Abstract
Estrogens have been linked to a higher female incidence of autoimmune diseases. The role of androgen and the androgen receptor (AR) in autoimmune diseases, however, remains unclear. Here we report that the lack of AR in B cells in different strains of mice, namely general AR knockout, B cell-specific AR knockout, and naturally occurring testicular feminization mutation AR-mutant mice, as well as castrated wild-type mice, results in increased B cells in blood and bone marrow. Analysis of the targeted mice, together with bone marrow transplantation using Rag1−/− recipients, overexpression of retrovirally encoded AR-cDNA, and small interfering RNA-mediated AR mRNA knockdown approaches also show that the B cell expansion results from resistance to apoptosis and increased proliferation of bone marrow precursor B cells, accompanied by changes in several key modulators related to apoptosis, such as Fas/FasL signals, caspases-3/-8, nuclear factor-κB, and Bcl-2. We also show that the effects of AR loss are, in part, B cell intrinsic. Mice bearing AR-deficient B cells show increased levels of serum IgG2a and IgG3 as well as basal double-stranded DNA-IgG antibodies and are more vulnerable to development of collagen-induced arthritis. Together, these data indicate that androgen/AR play a crucial role in B cell homeostasis and tolerance. Therapies targeting AR might provide an alternative strategy with which to battle autoimmune diseases.
Loss of B cells androgen receptor in mice results in increased blood/bone marrow B cells demonstrating a role for androgen/AR in B cell homeostasis/tolerance.
Androgen receptor (AR) (1,2) is a member of the nuclear receptor superfamily (3,4) the role of which in B cell development and function is unclear. Early studies suggest that both B cells and stromal cells within the bone marrow express AR (5,6) and that castration or AR mutations in mice result in increased B cells in the peripheral blood (7,8). The inhibitory effect of sex hormones on B cells is required for the function of the hormone receptors (8). The intrinsic effects of androgens and influence of AR mutations on B cell development within bone marrow, however, remain to be elucidated.
Many autoimmune diseases are known to display a strong association with the female, and sex hormones including androgen have been shown to exert a number of immunoregulatory activities in B lymphocytes (9,10). The causal relationship between sex hormones and susceptibility to autoimmunity is consistent with the observation that male rheumatoid arthritis patients display low levels of androgens (11,12,13) and that prostate cancer patients treated with androgen-ablation therapy were reported to have an increased incidence of rheumatoid arthritis (14).
To further address the role of AR function in B cell development and the relationship of AR to the incidence of autoimmune disease, we applied conditional knockout strategies to ablate AR function in B cells. We found that the lack of AR influenced B cell numbers in the bone marrow, peripheral blood, and spleen. B cell ontogeny studies revealed that loss of AR affected the development of immature B cells in the bone marrow. The increased number of B cells was associated with the inhibition of apoptosis via Fas/Fas ligand (FasL) and caspase pathways. In addition, we show that collagen-induced arthritis (CIA) is more severe in mice bearing AR-deficient B cells compared with wild-type (Wt) controls, suggesting that AR might play an important role in the regulation of B cell tolerance in specific autoimmune diseases.
Results
General AR knockout mice (G-ARKO) have increased B cells in blood and bone marrow
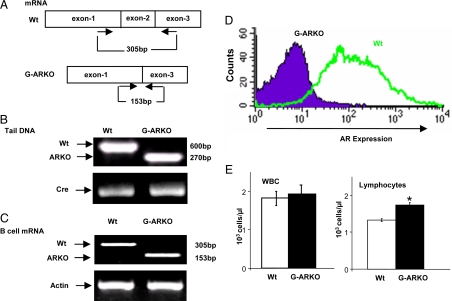
G-ARKO (C57BL6/FVB) mice, bearing a deletion of the AR DNA-binding domain (Fig. 1A), were obtained using a previously reported conditional Cre-lox recombination-based strategy (15) and confirmed through genotyping of tail snips (Fig. 1, A and B). RT-PCR analysis of G-ARKO bone marrow B cells showed the diagnostic alteration of AR mRNA (Fig. 1C). Loss of AR protein expression in bone marrow B cells was confirmed by flow cytometry analysis (Fig. 1D). Automated analysis of hematological parameters showed no significant difference between G-ARKO and Wt mice in red blood cells, platelets, hemoglobin, hematocrit (data not shown), and total white blood cells (Fig. 1E), but the total number of lymphocytes was significantly increased in G-ARKO mice compared with Wt mice (Fig. 1E).
Figure 1.
Characterization of G-ARKO mice (A) G-ARKO (C57BL6/FVB) mice, bearing a deletion of the AR DNA binding domain (exon 2). B, Genotyping of tissue DNA isolated from G-ARKO and Wt littermates using “select and 2–9” primers; 600 bp represents Wt AR and 270 bp represents G-ARKO. C, We used the primers 5′-AATGGGACCTTGGATGGAGAAC-3′ and 5′-TCCCTGCTTCATAACATTTCCG-3′ for RT-PCR, to obtain an AR transcript of 305 bp from Wt AR, but only 153 bp when exon 2 is deleted. Full-length AR mRNA is not expressed in B cells from the bone marrow of G-ARKO mice. D, FACS analysis shows intracellular AR protein is not detected in B cells from the bone marrow of G-ARKO mice. E, Automated blood analysis shows peripheral blood lymphocytes, but not total white blood cells (WBC), are increased in G-ARKO (n = 24) compared with Wt (n =18). Data are mean ± sd. *, P < 0.05.
