Abstract
Association between protein inclusions and neurodegenerative diseases, including Parkinson's and Alzheimer's diseases, and polyglutamine disorders, has been widely documented. Although ubiquitin is conjugated to many of these aggregated proteins, the 26S proteasome does not efficiently degrade them. Mutations in the ubiquitin-protein ligase Parkin are associated with autosomal recessive juvenile Parkinsonism. Although Parkin-positive inclusions are not detected in brains of autosomal recessive juvenile Parkinsonism patients, Parkin is found in Lewy bodies in sporadic disease. This suggests that loss of Parkin ligase activity via mutation, or sequestration to Lewy bodies, is a contributory factor to sporadic disease onset. We now demonstrate that decreased proteasomal activity causes formation of large, noncytotoxic inclusions within the cytoplasm of both neuronal and nonneuronal cells overexpressing Parkin. This is not a general phenomenon as there is an absence of similar inclusions when HHARI, a structural homolog of Parkin, is overexpressed. The inclusions colocalize with ubiquitin and with proteasomes. Furthermore, Parkin inclusions colocalize with γ-tubulin, acetylated α-tubulin, and cause redistribution of vimentin, suggesting aggresome-like properties. Our data imply that lower proteasomal activity, previously observed in brain tissue of Parkinson's disease patients, leads to Parkin accumulation and a concomitant reduction in ligase activity, thereby promoting Lewy body formation.
INTRODUCTION
Parkinson's disease (PD) is a major neurodegenerative disease of middle and old age. It is characterized by the loss of dopaminergic neurons in the substantia nigra of the midbrain and the presence of proteinaceous cytoplasmic inclusions termed Lewy bodies in many of the remaining cells (Lowe et al., 1988; Lang and Lozano, 1998). The majority of PD is apparently sporadic, with only 5–10% of all cases being familial (Olanow and Tatton, 1999). To date, genetic studies have identified mutations in the genes that code for α-synuclein, Parkin, and UCH-L1, in a relatively small number of cases (Polymeropoulos et al., 1997; Kitada et al., 1998; Leroy et al., 1998). More recently, mutations in the genes encoding DJ-1 and NR4A2 have also been associated with familial PD (Bonifati et al., 2003; Le et al., 2003). The defective gene(s) at other candidate loci remain to be identified (Scott et al., 2001; Valente et al., 2001). Although the molecular changes underlying the initiation and progression of sporadic disease remain unclear, there is strong evidence that implicates abnormal processing of a variety of cellular proteins via the ubiquitin/26S proteasomal system in PD development.
Parkin mutations account for ∼50% of cases of autosomal recessive juvenile Parkinsonism (AR-JP) (Lucking et al., 2000). Parkin is a really interesting new gene (RING) type ubiquitin-protein ligase (E3) (Shimura et al., 2000). Two RING (R) domains are separated by an additional Cys/Hisrich region termed the “in between RING-fingers” (I) domain (Morett and Bork, 1999) (see Figure 3A). Collectively, this RIR domain structure is required for interaction between Parkin and the ubiquitin-conjugating enzymes (E2s) UbcH7 or UbcH8, or the endoplasmic reticulum (ER)-associated E2s UBC6 and UBC7 (Imai et al., 2000, 2001; Shimura et al., 2000; Zhang et al., 2000). Disease-associated mutations within the RIR domain of Parkin preclude interaction with the E2, thereby nullifying its E3 activity (Shimura et al., 2000; Imai et al., 2000; Zhang et al., 2000). Consequently, the failure of Parkin to ubiquitylate target proteins may lead to their accumulation, resulting in the formation of aggregates that are toxic to neurons of the substantia nigra. To date, seven targets of Parkin-mediated degradation have been identified: CDCrel-1 (Zhang et al., 2000), the Pael receptor (Imai et al., 2001), synphilin-1 (Chung et al., 2001), α-synuclein (Choi et al., 2001), and/or a glycosylated form of α-synuclein, termed αSp22 (Shimura et al., 2001), cyclin E (Staropoli et al., 2003), α/β tubulin (Ren et al., 2003), and the p38 subunit of the aminoacyl-tRNA synthase complex (p38) (Corti et al., 2003).
Figure 3.
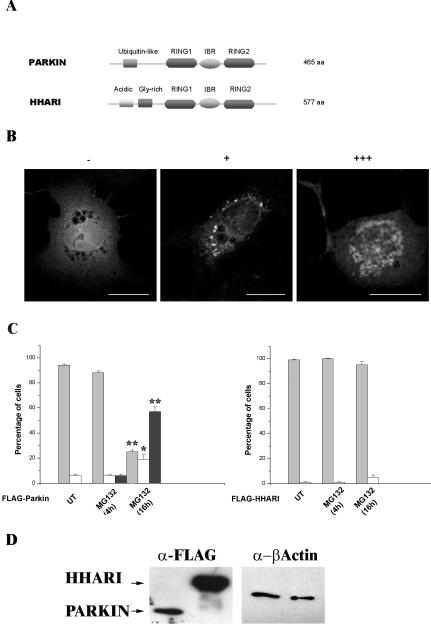
Inclusion body formation by Parkin is not a general response of RING-IBR-RING proteins to proteasome inhibition. (A) Schematic illustrating the domain structures of Parkin and HHARI. Parkin and HHARI demonstrate structural homology at the 3′ C-terminus. (B) COS-7 cells transfected with the FLAG-Parkin construct, stained with anti-FLAG antibodies as described for Figure 1. Cells were scored as either containing no inclusions (-), small individual inclusions (+), or large inclusions (+++). Bars, 20 μm. (C) COS-7 cells were transfected with FLAG-Parkin (left) or FLAG-HHARI (right). MG132 (20 μM) was added to cells for 4 or 16 h before fixation. The presence of Parkin or HHARI was assessed by immunofluorescence using the anti-FLAG antibody as described for Figure 1. Light gray bars, -; white bars, +; and dark gray bars, +++. Error bars indicate the SE from the mean. The asterisk (*) indicates a significant difference between the percentage of untreated cells with or without inclusions vs. the numbers of MG132-treated cells at each time point. *p < 0.005; ** p < 0.001. N-Terminal Myc-tagged and pDsRed1-C1 Parkin or HHARI constructs produced similar results. (D) Lack of HHARI inclusions is not due to poor expression of the construct. COS-7 cells were transfected with FLAG-Parkin or FLAG-HHARI. Forty-four hours post-transfection, cell lysates were prepared in Triton X-100 buffer. Ten micrograms of each soluble fraction was separated by SDS-PAGE and analyzed by Western blotting with anti-FLAG and anti-β-actin antibodies. Arrows indicate the presence of the respective FLAG construct.
Association of protein inclusions or aggregates with PD and other neurodegenerative diseases is widely documented (Kaytor and Warren, 1999; Sherman and Goldberg, 2001). In general, these inclusions consist of insoluble, unfolded, ubiquitylated polypeptides that fail to be degraded by the 26S proteasome. Their apparent stability may, in part, be due to the decreased levels of 26S proteasomal activity, which is associated with increasing age (Goto et al., 2001) and is notably lower in PD patients (McNaught and Jenner, 2001; McNaught et al., 2003).
Lewy bodies accumulate a range of normal and abnormal proteins, many of which are ubiquitylated (Pollen et al., 1993). α-Synuclein seems to be the main constituent of these inclusions (Spillantini et al., 1997). Other constituents include UCH-L1 (Lowe et al., 1990), proteasomal subunits (Ii et al., 1997), Parkin (Shimura et al., 1999), UbcH7 (Schlossmacher et al., 2002), synphilin-1 (Wakabayashi et al., 2000), and p38 (Corti et al., 2003). Whether CDCrel-1, Pael-R, αSp22, cyclin E, or α/β tubulin are deposited within Lewy bodies remains to be determined. At present, it is unclear whether these inclusions cause dysfunction or neurodegeneration directly or whether they are part of a neuronal cell's defensive response to toxic reagents. Indeed, Lewy bodies are found only in surviving neurons in PD, and inclusions in several polyglutamine disorders display protective properties (Saudou et al., 1998; Cummings et al., 1999).
Proteasome inhibitors are routinely used to study proteasome-mediated proteolysis in mammalian cells (Lee and Goldberg, 1998; Kisselev and Goldberg, 2001; Myung et al., 2001). Overexpression of Presenilin 1 or Huntingtin in proteasome-inhibited mammalian cells leads to inclusion formation. These inclusions have been characterized as “aggresomes” (Johnston et al., 1998; Garcia-Mata et al., 1999; Waelter et al., 2001). It is postulated that endogenous aggresomes occur when the capacity of the proteasome is exceeded by substrate expression. Lewy bodies may represent a specialized aggresome-related inclusion specific to dopaminergic neurons (McNaught et al., 2001, 2002). Although AR-JP patients with PARKIN mutations are characterized by the lack of Lewy body inclusions (Mori et al., 1998; Shimura et al., 1999), Parkin is found in Lewy bodies in sporadic disease. These observations suggest that loss of Parkin ligase activity through mutation in hereditary disease, or sequestration to Lewy bodies in sporadic disease, is a contributory factor to disease onset or progression. We now describe evidence that Parkin accumulates as a result of diminished cellular proteasomal activity to form noncytotoxic, cytoplasmic inclusions.
MATERIALS AND METHODS
Generation of Plasmid Constructs
A FLAG-pcDNA3 expression vector was generated by ligation of oligonucleotide coding for the FLAG epitope (DYKDDDDK), an additional upstream start codon, and BamHI and EcoRI restriction endonuclease sites (5′ and 3′, respectively) into pcDNA3 (Invitrogen, Carlsbad, CA). Parkin coding sequence was amplified by polymerase chain reaction (PCR) from brain cDNA by using Pfu Turbo DNA polymerase (Stratagene, San Diego, CA), purified by agarose gel electrophoresis and ligated into EcoR V digested FLAG-pcDNA3 to generate FLAG-Parkin. Red-Parkin was generated by digestion of FLAG-Parkin with restriction enzymes EcoRI and ApaI, purified by agarose gel electrophoresis and ligated into appropriately digested pDsRed1-C1 expression vector (BD Biosciences, San Jose, CA).
Myc-pcDNA3 was generated by ligation of an oligonucleotide coding for the Myc epitope (EQKLISEEDL), containing an additional upstream start codon, as well as HindIII and BamH I restriction endonuclease sites (5′ and 3′, respectively) into pcDNA3 (Invitrogen). An additional HpaI restriction endonuclease site was placed immediately downstream of the Myc epitope to aid future cloning. PCR generated full-length Parkin was cloned into the HpaI restriction endonuclease site to generate Myc-Parkin.
