Abstract
The relationship between sphingosine kinase (SPHK), cellular ceramide concentration and chemosensitivity was investigated in human colon cancer cell lines. Among nine colon cancer cell lines, SPHK1 and SPHK2 activity and protein expression was highest in RKO cells and lowest in HCT116 cells. A viability assay revealed that HCT116 cells were sensitive to the effects of oxaliplatin (l-OHP), whereas RKO cells were resistant to those of l-OHP. Treatment with 5μg/ml l-OHP induced a marked time-dependent increase in various ceramides (C16, C24, C24:1) in HCT116 cells but not in RKO cells, as indicated by liquid chromatography/mass spectrometry. The increase in ceramide and caspase activation induced by l-OHP in the sensitive HCT116 cells was abolished by pretreatment with a neutral sphingomyelinase inhibitor, suggesting that the ceramide formation was due to the activation of neutral, rather than acid, sphingomyelinase. In contrast, in l-OHP-resistant RKO cells, treatment with an SPHK inhibitor or SPHK1 and SPHK2 silencing by RNA interference suppressed cell viability and increased caspase activity and cellular ceramide formation after l-OHP treatment. The elevated ceramide formation induced by SPHK inhibition and l-OHP was inhibited by fumonisin B1 but not myriocin, suggesting that ceramide formation was through the salvage pathway. Endogenous phosphorylated Akt levels were much higher in the resistant RKO cells than in the sensitive HCT116 cells. Either SPHK1 or SPHK2 silencing in RKO cells decreased phosphorylated Akt levels and increased p53 and p21 protein levels as well as poly(ADP-ribose) polymerase cleavage in response to l-OHP treatment. These findings indicate that SPHK isoforms and neutral sphingomyelinase contribute to the regulation of chemosensitivity by controlling ceramide formation and the downstream Akt pathway in human colon cancer cells.
Sphingolipids have critical functions as signaling molecules. In particular, ceramide and sphingosine 1-phosphate (S1P)3 are involved in regulating cell responses such as proliferation and apoptosis (1–4). Ceramide, located at the central position of the sphingolipid metabolic pathway, is both the precursor and degradation product of sphingomyelin. Ceramide acts as a signaling molecule to activate a variety of signaling cascades that ultimately lead to cell responses such as apoptosis, growth arrest, cell differentiation, and various aspects of inflammation (5). The hydrolysis of sphingomyelin is catalyzed by sphingomyelinases (SMases) yielding ceramide and phosphorylcholine. To date, several SMase activities have been identified that can be classified in two main groups, the acidic and neutral forms. Ceramide can also be formed from sphinganine by ceramide synthase. This de novo synthesis of ceramide requires hours of stress exposure and can be induced by several factors including tumor necrosis factor-α, hypoxia, and various chemotherapeutics (6). Ceramide is also produced by the salvage pathway. Ceramide is involved in the activation of both downstream and upstream caspases (7). Ceramide also triggers the activation of various protein kinase cascades, including the classical MAPK/ERK cascade (8) and the more recently discovered stress-activated protein kinases of the SAPK/JNK subfamily (9). Another signaling pathway involved in the regulation of an apoptosis cell response that may be affected by ceramide is the survival phosphatidylinositol 3-kinase (PI3K)/Akt cascade (10).
On the other hand, the sphingolipid S1P acts as a potent mitogen for a variety of cell types (11, 12), mainly involving specific cell surface S1P receptors (13) that belong to the class of G protein-coupled receptors (S1P(1,2,3,4,5)) (14). S1P prevents apoptosis induced by serum withdrawal, Fas, and ceramide, among others. A critical factor regulating the availability of S1P is the activity of sphingosine kinase (SPHK), which phosphorylates sphingosine to S1P and is thought to act as an oncogene in tumorigenesis (12, 13, 15). Two SPHK subtypes have been identified, SPHK1 and SPHK2. SPHK1 activity is stimulated by various growth factors and cytokines (15), whereas the activation mechanism of SPHK2 is unclear, although epidermal growth factor enhances SPHK2 activity in the breast cancer cell line MCF-7 (16). Expression of SPHK1 enhances proliferation and protects cells from apoptosis, and induces tumor formation in mice (11, 12, 17). On the other hand, SPHK2 has been reported to suppress growth and enhance apoptosis (18). However, the role of endogenous SPHK2 has not been clearly defined, as down-regulation of SPHK2 in some cancer cells unexpectedly results in cell growth inhibition (19).
These two lipids, ceramide and S1P, together form a cellular “rheostat” regulating the balance between cell growth and cell death. SPHK1 is up-regulated in a variety of tumors (17, 20, 21), and ceramide levels are significantly reduced in colon carcinoma tissue when compared with normal tissue (22). Directing the balance in favor of ceramide may thus have beneficial effects in cancer therapy. Because of their opposing cellular functions, the balance between ceramide and S1P appears to be a critical determinant of cell death and proliferation and may therefore be an important factor to consider in therapeutic strategies against tumor progression (12, 21, 23). Our previous studies have shown that prostate cancer cells with high SPHK1 activity are resistant to the anticancer drug camptothecin (24), and leukemia cells with high SPHK1 activity are resistant to daunorubicin (25, 26). In those studies, however, the relationship between ceramide levels and SPHK activity was not examined.
In the present study, therefore, we compared ceramide levels and SPHK isozymes activity in drug-sensitive and -resistant colon cancer cells. Nine colon cancer cell lines had various levels of SPHK1 and SPHK2 activities. Colon cancer cells with high SPHK1 and SPHK2 expression were resistant to oxaliplatin (l-OHP) compared with those with low SPHK isozymes expression. Their high activities modulated the l-OHP-induced increase in ceramide, and inhibition of both SPHK isozymes increased ceramide levels, rendering the colon cancer cells sensitive to l-OHP. The regulation of sensitivity to anticancer drugs by the sphingolipid rheostat through Akt activation and p53 expression in the two types of colon cancer cells is discussed.
EXPERIMENTAL PROCEDURES
Materials—Oxaliplatin, myriocin, and fumonisin B1 were purchased from Sigma. Rabbit polyclonal antibodies against Ser473-phosphorylated Akt, Akt, poly(ADP-ribose) polymerase (PARP), and Bax were obtained from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antibodies against p21, nSMase2, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-human SPHK1 and SPHK2 antibodies were prepared as described previously (27). Mouse monoclonal anti-p53 antibody was obtained from Oncogene Research Products (San Diego). 2-(p-Hydroxyanilino)-4-(p-chlorophenyl) thiazole, a non-ATP competitive sphingosine kinase inhibitor (SKI), and GW4869, a neutral sphingomyelinase inhibitor, were purchased from Merck. The Caspase-3/7 assay kit (APO-One) was from Promega (Madison, WI). [choline-methyl-14C]Sphingomyelin and adenosine 5′-[γ-32P]triphosphate were from Amersham Biosciences.
Cell Lines and Cell Viability Measurement—Human colon cancer cell lines (COLO320DM, DLD1, HCT15, SW480, LoVo, HCT116, LS174T, RKO, CaCo2), purchased from American Type Culture Collection (ATCC, Manassas, VA), were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. For cell viability measurement, cells were plated at a density of 0.5 × 104 cells/well in 96-well plates. Cell viability of drug-treated and mock-treated cells was then analyzed for 48 h by standard MTT assays according to the manufacturer's instructions.
