Abstract
Nephrogenic syndrome of inappropriate antidiuresis is a recently identified genetic disease first described in two unrelated male infants with severe symptomatic hyponatremia. Despite undetectable arginine vasopressin levels, patients have inappropriately concentrated urine resulting in hyponatremia, hypoosmolality, and natriuresis. It was found that each infant had a different mutation of the vasopressin type II receptor (V2R) at codon 137 where arginine was converted to cysteine or leucine (R137C or R137L), resulting in constitutive signaling. Interestingly, a missense mutation at the same codon, converting arginine to histidine (R137H), leads to the opposite disease phenotype with a loss of the kidney’s ability to concentrate urine resulting in nephrogenic diabetes insipidus. This mutation is associated with impaired signaling, although whether this is predominantly due to impaired trafficking to the plasma membrane, agonist-independent internalization, or G protein uncoupling is currently unclear. Using bioluminescence resonance energy transfer and confocal microscopy, we demonstrate that both V2R-R137C and V2R-R137L mutants interact with β-arrestins in an agonist-independent manner resulting in dynamin-dependent internalization. This phenotype is similar to that observed for V2R-R137H, which is intriguing considering that it is accompanied by constitutive rather than impaired signaling. Consequently, it would seem that agonist-independent internalization per se is unlikely to be the major determinant of impaired V2R-R137H signaling. Our findings indicate that the V2R-R137C and V2R-R137L mutants traffic considerably more efficiently to the plasma membrane than V2R-R137H, identifying this as a potentially important mutation-dependent difference affecting V2R function.
Using bioluminescence resonance energy transfer and confocal microscopy, V2R-R137C and V2R-R137L were shown to interact with β-arrestins in the absence of agonist resulting in dynamin-dependent internalization.
Nephrogenic syndrome of inappropriate antidiuresis (NSIAD) is a genetic disease first described in 2005 in two unrelated infant boys whose bodies were overloaded with excess hypotonic fluid and who had undetectable arginine vasopressin (AVP) levels (1). Inappropriately concentrated urine results in hyponatremia, hypoosmolality, and natriuresis. It was found that each infant had a different mutation in the same codon of the gene encoding the vasopressin type II receptor (V2R) (1). After identification and characterization of NSIAD in these two infants, other NSIAD patients, including neonates and adults, have now been identified with similar V2R mutations (2,3,4,5). Furthermore, the number of diagnosed cases is likely to increase in the future, particularly as 10–20% of patients reported as presenting with symptoms of syndrome of inappropriate antidiuretic hormone secretion actually appear to have AVP levels at or below the limits of RIA detection (6), implying that they may be suffering from NSIAD instead (1,7). Indeed, the study of a family from which Decaux and colleagues (3) described three hemizygous males and four heterozygous females highlighted that although NSIAD is X-linked, penetrance is likely to be observed, with activating mutations of V2R affecting heterozygote women to some degree. Furthermore, NSIAD may go unrecognized for years due to variable expressivity (3), with the potential for subclinical NSIAD being unmasked upon excessive water loading (8). Consequently, it has been suggested as a contributing factor in some instances of exercise-associated hyponatremia (5).
V2R is a G protein-coupled receptor (GPCR) that is activated by AVP (also known as antidiuretic hormone). Normal fluid homeostasis depends on adequate water intake, regulated by an intact thirst mechanism, and on excretion of free water, mediated by AVP (9,10). The binding of AVP to V2R on the basolateral membrane of kidney collecting duct epithelial cells triggers activation of Gs-protein, leading to increased levels of cAMP. This in turn leads to trafficking of the water channel aquaporin-2 (AQP-2) to the apical membrane of collecting duct cells, resulting in increased water permeability and antidiuresis (11).
An important role in V2R signaling and regulation is played by β-arrestins, proteins that interact with GPCRs and block further signaling via G proteins in a process known as desensitization. After agonist binding, the active V2R is subsequently phosphorylated by a group of protein kinases (GPCR kinases) that increase receptor binding affinity for β-arrestins (12,13). The receptors are subsequently desensitized with respect to G protein-mediated signaling and internalized into clathrin-coated pits; thus receptors are no longer available at the cell surface for ligand stimulation unless or until they are recycled to the plasma membrane. There is also evidence for β-arrestin-mediated signaling after activation of the V2R by AVP, resulting in stimulation of ERK1/2 activity independently of G protein signaling (14).
The V2R, being a GPCR, has seven transmembrane domains. Missense mutations found in patients’ DNA coding for arginine (R) codon 137 leads to important functional disorders (1,7). This residue is part of a highly conserved DRY motif located at the junction of the third transmembrane domain and second intracellular loop, a region critical for stabilizing receptors in the active/inactive conformation (15,16). Interestingly, diverse missense mutations at the same site result in opposite disease phenotypes (1,7). Conversion of the arginine to a histidine (R137H) leads to a loss of the kidney’s ability to concentrate urine and results in the water-losing syndrome nephrogenic diabetes insipidus (NDI) (17,18,19). In contrast to R137H, conversion of the arginine to a cysteine or leucine (R137C or R137L) results in an inability to excrete a free water load with inappropriately concentrated urine and resultant hyponatremia, hypoosmolality, and natriuresis with nonelevated and potentially undetectable AVP levels, the syndrome termed NSIAD (1,2,3,4,5). To our knowledge, these cases are the only reported examples in which mutations at the DRY motif affect the same amino acid but cause two different genetic diseases. The molecular mechanisms by which these mutations elicit their effects still remain to be fully elucidated. However, it has been shown that the V2R-R137H mutant mediates negligible increases in cAMP, either constitutively or upon AVP stimulation (1,19,20). In contrast, V2R-R137C and V2R-R137L mutants exhibit constitutive signaling in the absence of ligand, as measured by increased CRE-luciferase activity (1).
Current explanations for the lack of V2R-R137H activity include agonist-independent β-arrestin interaction and internalization (20) and poor initial trafficking to the membrane (21,22). With these theories in mind, we compared interactions between β-arrestin and V2R-R137C or V2R-R137L with interactions between β-arrestin and V2R-R137H to better understand the reasons for the opposite clinical phenotypes.
We employed bioluminescence resonance energy transfer (BRET) and confocal microscopy to investigate the interactions of wild-type and mutant V2Rs with β-arrestins. The BRET procedure involves heterologous coexpression of fusion proteins linking one protein of interest (one of the V2Rs) to a bioluminescent donor enzyme, a variant of Renilla luciferase (Rluc8), and a second protein of interest (β-arrestin 2) to an acceptor fluorophore, a variant of green fluorescent protein (Venus or GFP10). If the Rluc8 and fluorophore are approximately 10 nm or less apart (23), energy resulting from the rapid oxidation of a cell-permeable coelenterazine substrate by Rluc8 will transfer to the fluorophore, which in turn fluoresces at a longer wavelength. BRET detection can be carried out using scanning spectrometry or dual-filter luminometry. Energy transfer implies that these molecules are in close proximity and therefore that the proteins of interest fused to the donor and acceptor interact directly or as part of a complex (24,25,26,27).
In this study, we have used recent advances in the BRET1 and BRET2 assay systems (24,25,27), in concert with confocal microscopy, to demonstrate agonist-independent interactions of β-arrestins with not only V2R-R137H, but also V2R-R137C and V2R-R137L. These findings have been supported by BRET data illustrating the ineffectiveness of a competitive antagonist to inhibit the agonist-independent receptor/β-arrestin interactions as well as data generated with C-terminally truncated receptors that do not interact with β-arrestins. Confocal microscopy data were obtained in the presence and absence of dominant-negative dynamin, dynamin(K44A), which allows interactions with β-arrestins but blocks subsequent internalization. These data demonstrate that despite agonist-independent interactions of all three mutant receptors with β-arrestins and a substantial proportion of receptor being localized in the cytosol at any given time, mutant receptors do appear to reach the plasma membrane for at least some of the time and therefore are available to interact with G protein in an agonist-induced and/or agonist-independent manner. Notably, when comparing V2R-R137H with V2R-R137C or V2R-R137L, BRET signals due to β-arrestin interactions are weaker and membrane localization as visualized by confocal microscopy appears less obvious. This is consistent with previous observations indicating that V2R-R137H trafficking is impaired (21,22) although suggesting that this is not the case for the V2R-R137C or V2R-R137L mutants.
