Abstract
Insulin receptor substrate-1 (IRS-1) is a docking protein tyrosine phosphorylated in response to insulin, IGF-1, GH, and other cytokines. IRS-1 has an N-terminal plekstrin homology domain (which facilitates membrane localization), a phosphotyrosine-binding domain [which associates with tyrosine-phosphorylated insulin receptor or IGF-1 receptor (IGF-1R)], and tyrosine residues that, when phosphorylated, bind signaling molecules. The role of IRS-1 in GH signaling is uncertain. We previously reported that IRS-1 and Janus kinase 2 associate independently of tyrosine phosphorylation via IRS-1’s N terminus and that IRS-1 reconstitution greatly enhances GH-induced ERK, but not STAT5, activation. We now use GH-responsive 3T3-F442A preadipocytes to study the influence of IRS-1 on GH action. We stably transfected cells with vector only (Control) or a vector encoding IRS-1 short hairpin RNA [knockdown (KD)] and compared representative clones. Immunoblotting confirmed more than 80% knockdown of IRS-1 in KD cells. GH caused characteristic Janus kinase 2 and STAT5 activation in both Control and KD cells, but ERK activation was dramatically reduced in KD cells in GH time course and dose-response experiments. Notably, GH-induced Src homology collagen (SHC) activation and SHC-Grb2 association in KD cells were also markedly diminished compared with Control cells. Subcellular fractionation revealed that IRS-1 in Control cells was largely cytosolic, but the component isolated with plasma membranes was highly enriched in lipid raft membranes (LR). In KD cells, GH-induced ERK activation in the LR fraction was particularly diminished compared with Control cells. These data suggest that LR-enriched IRS-1 contributes substantially to GH-induced ERK activation in LR in 3T3-F442A fibroblasts. Furthermore, our results are consistent with IRS-1 residing upstream of SHC in the GH-induced ERK-signaling pathway.
Deletion of insulin receptor substrate-1 (IRS-1) in 3T3-F442A cells results in markedly reduced GH-induced SHC and ERK signaling, suggesting IRS-1’s importance in this pathway.
GH is among the most powerful growth-promoting factors in humans and other vertebrates and it also exerts important metabolic regulatory effects (1,2). GH causes its biological actions largely by virtue of binding to the GH receptor (GHR), a glycoprotein that is displayed on the surface of GH target tissues (3). GHR engagement leads to rapid activation of several signaling pathways within the cell. Of keen importance is the activation of the latent cytoplasmic transcription factor, signal transducer and activator of transcription (STAT)5, which undergoes tyrosine phosphorylation, dimerization, and translocation to the nucleus where it impacts the expression of certain GH target genes, including IGF-I, acid-labile subunit of the IGF-binding protein complex, suppressor of cytokine signaling proteins, hepatic P450 enzymes, and serine protease inhibitor 2.1 (4,5,6,7,8,9,10,11,12,13,14). GH-induced STAT5 signaling requires activation of the GHR-associated cytoplasmic tyrosine kinase, Janus kinase 2 (JAK2), and tyrosine phosphorylation of the receptor intracellular domain (15,16,17,18,19).
GH also activates the ERK and phosphatidyinositol-3 kinase (PI3K) pathways. ERK activity is critical for GH-induced c-fos transcription (20), enhances GH-stimulated proliferation (21), and mediates cross talk with epidermal growth factor (EGF) signaling (22,23,24). GH-induced PI3K activity is implicated in antiapoptosis and/or proliferation and likely contributes to GH-induced ERK, p70 S6 kinase, and phosphodiesterase activation (20,21,25,26,27,28,29). Unlike STAT5, GH-induced ERK and PI3K activation does not require the presence of a large portion of the receptor’s intracellular domain; in fact, early work suggested that only the GHR region required for effective coupling to JAK2 and only JAK2 activation were necessary for initiating these signals (30,31,32,33,34,35). More recent work has introduced uncertainty as to the need for JAK2 activity to allow GH-induced ERK signaling (36,37,38); these studies suggest in some systems a more prominent role for src-family kinases and potentially for phospholipase C-γ. However, there is incomplete agreement as to whether src-family kinases exert this role in all cell types (39).
Although GH-induced STAT5 activation occurs in most cells that express GHR and JAK2, cells differ in their tendency to couple GH stimulation to ERK activation (40). ERK is strongly activated in response to GH in the murine 3T3-F442A preadipocyte fibroblast, a cell line that endogenously expresses GHR, JAK2, and its downstream signaling elements (16,20,24,27,34,35,41,42,43,44,45,46,47,48,49,50,51,52); in this system, GH stimulates tyrosine phosphorylation of the Src homology-collagen (SHC) adaptor proteins, SHC-Grb2 association, and resultant Ras-Raf-MEK1-ERK pathway activation (53,54). In 3T3-F442A cells, GHR, JAK2, ERKs, and Grb2 are substantially enriched in the cholesterol-rich lipid raft fraction of the plasma membrane, and depletion of cholesterol selectively diminishes GH-induced ERK activity (51). In contrast to 3T3-F442A, ERKs are not activated in response to GH in the human IM-9 lymphoblast, another cell line that endogenously expresses GHR, JAK2, and downstream signaling elements and robustly activates STAT5 (40,51). GHR and JAK2 are raft concentrated in IM-9 cells to the same degree as in 3T3-F442A cells; however, Grb2 and ERKs are not enriched in rafts in IM-9 cells, leading to the hypothesis that GH-induced activation of raft-specific pathways may be a key determinant of ERK activation in response to the hormone (51).
Our work has also implicated the docking protein, insulin receptor substrate-1 (IRS-1), in GH-induced ERK activation (29). IRS proteins are a family of large intracellular adaptors that interact with receptors and link to various signaling pathways, mainly by phosphorylation-dependent protein-protein association (55,56,57,58,59,60). In addition to being activated as an adaptor by insulin and IGF-I, IRS-1 is also tyrosine phosphorylated in response to GH, including in 3T3-F442A cells (21,34,61,62,63). Using a cellular reconstitution system, we previously found that expression of IRS-1 in cells devoid of IRS family members (32D-GHR cells) resulted in dramatically enhanced GH-dependent proliferation (21). Furthermore, GH-induced ERK and Akt activation were markedly increased in IRS-1-expressing cells, but this was unaccompanied by changes in the degree of STAT5 activation (29), suggesting pathway-specific effects of expression of IRS-1 on GH signaling. In the current study, we explore further the role of IRS-1 in GH signaling, using 3T3-F442A preadipocytes, which endogenously express IRS proteins. We find that silencing of IRS-1 expression renders these cells much less sensitive to GH for activation of the ERK and PI3K pathways without affecting GH-induced STAT5 activation. This decreased GH-induced ERK activation in cells deficient in IRS-1 is accompanied by markedly diminished GH-induced SHC tyrosine phosphorylation. This work further implicates IRS-1 as a specific participant in GH-induced ERK activation and suggests that SHC resides, at least in part, downstream of IRS-1 in this pathway.
Results
Knockdown of IRS-1 in 3T3-F442A preadipocytes does not impact GHR or JAK2 abundance or GH-induced JAK2 or STAT5 activation
To assess the role of IRS-1 in GH signaling in a nonreconstitution system, we used the 3T3-F442A preadipocyte fibroblast cell line. 3T3-F442A cells are endowed with GHRs (∼10,000 binding sites per cell) (64) and respond to GH with robust activation of JAK2, STAT5, ERKs, and Akt, making this cell line attractive for GH signaling studies. We prepared a plasmid that encoded a small hairpin insert with a validated small interfering RNA (siRNA) sequence targeting reading frame nucleotides 24–42 of murine IRS-1 (corresponding to residues 8–14 near the amino terminus of the protein) in the backbone of pRNAU6.1/Neo (GenScript, Piscataway, NJ). 3T3-F442A cells were transfected with either this siRNA plasmid or the vector (as a control), and stable clones were screened for IRS-1 expression by immunoblotting. Several clones from each transfection were selected for further study, including clones referred to as Control (vector-only) and KD (siRNA knockdown). All results shown in this study were verified with a second pair of clones (Control vs. KD).