Loss of AR in different strains of mice results in increased developing bone marrow B cells
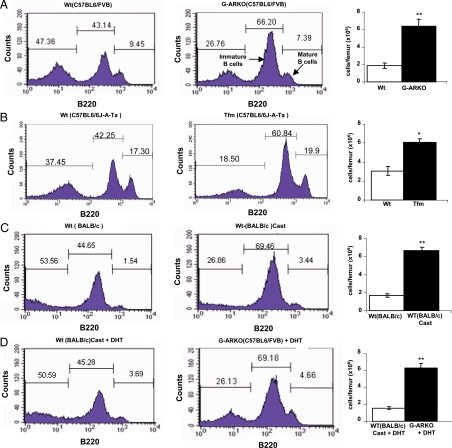
Flow cytometry analysis of the distribution and the absolute number of B cell subpopulations within the bone marrow showed that B220low (B220: antigen high-molecular weight glycoproteins uniquely expressed on the surface of B cells and their hemopoietic progenitors) developing B cells are increased in G-ARKO compared with Wt mice (Fig. 2A). This suggests that the observed increase in peripheral blood lymphocytes of G-ARKO mice (Fig. 1E) might be due to increased production of B cells in the bone marrow. The observed increase in the developing immature B cell fraction of G-ARKO mice is comparable to that observed in testicular feminization mutation (Tfm) mice (C57BL/6J-ATa) that carry a naturally defective AR gene (16) (Fig. 2B), and in castrated BALB/c mice (Fig. 2C). Implantation of dihydrotestosterone (DHT) pellets resulted in normal bone marrow B cell levels in castrated mice (Fig. 2D, left panel, vs. Fig. 2C, middle panel) but not in G-ARKO mice (Fig. 2D, middle panel, vs. Fig. 2A, middle panel) and summary in Table 1. Thus, data from three independent experimental mouse models demonstrate an essential role for AR in the maintenance of B cell development homeostasis.
Figure 2.
Expansion of developing B cells in distinct strains of mice lacking AR or with ablation of AR function after castration. A, High numbers of precursor B220low B cells in G-ARKO (C57BL6/FVB) (n = 5) compared with Wt (C57BL6/FVB) mice (n = 5). The absolute numbers of the B cells from BM (106 cells per femur) are increased in the G-ARKO mice (far right). B, High numbers of precursor B220low B cells in Tfm (C57B6/6J-A-Ta) (n = 4) compared with Wt mice (C57B6/6J-A-Ta) (n = 4). C, High numbers of precursor B220low B cells in castrated BALB/c (n = 5) compared with Wt BALB/c mice (n = 5). D, DHT supplementation reduces B cells in bone marrow of castrated BALB/c mice (n = 5) but not in G-ARKO mice (n = 5), suggesting that AR mediates this effect. Cast, Castration.
Table 1.
Phenotypic profiles of B cells (B220+) from bone marrow in various mice as determined by flow cytometry
| Mice | B220+% | Cell no. × 106 |
|---|---|---|
| Wt | 43.14 ± 1.7 | 2.0 ± 0.4 |
| ARKO | 66.20 ± 1.0 | 6.5 ± 0.6 |
| B-ARKO | 53.52 ± 2.0 | 5.9 ± 0.8 |
| Tfm | 60.84 ± 2.1 | 6.3 ± 0.3 |
| Wt-castration | 69.46 ± 2.3 | 7.1 ± 0.4 |
| Wt-castration + DHT | 45.28 ± 1.6 | 1.9 ± 0.2 |
| ARKO + DHT | 69.18 ± 3.1 | 6.7 ± 0.9 |
Intrinsic effects of AR ablation on developing B cells
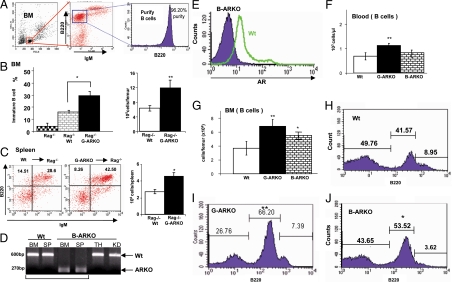
To determine whether the effect on immature B cell accumulation observed in the AR deficiency models described above was due only to an effect of AR on the bone marrow compartments and intercellular signals that direct B cell development or to an intrinsic effect of androgen/AR on developing B cells, we performed bone marrow transplantation experiments. We used Rag1−/− mice, in which B cell development is blocked due to loss of Ig gene rearrangements (17), but the bone marrow microenvironment is intact. B cells from bone marrow isolated from Wt and G-ARKO mice were transplanted into 5-wk-old Rag1−/− mice. After 8 wk, the bone marrow in the Rag−/− recipient mice transplanted with immature B cells from G-ARKO bone marrow showed increased numbers of developing B cells (Fig. 3, A and B) and increased B cells in spleen (Fig. 3C) compared with Rag1−/− mice transplanted with B cells from Wt bone marrow, although the difference is less pronounced than that between G-ARKO and Wt controls (see Fig. 2 above). These results suggest that AR ablation might have intrinsic, direct effects on B cell development in the bone marrow.
Figure 3.
Absence of AR in B cells results in an expansion of B cells in bone marrow (BM) and spleen. A, Purification and sorting the immature B cell (B220+ IgM−) cells for B cell BM transplantation. B, Distribution and absolute number of BM immature B cells in Rag1−/− recipients transplanted with BM cells from Wt (n = 5) and G-ARKO mice (n = 5). C, FACS analysis and absolute numbers of B cells in spleens of RAG1−/− recipient transplanted with BM cells from Wt (n = 5) and G-ARKO mice (n = 5). D, Genotyping of different tissue B cell DNA isolated from B-ARKO and Wt mice using “select and 2–9” primers, specific for sequences flanking the AR gene. Wt AR yields a PCR product of 600 bp, and the conditionally deleted region in the B-ARKO gene yields a fragment of 270 bp. B cells from B-ARKO BM and spleen (SP) only display the short AR 270 bp, suggesting specific ablation of the AR in B cells. E, Intracellular staining of AR in precursor B220low B cells (gated by FACS analysis) from BM of Wt and B-ARKO mice. Blood (panel F) and BM analyses (panel G) show expansion of B cells in G-ARKO (n = 18) mice as compared with those of Wt mice (n = 18). B-ARKO (n = 7) mice show expansion of B cells in BM but not in the blood. H–J, Distribution of B lymphocytes in the BM of Wt, G-ARKO, and B-ARKO mice. Data are mean ± sd. *, P < 0.05; **, P < 0.01. TH, Thymus; KD, kidney.