Results
Functional validation of the V2R/Rluc8 fusion protein
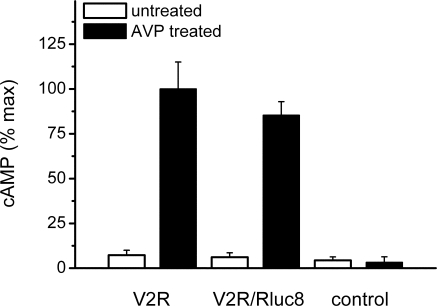
BRET fusion proteins need to be validated to ensure that addition of the fluorescent/luminescent tag does not impair their normal function (26,27). cAMP assays indicated that Rluc8-tagged receptor signaling was comparable to the untagged receptor (Fig. 1). It should be noted that the demonstration of a ligand-induced receptor/β-arrestin interaction shown below provides additional confirmation of functionality.
Figure 1.
Functional validation of V2R Rluc8-tagged BRET fusion protein. cAMP was measured after addition of AVP (treated) or vehicle (untreated) in COS-7 cells transfected with untagged V2R, V2R tagged with Rluc8 (V2R/Rluc8), or empty vector (control). Data shown are mean ± sem of three independent experiments.
Interactions of V2R wild type and mutants with β-arrestins detected by BRET1 and BRET2 assays using scanning spectrometry
The interactions of V2R wild type and the three mutants V2R-R137C, V2R-R137L, and V2R-R137H with β-arrestins were compared in the presence and absence of the agonist AVP using BRET1 (with coelenterazine h) and BRET2 (with DeepBlueC). HEK293 cells transfected with V2R-wild type/Rluc8 and one of the β-arrestin fusion proteins (β-arrestin 2/Venus for BRET1 or β-arrestin 2/GFP10 for BRET2) showed a single maximum peak at about 480 nm (Fig. 2A) for BRET1 and about 420 nm (Fig. 2B) for BRET2 in the absence of ligand. Upon treatment with 1 μm AVP, cells expressing V2R-wild type/Rluc8 and β-arrestin 2/Venus displayed a second peak at about 530 nm in addition to that at about 480 nm in the BRET1 assay (Fig. 2A). Similarly in the BRET2 assay, cells expressing V2R-wild type/Rluc8 and β-arrestin 2/GFP10 displayed a second peak at about 510 nm in addition to that at about 420 nm (Fig. 2B). The appearance of the second peak demonstrates a spectral shift resulting from resonance energy transfer, indicating that specific ligand-induced V2R/β-arrestin interactions had occurred. In contrast to wild-type V2R, cells expressing any of the three mutant fusion proteins V2R-R137C/Rluc8, V2R-R137L/Rluc8, or V2R-R137H/Rluc8 exhibited two peaks in the absence of ligand. Notably, the secondary peak was higher for V2R-R137C and V2R-R137L than for V2R-R137H in both the BRET1 and BRET2 assays. In the BRET1 assay, peaks were present at about 480 nm and at about 530 nm (Fig. 2, C, E, and G), and in the BRET2 assay, peaks were detected at about 420 nm and at about 510 nm (Fig. 2, D, F, and H). Upon treatment with 1 μm AVP, similar spectra were observed (Fig. 2, C–H). These results provide the first indication of agonist-independent interactions between V2R-R137C or V2R-R137L mutants and β-arrestins.
Figure 2.
Spectral analysis of wild-type and mutant (R137C, R137L, or R137H) V2Rs tagged with Rluc8 in untreated and AVP-treated HEK293 cells using coelenterazine h (A, C, E, and G) or DeepBlueC (B, D, F, and H) as Rluc8 substrate. Cells were cotransfected with cDNA for β-arrestin 2/Venus (A, C, E, and G) or β-arrestin 2/GFP10 (B, D, F, and H) and one of various V2R constructs tagged with Rluc8. Emission spectra were recorded immediately after substrate addition. In V2R wild-type untreated cells, an emission maximum of about 480 nm or about 420 nm corresponds to Rluc oxidizing coelenterazine h (A) or DeepBlueC (B), respectively. In V2R wild-type AVP-treated cells, an additional emission peak appears at about 530 nm (A) or about 510 nm (B) that corresponds to emission from Venus or GFP10, respectively, thereby demonstrating BRET due to ligand-induced V2R/β-arrestin 2 interactions. Emission spectra for the three V2R mutants, V2R-R137C (C and D), V2R-R137L (E and F), and V2R-R137H (G and H), exhibit two emission peaks in untreated as well as treated cells, thereby demonstrating BRET due to agonist-independent as well as agonist-induced V2R/β-arrestin 2 interactions. Data are representative of at least three independent experiments.
Interactions of V2R wild type and mutants with β-arrestins detected in real-time kinetic BRET1 and BRET2 assays
BRET signals were detected from cells cotransfected with different V2R/Rluc8 constructs and β-arrestin fusion protein (β-arrestin 2/Venus for the BRET1 assay or β-arrestin 2/GFP10 for the BRET2 assay). Measurements were taken before and after addition of ligand AVP or vehicle (PBS). The relative luminescence of the Rluc8-tagged V2R mutants compared with Rluc8-tagged V2R-wild type is presented in Table 1, showing that the V2R mutants were expressed at similar or slightly lower levels than the wild type in both real-time kinetic BRET1 and BRET2 assays. Consequently, the findings from these assays are not due to increased expression levels of the mutant receptors compared with wild type.
Table 1.
Relative luminescence intensities of the three V2R mutants investigated compared with the luminescence intensity of V2R-wild type transfected with the corresponding β-arrestin 2 construct (β-arrestin 2/Venus or β-arrestin 2/GFP10)
| Transfected constructs | Luminescence relative to V2R-wild type/Rluc8 (%) |
|---|---|
| β-Arrestin 2/Venus and V2R-R137C/Rluc8 | 84.9 ± 3.1 |
| β-Arrestin 2/Venus and V2R-R137L/Rluc8 | 101.0 ± 4.1 |
| β-Arrestin 2/Venus and V2R-R137H/Rluc8 | 77.7 ± 3.4 |
| β-Arrestin 2/GFP10 and V2R-R137C/Rluc8 | 95.7 ± 1.1 |
| β-Arrestin 2/GFP10 and V2R-R137L/Rluc8 | 99.8 ± 3.5 |
| β-Arrestin 2/GFP10 and V2R-R137H/Rluc8 | 85.3 ± 4.7 |
Luminescence intensities were detected as part of the BRET kinetic assay. Data shown are mean ± sem of four independent experiments.
