Abstract
Unlike most other matrix metalloproteinases (MMPs) MMP-19 is expressed in undifferentiated basal keratinocytes of healthy human skin. The human keratinocyte cell line HaCaT, which like basal keratinocytes constitutively expresses MMP-19, down-regulated the expression of MMP-19 at high calcium concentrations. Calcium-regulation occurred through E-cadherin mediated cell-cell contacts because neutralizing anti-E-cadherin antibodies restored MMP-19 expression in high calcium. Overexpression of MMP-19 in HaCaT cells (HaCaT-WT) increased cellular proliferation, as well as migration and adhesion on type I collagen. This was due to proteolysis of the insulin-like growth factor (IGF) binding protein-3 by MMP-19, which augmented signaling through the IGF-I receptor, as evidenced by its increased autophosphorylation. Conversely, these effects were not observed in cells transfected with MMP-2 or a catalytically inactive MMP-19 mutant. As further proof that increased IGF-signaling promoted adhesion and migration in HaCaT-WT cells, we reproduced these effects by treating parental HaCaT with IGF-I. We observed dephosphorylation of the focal adhesion kinase in HaCaT-WT as well as IGF-I–treated HaCaT cells, suggesting that inactivating focal adhesion kinase is a mechanism by which IGF-I enhances adhesion. Furthermore, IGF-I-triggered motility on type I collagen was mediated by MMP activity, which, however, was distinct from MMP-19. Considering the coexpression of IGFBP-3 and MMP-19 in the skin, we conclude that MMP-19 is a likely candidate to be the major IGFBP-3 degrading MMP in the quiescent epidermis. This activity might have widespread consequences for the behavior of epidermal keratinocytes.
INTRODUCTION
The epidermis is a stratified, squamous epithelium, which provides a barrier between the internal and external regions of the body. Tissue injury starts a complex program by the organism, eventually leading to reepithelialization of the epidermis. This process requires keratinocyte migration and proliferation, which is coordinated by the interaction of growth factors, proteinases, and components of the extracellular matrix (ECM).
The matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases that are responsible for the degradation of various proteins of the extracellular matrix, release and activation of some growth factors, and shedding of several cell surface proteins (Birkedahl-Hansen et al., 1993; Parks and Mecham, 1998; Vu and Werb, 2000). Expression, secretion, and activation of MMPs are tightly regulated across organs with low or even undetectable levels of most MMPs in normal, resting tissues. Induced expression or overexpression of MMPs is involved in the pathogenesis of diverse organ diseases (Birkedahl-Hansen et al., 1993; Parks and Mecham, 1998; Vu and Werb, 2000). Especially in the skin, expression of several MMPs occurs in response to tissue injury (Saarialho-Kere et al., 1993, 1994; Lund et al., 1999).
Insulin-like growth factors (IGFs) are potent mitogens that induce proliferation and differentiation in many tissues. In the skin, IGF-I is produced by dermal fibroblasts, epidermal melanocytes, and keratinocytes within the stratum granulosum (Tavakkol et al., 1992; Rudman et al., 1997). IGF-I acts through the IGF-I receptor (IGF-IR), which is expressed in the basal layer of the epidermis (Krane et al., 1992). The importance of the IGF system for epidermal growth and development is evident from mice deficient in the IGF-IR. These mice possess an abnormally thin epidermis devoid of a stratum spinosum (Liu et al., 1993). The observation that IGF also stimulates keratinocyte migration in vitro (Ando and Jensen, 1993) could explain the beneficial effects of IGF on wound healing in vivo (Koshizuka et al., 1997).
IGF action is modulated by a family of six known high-affinity IGF-binding proteins (IGFBP), that sequester IGFs and thereby prevent them from binding to IGF receptors (McCusker et al., 1991, Shimasaki and Ling, 1991). However, proteolytic cleavage of IGFBPs by aspartic, serine, and metalloproteinases has been shown to release IGFs, because IGFBP fragments are reduced in their affinity to IGFs (Cohen et al., 1992; Conover and De Leon, 1994; Fowlkes et al., 1994). Possible direct effects of IGFBPs, independent of their IGF binding capacity, have also been reported, but so far are less well understood (Oh, 1997).