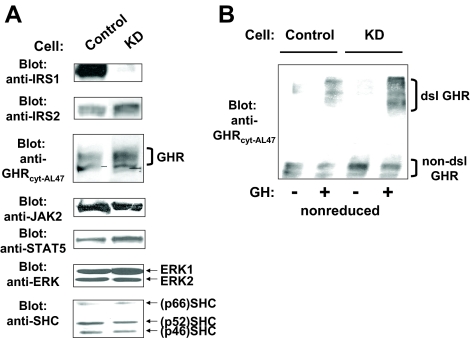
Control and KD cells were subjected to detergent extraction, and extracted proteins were resolved by SDS-PAGE. Dramatic knockdown of IRS-1 was evident by immunoblotting of equal amounts of protein from each cell line (Fig. 1A, top row). Densitometry indicated that IRS-1 abundance was lessened by more than 80% in KD cells compared with either Control cells or untransfected 3T3-F442A cells (data not shown). Also shown in Fig. 1A are immunoblotting comparisons of Control vs. KD cells for levels of IRS-2, GHR, JAK2, ERK1/ERK2, STAT5, and SHC. In each case, IRS-1 knockdown did not appreciably alter the levels of these proteins. We next examined whether the reduction of IRS-1 affected the most proximal aspects of GH action by assaying GH-induced GHR disulfide linkage in Control vs. KD cells. We previously demonstrated in a variety of cell types (including 3T3-F442A) that GH binding that engenders the receptor conformational changes required for signaling is associated with formation of a high Mr GHR form detected by immunoblotting of cell extracts after SDS-PAGE under nonreducing conditions (65,66,67,68,69). The disulfide-linked GHR arises from covalent bonding between receptors mediated by cysteine-241, the only unpaired extracellular cysteine, and its appearance can be used to monitor the GHR’s attainment of an activated conformation (66). As seen in Fig. 1B, both Control and KD cells acutely responded to GH treatment with formation of the disulfide-linked form of the GHR, indicating that IRS-1 depletion did not affect receptor engagement by GH.
Figure 1.
Characterization of the representative Control and KD clones. A, Serum-starved Control and KD cells were subjected to detergent extraction. Equal amounts of protein were resolved in SDS-PAGE and transferred to nitrocellulose membranes. Resolved proteins were serially immunoblotted with anti-IRS-1, anti-IRS-2, anti-GHR, anti-JAK2, anti-STAT5, anti-SHC, and anti-ERK. B, Serum-starved Control and KD cells were treated with vehicle or GH (125 ng/ml) for 10 min before detergent extraction. Equal amounts of protein were resolved by SDS-PAGE under nonreduced conditions and immunoblotted with anti-GHR. Positions of the non-disulfide-linked and disulfide-linked GHR are indicated. dsl GHR, Disulfide-linked GHR.
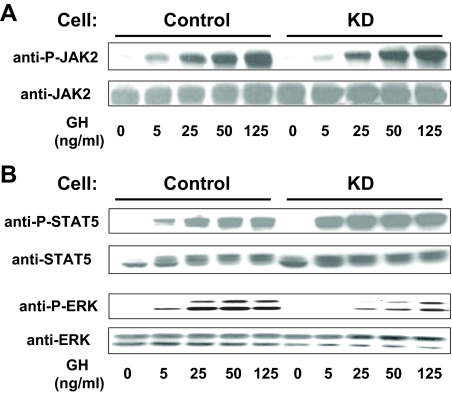
To assess downstream signaling, we first examined the activation of JAK2 and STAT5. Serum-starved Control and KD cells were treated with varying concentrations of GH (0–125 ng/ml) for 10 min, after which equal protein amounts from detergent extracts were resolved by SDS-PAGE and immunoblotted with antibodies that detect tyrosine-phosphorylated JAK2 (anti-P-JAK2) (Fig. 2A) or tyrosine-phosphorylated STAT5 (anti-P-STAT5) (Fig. 2B). The GH concentration dependence for JAK2 tyrosine phosphorylation was very similar between the two cell types, as was the GH concentration dependence for STAT5 tyrosine phosphorylation. Reprobing of each blot for JAK2 (Fig. 2A) and STAT5 (Fig. 2B) verified the similarity of abundance of each molecule between the two cells, as shown above in Fig. 1A). These observations of similar activation profiles between cells were confirmed by densitometric analysis of several similar experiments for anti-P-JAK2 (Fig. 3C) and anti-P-STAT5 (Fig. 3D). In other experiments, cells were treated with 125 ng/ml GH for 0, 1, 3, 5, and 10 min, and resulting JAK2 and STAT5 activation was similarly analyzed, revealing that the GH time courses for each did not vary between Control and KD cells (data not shown). Collectively, these data indicate that knockdown of IRS-1 in 3T3-F442A cells had no appreciable effect on the ability of GH to engage the GH receptor and cause acute JAK2-dependent STAT5 activation.
Figure 2.
GH-induced JAK2, STAT5, and ERK signaling in Control and KD cells. In two separate experiments (A and B), serum-starved cells were treated with GH (0–125 ng/ml) for 10 min before detergent extraction. Equal amounts of protein were resolved by SDS-PAGE and immunoblotted sequentially with anti-P-JAK2 and anti-JAK2 (A) and anti-P-STAT5, anti-STAT5, anti-P-ERK, and anti-ERK (B).
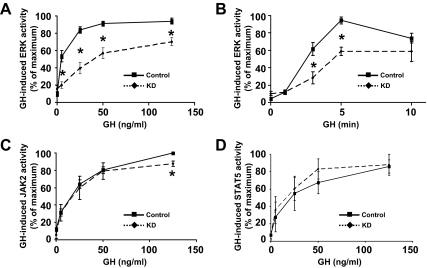
Figure 3.
Densitometric and statistical analysis of GH-induced JAK2, STAT5, and ERK signaling in Control and KD cells. Multiple dose-response experiments (A, C, and D) such as that shown in Fig. 2 and time course experiments (B) were analyzed densitometrically and are plotted as mean ± se relative to the maximal signal obtained within each experiment (100%). Asterisk indicates P < 0.03 for KD compared with Control. A, Dose response of GH-induced ERK activation (n = 9). B, Time course of GH-induced ERK activation (n = 7). C, Dose response of GH-induced JAK2 activation (n = 3). D, Dose response of GH-induced STAT5 activation (n = 3).
Unlike JAK2 and STAT5, GH-induced ERK and Akt activation is markedly impaired in cells depleted of IRS-1
We next examined GH-induced activation of the ERK pathway in Control vs. KD cells. Serum-starved cells treated with GH (0–125 ng/ml) for 10 min were lysed, and extracts were resolved and sequentially blotted with anti-P-ERK, an antibody that recognizes the activated forms of ERKs, and anti-ERK (Fig. 2B). In contrast to JAK2 and STAT5 activation, the pattern of GH-induced ERK phosphorylation differed markedly between the two cell lines, with substantially blunted ERK phosphorylation in KD cells. Densitometric analysis of several such concentration dependence experiments revealed highly significant reductions at each GH concentration and EC50 of approximately 4 ng/ml for Control cells and approximately 20 ng/ml for KD cells (Fig. 3A). Analysis of multiple time course experiments is shown in Fig. 3B. Maximum GH (125 ng/ml)-induced ERK activity was seen at 5 min in both cells but was decreased by roughly 40% in KD cells compared with Control cells.
