Abstract
We show here that IL-17-secreting CD4 T (Th)17 and CD8 T (Tc)17 effector cells are found in the lung following primary challenge with influenza A and that blocking Ab to IL-17 increases weight loss and reduces survival. Tc17 effectors can be generated in vitro using naive CD8 T cells from OT-I TCR-transgenic mice. T cell numbers expand 20-fold and a majority secretes IL-17, but little IFN-γ. Many of the IL-17-secreting cells also secrete TNF and some secrete IL-2. Tc17 are negative for granzyme B, perforin message, and cytolytic activity, in contrast to Tc1 effectors. Tc17 populations express message for orphan nuclear receptor γt and FoxP3, but are negative for T-bet and GATA-3 transcription factors. The FoxP3-positive, IL-17-secreting and IFN-γ-secreting cells represent three separate populations. The IFN-γ-, granzyme B-, FoxP3-positive cells and cells positive for IL-22 come mainly from memory cells and decrease in number when generated from CD44low rather than unselected CD8 T cells. Cells of this unique subset of CD8 effector T cells expand greatly after transfer to naive recipients following challenge and can protect them against lethal influenza infection. Tc17 protection is accompanied by greater neutrophil influx into the lung than in Tc1-injected mice, and the protection afforded by Tc17 effectors is less perforin but more IFN-γ dependent, implying that different mechanisms are involved.
Analysis of CD4 T cell lines showed that CD4 T cells could be separated into subsets termed Th1 and Th2 defined by the pattern of cytokines that they secreted (1) and the same subsets could be generated in vitro from normal CD4 T cells (2, 3). Corresponding subsets of CD8 T cells, Tc1 and Tc2, can also be made using similar polarizing cytokines in vitro (4, 5). Numerous studies demonstrated the significance of these two subsets in a large number of disease models. This dichotomy dominated thinking until recently, although some investigators showed that not all CD4 T cells fitted into these two categories. Kelso et al. (6) and others (e.g., 7, 8) have shown considerable heterogeneity in the cytokine profiles of individual cells and it can be argued that the division into the two alleged subsets is an artifact of the way they are prepared and that cytokine secretion profiles of in vivo-generated T cells represent a continuum of patterns. Nevertheless, certain patterns of cytokine secretion are strongly associated with functionally distinct subsets. Three new subsets of CD4 T cells have been recently characterized, regulatory T cells (9, 10), follicular helper T (11), and Th17 (12-14), and each has distinct requirements for generation and different functions.
The Th17 T cells represent a newly discovered subset of IL-17-secreting CD4 T cells (15-19) that has been implicated in exacerbating autoimmune responses (20, 21) and also participating in enhancing beneficial Th1 responses in tuberculosis (22, 23) rota virus (24), Klebsiella pneumoniae (25), and Candida albicans infections (26) and probably in responses to other infectious disease organisms. IL-17-producing T cells act in multiple ways including the induction of proinflammatory and hematopoietic cytokines (27) and in the recruitment of neutrophils (28).
The factors that control the generation of Th17 cells seem to be complex and are not yet completely understood. Th17 cells can be generated in vitro when naive murine CD4 T cells are stimulated in the presence of TGF-β and IL-6 (29, 30). IL-21 also stimulates Th17 development and is also secreted by Th17 cells (31), constituting a positive feedback loop. Th17 cells express IL-23 receptors and IL-23 has been shown to support the expansion and survival of already established Th17 cells (23, 32, 33). IL-23 has also been implicated in the induction of IL-22 in Th17 cells (34). IL-15 is another cytokine that has been shown to enhance the Th17 response (35) while IL-27 is a negative regulator of Th17 activity through the induction of IL-10 (36-39). IL-35 (40), made up of the EBV-induced gene 3 (EBI3) and the p35 subunit of IL-12, has also been shown to be suppressive of Th17 activity. Retinoic acid (RA)3 antagonizes Th17 development and the inclusion of RA inhibitors can enhance the generation of Th17 in vitro when dendritic cells from RA-secreting mesenteric lymph node cells are used as APCs (41).
Th17 populations of cells have been shown to express CCR6 on the cell surface (42) and respond to CCL20. Th17 cells express the orphan nuclear receptor γt (RORγt) which directs their differentiation program (43). FoxP3 is commonly thought to be primarily associated with regulatory T cells (44) but may also be expressed in Th17 cells (Ref. 45 and D. R. Littman, personal comment). The development of human Th17 appeared to differ from that in the mouse in that it does not require the presence of TGF-β (46) but this is now in dispute (47). It seems likely that there must be some redundancy in the factors that can lead to the induction of this subset and that more than one combination of cytokines may be able to cause the differentiation of CD4 T cells to Th17 effectors.
Th17 cells coexpress IL-17A, IL-17F, and IL-22 (48, 49) and also make IL-21 (31). These cytokines, acting singly or together, can induce a wide range of cytokines (50), a wide range of inflammatory mediators, including defensins (51), antimicrobial peptides, metalloproteinases (52), C reactive protein, cytokines, and chemokines, many of which are chemotactic for neutrophils. At least some of these effects are mediated via an Act1-dependent pathway (53) and at least some of these effects are brought about by message stabilization (54).
We had previously shown (5, 55) that it was possible to generate CD8 T cell subsets, Tc1 and Tc2, analogous to Th1 and Th2 (56) and showed that they had distinctive properties in vivo, both in response to tumors (57, 58) and to influenza (59, 60).
We have investigated, here, whether there was a corresponding subset of IL-17-secreting CD8 T cells with similar and contrasting characteristics to the Th17 subset of CD4 T cells. We show, for the first time, that IL-17-secreting CD4 and CD8 effector cells can be detected in the lung in response to challenge with influenza A. We refer to the IL-17-secreting CD8 T cells as Tc17 effectors following the convention that designates IL-17-secreting CD4 T cells as Th17. Injection of neutralizing Ab to IL-17 diminishes the protection resulting from priming with heterosubtypic influenza virus. We find that a population containing IL-17-secreting CD8 T cells effectors can be generated in vitro by 4-day culture of naive CD8 T cells from OT-I TCR-transgenic mice in the presence of IL-6, TGF-β, IL-21, and Abs to IFN-γ. The naive cell numbers expand up to 20-fold in culture, under these conditions, and a majority of the resulting cells secrete IL-17 upon restimulation. The majority of the IL-17-secreting effector cell population also secrete TNF and IL-2 but, in marked contrast to Tc1 effectors, they contain very few cells that secrete IFN-γ or granzyme B or express message for perforin and they exhibit no cytolytic activity, again in marked contrast to Tc1 or Tc2 effectors. RNA from populations containing a high proportion of Tc17 cells express message for IL-2, IL-10, IL-17A, IL-17F, and TGF-β but are negative for IL-4 and IFN-γ message. Tc17 prepared from unpurified CD8 T cells are positive for IL-22 message but those made from purified naive CD8 T cells make very little. RNA from Tc17 cells derived from naive CD8 populations express message for orphan nuclear receptor γt and FoxP3, but are negative for T-bet and GATA-3.
Preliminary studies suggest that Tc17 and IL-17 play a substantial though ill-defined role in vivo since populations of both Tc1 and Tc17 effectors from OT-1 mice can protect naive recipients against lethal infection with SIINFEKL-bearing influenza virus. Tc17 and Tc1 populations appear to protect by different mechanisms. Tc17 protection appears to be independent of perforin expression while Tc1 protection is perforin dependent. For IFN-γ, the situation is the reverse. Tc17 protection is less effective with effectors from IFN-γ-deficient mice, while Tc1 protection is unaffected. Tc17 protection is accompanied by an early enhanced influx of neutrophils into the lung.
Materials and Methods
Mice
OT-1 TCR-transgenic B6, B6.OT-1.Thy1.1, B6.OT-1.CD45.1, B6.OT-1.IFN-γ-/-, B6.OT-1.perforin-/-, P14 TCR-transgenic B6, C57BL/6 (Thy1.2), and C57BL/6.PL (B6.Thy1.1) mice were bred at the Trudeau Institute and were used at 3–22 wk of age. All animal procedures were approved by the Institute Animal Care and Use Committee the Trudeau Institute.
Influenza virus, infections
Influenza A/Puerto Rico (A/PR8) (H1N1) and A/PR8ova1 (provided by Dr. R. Webby) came from St. Jude Children’s Research Hospital (Memphis, TN). Cold-adapted A/Alaska/6/77 (H3N2) was a gift from Dr. S. Epstein (National Institutes of Health, Bethesda, MD). Viruses were grown in the allantoic cavity of embryonated hen eggs from virus stocks. Lightly anesthetized mice were infected with influenza by intranasal (i.n.) inoculation of 50 μl of virus in PBS. These viruses were used with the following doses: 2 × 104 EID50 (4 LD50) for lethal challenge or 1 × 103 EID50 (0.2 LD50) for sublethal challenge of A/PR8, 970 PFU (∼2 LD50) for lethal challenge or 145 PFU (∼0.3 LD50) for sublethal challenge of A/PR8ova1, and 2500 TCID50 for the priming dose of cold-adapted A/Alaska. These doses are based on the original titrations conducted at the time the batch was produced. The titers drift down with time but the doses cited are based on the original determination and thus should be considered nominal.
Measurement of the in vivo cellular response
B6.Thy1.1 mice were sacrificed at various time points before and after influenza infection, and spleen inguinal lymph nodes (ILN) were taken. Bronchoalveolar lavage (BAL) was collected by washing the airways five times with 0.5 ml of PBS. Lungs were removed following perfusion with 5 ml of PBS via the left ventricle of the heart and single-cell suspensions were prepared by collagenase treatment (2.5 mg/ml collagenase D). Finally, mediastinal lymph nodes (MedLN) were collected. Single-cell suspensions (5 × 105) were cultured in 24-well plate for 4 h with 10 ng/ml PMA, 500 ng/ml ionomycin, and 10 μg/ml brefeldin A or with 5 μg/ml NP366–374 peptide (ASNENMETM) for CD8 T cells, 5 μg/ml NP261–275 peptide (RSALILRGSVAHKSC) for CD4 T cells, and 10 μg/ml brefeldin A. Genetically different spleen cells (5 × 105) from naive B6.Thy1.2 mice were added as APCs for peptide stimulation. Cells were harvested and cell surface markers were stained with the following Abs: CD3-PE-Cy7 (145-2C11; eBioscience), CD4-FITC (RM4-4; BD Biosciences), CD8-allophy-cocyanin-Alexa Fluor750 (53-6.7, eBioscience), CD90.1-PerCP (OX-7; BD Biosciences), CD90.2-biotin (53-2.1; BD Biosciences), and γδ-TCR-biotin (GL3; BD Biosciences). Biotinylated Abs were counterstained with streptavidin-Pacific Orange (8 μg/ml; Invitrogen) and then fixed with 4% formalin. Cells were then incubated with Abs to intracellular cytokines, IFN-γ-Pacific Blue (XMG1.2; eBioscience), IL-17-PE (TC11-18H10; BD Biosciences), and granzyme B-allophycocyanin (GB11; Caltag Laboratories) in 0.1% saponin buffer. Cells were analyzed on the FACSCanto II (BD Biosciences). Cells were gated on CD3+, CD90.1+, CD90.2-, and γδ- and their staining profiles were analyzed using FlowJo (Tree Star).