We and others have recently reported on MMP-19 expression in human epidermis and various skin diseases (Sadowski et al., 2003; Impola et al., 2003; Suomela et al., 2003). A common observation among these different studies was that, unlike most other MMPs, MMP-19 is present in healthy epidermis and seems to be confined to undifferentiated keratinocytes. In the present manuscript we have extended these studies and report on functions of MMP-19 in keratinocytes. We used the HaCaT keratinocyte cell line, which shares many characteristics with basal keratinocytes, to study the regulation and function of MMP-19 expression. Using this cell line, we have found that an increase of calcium down-regulates MMP-19 and this effect is mediated by E-cadherin. We further provide evidence that overexpression of MMP-19 in HaCaT cells increases cellular proliferation as well as migration and adhesion to type I collagen. These effects of MMP-19 were mediated by providing bioavailable IGF through proteolysis of IGFBP-3. Considering the coexpression of the two molecules in epidermis, we conclude that MMP-19 is a likely candidate to be the major IGFBP-3 degrading MMP in the quiescent epidermis.
MATERIALS AND METHODS
Cells and Culture Conditions
The HaCaT keratinocyte cell line was generously provided by N. Fusenig (Deutsches Krebsforschungszentrum, Heidelberg, Germany). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in DMEM (PAA Laboratories, Linz, Austria) supplemented with 10% fetal bovine serum and 50 U/ml penicillin and 50 μg/ml streptomycin. For some experiments, HaCaT cells were grown in a serum-free keratinocyte growth medium (keratinocyte-SFM; Invitrogen, Carlsbad, CA) without CaCl2 but containing 50 μg/ml bovine pituitary extract and 5 ng/ml epidermal growth factor (EGF). Where mentioned, CaCl2 was added to a final concentration of 0.03 or 1.2 mM. cytochalasin D (Sigma-Aldrich, St. Louis, MO) was prepared as a 5 mg/ml stock solution in dimethyl sulfoxide and was used at a final concentration of 5 μg/ml.
Immunohistochemistry
Tissue specimens of human skin with normal morphology embedded in paraffin were kindly provided by E. Proksch (University of Kiel, Kiel, Germany). Before antibody staining, sections were processed as has been described previously (Sadowski et al., 2003). Monoclonal anti-MMP-19 antibody CK8/4 (Mauch et al., 2002) was diluted 1:100 and monoclonal anticytokeratin 14 antibody (ICN Pharmaceuticals, Irvine, CA) was diluted 1:10. Bound antibodies were detected using the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) following the manufacturer's instructions. Peroxidase activity was detected using 3-amino-9-ethylcarbazole as a chromogenic substrate (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin.
For immunofluorescent detection of proteins, human skin was embedded in tissue freezing medium (Leica Instruments, Nussloch, Germany), snap frozen in liquid nitrogen, and cut to a thickness of 4 μm on a cryostat. After drying and fixation, sections were blocked (Powerblock, Biogenex, San Ramon, CA) and then incubated with primary antibody in phosphate-buffered saline (PBS)/Triton X-100 (0.1%) containing 3% bovine serum albumin for 2 h at room temperature. Monoclonal antibody (mAb) CK8/4 was diluted 1:100 and anti-E-cadherin antibody (DECMA-1; Sigma-Aldrich) was diluted 1:500. Bound antibodies were detected with Alexa 488-conjugated goat anti-mouse IgG antibody (Molecular Probes, Eugene, OR) and Cy3-conjugated goat anti-rat IgG antibody (Dianova, Hamburg, Germany), respectively. Visualization was via a BX-500 fluorescent microscope (Olympus, Tokyo, Japan), and images were captured by video camera (Sony, Tokyo, Japan) and processed with the Analysis Soft Imaging system (Lakewood, CA).