HHARI constructs were generated by digestion of HHARI-Myc/His (Ardley et al., 2001) with appropriate restriction endonucleases and ligation into the expression vectors described above to create Myc-HHARI, FLAG-HHARI, and Red-HHARI. FLAG-Ubiquitin was generated via PCR amplification of ubiquitin from human brain cDNA and ligation into the EcoRV site of FLAG-pcDNA3. The Presenilin 1 construct was kindly provided by Prof. C.C.J. Miller (Irving and Miller, 1997).
All mutant Parkin and Ubiquitin constructs were generated using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. Deletion mutants were generated by PCR with the full-length construct as template (primer details available on request).
Antibodies
Mouse monoclonal antibodies to Myc (clone 9E10), FLAG (M2), β-actin (AC-15), γ-tubulin (GTU-88), acetylated α-tubulin (clone 6–11B-1), and vimentin (clone V9) were obtained from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal to BiP was obtained from BD Biosciences. Rabbit polyclonal antibodies to the 20S proteasome and ubiquitin were purchased from Affiniti Research Products (Exeter, United Kingdom) DAKO (Carpinteria, CA), respectively. Rat anti-α-tubulin was obtained from Serotec (Oxford, United Kingdom). The rabbit polyclonal antibody to UbcH7 C-terminal peptide was described previously (Moynihan et al., 1999). Polyclonal rabbit antiserum to Parkin C-terminal peptide [(C)EQARWEAASKETIK], was generated by Genosphere Biotechnologies (Paris, France) and affinity purified using the same peptide bound to Sulfogel (Pierce Chemical, Rockford, IL). Affinity-purified rabbit anti-Presenilin 1 peptide antibodies [MAEGDPEAQRRVSKNSKYNAE(C)] were produced by Cambridge Research Biochemicals (Northwich, United Kingdom). The MitoTracker probe was used to label mitochondria according to the manufacturer's protocol (Molecular Probes, Eugene, OR).
Cell Culture and Transfection
COS-7 cells were grown in DMEM with Glutamax (Invitrogen). The human glioblastoma cell line U-138MG was maintained using Eagle's minimal essential medium with nonessential amino acids and 1.0 mM sodium pyruvate. SH-SY5Y neuroblastoma cells were maintained in 1:1 mixture of Eagle's minimal essential medium and Ham's F-12 medium. All cells were grown at 37°C in 6% CO2 and supplemented with 10% (vol/vol) fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Transfections were performed using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's protocol, in medium appropriate for each particular cell type, but without antibiotics or serum with the exception of COS-7 cells, which were supplemented with 2% (vol/vol) fetal calf serum. Unless stated otherwise, stress-inducing reagents were added to cell cultures 28 h post-transfection for 16 h. Such reagents included 20 μM MG132 (Calbiochem), 400 μM hydrogen peroxide, 300 mM sorbitol, 10 μg/ml tunicamycin or 10 μg/ml nocodazole (Sigma-Aldrich). Control experiments using the resuspension solvents dimethyl sulfoxide (DMSO) or methanol were performed where appropriate.
Immunofluorescence and Confocal Microscopy
Cells were seeded onto sterile glass coverslips in a six-well culture plate. After attachment, cells were transfected as described below with 1.0 μg of the appropriate construct(s). Cells were fixed either with 4% (wt/vol) paraformaldehyde for 15 min, washed with phosphate-buffered saline (PBS) and permeablized in 0.1% (vol/vol) Triton X-100 in PBS for 10 min, or with methanol at -20°C for 15 min. Immunostaining of cells was performed as described previously (Ardley et al., 2001) by using appropriate dilutions of antibody solutions. Confocal images were obtained using a TCS SP confocal imaging system (Leica, Wetzlar, Germany).
Analysis of Inclusion Body Formation
An inverted microscope (Carl Zeiss, Jena, Germany) with 40× objective lens was used to observe inclusion body formation. The number of transfected cells with or without inclusion bodies was counted in three randomly chosen microscope fields in different areas of the slide. Cells were scored as either containing no inclusions (-), small individual inclusions (+), or large inclusions (+++) (see Figure 3B). A minimum of 300 transfected cells was analyzed for each sample. Experiments were repeated at least twice, and counts were made in a blinded manner. One-way analysis of variance (Bonferroni) statistical analysis was performed using SSPS 11.0 for Windows.
Preparation of Cell Lysates and Their Analysis
Transfections for immunoblotting analysis were performed in six-well dishes by using 1.0 μg of each DNA construct and 5.0 μl of LipofectAMINE 2000. COS-7 cells were either mock transfected or transfected with a FLAG-tagged construct. Forty-eight hours post-transfection, cells were washed twice in PBS before lysis in either 0.50 ml of radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris-HCl buffer, pH 8.0, containing 150 mM NaCl, 1% (vol/vol) NP-40, 0.5% (wt/vol) sodium deoxycholate, and 0.1% (wt/vol) SDS] or 2% (vol/vol) Triton X-100 in PBS. Buffers were supplemented with 1× Complete protease inhibitors (Roche Diagnostics, Indianapolis, IN). All subsequent steps were performed at 4°C. Cell lysates were passed 10 times through a 26.5-gauge needle. Insoluble material was recovered by centrifugation at 12,000 × g for 20 min. Pellets were resuspended in 200 μl of the appropriate lysis buffer. Protein lysates were quantified using the DC protein assay (Bio-Rad, Hercules, CA). For each sample, the presence of specific proteins in 10 μg of protein extract was determined by Western blot analysis essentially as described previously (Ardley et al., 2001).
RESULTS
Localization of Parkin in Neuronal and Nonneuronal Cell Lines
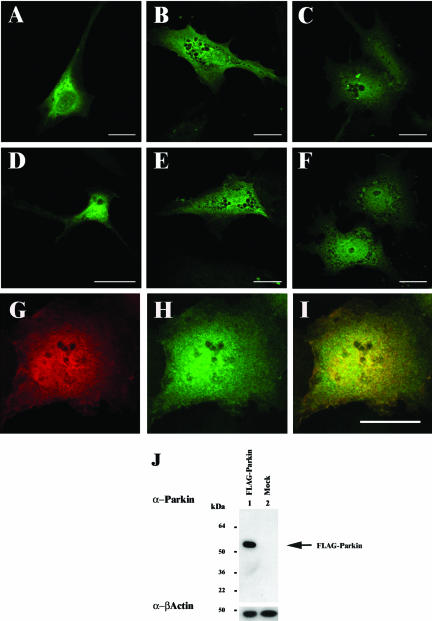
An anti-Parkin peptide antiserum was raised in rabbits to a sequence located at the C terminus of human Parkin, encompassing amino acid residues 399–412 (C-EQARWEAASKETIK). Anti-Parkin peptide antibodies were then affinity purified using the same peptide bound to Sulfogel. The specificity of these antibodies was ascertained by Western blotting of a lysate of COS-7 cells transfected with FLAG-Parkin (Figure 1J). A single band of the predicted size for FLAG-tagged Parkin (∼53 kDa) was detected in the lysate overexpressing FLAG-Parkin (Figure 1J, lane 1). Endogenous Parkin could not be detected in nontransfected COS-7 cell lysate (Figure 1J, lane 2), indicating that these antibodies could not be used to detect endogenous Parkin in this cell line.
Figure 1.
Localization of Parkin in neuronal and nonneuronal cell lines. FLAG-Parkin was transfected into SH-SY5Y (A and D), U138MG (B and E), or COS-7 (C and F–I). Forty-four hours post-transfection, the cells were fixed with 4% (wt/vol) paraformaldehyde, permeabilized, and immunostained with mouse monoclonal anti-FLAG (1:1000) (A–C and G), or affinity-purified rabbit anti-Parkin peptide (1:50) (D–F and H) antibodies. Anti-FLAG and anti-Parkin demonstrate overlapping staining patterns in COS-7 cells transfected with FLAG-Parkin (I, yellow staining indicates regions of colocalization within cells). Bars, 20 μm. (J) COS-7 cells lysates from FLAG-Parkin (lane 1) or mock (lane 2)-transfected cells were prepared in RIPA buffer 48 h post-transfection. Ten micrograms of each total cell lysate was separated by SDS-PAGE and analyzed by Western blotting with affinity-purified anti-Parkn C-terminal peptide (1: 500) and anti-β-actin (1:5000) antibodies. Arrow indicates the presence of FLAG-Parkin. A band corresponding to the expected position of endogenous Parkin was not observed in either lane.
The patterns of expression produced when SH-SY5Y, U138MG, and COS-7 cells were transfected with N-terminal FLAG-tagged Parkin and then immunostained using anti-FLAG (Figure 1, A–C) or anti-Parkin (Figure 1, D–F) antibodies were identical (Figure 1, G–L). Although FLAG-Parkin expression was mostly cytoplasmic, a small percentage of cells displayed additional nuclear and/or ER-like staining patterns. Similar staining patterns were obtained in each cell line analyzed. Unfortunately, the sensitivity of the assay was such that specific endogenous Parkin expression could not be confidently observed above background levels in either neuronal or nonneuronal cell lines (our unpublished data).
Inhibition of Proteasomal Activity Causes Inclusion Formation in Cells Overexpressing Parkin
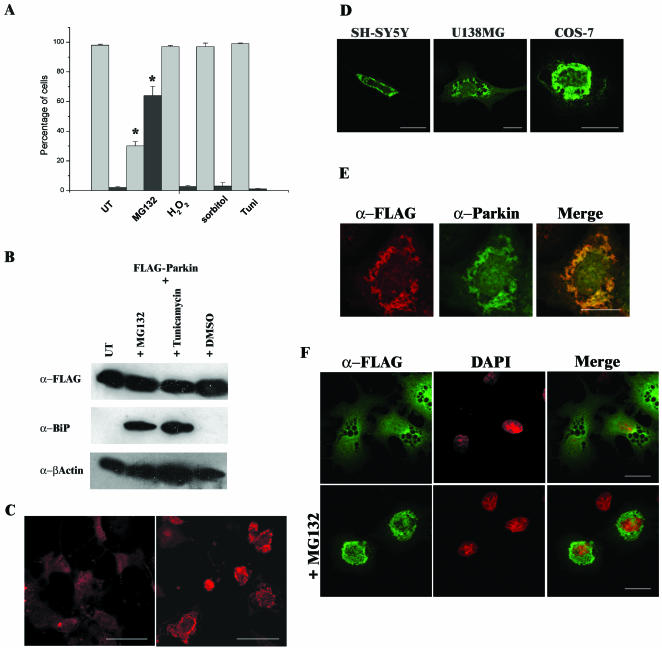
Imai et al. (2000) previously reported that elevated levels of Parkin that occurred as the result of the unfolded protein stress response (UPR) suppressed stress-induced cell death. Therefore, we subjected cells to a variety of stress-inducing agents to establish whether these effects were the result of an intracellular redistribution of Parkin. Twenty-four hours after transfection with FLAG-Parkin, COS-7 cells were treated with hydrogen peroxide (400 μM; an inducer of oxidative stress), 300 mM sorbitol (to increase osmotic stress), 10 μg/ml tunicamycin (N-glycosylation inhibitor, which induces the UPR), or treatment with the reversible proteasome inhibitor MG132 (20 μM) for 16 h. No significant change in Parkin expression patterns were observed after exposure to hydrogen peroxide, sorbitol, or tunicamycin, although treatment with hydrogen peroxide caused many of the cells to detach from the surface of the flask. Only occasional inclusions were found in a relatively few untreated transfected cells after these stress treatments (<3%). In contrast, Parkin expression patterns were significantly altered after MG132 treatment. At least 65% of MG132-treated cells contained inclusions (Figure 2A). Inclusions were also observed in COS-7 cells treated with 10 μM lactacystin for 16 h (our unpublished data). Because the synthetic peptide inhibitor MG132 and the natural inhibitor lactacystin caused Parkin inclusion formation, these data suggest that these protein aggregates resulted due to impairment of proteasome function.