Silencing of Sphingosine Kinase1, Sphingosine Kinase2, and Neutral Sphingomyelinase2—The siRNAs of human SPHK1, SPHK2, and nSMase2 were obtained from Sigma Genosys (Hokkaido, Japan) as described previously (26). Scrambled siRNA was purchased from Dharmacon (GE Healthcare). Sequences for the SPHK-specific siRNAs are as follows: SPHK1(1), 5′-GGGCAAGGCCUUGCAGCUC-3′ and 5′-GAGCUGCAAGGCCUUGCCC-3′; SPHK1(2), 5′-GGCUGAAAUCUCCUUCACG-3′ and 5′-CGUGAAGGAGAUUUCAGCC-3′; and SPHK2(1), 5′-GGGUAGUGCCUGAUCAAUG-3′ and 5′-CAUUGAUCAGGCACUACCC-3′; SPHK2(2), 5′-GCUGGGCUGUCCUUCAACCU-3′ and 5′-AGGUUGAAGGACAGCCCAGC-3′. Sequences for nSMase2-specific siRNA are 5′-AAUGCUACUGGCUGGUGGACC-3′ and 5′-GGUCCACCAGCCAGUAGCAUU-3′. Scrambled siRNA or siRNAs of SPHK1s and SPHK2s (50 nm) and nSMase2 (100 nm) were transfected to the tumor cells using Lipofectamine 2000 (Invitrogen). Changes in SPHK1, SPHK2, and nSMase2 expression were confirmed by Western blotting using anti-human SPHK1, anti-human SPHK2, and anti-human nSMase2 antibodies and by quantitative reverse transcription-PCR. Quantitative reverse transcription-PCR was performed according to the method reported previously (25). The relative mRNA level was expressed as the ratio of the respective mRNA divided by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Western Blotting—Cells were lysed by sonication in lysis buffer (1% Nonidet P-40, 0.5% sodium cholate, 1% SDS, 1 mm EDTA, 1 mm EGTA, 150 mm NaCl, 20 mm HEPES, 3 mm MgCl2, protease inhibitors, 0.5 mm 4-deoxypyridoxine, 20 mm β-glycerophosphate, 1 mm sodium fluoride, and 1 mm sodium orthovanadate, pH 7.4). The total cell lysate (10–30 μg of protein) was subjected to electrophoresis on 10%-13% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Danvers, MA). The membranes were blocked using 5% bovine serum albumin. Expression of SPHK1, SPHK2, phosphorylated-Akt, total Akt, p53, p21, Bax, and PARP was measured by immunoblotting with specific antibodies. Anti-β-actin antibody was used as the loading control. After repeated washes, bound antibodies were detected using the ECL Western blotting detection system (GE Healthcare). Protein band density was determined with a densitometer (Atto Densitograph, version 2.5; Atto, Ltd., Tokyo).
Sphingosine Kinase Enzyme Activity—The activity of the two SPHK isoforms, SPHK1 and SPHK2, was measured separately, as described previously (26). SPHK activity was determined either in the presence of 0.25% Triton X-100 (SPHK1) or in 1 m KCl (SPHK2). SPHK1 activity was examined in the presence of 50 μm sphingosine and [γ-32P]ATP (10 μCi, 1 mm) containing 10 mm MgCl2 in 0.25% Triton X-100, which inhibits SPHK2 (26). SPHK2 activity was determined with sphingosine added as a complex with 4 mg/ml bovine serum albumin and [γ-32P]ATP containing 10 mm MgCl2 in the presence of 1 m KCl, conditions in which SPHK2 activity is optimal and SPHK1 activity is strongly inhibited. Labeled S1P was extracted and separated by thin layer chromatography on silica gel G60 with 1-butanol/acetic acid/H2O (3:1:1) as the solvent. The spot of S1P on the thin layer chromatography plate was visualized by autoradiography and quantified using a densitometer. The activity was expressed as pmol/min/mg protein.
Sphingomyelinase Activity Assay—Acid and neutral SMase activities were measured as described previously (7).
Caspase Activity Assay—Caspase-3 and -7 activities were measured using an Apo-One™ homogenous caspase-3/7 assay kit (Promega). Experiments were performed according to the manufacturer's recommended procedures. Briefly, the cells were plated at 5 × 103 cells/well in 96-well plates and incubated overnight. Subsequently, the medium was replaced with new medium with or without Z-VAD-fluoromethyl ketone (Z-VAD; 100 μm) and preincubated for 1 h before treatment with 5 μg/ml l-OHP. After incubation for the indicated time, the caspase-Glo 3/7 reagents were added to the wells followed by measurements of luminescence 1 h later with a plate-reading luminometer (Turner Biosystems, Sunnyvale, CA).
Lipid Extraction and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)Analysis of S1P and Ceramides—Colon cancer cells were lysed by sonication in the lysis buffer. Cellular lipids were extracted using a modified Bligh and Dyer procedure under acidic conditions with 0.1 m HCl. C6-ceramide (50 ng) and C17-S1P (50 ng) (Avanti Polar Lipid, Inc., Alabaster, AL), used as internal standards, were added during the lipid extraction step. The lipid extracts were dissolved in 20% acetonitrile (100 μl) containing 0.1% trifluoroacetic acid and subjected to combined LC-MS/MS.
LC-MS/MS analyses were performed using a Waters 2695 high pressure liquid chromatography system (Milford, MA) coupled to a Micromass Quattromicro™ API triple-quadrapole mass spectrometer (Micromass, Manchester, United Kingdom) as described previously (26). Peak areas for all components were integrated automatically using Masslynx™ NT 4.0 software (Micromass). Losses were accounted for by dividing each sphingolipid peak area by the percent recovery of the C6-ceramide or C17-S1P internal standard in each sample. Each loss-adjusted sphingolipid peak area was converted into a concentration using a loss-adjusted standard curve for each sphingolipid, which was linear in the relevant range.
Ceramide Synthase Activity Assay—The synthesis of ceramide from sphingosine was measured by LC-MS/MS analysis using C17 backbone sphingosine as described previously (28). Briefly, 0.3 mg of C17 backbone sphingosine was evaporated and sonicated in the buffer (40 μl) containing 0.1 mm dithiothreitol, 0.2 mm MgCl2, 50 mm HEPES/HCl, pH 7.5. The cell lysate (40 μg) and 25 μm palmitoyl-CoA were added in the substrate buffer and incubated for 30 min at 37 °C. The reaction was stopped by pipetting the reaction mixture into the extraction solvent (100 μl of chloroform and methanol (1:1, v:v)). The lipids were extracted and subjected to LC-MS/MS analysis as described previously (29).
Statistical Analysis—Microsoft Excel and its affiliated software were used to calculate the correlation index. The statistical significance was analyzed by either Student's t test or one-way factorial analysis of variance and multiple comparison test (Fisher's method) using StatView version 5 (SAS Institute Inc., Cary, NC). A p value of less than 0.05 was considered significant.
RESULTS
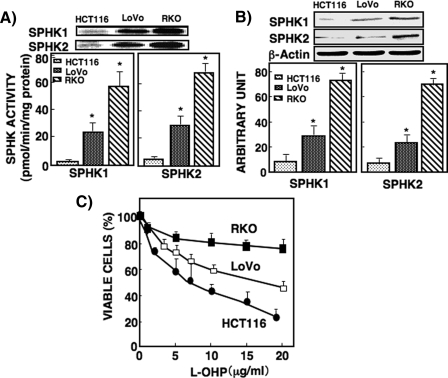
Relationship between Sphingosine Kinase Activity and Anticancer Drug Sensitivity of Colon Cancer Cells—SPHK1 is an anti-apoptotic enzyme, and the other SPHK isoform, SPHK2, has been reported to be pro-apoptotic (16). SPHK1 and SPHK2 activities were determined separately in nine human colon cancer cell lines. Their activities differed among the nine colon cancer cell lines (supplemental Table 1). Among these cell lines, RKO cells exhibited the highest SPHK1 and SPHK2 activities, whereas HCT116 cells exhibited the lowest activities, and LoVo cells showed the intermediate activities (Fig. 1A). SPHK1 and SPHK2 activities in RKO cells were ∼20 and 3 times higher than those in HCT116 and LoVo cells, respectively. Their protein expression levels were higher in RKO cells than in HCT116 and LoVo cells, which corresponded to their activity levels (Fig. 1B).
FIGURE 1.
Comparison of SPHK1 and SPHK2 activity and protein expression and the IC50 of l-OHP in HCT116, LoVo, and RKO cells. A, SPHK1 and SPHK2 activities were measured in HCT116, LoVo, and RKO cells as described under ”Experimental Procedures.“ The upper panel shows [32P]S1P bands visualized by autoradiography; the spots of the S1P band were quantified using a densitometer. SPHK activity was expressed as pmol/min/mg protein. B, SPHK1 and SPHK2 protein levels in HCT116, LoVo, and RKO cells were measured by Western blot with antibodies against SPHK1 and SPHK2. β-Actin was used as a loading control. C, HCT116, LoVo, and RKO cells were treated with various concentrations of l-OHP for 48 h and then subjected to an MTT assay as described under ”Experimental Procedures.“ Each point was measured in triplicate, and data are expressed as mean ± S.D. of three different experiments. *, p < 0.05 compared with HCT116.