Increased B cells in bone marrow in B cell-specific AR knockout (B-ARKO) mice
To further characterize the potential intrinsic role of androgen/AR during B cell maturation and development, we generated B cell-specific AR knockout mice (B-ARKO) by mating floxed-AR female mice with male mice expressing Cre recombinase under the control of the CD19 promoter (18). PCR amplification of the critical AR gene region from genomic DNA isolated from B cells from bone marrow and spleen, with thymus and kidney as control genomic DNA, revealed essentially complete Cre-mediated deletion at the single, X-linked AR locus in these mice (Fig. 3D); this was confirmed by loss of AR protein expression in bone marrow B cells, as established by flow cytometry analysis (Fig. 3E). Increased B cells were again observed in B-ARKO blood (Fig. 3F) and bone marrow (Fig. 3G). B220low developing B cells are specifically increased in the bone marrow of B-ARKO mice compared with Wt mice (Fig. 3, H–J, and Table 1). We also saw a slight increase of B cells in the blood and significant increase in bone marrow of B-ARKO. In all cases, however, these differences are less pronounced in B-ARKO than in G-ARKO mice.
Developmental stage distribution of B cells in bone marrow and periphery of G-ARKO, B-ARKO, and Wt mice
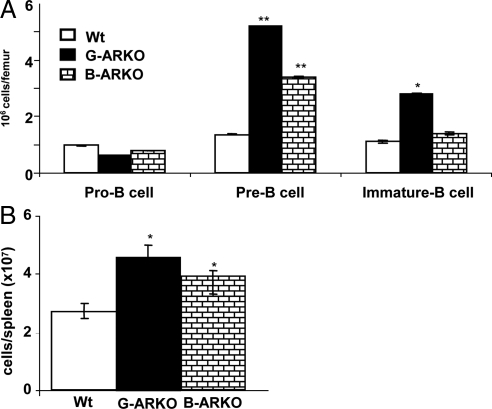
B cell development occurs in stages characterized by distinct combinations of cell surface markers and gene expression patterns (19). Thus, pro-B cells (B220+ CD2− IgM−) express B220+ but do not express either CD2 or IgM [immunoglobulin M (IgM) expresses in the mature B cells and the cluster of differentiation 2 (CD2) is a cell adhesion molecule expressed in the early development of B cells]. The expression of both B220 and CD2, but not IgM, characterizes pre-B cells (B220+ CD2+ IgM−) and cells expressing B220, CD2, and IgMlow are defined as immature B cells (B220+ CD2+ IgMlow). Bone marrow cells from G-ARKO and B-ARKO mice have a statistically higher proportion of pre-B cells (B220+ CD2+ IgM−) and a lower proportion of pro-B cells (B220+ CD2− IgM−) than bone marrow cells in Wt mice (Fig. 4A). The distribution of peripheral B cells (B220+), in spleens of G-ARKO, B-ARKO, was higher than in Wt mice (Fig. 4B).
Figure 4.
Stage of differentiation of B220+ cells in bone marrow and in periphery of G-ARKO, B-ARKO. A, Bone marrow cells from G-ARKO, B-ARKO, and Wt mice were analyzed for cell surface marker expression patterns using flow cytometry with markers B220, CD2, and IgM. Three populations of B220+ gated cells were observed: pro-B cells (B220+, CD2−, IgM−), pre-B cells (B220+, CD2+, IgM−) and immature B cells (B220+,CD2+, IgMlow). B, The subset distribution of B cells (B220+), in the spleens of G-ARKO, B-ARKO, and Wt mice.
Resistance to apoptosis in AR-deficient B cells
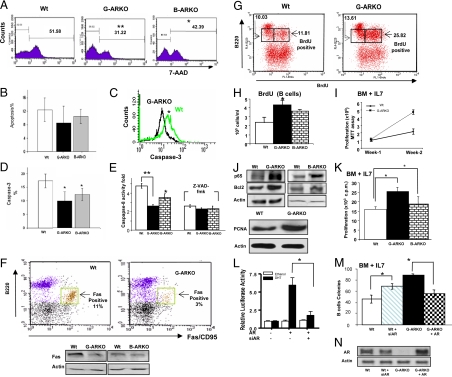
Apoptosis plays a crucial role during B cell development and in the establishment of B cell tolerance (20,21). To determine whether the observed increase in AR-deficient precursor B cells could be attributed to apoptosis resistance, we measured spontaneous apoptosis in Wt, G-ARKO, and B-ARKO bone marrow B cells cultured in vitro in the presence of IL-7. A significant decrease in the number of G-ARKO and B-ARKO apoptotic B cells compared with Wt B cells was observed after 14 d by 7-amino actinomycin D (7-AAD) staining (22) (Fig. 5A), and at 48 h, there was a significant decrease in the number of G-ARKO and B-ARKO apoptotic B cells as detected by TUNEL assays (23) (Fig. 5B). Concomitantly with diminished apoptosis, caspase-3 and caspase-8 activities were decreased in B cells from G-ARKO mice compared with Wt mice (Fig. 5, C–E). Furthermore, the expression of Fas protein (Fig. 5F) was decreased in B cells from G-ARKO and B-ARKO mice compared with Wt mice.
Figure 5.