ELISPOT assays
IL-17-secreting cells were assayed by ELISPOT. Five million of MACS-purified CD8 T cells from nonimmunized OT-1.Thy1.1 were transferred to B6 recipient mice (Thy1.2) i.v. on day -1. On day 0, mice were challenged with 2 LD50 of influenza A/PR8ova1. On day 6, cells from spleen and lung were treated with anti-Thy1.2 mAb (clone HO134) and complement to deplete host T cells. The number of IL-17-secreting cells in 8 × 105 of the remaining cells was determined after a 4-h stimulation with SIINFEKL peptide or PMA and ionomycin in a standard ELISPOT assay. The lymphocytic choriomeningitis virus (LCMV) glycoprotein gp33 (KAVYNFATM) was used as the negative control.
Anti-IL-17-blocking experiment
Mice were treated with four i.p. injections of 0.1 mg of anti-IL-17 Ab (clone 50104; R&D Systems) or four i.p. injections of 0.1 mg of isotype control IgG2b (clone LTF-2; R&D Systems) on days -1, 1, 3, and 5 after influenza challenge.
Generation of Tc17, Tc1, Tc2, and Tc0 CD8 effector cells in vitro
Tc1, Tc2, and Tc0 effectors were generated as previously described (59). T cell-depleted APCs (B cell blasts) were prepared by negative selection on MACS columns using FITC-labeled anti-Thy1.2 mAb (53-2.1; eBioscience) and anti-FITC-MACS beads (Miltenyi Biotec). The B cells were stimulated with LPS (25 μg/ml) and dextran sulfate (DXS; 25 μg/ml) for 3 days and were used as APCs. They were loaded with SIINFEKL peptide (10 μg/ml) or control LCMV gp33 peptide KAVTNFATM (10 μg/ml) at 37°C for 30 min, treated with mitomycin C (50 μg/ml) at 37°C for 30 min, and washed three times before use. CD8 T cells from spleens of OT-1 TCR-transgenic mice or P14 TCR-transgenic mice which were enriched by CD8 MACS beads (Miltenyi Biotec) and incubated with SIINFEKL or KAVYNFATM peptide-pulsed B cell blasts (T:B = 1:3) for 4 days. For Tc17 cultures, IL-2 (4.7 ng/ml), IL-1β (10 ng/ml; PeproTech), IL-6 (20 ng/ml; PeproTech), porcine TGF-β (3 ng/ml; R&D Systems), IL-21 (80 ng/ml; PeproTech), IL-23 (50 ng/ml; R&D Systems), anti-IL-4 mAb (11B11, 10 μg/ml), and anti-IFN-γ mAb (XMG1.2, 10 μg/ml) were added. For some experiments, TNF-α (10 ng/ml; PeproTech), anti-TNF-α mAb (MP6-XT22,10 μg/ml), and anti-IL-2 mAb (S4B6, 10 μg/ml) were added. For Tc1 cultures, IL-2 (4.7 ng/ml), IL-12 (9.2 U/ml, provided by S. Wolf, Genetics Institute, Cambridge, MA), and anti-IL-4 mAb (11B11, 10 μg/ml) were added. For Tc2 cultures, IL-2 (4.7 ng/ml), IL-4 (200 U/ml, X63.Ag.IL-4 supernatant), and anti-IFN-γ mAb (XMG1.2, 10 μg/ml) were added. For Tc0 cultures, IL-2 (4.7 ng/ml) was added. On day 2, the same amount of fresh medium containing IL-2 (4.7 ng/ml) was added. In some experiments, the RA inhibitor LE540 (Wako Pure Chemical) at 1 mM was added on day 0. Tc17 effector cells from naive CD8 T cells (nTc17) were prepared from naive CD44lowCD62Lhigh CD8 T cells obtained by FACS sorting.
Phenotype of Tc effectors
Tc effectors were prepared as described above. Cell suspensions were stained with the following Abs for cell surface analysis: CD8-PerCP (53-6.7; BD Biosciences), CD25-FITC (7D4; BD Biosciences), and CD44-allophycocyanin-Alexa Fluor 750 (IM7; eBioscience), CD62L-Pacific Blue (MEL-14; eBioscience), CD69-PE (H1.2F3; BD Biosciences), and CD90.1-PerCP. For intracellular cytokine staining, single Tc effector cell suspensions were cultured for 4 h with 10 ng/ml PMA, 500 ng/ml ionomycin, and 10 μg/ml brefeldin A. Cells were harvested and incubated with Ab to cell surface markers. Cells were then fixed with 4% formalin and incubated with Abs to intracellular cytokines: IFN-γ-Pacific Blue, IL-2-FITC (JES6-5H4; eBioscience), IL-17-PE, TNF-α-PE-Cy7 (MP6-XT22; BD Biosciences), IL-10-Alexa Fluor 647 (JES5-16E3; eBioscience), IL-17-Pacific Blue (TC11-18H10; BioLegend), or granzyme B-PE (GB11; Caltag Laboratories) in 0.1% saponin buffer. For FoxP3 and intracellular cytokine costaining, cells were also cultured for 4 h with PMA/ionomycin and brefeldin A. Cells were harvested and incubated with Ab to cell surface markers and then fixed with Fixation/Permeabilization solution (BD Biosciences) for 10 min. Cells were then washed with 1× Perm/Wash buffer (BD Biosciences) and then permeabilized with 1× Perm/Wash buffer with 0.05% Triton X-100 for 10 min. Then cells were incubated with Abs to cytokines and FoxP3 in 1× Perm/Wash buffer with 0.05% Triton X-100, FoxP3-PE (FJK-16s; eBioscience), IL-17-Pacific Blue, and IFN-γ-FITC (XMG1.2; BD Biosciences). Cells were analyzed on the CyAN LX9 laser flow cytometer (DakoCytomation). The staining profiles were analyzed using FlowJo.
Cytokine and chemokine message
RNAs were extracted using a RNeasy Mini kit (Qiagen) and treated with RNase-Free DNase (Qiagen) according to the manufacturer’s protocol. cDNAs were synthesized from 2 to 5 μg of total RNA using 100 U of Superscript II Reverse Transcriptase (Invitrogen) and 125 ng of random primer (Invitrogen). Quantitative PCR was performed using TaqMan Universal PCR Master Mix on the Applied Biosystems Prism 7700 sequence detection system. Primers and probes to IL-17F (Mm00521423_m1), IL-22 (Mm00444241_m1), RORγt (Rorc, Mm00441139_m1), and perforin (Prf1, Mm00812512_m1) were purchased from Applied Biosystems. Dr. S. Smiley and P. S. Adams designed the primers and probes for GAPDH, IL-2, IL-4, IL-10, IL-17, IFN-γ, TGF-β, T-bet, GATA-3, and FoxP3 (Trudeau Institute, Saranac Lake, NY). Levels were expressed as fold in comparison to levels for GAPDH.
Cytolytic activity
The cytolytic activity of the in vitro-generated OT-1 Tc1 and OT-1 Tc17 effector populations was measured against peptide pulsed EL-4 targets using the JAM assay (61). In brief, CD8 effectors were washed and plated at decreasing concentrations in 96-well round-bottom plates. EL-4 targets, labeled for 4 h with 4 μCi/ml [3H]thymidine (Amersham Biosciences) and pulsed for 4 h with peptide, were added to effectors at a concentration of 1 × 104 cells/well. SIINFEKL peptide, or irrelevant peptide (KAVYNFATM) as a control, was added at final concentration of 2 μg/ml. After 4 h at 37°C, plates were harvested, and incorporated [3H]thymidine was measured using a beta plate scintillation counter (Wallac). The percentage of specific cytotoxicity was calculated as ((spontaneous cpm - experimental cpm)/spontaneous cpm) × 100.
Adoptive transfers, lethal infection, weight changes and survival
C57BL/6 mice were injected i.v. with 4, 8, or 16 × 106 of Tc17 or Tc1, made from OT-I wild type, IFN-γ-/- or perforin-/- mice, or P14 Tc17 effector cells on day -1 and challenged on day 0 with an i.n. lethal dose of 2 LD50 influenza A/PR8ova1 virus or 3 LD50 influenza A/PR8 virus. Mice were weighed every second day and weight was expressed as percentage of initial. A cohort of mice was followed up to day 28 after challenge to determine percent survival. Control mice received Tc17 effectors from P14 mice.
Adoptive transfers, sublethal infection, and cellular response
B6.Thy1.1 mice were injected i.v. with 2 × 106 of OT-1.CD45.1 Tc17 effector cells on day -1 and challenged on day 0 with an i.n. sublethal dose of 0.3 LD50 influenza A/PR8ova1 virus. Mice were weighed every second day and weight was expressed as percentage of initial. Mice were sacrificed at various time points before and after influenza infection, and spleen, lung, MedLN, BAL, and ILN were taken as described. Single-cell suspensions (5 × 105) were cultured in 24-well plate for 4 h with 10 ng/ml PMA, 500 ng/ml ionomycin, and 10 μg/ml brefeldin A or with 5 μg/ml OVA peptide (SIINFEKL) and 10 μg/ml brefeldin A. Genetically different spleen cells (5 × 105) from naive B6 mice were added as APCs. Cells were harvested and cell surface markers were stained with the following Abs: CD8-allo-phycocyanin-Alexa Fluor 750, CD45.1-FITC (A20; eBioscience), CD45.2-biotin (104; BD Biosciences), CD90.1-PerCP and CD90.2-PE-Cy7 (53-2.1; eBioscience), CD11b-allophycocyanin-Alexa Fluor 750 (M1/70; eBioscience), CD11c-PE-Cy7 (N418; eBioscience), Gr-1-PerCP-Cy5.5 (RB6-8C5; BD Biosciences), F4/80-Pacific Blue (BM8; eBioscience), anti-neutrophils-FITC (7/4; Serotec), I-Ab-PE (AF6-120.1; BD Biosciences). Biotinylated Abs were counterstained with streptavidin-Pacific Orange and then fixed with 4% formalin. Cells were then incubated with Abs to intracellular cytokines, IFN-γ-Pacific Blue, IL-17-Alexa Fluor 647 (TC11-18H10; BioLegend), and granzyme B-PE in 0.1% saponin buffer. Cells were analyzed on the FACSCanto II. Transferred donor cells were gated on CD45.1+, CD45.2-, CD90.1-, CD90.2+, CD8+, and neutrophils were gated on Gr-1high, 7/4high, CD11b+, F4/80-, and I-Ab- and their staining profiles were analyzed using FlowJo.