Figure 2.
Addition of the proteasomal inhibitor MG132 causes inclusion body formation in cells overexpressing Parkin. (A) COS-7 cells were transfected with FLAG-Parkin. Twenty-eight hours post-transfection, cells were incubated for 16 h in the presence of either 20 μM MG132, 400 μM hydrogen peroxide (H2O2), 300 mM sorbitol, or 10 μg/ml tunicamycin. The presence of Parkin was then assessed by immunofluorescence with mouse monoclonal anti-FLAG antibody. Light gray bars represent the percentage of cells demonstrating a “wild-type” staining pattern, and dark gray bars represent the percentage of cells containing inclusion bodies. A minimum of 300 transfected cells was analyzed for each sample. Data shown represent the results of two independent sets of experiments. Error bars indicate the SE from the mean of these experiments. The asterisk (*) indicates a significant difference between the percentage of untreated cells versus the percentage of treated cells. *p < 0.01. (B) Tunicamycin and the proteasome inhibitor MG132 induce the UPR. COS-7 cell lysates from FLAG-Parkin–transfected cells left untreated (UT) or treated with 20 μM MG132, 10 μg/ml tunicamycin, or the carrier DMSO for 16 h were prepared in RIPA buffer 48 h post-transfection. Ten micrograms of each total cell lysate was separated by SDS-PAGE and analyzed by Western blotting with anti-FLAG, anti-BiP (1:250), and anti-β-actin antibodies. (C) COS-7 cells were transfected with FLAG-Parkin. Twenty-eight hours post-transfection, cells were grown in the presence or absence of 20 μM MG132 for 16 h before fixation. The presence of Parkin was assessed as described above. COS-7 cells containing MG132 induced inclusions (right) display a more intense staining pattern than untreated cells overexpressing Parkin (left). Confocal images of cells were scanned at the same fluorescent intensity. Bars, 50 μm. (D) Proteasome inhibition induces inclusion formation in SH-SY5Y, U138MG and COS-7 cells overexpressing FLAG-Parkin. (E) Immunostaining of inclusions with anti-FLAG (red) or anti-Parkin peptide (green) antibodies demonstrated similar staining patterns as indicated by yellow staining in merged panel. (F) Inclusions formed are within the cytosol. Transfected COS-7 cells were assessed by immunofluorescence by using the monoclonal anti-FLAG antibodies (green) and counterstained with 4,6-diamidino-2-phenylindole to visualize nuclei (red). Bars (D–F), 20 μm.
Because Imai et al. (2000) previously reported that levels of Parkin were increased on induction of the UPR, we analyzed the expression levels of FLAG-Parkin in the presence or absence of MG132, tunicamycin, or the carrier DMSO (Figure 2B). We did not observe an increase in the levels of FLAG-Parkin in the presence of these drug treatments (Figure 2B, top). In contrast, elevated levels of BiP (a chaperone that is up-regulated as part of the UPR) were clearly apparent in lysates prepared from cells cultured in the presence of either MG132 or tunicamycin, confirming that the UPR had been induced (Figure 2B, middle, compare treated cells in lanes 2 and 3 with untreated extracts in lanes 1 and 4) (Kuznetsov et al., 1996; Bush et al., 1997).
Cells containing inclusions demonstrated a much more intense staining pattern than cells demonstrating a “normal” Parkin distribution pattern (Figure 2C, compare the expression profile of untreated cells in the left-hand panel with MG132-treated cells in the right-hand panel). The former cells displayed strong generalized staining of the inclusions with diffuse staining elsewhere within the cell. Inclusion formation was also observed in MG132 treated SH-SY5Y and U138MG cells overexpressing Parkin (Figure 2D). Inclusions produced after MG132 treatment costained with both anti-Parkin and anti-FLAG antibodies (Figure 2E). For each cell line, a percentage of MG132-treated cells (∼15–25%) contained multiple small inclusions within the cytoplasm, whereas the remainder seemed to contain one large cytoplasmic inclusion. Inclusion bodies in MG132-treated cells were located at the nuclear periphery rather than within nuclei (Figure 2F).
Inclusion Body Formation Is Not a General Response of RING-IBR-RING Proteins to Inhibition of Proteasomal Activity
The domain structure of Parkin is highly similar to that of another putative E3, HHARI (human homolog of Drosophila Ariadne) (Moynihan et al., 1999). Both proteins contain the RIR motif that regulates their interactions with the E2, UbcH7 (Figure 3A). Therefore, we studied the localization of FLAG-HHARI in cells grown in the presence of MG132 to see whether inclusion formation was a general phenomenon of RIR domain proteins. For these experiments, cells were incubated with MG132 for either 4 or 16 h to assess the rate of inclusion formation. Because we had noted that different types of inclusion structures could be observed within cell populations, cells were classified as containing: no inclusions (-), multiple small inclusions (+), or large inclusions (+++) (Figure 3B). Although an increase in inclusion formation was observed 4 h after transfection with FLAG-Parkin compared with control cultures after incubation in the presence of MG132, a far more substantial increase in inclusion-containing cells, and in particular large inclusions, was observed after 16 h treatment (Figure 3C). In contrast, inclusions did not form in FLAG-HHARI–transfected cells cultured in the presence of MG132 either after 4- or 16-h incubation (Figure 3C). FLAG-HHARI displayed both cytoplasmic and nuclear staining as described previously for cells over expressing HHARI (Ardley et al., 2001).
The lack of inclusion formation with FLAG-HHARI was not a result of lower levels of expression because this construct repeatedly demonstrated higher levels of expression than the FLAG-Parkin construct (Figure 3D). Furthermore, both N-terminally Myc-tagged and pDsRed1-C1–tagged Parkin or HHARI constructs produced similar results to those of FLAG-Parkin or FLAG-HHARI, respectively, indicating that these effects were not a function of the attached tag (our unpublished data).
Parkin Inclusions Remain Stable after the Removal of Proteasome Inhibitor
Several recent studies have indicated that inclusion formation may be reversible (Martin-Aparicio et al., 2001; Lee et al., 2002). Therefore, we incubated cells transfected with Parkin in the presence or absence of MG132 for varying times to determine whether aggregation of Parkin could be reversed (Table 1). In cells overexpressing Parkin inclusion formation increased from only 4%, at 48 h post-transfection, to 21% after 68 h when cultured in the absence of MG132. In comparison, inclusion formation increased with time and to a far greater extent in the presence of MG132. The numbers of cells with inclusions were significantly higher (p < 0.01) with essentially all cells (96%) displaying inclusions after 40 h of growth in the presence of MG132 (Table 1). By this time, the total number of adherent cells was reduced and many displayed abnormalities in nuclear membrane structure.
Table 1.
Parkin inclusions remain stable on removal of proteasome inhibitor
| Time (h) | Incubation with MG132 (h) | Replacement media (h) | Mean no. inclusions (%) (± SE) |
|---|---|---|---|
| 44 | 4 ± 0.8 | ||
| 44 | 16 | 58 ± 1.4 | |
| 48 | 4 ± 0.6 | ||
| 48 | 16 | 4 | 50 ± 6.6 |
| 48 | 20 | 66 ± 0.8 | |
| 68 | 21 ± 3.8 | ||
| 68 | 16 | 24 | 50 ± 6.8 |
| 68 | 40 | 96 ± 1.2 |
COS-7 cells were transfected with the FLAG-Parkin expression construct and incubated for a total transfection time of either 44 h, 48 h, or 68 h. At 28 h post-transfection cells were incubated in the presence or absence of MG132 for the times indicated. The percentage of cells containing inclusions was assessed by immunofluoresence using anti-FLAG antibodies as described for Figure 3. A minimum of 300 transfected cells were analyzed for each sample. “Incubation with MG132” indicates the time cells were incubated in presence of MG132; “replacement media” indicates the time cells were cultured in MG132-free media, post-incubation with MG132. ±SE, ± standard error of the mean.
Replacement of MG132-containing media with fresh medium 4 h before the end of the experiment did not significantly reduce the total number of cells with inclusions after a total experiment time of either 44 or 48 h compared with those without replacement media (Table 1; 50% compared with 58 or 66%, respectively; p > 0.05). In contrast, there was a significant decrease between the numbers of cells containing inclusions 24 h after removal of MG132 and replenishment with fresh media after a total experiment time of 68 h (Table 1; 50% compared with 96%; p <0.001). This percentage of cells was comparable with that observed in cells grown in the presence of MG132 for either 16 or 20 h for a total experiment time up to 48 h (Table 1; 50% compared with 58%, 50 and 66% respectively; p > 0.05).
Proteasome Inhibition Promotes Formation of Insoluble Parkin Inclusion Bodies
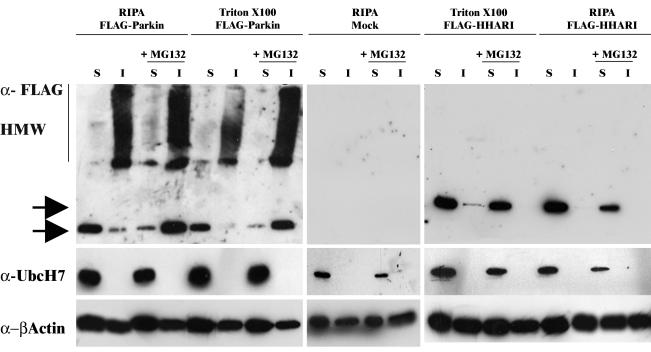
Inhibition of proteasome activity leads to accumulation of detergent-insoluble ubiquitylated proteins such as CFTR (Ward et al., 1995) and Parkin substrates such as Pael-R (Imai et al., 2001) and α-synuclein (Rideout et al., 2001). Therefore, COS-7 cells were transfected with FLAG-Parkin and cultured in the presence or absence of MG132 to establish whether MG132-induced Parkin inclusions were also detergent insoluble (Figure 4).
Figure 4.