G-ARKO B cells are resistant to apoptosis. A, Bone marrow (BM) immature B cells from G-ARKO and B-ARKO mice were more resistant to apoptosis than cells from Wt littermates. Total BM cells were cultured in vitro for 14 d in MesenCult medium supplemented with IL-7 and then stained with fluorochrome-conjugated anti-B220, anti-IgM, and 7-AAD and analyzed by flow cytometry. Histograms show 7-AAD staining profiles of gated B220low IgMlow immature B cells. B, Total BM cells were cultured in vitro for 48 h in MesenCult medium supplemented with IL-7. The TUNEL assay was used to detect apoptotic cells, with these events gated on B220low. C and D, Caspase-3 activity was analyzed in B cells from Wt and G-ARKO BM cultures cultured in vitro for 14 d in MesenCult medium supplemented with IL-7, stained with fluorochrome-conjugated anti-B220 and anti-caspase-3, and then analyzed by FACS gating on B220low developing B cells. E, Caspase-8 analysis of immature B cells from Wt, G-ARKO, and B-ARKO BM cells cultured in vitro for 14 d in MesenCult medium containing IL-7. We analyzed levels of caspase-8 in BM B cell lysates by ELISA and used general caspase inhibitor Z-VAD-fmak to reduce caspase-8 activity. F, Distribution of Fas (CD95)-positive B220+lowcells in Wt and G-ARKO BM cells cultured in vitro for 14 d in MesenCult medium containing IL-7 and then stained with fluorochrome-conjugated anti-B220 and anti-CD95 (Fas). Flow cytometry analysis and Western blot revealed that BM B220low cells from G-ARKO, B-ARKO mice have less Fas-positive cells than Wt littermates. G, BrdU staining of B cells from G-ARKO and Wt littermates. G-ARKO and Wt littermates were injected ip with 1 mg of BrdU twice a day for 4 d. BM cells were stained with fluorochrome-conjugated anti-B220 and anti-BrdU and analyzed by FACS for B220low BrdU+ cells. H, The absolute number of BrdU-positive B220low cells was increased in G-ARKO (n = 9) and B-ARKO (n = 6) compared with Wt mice (n = 9). I, Proliferation analysis for bone marrow B cells from Wt and G-ARKO littermates in the presence of 20 nm IL-7. J, Western blot analysis of NF-kB/p65 and Bcl2 from BM B cell lysates of Wt, G-ARKO, and B-ARKO mice littermates. K, BM B cell proliferation was measured by [3H]thymidine incorporation in the presence of 20 nm IL-7 (see Materials and Methods). Thymidine incorporation is greater in immature B cells from G-ARKO (n = 5) and B-ARKO mice (n = 5) compared with Wt (n = 5). L, AR activity is knocked down by AR siRNA and restored by AR-cDNA. CV-1 cells were cotransfected with ARE4-Luc, pBabe-AR (or pBabe vector), and AR-siRNA using SuperFect. phRL-TK was cotransfected as the control for normalization. Total plasmid DNA amounts were balanced by empty vectors. After 24 h transfection, cells were treated with or without 10 nm DHT. After 16–18 h incubation, cells were harvested for the luciferase reporter assay. Each Luc activity is presented relative to the transactivation observed in the absence of DHT and is the mean ± sd of four experiments. M, We plated BM cells infected with retroviruses harboring pBabe-AR, pSuperior-ARsiRNA, and vectors in methylcellulose-containing media supplemented with IL-7. Colonies containing more than 30 cells were counted after 14 d. AR knockdown in Wt BM cells by siRNA induces colony forming, and AR restoration in G-ARKO BM cells reduces colony forming. N, Western blot revealed AR restored by AR-cDNA in G-ARKO BM cells. Data are presented as mean ± sd *, P < 0.05; **, P < 0.01. PCNA, Proliferating cell nuclear antigen.
Next, we tested whether the absence of AR signaling might affect B cell proliferative capacity in vivo. G-ARKO, B-ARKO, and Wt (control mice) were injected ip with bromodeoxyuridine (BrdU) and B cells were analyzed for BrdU incorporation by fluorescence-activated cell sorting (FACS) (24). B220-low bone marrow precursor B cells from G-ARKO mice were found to have substantially higher (11.81 to 25.82%) BrdU incorporation compared with Wt mice (Fig. 5, G and H). G-ARKO, B-ARKO, and B-ARKO mice bone marrow B cells cultured in vitro in the presence of IL-7, which is positively linked to proliferation, also had higher numbers of B cells than similarly cultured Wt cells (Fig. 5, I and K). Antiapoptotic markers, such as nuclear factor-κB (NF-κB) (p65) (25) and Bcl-2 (26), were up-regulated in G-ARKO and B-ARKO B cells compared with Wt mice (Fig. 5J). These results were further confirmed by higher expression of proliferating cell nuclear antigen (27) in G-ARKO and B-ARKO precursor B cells (Fig. 5J). G-ARKO and B-ARKO mice bone marrow B cells cultured in vitro in the presence of IL-7 also had higher thymidine incorporation (28) than similarly cultured Wt cells (Fig. 5K). Together, these results suggest that both resistance to apoptosis via modulation of several apoptotic factors and increased proliferation in developing B cells are likely to account for the expansion of developing B cells observed in G-ARKO and B-ARKO mice.
Bone marrow retroviral transfection with either AR-small interfering RNA (siRNA) or AR-cDNA confirms the role of AR in B cell homeostasis
We transfected CV-1 cells (used to test siRNA efficiency) with pBabe-AR and pSuperior-AR-siRNA to demonstrate increased or suppressed endogenous AR function via transactivation assays (Fig. 5L). We then used the same vectors to transduce bone marrow B cell precursors from normal and G-ARKO mice and performed in vitro colony formation assays in the presence of IL-7 (16) (Fig. 5M) and AR expression by Western blot (Fig. 5N).
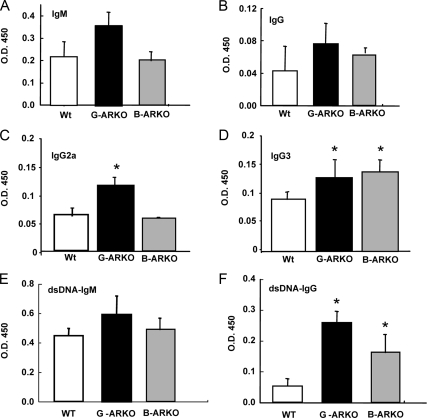
Increased immunoglobulin concentration in the serum of G-ARKO and B-ARKO mice
A potential consequence of alteration of B cell homeostasis is the development of autoimmune disease (29). Serum concentrations of IgG3 and IgG2a in G-ARKO mice and IgG3 in B-ARKO mice were significantly increased compared with Wt mice (Fig. 6, A–D). G-ARKO and B-ARKO mice also displayed little difference in the double-stranded DNA (dsDNA)-IgM (Fig. 6E) and significantly elevated levels of serum IgG autoantibodies to dsDNA, a hallmark of autoimmune susceptibility (Fig. 6F).
Figure 6.