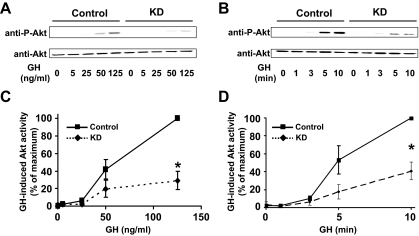
In several systems, IRS proteins provide a crucial link to activation of PI3K, which serves as an upstream activator of the Akt kinase (70). Indeed, previous work by Argetsinger et al. (34) demonstrated that GH treatment of 3T3-F442A cells with GH promotes association of IRS-1 with the p85-regulatory subunit of PI3K, and the same results were seen in GHR- and JAK2-expressing cell lines by Yamauchi et al. (63). In our previous reconstitution experiments, expression of IRS-1 in IRS-1-deficient promonocytes resulted in greatly augmented GH-induced Akt phosphorylation, a marker of its activation (29). To determine the effect of reduction of endogenous IRS-1 on this pathway, we compared GH-induced Akt activation in Control vs. KD cells (Fig. 4). GH concentration-dependence (Fig. 4, A and C) and time course (Fig. 4, B and D) experiments were carried out on serum-starved cells, and detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-P-Akt, which recognizes the activated form of the molecule phosphorylated at serine-473, and anti-Akt to detect total Akt levels. GH promoted diminished Akt phosphorylation in both types of experiments. Densitometric analysis (Fig. 4D) indicated that stimulation with GH (125 ng/ml) for 10 min resulted in a 60–70% reduction in Akt phosphorylation in KD cells compared with Control cells. This reduction could not be accounted for by alteration in the level of Akt. Rather, the IRS-1 deficiency apparently leads to reduced phosphorylation of the available Akt molecules.
Figure 4.
GH-induced Akt signaling in Control and KD cells. Serum-starved cells were treated with GH with varying doses (0–125 ng/ml) for 10 min (A) and with a single dose (125 ng/ml) for varying durations (0–10 min) (B) before detergent extraction and sequential blotting with anti-P-Akt and anti-Akt. Data from multiple dose-response (C) and time course (D) experiments (n = 3) including those in A and B were analyzed densitometrically and are plotted as mean ± se relative to the maximal signal obtained within each experiment (100%). Asterisk indicates P < 0.03 for KD compared with Control.
IRS-1 knockdown does not affect platelet-derived growth factor (PDGF)-induced ERK activation
The data in Figs. 2–4 indicate that knockdown of IRS-1 impairs GH-induced activation of both ERK and Akt. In principle, these defects could represent more global signaling consequences somehow related to knockdown of IRS-1 or arising as an artifact of the generation of the clones. To address this issue, we sought to assess the activation of the ERK and Akt signaling pathways in response to a stimulus that does not signal via engagement of IRS proteins. PDGF is such a stimulus; PDGF-induced activation of ERK and PI3K is believed to be independent of IRS proteins (71,72,73).
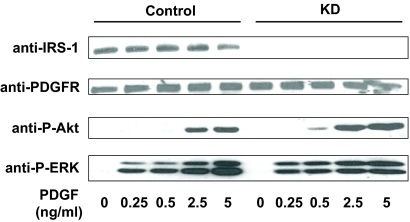
In the experiment shown in Fig. 5, serum-starved Control and KD cells were treated for 10 min with PDGF-BB (0–5 ng/ml). Cell lysates were resolved by SDS-PAGE and blotted with anti-P-Akt and anti-P-ERK. This revealed no difference in PDGF-induced activation of either pathway. Blotting of the same extracts with anti-IRS-1 verified its knockdown in KD cells and reprobing with anti-PDGF receptor (PDGFR) revealed similar PDGFR abundance in each cell and verified similar protein loading in each sample. This experiment indicates that the diminished GH-induced ERK and Akt activation seen in KD cells cannot be explained by a global defect in these pathways resulting from knockdown of IRS-1 expression and suggests specificity in the effects of that knockdown.
Figure 5.
PDGF-induced Akt and ERK activation in Control and KD cells. Serum-starved cells were treated with PDGF (0–5 ng/ml) for 10 min before detergent extraction. Equal amounts of protein were resolved by SDS-PAGE and sequentially blotted with anti-IRS-1, anti-PDGFR, anti-P-Akt, and anti-P-ERK.
Knockdown of IRS-1 in 3T3-F442A preadipocytes results in markedly reduced GH-induced tyrosine phosphorylation of SHC
GH promotes JAK2 tyrosine phosphorylation in 3T3-F442A cells within seconds (41). ERK activation in response to GH, although lagging slightly behind JAK2 activation, is also quite acute (43,44) in these cells. Several elegant studies have shown that GH-induced tyrosine phosphorylation of SHC, likely catalyzed by JAK2, and consequent SHC-Grb2 association are among the earliest detectable events that promote ERK activation in 3T3-F442A cells (40,53,54). We thus asked whether the diminished GH-induced ERK activation in KD cells was related to altered SHC tyrosine phosphorylation (Fig. 6).
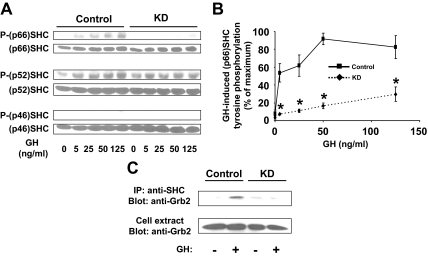
Figure 6.
GH-induced SHC activation in Control and KD cells. Serum-starved cells were treated with GH (0–125 ng/ml) for 10 min (A and B) or with 125 ng/ml for 10 min (C) before detergent extraction. Extracts were resolved by SDS-PAGE and sequentially blotted with anti-P-SHC and anti-SHC (A and B) or were immunoprecipitated with anti-SHC (C). In the representative experiment shown in panel A, regions of the blots including each of the SHC isoforms are shown. In B, data for phosphorylated p66 SHC from three experiments such as in A were analyzed densitometrically and are plotted as mean ± se relative to the maximal signal obtained within each experiment (100%). Asterisk indicates P < 0.03 for KD compared with Control. In C, eluates from anti-SHC precipitates (upper panel) or unprecipitated extracts (lower panel) were resolved by SDS-PAGE and blotted with anti-Grb2. Data in C are representative of three such experiments. IP, Immunoprecipitation.
Serum-starved Control and KD cells were treated with varying concentrations of GH (0–125 ng/ml) for 10 min, after which equal protein amounts from detergent extracts were resolved by SDS-PAGE and immunoblotted with an antibody that recognizes the tyrosine-phosphorylated form of SHC proteins (Fig. 6A). As seen in previous studies in 3T3-F442A cells (40,53), GH treatment of Control cells caused markedly increased tyrosine phosphorylation of the p66 isoform of SHC, but not of the p46 isoform. Similar to the findings of VanderKuur et al. (53), we found that p52 SHC was basally tyrosine phosphorylated and that GH caused some further phosphorylation of this isoform in Control cells. Notably, SHC tyrosine phosphorylation in response to GH was dramatically reduced in KD cells compared with Control cells, in particular for the p66 isoform. Reprobing with anti-SHC confirmed that this difference in GH-induced SHC tyrosine phosphorylation was not explained by disparate levels of SHC in the two cells, as is also shown in Fig. 1A above. Data in four such experiments were analyzed densitometrically and graphically displayed in Fig. 6B, verifying that the KD cells displayed a marked desensitization to GH-induced p66 SHC tyrosine phosphorylation compared with Control cells.
The ability of SHC to associate with Grb2 in response to GH was examined in the experiment shown in Fig. 6C. Serum-starved Control and KD cells were treated with GH (125 ng/ml) for 10 min, after which detergent-extracted proteins were precipitated with anti-SHC. Eluates were resolved by SDS-PAGE and immunoblotted sequentially with anti-Grb2. As expected, GH induced Grb2-SHC association in Control cells. Notably, GH-induced SHC-Grb2 association was markedly reduced in KD cells, despite the similar abundance of Grb2 in extracts from each cell. The data in Fig. 6 are consistent with the interesting conclusion that loss of IRS-1 renders the KD cells much less sensitive with regard to GH-induced SHC activation and strongly suggest that this insensitivity underlies the reduced GH-induced ERK activation observed in these cells.