Results
IL-17-secreting CD8 T cells are detected in primary responses to influenza A
C57BL/6 mice were challenged with a sublethal dose of A/PR8 influenza virus. Groups of mice (n = 3) were sacrificed just before challenge (day 0) and on days 2, 4, 8, 12, 22, and 34 after challenge. Spleen, lung, MedLN, ILN, and BAL cell suspensions were prepared and analyzed for intracellular IL-17 and IFN-γ after restimulation with PMA and ionomycin or NP peptides as described in Materials and Methods and counterstained with anti-CD4 and anti-CD8.
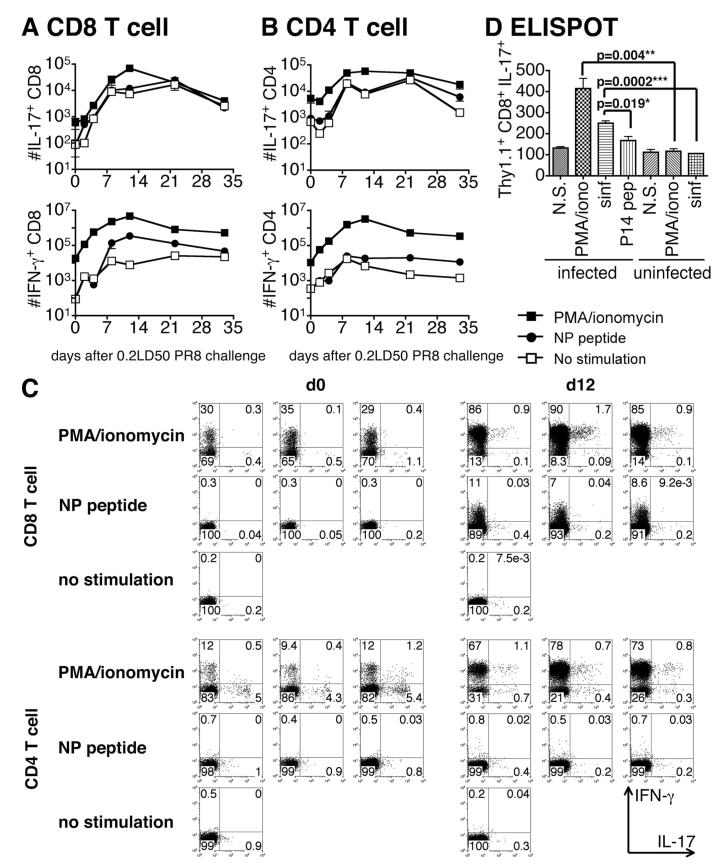
The number of IL-17-producing CD8 (Fig. 1A) and CD4 T cell (Fig. 1B) in the lung of the infected mice after restimulation with PMA and ionomycin was slightly increased at day 2 after infection and rose almost 100-fold to a peak at day 12 for IL-17+ CD8 T cells and at days 8–12 for IL-17+ CD4 T cells. The number of IL-17+ CD8 T cells, at 60,000, on day 12 after infection was slightly higher than that of CD4 T cells. The number of IL-17+ CD4 T cells was still 30% of that seen at the peak at day 33, but only 6% of IL-17+ CD8 T cells still remained. Representative flow data are shown in Fig. 1C for days 0 and 12 but similar data were collected for all time points.
FIGURE 1.
IL-17-secreting CD8 T cells in the influenza A-infected mouse. A and B, B6.Thy1.1 mice were infected with 0.2 LD50 influenza A/PR8. Mice were sacrificed at the times indicated, before and following challenge, and cells suspensions were prepared from lung and restimulated with PMA/ionomycin (■) or NP peptides (○) for 4 h or left unstimulated (□). Naive spleen cells from B6 mice were added as APCs. After restimulation, cells were stained with Abs to intracellular IL-17 (top) and IFN-γ (bottom) and surface CD8 (A) and CD4 (B). To exclude other type of cells, we gated on CD3+CD90.1+CD90.2-γδ- cells. Representative flow data are shown in C for days 0 and 12. D, Naive C57BL/6 mice were injected with 106 naive SIINFEKL/Kb-specific CD8 T cells from OT-1 mice carrying the Thy1.1 allele and were exposed to ∼2 LD50 of influenza A/PR8ova1. Uninfected mice served as a control. Mice were sacrificed on day 6 and cell suspensions were prepared from lung and spleen. Recipient T cells were removed by treatment with anti-Thy1.2 and complement and 8 × 105 of the resulting cells, derived from the donor CD8 T cells and host non-T cells, were plated per well in an ELISPOT assay. Cells were left unstimulated (N.S.) or restimulated with PMA and ionomycin (PMA/iono) to assay all IL-17-secreting cells, or the albumin-derived SIINFEKL peptide (sinf) to assay IL-17-secreting OT-1 CD8 T cells, or the P14 peptide of LCMV (P14 pep) as the unrelated control.
In comparison, the number of IFN-γ+CD8 (Fig. 1A) and CD4 (Fig. 1B) T cells in the lung after restimulation with PMA/ionomycin or NP peptides peaked on day 8–12 and was much greater that that seen for IL-17. Most of the IL-17+ CD8 T cells at day 2 after infection were IL-17+IFN-γ-, but 40∼80% of IL-17+ CD8 T cells were IL-17+IFN-γ+ by day 8 (Table I).
Table I.
The number of T cells and IFN-γ+IL-17+ producing cells (DP) in lunga
| Days after Infection |
Total (×10-5) |
Total CD8 (×10-4) |
DP CD8 (×10-2) |
Total CD4 (×10-4) |
DP CD4 (×10-2) |
|---|---|---|---|---|---|
| 0 | 18.7 ± 1.4 | 7.5 ± 0.7 | 1.6 ± 0.6 | 10.5 ± 2.1 | 7.5 ± 4.5 |
| 2 | 73.5 ± 9.7 | 28 ± 1.8 | 2.3 ± 1.4 | 33.0 ± 0.8 | 6.5 ± 1.2 |
| 4 | 162 ± 33.7 | 69 ± 19.1 | 11.6 ± 3.2 | 49.1 ± 11.9 | 15.3 ± 4.3 |
| 8 | 161 ± 17.6 | 248 ± 39.0 | 221 ± 85.7 | 154 ± 34.2 | 383 ± 74.8 |
| 12 | 230 ± 52.7 | 401 ± 32.9 | 639 ± 174 | 273 ± 11.3 | 386 ± 47.7 |
| 22 | 98.5 ± 19.1 | 112 ± 26.6 | 85.5 ± 42.7 | 99.9 ± 24.2 | 109 ± 47.4 |
This table provides the actual number of total CD8 and CD4 cells at each time point for the data portrayed in Fig. 1. It also lists the number of CD8 and CD4 cells that were positive for both IL-17 and IFN-γ by restimulation of PMA/ionomycin for 4 h. For further details of the experiment, see legend to Fig. 1.
A similar pattern of CD8 and CD4 of IL-17-producing cells was also seen in the MedLN and BAL but the responses in the spleen and ILN were less marked (data not shown).
It was of interest that a considerably higher percentage of CD4 cells with the potential to make IL-17 was present before infection than was the case for CD8 and this is reflected in the higher percentage seen at day 0 in Fig. 1C. It can be seen, in Table I, that the number of total CD4 increased greatly during the response, while the number of IL-17-secreting CD4 cells increased <10-fold (Fig. 1B) and as a consequence the percentage of CD4 cells (Fig. 1C) that had the potential to make IL-17 actually fell. In contrast, the number of CD8 IL-17-secreting cells at day 0 started much lower than for CD4 and increased 100-fold, while the total number rose <15-fold and the percentage of cells making IL-17 rose. In the early phase of the response, most IL-17-secreting cells are IFN-γ negative, but double producers emerge as the response proceeds. The number of double producers is indicated in Table I.
The number of IL-17-producing CD4 and CD8 T cell in the lung after restimulation with influenza NP peptides, as judged by intracellular cytokine staining, was almost the same as that without stimulation. This could be taken to indicate that cells specific for these peptides were not a major component of the response.
To confirm the generation of Ag-specific Tc17 cells, we used ELISPOT assays to measure cytokine secretion. CD8 T cells from OT-1.Thy1.1 mice were injected into C57BL/6.Thy1.2 mice that were then given i.n. 2 LD50 of A/PR8ova1, carrying the SIINFEKL OVA peptide insert in the neuraminidase stalk. Lungs were harvested at day 6 and the cell suspension was treated with anti-Thy1.2 and complement to remove host T cells. ELISPOT assays were performed on 8 × 105 of the resulting population (depleted of host T cells) which were stimulated with SIINFEKL peptide (sinf) or PMA/ionomycin as shown in Fig. 1D. Cells from lung stimulated with PMA and ionomycin (donor CD8 and host non- T cells) or with OVA peptide SIINFEKL (donor CD8 only) gave 400 or 250 IL-17-secreting cells per 8 × 105 cells assayed, respectively. Cells that were not stimulated in vitro, or stimulated with an unrelated LCMV peptide, or stimulated cells from uninfected mice gave lower numbers of spots. Parallel assays on cells from the spleen showed only background levels of secretion (data not shown). Values of p values for the differences between stimulation with specific and nonspecific peptide and for cells from infected and control mice are indicated in Fig. 1C, and it can be seen that the response to the A/PR8ova1 influenza virus includes a statistically significant increase in Ag-stimulated IL-17-secreting Tc17 cells in the infected lung.