MG132 induces the formation of insoluble, high-molecular-weight Parkin species. COS-7 cells were transfected with FLAG-Parkin (left), FLAG-HHARI (right), or mock-transfected (middle) and grown in the presence or absence of MG132 for 16 or 28 h post-transfection. Cell lysates were prepared in buffer as indicated, and 10 μg of each soluble (S) and insoluble (I) fractions was separated by SDS-PAGE and analyzed by Western blotting. Blots were probed with mouse anti-FLAG (top), affinity-purified rabbit anti-UbcH7 peptide (1:5000) (middle), and anti-β-actin (bottom) antibodies. Arrows indicate the presence of each respective FLAG construct.
Before proteasome inhibition, the majority of monomeric Parkin detected was found in soluble fractions when cell lysates were prepared using either 2% (vol/vol) Triton X-100 in PBS or RIPA lysis buffer. However, high-molecular-weight species, as well as the monomeric form, of Parkin were detected in the insoluble fractions (Figure 4, left). Addition of MG132, increased the amount of both monomeric and high-molecular-weight species of insoluble Parkin. In particular, an increase in the high-molecular-weight species was observed in lysates prepared in 2% (vol/vol) Triton X-100 in PBS. These data indicated that MG132 caused a stabilization of Parkin and the formation of the high-molecular-weight species is likely to represent ubiquitylated Parkin. Interestingly, in parallel experiments, the majority of HHARI was found as a monomer in the soluble fractions (Figure 4, right). Although prolonged exposure did reveal the presence of a relatively low amount of high-molecular-weight HHARI species in the insoluble fraction, this level of expression was not affected by the addition of MG132 (our unpublished data).
Because Parkin displays efficient E3 ligase activity in the presence of UbcH7 (Shimura et al., 2000), we carried out an investigation to determine whether Parkin also sequestered UbcH7 into the insoluble fraction after inhibition of proteasome activity. UbcH7 was only detected in the soluble fractions in the presence or absence of MG132 in Parkin-transfected cells. Probing the blot with antibodies to β-actin confirmed equivalent loading of protein extracts in all lanes. UbcH7 distribution was identical in HHARI or mock-transfected cells.
Parkin Inclusions Have Aggresome-like Properties
We next wished to establish whether Parkin inclusions displayed the microtubule-dependent properties characteristic of aggresome formation (Johnston et al., 1998; Garcia-Mata et al., 1999). The expression patterns of the familial Alzheimer's disease-associated protein Presenilin 1 (PS1) were performed, in parallel, as a control. Overexpression of PS1 in cells leads to the formation of aggresomes (Johnston et al., 1998).
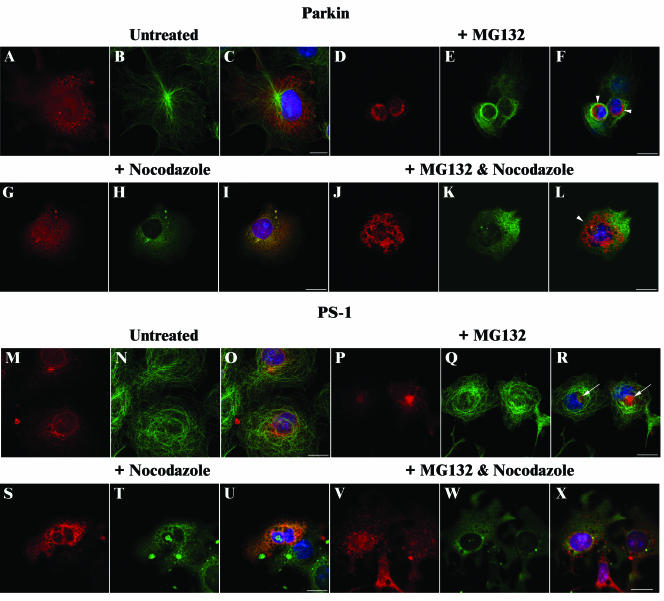
COS-7 cells overexpressing Parkin or PS1 were treated with MG132, the microtubule disruption reagent nocodazole, or both, for 16 h before fixation and immunostaining. Costaining with α-tubulin antibodies was performed to confirm disruption of microtubules. As previously reported, we demonstrated that cells overexpressing PS1 in the presence of MG132 contained aggresomes (Figure 5, P–R, arrows in R). Normal patterns of α-tubulin expression were observed in these cells. In contrast, bundling of α-tubulin around the aggregate was observed in the majority of cells containing Parkin inclusions (Figure 5, D–F, arrowhead in F). Colocalization of α-tubulin with inclusions was observed in a small number of cells (our unpublished data; see below). In nocodazole-treated cells, neither Parkin nor PS1 inclusions were detected in the majority of cells (Figure 5, G–I and S–U, respectively). However, large (+++) type inclusions were observed in cells overexpressing Parkin after the addition of both MG132 and nocodazole (Figure 5, J–L, arrowhead in L). Aggresome structures were not detected in PS1-overexpressing cells treated with both drugs, but small inclusions occurred that were dispersed throughout the cytoplasm (Figure 5, V–X).
Figure 5.
Effect of nocodazole on Parkin and PS-1 inclusions. COS-7 cells were transfected with FLAG-Parkin (A–L) or PS1 (M–X) constructs and grown in the absence (A–C and M–O) or presence of 20 μM MG132 (D–F and P–R) or 10 μg/ml nocodazole (G–I and S–U) or both (J–L and V–X) for 16 h. After methanol fixation, cells were probed with affinity-purified rabbit anti-Parkin peptide (1:50) (A–L; red), or affinity-purified rabbit anti-PS1 peptide (1:50) (M–X; red) antibodies, and rat anti-α-tubulin (1:500) (A–X; green) antibodies. Overlays of each set of three include 4,6-diamidino-2-phenylindole staining (blue) to identify nuclei (C, F, I, L, O, R, U, and X). Regions of colocalization within cells stain yellow. Arrowheads indicate Parkin inclusions; arrows indicate PS1 aggresomes. Bars, 20 μm.
Aggresome formation routinely causes disruption of γ-tubulin localization (Johnston et al., 1998; Garcia-Mata et al., 1999). Therefore, Parkin-transfected cells cultured in the presence (Figure 6, E–G) or absence (Figure 6, A–C) of proteasome inhibitors were stained with γ-tubulin antibodies to assess the effect of Parkin inclusions on γ-tubulin distribution. γ-Tubulin staining was clearly observed at the centrosome in the absence of inhibitors (Figure 6, C and D, arrowheads). There was no evidence of its colocalization with Parkin. However, γ-tubulin distribution was radically altered in cells transfected with Parkin and cultured in the presence of MG132. It colocalized with Parkin inclusions (Figure 6G, arrowheads). Staining of nontransfected cells confirmed aggregation of γ-tubulin only occurred in cells overexpressing Parkin (Figure 6H, arrowheads).
Figure 6.
Parkin inclusion bodies display aggresome-like characteristics. (A–H) Parkin inclusions cause disruption of γ-tubulin localization. COS-7 cells were transfected with FLAG-Parkin (A–C and E–G) in the presence (E–H) or absence (A–D) of MG132. Cells were stained with affinity-purified rabbit anti-Parkin (red) and mouse γ-tubulin (1:1000) (green) antibodies. Staining of untransfected cells confirmed aggregation of γ-tubulin only occurs in cells overexpressing Parkin (D and H). Red staining in D and H represents 4,6-diamidino-2-phenylindole staining of the nucleus. Arrowheads indicate γ-tubulin localization. (I–N) Partial colocalization of Parkin and vimentin near the centrosome. Cells overexpressing Parkin were stained with affinity-purified rabbit anti-Parkin (red) and anti-vimentin (1:40) antibodies (green) in the presence (L–N) or absence (I–K) of MG132. Arrow indicates region of colocalization in N. (O–Q) Acetylated α-tubulin localizes to Parkin inclusions. Cells overexpressing Parkin were stained with affinity-purified rabbit anti-Parkin (red) and mouse anti-acetylated α-tubulin (1:2000) antibodies (green). Panels show untreated (O), MG132-treated (P), or combined MG132 and nocodazole-treated (Q) cells. Arrows indicate colocalization within the inclusion in P and Q. The asterisk (*) indicates an untransfected cell with intact acetylated α-tubulin staining in Q. Overlays of each set include 4,6-diamidino-2-phenylindole staining (blue) (C, G, K, and N) to identify the nucleus. Mitochondria localize with Parkin inclusions. In R and S, Parkin expression is indicated by the green fluorescence and the mitochondria were labeled using MitoTracker (used at 300 nM; red fluorescence). With the exception of (R and S), which were fixed with 4% (wt/vol) paraformaldehyde, all cells were fixed with methanol. Bars, 20 μm.
Aggresomes (Johnston et al., 1998; Garcia-Mata et al., 1999) and aggresome-like inclusions (Meriin et al., 2001; Lee et al., 2002) commonly cause disruption of vimentin intermediate filaments, such that aggregates are surrounded by a cage-like structure. Coimmunostaining of Parkin-transfected cells with anti-Parkin and anti-vimentin antibodies (Figure 6, I–N) revealed that Parkin partially colocalized with vimentin close to the centrosome in inclusion containing cells (Figure 6, L–N). However, Parkin inclusions were only loosely surrounded by a vimentin “cage-like” structure (Figure 6N, arrow).
Parkin inclusions seem to be stable in the presence of nocodazole and partially colocalize with α-tubulin, yet cause disruption of γ-tubulin. These observations suggest Parkin inclusions share only some of the properties of classical aggresomes similar to other aggregate forming proteins (Meriin et al., 2001; Lee et al., 2002). Acetylation of α-tubulin is reported to promote microtubule stability, even in the presence of microtubule-depolymerizing reagents (Takemura et al., 1992). Therefore, we hypothesized that acetylated α-tubulin may also contribute to the stability of Parkin inclusions. We found that acetylated α-tubulin localized to Parkin inclusions in the presence of MG132 (Figure 6P, arrow) or MG132 and nocodazole-treated cells (Figure 6Q, arrow). Only in those cells treated with proteasome inhibitor and overexpressing Parkin was acetylated α-tubulin colocalized to inclusions (e.g., Figure 6Q, compare the cell indicated by an arrow with that indicated by *). Acetylated α-tubulin did not colocalize with Parkin in untreated cells (Figure 6O).
Disruption of mitochondria is also often associated with protein aggregates (Garcia-Mata et al., 1999; Kegel et al., 2000). In cells overexpressing Parkin, staining of mitochondria revealed that they condense and become clustered within inclusion bodies after the addition of MG132 (Figure 6S). However, they are distinct from Parkin staining in untreated cells (Figure 6R).
Colocalization of UPS Components with Parkin Inclusion Bodies
Many neurological-associated inclusions are both ubiquitin-positive and stain for other components of the UPS (Lowe et al., 1988; Pollen et al., 1993; Saudou et al., 1998; Cummings et al., 1999). Furthermore, Parkin inclusions may be expected to be ubiquitylated as Parkin undergoes autoubiquitylation (Shimura et al., 2000). Therefore, we investigated the expression patterns of Parkin and other components of the UPS in cells overexpressing FLAG-Parkin in the absence and presence of MG132.