IRS-1 is enriched in lipid rafts and its knockdown principally affects GH-induced raft-associated ERK activation
We previously examined the distribution of GHR and several of its signaling proteins within membrane fractions in 3T3-F442A cells (51). Using a well-validated nondetergent extraction and multistep fractionation scheme, we isolated four fractions: postnuclear supernatant (PNS); plasma membranes (PM); and from the plasma membranes, the nonlipid raft membranes (NLR) and lipid raft membranes (LR). The GHR was greatly enriched within the PM fraction; interestingly, within that fraction the receptor was highly concentrated in LR. In fact, when normalized for protein content, the GHR concentration in LR was nearly 7-fold higher than in bulk PM. Given the low abundance of LR relative to NLR within the PM, we estimated that roughly one-third of PM GHR was associated with cholesterol-rich raft microdomains (51). The same analysis indicated that in these cells JAK2 and ERKs were also quite enriched (2.5- and 2.3-fold, respectively) in the raft fraction relative to the PM as a whole, and most of the ERK activated by GH treatment was found associated with LR (51). In 3T3-F442A cells, roughly 20% of total PNS protein was recovered in the PM and approximately 5% of protein in PM was recovered in LRs; because ERKs were roughly 1.8-fold enriched in the LR vs. PNS, we estimated that less than 2% of total PNS ERK was found associated with the LR fraction, further emphasizing that ERK activation was quite enriched in this fraction (Ref. 51 and our unpublished results).
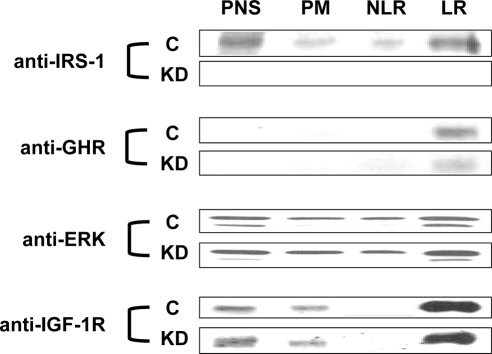
We applied the same fractionation analysis to the Control and KD cells. As seen in Fig. 7, the GHR was highly LR enriched in Control cells, as expected, and was similarly localized in KD cells. As reported previously in other cell types (74,75,76,77,78), IGF-1R was also strongly LR enriched in Control cells. Depletion of IRS-1 had no effect on the distribution of IGF-1R in KD cells. (In data not shown, IGF-I-induced IGF-1R activation was also unchanged by loss of IRS-1.) Despite the substantially reduced GH-induced ERK activity in KD cells, the subcellular distribution of ERKs did not differ between Control and KD cells, displaying an LR enrichment in both. Although it could not be evaluated in the KD cells (because of its knockdown), the distribution of IRS-1 was assessed in the Control cells. Interestingly, although IRS-1 was largely cytosolic (PNS), the component that was isolated with plasma membranes was highly enriched in LR. This is consistent with the only previous study to evaluate IRS-1 raft distribution, in which a different raft isolation procedure was followed and a different cell system (H35 hepatoma cells) was used (79).
Figure 7.
Subcellular distributions of GHR and downstream signaling elements in Control and KD cells. PNS, PM, NLR, and LR fractions were isolated from serum-starved cells, as in Materials and Methods. Equal amounts of protein (5 μg) from each fraction were resolved by SDS-PAGE and sequentially immunoblotted with anti-IRS-1, anti-GHR, anti-ERK and anti-IGF-1R β-chain. The experiments shown are representative of three such experiments. C, Control.
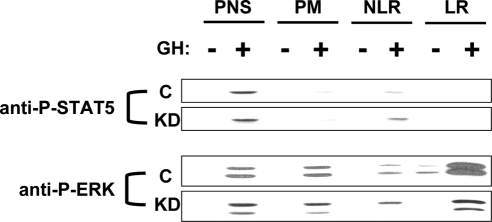
The pattern of distribution of tyrosine-phosphorylated STAT5 and ERK that appeared in response to GH (125 ng/ml; 10 min) was also examined (Fig. 8). Consistent with our previous data in the parental 3T3-F442A cells (51), tyrosine phosphorylated STAT5 was found almost exclusively in the PNS fraction in Control cells. This pattern was unchanged in the KD cells, which agrees with our findings in Figs. 2 and 3D that depletion of IRS-1 had no effect on GH-induced STAT5 activation. In contrast, the pattern of subcellular localization of ERK activation markedly differed between the two cells. As expected, in Control cells, GH induced some ERK phosphorylation in all fractions, but it was markedly enriched in the LR fraction. Notably, GH-induced relative ERK phosphorylation in the PNS, PM, and NLR fractions in KD cells did not differ greatly compared with Control cells. However, relative GH-induced ERK phosphorylation in the raft fraction was substantially diminished in KD cells in comparison with Control cells. These data suggest that the decreased GH-induced ERK activity in KD vs. Control cells can be accounted for largely by diminished ERK phosphorylation associated with the raft fraction of the plasma membrane, in particular.
Figure 8.
Subcelluar distribution of the P-STAT5 and P-ERK in Control and KD cells. Serum-starved cells were treated with GH (500 ng/ml) or vehicle for 10 min at 37 C before harvesting and subcellular fractionation, as in Materials and Methods. Equal amounts of protein (5 μg) from the PNS, PM, NLR, and LR fractions were resolved by SDS-PAGE and sequentially immunoblotted with anti-P-STAT5 and anti-P-ERK. The experiments shown are representative of three such experiments. C, Control.
Discussion
In this study, we examined the consequences of markedly reducing the cellular content of the IRS-1 docking protein on acute GH-induced signal pathway activation in 3T3-F442A preadipocyte fibroblasts. We found that stable expression of a siRNA directed at a sequence specific for IRS-1 dramatically reduced the endogenous IRS-1 level without affecting the levels of GHR, JAK2, IRS-2, STAT5, SHC, Akt, or ERKs. GH-induced GHR disulfide linkage (reflecting GH-induced receptor conformational changes required for signaling), JAK2 activation, and STAT5 tyrosine phosphorylation were not substantially affected by knockdown of IRS-1. In contrast, GH-induced ERK activation and Akt phosphorylation were diminished in IRS-1 knockdown cells compared with control cells, but these pathways were similarly activated in each cell by a different stimulus, PDGF, indicating that the effect of IRS-1 knockdown on GH-induced ERK and Akt activation did not reflect a global dysfunction of these pathways related to clonal factors and/or loss of IRS-1 in 3T3-F442A cells. Notably, knockdown of IRS-1 in these cells also resulted in markedly diminished GH-induced SHC activation. Finally, we demonstrate for the first time in cells of nonhepatic origin that IRS-1 is found to be highly enriched in the lipid raft fraction of the plasma membrane and that deletion of IRS-1 resulted in markedly diminished GH-induced ERK activation, particularly within rafts.
GH was first shown to activate ERKs by several groups at much the same time in 1992 (30,43,44,80). Mechanisms by which GH couples to this pathway have been examined in substantial detail since that time. Much of this work is consistent with the familiar SHC-Grb2-SOS-Ras-Raf-MEK1-ERK scheme as being a major route of GH-induced ERK activation (53,54). At the proximal aspect of this cascade, VanderKuur et al. (53) provided evidence that the SHC Src homology-2 domain fused to glutathione-S-transferase directly associates with JAK2, which was consistent with GHR deletional analysis, indicating that only the region of the receptor intracellular domain required for JAK2 activation was required for ERK activation (31,32). Notably, however, other pathways and molecules have been implicated in GH-induced ERK activation. PI3K inhibitors, for example, prevent GH-induced ERK activation in certain cell systems (20,27,29), suggesting involvement of PI3K upstream of ERK, although the mechanism for this involvement is not clear. EGF receptor (EGFR) was implicated in GH-induced ERK activation by Yamauchi et al. (81), with GH-induced (JAK2-mediated) phosphorylation of EGFR at a particular tyrosine residue being thought to allow Grb2 association with the EGFR, which, in essence, functions as an adaptor protein in this scheme. Likewise, we implicated another large adaptor, Gab-1, and an SH2-containing protein tyrosine phosphatase, Src homology-2, as important for enhanced GH-induced ERK activation (47,49).