Injection of Ab to IL-17 reduces heterosubtypic protection to influenza A
We sought next to determine whether IL-17 secretion played a role in the protection against influenza. C57BL/6 mice were primed with 2500 TCID50 of cold-adapted A/Alaska6/77 on day -35 and were challenged with 4 LD50 of A/PR8 on day 0. Injections of depleting Ab to IL-17 or isotype control were performed 1 day before and 2, 3, and 5 days after challenge. One group of five mice was treated with four injections of 0.1 mg of anti-IL-17 Ab. A second group of five mice was treated with four injections of 0.1 mg of isotype control IgG2b. A third control group was not primed and all died upon challenge. It can be seen in Fig. 2, experiment 1, that four of five of the isotype control-injected mice were protected from lethal challenge, lost only 20% weight, and started to regain weight by day 8. The anti-IL-17 Ab-treated group suffered severe weight loss and only one of five survived, and mice in this group did not start to regain weight until after day 10. In a second experiment (Fig. 2, experiment 2) with 2-fold lower viral challenge, all of the mice survived in both groups but weight loss was significantly increased in the anti-IL-17 Ab-injected group from days 9 through 12 (p = 0.066, day 8; 0.033, day 9; 0.029, day 10; 0.022, day 11; 0.039, day 12; and 0.30, day 14).
FIGURE 2.
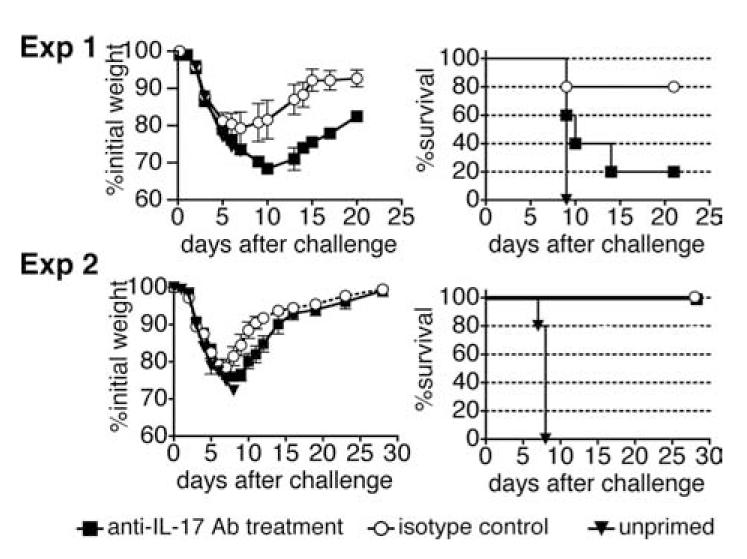
Ab to IL-17 reduces heterosubtypic protection. In experiment 1, C57BL/6 mice were primed with 2500 TCID50 of cold-adapted A/Alaska6/77 on day -35 and were challenged with 4 LD50 of A/PR8 on day 0. Injections of depleting Ab or isotype control were given 1 day before and 1, 3, and 5 days after challenge. One group of five mice was treated with four injections of 0.1 mg of anti-IL-17 Ab. A second group of five was treated with four injections of 0.1 mg of isotype control IgG2b. A third control group was not primed. A second experiment of the same design but with 2-fold lower challenge dose is shown in experiment 2.
IL-17-secreting CD8 T cells can be generated in vitro
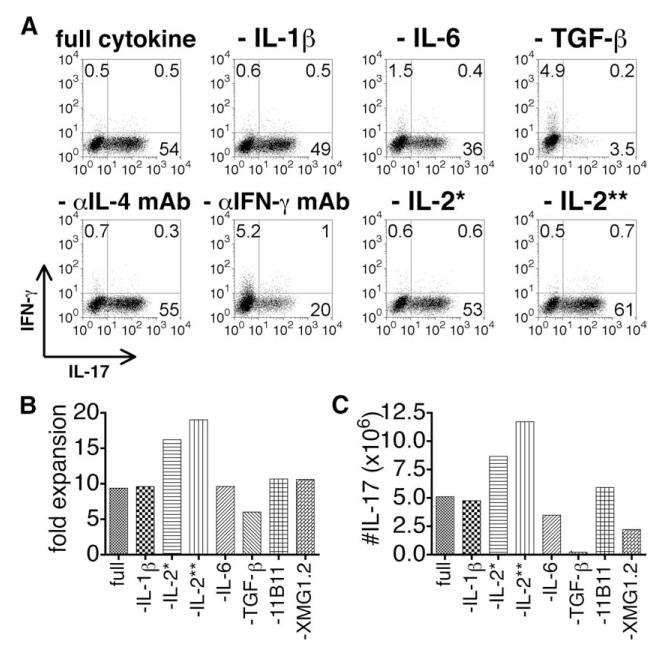
The conditions for the generation of Th17 in vitro are well documented but we sought here to determine what cytokines might be involved in the generation of Tc17 effectors. We tested a variety of combinations to establish the optimal conditions for the generation of IL-17-secreting CD8 T cells. In brief, 106 CD8 T cells from OT-1 mice, prepared by positive selection, were cultured with (the OVA-derived peptide) SIINFEKL-pulsed B blasts (one CD8 cell to three B cells) obtained by 3-day culture in LPS and DXS and the following cytokines: IL-2, IL-1β, IL-6, IL-21, IL-23, and TGF-β. Abs to IL-4 and IFN-γ were also added. Cultures were fed with additional medium containing IL-2 on day 2. It can be seen (Fig. 3A) that the naive cells proliferated extensively under each of these conditions and expanded up to 15-fold (left-hand panels). IL-21 was critical for the generation of Tc17 cells. The inclusion of IL-23 in addition to IL-21 only marginally increased the yield of Tc17 cells. The maximum percentage (Fig. 3A, right-hand panels) and maximum numbers (Fig. 3A, middle panels) of IL-17-secreting cells were seen when IL-21 and IL-23 were both present in the culture medium. The addition of TNF or anti-TNF had no effect on the recovery of Tc17 cells (data not shown).
FIGURE 3.
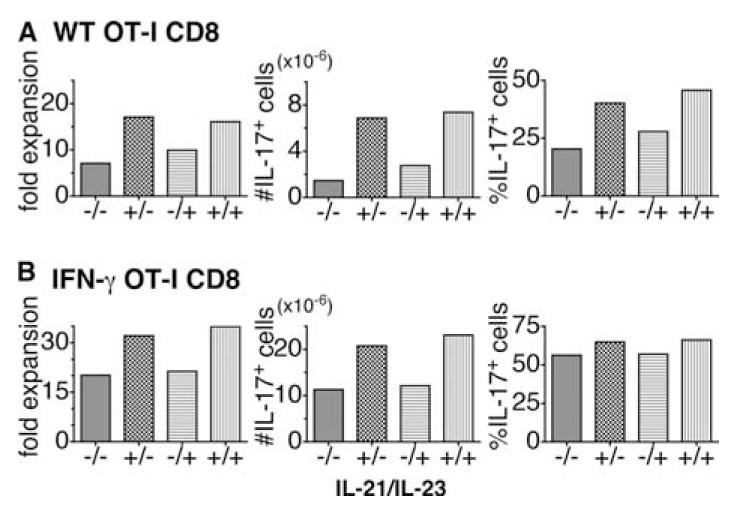
Generation of Tc17 effectors by in vitro culture. CD8 T cells were prepared from OT-1 (A) or OT-1.IFN-γ-/- mice (B), as described in Materials and Methods. In brief, 106 CD8 T cells from OT-1 mice were cultured for 4 days with (the OVA-derived peptide) SIINFEKL (10 μg/ml) pulsed onto mitomycin C-treated B blasts (one CD8 cell to three B cells) obtained by 3-day culture of T-depleted spleen cells in LPS and DXS. The following cytokines were added to the culture medium: IL-2 (4.7 ng/ml), IL-1β (10 ng/ml), IL-6 (20 ng/ml), and TGF-β (3 ng/ml). Abs to IL-4 (10 μg/ml) and IFN-γ (10 μg/ml) were also added. IL-21 (80 ng/ml) and IL-23 (50 ng/ml) were not added or were added singly or together in various combinations as indicated by plusses and minuses for IL-21/IL-23. Cultures were fed with additional medium containing IL-2 on day 2. Cells were harvested at day 4 and the fold increase (left-hand panel), the percent IL-17-secreting cells determined by intracellular cytokine staining (right-hand panel), and the calculated number of IL-17-secreting cells (middle panel). WT, Wild type.
IFN-γ added to naive CD4 T cells suppressed the generation of Th17; therefore, we tested the potential inhibitory role of IFN-γ on the generation of Tc17 cells by comparing the response of OT-1 cells from wild-type (Fig. 3A) with that from IFN-γ deficient mice (Fig. 3B). It can be seen that the yields and numbers of IL-17-positive cells were almost double in Tc17 populations derived from IFN-γ-deficient mice compared with wild-type mice. However, when we compared Tc17 from wild-type-sorted naive CD8 T cells (which secrete less IFN-γ than Tc17 generated from unselected CD8 cells (see Fig. 5B) with Tc17 from naive IFN-γ-deficient CD8 T cells, their fold expansion and ratio of IL-17-producing cells were almost the same (data not shown). The characteristics of Tc17 generated from TNF-deficient or perforin-deficient mice were no different from those generated from wild-type mice (data not shown). In a separate experiment, we found that the addition of LE540, a RA inhibitor, did not affect the percentage and number of IL-17-secreting cells (data not shown).
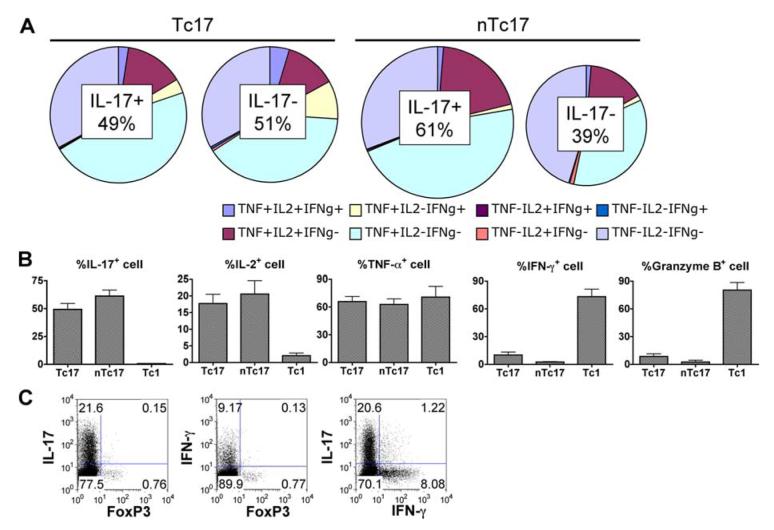
FIGURE 5.