Interactions of the wild-type and mutant V2Rs (V2R-R137C, V2R-R137L, and V2R-R137H) with β-arrestin 2 were detected in cells that were treated with vehicle or one of three doses of AVP (final concentrations 1, 0.1, and 0.01 μm), to elucidate the dose dependency of these interactions (Fig. 3). In comparison with wild-type V2R, all three mutants showed elevated BRET signals for untreated cells, indicating agonist-independent interactions of all of the V2R mutants with β-arrestin 2. Notably, V2R-R137C and V2R-R137L exhibited higher BRET signals than V2R-R137H. With BRET1, the BRET ratio above wild-type baseline ± sem immediately before ligand treatment was 0.223 ± 0.002, 0.263 ± 0.004, and 0.129 ± 0.003, respectively. The distinction was also observed with BRET2, the BRET ratio above wild-type baseline ± sem immediately before ligand treatment being 0.0607 ± 0.0003, 0.0793 ± 0.0005, and 0.0252 ± 0.0004 for V2R-R137C, V2R-R137L, and V2R-R137H, respectively. Moreover, despite the V2R mutants already eliciting a BRET signal in the absence of ligand, their interactions with β-arrestin 2 still increased slightly after treatment with increasing doses of agonist AVP. This can be seen more clearly with dual-filter luminometry than with scanning spectrometry because the former is a more sensitive BRET detection method.
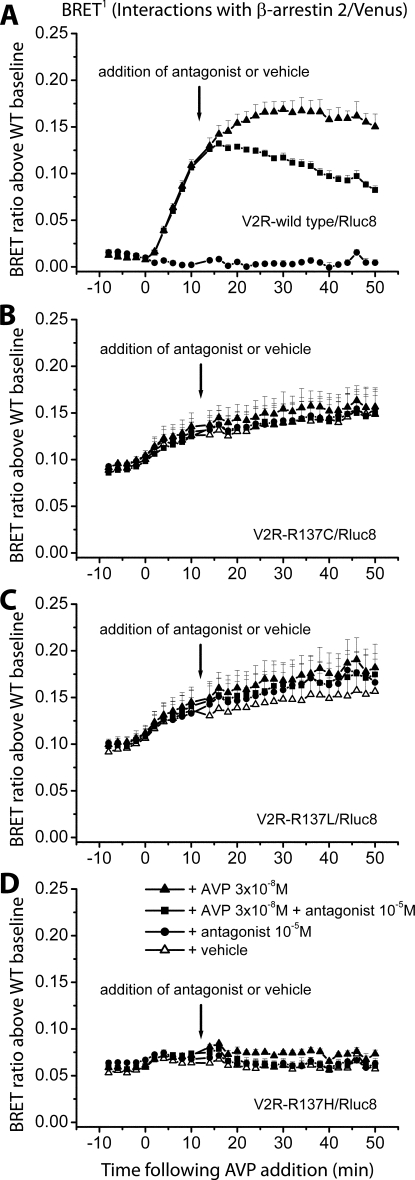
Figure 3.
Detection of protein-protein interactions by real-time BRET1 (A, C, E, and G) and BRET2 (B, D, F, and H) assays. Kinetics data comparing V2R-wild type (A and B) and the three different mutants V2R-R137C (C and D), V2R-R137L (E and F), and V2R-R137H (G and H) were generated by monitoring the interaction with β-arrestin 2/Venus (BRET1) or β-arrestin 2/GFP10 (BRET2). The Rluc8 substrate coelenterazine h for BRET1 (A, C, E, and G) or DeepBlueC for BRET2 (B, D, F, and H) was added immediately before real-time measurements at 37 C. The transiently cotransfected HEK293 cells were assayed before and after treatment with ligand (AVP final concentrations of 1, 0.1, and 0.01 μm) or vehicle (PBS). BRET ratio above wild-type baseline was calculated as described in Materials and Methods. Data shown are mean ± sem of four independent experiments.
BRET1 assays were repeated with measurements taken before and after addition of a submaximal dose of AVP (0.03 μm) or vehicle (Fig. 4). After 10 min, the V2R antagonist [adamantaneacetyl1, O-Et-d-Tyr2, Val4, aminobutyryl6, Arg8,9]-vasopressin or vehicle was added. Inhibition of the interaction between V2R wild type and β-arrestin 2 was observed to occur rapidly and gradually increase over time (Fig. 4A). This is likely to reflect competition with agonist binding to receptors at the plasma membrane, thereby inhibiting receptor activation and therefore β-arrestin binding. We would not expect the peptide antagonist to affect those receptors already internalized and interacting with β-arrestin in the cytosol. The gradual increase in inhibition over time is therefore consistent with receptors being continually trafficked to the plasma membrane where they are exposed to antagonist. The lack of BRET signal inhibition by antagonist with any of the mutant receptors (Fig. 4, B–D) is consistent with these receptors interacting with β-arrestin 2 in an agonist-independent manner.
Figure 4.
Detection of protein-protein interactions by real-time BRET1 assays to assess the effect of an antagonist. Kinetics data comparing V2R-wild type (A) and the three different mutants V2R-R137C (B), V2R-R137L (C), and V2R-R137H (D) were generated by monitoring the interaction with β-arrestin 2/Venus. The Rluc8 substrate coelenterazine h was added immediately before real-time measurements at 37 C. The transiently cotransfected HEK293 cells were firstly assayed before and after treatment with a submaximal dose of agonist (AVP final concentration of 0.03 μm) or vehicle (PBS). At 10 min after addition of agonist/vehicle, cells were treated with antagonist (final concentration of 10 μm) or vehicle (PBS). BRET ratio above wild-type baseline was calculated as described in Materials and Methods. Data shown are mean ± sem of four independent experiments.
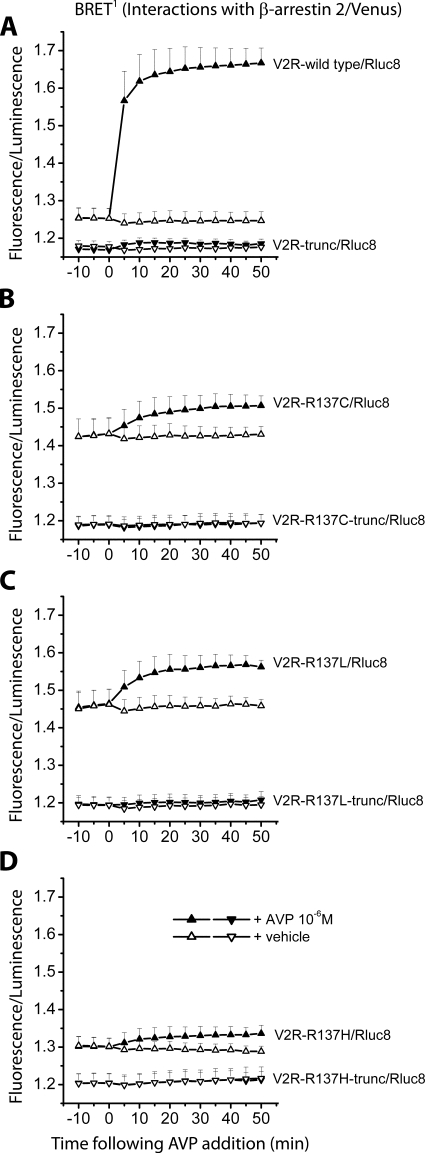
Specificity of BRET data for V2R/β-arrestin interactions is supported by the lack of BRET signals observed with C-terminally truncated V2Rs
Truncated receptors lacking the C terminus (the binding region for β-arrestin) were generated and tested with the BRET1 assay in parallel with nontruncated receptors to further demonstrate the specificity of BRET signals observed for V2R/β-arrestin interactions (Fig. 5). These BRET signal data have been presented as fluorescence/luminescence without background subtraction (27) to allow direct comparison between truncated and nontruncated receptors. After addition of AVP, V2R wild type showed an increase in BRET signal, indicating activation of the receptor followed by β-arrestin binding (Fig. 5A). Untreated V2R wild type maintained a baseline level of BRET that was slightly higher than that observed with the truncated receptor. Comparison of luminescence intensities implies that this is not due to differences in expression levels (Table 2). It may be due to a low (basal) level of agonist-independent β-arrestin interaction even with the wild-type receptor. However, regardless of the reason, additional differences observed between mutant truncated and nontruncated receptors are likely to be due to the mutation of R137. The mutant receptors V2R-R137C (Fig. 5B), V2R-R137L (Fig. 5C), and V2R-R137H (Fig. 5D) showed elevated BRET signals in the presence and absence of ligand that were higher than those for the respective truncated receptors and higher than the baseline for the nontruncated V2R wild type (Fig. 5A). This was particularly clear for V2R-R137C and V2R-R137L. The lack of BRET signal modulation by ligand was also consistent with the lack of interaction of truncated receptors with β-arrestin 2.