Interestingly, work from two laboratories has suggested that ERKs can be activated by GH independent of JAK2 activity (36,38). These studies were carried out in NIH-3T3 cells (36) and in FDC-P1 cells and JAK2-deficient human fibrosarcoma cells transfected with rabbit GHR (38). In both, it was concluded that JAK2-independent GH-induced ERK activation was dependent on GH-induced src family kinase activation, and in one (38) this was believed to be unrelated to SHC tyrosine phosphorylation. Furthermore, Rowlinson et al. (38) concluded that GH, by causing a specific conformational change in the GHR extracellular domain in the region of the subdomain 2 F′G′ loop, activates src and thus phospholipase C-γ, allowing ERK activation. It is important to note, however, that a very recent report from Jin et al. (39) demonstrated that GH-induced ERK activation in 3T3-F442A cells and liver-derived H4IIE cells was not inhibited by src kinase inhibitors and that GH did not induce src family kinase activation in these cells. Additionally, GH-induced ERK activation was not reduced in murine embryonic fibroblasts that genetically lack src family kinases. Thus, the degree to which src family kinase, rather than JAK2, activation underlies GH-induced ERK activation may vary among cell types and, in any case, JAK2 appears the major mediator of this GH effect in 3T3-F442A cells such as those used in our current study.
Our findings that IRS-1 knockdown in 3T3-F442A cells reduces GH-induced ERK and Akt activation without affecting GH-induced JAK2 or STAT5 activation compliment our previous findings in 32D-GHR cells (29). IRS proteins are not endogenously expressed in 32D-GHR, but stable expression of IRS-1 conferred markedly enhanced GH-induced ERK and Akt activation without changing GH-induced STAT5 tyrosine phosphorylation. Furthermore, in that system, whereas expression of IRS-1 did not affect the abundance of GHR, JAK2, SHP-2, Raf, MEK1, or ERKs, it did enhance GH-induced MEK1 tyrosine phosphorylation and basal and GH-induced Ras activation. These data suggested that at least part of IRS-1’s effects on GH-induced ERK activation might reside at or above the level of Ras.
Regarding Akt activity, our current findings in 3T3-F442A cells are interesting in that they confirm a role for IRS-1 in mediation of PI3K activity in response to GH. Previous studies in rat adipocytes and in 3T3-F442A cells demonstrated GH-induced tyrosine phosphorylation of IRS-1 and its subsequent association with the PI3K-regulatory subunit and/or PI3K activity (27,34,61,63,82). Mutagenesis and coimmunoprecipitation suggest that IRS-1 may interact with JAK2 (29,34); furthermore, JAK2 could be involved in PI3K activation even in the absence of IRS-1 by virtue of PI3K association with JAK2 itself, because there are putative consensus sequences for p85 association with the tyrosine-phosphorylated JAK2.
There are multiple potential mechanisms by which knockdown of endogenous IRS-1 in 3T3-F442A cells might reduce GH-induced ERK activity. Beginning at proximal aspects of the GH signaling pathway, GHR abundance, GH binding capacity, or GH-induced JAK2 activation could, in principle, be impaired. However, our data do not support such effects in KD cells, because immunoblottable GHR levels, GH-induced GHR disulfide linkage, and GH-induced JAK2 and STAT5 tyrosine phosphorylation were not detectably different between KD and Control cells. Instead, we made the interesting observation that GH-induced SHC tyrosine phosphorylation and its association with Grb2 were markedly reduced in the KD cells compared with Control cells, despite the lack of difference in SHC and Grb2 levels. This lessening of GH-induced SHC phosphorylation in cells deficient in IRS-1 was an unexpected result, in that our initial hypothesis was that SHC and IRS-1 likely function independently from each other in coupling to Ras activation and downstream ERK signaling. Indeed, to our knowledge, this issue has not been explored in previous studies of the role of IRS proteins in GH signaling.
In contrast to our understanding of its role in GH signaling, the role of IRS-1 in insulin signaling has been extensively examined. Notably, its effects on insulin-induced ERK activation may vary according to cell types and context. For example, in IRS-1-deficient mice compared with wild-type mice, insulin-induced ERK activation in vivo in muscle was markedly impaired but was unaffected in liver (83). Similarly, in fibroblasts derived from IRS-1-deficient mice, insulin-induced ERK activation was not reduced compared with those derived from wild-type mice (84), a finding also noted for IGF-I stimulation of these cells (85). However, in brown adipocytes from IRS-1-deficient mice, insulin-induced MEK1 and ERK activation were severely impaired in comparison with wild-type brown adipocytes (86). Notably, in the same study, insulin-induced SHC tyrosine phosphorylation and SHC-Grb2 association were also quite reduced in the IRS-1-deficient brown adipocytes (86). In wild-type brown adipocytes or in IRS-1-deficient brown adipocytes reconstituted with IRS-1, the authors observed that insulin caused coimmunoprecipitation of IRS-1 with SHC. This result complemented a prior yeast two-hybrid study that also defined a direct interaction between IRS-1 and SHC (87).
In the context of these insulin and IGF-I signaling studies, it is clear that the effects of IRS-1 on GH-induced ERK activation may be equally diverse among cells and tissues, depending on variables such as coexpression of other ERK activator molecules, compensatory changes in the levels of these molecules when IRS-1 levels are experimentally modulated, and other unknown factors. However, we are intrigued by the possibility that in 3T3-F442A cells, which respond robustly to GH, IRS-1 and SHC may act in partnership, rather than independently, to transmit GH engagement of GHR and activation of JAK2 to the ERK activation pathway. Whether or not GH-induced IRS-1-SHC interaction underlies this partnership remains to be studied. We have not yet been able to detect specific IRS-1-SHC coimmunoprecipitation in 3T3-F442A or Control cells, however. Furthermore, we do not yet know whether the dramatic decline in tyrosine phosphorylation of the p66 SHC isoform in KD cells suggests that this isoform is disproportionately important in GH-induced IRS-1-mediated ERK signaling compared with other isoforms; this question merits further investigation. The decrease in GH-induced SHC-associated Grb2 in KD cells suggests that IRS-1 knockdown affects this property of all immunoprecipitable SHC isoforms under these conditions.
Our results also raise interesting issues regarding the spatial compartmentalization of GH signaling. In concordance with our prior studies, we found GH-induced ERK activation associated largely with the LR fraction of the plasma membrane. Notably, the reduction of this activity in the KD cells was almost completely found in the LR fraction. As IRS-1 (present study) and GHR and JAK2 (51) are highly LR concentrated and GH causes SHC to become increased in the LR fraction of these cells (51), it is tempting to speculate that a putative GH-induced JAK2-IRS-1-SHC interaction in the LR fraction may underlie a substantial component of GH-induced ERK activation. This will be a topic for future studies made possible with the cell model created in this study. Furthermore, it is of interest to attempt to account for the remaining GH-induced ERK activation in these IRS-1-deficient cells, because it will contribute to a more complete understanding of the complex pathways that apparently exist to transduce this signal.
Materials and Methods
Materials
Routine reagents were purchased from Sigma Aldrich Corp. (St. Louis, MO) unless otherwise noted. G418 was from Mediatech (Herndon, VA). Fetal bovine serum, gentamicin sulfate, penicillin, and streptomycin were purchased from BioFluids (Rockville, MD). Recombinant hGH was kindly provided by Eli Lilly &Co. (Indianapolis, IN).
Antibodies
Polyclonal anti-IRS-1 antibody, affinity-purified polyclonal anti-MAPK antibody (anti-ERK, recognizing both ERK1 and ERK2), polyclonal anti-SHC antibody, polyclonal anti-PDGFR antibody (recognizing both PDGF type A and B receptors), and polyclonal anti-p-JAK2 antibody (Tyr1007/1008) were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Affinity-purified polyclonal antiactive MAPK antibody (antiactive ERK, recognizing the dually phosphorylated Thr-183 and Tyr-185 residues corresponding to the active forms of ERK1 and ERK2) was from Promega Corp. (Madison, WI). Anti-JAK2AL33 (directed at residues 746-1129 of murine JAK2) polyclonal serum has been described elsewhere (88). The rabbit polyclonal antiserum, anti-GHRcyt-AL47, was raised against a bacterially expressed N-terminally His-tagged fusion protein incorporating human GHR residues 271-620 (the entire cytoplasmic domain) and has been previously described (89). Polyclonal anti-Akt and polyclonal anti-p-Akt (Ser 473) were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Polyclonal anti-p-SHC antibody (Tyr 239) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Grb2 monoclonal antibody was obtained from Transduction Laboratories (Lexington, KY). Polyclonal anti-p-STAT5 antibody was obtained from Zymed Laboratories, Inc. (South San Francisco, CA).