Immunoblotting
Immunoblotting and detection of proteins was done as described previously (Mueller et al., 2000). MMP-19 was detected with polyclonal anti-MMP-19 antibodies (Chemicon International, Temecula, CA) and IGFBP-3 by using a mAb (R&D Systems, Minneapolis, MN). Primary antibodies were diluted 1:1000. The bound antibodies were detected using peroxidase-conjugated goat anti-rabbit and anti-mouse IgG antibodies (Pierce Chemical, Rockford, IL) and the chemiluminescence luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA). Signals were recorded with a luminescent image analyzer (FujiFilm, Tokyo, Japan).
Analysis of Phosphorylation
HaCaT cells were scraped from the tissue culture vessel into ice-cold PBS and lysed for 45 min on ice in Tris buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM sodium orthovanadate, 25 mM NaF, Complete proteinase inhibitor; Roche Diagnostics, Mannheim, Germany). Equal protein amounts were subjected to immunoprecipitation by incubating with a mAb recognizing IGF-IR (clone aIR-3; Calbiochem, Bad Soden, Germany), or with a polyclonal antibody recognizing FAK (Santa Cruz Biotechnology) overnight at 4°C, followed by the addition of protein G-Sepharose, and a further incubation for 1 h at 4°C. The precipitates were washed three times with cold RIPA buffer and immunoblotted. Membranes were probed sequentially with anti-phosphotyrosine (clone 4G10; Upstate Biotechnology, Lake Placid, NY) and polyclonal anti-IGF-IR antibodies (Santa Cruz Biotechnology) or anti-FAK antibodies.
Gelatinolytic Zymogram
Conditioned media standardized for cell number were mixed with a nonreducing SDS sample buffer and loaded onto 8% polyacrylamide gels containing 1 mg/ml gelatin. After electrophoresis gels were soaked in 2.5% Triton X-100 to remove the SDS. Gelatinolytic activities were developed in Tris buffer containing 50 mM Tris, 5 mM CaCl2, and 0.05% Triton X-100, pH 7.5, at 37°C for 24 h. Lytic bands were visualized by staining the gels with Coomassie Blue R-250 and destaining in 10% acetic acid.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Isolation of RNA and synthesis of cDNA was done as described previously (Mauch et al., 2002). Depending on the cDNA to be amplified, PCR was performed for 22 cycles (GAPDH), 30 cycles (β1 and α2 integrin), and 32 cycles (MMP-19, α3 integrin), respectively (denaturing at 95°C for 45 s, annealing for 30 s at 62°C [MMP-19], 50°C [GAPDH], or 56°C [integrins], extension at 72°C for 60 s). The reaction was thereby kept in the exponential range of amplification to obtain semiquantitative results (our unpublished data). All primer sequences were determined using established European Molecular Biology Laboratory database sequences (accession numbers and primer binding sites are given in parentheses): MMP-19 (X92521), 5′-TGCCCACAGAACCCAGTCC-3′ (bp 934–952) and 5′-GGTATTCCCACCTGATGGGGTAG-3′ (bp 1560–1538); GAPDH (M33197), 5′-GTGAAGGTCGGAGTCAACG-3′ (bp 70–88) and 5′-GAGATGATGACCCTTTTGG-3′ (bp 426–408); β1 integrin (X07979), 5′-GAGAAGCTCAAGCCAGAGGA-3′ (bp 413–432) and 5′-ACATTCCTCCAGCCAATCAG-3′ (bp 913–894); α2 integrin (X17033), 5′-TATTCTGATGCTGGGTGCAG-3′ (bp 1224–1243) and 5′-TTGCTGAACCAAATCGAGTG-3′ (bp 1721–1701); α3 integrin (M59911), 5′-TGAGGTCCAGTTCCAGAAGG-3′ (bp 1894–1913) and 5′-AGAAGCTTTGTAGCCGGTGA-3′ (bp 2397–2378). PCR products were resolved in 1% agarose gels, followed by staining with ethidium bromide and recording with a digital camera.
Cadherin Blocking Experiments
HaCaT keratinocytes were seeded into chamber slides (Nalge Nunc, Naperville, IL) in keratinocyte-SFM containing 1.2 mM CaCl2. Antibodies against E-cadherin (DECMA-1; Sigma-Aldrich) or nonimmune rat IgG (Sigma-Aldrich) was added to the cells at a final dilution of 1:15. After 24 h, cells were washed in PBS and fixed in 4% paraformaldehyde for 15 min at room temperature. Afterward, cells were processed for immunofluorescent detection of MMP-19 as described above.