Levels of Ig isotypes by ELISAs. Relative serum concentrations of (A) IgM, (B) IgG, (C) IgG2a, (D) IgG3, (E) dsDNA-IgM, and (F) dsDNA-IgG were higher in G-ARKO mice compared with Wt mice. IgG, IgG3, and dsDNA-IgM are higher in B-ARKO mice than in Wt mice. The data were obtained from six pairs of mice. Data are presented as mean ± sd. *, P < 0.05.
Loss of AR in B cells promotes development of CIA
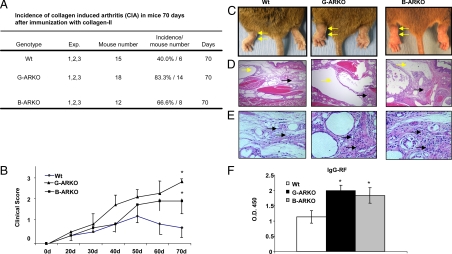
Previously, a report showed that ARKO might contribute to the development of type 2 diabetes (30). Thus, we hypothesize that ARKO mice might develop spontaneous autoimmune diseases such as lupus. The sera levels of IgG-dsDNA were positive, but the clinical scores were negative. Therefore, we immunized ARKO mice with ds-DNA (31) and the clinical score for lupus (IgG glomerular deposits and glomerulonephritis) were negative (data not shown). Furthermore, we tested whether B cell expansion and increase in basal IgG-rheumatoid factor (IgG-RF) autoantibody levels in G-ARKO and B-ARKO mice are associated with developing RA. Both G-ARKO and B-ARKO mice were observed to have an increase in the IgG-RF, but no rheumatoid arthritis clinical score has been observed (data not shown). Therefore, we immunized ARKO and B-ARKO mice with bovine (b) collagen II (CII) and followed them for 10 wk to assess development of CIA (32).
As shown in Fig. 7, we found that CIA incidence (Fig. 7A) and clinical scores (Fig. 7B) were increased in G-ARKO, B-ARKO, and Wt mice. Figure 7C shows external joint phenotype after inducing arthritis, and Fig. 7, D and E, shows histological evidence of significant CIA manifestations in both G-ARKO and B-ARKO mice compared with Wt mice, including enlarged synovial membrane and lymphocyte infiltration. Also, concentrations of IgG-RF, a marker of ongoing autoimmune disease, were significantly elevated in both G-ARKO and B-ARKO mice compared with Wt mice (Fig. 7F).
Figure 7.
G-ARKO and B-ARKO mice are more vulnerable to CIA than their Wt littermates. A, Incidence of CIA in mice 70 d after immunization with CII. B, Average clinical scores per paw during disease progression. C, External joint phenotype after inducing arthritis is indicated by arrows. D, Histological features of joint sections. Yellow arrows indicate extended synovial bursa. Black arrows indicate hypertrophic synovial membrane. E, Infiltration of lymphocytes in synovial membrane of joints after CIA (arrows). F, Relative serum IgG-RF levels at the end of the experiment were increased in G-ARKO (n = 5) and B-ARKO (n = 3), but not in Wt mice (n = 5). Data, mean ± sd; *, P < 0.05; **, P < 0.01.
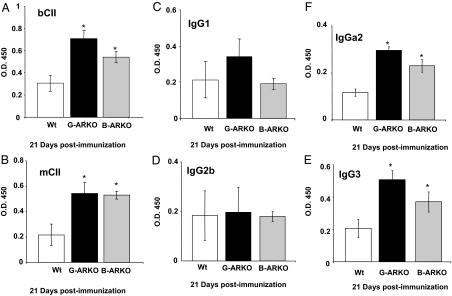
Moreover, after immunization, we measured the CII-specific antibody (Ab) by ELISA. Mice were immunized with CII and, after 21 d immunization, the sera were collected and analyzed for the presence of total Ab of bCII (bCII-specific) (Fig. 8A), murine (m) CII (mCII-specific) (Fig. 8B), and analysis of isotype expression in the mCII-specific Ab response (Fig. 8, C–F). mCII-specific isotypes were quantitated using the sera described in Fig. 8A and a solid-phase ELISA in which the detection Abs were specific for the isotypes listed. These results show that loss of AR in B cells renders mice more vulnerable to develop autoimmune diseases, although this effect is less pronounced than in mice with ubiquitous loss of AR.
Figure 8.
Measurement of CII-specific Ab by ELISA after immunization. Sera from G-ARKO, B-ARKO, and Wt mice. Mice were immunized with collagen II, and after 21 d the sera were collected and analyzed for the presence of total anti-body of (A) bCII (bCII-specific), (B) mCII (mCII-specific), and (C–F) analysis of isotype expression in the mCII-specific Ab response. mCII-specific isotypes were quantitated using the sera described in panel A and a solid phase ELISA in which the detection Abs were specific for the isotypes listed. The quantity of Ab detected is expressed as relative OD450 based on a CII-specific reference serum. Data are presented as mean ± sd. *, P < 0.05.
Discussion
Defects in androgen/AR have been shown to cause a variety of effects on B cell development and maturation (8,9). Here, we show, for the first time, that some of these defects are, in part, intrinsic to B cells and can result in increased susceptibility to autoimmune disease. Using three different strains of mice (B-ARKO, G-ARKO, and natural AR gene defect in Tfm mice), as well as castrated Wt mice, we demonstrated that the loss of AR results in expansion of bone marrow and peripheral B cell compartments. Furthermore, bone marrow transplantation using Rag1−/− mice and transduction of pSuperior-AR-siRNA, or pBabe-AR-cDNA into B cells of Wt mice or G-ARKO mice, respectively, clearly demonstrated that loss of AR in B cells within the bone marrow results in increased developing B cells in the bone marrow. Earlier reports have shown that loss of estrogen receptor results in increased B cells in female, but not male, mice (33). In addition, estrogen treatment of castrated male mice has been shown to increase the production of autoantibodies (9), which together with our data suggests that androgen/AR might be sufficient for maintaining normal B cell homeostasis in estrogen receptor-deficient male mice.