In the absence of MG132, anti-UbcH7, anti-ubiquitin and anti-20S proteasome antibodies all produced similar, overlapping staining patterns to Parkin (Figure 7). In general, a diffuse pattern was observed within the nucleus and cytoplasm, with Parkin colocalizing with the other UPS components mostly in the cytoplasm (Figure 7, C, I, and O). After the addition of MG132, components of the ubiquitin-proteasome system localized to different regions of inclusion-containing cells. The majority of UbcH7 expression was nuclear and did not colocalize with Parkin inclusions (Figure 7, D–F). Similarly, a nuclear staining pattern was observed with anti-20S proteasomal antibodies in the presence of MG132. However, staining was also observed within the inclusion (Figure 7, J–L, arrow in L). Ubiquitin expression was observed throughout the inclusion, with intense staining at the inclusion periphery (Figure 7, P–R, arrow in R).
Figure 7.
Colocalization of Parkin inclusions with other components of the ubiquitin-proteasome pathway. (A–R) COS-7 cells were transfected with the FLAG-Parkin expression construct and cultured in the presence or absence of MG132 for 16 h. Cells were probed with the following: A–F, anti-FLAG and affinity-purified rabbit anti-UbcH7 peptide (1:100) antibodies; ubiquitin (1:50) antibodies; G–L, anti-FLAG and anti-20S (1:100) antibodies; or M–R, anti-FLAG and anti-ubiquitin (1:50) antibodies. Staining was as follows: left, FLAG, to identify Parkin (red); middle, UbcH7 (B and E), 20S (H and K), or ubiquitin (N and Q) (green); and right, overlay of each set with the addition of 4,6-diamidino-2-phenylindole staining (blue) to identify the nucleus. Regions of colocalization within cells stain yellow. Arrows indicate colocalization within an inclusion. Bars, 20 μm. (S) COS-7 cells were transfected with the Myc-Parkin expression construct alone or cotransfected with the FLAG-tagged ubiquitin construct as indicated and cultured in the absence of MG132. Cells were analyzed by immunofluorescence by using anti-Parkin antibodies as described for Figure 1 and assessed as described in Figure 3. Error bars indicate the SE from the mean. The asterisk (*) indicates a significant difference between the number of Myc-Parkin/FLAG-ubiquitin construct-transfected cells containing inclusions versus the number found in Myc-Parkin alone transfected cells; p < 0.01. Costaining with anti-FLAG antibody revealed that exogenous ubiquitin had an identical staining pattern to the endogenous protein (our unpublished data).
The choice of lysine residue for the formation of a polyubiquitin chain determines the fate of an ubiquitylated protein (Weissman, 2001; Hartmann-Petersen et al., 2003). Ubiquitin chains of four or more ubiquitin molecules linked through K48 are targeted to the 26S proteasome for degradation (Gregori et al., 1990; Thrower et al., 2000). In contrast, K63-linked polyubiquitin chains have important functions in DNA repair, protein translation, and endocytosis (Spence et al., 1995, 2000; Galan and Haguenauer-Tsapis, 1997). Overexpression of Myc-Parkin with FLAG-tagged ubiquitin or the ubiquitin mutants K48R or K63R (either of which, once incorporated, prevent ubiquitin chain formation), was performed to establish whether these lysine residues were important for Parkin inclusion formation. FLAG-Ubiquitin K48R, but not FLAG-Ubiquitin K63R mutant, produced significantly more inclusions without MG132 treatment (Figure 7S; p < 0.01). Overexpression of ubiquitin constructs generated similar staining patterns to that of endogenous ubiquitin (our unpublished data).
Effects of Mutant Forms of Parkin on Inclusion Body Formation
From the limited numbers of studies undertaken, it seems that brain tissue from AR-JP patients do not contain Lewy bodies (Mori et al., 1998; Shimura et al., 1999). Therefore, we analyzed the effects produced by a series of Parkin deletion, and point, mutants to establish whether disease-related mutations behaved differently to wild-type or nondisease-related mutations in our assays (Figure 8A). Potential functional roles of the different domains of Parkin have been proposed. Whereas the N-terminal ubiquitin-like domain (Ubl) domain is required for interaction with the proteasome (Sakata et al., 2003; Tsai et al., 2003), the C-terminal RIR domain is required for interaction with an E2 (Imai et al., 2000, 2001; Shimura et al., 2000; Zhang et al., 2000). Mutations in either domain can affect its ligase activity.
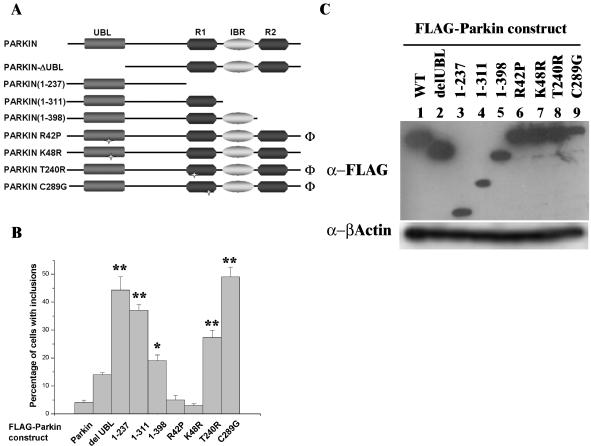
Figure 8.
Effects of truncation and point mutations of Parkin on inclusion body formation. (A) Schematic illustrating the positions of the point mutations and truncations of Parkin constructs used in B. Φ, AR-JP–associated mutation. (B) COS-7 cells were transfected with FLAG-Parkin or deletion/mutation constructs and cultured in the absence of MG132. The expression of Parkin was analyzed by immunofluorescence with anti-FLAG antibodies. Cells were scored for the presence of inclusion bodies. Error bars indicate the SE from the mean. Asterisk(s) indicate a significant difference between the number of inclusions observed with each construct compared with wild type, FLAG-Parkin, without addition of MG132. *p < 0.05; **p < 0.001. (C) Inclusion formation is not related to expression levels of the Parkin constructs. COS-7 cells were transfected with each FLAG-Parkin construct as indicated. Forty-four hours post-transfection, cell lysates were prepared in RIPA buffer. Ten micrograms of total cell lysate was separated by SDS-PAGE and analyzed by Western blotting with anti-FLAG (top) and anti-β-actin (bottom) antibodies.
In the absence of MG132, deletions in both the Ubl and RIR domains of Parkin produced more inclusions than wild-type Parkin. FLAG-Parkin (1–237) produced the greatest increase in inclusions (44% compared with 4%; p <0.001). The effect of point mutations was more varied. The R42P or K48R mutations, which lay within the Ubl domain, produced similar levels of inclusion formation to wild-type Parkin. In contrast, the T240R and C289G mutations that lay within the RIR domain significantly increased inclusion formation (both p < 0.001). In the presence of MG132, all constructs gave similar high levels of inclusions to those cells overexpressing native Parkin (74.9 ± 18.1%). We wished to confirm that the number of inclusion-containing cells observed from each Parkin construct was not related to the transfection and/or expression efficiency of the construct because previous reports have described differences in expression levels from Parkin mutant/deletion constructs (Finney et al., 2003). Western blotting of cell lysates overexpressing each of the Parkin constructs indicated that any elevation in the number of inclusions observed by immunofluorescence was not due to differential expression patterns (Figure 8C).
DISCUSSION
We have demonstrated that under normal cell culture conditions, cells overexpressing Parkin display mostly cytoplasmic staining, although some cells showed nuclear and/or ER staining patterns. These findings are similar to the localization of endogenous Parkin reported by others. Although the majority of staining is cytoplasmic (Shimura et al., 1999; Gu et al., 2000; Horowitz et al., 2001), nuclear (Stichel et al., 2000; Horowitz et al., 2001), ER (Imai et al., 2002), Golgi (Shimura et al., 1999), trans-Golgi network, and vesicular staining (Kubo et al., 2001) have also been reported. The reasons for the varied distribution of Parkin within cells are not known but may, for example, reflect their proliferation or differentiation status. It may also indicate distinct roles for Parkin in different regions of different cell types. Although we could detect Parkin in cells overexpressing it, we were unable to detect endogenous Parkin with our purified antibodies by either the immunofluorescence or Western blotting techniques described, probably reflecting the low levels of endogenous expression.
Parkin has previously been reported to suppress cell death induced by the UPR and is associated with ER-associated protein degradation (ERAD) (Imai et al., 2000, 2001). Therefore, we investigated the effects of different cellular stresses on the cellular localization of the protein. In this study, induction of the unfolded protein stress response by tunicamycin had little effect on the localization of Parkin. Moreover, induction of the UPR by either MG132 or tunicamycin did not increase Parkin levels. In the presence of MG132, a potent inhibitor of the proteasome (Bush et al., 1997), at least 65% of cells overexpressing Parkin displayed some evidence of insoluble, cytoplasmic protein aggregation. This correlated with a propensity for Parkin to migrate as detergent-insoluble high-molecular-weight species when analyzed by Western blotting. The apparent discrepancies between our study and those of Imai et al. (2000) remain unclear. However, the link between Parkin expression and the UPR remains controversial. West et al. (2003) recently reported that they also could not detect increased levels of Parkin in response to the UPR. This indicates that in addition to UPR, other factors such as cell culture conditions may influence the expression levels of Parkin. Furthermore, Ledesma et al. (2002) reported brain cell-specific regulation of Parkin expression and distribution during the UPR.
The formation of inclusions were due to some inherent property of Parkin because its structural homolog, HHARI, did not produce similar inclusions after the inhibition of proteasome activity. In addition, high-molecular-weight HHARI complexes were not easily detected by Western blot analysis.
Parkin containing inclusions are similar to those observed in cells overexpressing synphilin-1 (O'Farrell et al., 2001) and the Lewy body-like, ubiquitin-positive, cytosolic inclusions observed when α-synuclein and synphilin-1 were coexpressed with Parkin in human embryonic kidney 293 cells (Chung et al., 2001). Interestingly, these authors demonstrated that the inclusions generated by coexpression of synphilin-1 and α-synuclein were not ubiquitin positive without additional expression of wild-type Parkin. The results of a more recent study demonstrated that Parkin promoted ubiquitylated p38-positive inclusions in SH-SY5Y cells and protected them from apoptotic cell death (Corti et al., 2003). Furthermore, Pael-R becomes unfolded and insoluble accumulating in the ER when overexpressed and eventually causes UPR-induced cell death (Imai et al., 2001). However, Parkin prevented its accumulation and subsequent cell death via ER-associated UBC6/UBC7 ubiquitylation and cytoplasmic targeting of Pael-R to the proteasome (Imai et al., 2001, 2002). Disease-associated Parkin mutations, or inhibition of proteasome activity, inhibited these protective properties of Parkin resulting in UPR mediated cell death (Imai et al., 2001).