We then examined sensitivity to an anticancer drug, l-OHP, in the three cell lines. HCT116 cells were the most sensitive to l-OHP (IC50 was 7.5 ± 0.5 μg/ml), and RKO cells were the least sensitive to l-OHP (IC50 = 71.2 ± 3.4 μg/ml); LoVo cells showed intermediate sensitivity (IC50 = 19.3 ± 5.4 μg/ml) (Fig. 1C). These results suggest that the activities and protein expressions of these SPHK isoforms may be related to chemosensitivity for l-OHP. RKO cells with higher SPHK activity and expression were much less sensitive (resistant) to the drug compared with HCT116 cells with lower SPHK activity and expression, which were sensitive to the drug. These results prompted us to investigate further how SPHK activity regulates chemosensitivity in these cells.
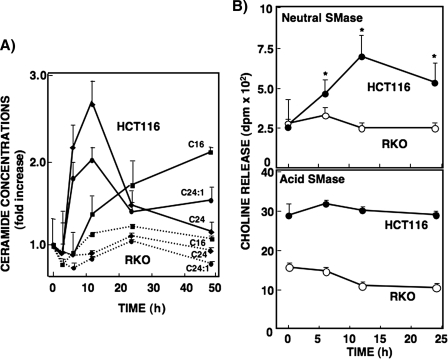
Changes in Cellular Levels of Ceramides after l-OHP Treatment—Changes in C16-ceramide, C18-ceramide, and C24-ceramide levels have been involved in apoptosis of various cancer cells (30–32). To examine whether ceramide synthesis/metabolism is involved in the mechanism of sensitivity to l-OHP, LC-MS/MS was used to measure endogenous ceramide levels in HCT116, LoVo, and RKO cells, with or without l-OHP treatment. Endogenous ceramide levels and ceramide N-acyl chain compositions were not significantly different among these three cell lines, showing abundant C16-, C24-, and C24: 1-ceramide species and lower levels of C18- and C22-ceramides, but no detectable C20-ceramide (supplemental Fig. 1A). Quantitative analysis of these molecular species in HCT116 cells treated with l-OHP revealed that the levels of both C24- and C24:1-ceramides (2.7- and 2.0-fold, respectively) increased transiently and markedly, and C16-ceramide (2.0-fold after 48 h) showed a gradual increase (Fig. 2A). Changes in C18- or C22-ceramide were modest (data not shown). On the other hand, treatment of RKO cells with l-OHP (5 μg/ml) did not induce significant changes in any of the ceramide species. No cell death was observed at this l-OHP concentration in RKO cells. The treatment of LoVo cells with l-OHP (5 μg/ml) for 12 h induced increases in the levels of C16-, C24- and C24:1-ceramides, which were smaller than those of HCT116 cells (supplemental Fig. 1B). These results suggest that the changes in these endogenous ceramide levels are associated with l-OHP sensitivity.
FIGURE 2.
Changes in ceramide species and sphingomyelinase activity upon treatment with l-OHP in HCT116 and RKO cells. A, HCT116 and RKO cells were treated with 5 μg/ml l-OHP, and the cells were lysed at the indicated times. Lipid extracts from the cell lysates corresponding to 0.5 mg of protein were dissolved in 20% acetonitrile containing 0.1% trifluoroacetic acid (100 μl) as described under ”Experimental Procedures,“ and 10 μl was injected for analysis of the ceramide species (C16, C24, and C24:1). C6-ceramide (50 ng) was used as the internal standard. Results are shown as mean ± S.D. of three different experiments. B, HCT116 and RKO cells were treated with 5 μg/ml l-OHP for various time periods, and acid and neutral SMase activities were measured at the indicated times as described under ”Experimental Procedures.“ Results are shown as mean ± S.D. of three different experiments. *, p < 0.05.
To validate whether the changes in endogenous ceramide levels are due to hydrolysis of sphingomyelin, we measured acid and neutral SMase activities in both HCT116 and RKO cell lines after various durations of l-OHP (5 μg/ml) treatment. Acid SMase activity did not change significantly during l-OHP treatment in either cell line (Fig. 2B). In contrast, however, neutral SMase activity was markedly increased (maximum: 3-fold at 12 h) in the l-OHP-sensitive HCT116 cells but not in the resistant RKO cells. These findings suggested that the increased ceramide generation was due to neutral SMase-catalyzed hydrolysis of sphingomyelin.
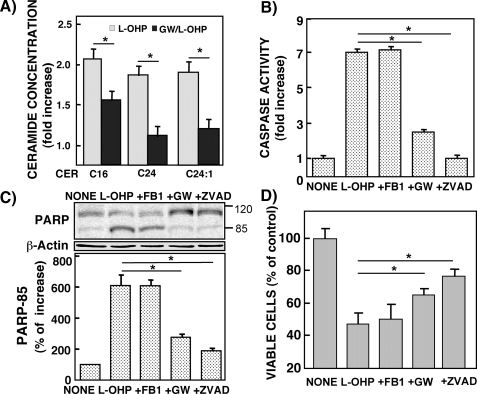
Modulation of Ceramide Generation by Neutral Sphingomyelinase Inhibitor and Its Effect on l-OHP-induced Apoptosis in HCT116 Cells—l-OHP-sensitive HCT116 cells pretreated with 10 μm GW4869, a neutral SMase inhibitor, significantly reduced the l-OHP-induced accumulation of C16-, C24-, and C24:1-ceramides (Fig. 3A). Caspase-3/7 activity increased in response to l-OHP (5 μg/ml for 24 h) but was completely abolished by pretreatment of the cells with the caspase inhibitor Z-VAD (Fig. 3B). The l-OHP treatment-induced increase in caspase-3/7 activity was suppressed by GW4869 pretreatment but not by the de novo ceramide synthase inhibitor fumonisin B1. l-OHP treatment of HCT116 cells induced PARP cleavage, which was suppressed by Z-VAD and GW4869, compatible with the inhibitory profile of caspase-3/7 activity (Fig. 3C). Fumonisin B1 did not affect l-OHP-induced PARP cleavage. The l-OHP-induced cytotoxicity of HCT116 cells was significantly reduced by treatment with Z-VAD and GW4869 but not fumonisin B1 (Fig. 3D). These results suggested that ceramide generation from sphingomyelin hydrolysis induced by neutral SMase was involved in the l-OHP-induced caspase-dependent apoptosis of HCT116 cells.
FIGURE 3.
Effects of various inhibitors on l-OHP-induced ceramide formation, caspase activity, PARP degradation, and cell viability in HCT116 cells. HCT116 cells were pretreated with or without fumonisin B1 (FB1; 100 μg/ml), neutral SMase inhibitor GW4869 (GW; 10 μm), or Z-VAD (100 μg/ml) for 1 h and then treated with or without 5 μg/ml l-OHP for 24 h. The treated cells were lysed, and protein concentrations were measured. A, the lipids were extracted from the treated cells, and ceramide species were determined by LC-MS/MS as described under ”Experimental Procedures.“ The results are expressed as -fold increase of the ceramide concentrations in the cells without l-OHP treatment and shown as means ± S.D. of three different experiments. *, p < 0.01. B, caspase-3/7 activity was measured as described under ”Experimental Procedures.“ The results are represented as -fold increase of the activity of the cells without l-OHP treatment and shown as means ± S.D. of three different experiments. *, p < 0.01 C, PARP cleavage was examined by Western blot with antibody against PARP. β-Actin was used as a loading control. The 85-kDa band of the PARP-cleaved product was determined by a densitometer, and the results are shown as the percentage of increase in band density in the cells without l-OHP treatment. Data are shown as means ± S.D. of three different experiments. *, p < 0.01. D, viable cells were measured by MTT assay. The results are expressed as the percentage of cells without l-OHP treatment and shown as means ± S.D. of three different experiments. *, p < 0.05.