Cell Proliferation Assays
For assessing the proliferation after calcium treatment and transfection of MMP-19 constructs, HaCaT keratinocytes were seeded at an initial number of 15,000 cells into the wells of microtiter plates. After a 24-h incubation under standard culture conditions, cells were pulsed with 0.25 μCi/well [3H]thymidine (Amersham Biosciences, Freiburg, Germany) for an additional 4 h. To determine the effect of IGFBP-3, HaCaT keratinocytes were seeded as described above but incubated for 48 h under standard culture conditions in the presence of human recombinant IGFBP-3 (rhIGFBP-3; kindly provided by Y Ogawa, Celtrix Pharmaceuticals, Santa Clara, CA). After the radioactive labeling, cells were briefly frozen to detach them from the plates and harvested by a cell harvester (Inotech, Dottikon, Switzerland). The incorporated radioactivity was quantitated on a liquid scintillation counter (Wallac, Turku, Finland).
Adhesion Assay
Keratinocytes were collected by trypsinization, washed in serum-free DMEM, and seeded at 5 × 104 cells in the wells of microtiter plates coated with monomeric rat tail type I collagen (10 μg/cm2; BD Biosciences, Bedford, MA), vitronectin, and fibronectin (5 μg/well; Invitrogen). For evaluating the effect of IGF-I (Sigma-Aldrich) on adhesion, keratinocytes were grown for 24 h in serum-free DMEM containing various amounts of the growth factor before they were seeded on type I collagen-coated microtiter plates. After incubation for 2 h at 37°C nonadherent cells were removed by washing with PBS, and the remaining cells were fixed in 4% paraformaldehyde and stained with methylene blue as described previously (Oliver et al., 1989). The dye was eluted in 1:1 (vol/vol) ethanol and 0.1 M HCl, and absorbance at 650 nm was read in a microplate photometer.
In Vitro Wound Healing
Keratinocytes were cultured in type I collagen-coated dishes until they reached confluence. To avoid a proliferative effect, cells were then treated with 100 mM hydroxyurea for 24 h (Sigma-Aldrich). Media were then changed to DMEM with 0.5% fetal bovine serum and a cell-free area was introduced by scraping the monolayer with a blue pipette tip. After 48 h under standard culture conditions, cells were fixed in 4% paraformaldehyde and stained with methylene blue as described above. Photographs were taken using an inverted phase-contrast microscope (Carl Zeiss, Jena, Germany).
Plasmid Constructs and Transfection
The full-length cDNA of human MMP-19 was subcloned into the Eco47III/XhoI site of pIRES2-EGFP (BD Biosciences Clontech, Palo Alto, CA). In addition, site-directed mutagenesis was used to exchange the glutamine at position 213 in the zinc-binding motif HExxHxxGxxH for an alanine (E213A). The oligonucleotide CGCATCATTGCAGCGCATGCAGTGGGCCATGCTCTG was used for the generation of the mutant. The exchange GAA → GCA led to the exchange E213A, whereas the silent mutation GCC → GCG generated a new PaeI-site needed for analytical reasons. The full-length cDNA of MMP-19 contained in pBluescript II served as template. All further steps were done with the GeneEditor-system as described by the manufacturer (Promega, Mannheim, Germany). The mutated MMP-19 cDNA was then subcloned into the Eco47III/XhoI site of pIRES2-EGFP. An EcoRI fragment of pSG5-MMP2 (kindly donated by H. Sato, Meiji Pharmaceutical University, Tokyo, Japan) representing the full-length cDNA of MMP-2 was subcloned between EcoRI site of pIRES2-EGFP.
For transfection of HaCaT keratinocytes we used FuGENE 6 transfection reagent according to the manufacturer's instructions (Roche Diagnostics). Stable cell lines were selected in 0.6 mg/ml G418 (Invitrogen).