Cytokine secretion pattern, granzyme B expression, and FoxP3 expression of Tc17 effectors. In vitro-generated Tc17 from unselected and from CD44lowCD62Lhigh selected CD8 and Tc1 effectors were prepared as described in the legend to Fig. 3. The effectors harvested at day 4 were stimulated with PMA and ionomycin for 4 h and stained with a panel of Abs for intracellular cytokines, including IL-2, IL-17, IFN-γ, and TNF, and the results are shown in the pie diagrams (A). The graphs in B summarize the salient features of the staining of Tc17 compared with Tc1 and show also staining for granzyme B. Double staining for IL-17 and FoxP3, IFN-γ and FoxP3, and IL-17 and IFN-γ is shown in C.
The optimal T:B ratio for generating IL-17-producing CD8 T cells was determined to be one CD8 T cell to three B blasts (data not shown). We also titrated the optimal concentration of peptide for pulsing B blasts and the highest numbers of IL-17-producing cells were induced when B blasts were pulsed with 10 μg/ml peptide (data not shown).
To determine whether each cytokine is required to induce IL-17-producing CD8 T cells, Tc17 effectors were prepared using protocols that removed one of each cytokine or Ab from the full cytokine and Ab mixture (Fig. 4). IL-6, TGF-β, and anti-IFN-γ Ab and, to a less extent, IL-1β were critical for generation of Tc17 cells. Removal of anti-IL-4 Ab was without effect. It should be pointed out that the “B blasts” used as APC are also a source of several factors including IL-1 and IL-6; therefore, omission of these factors from the mixture did not eliminate their presence from the medium. IL-17-producing cells were enhanced in the absence of IL-2 (Fig. 4B). To determine whether these minimum critical reagents were sufficient for Tc17 generation, Tc17 were prepared with IL-6, TGF-β, IL-21, and anti-IFN-γ. The Tc17 prepared with the minimum set of reagents were identical to Tc17 prepared when the full set of reagents was added, as assessed by cytokine production, mRNA expression, and protective activity against lethal influenza virus challenge (data not shown). In agreement with the lack of requirement for IL-2, IL-17-producing CD8 T cells were enhanced by IL-2 blocking (data not shown).
FIGURE 4.
Determination of critical cytokines for Tc17 induction. Tc17 effectors were prepared using protocols as in Fig. 3 or with removal of each cytokine or Ab from the full cytokine and Ab mixture (IL-1β, IL-2, IL-6, IL-21, IL-23, TGF-β, anti-IL-4, and anti-IFN-γ). Cultures were fed with additional medium containing IL-2 on day 2 except IL-2 (**), which were not fed. Cells were harvested at day 4 and the number of cells and the number of IL-17-secreting cells was determined by intracellular cytokine staining (A). The fold increase is shown in B and the calculated number of IL-17-secreting cells is shown in C. One asterisk (*) indicates that this group had no IL-2 at the beginning of incubation, but fed only on day 2, and two asterisks (**) indicates no IL-2 throughout the entire experiment.
To determine whether contaminating memory cells might influence polarization, Tc17 effector cells generated from unselected CD8 T cells (Tc17) were compared with Tc17 effector cells from naive CD44lowCD62Lhigh CD8 T cells obtained by fluorescent-activated cell sorting (designated nTc17). We found that Tc17 effector cells derived from naive cells expanded more than those from unselected cells (average 21.0-fold compared with 13.4-fold; data not shown). Sorted naive CD8 cells also gave rise to higher percentages of IL-17-secreting cells (61.3%) than unselected CD8 (49.3%) as shown in Fig. 5B and higher yields were also seen when the naive CD8 T cells used were obtained from younger mice (data not shown).
The Tc17 cells generated using peptide-pulsed B blasts, IL-2, IL-1β, IL-6, IL-21, IL-2,3 and TGF-β and Abs to IL-4 and IFN-γ were stained with a panel of fluorescence-labeled Abs to determine their cell surface phenotype. It can be seen that the Tc17 population was similar to that of Tc1, Tc2, and Tc0 cells generated in parallel in that they were CD69highCD44highCD25high and had the same forward scatter and side scatter (data not shown). CD62L expression on Tc17 cells was very low and CD8α expression was repeatedly seen to be somewhat down-regulated (data not shown). All live cells were CD90+ and there were no CD19+ cells present, indicating that the mitomycin-treated B cells used as APCs were gone by the time of harvest (data not shown). Thus, despite dramatic differences in function, the cell surface phenotype differed little among CD8 subsets.
Cytokines secreted, granzyme B expression, and FoxP3 expression of Tc17 CD8 effector populations compared with other subsets
In vitro-generated Tc17 and Tc1 effectors from wild-type OT-1 mice, prepared as above, were stimulated with PMA and ionomycin for 4 h and stained with a panel of Abs for intracellular cytokines. The pie diagrams in Fig. 5A show the cytokine secretion patterns of IL-17+ and IL-17- cells in Tc17 effectors generated from unselected CD8 populations “Tc17” or selected naive CD8 “nTc17.” It can be seen (Fig. 5A, Tc17) that 49.3% of the cells prepared from unselected CD8 T cells made IL-17 while 51% did not. The percentage of IL-17+ cells was somewhat higher (at 61%) when cells were generated from naive CD8 (nTc17). In either case, many of the IL-17+ or IL-17- cells also made TNF-α and a smaller percentage also made IL-2. Very few cells were positive for IFN-γ secretion and IFN-γ production in the Tc17 cells prepared from unselected CD8 T cells (10%) was higher than that in Tc17 made from naive CD8 (2.6%). Only a very small fraction of the Tc17 effector population was positive for granzyme B in contrast to populations of Tc1 effectors in which >75% were positive. Tc17 effector cells from naive CD44lowCD62Lhigh CD8 T cells (nTc17) contained more IL-17-secreting (61.3%) and fewer IFN-γ (2.6%)- and granzyme B-secreting (0.8%) cells (Fig. 5, A and B). Unstimulated controls were negative for all cytokines, except that measurable amounts of IL-17 were secreted without restimulation (data not shown). Thus, Tc17 effectors exhibited a unique pattern of cytokine secretion and granzyme B expression that differed from that of Tc1.
In additional experiments, we stained the Tc17 populations for IL-10 and IL-17 and found that the majority of IL-10-secreting cells were IL-17 negative but that a significant number of cells were double producers (data not shown).
Tc17 effectors prepared from IFN-γ-deficient OT-1, TNF-deficient OT-1, or perforin-deficient OT-1 mice exhibited very similar cytokine profiles upon stimulation but were completely negative for IFN-γ, TNF, or perforin, respectively, as expected (data not shown).
A very small FoxP3-positive fraction was also detected in Tc17 effector populations (0.88%) from unselected CD8, but this fraction did not coproduce either IL-17 or IFN-γ (Fig. 5C). As was true for IFN-γ and granzyme B expression, Tc17 effectors from sorted naive CD8 T cells contained lower levels of FoxP3 expression (0.35%; data not shown).
Cytokine and transcription factor message profiles of Tc17 CD8 effector populations
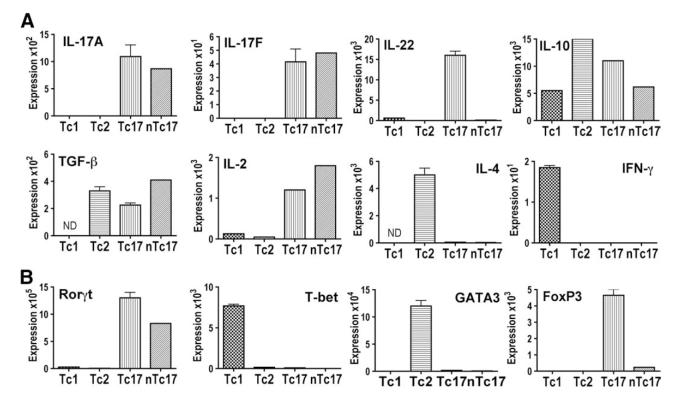
To further evaluate the unique properties of Tc17 effectors, we prepared populations of cells containing predominantly Tc17 (from unselected CD8 T cells), nTc17 (from sorted naive CD8 T cells), and Tc1 or Tc2 CD8 effectors prepared as before. The cells were harvested at day 4 and RNA was extracted from the effector populations and assayed for message expression by TaqMan PCR. Message expression was compared with GAPDH. It can be seen in Fig. 6A that the Tc17 population of effectors was positive for cytokines IL-2, IL-17A, IL-17F, IL-22, IL-10, and TGF-β. It was negative for IL-4 and IFN-γ. Tc1 effector populations were positive for IFN-γ and Tc2 were positive for IL-4, as expected. These same effector populations were assayed for transcription factor message. It can be seen in Fig. 6B that Tc1 effector populations were positive for T-bet and Tc2 for GATA-3 and both Tc1 and Tc2 were negative for RORγt and for FoxP3. Tc17 effector populations were negative for T-bet and GATA-3 but were positive for both RORγt and for FoxP3. Interestingly, when we prepared Tc17 from naive CD8 T cells (nTc17), the expression of IL-22 and FoxP3 both disappeared, suggesting that effectors expressing these characteristics were derived from the CD44high memory cells in the unselected CD8 population.
FIGURE 6.
Cytokine and transcription factor message profiles of Tc17 CD8 effector populations. Tc1, Tc2, and Tc17 effector cells from unselected CD8 T cells (Tc17) and from naive CD44lowCD62Lhigh CD8 T cells (nTc17) were prepared as in Fig. 3. The cells were harvested at day 4 and RNA was extracted from the effector populations and assayed for message expression by TaqMan PCR (see Materials and Methods). Message expression was compared with GAPDH. A, Cytokine message for IL-2, IL-4, IL-17, IL-17F, IL-22, IL-10, IFN-γ, and TGF-β. B, Transcription factor message for RORγt, T-bet, GATA-3, and FoxP3.
It should be pointed out that an absence of message provided evidence that cells expressing that message were not present. The presence of message showed that cells expressing that message were present but cannot reveal what fraction of the cells expressed message or whether the same cell or different cells expressed each of the messages identified.