We have demonstrated that prolonged overexpression of Parkin without inhibitor also leads to an increase in the number of cells with inclusions, suggesting that inclusion formation occurs naturally with time in cells expressing high levels of Parkin regardless of the levels of its endogenous targets. Furthermore, in cultures grown in the presence of MG132, the percentage of cells with inclusions remained constant after removal of the proteasome inhibitor, at least for the 24-h “washout” tested. These data suggest that once formed these inclusions remain resistant to proteasomal breakdown.
Formation of perinuclear aggresomes is a protective cellular response to overloading of the proteasome (Johnston et al., 1998). Prolonged aggresome formation results in almost complete inhibition of the ubiquitin-proteasome system (Bence et al., 2001). They may contain chaperones, components of the ubiquitin-proteasome system, centrosomal material, and cytoskeletal proteins. Aggresomes are delivered in a microtubule-dependent manner to the microtubule organizing center, where they become surrounded by a vimentin “cage” (Johnston et al., 1998). Although the aggresome seems to be a specialized form of inclusion body, an increasing number of other protein aggregates share overlapping aggresome-like properties (Meriin et al., 2001; Lee et al., 2002).
During the preparation of this manuscript, Junn et al. (2002) reported that Parkin forms aggresomes in response to proteasomal inhibition. Similarly, we found that Parkin inclusion bodies are aggresome-like structures. In both studies, the formation of Parkin inclusions resulted in redistribution of γ-tubulin and vimentin. However, in contrast to Junn et al. (2002), we demonstrated that microtubule disruption did not affect the formation or maintenance of the Parkin-containing inclusions. Furthermore, aggregated Parkin caused disruption of the cytoskeletal protein α-tubulin and was only loosely associated with vimentin in inclusioncontaining cells. The reasons for these apparent discrepancies are not clear. Differences in the levels of Parkin expression or fixation technique may have contributed. For example, we routinely used methanol fixation for cytoskeletal staining of cells to maximize the integrity of the cytoskeleton.
Interestingly, Ren et al. (2003) found that wild-type, but not mutant, Parkin targets misfolded tubulin monomers for degradation in neuronal cells. In this study, we observed that Parkin inclusions were found to colocalize with acetylated α-tubulin. Nerve cell microtubules are highly enriched in acetylated tubulin (Cambray-Deakin and Burgoyne, 1987; Baas and Black, 1990), and neurite microtubules are resistant to depolymerization (Black and Greene, 1982; Baas and Black, 1990). Furthermore, cells overexpressing the microtubule-associated proteins MAP1B, MAP2, and Tau are associated with enrichment of α-tubulin acetylation and subsequent microtubule stability (Takemura et al., 1992) and have been detected in Lewy bodies (Arima et al., 2000; Jensen et al., 2000; D'Andrea et al., 2001), with mitogen-activated protein 1B interacting with α-synuclein (Jensen et al., 2000). α-Tubulin is acetylated at Lys40 (Polevoda and Sherman, 2002). By blocking access to this lysine residue, acetylation of this residue may protect the α-tubulin from ubiquitin-proteasome–mediated degradation. Together, our results suggest that as well as α/β-tubulin, acetylated α-tubulin may also interact with Parkin. The maintenance of intact microtubules may result in stabilization of the Parkin inclusions.
Similar to other aggresome-like aggregates, Parkin inclusion bodies stained with anti-20S proteasome and anti-ubiquitin antibodies, suggesting Parkin inclusions contained ubiquitylated protein(s). Polyubiquitylation via K48 targets proteins to the 26S proteasome for degradation (Gregori et al., 1990; Thrower et al., 2000). Cotransfection studies with Myc-Parkin and the ubiquitin mutant K48R, which prevents ubiquitin chain extension at this residue, produced significantly more inclusions without proteasome inhibitor treatment compared with wild-type ubiquitin. This indicates the importance of K48-linked (poly)ubiquitin chains for Parkin ubiquitylation and subsequent recognition and degradation by the proteasome. Our observation that UbcH7 does not localize with insoluble Parkin inclusions, either by immunofluorescence or Western blot analysis, may be due either to masking of the binding site due to protein aggregation or to protein denaturation. In both cases, the consequence would be a loss of ligase activity. Alternatively, UbcH7 may not be involved in the ubiquitylation step that promotes Parkin inclusion formation. For example, if the formation of these inclusions is due to inhibition of Parkin-associated ERAD substrate ubiquitylation such as that of Pael-R, then UBC6 and UBC7 are more likely to be associated with these deposits.
Similar to aggresomes (Garcia-Mata et al., 1999; Waetler et al., 2001), and proteasome inhibition studies (Tanaka et al., 2001; Wright et al., 2001), we observed disruption of mitochondria that resulted in perinuclear mitochondrial clustering and condensation. Similarly, mitochondrial pathology has been observed in Parkin null mutants in Drosophila (Greene et al., 2003). This is likely to disrupt ATP production, further inhibiting protein clearance by the ubiquitin pathway, which is ATP dependent.
It had been proposed that AR-JP patients do not have Lewy body pathology from the analysis of relatively few autopsies (Mori et al., 1998; Shimura et al., 1999). However, more recently, one family with Parkin mutations and Lewy bodies has been described (Farrer et al., 2001). From the work described above, Parkin characterized by different mutations displayed variable degrees of aggregation potential. For example, whereas Parkin K48R or R42P mutants did not form inclusions in the absence of proteasome inhibitors, the disease-associated T240R (Hattori et al., 1998) and C289G (Lucking et al., 2000) mutants were very prone to aggregate. This latter observation is interesting because coexpression of Pael-R with T240R does not prevent accumulation and subsequent Pael-R–mediated cell death (Imai et al., 2001), a process, in part, impeded by inhibition of the interaction between the mutant Parkin and CHIP (Imai et al., 2002). Data here may indicate other ERAD substrates for Parkin exist, hence the accumulation of these as yet unidentified proteins and Parkin in the presence of inhibitors of proteasome activity.
Ubl domains may promote a direct interaction between E3s and the 26S proteasome (Kleijnen et al., 2000). Indeed, Parkin interacts with proteasome subunits Rpn10 (S5a in mammals) (Sakata et al., 2003) and Rpt6 (Tsai et al., 2003). Although the R42P mutation has been described as “a naturally occurring point mutation” (Shimura et al., 2000), patients with the R42P mutation do not display the clinical hallmarks of AR-JP and are indistinguishable from those with sporadic disease (Terreni et al., 2001). This mutation may prevent proteasomal targeting of proteins by inhibiting binding to Rpn10 (Sakata et al., 2003), because it can interact with UbcH7 but lacks E3 activity (Shimura et al., 2000). Intriguingly, the FLAG-Parkin R42P and K48R mutants displayed inclusion formation comparable with wild-type Parkin in untreated COS-7 cells. These data suggested that R42P might reduce ubiquitylation by Parkin rather than totally abolishing ligase activity. In addition, analysis of the FLAG-Parkin K48R mutation (an artificial mutation which has not been implicated in disease) indicated that although highly conserved with that of ubiquitin itself, this residue is not required for ubiquitin recognition and polyubiquitin chain formation.
McNaught et al. (2001) proposed that accumulation of intracellular proteins due to failure of the ubiquitin-proteasome pathway will lead to death of neurons in both familial and sporadic PD. Indeed, Lewy bodies form via an aggresome-related mechanism (McNaught et al., 2002), which upon failure or overloading is likely to cause cell death. Our current study highlights the importance of proteasome function in Parkin turnover and ubiquitylation and may represent an accelerated model of naturally occurring Parkin aggregation in cells. It implies a role for Parkin in progression of nigrostriatal degeneration in all manifestations of PD. Formation of aggresomes and other types of inclusion bodies generates homogenous foci of specific protein to which binding partners are recruited via “native coaggregation” (Rajan et al., 2001). We postulate that aggregation-prone proteins such as Pael-R, α-synuclein, and its associated protein synphilin-1, p38, or other protein(s) may accumulate to form aggresome-like structures. Parkin, and possibly other components of the ubiquitin-proteasome machinery, then become independently recruited into this structure. Formation of this mature Lewy body would normally allow ubiquitylation and subsequent 26S-mediated degradation of these proteins. In sporadic PD, impaired ubiquitin-proteasome function due to age-related decreases in proteasomal activity, increased tubulin acetylation of neuronal cells, decreased ATP production by mitochondria, and increased UPR, would prevent clearance of the proteins and eventually lead to cell death.
In AR-JP, the lack of Lewy bodies could be as a result of aggresome-like structures not being targeted to the proteasome by the mutant form of Parkin. Mutations or deletions in the RIR prevent E2 recognition but may still allow recognition of the proteasome. This would result in Parkin-target conjugates becoming aggregated at the proteasome; however, the lack of ubiquitylated substrate would prevent degradation and cause “clogging up” of the proteasome. Conversely, mutations or deletions of the Ubl domain may allow ubiquitylation of a target protein(s) that never reach the proteasome and subsequently form ubiquitylated inclusions. These “pre-Lewy bodies” may be more toxic to the cell than the mature inclusion. Indeed, two recent reports demonstrated that early, misfolded intermediates generated during the production of amyloid fibrils (which may subsequently form amyloid plaques, pathologically characteristic of Alzheimer's disease) are highly toxic, possibly more so than the fibril itself (Bucciantini et al., 2002; Walsh et al., 2002). In this scenario, the combination of substrate proteins recruitment of Parkin to the pre-Lewy body, but the lack of cytosolic protein clearance, together with accelerated UPR by Pael-R and other protein(s), may cause increased toxicity and cell death. Supporting this idea, Corti et al. (2003) demonstrated that Parkin is recruited to p38-containing inclusions and is required for both p38 proteasomal degradation and aggregation. Furthermore, a pathological mutant and a deletion of the Ubl domain mutant were incorporated into the p38 positive inclusions, despite the fact that they lacked E3 activity.
Future studies to generate wild-type or disease associated mutant cell lines will allow us to investigate whether these mutant proteins have properties akin to their wild-type counterparts. In addition, such cell lines will allow the screening of agents that can eliminate Lewy bodies in a similar manner to studies recently described for Huntingtin inclusions (Apostol et al., 2003).
Acknowledgments
We thank Dr. R. Layfield and C. Houghton (School of Biomedical Sciences, University of Nottingham, Nottingham, United Kingdom) for site-directed mutagenesis primers to generate the Parkin R42P construct, and Prof. C.C.J. Miller (Institute of Psychiatry, University of London, London, United Kingdom) for the PS1 construct. We also thank Drs. E.E. Morrison (Cancer Research UK Clinical Centre, Leeds, United Kingdom) and R. Layfield for helpful discussions. This study was supported by The Wellcome Trust, The Royal Society, and Yorkshire Cancer Research. H.C.A. was a Lloyds of London Tercentenary Foundation Research Fellow for the majority of this work and is now supported by a Research into Ageing Fellowship Award.