Figure 5.
Detection of protein-protein interactions by real-time BRET1 assays comparing C-terminally truncated and nontruncated receptors. Kinetics data comparing V2R-wild type (A) and the three different mutants V2R-R137C (B), V2R-R137L (C), and V2R-R137H (D) and corresponding C-terminally truncated receptors V2R-trunc (A), V2R-R137C-trunc (B), V2R-R137L-trunc (C), and V2R-R137H-trunc (D) were generated by monitoring the interaction with β-arrestin 2/Venus. The Rluc8 substrate coelenterazine h was added immediately before real-time measurements at 37 C. The transiently cotransfected HEK293 cells were assayed before and after treatment with ligand (AVP final concentration of 1 μm) or vehicle (PBS). BRET signals are expressed as fluorescence/luminescence without background subtraction as described in Materials and Methods. Data shown are mean ± sem of four independent experiments.
Table 2.
Relative luminescence intensities of the V2R-wild type and three V2R mutants investigated compared with the luminescence intensity of the corresponding Rluc8-tagged nontruncated receptors cotransfected with β-arrestin 2/Venus
| Transfected constructs | Luminescence relative to Rluc8-tagged nontruncated receptors (%) |
|---|---|
| β-Arrestin 2/Venus and V2R-trunc/Rluc8 | 108.2 ± 2.0 |
| β-Arrestin 2/Venus and V2R-R137C-trunc/Rluc8 | 86.5 ± 3.6 |
| β-Arrestin 2/Venus and V2R-R137L-trunc/Rluc8 | 128.6 ± 9.2 |
| β-Arrestin 2/Venus and V2R-R137H-trunc/Rluc8 | 111.2 ± 16.2 |
Luminescence intensities were detected as part of the BRET kinetic assay. Data shown are mean ± sem of four independent experiments.
Distribution and trafficking of wild-type and mutant V2Rs
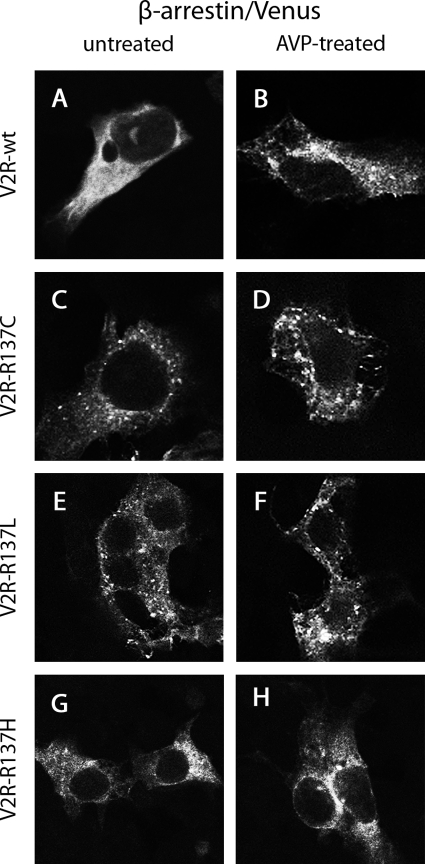
HEK293 cells were cotransfected with Venus-tagged V2R wild type or mutants (V2R-R137C, V2R-R137L, or V2R-R137H) and untagged β-arrestin 2. Confocal microscopy indicated membrane localization of V2R wild type in untreated cells (Fig. 6A). Upon treatment with 1 μm AVP, the receptor internalized, localizing to punctate intracellular vesicles (Fig. 6B). In contrast to V2R wild type, the mutant receptors exhibited intracellular localization in the absence of agonist (Fig. 6, C, E, and G) as well as upon treatment with 1 μm AVP (Fig. 6, D, F, and H). Notably, V2R-R137H appeared to be observed in structures peripheral to and branching out from the nucleus in a pattern more consistent with endoplasmic reticulum localization (Fig. 6, G and H). In contrast, the more punctate cytosolic distribution of V2R-R137C and V2R-R137L (Fig. 6, C–F) appears more consistent with agonist-independent internalization of these mutant receptors into intracellular vesicles.
Figure 6.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed untagged β-arrestin 2 with V2R-wild type/Venus (A and B), V2R-R137C/Venus (C and D), V2R-R137L/Venus (E and F), or V2R-R137H/Venus (G and H).
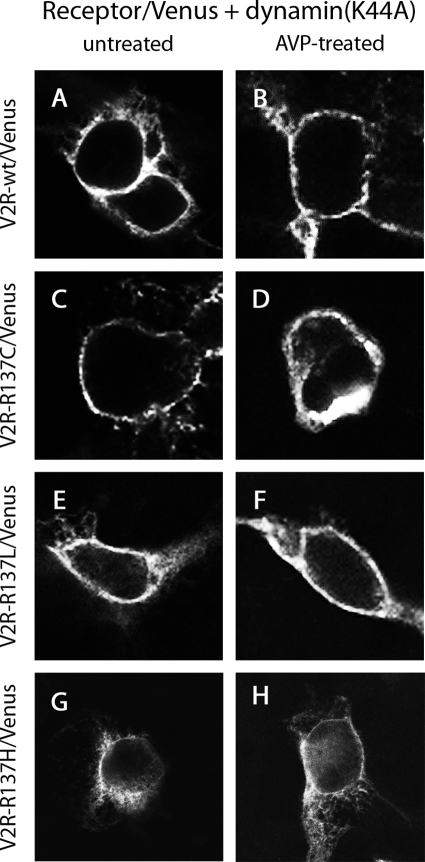
In HEK293 cells in the absence of ligand, the expression of dynamin(K44A) with the V2R wild type did not alter membrane localization of the receptor (Fig. 7A). However, in contrast to that observed in Fig. 6B, the receptor remained at the plasma membrane and did not internalize into endocytic vesicles after 30 min treatment with the agonist AVP (Fig. 7B). Coexpression of dynamin(K44A) with the V2R mutants V2R-R137C or V2R-R137L resulted in plasma membrane localization in the absence as well as in the presence of AVP (Fig. 7, C–F), in contrast to that observed in Fig. 6, C–F. Plasma membrane localization of V2R-R137H in the absence and presence of AVP (Fig. 7, G and H), although greater than that observed in the absence of dynamin(K44A) (Fig. 6, G and H), appeared less extensive than that observed with V2R-R137C or V2R-R137L (Fig. 7, C–F).
Figure 7.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed untagged β-arrestin 2 with V2R-wild type/Venus (A and B), V2R-R137C/Venus (C and D), V2R-R137L/Venus (E and F), or V2R-R137H/Venus (G and H) in the presence of dominant-negative dynamin, dynamin(K44A).