Plasmid construction
A plasmid encoding a small hairpin insert with a validated siRNA sequence targeting reading frame nucleotides 24-42 of murine IRS-1 (corresponding to residues 8-14 near the amino terminus of the protein) in the backbone of pRNAU6.1/Neo (GenScript) was constructed. The target hairpin insert was synthesized, inserted into pRNAU6.1/Neo between BamHI and HindIII, and sequenced by GenScript.
Cell culture, stable transfection, and clone selection
3T3-F442A cells, kindly provided by Drs. H. Green (Harvard University, Boston, MA) and C. Carter-Su (University of Michigan, Ann Arbor, MI), were maintained in DMEM (4.5 g/liter glucose) (Cellgro, Inc., Herndon, VA) supplemented with 5% calf serum (Atlanta Biological, Lawrenceville, GA) and 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Biofluids). Stable transfection was achieved by introducing 3 μg vector (pRNAU6.1/Neo) or IRS-1 siRNA plasmid, as described above, using Lipofectamine Plus (Invitrogen, San Diego, CA) according to the manufacturer’s instructions. After transfection, cells either with vector or with siRNA plasmid were trypsinized and aliquoted into four 10-cm2 dishes. After G418 (500 μg/ml) drug selection, clones were isolated by dilution and screened by anti-IRS-1 immunoblotting. The selected clones vector (Control) and IRS-1 siRNA (KD) were maintained in 400 μg/ml G418.
Cell stimulation, protein extraction, immunoprecipitation, electrophoresis, and immunoblotting
Serum starvation of 3T3-F442A cell derivatives (Control or KD cells) was accomplished by substitution of 0.5% (wt/vol) BSA (fraction V, Roche Molecular Biochemicals, Indianapolis, IN) for calf serum in the culture medium (starvation medium) for 16–20 h before stimulation. Starvation and stimulations were carried out at 37 C in starvation medium. Serum-starved cells were treated for 10 min with GH (5, 25, 50, or 125 ng/ml), PDGF (0.25, 0.5, 2.5, or 5 ng/ml), or vehicle, as specified for each experiment. For time course experiments, serum-starved cells were treated with GH (50 ng/ml or 125 ng/ml) for varying duration (0, 1, 3, 5, or 10 min). Stimulations were terminated by washing the cells once with ice-cold PBS supplemented with 0.4 mm sodium orthovanadate (PBS-vanadate) before harvesting by scraping in PBS-vanadate. Cells were collected by brief centrifugation and solubilized for 15 min at 4 C in lysis buffer [1% (vol/vol) Triton X-100 (0.5% Triton X-100 for immunoprecipitation), 150 mm NaCl, 10% (vol/vol) glycerol, 50 mm Tris-HCl (pH 7.3), 100 mm NaF, 1 mm EDTA, 1 mm phenylmethylsulfonylfluoride, 1 mm sodium orthovanadate, 10 mm benzamidine, 5 μg/ml aprotinin, and 5 μg/ml leupeptin]. After centrifugation at 15,000 × g for 15 min at 4 C, the detergent extracts (supernatant) were protein assayed (using Pierce BCA Protein assay Kit; Pierce Chemical Co., Rockford, IL) and directly electrophoresed and immunoblotted. For immunoprecipitation, cell extracts (400–800 μg) were mixed with 7 μl anti-SHC antibody and incubated at 4 C for 2 h with continuous agitation. Protein A-Sepharose (Amersham Pharmacia Biotech, Arlington Heights, IL) was added and incubated at 4 C for an additional 1 h, after which the beads were washed four times with lysis buffer. Laemmli sample buffer eluates were resolved by SDS-PAGE and immunoblotted.
Proteins resolved by SDS-PAGE under reducing or nonreducing conditions were transferred to Hybond ECL Nitrocellulose membranes (Amersham Pharmacia Biotech). The membranes were blocked with TBST buffer [20 mm Tris-HCl (pH 7.6), 150 mm NaCl, and 0.1% (vol/vol) Tween 20] containing 2% BSA and incubated with primary antibodies (0.5–1 μg/ml or 1:1000 dilution), as specified in each experiment. After three washes with TBST, the membranes were incubated with appropriate secondary antibodies (1:10,000 dilution) and washed. The bound antibodies were detected with SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). Membrane stripping was performed according to manufacturer’s suggestions (Amersham Pharmacia Biotech).
Preparation of lipid rafts
Lipid rafts (LR) were prepared as described previously (51). Briefly, pelleted cells were resuspended in buffer A (0.25 m sucrose, 1 mm EDTA, and 20 mm Tricine; pH 7.8), including inhibitors, and Dounce homogenized (20 strokes). The lysate was centrifuged at 1000 × g for 10 min to remove nuclei and unbroken cells. The resulting pellet was resuspended in buffer A, homogenized, and centrifuged. Supernatants from both centrifugations were combined [referred to as the postnuclear supernatant (PNS)] and loaded onto 23 ml of 30% (wt/vol) Percoll in buffer A before centrifugation at 26,000 rpm for 35 min in a Beckman SW28 rotor. The plasma membrane (PM) fraction, a visible band roughly 5.5 cm from the bottom of the centrifuge tube, was collected and sonicated (six bursts of 10 sec each). This sonicate was mixed with 50% (wt/vol) Opti-Prep in buffer B (0.25 m sucrose, 6 mm EDTA, and 120 mm Tricine; pH 7.8) to yield 4 ml of 23% Opti-Prep and loaded at the bottom of a centrifuge tube, on top of which 6 ml of a 10–20% (wt/vol) gradient was layered. The sample was centrifuged at 22,000 rpm for 90 min in a Beckman SW41 rotor. The resulting nonfloating fraction (the bottom 3.5 ml of the tube) was collected and is referred to as the NLR fraction. The top 5 ml (the floating fraction) was collected, mixed with 4 ml of 50% (wt/vol) Opti-Prep, and loaded at the bottom of another tube. On top of this was layered 2 ml of 5% (wt/vol) Opti-Prep, and the sample was centrifuged at 22,000 rpm for 90 min in a Beckman SW41 rotor. The lipid raft (LR) fraction was collected just above the interface of these two gradients.
Densitometric and statistical analysis
Immunoblots were scanned using a high-resolution scanner (Hewlett-Packard Co., Cupertino, CA). Densitometric quantification of images exposed in the linear range was performed using an image analysis program, Image J (developed by W. S. Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD). Pooled data of densitometry from several experiments are displayed as mean ± se. The significance (P value) of differences of pooled results was estimated by t tests.
Acknowledgments
We thank Drs. Y. Huang, K. He, K. Loesch, J. Xu, L. Liu, and Y. Zhang for helpful conversations.
Footnotes
This work was supported by National Institutes of Health Grant DK58259 (to S.J.F.).
Parts of this work were presented at the 87th Annual Meeting of The Endocrine Society in San Diego, CA, 2005.
Disclosure Summary: X.W., N.Y., L.D., X.L., J.J., and S.J.F. have nothing to declare.
First Published Online January 22, 2009
Abbreviations: EGF, epidermal growth factor; EGFR, EGF receptor; GHR, GH receptor; IRS-1, insulin receptor substrate-1; JAK2, Janus kinase 2; KD, knockdown; LR, lipid raft membrane; NLR, nonlipid raft membranes; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PM, plasma membrane; PNS, postnuclear supernatant; SHC, Src homology-collagen; siRNA, small interfering RNA; STAT, signal transducer and activator of transcription.