Tc17 effectors lack both perforin message expression and cytolytic activity
Tc1 and Tc17 effector-containing populations were also assayed for expression of perforin mRNA and for cytolytic activity in the JAM assay. It can be seen in Fig. 7A that the Tc17 populations did not express perforin message in comparison to Tc1. Tc1 (■ and □), Tc17 (● and ○), or IFN-γ-deficient Tc17 (◆ and ◇ effectors were incubated with tritium-labeled EL-4 targets that were pulsed with SIINFEKL peptide (Fig. 7A, ■, ●, and ◆)or P14 peptide (□, ○, and diao]). It can be seen in Fig. 7B that Tc17 effectors had less than one hundredth of the lytic activity seen with Tc1 effectors and that the lytic activity of Tc17 cells against specific peptide-pulsed targets was almost the same as that against control targets.
FIGURE 7.
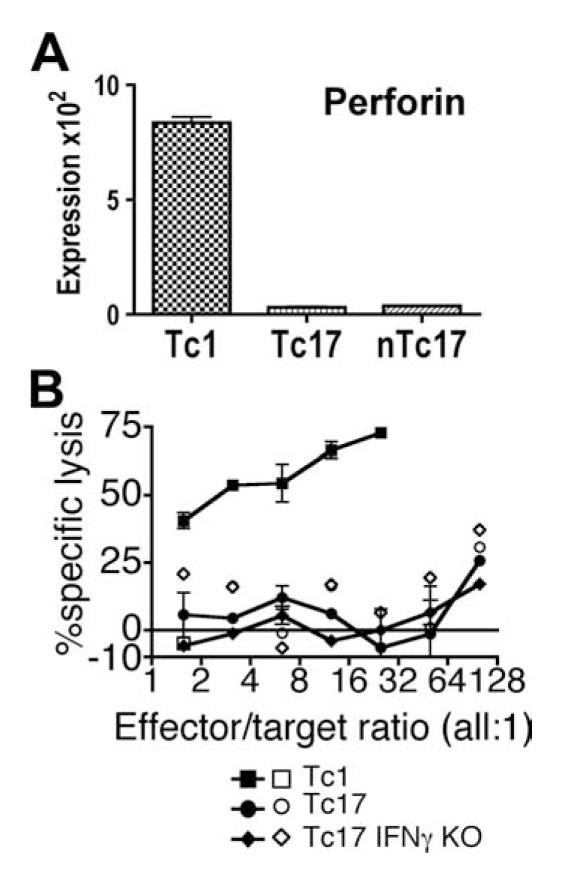
Perforin message expression and cytolytic activity of Tc17 CD8 effector populations. Tc1 and Tc17 effectors from unselected CD8 T cells (Tc17) and from naive CD44lowCD62Lhigh CD8 T cell (nTc17) populations prepared as in Fig. 3 were also assayed for perforin message expression (A). B, Cytolytic activity of OT-1 Tc1 (■ and □), OT-1 Tc17 (● and ○), and IFN-γ-deficient OT-1 Tc17 (◆ and ◇) effectors against specific SIINFEKL peptide-pulsed EL-4 (■, ●, and ◆) and nonspecific KAVYNFATM peptide-pulsed EL-4 (□, ○, and ◇) were assessed in the JAM assay, as described in Materials and Methods.
Injection of Tc17 effectors protects naive mice from lethal influenza A challenge
We next looked to see whether the Tc17 effector populations had any function in vivo. The various CD4 T cell subsets have been shown to have differing biological functions and a similar diversity has been seen between Tc1 and Tc2, which we have shown can reject tumors by different mechanisms (58, 59, 62). We had also shown that Tc1 and Tc2 are both cytolytic and that adoptive transfer of either the OVA-specific effector population can protect against influenza infection using A/PR8 (60).
Tc17 and Tc1 effectors were generated from naive CD8 T cells obtained from OT-1 mice. Four, 8, or 16 million effector cells were injected into naive C57BL/6 recipients that were challenged 1 day later with ∼2 LD50 of influenza A/PR8ova1. It can be seen (Fig. 8) that both Tc17 and Tc1 effectors brought about a reversal in weight loss (Fig. 8A). Four of five of the mice injected with 4 million Tc17 effectors survived, in contrast to uninjected mice that continued to lose weight and all died. Four million of Tc1 effectors were almost as protective as Tc17, protecting three of five mice (Fig. 8B). Eight million of either Tc17 or Tc1 effector cells gave complete protection against lethal influenza virus. Four million Tc17 effectors generated from OT-1 mice were not able to protect mice that were challenged with 3 LD50 of influenza A/PR8. Neither 16 million naive OT-I CD8 T cells nor 4 million Tc17 effectors generated from P14-transgenic mice protected mice that were challenged with ∼2 LD50 of influenza A/PR8ova1 (data not shown).
FIGURE 8.
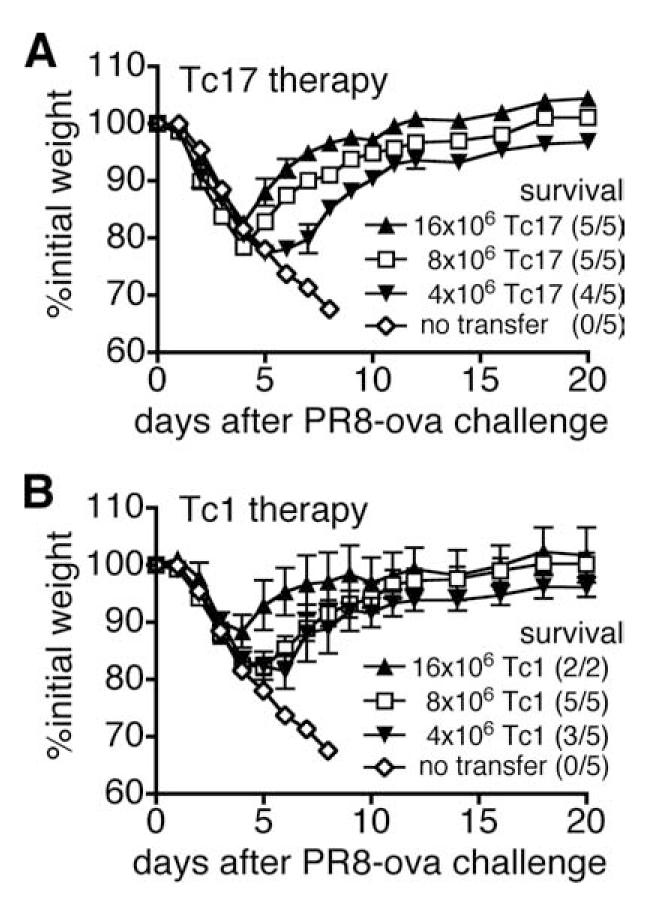
Tc17 effectors provide protection from lethal challenge with influenza A. Tc1 and Tc17 effector populations were prepared and 4, 8, or 16 × 106 cells of Tc17 (A) or Tc1 (B) were injected into naive C57BL/6 recipients that were challenged 1 day later with ∼2 LD50 A/PR8ova1. Control mice received no effector cells. Weight changes of mice were expressed as the percentage of initial weight.
The protective effect of Tc17 effectors is relatively independent of perforin compared with that of Tc1 effectors
Tc1 and Tc17 effectors were prepared from CD8 T cells from wild-type OT-1, OT-1.perforin-/-, and OT-1.IFN-γ-/- mice. Four or 8 × 106 cells were injected into naive mice that were then challenged with 2 LD50 A/PR8ova1. It can be seen in Fig. 9 that uninjected control mice all lost weight and died. Mice injected with wild-type Tc17 (Fig. 9B, upper part, or wild-type Tc1, lower half, recovered weight early. All survived in mice injected with 8 × 106 wild-type Tc17 effector cells, while survival was only 70% when injected with only 4 × 106 wild-type Tc17 effector cells. Mice protected with 8 × 106 Tc17 effector cells from perforin-deficient donors survived as well as those injected with effector cells from wild-type mice. Mice injected with only 4 × 106 perforin-deficient Tc17 cells lost slightly more weight and only 50% survived. Mice injected with comparable numbers of Tc17 effectors from IFN-γ-/- mice were slightly less well protected with 70% survival in mice receiving 8 × 106 effectors and only 20% in mice receiving 4 × 106 cells.
FIGURE 9.
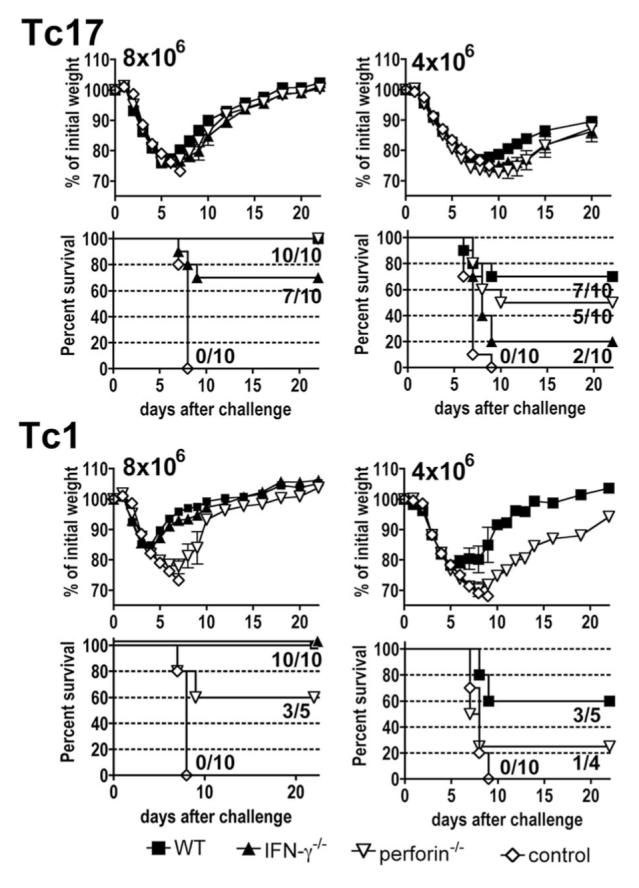
Effect of IFN-γ and perforin deficiencies on Tc17- and Tc1-mediated protection against influenza A. Tc1 and Tc17 effector populations from wild-type (WT), IFN-γ-/-, or perforin-/- were prepared and 4 or 8 × 106 cells of Tc17 or Tc1 were injected into naive C57BL/6 recipients that were challenged 1 day later with ∼2 LD50 A/PR8ova1. Control mice received no effector cells. Weight changes of mice were expressed as the percentage of initial weight and percent survival is shown. The values inside the survival graphs indicate the number of surviving mice/the number of total mice.