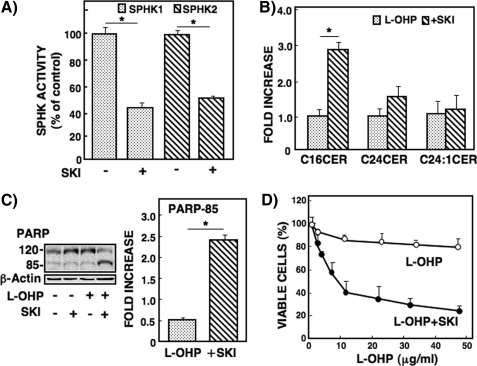
Effects of Inhibition of SPHK on l-OHP Sensitivity of RKO Cells and Caspase Activity in RKO Cells—Modulation of the sphingolipid-metabolizing enzyme affects chemosensitivity (20, 21), and SPHK1 activity inversely correlates with ceramide levels (1, 3). Therefore, we used an SPHK inhibitor, SKI, to investigate how SPHK activity affects l-OHP sensitivity in the resistant RKO cells. We examined the specificity of SKI for SPHK1 and SPHK2 activities. Treatment of RKO cells with SKI (3 μm) inhibited both SPHK1 and SPHK2 activity by ∼50% (Fig. 4A), indicating a similar level of inhibitory potency for the two SPHK isozymes. Under these conditions, the effect of SKI was examined on l-OHP-induced ceramide accumulation in RKO cells. When RKO cells were pretreated with SKI, l-OHP treatment induced increases in the levels of C16-ceramide but not C24:1-ceramide (Fig. 4B). Moreover, only the combined treatment with SKI and l-OHP stimulated PARP degradation in RKO cells (Fig. 4C); SKI (3 μm) alone had no effect on ceramide formation and PARP degradation. l-OHP-induced cell cytotoxicity was significantly increased in the presence of SKI (3 μm) in RKO cells (IC50 = 8.9 ± 1.3 μg/ml; Fig. 4D). These results indicated that inhibition of SPHK activity enhanced the ceramide (C16) level and l-OHP sensitivity in the resistant RKO cells.
FIGURE 4.
Effect of SPHK inhibitor on l-OHP-induced ceramide formation, PARP cleavage, and cell viability in RKO cells. A, RKO cells were pretreated with the SPHK inhibitor SKI (2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole) (3 μm) for 2 h, and SPHK1 and SPHK2 activities were measured in the cell lysates in triplicate as described under ”Experimental Procedures.“ Results are expressed as the percentage of activity in the cells without SKI treatment. *, p < 0.01. B, RKO cells were pretreated with or without SKI (3 μm) for 2 h and then treated with l-OHP (5 μg/ml). After 24 h, the lipids were extracted, and ceramide species were determined by LC-MS/MS as described under ”Experimental Procedures.“ The results are expressed as -fold increase of l-OHP-induced ceramide in cells without SKI and shown as means ± S.D. of three different experiments. *, p < 0.01. C, RKO cells were pretreated with or without 3 μm SKI for 2 h and then treated with or without l-OHP (5 μg/ml) for 24 h. The cell lysates were subjected to Western blot analysis using the antibody against PARP, and the band of PARP-cleaved product p85 was measured by a densitometer. The results are expressed as -fold increases of the band in the cells without SKI. *, p < 0.01. D, RKO cells were pretreated with or without 3 μm SKI for 2 h and then treated with various concentrations of l-OHP for 48 h. The viable cells were measured by MTT assay and expressed as the percentage of cells without l-OHP and SKI. Data are shown as the means ± S.D. of three different experiments.
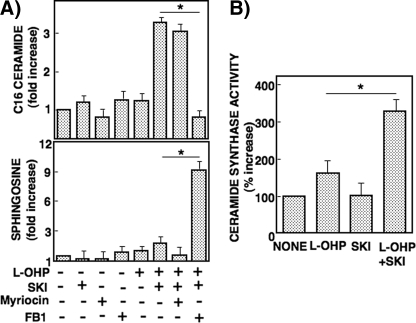
As SPHK inhibition was shown here to increase ceramide accumulation, we examined its mechanism. RKO cells were pretreated with SKI (3 μm) or the inhibitors of enzymes involving de novo ceramide generation: myriocin (10 μm) for serine palmitoyltransferase and fumonisin B1 (50 μm) for ceramide synthase. Accumulation of C16-ceramide elicited by the combined treatment with SKI and l-OHP was abolished by pretreatment with fumonisin B1 but not myriocin (Fig. 5A). On the other hand, fumonisin B1 pretreatment induced accumulation of sphingosine in the cells treated with SKI and l-OHP. When ceramide synthase activity was examined by using C17-based sphingosine as the substrate, the combined treatment of RKO cells with SKI and l-OHP caused a significant increase in C17-based C16-ceramide level (Fig. 5B). Furthermore, to examine the implication of sphingomyelin hydrolysis on the ceramide accumulation in RKO cells treated with SKI and l-OHP, nSMase was knocked down. The transfection of RKO cells with siRNA of nSMase2 appreciably reduced nSMase2 expression but did not change C16-ceramide accumulation (supplemental Fig. 2). These results indicated that the ceramide formation induced by combined treatment with SKI and l-OHP in RKO cells was most likely due to the increase in ceramide synthase activity.
FIGURE 5.
Effect of SPHK inhibitor and de novo ceramide synthesis inhibitors on l-OHP-induced ceramide and sphingosine formation and ceramide synthase activity in RKO cells. A, RKO cells were pretreated with or without SKI (3 μm) or de novo ceramide synthesis inhibitors (10 μm myriocin and 50 μm fumonisin B1 (FB1)) for 2 h and then treated with or without l-OHP (5 μg/ml). After 24 h, the lipids were extracted, and C16-ceramide and sphingosine were measured by LC-MS/MS as described under ”Experimental Procedures.“ The results are expressed as -fold increase of the cells not treated with l-OHP or inhibitors and are shown as means ± S.D. of three different experiments. *, p < 0.01. B, RKO cells were pretreated with or without SKI (3 μm) for 2 h and then treated with or without l-OHP (5 μg/ml). After 24 h, cells were collected, and cell lysates were subjected to ceramide synthase activity as described under ”Experimental Procedures.“ Results are expressed as the percentage of increase in activity in the cells without l-OHP and SKI, shown as means ± S.D. of three independent experiments. *, p < 0.05.
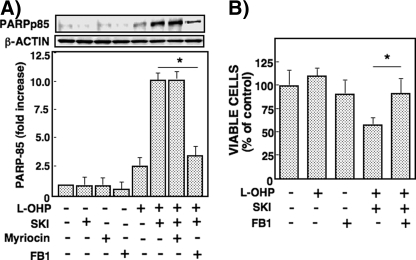
We then examined the involvement of ceramide accumulation in cytotoxicity in RKO cells. PARP cleavage caused by SKI and l-OHP was largely reduced by pretreatment with fumonisin B1 (50 μm) but not myriocin (Fig. 6A). In this context, fumonisin B1 suppressed the cytotoxicity inferred by the viability assay (Fig. 6B).
FIGURE 6.
Effects of SPHK and de novo ceramide synthesis inhibitors on PARP cleavage and cell viability in RKO cells. A, RKO cells were pretreated with or without SKI (3 μm) or de novo ceramide synthesis inhibitors (10 μm myriocin and 50 μm fumonisin B1 (FB1)) for 2 h and then treated with or without l-OHP (5 μg/ml) for 24 h. The cell lysates were subjected to Western blot analysis using the antibody against PARP, and the band of PARP-cleaved product p85 was measured by a densitometer. The results are expressed as -fold increase of the band density in the cells without l-OHP and inhibitors. *, p < 0.01. B, RKO cells were pretreated with or without 3 μm of SKI and fumonisin B1 (50 μm) for 2 h and then treated with various concentrations of l-OHP for 48 h. The viable cells were measured by MTT assay and expressed as the percentage of cells without l-OHP and inhibitors. Data are shown as means ± S.D. of three different experiments. *, p < 0.05.