References
- Kaplan S 1999 Hormonal regulation of growth and metabolic effects of growth hormone. In: Kostyo J, Goodman HM, eds. Handbook of physiology. Chap 5. New York: Oxford University Press; 129–143 [Google Scholar]
- Isaksson OG, Eden S, Jansson JO 1985 Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol 47:483–499 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Messina JL 2002 Growth hormone receptor. In: Oppenheim JJ, Feldman M, eds. Cytokine reference on-line. London: Academic Press, Harcourt; 1–21 [Google Scholar]
- Carter Su C, Schwartz J, Smit LS 1996 Molecular mechanism of growth hormone action. Annu Rev Physiol 58:187–207 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Frank SJ 2000 Growth hormone action: signaling via a JAK/STAT-coupled receptor. In: Conn PM, Means A, eds. Principles of molecular regulation. Totowa, NJ: Humana Press; 55–83 [Google Scholar]
- Frank SJ, O'Shea JJ 1999 Recent advances in cytokine signal transduction: lessons from growth hormone and other cytokines. In: LeRoith D, ed. Advances in molecular and cellular endocrinology. Vol 3. Greenwich, CT: JAI Press; 1–42 [Google Scholar]
- Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ 2006 New insights into growth hormone action. J Mol Endocrinol 36:1–7 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Bergad PL, Shih HM, Towle HC, Schwarzenberg SJ, Berry SA 1995 Growth hormone induction of hepatic serine protease inhibitor 2.1 transcription is mediated by a Stat5-related factor binding synergistically to two γ-activated sites. J Biol Chem 270:24903–24910 [DOI] [PubMed] [Google Scholar]
- Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE 1999 STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol 158:111–116 [DOI] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR 2001 STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142:3836–3841 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR 1998 Binding of STAT5a and STAT5b to a single element resembling a γ-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol Endocrinol 12:675–687 [DOI] [PubMed] [Google Scholar]
- Woelfle J, Chia DJ, Rotwein P 2003 Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278:51261–51266 [DOI] [PubMed] [Google Scholar]
- Hansen LH, Wang X, Kopchick JJ, Bouchelouche P, Nielsen JH, Galsgaard ED, Billestrup N 1996 Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J Biol Chem 271:12669–12673 [DOI] [PubMed] [Google Scholar]
- Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C 1996 The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol 10:519–533 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Moutoussamy S, Renaudie F, Clauss M, Kayser C, Gouilleux F, Kelly PA, Finidori J 1996 Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor. Mol Endocrinol 10:998–1009 [DOI] [PubMed] [Google Scholar]
- Wang X, Darus CJ, Xu BC, Kopchick JJ 1996 Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol Endocrinol 10:1249–1260 [DOI] [PubMed] [Google Scholar]
- Yi W, Kim SO, Jiang J, Park SH, Kraft AS, Waxman DJ, Frank SJ 1996 Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol 10:1425–1443 [DOI] [PubMed] [Google Scholar]
- Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J 1998 Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem 273:31327–31336 [DOI] [PubMed] [Google Scholar]
- Liang L, Zhou T, Jiang J, Pierce JH, Gustafson TA, Frank SJ 1999 Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology 140:1972–1983 [DOI] [PubMed] [Google Scholar]
- Kim SO, Houtman JC, Jiang J, Ruppert JM, Bertics PJ, Frank SJ 1999 Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3-F442A fibroblasts. J Biol Chem 274:36015–36024 [DOI] [PubMed] [Google Scholar]
- Huang Y, Chang Y, Wang X, Jiang J, Frank SJ 2004 Growth hormone alters epidermal growth factor receptor binding affinity via activation of ERKs in 3T3-F442A cells. Endocrinology 145:3297–3306 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim SO, Jiang J, Frank SJ 2003 Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. Modulation of EGF-induced trafficking and signaling. J Biol Chem 278:18902–18913 [DOI] [PubMed] [Google Scholar]
- Costoya JA, Finidori J, Moutoussamy S, Searis R, Devesa J, Arce VM 1999 Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway. Endocrinology 140:5937–5943 [DOI] [PubMed] [Google Scholar]
- Jeay S, Sonenshein GE, Kelly PA, Postel-Vinay MC, Baixeras E 2001 Growth hormone exerts antiapoptotic and proliferative effects through two different pathways involving nuclear factor-κB and phosphatidylinositol 3-kinase. Endocrinology 142:147–156 [DOI] [PubMed] [Google Scholar]
- Kilgour E, Gout I, Anderson NG 1996 Requirement for phosphoinositide 3-OH kinase in growth hormone signalling to the mitogen-activated protein kinase and p70s6k pathways. Biochem J 315:517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie SJ, Yarwood SJ, Peden AH, Bolger GB, Vernon RG, Houslay MD 1998 Stimulation of p70S6 kinase via a growth hormone-controlled phosphatidylinositol 3-kinase pathway leads to the activation of a PDE4A cyclic AMP-specific phosphodiesterase in 3T3-F442A preadipocytes. Proc Natl Acad Sci USA 95:3549–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Jiang J, Frank SJ 2000 Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation. Endocrinology 141:3328–3336 [DOI] [PubMed] [Google Scholar]
- Moller C, Hansson A, Enberg B, Lobie PE, Norstedt G 1992 Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem 267:23403–23408 [PubMed] [Google Scholar]
- Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay MC, Finidori J, Kelly PA 1994 Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology 135:1292–1298 [DOI] [PubMed] [Google Scholar]
- VanderKuur JA, Wang X, Zhang L, Campbell GS, Allevato G, Billestrup N, Norstedt G, Carter-Su C 1994 Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem 269:21709–21717 [PubMed] [Google Scholar]
- Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiang J, Sakai I, Kraft AS 1995 Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem 270:14776–14785 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Hsu GW, Myers MGJ, Billestrup N, White MF, Carter-Su C 1995 Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem 270:14685–14692 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C 1996 Growth hormone, interferon-γ, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J Biol Chem 271:29415–29421 [DOI] [PubMed] [Google Scholar]
- Zhu T, Ling L, Lobie PE 2002 Identification of a JAK2-independent pathway regulating growth hormone (GH)-stimulated p44/42 mitogen-activated protein kinase activity. GH activation of Ral and phospholipase D is Src-dependent. J Biol Chem 277:45592–45603 [DOI] [PubMed] [Google Scholar]
- Ling L, Zhu T, Lobie PE 2003 Src-CrkII-C3G-dependent activation of Rap1 switches growth hormone-stimulated p44/42 MAP kinase and JNK/SAPK activities. J Biol Chem 278:27301–27311 [DOI] [PubMed] [Google Scholar]
- Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, Millard K, Palethorpe K, Nielsen K, Clyde-Smith J, Hancock JF, Waters MJ 2008 An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat Cell Biol 10:740–747 [DOI] [PubMed] [Google Scholar]
- Jin H, Lanning NJ, Carter-Su C 2008 JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol 22:1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DW, Whatmore AJ, Clayton PE, Silva CM 1998 Growth hormone stimulation of the mitogen-activated protein kinase pathway is cell type specific. Endocrinology 139:1965–1971 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Carter-Su C 1996 Mechanism of signaling by growth hormone receptor. Physiol Rev 76:1089–1107 [DOI] [PubMed] [Google Scholar]
- Campbell GS, Pang L, Miyasaka T, Saltiel AR, Carter-Su C 1992 Stimulation by growth hormone of MAP kinase activity in 3T3-F442A fibroblasts. J Biol Chem 267:6074–6080 [PubMed] [Google Scholar]
- Winston LA, Bertics PJ 1992 Growth hormone stimulates the tyrosine phosphorylation of 42- and 45-kDa ERK-related proteins. J Biol Chem 267:4747–4751 [PubMed] [Google Scholar]
- Smit LS, VanderKuur JA, Stimage A, Han Y, Luo G, Yu-Lee LY, Schwartz J, Carter-Su C 1997 Growth hormone-induced tyrosyl phosphorylation and deoxyribonucleic acid binding activity of Stat5A and Stat5B. Endocrinology 138:3426–3434 [DOI] [PubMed] [Google Scholar]