Expression and Purification of Recombinant MMP-19
We constructed fusion proteins of glutathione S-transferase (GST) and MMP-19 terminating after the hemopexin-like domain of MMP-19. For this purpose, PCR products of the wild-type MMP-19 and its E213A-mutant were synthesized using the primers 5′-TTCCAAGATCTCGGGTCCTGGGGCTTGCAGAGGTG-3′ and 5′-TGATGAATTCTCAACGACAGTGCATCCAGTTGTGG-3′ (MMP-19 sequence in bold). The PCR product was digested with BglII and EcoRI and cloned into the pGEX-2T vector (Amersham Biosciences). Expression of the fusion protein in the BLR(DE3) strain of Escherichia coli (Novagen, Darmstadt, Germany) was induced by adding 0.1 mM isopropyl-1-thio-β-d-galactopyranoside followed by a further incubation at 25°C for 6 h. GST-MMP-19 was purified from the soluble fraction with reduced glutathione-Sepharose beads and incubated for 8 h at 37°C. This incubation led to an activation of the enzyme, possibly due to the opening of the cysteine switch by glutathione, which was still present in the incubation buffer. Proteolytic activity was detected using the synthetic fluorescent substrate McaPLAN-vaARNH2 (a kind gift of G. Murphy, University of East Anglia, Norwich, United Kingdom). Routine assays were performed at 37°C at a substrate concentration of 1 mM in TNC buffer. Inhibition of activated GST-MMP-19 by BB94 was demonstrated using the above-mentioned assay.
Statistical Analysis
Groups of data were analyzed using Student's two-tailed paired t test. Significance was set p ≤ 0.05. Data are presented as mean ± SEM.
RESULTS
MMP-19 Expression in Human Skin and HaCaT Keratinocytes Is Dependent on Cellular Differentiation
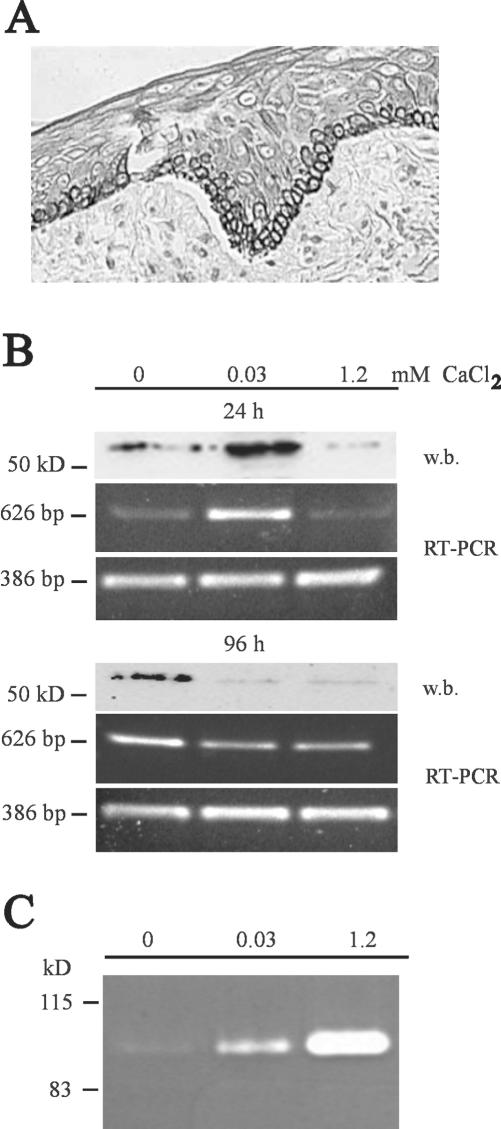
Immunohistochemical staining of skin samples with a mAb against MMP-19 revealed a constitutive expression in the basal cell layer of the epidermis, whereas the dermal compartment was negative for MMP-19 (Figure 1A). Because the staining for MMP-19 matched with that of cytokeratine 14 (our unpublished data), which is typically expressed in the stratum basale containing stem cells and transit amplifying cells, the expression of MMP-19 seemed to be confined to undifferentiated keratinocytes.
Figure 1.