Using reciprocal bone marrow transplantation experiments in Tfm mice, Olsen and colleagues (8,9,34) have shown that AR deficiency in the bone marrow stroma, rather than B cells, is responsible for the expansion of precursor B cell populations in that strain. Although those results are different from ours, the finding that B-ARKO mice display a similar phenotype to that of G-ARKO mice quite clearly demonstrates that AR has intrinsic effects on B cells. Nevertheless, because all observed phenotypes are milder in B-ARKO than in G-ARKO mice, the evidence clearly supports a role for non-B cell components, including stromal cells, but also possibly T cells and nonlymphoid populations. Targeted inactivation of AR in candidate cellular subsets, using strategies analogous to those used in this report, will be required to unravel the pleiotropic role of AR in immune homeostasis.
As far as the B cell-specific effects are concerned, several mechanisms seem to contribute to the increased B lymphopoiesis in the absence of AR. First, our results suggest an increased number of IL-7-responsive early B cell precursors in bone marrow from AR-deficient mice. Second, based on BrdU incorporation experiments, AR-deficient developing B cells display higher proliferative rates compared with controls. Finally, a number of factors involved in the regulation of apoptosis, including Fas/FasL, caspases-3/-8, and antiapoptotic mediators appear dysregulated in AR-deficient B cells. Moreover, it is possible that the AR up-regulates apoptosis signal pathways such as caspases or Fas/FasL pathways (Fig. 5, C–F) or down-regulates the proliferative signal such as NF-kB/p65, Bcl2, and proliferating cell nuclear antigen (Fig. 5, I and J). The expansion of B cells in the periphery of G-ARKO mice could be due to the failure of the peripheral compartment to compensate for the increased influx of newly developed B cells from the bone marrow.
Overall, the results of the present study suggest that androgen/AR might influence immune function, in part, by affecting the primary B cell repertoire, because removal of AR results in a larger and possibly more diverse population of B lymphocytes in terms of androgen specificities. Because of reduced apoptosis, the expanded population of B cells in AR-deficient mice could include cells with autoreactive specificities, thus providing a conceptual mechanistic link between loss of AR and development of autoimmune diseases. This hypothesis is consistent with our findings of enhanced basal anti-dsDNA-IgG levels in G-ARKO mice. Most strikingly, increased susceptibility to CIA in G-ARKO mice underscores the essential role of androgen in preventing autoimmune pathologies.
Rheumatoid arthritis is believed to be triggered by exposure of a genetically susceptible host to an arthrogenic antigen and subsequent autoimmune chronicity and progression (35,36). Ultimately, joint destruction results from activation of T and B lymphocytes and local release of inflammatory mediators and cytokines. The target antigens and the mechanisms of lymphocyte activation are still largely unknown. Increasing evidence suggests that immune complex deposition might play a role in joint destruction (37). Rheumatoid arthritis patients, as well as models of autoimmune arthritis such as murine CIA, demonstrate defective B cell apoptotic pathways and tolerance checkpoints that result in the accumulation of autoreactive naïve B cells in the blood (26). Impaired B cell tolerance checkpoints might be a common feature in many rheumatic diseases (38). Low levels of androgens have been found in male rheumatoid arthritis patients (12,13) and case reports have suggested that androgen ablation therapy to suppress AR function in prostate cancer might be a risk factor for development of rheumatoid arthritis (14). Moreover, Cutolo et al. (39) found that androgen replacement therapy improved clinical and immunological parameters associated with rheumatoid arthritis. Ellis et al. (40) also showed similar results after androgen ablation.
Together, these human clinical data support our finding that loss of AR in B cells, using G-ARKO and B-ARKO mice, results in the expansion of B cells that can contribute to the development of selective autoimmune disease, such as CIA. Therefore, AR might become a new target to treat these selective autoimmune disease.
Materials and Methods
Mice
Construction of targeting vectors and generation of the chimera founder mice have been previously described (15). We generated floxed AR mice with a nearly pure C57BL/6 background by backcrossing the original C57BL6/129 background floxed AR mice with pure C57BL/6 background mice for more than eight generations. β-Actin is constitutively and ubiquitously expressed; thus, the β-actin promoter-driven ACTB Cre in the FVB background, and CD19Cre in the B6 background (The Jackson Laboratory, Bar Harbor, ME) will express and delete floxed AR fragments in all the tissues and B-cells, respectively. Wt and ARKO mice were genotyped by PCR as described previously (15). Male BALB/c mice, Rag−/−, and male Tfm mice, as well as Wt control mice [C57BL/6J-Ata 6J)+/Y], were purchased from The Jackson Laboratory.
Androgen supplementation
We achieved androgen supplementation in castrated Wt mice and G-ARKO littermates by sc implantation of 21-d time-release pellets (Innovative Research of America) containing DHT (5 mg per pellet); mice were killed 14 d after androgen supplementation. Levels of plasma DHT were determined by a DHT ELISA Kit (Alpha Diagnostic International, San Antonio, TX) according to the manufacturer’s protocol.
Cytology and automated hematology analysis
We measured hematological parameters from blood samples obtained from 8- to 12-wk-old mice using an Abbott CELL-DYN 4000 system.
Cell staining and isolation using flow cytometry
We performed immunofluorescence analysis using fluorochrome-conjugated antibodies purchased from eBiosciences, including APC-anti-B220 and PE-anti-IgM, as well as fluorescein isothiocyanate (FITC)-anti-CD45 from Pharmingen (San Diego, CA). We incubated 1–5 × 105 cells on ice for 30 min with saturating amounts of Ab and then isolated B cells from bone marrow, spleen, and peripheral blood by the EasySep FITC selection kit (Stem Cell Technologies).
Adoptive transfer of B cells
We used ARKO and Wt mice at 8–10 wk of age as bone marrow B cell donors. The B cells from the bone morrow were purified by using B220+ markers, after which the purity of the B cell bone marrow was tested (see Fig. 3A). The purified G-ARKO and Wt bone marrow B cells (B220+) (1.5 × 107) in 200 μl PBS were injected iv into the orbital venous plexus of recipient Rag1−/− mice (The Jackson Laboratory) at 8 wk of age, and the recipient mice were killed 6–8 wk after B cell transfer for analyses.