Abbreviations used: PD, Parkinson's disease; AR-JP, autosomal recessive Juvenile Parkinsonism; RING, really interesting new gene; IBR in between RING-fingers; RIR, RING-IBR-RING; PS1, Presenilin 1; Ubl, ubiquitin-like domain.
References
- Apostol, R.L., et al. (2003). A cell-based assay for aggregation inhibitors as therapeutics of polyglutamine-repeat disease and validation in Drosophila. Proc. Natl. Acad. Sci. USA 100, 5950-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardley, H.C., Tan, N.G.S., Rose, S.A., Markham, A.F., and Robinson, P.A. (2001). Features of the Parkin/Ariadne-like ubiquitin ligase, HHARI, that regulate its interaction with the ubiquitin-conjugating enzyme, UbcH7. J. Biol. Chem. 276, 19640-19647. [DOI] [PubMed] [Google Scholar]
- Arima, K., Mizutani, T., Alim, M.A., Tonozuka-Uehara, H., Izumiyama, Y., Hirai, S., and Ueda, K. (2000). NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies. Acta Neuropathol. 100, 115-121. [DOI] [PubMed] [Google Scholar]
- Baas, P.W., and Black, M.M. (1990). Individual microtubules in the axon consist of domains that differ in both composition and stability. J. Cell Biol. 111, 495-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence, N.F., Sampat, R.M., and Kopito, R.R. (2001). Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552-1555. [DOI] [PubMed] [Google Scholar]
- Black, M.M., and Greene, L.A. (1982). Changes in colchicines susceptibility of microtubules associated with neurite outgrowth: studies with nerve growth factor-responsive PC12 pheochromocytoma cells. J. Cell Biol. 95, 379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati, V., et al. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 299, 256-259. [DOI] [PubMed] [Google Scholar]
- Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C. M., and Stefani, M. (2002). Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507-511. [DOI] [PubMed] [Google Scholar]
- Bush, K.T., Goldberg, A.L., and Nigam, S.K. (1997). Proteasome inhibition leads to heat-shock response, induction of endoplasmic reticulum chaperones and thermotolerance. J. Biol. Chem. 272, 9086-9092. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin, M.A., and Burgoyne, R.D. (1987). Posttranslational modifications of alpha-tubulin: acetylated and detryrosinated forms in axons of rat cerebellum. J. Cell Biol. 104, 1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, P., Golts, N., Snyder, H., Chong, M., Petrucelli, L., Hardy, J., Sparkman, D., Cochran, E., Lee, J. M., and Wolozin, B. (2001). Co-association of parkin and alpha-synuclein. Neuroreport 1213, 2839-2843. [DOI] [PubMed] [Google Scholar]
- Corti, O., et al. (2003). The p38 subunit of the aminoacyl-tRNA synthase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum. Mol. Genet. 12, 1427-1437. [DOI] [PubMed] [Google Scholar]
- Cummings, C.J., Reinstein, E., Sun, Y., Antalffy, B., Jiang, Y., Ciechanover, A., Orr, H.T., Beaudet, A.L., and Zoghbi, H.Y. (1999). Mutation in the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine induced pathology in SCA1 mice. Neuron 24, 879-892. [DOI] [PubMed] [Google Scholar]
- Chung, K.K.K., Zhang, Y., Lim, K.L., Tanaka, Y., Huang, H., Gao, J., Ross, C.A., Dawson, V.L., and Dawson, T.M. (2001). Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1, implications for Lewy-body formation in Parkinson disease. Nat. Med. 7, 1144-1150. [DOI] [PubMed] [Google Scholar]
- D'Andrea, M.R., Ilyin, S., and Plata-Salaman, C.R. (2001). Abnormal patterns of microtubule-associated protein-2 (MAP-2) immunolabeling in neuronal nuclei and Lewy bodies in Parkinson's disease substantia nigra brain tissues. Neurosci. Lett. 306, 137-140. [DOI] [PubMed] [Google Scholar]
- Farrer, M., J. et al. (2001). Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 50, 293-300. [DOI] [PubMed] [Google Scholar]
- Finney, N., Walther, F., Mantel, P.-Y., Stauffer, D., Rovelli, G., and Dev, K.K. (2003). The cellular protein level of Parkin is regulated by its ubiquitin-like domain. J. Biol. Chem. 278, 16054-16058. [DOI] [PubMed] [Google Scholar]
- Galan, J.M., and Haguenauer-Tsapis, R. (1997). Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16, 5847-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata, R., Bebok, Z., Sorscher, E.J., and Sztul, E.S. (1999). Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell Biol. 146, 1239-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, S., Takahashi, R., Kumiyama, A., Radak, Z., Hayashi, T., Takenouchi, M., and Abe, R. 2001. Implications of protein degradation in aging. Ann. NY Acad. Sci. 928, 54-64. [DOI] [PubMed] [Google Scholar]
- Greene, J.C., Whitworth, A.J., Kuo, I., Andrews, L.A., Feany, M.B., and Pallanck, L.J. (2003). Mitochondrial pathology and apoptotic muscle degeneration in Drosophila Parkin mutants. Proc. Natl. Acad. Sci. USA 100, 4078-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori, L., Poosch, M.S., Cousins, G., and Chau, V. (1990). A uniform isopeptide-linked multiubiquitin chain is sufficient to target substrate for degradation in ubiquitin-mediated proteolysis. J. Biol. Chem. 265, 8354-8357. [PubMed] [Google Scholar]
- Gu, W.-J., Abbas, N., Lagunes, M.Z., Parent, A., Pradier, L., Bohme, G.A., Agid, Y., Hirsch, E.C., Raisman-Vozari, R., and Brice, A. (2000). Cloning of rat Parkin cDNA and distribution of Parkin in rat brain. J. Neurochem. 74, 1773-1776. [DOI] [PubMed] [Google Scholar]
- Hartmann-Petersen, R., Seeger, M. and Gordon, C. (2003). Transferring substrates to the 26S proteasome. Trends Biochem. Sci. 28, 26-31. [DOI] [PubMed] [Google Scholar]
- Hattori, N., et al. (1998). Point mutations (thr240arg and gln311stop) in the parkin gene. Biochem. Biophys. Res. Commun. 249, 754-758. [DOI] [PubMed] [Google Scholar]
- Horowitz, J.M., Vernace, V.A., Myers, J., Stachowiak, M.K., Hanlon, D.W., Fraley, G.S., and Torres, G. (2001). Immunodetection of Parkin in vertebrate and invertebrate brains: a comparative study using specific antibodies. J. Chem. Neuroanat. 21, 75-93. [DOI] [PubMed] [Google Scholar]
- Ii, K., Ito, H., Tanaka, K., and Hirano, A. (1997). Immunocytochemical colocalization of the proteasome in ubiquitinated structures in neurodegenerative diseases and the elderly. J. Neuropathol. Exp. Neurol. 56, 125-131. [DOI] [PubMed] [Google Scholar]
- Imai, Y., Soda, M., and Takahashi, R. (2000). Parkin suppresses protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J. Biol. Chem. 275, 35661-35664. [DOI] [PubMed] [Google Scholar]
- Imai, Y., Soda, M., Inoue, H., Hattori, N., Mizuno, Y., and Takahashi, R. (2001). An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate for Parkin. Cell 105, 891-902. [DOI] [PubMed] [Google Scholar]
- Imai, Y., Soda, M., Hatakeyama, S., Akagi, T., Hashikawa, T., Nakayama, K.-I., and Takahashi, R. (2002). CHIP is associated with Parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity. Mol. Cell 10, 55-67. [DOI] [PubMed] [Google Scholar]
- Irving, N.G., and Miller, C.C.J. (1997). Tau phosphorylation in cells transfected with wild-type or an Alzheimer's disease mutant Presenilin 1. Neurosci. Lett. 222, 71-74. [DOI] [PubMed] [Google Scholar]
- Johnston, J.A., Ward, C.L., and Kopito, R.R. (1998). Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143, 1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, P.H., Islam, K., Kenney, J., Nielsen, M.S., Power, J., and Gai, W.P. (2000). Microtubule-associated protein 1B is a component of cortical Lewy bodies and binds alpha-synuclein filaments. J. Biol. Chem. 275, 21500-21507. [DOI] [PubMed] [Google Scholar]
- Junn, E., Lee, S.S., Suhr, U.T., and Mouradian, M.M. (2002). Parkin accumulation in aggresomes due to proteasome impairment. J. Biol. Chem. 277, 47870-47877. [DOI] [PubMed] [Google Scholar]
- Kaytor, M.D., and Warren, S.T. (1999). Aberrant protein deposition and neurological disease. J. Biol. Chem. 274, 37507-37510. [DOI] [PubMed] [Google Scholar]
- Kegel, K.B., Kim, M., Sapp, E., McIntyre, C., Castano, J.G., Aronin, N., and DiFiglia, M. (2000). Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J. Neurosci. 20, 7268-7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev, A.F., and Goldberg, A.L. (2001). Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8, 739-758. [DOI] [PubMed] [Google Scholar]
- Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., Yokochi, M., Mizuno, Y., and Shimizu, N. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605-608. [DOI] [PubMed] [Google Scholar]
- Kleijnen, M.F., Shih, A.H., Zhou, P., Kumar, S., Soccio, R.E., Kedersha, N.C., Gill, G., and Howley, P.M. (2000). The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol. Cell 6, 409-419. [DOI] [PubMed] [Google Scholar]
- Kubo, S.-I., Kitami, T., Noda, S., Shimura, H., Uchiyama, Y., Asakawa, S., Minoshima, S., Shimizu, N., Mizuno, Y., and Hattori, N. (2001). Parkin is associated with cellular vesicles. J. Neurochem. 78, 42-54. [DOI] [PubMed] [Google Scholar]
- Kuznetsov, G., Bush, K.T., Zhang, P.L., and Nigam, S.K. (1996). Perturbations in maturation of secretory proteins and their association with endoplasmic reticulum chaperones in a cell culture model for epithelial ischemia. Proc. Natl. Acad. Sci. USA 93, 8584-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, A.E., and Lozano, A.M. (1998). Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044-1053. [DOI] [PubMed] [Google Scholar]
- Le, W.-D., Xu, P., Jankovic, J., Jiang, H., Appel, S.H., Smith, R.G., and Vassilatis, D.K. (2003). Mutations in NR4A2 associated with familial Parkinson disease. Nat. Genet. 33, 85-89. [DOI] [PubMed] [Google Scholar]
- Ledesma, M.D., Galvan, C., Hellias, B., Dotti, C., and Jensen, P.H. (2002). Astrocytic but not neuronal increased expression and redistribution of Parkin during unfolded protein stress. J. Neurochem. 83, 1431-1440. [DOI] [PubMed] [Google Scholar]
- Lee, D.H., and Goldberg, A.L. (1998). Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8, 397-403. [DOI] [PubMed] [Google Scholar]