MMP-19 expression in keratinocytes is dependent on cellular differentiation. (A) Sections of paraffin-embedded samples of human skin with normal morphology were analyzed with antibodies against MMP-19. The mAb CK8/4 detects MMP-19 specifically in basal keratinocytes. Bars, 50 μm. (B) MMP-19 protein and mRNA expression of HaCaT after 24 h and 96 h in keratinocyte-SFM with 0, 0.03, and 1.2 mM calcium. Cell lysates were subjected to Western blotting (w.b.) and probed with polyclonal antibodies against human MMP-19. A specific signal was detected at the expected size of ∼57 kDa (top). For evaluating the mRNA expression of MMP-19, total RNA was isolated and analyzed by RT-PCR with MMP-19-specific primers (middle). GAPDH was used as an internal control (bottom). Results are representative of three experiments. (C) The proform of MMP-9 is detected in conditioned media of HaCaT grown for 96 h in keratinocyte-SFM with 0, 0.03, and 1.2 mM calcium. Shown is a gelatinolytic zymogram. Results are representative of three experiments.
As a first step in understanding the role of MMP-19 in human epidermis, we examined its regulation in proliferating and differentiation-induced HaCaT keratinocytes. We therefore exposed HaCaT keratinocytes to increasing concentrations of CaCl2 (Paramio and Jorcano, 1997; Todd and Reynolds, 1998) and determined their proliferation and MMP-19 expression. Measurement after 24 and 96 h revealed that [3H]thymidine incorporation at both time points was highest in the absence of CaCl2, whereas 1.2 mM CaCl2 decreased proliferation by 50 ± 3.8 and 56 ± 1.0% after 24 h and 4 d, respectively (our unpublished data). In accordance to this, expression of MMP-19 at the mRNA and protein level was down-regulated by the high CaCl2 concentration (Figure 1B). A slight difference was observed after 24 h when maximal expression of MMP-19 was determined at 0.03 mM CaCl2, whereas proliferation was highest in the complete absence of CaCl2. This suggests that calcium-dependent adhesion to the culture vessels was initially necessary to support production of MMP-19. Indeed, when we coated culture dishes with Matrigel, which promotes cellular adhesion, maximum MMP-19 expression after 24 h was determined at 0 mM CaCl2 (our unpublished data). To illustrate that the observed calcium-regulation was specific for MMP-19, we also determined the secretion of MMP-9 in response to different calcium concentrations. In agreement with previous studies (Kobayashi et al., 1996), we detected increased amounts of MMP-9 at 1.2 mM CaCl2 by zymography (Figure 1C).
Suppression of MMP-19 Expression by High Calcium Is Mediated by E-Cadherin
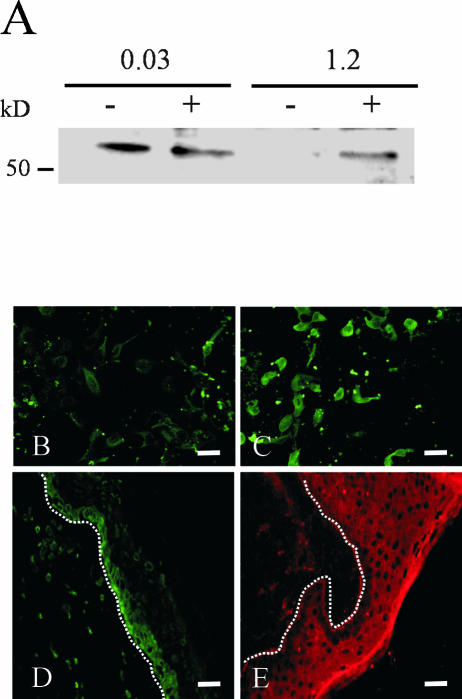
The most prominent calcium effects on MMP-19 expression were observed at high cell densities (our unpublished data). Because homophilic interactions among E-cadherins on epithelial cells are calcium-dependent and a role of adherens junctions in the control of cell proliferation has been postulated, we investigated whether the calcium regulation of MMP-19 expression takes place at the level of cell-cell contacts. Cytochalasin D treatment led to a rounded appearance of keratinocytes and restored expression of MMP-19 protein in high calcium, whereas it had no effect at low calcium concentrations, as we expected (Figure 2A). Next, we added the anti-E-cadherin antibody DECMA-1 to cells that were grown in high calcium, thereby specifically blocking E-cadherin–mediated cell-cell contacts, as has been described previously (Pece and Gutkind, 2000). HaCaT cells were subsequently stained for MMP-19. Those cells that were treated with DECMA-1 exhibited a rounded appearance and expressed significantly higher levels of MMP-19 than those treated with the isotype control (Figure 2, B and C).