B cell proliferation assays
Cells were cultured for 4 d in MesenCult medium supplemented with IL-7 and then we added 1 mCi [methyl-3H]thymidine (Dupont NEN Products, Boston, MA) in 25 μl in MesenCult medium supplemented with IL-7 for the last 18 h. Then we harvested cells onto glass fiber filters using a Packard Micromate 196 cell harvester (Packard Co., Meriden, CT) and counted 3H incorporation with a Packard Matrix 96 direct β-counter (28). To assess levels of in vivo proliferation, mice received a 1 mg ip injection of BrdU suspended in PBS. B cells (B220+) were fixed and then incubated with an anti-BrdU Ab conjugated to FITC (anti-BrdU-FITC) for flow cytometry. Cells were cultured for 4 d in MesenCult medium supplemented with IL-7 after which cell viability by dimethyl thiazolyl blue tetrazolium bromide assay was performed. At the indicated time points, media were removed and serum-free media containing dimethyl thiazolyl blue tetrazolium bromide (0.5 mg/ml; Sigma Chemical Co., St. Louis, MO) were then added into each well. After 4 h incubation at 37 C, cellular formazan product was dissolved with acidic isopropanol, and the absorbance at OD595 was measured by spectrophotometry (Beckman Du640B).
Construction of mouse pBabe-AR and pSuperior-ARsiRNA
Oligonucleotides (5′-CGGAATTCGTGGAAGCT-3′ and 5′-GAAAGATCTACA-TCAGTAGAGG-3′) were used to clone mouse AR fragments from mouse prostate cDNA library using PCR. The retroviral vector pBabe-AR was constructed by ligating the BamHI/Bgl II-digested PCR product into BamHI/BglII-digested pBabe-puro vector. The pSuperior-green fluorescent protein (GFP) vector for expression of siRNA was purchased from OligoEngine (Seattle, WA). To generate pSuperior-GFP-ARsiRNA, the pSuperior-GFP vector was digested with BglII and HindIII, and the annealed oligonucleotides were ligated into the vector. The 19-nucleotide sequences for targeting mouse AR were: 5′-GATCCC-CTCTAGCCTCAATGAGCTTGTTCAAGAGACAAGCTCATTGAGGCTAGATTTTTA-3′ and 5′-AGCTTAAAAATCTAGCCTCAATGAGCTTGTCTCTTGAACAAG-CTCATTGAGGCTAGA- GGG-3′; pSuperior-GFP-AR scramble siRNA control plasmid will be constructed.
Transient transfections and reporter gene assays
We performed transfections using SuperFect and carried out Luciferase (Luc) assays as described previously (25). Briefly, we plated 1–4 × 105 cells on 35- or 60-mm dishes 24 h before adding the DNA precipitation mix containing androgen response element-Luc reporter plasmid DNA. We changed the medium to phenol-red-free RPMI 1640 with 10% charcoal-stripped fetal calf serum 1 h before transfection. In each experiment, we equalized the total amount of transfected DNA per dish by the addition of empty expression vector (pCMV) to make a total of 10 μg/60-mm dish. We determined Luc activity using a Dual-Luciferase Reporter Assay System (Promega Corp., Madison, WI) and a luminometer.
Collagen-induced arthritis (CIA)
ARKO mice and Wt littermates were injected intradermally at four sites on the flanks with 200 μl containing 200 μg of bCII (32). The incidence of arthritis reflects the development of disease in any paw (limb). Between d 21 and d 70, two trained laboratory personnel examined the mice three times weekly (blinded as to strain of mouse). A three-point scale was used for each paw: 0 = normal joint; 1 = slight inflammation and redness; 2 = severe erythema and swelling affecting the entire paw; and 3 = deformed paw or joint with ankylosis, joint rigidity, and loss of function. Our data present the clinical score from the average of all four limbs per animal.
Histological examination
We prepared the tissue samples for histological examination as previously described (15). We scored the joint sections for changes in synovial inflammation, pannus, cartilage damage, and bone damage, all on a scale of 0–5, with an overall score calculated as the total of the four individual parameters. We present the compiled data for six G-ARKO mice and six Wt mice as the mean ± sd based upon a set of four joints per animal.
ELISA
We used a sandwich ELISA for Ig isotype determination in serum for determination of specific antibodies after immunization (30).
Statistics
We presented data as the mean ± sd, making comparisons between groups using a two-sided Student’s t test. P values < 0.05 were considered significant.
Other methods
We performed RT-PCR and Western blotting assays as previously described (1).
Acknowledgments
We thank Drs. P. Anthony di Sant’Agnese and Edward M. Messing and Dr. Chang’s laboratory for thoughtful comments on this manuscript. We also thank Pharm Research MD Co. (www.pharmresearchmd.com) for providing us reagents and Dr. Mhohammed Elkhalifa, King Faisal Specialist Hospital, Riyadh, Saudi Arabia. We thank Karen Wolf for manuscript preparation.
Footnotes
This work was supported by National Institutes of Health Grant DK073414, George Whipple Professorship Endowment, and King Abdulaziz City for Science and Technology (KACST) Grant ARP-28-74.
Disclosure Summary: The authors have nothing to declare.
First Published Online January 22, 2009
Abbreviations: 7-AAD, 7-Amino actinomycin D; Ab, antibody; AR, androgen receptor; B-ARKO, B cell-specific AR knockout; BrdU, bromodeoxyuridine; CIA, collagen-induced arthritis; CII, collagen II; DHT, dihydrotestosterone; dsDNA, double-stranded DNA; FACS, fluorescence-activated cell sorting; FasL, Fas/Fas ligand; FITC, fluorescein isothiocyanate; G-ARKO, general AR knockout; GFP, green fluorescent protein; IgG-RF, IgG-rheumatoid factor; NF-κB, nuclear factor-κB; siRNA, small interfering RNA; Tfm, testicular feminization mutation; Wt, wild type.