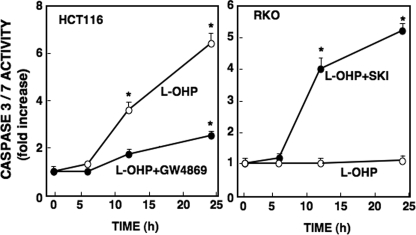
Changes in caspase-3/7 activity were well correlated with ceramide generation induced by l-OHP. In HCT116 cells, caspase-3/7 activity was enhanced by treatment with l-OHP and suppressed in the presence of the neutral SMase inhibitor GW4869 (Fig. 7). In contrast, although l-OHP alone did not increase caspase-3/7 activity in RKO cells, the combined treatment with SKI and l-OHP markedly enhanced caspase activity in RKO cells. These results suggested that sphingolipid-metabolizing enzymes have a pivotal role in l-OHP-induced caspase-3/7 activation and chemosensitivity.
FIGURE 7.
Effects of SPHK and neutral SMase inhibitors on caspase-3/7 activity in HCT116 and RKO cells. HCT116 cells and RKO cells were pretreated with or without neutral SMase inhibitor (GW4869, 10 μm) and SKI (3 μm), respectively, for 2 h and then treated with l-OHP (5 μg/ml). After the indicated times, cells were collected, and cell lysates were subjected to caspase-3/7 activity assay as described under ”Experimental Procedures.“ Results are expressed as -fold increase in activity in cells without l-OHP and inhibitors, shown as means ± S.D. of three independent experiments. *, p < 0.01.
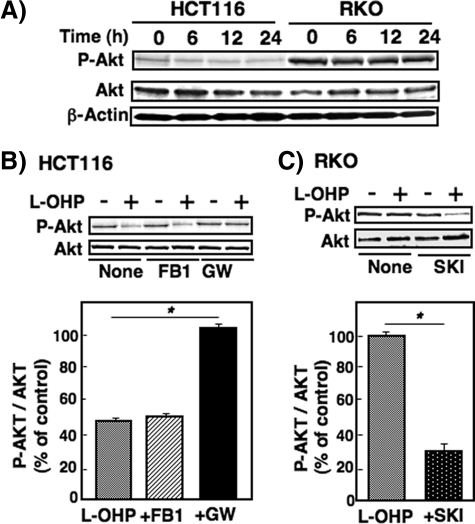
Effects of Modulation of Sphingolipid Metabolite on Akt Phosphorylation in Colon Cancer Cells—The activation of Akt is crucially involved in survival signaling in various cell types (33). To investigate the role of Akt in l-OHP sensitivity, Akt phosphorylation was compared in both sensitive HCT116 and resistant RKO cells. In the untreated cells, the Akt phosphorylation levels were much higher in RKO cells than in HCT116 cells, although total Akt levels were unchanged (Fig. 8A). Akt phosphorylation was reduced to ∼50% in HCT116 cells treated with l-OHP (5 μg/ml; Fig. 8B). Pretreatment of HCT116 cells with GW4869, but not fumonisin B1, prevented the cells from the l-OHP-induced decrease in Akt phosphorylation. On the other hand, Akt phosphorylation was found not to be affected by treatment with l-OHP in RKO cells (Fig. 8C). The Akt phosphorylation level in RKO cells pretreated with SKI (3 μm), however, was considerably suppressed by l-OHP (5 μg/ml) treatment. SKI alone did not affect Akt phosphorylation. These changes in Akt phosphorylation levels caused by l-OHP were shown to be quite compatible with those in caspase-3/7 activation (Figs. 3 and 7).
FIGURE 8.
Inhibition of Akt phosphorylation by l-OHP treatment and the effects of SPHK and neutral SMase inhibitors on Akt phosphorylation in HCT116 and RKO cells. A, HCT116 and RKO cells were treated with 5 μg/ml l-OHP, and the cells were collected at the indicated times. The cell lysates were subjected to Western blot analysis using anti-phosphospecific Akt and total Akt antibodies. β-Actin was used as the loading control. B and C, HCT116 cells were pretreated with or without fumonisin B1 (FB1; 50 μm) and neutral SMase inhibitor GW4869 (GW; 10 μm) for 2 h (B) and RKO cells pretreated with or without SKI (5 μm) for 2 h (C), and then they were treated with or without l-OHP (5 μg/ml) for 24 h. Cell lysates were subjected to Western blot analysis using the two Akt antibodies. Bands were quantified with a densitometer, and the ratio of phosphospecific Akt (P-Akt) to total Akt (Akt) was calculated. Results are expressed as percent of the ratio in the cells with l-OHP compared with the cells (control) without l-OHP treatment and shown as means ± S.D. of three different experiments. *, p < 0.01.
Changes in the other pro-apoptotic proteins caused by l-OHP treatment were also examined. Phosphorylation levels of MAP kinase families SAPK/JNK, ERK, and p38 MAPK were not significantly distinct between the two cell lines and also were not changed after l-OHP treatment (data not shown). l-OHP treatment of HCT116 cells induced remarkable increases in the expression of p53, p21, and Bax in a time-dependent manner compared with RKO cells (supplemental Fig. 3), which suggests their possible role in l-OHP-induced apoptosis.
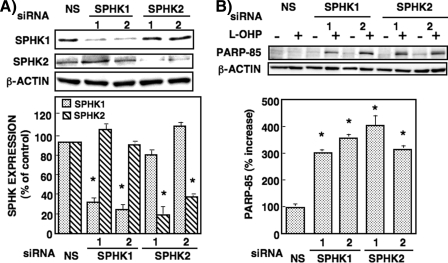
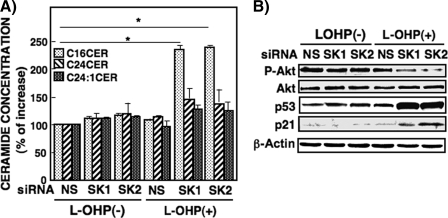
Effects of Knockdown of Sphingosine Kinase Isozymes on Ceramide Generation, Akt Phosphorylation, and Pro-apoptotic Protein Expression in RKO Cells—To examine the role of SPHK isoforms in these cellular responses, we used two transfectants each of siRNAs of SPHK1 and SPHK2. In RKO cells transfected with two SPHK1(1,2) and two SPHK2(1,2) siRNAs their protein expressions were reduced 20–40% (Fig. 9A). Knockdown of SPHK1 by two specific siRNAs, but not nonspecific siRNA, greatly enhanced l-OHP-induced PARP cleavage (Fig. 9B). However, unexpectedly and interestingly, knockdown of SPHK2 by two specific siRNAs was found to increase l-OHP-induced PARP cleavage to a similar or greater extent than for SPHK1 knockdown. Either SPHK1 or SPHK2 knockdown increased C16-ceramide generation by l-OHP (5 μg/ml) treatment (Fig. 10A). These results provide additional support for the finding shown in Fig. 4, where the inhibition of SPHK activity by SKI caused the increased accumulation of C16-ceramide and PARP cleavage. Moreover, Akt phosphorylation by l-OHP was significantly reduced in both SPHK1 and SPHK2 knockdown RKO cells (Fig. 10B). Also, l-OHP-induced p53 and p21 protein expressions were enhanced in both SPHK1 and SPHK2 knockdown cells. Similar profiles of increased C16-ceramide accumulation and changes of protein expression induced by l-OHP were observed in RKO cells transfected with other siRNAs targeting different regions of either SPHK1 or SPHK2 (data not shown). These findings suggested that both SPHK1 and SPHK2 are involved in the regulation of ceramide accumulation and chemosensitivity in l-OHP-treated colon cancer cells.
FIGURE 9.
Effect of SPHK1 and SPHK2 knockdown on l-OHP-induced PARP cleavage in RKO cells. A, RKO cells were transfected with siRNA of SPHK1(1), SPHK1(2), SPHK2(1), SPHK2(2), or nonspecific (NS) siRNA with Lipofectamine 2000 according to the manufacturer's instructions. After 48 h, the cell lysates were subjected to Western blot analysis using the antibodies against SPHK1 and SPHK2. Bands were quantified with a densitometer. Results are expressed as percent NS siRNA. *, p < 0.01. B, cells transfected with siRNA of SPHK1(1), SPHK1(2), SPHK2(1), SPHK2(2), or NS siRNA were treated with or without l-OHP (5μg/ml). After 24 h, cells lysates were subjected to Western blot analysis using the antibody against PARP, and the band of PARP-cleaved product p85 was measured by a densitometer. β-Actin was used as the loading control. The ratio of l-OHP-stimulated PARP-85 to the band without l-OHP treatment was calculated. Results are expressed as percent increase of the ratio in cells transfected with NS siRNA, shown as means ± S.D. of three different experiments. *, p < 0.01 compared with NS.
