the dynamic three-dimensional relationships of the outer surface, nucleus and pseudopods of crawling cells. Cell Motil. Cytoskeleton 41, 225-246. [DOI] [PubMed] [Google Scholar]
- Zang, J.H., Cavet, G., Sabry, J.H., Wagner, P., Moores, S.L., and Spudich, J.A. (1997). On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell 8, 2617-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zischka, H., Oehme, F., Pintsch, T., Ott, A., Keller, H., Kellermann, J., and Schuster, S.C. (1999). Rearrangement of cortex proteins constitutes an osmoprotective mechanism in Dictyostelium. EMBO J. 18, 4241-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]