References
- Chang CS, Kokontis J, Liao ST 1988 Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324–326 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2004 Androgen receptor in prostate cancer. Endocr Rev 25:276–308 [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C 2005 Dynamic combinatorial networks in nuclear receptor-mediated transcription. J Biol Chem 280:32565–32568 [DOI] [PubMed] [Google Scholar]
- Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE 1997 The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82:3493–3497 [DOI] [PubMed] [Google Scholar]
- Mantalaris A, Panoskaltsis N, Sakai Y, Boume P, Chang C, Messing EM, Wu JHD 2001 Localization of androgen receptor expression in human bone marrow. J Pathol 193:361–366 [DOI] [PubMed] [Google Scholar]
- Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW 1998 The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol 161:27–34 [PubMed] [Google Scholar]
- Wilson CA, Morse SA, Thomas DW 1995 Enhanced production of B lymphocytes after castration. Blood 85:1535–1539 [PubMed] [Google Scholar]
- Viselli SM, Stanziale S, Shults SK Kovacs WJ, Olsen NJ 1995 Castration alters peripheral immune function in normal male mice. Immunology 84:337–342 [PMC free article] [PubMed] [Google Scholar]
- Kanda N, Tsuchida T, Tamaki K 1996 Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol 106:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Watson MB, and Kovacs WJ 1991 Studies of immunological function in mice with defective androgen action. Distinction between alterations in immune function due to hormonal insensitivity and alterations due to other genetic factors. Immunology 73:52–57 [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Balleari E, Accardo S 1984 Preliminary results of serum androgen level testing in men with rheumatoid arthritis. Arthritis Rheum 27:958–959 [DOI] [PubMed] [Google Scholar]
- Spector TD, Perry LA, Tubb G 1988 Low free testosterone levels in rheumatoid arthritis. Ann Rheum Dis 47:65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope JE, Joneja M, Hong P 2002 Anti-androgen treatment of prostatic carcinoma may be a risk factor for development of rheumatoid arthritis. J Rheumatol 29:2459–2462 [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C 2002 Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA 99:13498–13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS 1991 A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol 5:573–581 [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE 1992 RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877 [DOI] [PubMed] [Google Scholar]
- Rickert RC, Roes J, Rajewsky K 1997 B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 25:1317–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K 2001 B cell development pathways. Annu Rev Immunol 19:595–621 (Review) [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Sliver J, Hardy RR 1999 Positive selection of natural autoreactive B cells. Science 285:113–126 [DOI] [PubMed] [Google Scholar]
- Montes CL, Maletto BA, Acosta Rodriguez EV, Gruppi A, Pistoresi-Palencia MC 2006 B cells from aged mice exhibit reduced apoptosis upon B-cell antigen receptor stimulation and differential ability to up-regulate survival signals. Clin Exp Immunol 143:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott NJ, Turner AJ, Scopes J, Westby M, Marsh JC, Gordon-Smith EC, Dalgleish AG, Gibson FM 1996 The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood 87:2244–2251 [PubMed] [Google Scholar]
- Lin HK, Altuwaijri S, Lin WJ, Kan PY, Collins LL, Chang C 2002 Proteasome activity is required for androgen receptor transcriptional activity via regulation of androgen receptor nuclear translocation and interaction with coregulators in prostate cancer cells. J Biol Chem 277:36570–36576 [DOI] [PubMed] [Google Scholar]
- Rothaeusler K, Baumgarth N 2006 Evaluation of intranuclear BrdU detection procedures for use in multicolor flow cytometry. Cytometry A 69:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C 2003 Interruption of nuclear factor κB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res 63:7106–7112 [PubMed] [Google Scholar]
- Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, de Jong D, Maartense E, Schuuring E, Kluin PM 1998 Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood 92:3152–3162 [PubMed] [Google Scholar]
- Balajee AS, Dianova I, Bohr VA 1999 Oxidative damage-induced PCNA complex formation is efficient in xeroderma pigmentosum group A but reduced in Cockayne syndrome group B cells. Nucleic Acids Res 27:4476–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher TH, O'Brien DP, Altuwaijri S, Barth RK 2000 Increasing the frequency of T-cell precursors specific for a cryptic epitope of hen-egg lysozyme converts it to an immunodominant epitope. Immunology 99:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Tuckova L, Stepankova R, Hudcovic T, Palova-Jelinkova L, Kozakova H, Rossmann P, Sanchez D, Cinova J, Hrncir T, Kverka M, Frolova L, Uhlig H, Powrie F, Bland P 2005 Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann NY Acad Sci 1051:787–798 [DOI] [PubMed] [Google Scholar]
- Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD, Chang C 2005 Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54:1717–1725 [DOI] [PubMed] [Google Scholar]
- Voynova EN, Tchorbanov AI, Todorov TA, Vassilev TL 2005 Breaking of tolerance to native DNA in nonautoimmune mice by immunization with natural protein/DNA complexes. Lupus 14:543–550 [DOI] [PubMed] [Google Scholar]
- Campbell IK, Hamilton JA, Wicks IP 2000 Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol 30:1568–1575 [DOI] [PubMed] [Google Scholar]
- Peeva E, Venkatesh J, Diamond B 2005 Tamoxifen blocks estrogen-induced B cell maturation but not survival. J Immunol 175:1415–1422 [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Gu X, Kovacs WJ 2001 Bone marrow stromal cells mediate androgenic suppression of B lymphocyte development. J Clin Invest 108:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli E, Gonzalez-Gay MA David CS 1995 Could HLADRB1 be the protective locus in rheumatoid arthritis? Immunol Today 16:274–278 [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Andersson M, Larsson E 1988 Immunogenetics of type II collagen autoimmunity and susceptibility to collagen arthritis. Immunology 65:305–310 [PMC free article] [PubMed] [Google Scholar]
- Myers LK, Rosloniec EF, Cremer MA, Kang AH 1997 Collagen-induced arthritis, an animal model of autoimmunity. Life Sci 61:1861–1878 [DOI] [PubMed] [Google Scholar]
- Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E 2005 Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med 201:1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Balleari E, Giusti M 1991 Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum 34:1–5 [DOI] [PubMed] [Google Scholar]
- Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED 2001 Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol 13:553–558 [DOI] [PubMed] [Google Scholar]