In marked contrast, although wild-type Tc1 effectors protected as well as comparable numbers of Tc17, the protective effect was much more dependent on the ability to express perforin, with only 60% survival after transfer of 8 × 106 Tc1 cells and 25% survival with transfer of 4 × 106 perforin-deficient Tc1 cells. Protection was independent of IFN-γ at 8 × 106 cells and was not tested at 4 × 106 cells. We conclude that Tc17 and Tc1 effectors protected by different mechanisms.
The numbers of adoptively transferred Tc17 effectors expand considerably in the lungs and lymphoid organs of recipient mice following sublethal challenge
To follow the response of the Tc17 cells, we transferred 2 × 106 Tc17 effector T cells into naive recipients that were given a sublethal i.n. challenge of 0.3 LD50 A/PR8ova1 1 day later and the numbers of IL-17-secreting cells followed for 17 days. It can be seen in Fig. 10 that the number of cells in all locations expanded ∼100-fold peaking at day 8 and then declined again to near the initial value by day 17. Highest numbers were seen in the lung, with 5 million at day 8, followed by 10-fold lower numbers in the spleen and lymph nodes and the lowest numbers were seen in the BAL. Donor cells remained IL-17+IFN-γ- at the initial time points but most became double producers by day 7 (data not shown).
FIGURE 10.
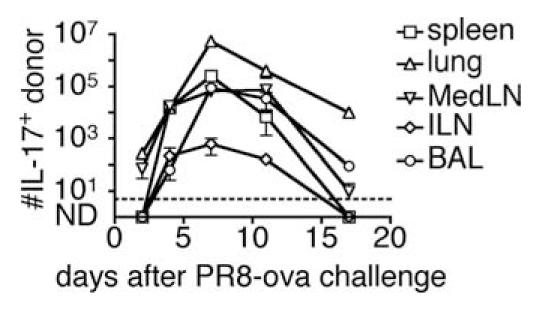
The number of transferred Tc17 after influenza A infection. Tc17 effectors from OT-1.CD45.1 were prepared and 2 × 106 of Tc17 were i.v. injected into naive B6.Thy1.1 recipients. One day after Tc17 injection, B6.Thy1.1 mice were i.n. infected with ∼0.3 LD50 of influenza A/PR8oval. Mice were sacrificed at the times indicated following challenge, and cells suspensions were prepared from spleen (□), lung (△), MedLN (▽), ILN (◇), and BAL (○) and restimulated with PMA/ionomycin. After restimulation, cells were stained with Abs to intracellular IL-17 and surface CD8. The analysis was gated on CD45.1+CD45.2-CD90.1-CD90.2+ CD8 T cells to exclude other cell types.
Protection conferred by Tc17 effectors correlated with the recruitment of significantly greater numbers of neutrophils shortly after challenge
Tc17 are known for their ability to induce an inflammatory reaction. We therefore examined whether the mice protected with Tc17 showed any evidence of increased neutrophil recruitment into the lung. Mice were injected with 4 × 106 of Tc1 or Tc17 effectors and challenged with 0.3 LD50 A/PR8oval. Mice were sacrificed at the time shown and cell suspensions were prepared from the perfused lungs and the numbers of neutrophils were determined. It can be seen in Fig. 11 that there was an earlier increase in the number of neutrophils in the Tc17-injected mice that was statistically greater than that seen in uninjected control mice at day 2 and higher than both Tc1 and control mice by day 5. Neutrophil recruitment into the lungs of control mice was delayed but was eventually higher than in both the Tc1- and Tc17-injected mice by days 8 and 15.
FIGURE 11.
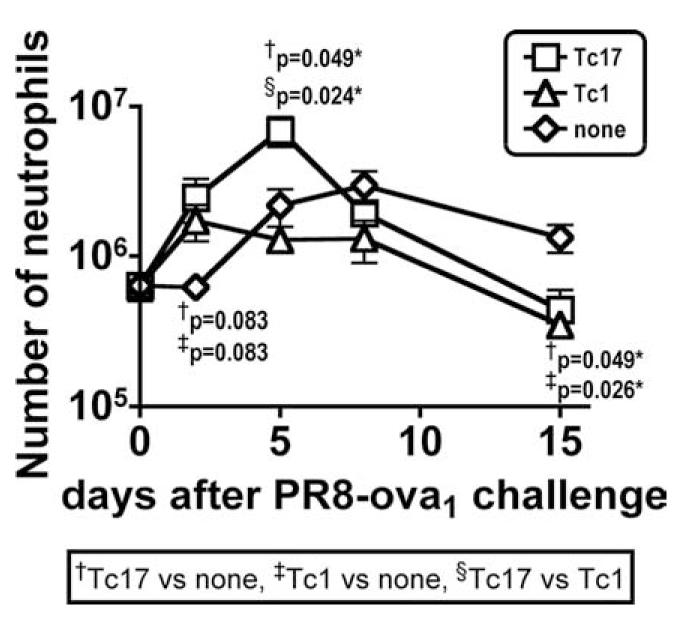
The number of lung neutrophils in protected and control mice after influenza A infection. Tc17 effectors from OT-1.CD45.1 were prepared and 4 × 106 of Tc17 (□) or Tc1 (△) were i.v. injected into naive B6.Thy1.1 recipients. Control mice were not injected (◇). One day after Tc17 or Tc1 injection, B6.Thy1.1 mice were i.n. infected with ∼0.3 LD50 of influenza A/PR8oval. Mice were sacrificed at the times indicated following challenge, and cells suspensions were prepared from lung. Neutrophils were gated on Gr-1high7/4highCD11b+F4/80-I-Ab-.
Discussion
Th17 cells have been implicated in the response to a number of pathogens and we show here that both CD4 and CD8 IL-17-secreting cells can be detected in the lungs of mice responding to influenza A infection. Somewhat higher levels of IL-17-secreting cells were seen in the secondary response to influenza (data not shown).
We speculate that Tc17 cells represent highly differentiated effector cells that are recruited to the lung during a pulmonary infection.
The injection of blocking Abs to IL-17 enhanced weight loss and diminished survival in response to lethal heterosubtypic challenge, suggesting that IL-17 plays some role in protection. The blocking of IL-17A does not block IL-17F or IL-22 and it may be that these factors can make up for the lack of IL-17A.
IL-17-secreting CD8 effector T cells can be generated in vitro using similar strategies to those used to generate Th17 effectors. In critical experiments, we used cell-sorted naive CD44low populations as our starting cell population, but in other experiments using unselected CD8 there were only modest differences in the resulting population. Tc17 prepared from naive cells had higher levels of IL-17-secreting cells and lower levels of IFN-γ and granzyme B. They were devoid of message for IL-22, which we presume must be derived from effectors derived from memory cells. We used a basic mixture of cytokines modeled after studies with CD4 T cells, including IL-1β, IL-6, and TGF-β with additional IL-2 and Abs to IL-4 and IFN-γ, two cytokines that had been shown to be inhibitory to the development of Th17. In later studies, we omitted IL-2 and added Ab to IL-2 which improved yields. The inclusion of IL-21 appeared to be crucial and enhanced proliferation and the development of the capacity to secrete IL-17 upon restimulation. The addition of IL-23, either in the presence or absence, of IL-21, led to only a modest improvement in yield and differentiation.
RA has been shown to antagonize the effect of TGF-β in the generation of Th17 effectors (41). In our studies here, we found that the addition of the RA inhibitor LE540 had relatively little effect (data not shown). Dendritic cells from MedLN (used in the Mucida study (41)) have been shown to secrete RA (63) and it seems likely that the lack of effect in our studies is due to the fact that we used LPS-stimulated B cells for Ag presentation, which we presume secrete no RA. IFN-γ and IL-4 inhibit Th17 generation by directing development toward Th1 or Th2 effectors (12) and we found that the yield of Tc17 effectors was significantly increased when effectors were generated using CD8 T cells from IFN-γ-deficient rather than wild-type OT-1 mice when effectors were prepared from unselected CD8 cells. In other experiments (data not shown), we found that the T cells had already differentiated into IL-17-producing cells in the first 2 days of incubation and their messenger RNA expression was almost the same as day 4 Tc17 effector cells. The number of Tc17 effector cells expanded linearly through days 2–5 with a similar ratio fraction of IL-17-secreting cells.
The cell surface phenotype of the Tc17 effector population was very similar to that of Tc1 and Tc2 effectors. CD25, CD44, and CD69 were all up-regulated. CD62L was down-regulated as in Tc1 and Tc2 but expression was unusually low. We have previously shown that only the most highly differentiated, CD62Llow CD4 (64) or CD8 (65) effectors migrate to the lung and the finding here supports the idea that this is also true for Tc17 cells.
Several features of effector function of the Tc17 population emerged from the analysis of intracellular cytokine staining and by PCR assays of effector cell RNA. Fifty to 70% of the in vitro-generated effector population secreted IL-17 upon restimulation as judged by intracellular cytokine staining. A large fraction of the IL-17-secreting cells also secreted TNF and a smaller fraction also secreted IL-2. It was striking that there were very few cells in the IL-17-positive or negative effector population that secreted IFN-γ or stained for granzyme B. Only 2–5% of the IL-17-positive cells were also positive for IFN-γ. This appears to be a consequence of the special conditions used for in vitro generation and was not characteristic of IL-17-secreting cells in vivo. The IL-17-negative population also secreted TNF but still only a few cells were positive for IFN-γ. Tc17 effector cells from sorted naive CD44low CD62Lhigh CD8 T cells (nTc17) had fewer IFN-γ and granzyme B- and FoxP3-positive cells than Tc17 effector cells from unselected CD8 T cells (Tc17), and we conclude that these cells maybe come from memory CD8 (CD44high) T cells.