FIGURE 10.
Effect of SPHK1 and SPHK2 knockdown on l-OHP-induced ceramide formation, Akt phosphorylation, and pro-apoptotic protein expression in RKO cells. A, RKO cells were transfected with siRNA of SPHK1(1), siRNA of SPHK2(1), or nonspecific (NS) siRNA with Lipofectamine 2000 according to the manufacturer's instructions. After 24 h, the transfected cells were treated with or without l-OHP (5 μg/ml) for 24 h. The lipids were extracted from the cells, and the cellular ceramide concentration was measured by LC-MS/MS as described under ”Experimental Procedures.“ Results are expressed as percent increase of cells transfected with NS siRNA without l-OHP treatment, shown as means ± S.D. of three different experiments. *, p < 0.05. B, RKO cells transfected with siRNAs of SPHK1(1), SPHK2(1), or NS siRNA were treated with or without l-OHP (5 μg/ml). After 24 h, the cell lysates were subjected to Western blot analysis using the antibodies against phosphospecific-Akt, total Akt, p53, and p21. β-Actin was used as the loading control.
DISCUSSION
Our previous studies demonstrated that the human prostate cancer cell line PC3, which has high SPHK1 activity, is resistant to camptothecin (24) and also that the SPHK1 message level in bone marrow from patients with myelodysplastic syndromes and acute leukemia is well correlated with sensitivity to daunorubicin (25). In the present study, we have shown that SPHK activity and protein expression are inversely related to l-OHP sensitivity in three representative colon cancer cell lines that are distinct in SPHK expression and activity. Pretreatment of l-OHP-resistant RKO cells with SPHK inhibitor or knockdown of endogenous SPHK1 and SPHK2 induced a marked increase in l-OHP sensitivity. Furthermore, our preliminary data showed that an increased SPHK expression level was found in the resistant cell line (HCT116-OR) isolated from the parental cells, indicating chemosensitivity and low SPHK levels (data not shown). These observations lead us to propose that SPHK2, as well SPHK1, is involved in chemoresistance in colon cancer cells.
The endogenous ceramide level is thought to determine cell fate, i.e. differentiation, senescence, and apoptosis (2, 5), and treatments that increase the ceramide levels in cancer cells lead to the induction of cell death. In the present study, we demonstrated that in sensitive HCT116 cells, l-OHP treatment induced an increase in the cellular ceramide levels, resulting in cell death, and combined treatment with a neutral SMase inhibitor suppressed the increase in ceramide generation leading to the recovery of cell viability. In contrast, in l-OHP-resistant RKO cells, although low l-OHP treatment did not change the cellular ceramide levels or induce cell death, combined treatment with SKI and l-OHP induced cell death and was accompanied by a significant increase in ceramide generation. These results imply that an increase in cellular ceramide levels is important for the induction of cell death by the anticancer drug l-OHP and that cellular ceramide levels are negatively regulated by SPHK activity in colon cancer cells. Among the ceramide species analyzed, an increase in C24-ceramide, but not in C16- or C18-ceramide, induces all-trans-retinoic acid-induced cell death in NB4 cells (30). Our previous study also demonstrated that the C24-ceramide/S1P ratio was most closely related to chemosensitivity against daunorubicin in leukemia cells (26). In contrast, other reports demonstrate that an increase in C18- or C16-ceramide is important for inducing apoptosis (31, 32). Changes in the C24-ceramide levels are related to neutral SMase activity, whereas changes in the C16- or C18-ceramide levels are related to the de novo ceramide synthesis. Our study using l-OHP-sensitive HCT116 cells indicated that l-OHP treatment caused a transient increase in C24- and C24: 1-ceramide levels, whereas C16-ceramide levels were continuously gradually increased. These increases in the ceramide species were blocked by treatment with the neutral SMase inhibitor but not by fumonisin B1, indicating that l-OHP-induced generation of these ceramides resulted from sphingomyelin hydrolysis rather than de novo synthesis. On the other hand, in the l-OHP-resistant RKO cells, there was no significant accumulation of any of the ceramide species by l-OHP treatment alone. This could be due to the high SPHK activity, which results in inhibition of de novo ceramide biosynthesis (34, 35). Indeed, C16-ceramide levels were increased by l-OHP treatment in RKO cells pretreated with SKI or transfected with SPHK1 or SPHK2 siRNA. The ceramide accumulation induced by inhibition of the SPHK activity or SPHK knockdown would be mediated via the salvage pathway, because fumonisin B1, but not myriocin, inhibited l-OHP-induced ceramide accumulation and instead increased sphingosine in SKI-pretreated RKO cells (Fig. 5). In addition, the silencing of nSMase2 had no effect on the ceramide accumulation (supplemental Fig. 2). These studies together suggest that the ceramide accumulation induced by SKI or SPHK1 and SPHK2 knockdown is attributed to increased ceramide synthase activity and via a sphingosine salvage pathway (36). Although at present we have no evidence for the preferential importance of ceramide species in apoptosis, changes in the profile of ceramide molecular species by different treatments may possibly depend on the cell type and/or anticancer drug.
The sphingolipid rheostat is defined as the ratio of total cellular ceramide/S1P. Using imatinib-resistant K562 cells, Baran et al. (23) report that the acquisition of imatinib resistance is associated with increased SPHK1 expression and S1P production, resulting in changes in the ceramide/S1P ratio. Previous studies also have suggested that the suppression of the ceramide content is attributable to the activation of glucosylceramide synthase and sphingomyelin synthase in apoptosis-resistant leukemic cells (37). We previously demonstrated that expression of SPHK1, but not sphingomyelin synthase, decreases the intracellular ceramide content and is associated with resistance to daunorubicin (25, 26). Consistent with the decreased ceramide levels induced by overexpression of SPHK1, SPHK1 activity inversely correlates with ceramide levels. The present findings demonstrated that combined l-OHP and SKI treatment of RKO cells, which exhibit high SPHK1 and SPHK2 activity, increased ceramide levels and induced apoptosis. These data together suggest that SPHK overexpression is associated with anticancer drug resistance due to a decrease in ceramide formation.
Two isoforms of mammalian SPHK, SPHK1 and SPHK2, have been cloned and characterized. SPHK1 is critical for cell growth, and cancer cells overexpressing SPHK1 are more resistant to apoptosis induced by anticancer drugs (1, 15). In contrast, much less is known about the function of endogenous SPHK2. Its enforced overexpression suppresses cell growth and enhances apoptosis, suggesting that SPHK1 and SPHK2 have opposing roles in the apoptotic process (18). Although SPHK1 has been shown to be associated with daunorubicin resistance, SPHK2 is less likely to be involved in this drug sensitivity in human leukemic cells (25). However, SPHK2 knockdown renders cancer cells susceptible to doxorubicin-induced apoptosis, suggesting that endogenous SPHK2 influences the balance between cytostasis and apoptosis (38). Similarly, in the present study, the inhibition of either SPHK1 or SPHK2 activity showed an additive effect with l-OHP to induce apoptosis in l-OHP-resistant RKO cells (Fig. 9). Furthermore, our results show that knockdown of SPHK1 and SPHK2 augments the cytotoxic effect of l-OHP, as reflected in decreased Akt phosphorylation and increased p53 expression and PARP cleavage in RKO cells (Fig. 10). These results suggest that l-OHP sensitivity is regulated by both endogenous SPHK1 and SPHK2 in colon cancer cells.