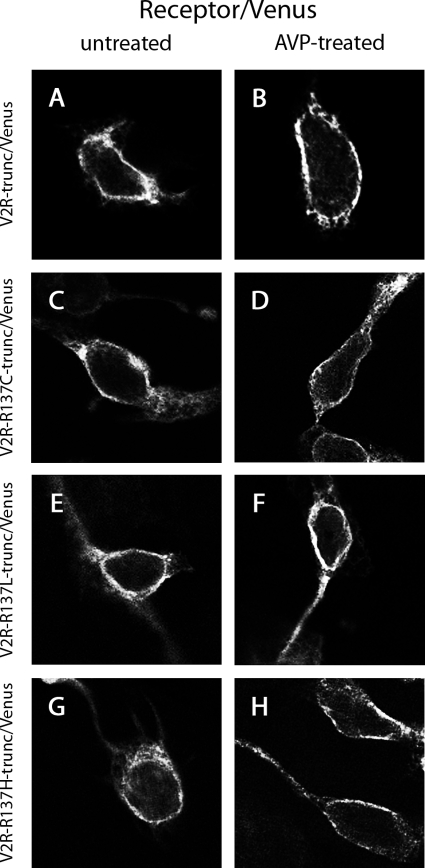
Additionally, HEK293 cells were cotransfected with Venus-tagged C-terminally truncated wild-type or mutant V2Rs (V2R-trunc/Venus, V2R-R137C-trunc/Venus, V2R-R137L-trunc/ Venus, or V2R-R137H-trunc/Venus) and untagged β-arrestin 2. In both the presence and absence of AVP stimulation, these truncated receptors were localized at the plasma membrane as observed using confocal microscopy (Fig. 8). In contrast to cells expressing nontruncated receptors (Fig. 6), no vesicle formation was observed.
Figure 8.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed untagged β-arrestin 2 with V2R-trunc/Venus (A and B), V2R-R137C-trunc/Venus (C and D), V2R-R137L-trunc/Venus (E and F), or V2R-R137H-trunc/Venus (G and H).
Distribution and trafficking of β-arrestins in cells expressing wild-type and mutant V2Rs
After AVP binding, the active V2R wild type is subsequently desensitized and internalized by β-arrestins (12). To examine whether the intracellular localization of the receptor mutants in the absence and presence of AVP is consistent with β-arrestin-mediated endocytosis, untagged wild-type or mutant V2Rs were coexpressed with β-arrestin 2/Venus. Homogeneous cytosolic fluorescence was observed after expression of β-arrestin 2/Venus with V2R wild type in the absence of agonist (Fig. 9A). After 30 min treatment with AVP, punctate intracellular distribution of Venus fluorescence was observed (Fig. 9B), suggesting the relocalization of β-arrestins into clathrin-coated vesicles presumably as complexes with activated V2R. In contrast to that observed with V2R wild type, β-arrestin 2/Venus exhibited punctate intracellular distribution in cells coexpressing mutant V2R-R137C or V2R-R137L with and without AVP treatment (Fig. 9, C–F). The distribution of β-arrestin 2/Venus when coexpressed with V2R-R137H was less clear in untreated (Fig. 9G) and AVP-treated cells (Fig. 9H). In both cases, localization appeared to be cytosolic yet less punctate than that observed with V2R-R137C and V2R-R137L.
Figure 9.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed β-arrestin 2/Venus with V2R-wild type (A and B), V2R-R137C (C and D), V2R-R137L (E and F), or V2R-R137H (G and H).
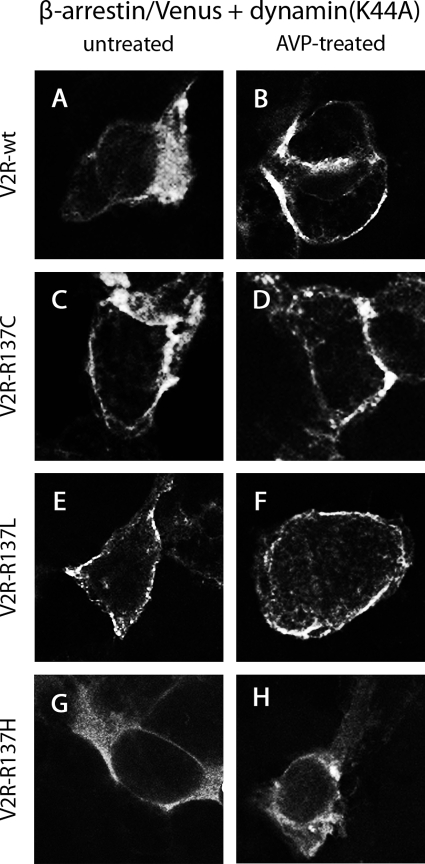
The homogeneous cytosolic distribution of β-arrestin 2/Venus when expressed with V2R wild type remained upon coexpression of dynamin (K44A) (Fig. 10A). However, in contrast to that observed in Fig. 9B, β-arrestin 2/Venus was observed at the plasma membrane and not within intracellular endocytic vesicles after 30 min AVP treatment (Fig. 10B). When dynamin(K44A) was expressed simultaneously with V2R-R137C or V2R-R137L, β-arrestin 2/Venus exhibited plasma membrane localization in both untreated (Fig. 10, C and E) and treated cells (Fig. 10, D and F). In contrast to β-arrestin 2/Venus localization in cells expressing V2R wild type, V2R-R137C, or V2R-R137L with dynamin(K44A), the distribution of β-arrestin 2/Venus when coexpressed with mutant V2R-R137H and dynamin(K44A) did not show such distinct plasma membrane localization in the absence or presence of AVP (Fig. 10, G and H).
Figure 10.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed β-arrestin 2/Venus with V2R-wild type (A and B), V2R-R137C (C and D), V2R-R137L (E and F), or V2R-R137H (G and H) in the presence of dominant-negative dynamin, dynamin(K44A).
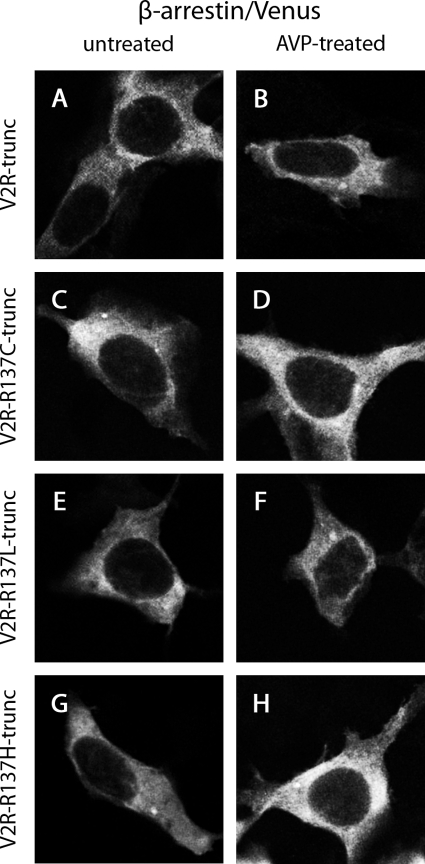
Additionally, we examined the intracellular localization of β-arrestin 2/Venus when coexpressed with untagged C-terminally truncated wild-type or mutant V2Rs (V2R-trunc, V2R-R137C-trunc, V2R-R137L-trunc, or V2R-R137H-trunc) in the absence and presence of AVP. Homogeneous cytosolic fluorescence was observed in each case (Fig. 11). In contrast to cells expressing β-arrestin 2/Venus with nontruncated receptors (Fig. 9), no vesicle formation was observed.
Figure 11.
Confocal microscopy using HEK293 cells that were untreated (A, C, E, and G) or treated with AVP for 30 min (B, D, F, and H). Cells expressed β-arrestin 2/Venus with V2R-trunc (A and B), V2R-R137C-trunc (C and D), V2R-R137L-trunc (E and F), or V2R-R137H-trunc (G and H).