Figure 2.
Suppression of MMP-19 expression by high calcium is mediated by cell-cell contacts. (A) Cytochalasin D (5 μg/ml) was used to disrupt adherens junctions. HaCaT keratinocytes were grown for 24 h in keratinocyte-SFM containing 0.03 and 1.2 mM calcium. Lysates of cytochalasin-treated (+) and untreated (-) cells were subjected to Western blotting for MMP-19. (B and C) The E-cadherin–specific antibody DECMA-1 down-regulated the expression of MMP-19. The DECMA-1 antibody (C) and an isotype control (B) were added at a final concentration of 1:15. HaCaT keratinocytes were grown in keratinocyte-SFM containing 1.2 mM calcium for 24 h. Afterwards, cells were fixed in 4% paraformaldehyde and processed for immunofluorescent detection of MMP-19 as described in Materials and Methods. Bars, 50 μm. The image shown is representative of three independent experiments. (C and D) Frozen sections of normal human epidermis were analyzed with antibodies against MMP-19 (C) and E-cadherin (D). Immunofluorescent detection was by Alexa 488 (MMP-19) and Cy 3 (E-cadherin)-conjugated secondary antibodies. Dotted lines indicate the basement membrane. Bars, 50 μm. Note that the expression of these molecules localizes to distinct compartments of human epidermis.
Having established that calcium-dependent cellular adhesion mediated by E-cadherin suppresses MMP-19 expression, we addressed the cellular distribution of MMP-19 (Figure 2D) and E-cadherin (Figure 2E) in human epidermis. As can be seen from the immunohistochemical staining, E-cadherin is only weakly expressed in basal keratinocytes, whereas strong expression was observed in suprabasal cells (Figure 2E). Thus, the formation of E-cadherin–mediated cell-cell contacts might be one of the mechanisms by which MMP-19 expression is down-regulated in suprabasal keratinocytes.
Overexpression of MMP-19 Promotes Proliferation in HaCaT Keratinocytes by Proteolysis of IGFBP-3
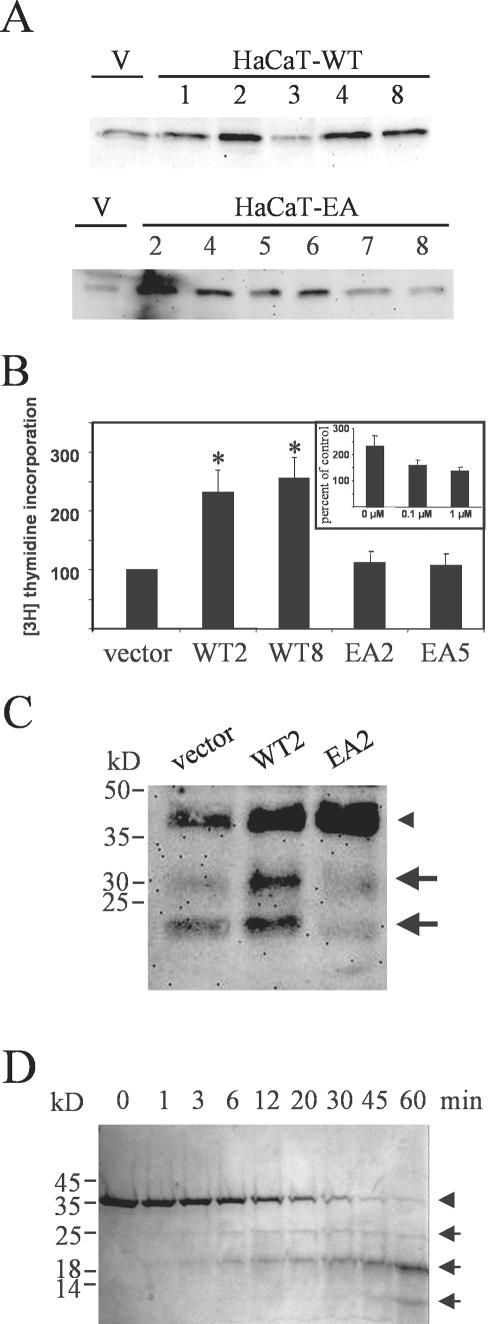
To study the functional role MMP-19 might play in epidermal keratinocytes, we stably transfected HaCaT cells with plasmids coding for the wild-type (HaCaT-WT) and for a functional knockout mutant of MMP-19 carrying an E213A mutation (HaCaT-EA). The E213 in the zinc-binding motif HExxHxxGxxH has been shown to be essential for catalytic activity of zinc-metalloproteinases (Hangauer et al., 1984; Morgunova et al., 1999). Accordingly, a recombinant form of MMP19EA, expressed in bacteria, did not display any catalytic activity against synthetic peptide substrates and the general protease substrate