Ceramide enhances pro-apoptotic molecules, such as caspase-3, and reactive oxygen species (5, 10), and suppresses anti-apoptotic molecules, such as heat shock protein-70 (HSP-70) and phosphatidylinositol 3-kinase (11). p53 is a well established tumor suppressor protein, and mutations in the p53 gene are the most common genetic alterations observed in human tumor samples (39). A number of in vitro studies have shown that p53-deficient cancer cells are relatively resistant to l-OHP and 5-fluorouracil (40–44). In this study we compared various pro-apoptotic and anti-apoptotic signals in l-OHP-sensitive HCT116 cells and l-OHP-resistant RKO cells. Time-dependent increases in p53, p21, and Bax expression were induced by l-OHP treatment in HCT116 cells, whereas expression of these proteins was marginal in RKO cells (supplemental Fig. 3), suggesting that these proteins have roles in promoting apoptosis induced by l-OHP in colon cancer cells.
In addition, recent studies have shown that the phosphoinositide 3-kinase/Akt pathway is related to chemoresistance, mainly through escape from cellular apoptosis (45). This signaling promotes cell survival and proliferation. Constitutively active Akt protects cells from apoptosis by inhibiting caspase-3 activation and reduces the sensitivity of tumor cells to pro-apoptotic agents (34, 45). Additionally, endogenous active Akt is appreciably present in human cancer cells throughout cancer progression, and therefore, Akt likely has a role in the resistance to anticancer drugs in vivo. These roles of active Akt are consistent with our data showing that Akt is highly activated in l-OHP-resistant RKO cells compared with l-OHP-sensitive HCT116 cells, suggesting that the Akt activation pathway is associated with l-OHP resistance.
We have demonstrated in this study that Akt activation through SPHK/S1P signaling is associated with l-OHP resistance in colon cancer cells. SPHK has been described as a key regulating factor for the sphingolipid rheostat that modulates the balance of proliferative and apoptotic signals through the conversion of ceramide and sphingosine to S1P (12). Furthermore, ceramide causes growth arrest and apoptosis by inhibiting Akt (10, 34, 46). In contrast, SPHK activation induces Akt phosphorylation, which may or may not be mediated by Gi-coupled S1P receptors (47, 48). In our study, when RKO cells were pretreated with pertussis toxin (Gi inhibitor), no significant inhibition of Akt phosphorylation was observed (data not shown). Therefore, data from our study suggested that the activation of SPHK1 and SPHK2 decreases ceramide levels, thereby enhancing Akt activation and, conversely, that pharmacologic inhibition or knockdown of SPHK1 and SPHK2 induces the accumulation of ceramides, leading to suppression of Akt activation evolved by l-OHP treatment (Figs. 8 and 10). Moreover, recent studies have implicated Akt in the down-regulation of p53 via MDM2, which is phosphorylated by Akt at different sites resulting in its activation (49). This view was, in part, addressed in a recent study by Ju et al. (43), which demonstrates that the induced expression of p53 in RKO cells is controlled at the post-translational level after treatment with doxorubicin, whereas p53 mRNA levels do not change by exposure to the anticancer drug. Our results also identify the SPHK-mediated activation of the Akt pathway as a mechanism of cellular survival and chemoresistance in colon cancer cells, which suggests that inhibition of the Akt pathway by an SPHK inhibitor or SPHK siRNAs is a possible strategy to overcome l-OHP resistance. Thus, the SPHK-dependence of l-OHP sensitivity is likely a result of SPHK-mediated regulation of Akt phosphorylation.
Taken together, our results demonstrate that colon cancer cell sensitivity against l-OHP is dependent on SPHK1 and SPHK2 activities, which regulate ceramide formation and Akt phosphorylation. Thus, in chemotherapy for anticancer drug-resistant tumors, the combination of an SPHK inhibitor (including siRNA) and l-OHP could be effective. Also, SPHK activity in colon cancers may be a useful marker for predicting chemoresistance.
Supplementary Material
Acknowledgments
We thank Kana Fukuda and Miki Takeshita (Gifu Pharmaceutical University) for technical assistance.
This work was supported in part by Grant-in-aid for Scientific Research (C) 20590055 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
Footnotes
The abbreviations used are: S1P, sphingosine 1-phosphate; l-OHP, oxaliplatin (cis-(oxalato(1R,2R-cyclohexanediamine)platinum); SPHK, sphingosine kinase; SMase, sphingomyelinase; nSMase, neutral sphingomyelinase; LC-MS/MS, liquid chromatography-tandem mass spectrometry; PARP, poly(ADP-ribose) polymerase; SKI, sphingosine kinase inhibitor (2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole); MTT, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; ERK, extracellular signal-regulated kinase; mitogen-activated protein kinase; SAPK, stress-activated protein kinase; JNK, c-Jun NH2-terminal kinase; siRNA, small interfering RNA; Z, benzyloxycarbonyl.
References
- 1.Taha, T. A., Hannun, Y. A., and Obeid, L. M. (2006) J. Biochem. Mol. Biol. 39 113–131 [DOI] [PubMed] [Google Scholar]
- 2.Ogretmen, B., and Hannun, Y. A. (2004) Nat. Rev. Cancer 4 604–616 [DOI] [PubMed] [Google Scholar]
- 3.Olivera, A., Kohama, T., Edsall, L. C., Nava, V., Cuvillier, O., Poulton, S., and Spiegel, S. (1999) J. Cell Biol. 147 545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia, P., Wang, L., Gamble, J. R., and Vadas, M. A. (1999) J. Biol. Chem. 274 34499–34505 [DOI] [PubMed] [Google Scholar]
- 5.Pettus, B. J., Chalfant, C. E., and Hannun, Y. A. (2002) Biochim. Biophys. Acta 1585 114–125 [DOI] [PubMed] [Google Scholar]
- 6.Linn, S. C., Kim, H. S., Keane, E. M., Andrai, L. M., Wang, E., and Merrill, A. H., Jr. (2001) Biochem. Soc. Trans. 29 831–835 [DOI] [PubMed] [Google Scholar]
- 7.Takeda, Y., Tashima, M., Takahashi, A., Uchiyama, T., and Okazaki, T. (1999) J. Biol. Chem. 274 10654–10660 [DOI] [PubMed] [Google Scholar]
- 8.Raines, M. A., Kolesnick, R. N., and Golde, D. W. (1993) J. Biol. Chem. 268 14572–14575 [PubMed] [Google Scholar]
- 9.Verheij, M., Bose, R., Lin, X. H., Yao, B., Jarvis, W. D., Grant, S., Birrer, M. J., Szabo, E., Zon, L. I., Kyriakis, J. M., Haimovitz-Friedman, A., Fuks, Z., and Kolesnick, R. N. (1996) Nature 380 75–79 [DOI] [PubMed] [Google Scholar]
- 10.Schubert, K. M., Scheid, M. P., and Duronio, V. (2003) J. Biol. Chem. 275 13330–13335 [DOI] [PubMed] [Google Scholar]
- 11.Spiegel, S., and Milstein, S. (2003) Nat. Rev. Mol. Cell Biol. 4 397–407 [DOI] [PubMed] [Google Scholar]