Discussion
We have investigated the molecular basis of clinical cases attributed to distinct point mutations of the same arginine residue in the V2R DRY motif. Interestingly and importantly, the V2R-R137H mutant causes the opposite disease phenotype (NDI) to the V2R-R137C and V2R-R137L mutants (causing NSIAD). Despite this, we were able to detect agonist-independent interactions of all three V2R mutants (V2R-R137H, V2R-R137C, and V2R-R137L) with β-arrestins using both BRET1 and BRET2, detected by both scanning spectrometry and dual-filter luminometry kinetic assays. The specificity of BRET signals reporting interactions with β-arrestin was additionally demonstrated by comparison with C-terminally truncated receptors that do not interact with β-arrestin.
Published clinical trial data indicate that the V2R antagonists tolvaptan and satavaptan are ineffective at treating NSIAD, in contrast to syndrome of inappropriate antidiuretic hormone secretion (3). Therefore, our findings with a peptide V2R antagonist at the cellular level are consistent with these observations in patients. Furthermore, our results are consistent with the notion that a competitive antagonist would be ineffective in treating a condition caused by agonist-independent receptor activation, whether the lack of effect be due to compromised binding or the absence of inverse agonistic properties. Characterization of effective V2R inverse agonists is clearly the next goal. It is intriguing that satavaptan appears to exhibit inverse agonist properties with the V2R-D136A constitutively active receptor (28) yet did not alleviate the symptoms of NSIAD in the aforementioned clinical trial (3). Investigating the reasons for this by studying the compound’s effect on V2R-R137C and V2R-R137L at the cellular level will undoubtedly provide further important insights into the molecular mechanisms behind NSIAD and strategies for treatment.
We have been able to demonstrate that V2R-R137C and V2R-R137L, in addition to V2R-R137H, internalize in an agonist-independent manner that is both β-arrestin and dynamin dependent after trafficking to and expression at the plasma membrane. Evidence for this is provided by confocal microscopy using the C-terminally truncated receptors or coexpression of the dominant-negative dynamin, dynamin(K44A). Dynamin is a large GTPase that acts as a nanospring to pinch off clathrin-coated pits to form clathrin-coated vesicles (13). Dynamin(K44A) inhibits this process so that receptors interacting with β-arrestins are trapped at the plasma membrane within clathrin-coated pits (29).
Our findings indicate that all three mutant receptors are trafficked to the plasma membrane to some degree because coexpression of dynamin(K44A) or C-terminal truncation increased the expression of fluorescently labeled receptor at the plasma membrane. Furthermore, some ligand-dependent increases in BRET signal were observed in addition to the signals due to agonist-independent interactions, implying that at least a subpopulation of receptors were exposed to ligand at the plasma membrane for at least some of the time. However, both the BRET and the confocal microscopy results imply that initial trafficking to the membrane is considerably more efficient for V2R-R137C and V2R-R137L than for V2R-R137H. This is supported by the higher secondary peak in both BRET1 and BRET2 scanning spectrometry, the higher BRET ratio above wild-type baseline in both BRET1 and BRET2 dual-luminometry kinetic assays, and the stronger and more defined plasma membrane fluorescence observed with confocal microscopy in the presence of dynamin(K44A), whether monitoring receptor/Venus or β-arrestin/Venus. Previous studies have shown impairment of trafficking for V2R-R137H (21,22) and our results are consistent with these findings. Interestingly, however, mutation to cysteine or leucine (C or L) does not appear to impair trafficking. This indicates a major mutation-dependent difference in receptor functionality at the cellular level that may well reflect significant differences in receptor misfolding and consequent endoplasmic reticulum retention. Cysteine and leucine are both hydrophobic residues. In contrast, histidine is a polar residue with a neutral or positive charge depending on local environment. Therefore, at the biochemical level, it is perhaps unsurprising that these two different types of mutations both affect receptor structure, but in different ways.
Our results provide the first evidence of naturally occurring V2R mutations that lead to agonist-independent receptor internalization as well as constitutive activity. The constitutive signaling activity of the gain-of-function mutants V2R-R137C and V2R-R137L has been demonstrated previously (1). In light of our current findings, this appears to occur despite the receptors exhibiting agonist-independent internalization and thus probably being present at the cell surface for G protein coupling for only a limited time period.
Our BRET assays monitor the real-time interactions of populations of receptors and β-arrestins in tens of thousands of cells. Consequently, this reflects the steady state of multiple receptors and β-arrestins associating, dissociating, and potentially reassociating over time (30). With most GPCRs, internalization is followed by some degree of either rapid or slow recycling. Indeed, the concept that constitutively active receptors can be internalized in an agonist-independent manner and continuously recycled has been described previously, a good example being a mutant angiotensin II AT1A receptor (AT1AR): The constitutively active AT1AR mutant L305Q was largely localized in intracellular vesicles, although a nonnegligible fraction of the receptors were localized to the plasma membrane and were continuously replaced by recycling receptors after their internalization (31). This scenario does not appear to be as likely for V2R because there is evidence for this receptor being prevented from recycling (12,32,33) or at least being held in the perinuclear recycling compartment for extended time periods (33). It has been proposed that this has evolved due to the hypertonic and relatively acidic environment of the renal medulla. To function in this environment, it is thought that the ligand-receptor interaction needs to be abnormally resistant to low pH, exposure to which usually facilitates recycling by causing ligand dissociation in acidic endosomes (33). Interestingly, however, Bouley and co-workers (33) did identify a pool of receptors (equivalent to 20% of prestimulation levels of cell surface receptor) that either recycled or preexisted in the cytoplasm ready for plasma membrane insertion. Therefore, a low but significant level of recycling may be involved in presentation of the NSIAD phenotype. Alternatively, a process of receptor synthesis, trafficking, signaling, internalization, and degradation may occur, with V2Rs being replaced rather than recycled. Using BRET, we observed inhibition of the interaction between V2R wild type and β-arrestin 2 occurring rapidly upon addition of peptide V2R antagonist. Importantly, this inhibition increased gradually over time, consistent with V2Rs continually appearing at the plasma membrane, either because of recycling or replacement. Regardless of which is the true scenario, transient agonist-independent signaling of multiple mutant receptors before internalization may well be sufficient to generate the disease phenotype, analogous to a chronically dripping tap causing a sustained increase in water reabsorption and consequent antidiuresis.
Our findings, in addition to providing new insights into V2R-R137C and V2R-R137L function, also have implications for interpreting V2R-R137H functional data, both past and present. It was originally suggested by Barak et al. (20) that this mutant interacts with β-arrestin and is internalized in an agonist-independent manner, a conclusion confirmed by Bernier et al. (21) and our current study. However, the degree to which this agonist-independent internalization is responsible for the NDI disease phenotype remains controversial. Two publications by Bernier and co-workers (21,22) have highlighted that the majority of R137H mutant is misfolded and unable to reach the plasma membrane after synthesis. This is also supported by our findings. Importantly, however, it has been difficult to ascertain whether the subpopulation of functional V2R-R137H that is able to reach the plasma membrane would be sufficient to avoid the NDI phenotype if it was not internalized in an agonist-independent manner. Barak et al. (20) demonstrated some restoration of cAMP signaling with inhibition of β-arrestin interaction upon C-terminal truncation of V2R-R137H [V2R(R137H,T362)] or mutation to remove GPCR kinase phosphorylation sites in the C terminus [V2R(R137H,Ala6)]. However, this restored function to only about 17% of that achieved by wild-type receptors.