- Lee, H.-J., Shin, S.Y., Choi, C., Lee, Y.H., and Lee, S.-J. (2002). Formation and removal of á-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. 277, 5411-5417. [DOI] [PubMed] [Google Scholar]
- Leroy, E., et al. (1998). The ubiquitin pathway in Parkinson's disease. Nature 395, 451-452. [DOI] [PubMed] [Google Scholar]
- Lowe, J., Blanchard, A., Morrell, K., Lennox, G., Reynolds, L., Billett, M., Landon, M., and Mayer, R.J. (1988). Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease, and Alzheimer's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and Mallory bodies in alcoholic liver disease. J. Pathol. 155, 9-15. [DOI] [PubMed] [Google Scholar]
- Lowe, J., McDermontt, H., Landon, M., Mayer, R.J., and Wilkinson, K.D. (1990). Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of neurodegenerative diseases. J. Pathol. 161, 153-160. [DOI] [PubMed] [Google Scholar]
- Lucking, C.B., et al. (2000). Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 342, 1560-1567. [DOI] [PubMed] [Google Scholar]
- Martin-Aparicio, E., Yamamoto, A., Hernandez, F., Hen, R., Avila, J., and Lucas, J.J. (2001). Proteasomal-dependent aggregation reversal and absence of cell death in a conditional mouse model of Huntington's disease. J. Neurosci. 21, 8772-8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught, K., and Jenner, P. (2001). Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci. Lett. 297, 191-194. [DOI] [PubMed] [Google Scholar]
- McNaught, K., Olanow, C.W., Halliwell, B., Isacson, O., and Jenner, P. (2001). Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat. Rev. Neurosci. 2, 589-594. [DOI] [PubMed] [Google Scholar]
- McNaught, K., Shashidharan, P., Perl, D.P., Jenner, P., and Olanow, C.W. (2002). Aggresome-related biogenesis of Lewy bodies. Eur. J. Neurosci. 16, 2136-2148. [DOI] [PubMed] [Google Scholar]
- McNaught, K., Belizaire, R., Isacson, O., Jenner, P., and Olanow, C.W. (2003). Altered proteasome function in sporadic Parkinson's disease. Exp. Neurol. 179, 38-46. [DOI] [PubMed] [Google Scholar]
- Meriin, A.B., Mabuchi, K., Gabai, V.L., Yaglom, J.A., Kazantsev, A., and Sherman, M.Y. (2001). Intracellular aggregation of polypeptides with expanded domain is stimulated by stress-activated kinase MEKK1. J. Cell Biol. 153, 851-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morett, E., and Bork, P. (1999). A novel transactivation domain in parkin. Trends Biochem. Sci. 24, 229-231. [DOI] [PubMed] [Google Scholar]
- Mori, H., Kondo, T., Yokochi, M., Matsumine, H., Nakagawa-Hattori, Y., Miyake, T., Suda, K., and Mizuno, Y. (1998). Pathological and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology 51, 890-892. [DOI] [PubMed] [Google Scholar]
- Moynihan, T.P., Ardley, H.C., Nuber, U., Rose, S.A., Jones, P.F., Scheffner, M., Markham, A.F., and Robinson, P.A. (1999). RING finger and IBR motifs characterize the interaction domains of HHARI and H7-AP1 with the ubiquitin-conjugating enzymes UbcH7 and UbcH8. J. Biol. Chem. 274, 30963-30967. [DOI] [PubMed] [Google Scholar]
- Myung, J., Kim, K.B., and Crews, C.M. (2001). The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 21, 245-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell, C., Murphy, D.D., Petrucelli, L., Singleton, A.B., Hussey, J., Farrer, M., Dickson, D.W., and Cookson, M.R. (2001). Transfected synphilin-1 forms cytoplasmic inclusions in HEK293 cells. Mol. Brain Res. 97, 94-102. [DOI] [PubMed] [Google Scholar]
- Olanow, C.W., and Tatton, W.G. (1999). Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22, 123-144. [DOI] [PubMed] [Google Scholar]
- Pollen, M.S., Dickson, D.W., and Bergeron, C. (1993). Pathology of the Lewy body. J. Neuropathol. Exp. Neurol. 52, 183-191. [DOI] [PubMed] [Google Scholar]
- Polevoda, B., and Sherman, F. (2002). The diversity of acetylated proteins. Genome Biol. 3, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos, M.H., et al. (1997). Mutations in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045-2047. [DOI] [PubMed] [Google Scholar]
- Rajan, R.S., Illing, M.E., Bence, N.F., and Kopito, R.R. (2001). Specificity in intracellular protein aggregation and inclusion formation. Proc. Natl. Acad. Sci. USA 98, 13060-13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Y., Zhao, J., and Feng, J. (2003). Parkin binds α/β tubulin and increases their ubiquitination and degradation. J. Neurosci. 23, 3316-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout, H.J., Larsen, K.E., Sulzer, D., and Stefanis, L. (2001). Proteasomal inhibition leads to formation of ubiquitin/synuclein-immunoreactive inclusions in PC12 cells. J. Neurochem. 78, 899-908. [DOI] [PubMed] [Google Scholar]
- Sakata, E., et al. (2003). Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 4, 301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou, F., Finkbeiner, S., Devys, D., and Greenberg, M.E. (1998). Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55-66. [DOI] [PubMed] [Google Scholar]
- Schlossmacher, M.G., et al. (2002). Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am. J. Pathol. 160, 1655-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, W. K., et al. (2001). Complete genomic screen in Parkinson disease: evidence for multiple genes. J. Am. Med. Assoc. 286, 2239-2244. [DOI] [PubMed] [Google Scholar]
- Sherman, M.Y., and Goldberg, A.L. (2001). Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29, 15-32. [DOI] [PubMed] [Google Scholar]
- Shimura, H., et al. (2000). Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302-305. [DOI] [PubMed] [Google Scholar]
- Shimura, H., et al. (1999). Immunohistochemical and subcellular localization of parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann. Neurol. 45, 668-672. [DOI] [PubMed] [Google Scholar]
- Shimura, H., Schlossmacher, M.G., Hattori, N., Frosch, M.P., Trockenbacher, A., Schneider, R., Mizuno, Y., Kosik, K.S., and Selkoe, D.J. (2001). Ubiquitination of a new form of α-synuclein by Parkin from human brain: implications for Parkinson's disease. Science 293, 263-269. [DOI] [PubMed] [Google Scholar]
- Spence, J., Gali, R.R., Dittmar, G., Sherman, F., Karin, M., and Finley, D. (2000). Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102, 67-76. [DOI] [PubMed] [Google Scholar]
- Spence, J., Sadis, S., Haas, A.L., and Finley, D. (1995). A ubiquitin mutant with specific defects in DNA repair and multiubiquitination. Mol. Cell. Biol. 15, 1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini, M.G., Schmidt, G., Lee, V.M.Y., Trojanowski, J.Q., Jakes, M., and Goedert, M. (1997). Alpha-synuclein in Lewy bodies. Nature 388, 839-840. [DOI] [PubMed] [Google Scholar]
- Staropoli, J.F., McDermott, C., Martinat, C., Schulman, B., Demireva, E., and Abeliovich, A. (2003). Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainite excitotoxicity. Neuron 37, 735-749. [DOI] [PubMed] [Google Scholar]
- Stichel, C.C., Augustin, M., Kuhn, K., Zhu, X.-R., Engels, P., Ullmer, C., and Lubbert, H. (2000). Parkin expression in the adult mouse brain. Eur. J. Neurosci. 12, 4181-4194. [PubMed] [Google Scholar]
- Takemura, R., Okabe, S., Umeyama, T., Kanai, Y., Cowan, N.J., and Hirokawa, N. (1992). Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci. 103, 953-964. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y., Engelender, S., Igarashi, S., Rao, R.K., Wanner, T., Tanzi, R.E., Sawa, A., Dawson, V.L., Dawson, T.M., and Ross, C.A. (2001). Inducible expression of α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 10, 919-926. [DOI] [PubMed] [Google Scholar]
- Terreni, L., Calabrese, E., Calella, A.M., Forloni, G., and Mariani, C. (2001). New mutation (R42P) of the parkin gene in the ubiquitinlike domain associated with parkinsonism. Neurology 56, 463-566. [DOI] [PubMed] [Google Scholar]
- Thrower, J.S., Hoffman, L., Rechsteiner, M., and Pickart, C.M. (2000). Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, Y.C., Fishman, P.S., Thakor, N.V., and Oyler, G.A. (2003). Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J. Biol. Chem. 278, 22044-22055. [DOI] [PubMed] [Google Scholar]
- Valente, E.M., Bentivoglio, A.R., Dixon, P.H., Ferraris, A., Ialongo, T., Frontali, M., Albanese, A., and Wood, N.W. (2001). Localization of a novel locus for autosomal recessive early-onset Parkinsonism, PARK6, on human chromosome 1p35–p36. Am. J. Hum. Genet. 68, 895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelter, S., Boeddrich, A., Lurz, R., Scherzinger, E., Lueder, G., Lehrach, H., and Wanker, E.E. (2001). Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol. Biol. Cell 12, 1393-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi, K., Engelender, S., Yoshimoto, M., Tsuji, S., Ross, C.A., and Takahashi, H. (2000). Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann. Neurol. 47, 521-523. [PubMed] [Google Scholar]
- Walsh, D.M., Klyubin, I., Fadeeva, J.V., Cullen, W.K., Anwyl, R., Wolfe, M.S., Rowan, M.J., and Selkoe, D.J. (2002). Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535-539. [DOI] [PubMed] [Google Scholar]
- Ward, C.L., Omura, S., and Kopito, R.R. (1995). Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83, 121-127. [DOI] [PubMed] [Google Scholar]
- Weissman, A.M. (2001). Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell. Biol. 2, 169-178. [DOI] [PubMed] [Google Scholar]
- West, A.B., Gonzalez-de-Chavez, F., Wilkes, K., O'Farrell, C., and Farrer, M.J. (2003). Parkin is not regulated by the unfolded protein response in human neuroblastoma cells. Neurosci. Lett. 341, 139-142. [DOI] [PubMed] [Google Scholar]
- Wright, G., Terada, K., Yano, M., Sergeev, I., and Mori, M. (2001). Oxidative stress inhibits the mitochondrial import of preproteins and leads to their degradation. Exp. Cell Res. 293, 107-117. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Gao, J., Chung, K.K.K., Huang, H., Dawson, V.L., and Dawson T.M. (2000). Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CD-Crel-1. Proc. Natl. Acad. Sci. USA 97, 13354-13359. [DOI] [PMC free article] [PubMed] [Google Scholar]