- 12.Milstien, S., and Spiegel, S. (2006) Cancer Cell 9 148–150 [DOI] [PubMed] [Google Scholar]
- 13.Hla, T. (2003) Pharmacol. Res. 47 401–407 [DOI] [PubMed] [Google Scholar]
- 14.Young, N., and van Brocklyn, J. R. (2006) Scientific World Journal 6 946–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hait, N. C., Oskeritzian, C. A., Paugh, S. W., Milstien, S., and Spiegel, S. (2006) Biochim. Biophys. Acta 1758 2016–2026 [DOI] [PubMed] [Google Scholar]
- 16.Hait, N. C., Sarkar, S., Le Stunff, H., ikami A., Maceyka, M., Milstien, S., and Spiegel, S. (2005) J. Biol. Chem. 280 29462–29469 [DOI] [PubMed] [Google Scholar]
- 17.Kawamori, T., Osta, W., Johnson, K. R., Pettus, B. J., Bielawski, J., Tanaka, T., Wargovich, M. J., Reddy, B. S., Hannun, Y. A., Obeid, L. M., and Zhou, D. (2006) FASEB J. 20 386–388 [DOI] [PubMed] [Google Scholar]
- 18.Liu, H., Toman, R. E., Goparaju, S., Maceyka, M., Milstien, S., and Spiegel, S. (2003) J. Biol. Chem. 278 40330–40336 [DOI] [PubMed] [Google Scholar]
- 19.Van Brocklyn, J. R., Jackson, C. A., Pearl, D. K., Kotur, M. S., Snyder, P. J., and Prior, T. W. (2005) J. Neuropathol. Exp. Neurol. 64 695–705 [DOI] [PubMed] [Google Scholar]
- 20.French, K. J., Schrecengost, R. S., Lee, B. D., Zhuang, Y., Smith, S. N., Eberly, J. L., Yun, J. K., and Smith, C. D. (2003) Cancer Res. 63 5962–5969 [PubMed] [Google Scholar]
- 21.Pchejetski, D., Golzio, M., Bonhoure, E., Calvet, C., Doumerc, N., Garcia, V., Mazerolles, C., Rischmann, P., Teissie, J., Malavaud, B., and Cuvillier, O. (2005) Cancer Res. 65 11667–11675 [DOI] [PubMed] [Google Scholar]
- 22.Selzner, M., Bielawska, A., Morse, M. A., Rudiger, H. A., Sindram, D., Hannun, Y. A., and Clavien, P. A. (2001) Cancer Res. 61 1233–1240 [PubMed] [Google Scholar]
- 23.Baran, Y., Salas, A., Senkal, C. E., Gunduz, U., Bielawski, J., Obeid, L. M., and Ogretmen, B. (2007) J. Biol. Chem. 282 10922–10934 [DOI] [PubMed] [Google Scholar]
- 24.Akao, Y., Banno, Y., Nakagawa, Y., Hasegawa, N., Kim, T.-J., Murate, T., Igarashi, Y., and Nozawa, Y. (2006) Biochem. Biophys. Res. Commun. 342 1284–1290 [DOI] [PubMed] [Google Scholar]
- 25.Sobue, S., Iwasaki, T., Sugisaki, C., Nagata, A., Kikuchi, R., Murakami, M., Takagi, A., Kojima, T., Banno, Y., Akao, Y., Nozawa, Y., Kannagi, R., Suzuki, M., Abe, A., Naoe, T., and Murate, T. (2006) Leukemia (Basingstoke) 20 2042–2046 [DOI] [PubMed] [Google Scholar]
- 26.Sobue, S., Nemoto, S., Murakami, M., Ito, H., Kimura, A., Gao, S., Furuhata, A., Takagi, A., Kojima, T., Nakamura, M., Ito, Y., Suzuki, M., Banno, Y., Nozawa, Y., and Murate, T. (2008) Int. J. Hematol. 87 266–275 [DOI] [PubMed] [Google Scholar]
- 27.Koda, M., Murate, T., Koizumi K., Igarashi, Y., Nozawa, Y., and Banno, Y. (2005) Biochim. Biophys. Acta 1733 101–110 [DOI] [PubMed] [Google Scholar]
- 28.Spassieva, S., Bielawski, J., Anelli, V., and Obeid, M. (2007) Methods in Enzymol. 434 232–241 [DOI] [PubMed] [Google Scholar]
- 29.Bielawski, J., Szulc, Z. M., Hannun, Y. A., and Bielawska, A. (2006) Methods (Amst.) 39 82–91 [DOI] [PubMed] [Google Scholar]
- 30.Murate, T., Suzuki, M., Hattori, M., Takagi, A., Kojima, T., Tanizawa, T., Asano, H., Hotta, T., Saito, H., Yoshida, S., and Tamiya-Koizumi, K. (2002) J. Biol. Chem. 277 9936–9943 [DOI] [PubMed] [Google Scholar]
- 31.Kobasi, S., Sekal, C. E., Sundararaj, K., Spassieva, S., Bielawski, J., Osta, W., Day, T. A., Jiang, J. C., Jazwinski, S. M., Hannun, Y. A., Obeid, L. M., and Ogretmen, B. (2004) J. Biol. Chem. 279 44311–44319 [DOI] [PubMed] [Google Scholar]
- 32.Osawa, Y., Uchinami, H., Bielawski, J., Schwabe, R. F., Hannun, Y. A., and Brenner, D. A. (2005) J. Biol. Chem. 280 27879–27887 [DOI] [PubMed] [Google Scholar]
- 33.Chan, T. O., Rittenhouse, S. E., and Tsichlis, P. N. (1999) Annun. Rev. Biochem. 68 965–1014 [DOI] [PubMed] [Google Scholar]
- 34.van Echten-Deckert, G., Zschoche, A., Bar, T., Schmidt, R. R., Raths, A., Heinemann, T., and Sandhoff, K. (1997) J. Biol. Chem. 272 15825–15833 [DOI] [PubMed] [Google Scholar]
- 35.Min, J., Mesika, A., Sivaguru, M., Van Veldhoven, P. P., Alexander, H., Futerman, A. H., and Alexander, S. (2007) Mol. Cancer Res. 5 801–812 [DOI] [PubMed] [Google Scholar]
- 36.Becker, K. P., Kitatani, K., Idkowiak-Baldys, J., Bielawski, J., and Hannun, Y. A. (2005) J. Biol. Chem. 280 2606–2612 [DOI] [PubMed] [Google Scholar]
- 37.Uchida, Y., Itoh, M., Taguchi, Y., Yamaoka, S., Umehara, H., Ichikawa, S., Hirabayashi, Y., Holleran, W. M., and Okazaki, T. (2004) Cancer Res. 64 6271–6279 [DOI] [PubMed] [Google Scholar]
- 38.Sankala, H. M., Hait, N. C., Paugh S. W., Shida, D., Leptine, S., Elmore, L. W., Dent, P., Milsten, S., and Spiegel, S. (2007) Cancer Res. 67 10466–10474 [DOI] [PubMed] [Google Scholar]
- 39.Hollstein, M., Sidransky, D., Vogelstein, B., and Harris, C. C. (1991) Science 253 49–53 [DOI] [PubMed] [Google Scholar]
- 40.Manic, S., Gatti, L., Carenini, N., Fumagalli, G., Zunin, F., and Perego, P. (2003) Curr. Cancer Drug Targets 3 21–29 [DOI] [PubMed] [Google Scholar]
- 41.Koivusalo, R., Krausz, E., Ruotsalainen, P., Helenius, H., and Hietanen, S. (2002) Cancer Res. 62 7364–7371 [PubMed] [Google Scholar]
- 42.Boyer, J., McLean, E. G., Aroori, S., Wilson, P., McCulla, A., Carey, P. D., Longley, D. B., and Johnston, P. G. (2004) Clin. Cancer Res. 10 2158–2167 [DOI] [PubMed] [Google Scholar]
- 43.Ju, J., Schmitz, J. C., Song, B., Kudo, K., and Chu, E. (2007) Clin. Cancer Res. 13 4245–4251 [DOI] [PubMed] [Google Scholar]
- 44.Arango, D., Wilson, A. J., Shi, Q., Corner, G. A., Aranes, M. J., Nicholas, C., Lesser, M., Mariadason J. M., and Augenlicht L. H. (2004) Br. J. Cancer 91 1931–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fresno Vara, J. A., Casado, E., Castro, J., Cejas, P., Belda-Iniesta, C., and Gonzalez-Baron, M. (2004) Cancer Treat. Rev. 30 193–204 [DOI] [PubMed] [Google Scholar]
- 46.Charles, S., Michela, M., Plivier, P., Patricia, H. W., Gavin, K., Erik, S., Frederic, E., Jenny, C., Jillian, T., Ahemed, A., James, D. B., and Julian, D. (2007) Cancer Cell 11 498–51217560332 [Google Scholar]
- 47.Osawa, Y., Banno, Y., Nagaki, M., Brenner, D. A., Naiki, T., Nozawa, Y., Nakashima, S., and Moriwaki, H. (2001) J. Immunol. 167 173–180 [DOI] [PubMed] [Google Scholar]
- 48.Radeff-Huang, J., Seasholtz, T. M., Chang, J. W., Smith, J. M., Walsh, C. T., and Brown, J. H. (2007) J. Biol. Chem. 282 863–870 [DOI] [PubMed] [Google Scholar]
- 49.Feng, J., Tamaskovic, R., Yang, Z., Brazil, D. P., Merlo, A., Hess, D., and Hemmings, B. A. (2004) J. Biol. Chem. 279 35510–35517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.