CD8 T cells of this Tc17 phenotype are unique in that they are able to produce IL-17A and IL-17F and lack perforin and granzyme B expression and have no cytolytic activity. The coexpression of TNF is probably part of the same phenotype, but how far TGF-β and IL-2 secretion are linked or come from cells of a separate subset is not clear. FoxP3 and IL-10 appeared to be made by IL-17-negative cells. Th17 have been shown to secrete IL-22. We found, however, that although Tc17 effectors from unselected CD8 T cells had a significant amount of message for IL-22, Tc17 effector cells made from naive CD8 T cells were negative. This result suggests that IL-22 detected in Tc17 effector populations also comes from effectors derived from CD44high memory cells. A similar observation has been made for CD4 T cells by Kreymborg et al. (34) who showed that IL-22 was induced by IL-23 but only in the CD62Llow population.
One can make a Venn diagram of overlapping circles, one for each cytokine and define a considerable number of cytokine secretion patterns but it is questionable that these should be considered discrete subsets.
The presence of IL-17-negative cells in the effector population could be taken as evidence that the population was incompletely polarized. Arguing against contamination, at least with Tc1, was the finding that almost none of the cells secreted IFN-γ or stained for granzyme B. The effector population also lacked any detectable cytolytic activity, also arguing against contamination. An absence of cytolytic activity in IL-17-secreting CD8 T cells was also observed by Liu et al. (66) who termed them Tnc17 cells (for non-cytolytic). It is of interest that stimulation of naive CD8 T cells under rather different conditions (in the presence of IFN-γ and absence of IL-6 and TGF-β) led to the development of strong cytolytic activity (67).
The PCR analyses showed that the Tc17 effector population contained message for IL-17A and IL-17F, as expected, but was negative for perforin message, again in contrast with Tc1. The Tc17 effector RNA was negative for IL-4 message, arguing against contamination with Tc2 but showed some message level for IL-10 and TGF-β, raising the possibility of contamination with some sort of CD8 equivalent of regulatory T cells or contaminating CD4 T cells. However, Tc17 effector cells from FACS-sorted naive CD8 T cells still showed some message level for IL-10 and TGF-β, and this did not come from CD4 T cells. This was further supported by the presence of message for FoxP3 in addition to RORγt while the absence of message for T-bet and GATA-3 reenforced that concept that there Tc1 and Tc2 were both absent. Alternatively, it is possible that T cell subsets should be regarded more as a continuum rather than solely as a limited number of discrete subsets and we are inclined to the latter belief.
We have made a preliminary investigation of the role of Tc17 cells in vivo. The fact that they can be seen in the lungs of flu-infected mice is consistent with that they do play some role. In a second model, we transferred Tc17 or Tc1 effectors from OT-1 mice into naive recipient mice that were then challenged with ∼2 LD50 of influenza A/PR8ova1. The Tc17 effectors reversed weight loss and increased survival after what would otherwise have been a lethal challenge. Tc17 effectors prepared from perforin-deficient mice were equally as effective as those prepared from wild-type mice, while Tc1 effectors from perforin-/- mice were less effective. Tc17 effectors, however, were somewhat less effective if made from IFN-γ-/- mice while Tc1 effectors were unchanged. Tc17 effectors in vivo are double producers, making IL-17 and IFN at later stages in the response, as noted in the description of Figs. 1 and 10, and it would appear that this IFN can play some role in the protection afforded by Tc17 effectors. The results indicate that Tc17 and Tc1 effectors protect by different mechanisms. The Tc17 effectors were able to protect the recipient mice from lethal challenge despite the fact that they lacked any cytolytic activity, perforin message, or granzyme B expression in vitro. The Tc17 were as potent as equivalent numbers of Tc1 effectors and it is thus unlikely that a small contaminating subset of other cells was responsible for the protection. Cytolytic destruction of infected epithelial cells by CD8 T cells is generally considered to play a key role in the control of influenza virus and we did not exclude the possibility that the Tc17 effectors developed cytolytic activity after injection, although the protective ability of Tc17 prepared from perforin-deficient mice argues against this. Tc17 effectors generated from sorted naive cells also protected against lethal infection and the protection was Ag specific. Tc17 effector cells from OT-1 mice did not protect against lethal influenza using A/PR8 (without the SIINFEKL insert) and the Tc17 effector cells from P14 TCR-transgenic mice did not protect lethal influenza A/PR8ova1-infected mice (data not shown).
The protection afforded by transfer of Tc17 effectors was accompanied by an increased influx of neutrophils into the lung, suggesting one mechanism of protection.
Tc17 effector and memory cells can make several potent cytokines upon restimulation including IL-17A, IL-17F, IL-21, TNF, and IL-22 and several chemokines including CCL2, CXCL9, CXCL10, CXCL11, and CXCL13. They can also interact with other cells such as dendritic cells, macrophages, and epithelial cells and induce a further cascade of events.
We believe that Tc17 cells may provide protection by recruiting host T cells, as shown by Khader et al. (23), by recruiting host neutrophils, macrophages, and other cells, as shown by a number of investigators (reviewed in Ref. 17), by enhancing B cell responses in a number of ways including the secretion of IL-21, stimulating B cell proliferation, and differentiation (reviewed in Ref. 68) and by the B cell chemoattractant CXCL13, the ligand for CXCR5, which is preferentially expressed by human Th17 cell clones (69) and finally by IL-17A which has been shown to promote growth of airway epithelial cells (70).
Acknowledgments
We thank the Flow Cytometry Core Facility and the Molecular Biology Core Facility of the Trudeau Institute for assistance.
Footnotes
This work was supported by National Institutes of Health Grant P01AI46530 and by the Trudeau Institute.
- RA
- retinoic acid
- BAL
- bronchoalveolar lavage
- DXS
- dextran sulfate
- EID50
- 50% egg infective dose
- ILN
- inguinal lymph node
- i.n.
- intranasal
- LCMV
- lymphocytic choriomeningitis virus
- MedLN
- mediastinal lymph node
- NP
- nucleoprotein
- RORγt
- orphan nuclear receptor γt
- Tc
- CD8 T cytotoxic
- TCID50
- 50% tissue culture infective dose
- nTc17
- naive Tc17
Disclosures: The authors have no financial conflict of interest.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Swain SL, Bradley LM, Croft M, Tonkonogy S, Atkins G, Weinberg AD, Duncan DD, Hedrick SM, Dutton RW, Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol. Rev. 1991;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 3.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 4.Seder RA, Boulay JL, Finkelman F, Barbier S, Ben-Sasson SZ, Le Gros G, Paul WE. CD8+ T cells can be primed in vitro to produce IL-4. J. Immunol. 1992;148:1652–1656. [PubMed] [Google Scholar]
- 5.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J. Exp. Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelso A, Troutt AB, Maraskovsky E, Gough NM, Morris L, Pech MH, Thomson JA. Heterogeneity in lymphokine profiles of CD4+ and CD8+ T cells and clones activated in vivo and in vitro. Immunol. Rev. 1991;123:85–114. doi: 10.1111/j.1600-065x.1991.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 7.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 8.Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J. Exp. Med. 2007;204:1193–1205. doi: 10.1084/jem.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 10.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr. Opin. Immunol. 2007;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locksley RM. The roaring twenties. Immunity. 2008;28:437–439. doi: 10.1016/j.immuni.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Weaver CT, Murphy KM. T-cell subsets: the more the merrier. Curr. Biol. 2007;17:R61–63. doi: 10.1016/j.cub.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 20.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 23.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 24.Smiley KL, McNeal MM, Basu M, Choi AH, Clements JD, Ward RL. Association of γ interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice i.n. immunized with VP6 and the adjuvant LT(R192G) J. Virol. 2007;81:3740–3748. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 27.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 29.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 33.Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 34.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 35.Yoshihara K, Yamada H, Hori A, Yajima T, Kubo C, Yoshikai Y. IL-15 exacerbates collagen-induced arthritis with an enhanced CD4+ T cell response to produce IL-17. Eur. J. Immunol. 2007;37:2744–2752. doi: 10.1002/eji.200737229. [DOI] [PubMed] [Google Scholar]
- 36.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 38.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 39.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 40.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 41.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 42.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 45.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl. Acad. Sci. USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 47.O’Garra A, Stockinger B, Veldhoen M. Differentiation of human TH-17 cells does require TGF-β! Nat. Immunol. 2008;9:588–590. doi: 10.1038/ni0608-588. [DOI] [PubMed] [Google Scholar]
- 48.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 50.Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int. Rev. Immunol. 1998;16:541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- 51.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 52.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-β, NF-κB, and AP-1 activation. Am. J. Physiol. 2007;293:H3356–H3365. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 53.Linden A. A role for the cytoplasmic adaptor protein Act1 in mediating IL-17 signaling. Sci. STKE. 2007;2007:re4. doi: 10.1126/stke.3982007re4. [DOI] [PubMed] [Google Scholar]
- 54.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 55.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr. Opin. Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 56.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 57.Dobrzanski MJ, Reome JB, Dutton RW. Therapeutic effects of tumor-reactive type 1 and type 2 CD8+ T cell subpopulations in established pulmonary metastases. J. Immunol. 1999;162:6671–6680. [PubMed] [Google Scholar]
- 58.Dobrzanski MJ, Reome JB, Dutton RW. Role of effector cell-derived IL-4, IL-5, and perforin in early and late stages of type 2 CD8 effector cell-mediated tumor rejection. J. Immunol. 2001;167:424–434. doi: 10.4049/jimmunol.167.1.424. [DOI] [PubMed] [Google Scholar]
- 59.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J. Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 60.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 1999;189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J. Immunol. Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 62.Helmich BK, Dutton RW. The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and homing properties of Tc1 and Tc2 effectors. J. Immunol. 2001;166:6500–6508. doi: 10.4049/jimmunol.166.11.6500. [DOI] [PubMed] [Google Scholar]
- 63.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 2002;196:957–968. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, Dutton RW. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clin. Immunol. 2004;113:89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-β and interleukin-6. J. Leukocyte Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 67.Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 68.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol. Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 69.Takagi R, Higashi T, Hashimoto K, Nakano K, Mizuno Y, Okazaki Y, Matsushita S. B cell chemoattractant. CXCL13 is preferentially expressed by human Th17 cell clones. J. Immunol. 2008;181:186–189. doi: 10.4049/jimmunol.181.1.186. [DOI] [PubMed] [Google Scholar]
- 70.Inoue D, Numasaki M, Watanabe M, Kubo H, Sasaki T, Yasuda H, Yamaya M, Sasaki H. IL-17A promotes the growth of airway epithelial cells through ERK-dependent signaling pathway. Biochem. Biophys. Res. Commun. 2006;347:852–858. doi: 10.1016/j.bbrc.2006.06.137. [DOI] [PubMed] [Google Scholar]