It would appear the basis of the theory that agonist-independent β-arrestin interaction and internalization plays the major role in perturbing V2R-R137H signaling needs to be reexamined as a result of our observations that the constitutively active V2R-R137C and V2R-R137L, responsible for the opposite disease phenotype of NSIAD, also interact with β-arrestins and internalize in an agonist-independent manner. Indeed, our findings are more in keeping with attributing the clinical NDI phenotype to impaired trafficking of V2R-R137H to the plasma membrane, as indicated subsequently (21,22). However, we feel it is also important not to discount impaired G protein coupling entirely, this being the original suggestion of Rosenthal et al. (19). Conceptually, it is hard to imagine that mutation of the arginine residue in the DRY motif, which is both incredibly well conserved across family A GPCRs and has consistently been shown to be involved in G protein coupling (16), does not affect the receptor-G-protein interaction in the V2R when substituted for histidine, particularly considering that it is capable of changing receptor conformation enough to facilitate β-arrestin binding and considering mutation to cysteine or leucine results in constitutive activity. Indeed, the elegant approach of using pharmacological chaperones to facilitate delivery of mutant V2Rs to the plasma membrane was notably ineffective at increasing AVP-stimulated cAMP accumulation with V2R-R137H compared with other missense and in-frame deletion mutants, achieving only about 7% of wild-type receptor response with SR49059 and about 3% of wild-type receptor response with YM087 (22).
In conclusion, we have further characterized V2R-R137C and V2R-R137L, mutant receptors previously identified as being constitutively active and responsible for the clinical condition termed NSIAD (1). We have now demonstrated that these mutations not only result in constitutive activity, but also agonist- independent β-arrestin binding and internalization, the first time such a combination of functional phenotypes has been observed with naturally occurring mutant V2Rs. Furthermore, our findings have important implications for interpreting results obtained with V2R-R137H, a mutant receptor causing the opposite disease phenotype of NDI.
Materials and Methods
Materials
AVP and [adamantaneacetyl1, O-Et-d-Tyr2, Val4, aminobutyryl6, Arg8,9]-vasopressin were from Sigma-Aldrich (Castle Hill, Australia). The β-arrestin 2/Venus construct was prepared from pC2-Venus kindly provided by Atsushi Miyawaki (RIKEN Brain Science Institute, Wako-city, Japan). A PCR product for the Venus coding sequence was substituted in-frame into pcDNA3-β-arrestin 2/Rluc replacing the Rluc cDNA as described previously (27). The β-arrestin 2/GFP10 construct was prepared from the vector pcDNA3.1/GFP10 kindly provided by Michel Bouvier (Department of Biochemistry, Université de Montréal, Canada). A PCR product for the GFP10 coding sequence was substituted in-frame into pcDNA3-β-arrestin 2/Venus replacing the Venus cDNA as described previously (27). Rluc8 cDNAs were amplified from the construct pcDNA3.1-Rluc8. This was kindly provided by Andreas Loening and Sanjiv Gambhir (Stanford University, Stanford, CA) (34). Human V2R wild type and its mutants V2R-R137C, V2R-R137L, and V2R-R137H cDNAs were amplified from the vectors carrying corresponding gene sequences with pcDNA3 as a backbone (1). Subsequently, PCR products for V2R wild type and mutants V2R-R137C, V2R-R137L, and V2R-R137H coding regions were substituted in-frame into pcDNA3-TRHR/Rluc8 described previously (27). The TRHR cDNA was replaced to generate V2R-wild type/Rluc8, V2R-R137C/Rluc8, V2R-R137L/Rluc8, and V2R-R137H/Rluc8, which were then also used for the cloning of the V2R-wild type/Venus, V2R-R137C/Venus, V2R-R137L/Venus, and V2R-R137H/Venus constructs. C-terminally truncated constructs of V2R wild type and the three mutants were amplified using PCR with nontruncated receptors as templates. These constructs truncated at codon 342 (V2R-trunc, V2R-R137C-trunc, V2R-R137L-trunc, and V2R-R137H-trunc) were subsequently subcloned into the previously generated constructs containing Rluc8 or Venus replacing the nontruncated receptors. Dynamin(K44A) cDNA was kindly provided by Marc Caron (Duke University Medical Center, Durham, NC). All construct sequences were confirmed by DNA sequencing at the Australian Genome Research Facility (Brisbane, Australia).
Cell culture and transfection
COS-7 and HEK293 cells were maintained at 37 C, 5% CO2 in complete medium (DMEM containing 0.3 mg/ml glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin) (GIBCO BRL, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS; GIBCO). Transient transfections were carried out 24 h after seeding using GeneJuice (Merck, Kilsyth, Australia) according to the manufacturer’s instructions.
Measurement of cAMP production
COS-7 cells were seeded in six-well plates at a density of 120,000 cells per well. At 24 h after transfection, cells were harvested in complete medium, added to a poly-l-lysine-coated 48-well plate and incubated at 37 C, 5% CO2. At 48 h after transfection, the cAMP production from cells stimulated with 1 μm AVP (treated sample) or vehicle (untreated sample) was measured using the cAMP AlphaScreen assay kit (PerkinElmer Life and Analytical Sciences, Waltham, MA) according to the manufacturer’s instructions. The AlphaScreen signal (counts per second) was measured in 384-well microplates on an EnVision Multilabel Plate Reader (PerkinElmer) and was used to calculate the concentration of cAMP using a standard curve.
Scanning spectrometry BRET assays
HEK293 cells were seeded in six-well plates at a density of 630,000 cells per well. At 24 h after transfection, cells were harvested without trypsin in HEPES-buffered phenol red-free complete medium containing 5% FCS and added to a poly-l-lysine-coated white 96-well plate (Nunc, Rochester, NY). Subsequently, the plate was incubated at 37 C, 5% CO2. At 48 h after transfection, medium in the plate was replaced with medium containing 1 μm AVP (treated sample) or vehicle (PBS; untreated sample). After about 1 h, emission spectral scans were performed with 5 μm coelenterazine h (for BRET1) or 10 μm DeepBlueC (for BRET2) using a Cary Eclipse spectrophotometer (Varian, Palo Alto, CA).
Real-time kinetic BRET assays
HEK293 cells were seeded in six-well plates at a density of 630,000 cells per well. At 24 h after transfection, cells were harvested in HEPES-buffered phenol red-free complete medium containing 5% FCS and added to a poly-l-lysine-coated white 96-well plate (Nunc) that was then incubated at 37 C, 5% CO2. Medium in the plate was replaced with PBS containing 5 μm coelenterazine h (for BRET1) or 10 μm DeepBlueC (for BRET2) and assays carried out immediately. BRET measurements were taken at 37 C using the VICTOR Light plate reader with Wallac 1420 software (PerkinElmer). Filtered light emissions were sequentially measured through both the donor wavelength window (370–450 nm for luciferase with DeepBlueC or 400–475 nm for luciferase with coelenterazine h) and acceptor wavelength window (500–525 nm for GFP10 or 520–540 nm for Venus). The BRET ratio above wild-type baseline was calculated as the ratio of emission through the acceptor wavelength window over emission through the donor wavelength window for each cell sample minus the same ratio for the vehicle-treated V2R-wild-type cell sample. This calculation is similar to the ligand-induced BRET ratio described previously (25,26), except that the background is determined using the vehicle-treated V2R-wild-type cell sample to observe agonist-independent BRET signals compared with V2R-wild type. The final pretreatment reading is presented at the zero time point (time of ligand/vehicle addition). When comparing truncated and nontruncated receptors, the BRET data are expressed as fluorescence/luminescence, meaning emission through the acceptor wavelength window over emission through the donor wavelength window, as described previously (27). These are not BRET ratios as normally defined because the background is not subtracted. This enables the differences between BRET data for truncated and nontruncated receptors to be evaluated directly.
Confocal microscopy
HEK293 cells were seeded on poly-l-lysine-coated coverslips in 12-well plates at a density of 250,000 cells per well. Treatments were carried out 48 h after transfection and cells fixed in 4% paraformaldehyde. Samples were coverslipped and sealed. Cells were examined using a confocal laser microscope (Bio-Rad, Hercules, CA) with an oil immersion ×60 objective (Nikon, Tokyo, Japan). Venus-tagged proteins were excited at 488 nm, and the emitted light was detected in the 500- to 550-nm range.