- Rui L, Mathews LS, Hotta K, Gustafson TA, Carter-Su C 1997 Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol Cell Biol 17:6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ 1998 Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem 273:2344–2354 [DOI] [PubMed] [Google Scholar]
- Stofega MR, Wang H, Ullrich A, Carter-Su C 1998 Growth hormone regulation of SIRP and SHP-2 tyrosyl phosphorylation and association. J Biol Chem 273:7112–7117 [DOI] [PubMed] [Google Scholar]
- Kim SO, Loesch K, Wang X, Jiang J, Mei L, Cunnick JM, Wu J, Frank SJ 2002 A role for Grb2-associated binder-1 in growth hormone signaling. Endocrinology 143:4856–4867 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim S-O, Yang N, Jiang J, Frank SJ 2004 Physical and functional interaction of GH and IGF-1 signaling elements. Mol Endocrinol 18:1471–1485 [DOI] [PubMed] [Google Scholar]
- Yang N, Huang Y, Jiang J, Frank SJ 2004 Caveolar and lipid raft localization of GH receptor and its signaling elements: impact on GH signaling. J Biol Chem 279:20898–20905 [DOI] [PubMed] [Google Scholar]
- Jin H, Lanning NJ, Carter-Su C 2008 JAK2, but not Src family kinases, is required for STAT, ERK and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol 22:1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderKuur J, Allevato G, Billestrup N, Norstedt G, Carter-Su C 1995 Growth hormone-promoted tyrosyl phosphorylation of SHC proteins and SHC association with Grb2. J Biol Chem 13:7587–7593 [DOI] [PubMed] [Google Scholar]
- Vanderkuur JA, Butch ER, Waters SB, Pessin JE, Guan KL, Carter-Su C 1997 Signaling molecules involved in coupling growth hormone receptor to mitogen-activated protein kinase activation. Endocrinology 138:4301–4307 [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF 1991 Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352:73–77 [DOI] [PubMed] [Google Scholar]
- Sun XJ, Wang LM, Zhang Y, Yenush L, Myers Jr MG, Glasheen E, Lane WS, Pierce JH, White MF 1995 Role of IRS-2 in insulin and cytokine signalling. Nature 377:173–177 [DOI] [PubMed] [Google Scholar]
- Lavan BE, Fantin VR, Chang ET, Lane WS, Keller SR, Lienhard GE 1997 A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J Biol Chem 272:21403–21407 [DOI] [PubMed] [Google Scholar]
- Lavan BE, Lane WS, Lienhard GE 1997 The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J Biol Chem 272:11439–11443 [DOI] [PubMed] [Google Scholar]
- Smith-Hall J, Pons S, Patti ME, Burks DJ, Yenush L, Sun XJ, Kahn CR, White MF 1997 The 60 kDa insulin receptor substrate functions like an IRS protein (pp60IRS3) in adipose cells. Biochemistry 36:8304–8310 [DOI] [PubMed] [Google Scholar]
- Cai D, Dhe-Paganon S, Melendez PA, Lee J, Shoelson SE 2003 Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J Biol Chem 278:25323–25330 [DOI] [PubMed] [Google Scholar]
- Souza SC, Frick GP, Yip R, Lobo RB, Tai LR, Goodman HM 1994 Growth hormone stimulates tyrosine phosphorylation of insulin receptor substrate-1. J Biol Chem 269:30085–30088 [PubMed] [Google Scholar]
- Ridderstrale M, Degerman E, Tornqvist H 1995 Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes. J Biol Chem 270:3471–3474 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark GR, Kerr IM, Tsushima T, Akanuma Y, Komuro I, Tobe K, Yazaki Y, Kadowaki T 1998 Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem 273:15719–15726 [DOI] [PubMed] [Google Scholar]
- Nixon T, Green H 1983 Properties of growth hormone receptors in relation to the adipose conversion of 3T3 cells. J Cell Physiol 115:291–296 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Gilliland G, Van Epps C 1994 Treatment of IM-9 cells with human growth hormone (GH) promotes rapid disulfide linkage of the GH receptor. Endocrinology 135:148–156 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Kopchick JJ, Frank SJ 1999 Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem 274:33072–33084 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ 2004 A conformationally-sensitive GHR (growth hormone (GH) receptor) antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- Yang N, Wang X, Jiang J, Frank SJ 2007 Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol 21:1642–1655 [DOI] [PubMed] [Google Scholar]
- Yang N, Langenheim JF, Wang X, Jiang J, Chen WY, Frank SJ 2008 Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol Endocrinol 22:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF 2002 IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422 [DOI] [PubMed] [Google Scholar]
- Myers Jr MG, Cheatham B, Fisher TL, Jachna BR, Kahn CR, Backer JM, White MF 1995 Common and distinct elements in insulin and PDGF signaling. Ann NY Acad Sci 766:369–387 [DOI] [PubMed] [Google Scholar]
- Sorokin A, Reed E, Nnkemere N, Dulin NO, Schlessinger J 1998 Crk protein binds to PDGF receptor and insulin receptor substrate-1 with different modulating effects on PDGF- and insulin-dependent signaling pathways. Oncogene 16:2425–2434 [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Chen JJ, Birnbaum MJ 2003 Platelet-derived growth factor (PDGF) stimulates glucose transport in 3T3-L1 adipocytes overexpressing PDGF receptor by a pathway independent of insulin receptor substrates. Endocrinology 144:3811–3820 [DOI] [PubMed] [Google Scholar]
- Maggi D, Biedi C, Segat D, Barbero D, Panetta D, Cordera R 2002 IGF-I induces caveolin 1 tyrosine phosphorylation and translocation in the lipid rafts. Biochem Biophys Res Commun 295:1085–1089 [DOI] [PubMed] [Google Scholar]
- Biedi C, Panetta D, Segat D, Cordera R, Maggi D 2003 Specificity of insulin-like growth factor I and insulin on Shc phosphorylation and Grb2 recruitment in caveolae. Endocrinology 144:5497–5503 [DOI] [PubMed] [Google Scholar]
- Podar K, Tai YT, Cole CE, Hideshima T, Sattler M, Hamblin A, Mitsiades N, Schlossman RL, Davies FE, Morgan GJ, Munshi NC, Chauhan D, Anderson KC 2003 Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem 278:5794–5801 [DOI] [PubMed] [Google Scholar]
- Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K 2003 Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem 278:11561–11569 [DOI] [PubMed] [Google Scholar]
- Lu X, Kambe F, Cao X, Yamauchi M, Seo H 2008 Insulin-like growth factor-I activation of Akt survival cascade in neuronal cells requires the presence of its cognate receptor in caveolae. Exp Cell Res 314:342–351 [DOI] [PubMed] [Google Scholar]
- Smith RM, Harada S, Smith JA, Zhang S, Jarett L 1998 Insulin-induced protein tyrosine phosphorylation cascade and signalling molecules are localized in a caveolin-enriched cell membrane domain. Cell Signal 10:355–362 [DOI] [PubMed] [Google Scholar]
- Anderson NG 1992 Growth hormone activates mitogen-activated protein kinase and S6 kinase and promotes intracellular tyrosine phosphorylation in 3T3-F442A preadipocytes. Biochem J 284:649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T 1997 Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature 390:91–96 [DOI] [PubMed] [Google Scholar]
- Ridderstrale M, Tornqvist H 1994 PI-3-kinase inhibitor Wortmannin blocks the insulin-like effects of growth hormone in isolated rat adipocytes. Biochem Biophys Res Commun 203:306–310 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, Takahashi Y, Yoshizawa F, Aizawa S, Akanuma Y, Sonenberg N, Yazaki Y, Kadowaki T 1996 Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol 16:3074–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Cheatham B, Kahn CR 1997 Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol 17:1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnay JN, Bruning JC, Burks DJ, Kahn CR 2000 Gab-1-mediated IGF-1 signaling in IRS-1-deficient 3T3 fibroblasts. J Biol Chem 275:10545–10550 [DOI] [PubMed] [Google Scholar]
- Valverde AM, Mur C, Pons S, Alvarez AM, White MF, Kahn CR, Benito M 2001 Association of insulin receptor substrate 1 (IRS-1) y895 with Grb-2 mediates the insulin signaling involved in IRS-1-deficient brown adipocyte mitogenesis. Mol Cell Biol 21:2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasus-Jacobi A, Perdereau D, Tartare-Deckert S, Van Obberghen E, Girard J, Burnol AF 1997 Evidence for a direct interaction between insulin receptor substrate-1 and Shc. J Biol Chem 272:17166–17170 [DOI] [PubMed] [Google Scholar]
- Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ 1998 Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem Biophys Res Commun 253:774–779 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ 2001 Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]