Acknowledgments
We thank Ruth Seeber and Matthew Dalrymple for critical reading of the article. The authors acknowledge the facilities, scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterization and Analysis, University of Western Australia, a facility funded by the University, State and Commonwealth Governments. We especially thank Paul Rigby for assistance with analysis of confocal microscopy images. We are grateful to Andreas Loening and Sanjiv Gambhir (Stanford University, Stanford, CA), Atsushi Miyawaki (RIKEN Brain Science Institute, Wako-city, Japan), Michel Bouvier (Department of Biochemistry, Université de Montréal, Canada), and Marc Caron (Duke University Medical Center, Durham, NC) for providing cDNA constructs.
Footnotes
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project Grant 404087). K.D.G.P. was supported by an NHMRC Peter Doherty Fellowship (353709). B.J.F. was supported by National Institutes of Health Grant DK073697.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 29, 2009
Abbreviations: AVP, Arginine vasopressin; BRET, bioluminescence resonance energy transfer; FCS, fetal calf serum; GPCR, G protein-coupled receptor; NDI, nephrogenic diabetes insipidus; NSIAD, nephrogenic syndrome of inappropriate antidiuresis; V2R, vasopressin type II receptor.
References
- Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE 2005 Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352:1884–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bes DF, Mendilaharzu H, Fenwick RG, Arrizurieta E 2007 Hyponatremia resulting from arginine vasopressin receptor 2 gene mutation. Pediatr Nephrol 22:463–466 [DOI] [PubMed] [Google Scholar]
- Decaux G, Vandergheynst F, Bouko Y, Parma J, Vassart G, Vilain C 2007 Nephrogenic syndrome of inappropriate antidiuresis in adults: high phenotypic variability in men and women from a large pedigree. J Am Soc Nephrol 18:606–612 [DOI] [PubMed] [Google Scholar]
- Marcialis MA, Faà V, Fanos V, Puddu M, Pintus MC, Cao A, Rosatelli MC 2008 Neonatal onset of nephrogenic syndrome of inappropriate antidiuresis. Pediatr Nephrol 23:2267–2271 [DOI] [PubMed] [Google Scholar]
- Soule S, Florkowski C, Potter H, Pattison D, Swan M, Hunt P, George P 2008 Intermittent severe, symptomatic hyponatraemia due to the nephrogenic syndrome of inappropriate antidiuresis. Ann Clin Biochem 45:520–523 [DOI] [PubMed] [Google Scholar]
- Zerbe R, Stropes L, Robertson G 1980 Vasopressin function in the syndrome of inappropriate antidiuresis. Annu Rev Med 31:315–327 [DOI] [PubMed] [Google Scholar]
- Rosenthal SM, Gitelman SE, Vargas GA, Feldman BJ 2007 Gain-of-function mutations in the V2 vasopressin receptor. Horm Res 67:121–125 [Google Scholar]
- Gitelman SE, Feldman BJ, Rosenthal SM 2006 Nephrogenic syndrome of inappropriate antidiuresis: a novel disorder in water balance in pediatric patients. Am J Med 119:S54–S58 [DOI] [PubMed] [Google Scholar]
- Robertson GL 2001 Antidiuretic hormone: normal and disordered function. Endocrinol Metab Clin North Am 30:671–694 [DOI] [PubMed] [Google Scholar]
- Verbalis JG 2003 Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab 17:471–503 [DOI] [PubMed] [Google Scholar]
- Schrier RW, Cadnapaphornchai MA 2003 Renal aquaporin water channels: from molecules to human disease. Prog Biophys Mol Biol 81:117–131 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG 1999 Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274:32248–32257 [DOI] [PubMed] [Google Scholar]
- Dromey JR, Pfleger KD 2008 G protein coupled receptors as drug targets: the role of β-arrestins. Endocr Metab Immune Disord Drug Targets 8:51–61 [DOI] [PubMed] [Google Scholar]
- Charest PG, Oligny-Longpré G, Bonin H, Azzi M, Bouvier M 2007 The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal 19:32–41 [DOI] [PubMed] [Google Scholar]
- Filipek S, Stenkamp RE, Teller DC, Palczewski K 2003 G protein-coupled receptor rhodopsin: a prospectus. Annu Rev Physiol 65:851–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR 2007 The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol 71:959–964 [DOI] [PubMed] [Google Scholar]
- Birnbaumer M 1995 Mutations and diseases of G protein coupled receptors. J Recept Signal Transduct Res 15:131–160 [DOI] [PubMed] [Google Scholar]
- Bichet DG 1998 Nephrogenic diabetes insipidus. Am J Med 105:431–442 [DOI] [PubMed] [Google Scholar]
- Rosenthal W, Antaramian A, Gilbert S, Birnbaumer M 1993 Nephrogenic diabetes insipidus. A V2 vasopressin receptor unable to stimulate adenylyl cyclase. J Biol Chem 268:13030–13033 [PubMed] [Google Scholar]
- Barak LS, Oakley RH, Laporte SA, Caron MG 2001 Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA 98:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier V, Lagacé M, Lonergan M, Arthus MF, Bichet DG, Bouvier M 2004 Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol 18:2074–2084 [DOI] [PubMed] [Google Scholar]
- Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperrière A, Brouard R, Bouvier M, Bichet DG 2006 Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol 17:232–243 [DOI] [PubMed] [Google Scholar]
- Wu P, Brand L 1994 Resonance energy transfer: methods and applications. Anal Biochem 218:1–13 [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Eidne KA 2006 Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat Methods 3:165–174 [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Seeber RM, Eidne KA 2006 Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat Protoc 1:337–345 [DOI] [PubMed] [Google Scholar]
- Pfleger KD, Dromey JR, Dalrymple MB, Lim EM, Thomas WG, Eidne KA 2006 Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein-protein interactions in live cells. Cell Signal 18:1664–1670 [DOI] [PubMed] [Google Scholar]
- Kocan M, See HB, Seeber RM, Eidne KA, Pfleger KD 2008 Demonstration of improvements to the bioluminescence resonance energy transfer (BRET) technology for the monitoring of G protein-coupled receptors in live cells. J Biomol Screen 13:888–898 [DOI] [PubMed] [Google Scholar]
- Morin D, Cotte N, Balestre MN, Mouillac B, Manning M, Breton C, Barberis C 1998 The D136A mutation of the V2 vasopressin receptor induces a constitutive activity which permits discrimination between antagonists with partial agonist and inverse agonist activities. FEBS Lett 441:470–475 [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock, DE, Schmid SL 1994 Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127:915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger KD, Dalrymple MB, Dromey JR, Eidne KA 2007 Monitoring interactions between G-protein-coupled receptors and β-arrestins. Biochem Soc Trans 35:764–766 [DOI] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Parnot C, Bardin S, Corvol P, Clauser E 2002 Constitutive internalization of constitutively active angiotensin II AT1A receptor mutants is blocked by inverse agonists. J Biol Chem 277:5891–5901 [DOI] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi HM, Tran NT, Birnbaumer M 1998 A serine cluster prevents recycling of the V2 vasopressin receptor. Proc Natl Acad Sci USA 95:2222–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley R, Lin HY, Raychowdhury MK, Marshansky V, Brown D, Ausiello DA 2005 Downregulation of the vasopressin type 2 receptor after vasopressin-induced internalization: involvement of a lysosomal degradation pathway. Am J Physiol Cell Physiol 288:C1390–C1401 [DOI] [PubMed] [Google Scholar]
- Loening AM, Fenn TD, Wu AM, Gambhir SS 2006 Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel 19:391–400 [DOI] [PubMed] [Google Scholar]