casein, which was readily cleaved by the wild-type MMP-19 (our unpublished data). Several clones were assayed for overexpression of MMP-19 by comparison with HaCaT carrying the empty vector (HaCaT-pIRES), and those that displayed highest expression (HaCaT-WT#2,4,8 and HaCaT-EA#2,5) were used in the following studies (Figure 3A). Stable transfectants were then assayed for proliferation by [3H]thymidine incorporation. We observed a twofold increase in proliferation of HaCaT-WT clones compared with HaCaT-pIRES and HaCaT-EA clones (Figure 3B). Moreover, we could reverse the enhanced proliferation with the MMP inhibitor BB94 (Figure 3B, inset).
Figure 3.
Increased proliferation and proteolysis of IGFBP-3 in keratinocytes overexpressing wild-type MMP19. (A) Several clones of HaCaT transfected with plasmids coding for the wild-type (HaCaT-WT) and for a functional knockout mutant of MMP-19 (HaCaT-EA) were assayed for overexpression of MMP-19 by comparison with HaCaT carrying the empty vector (V) by using Western blotting. (B) Independent clones of HaCaT-WT (WT), HaCaT-EA (EA), and HaCaT-pIRES (vector) were seeded at 15,000 cells/well in microtiter plates and grown for 24 h in standard culture medium. At the end of the incubation period, cells were pulsed with 0.25 μCi/well [3H]thymidine for an additional 4 h. Values obtained for cells carrying the empty vector were considered 100%. Given are the means ± SEM (n = 3). *, significantly different from HaCaT-pIRES with p ≤ 0.05. Inset, effect of the hydroxamate MMP-inhibitor BB94 on the proliferation of HaCaT-WT2 cells. (C) HaCaT-WT2 (WT2), HaCaT-EA2 (EA2), and HaCaT-pIRES (vector) were grown for 72 h in keratinocyte-SFM. Conditioned media were concentrated 10-fold and analyzed for IGFBP-3 proteolysis by Western blotting. The arrowhead indicates the position of intact IGFBP-3, whereas arrows point to 30- and 19-kDa IGFBP-3 proteolytic fragments. This experiment is representative of three independent experiments. (D) rhIGFBP-3 (240 μg/ml) was incubated with GST-MMP-19 (300 ng/ml) in TNC buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, 0.05% Brij 35, 20 μM ZnCl2, pH 7.5) at 37°C. Before starting the reaction, a 10-μl sample was taken, whereas the reaction-tube was still on ice, representing the intact IGFBP-3. At the indicated time points, samples were taken and immediately mixed with Laemmli buffer. The samples were run on a 12% SDS-polyacrylamide gel under reducing conditions. The arrowhead indicates the position of intact IGFBP-3, whereas arrows point to IGFBP-3 proteolytic fragments.
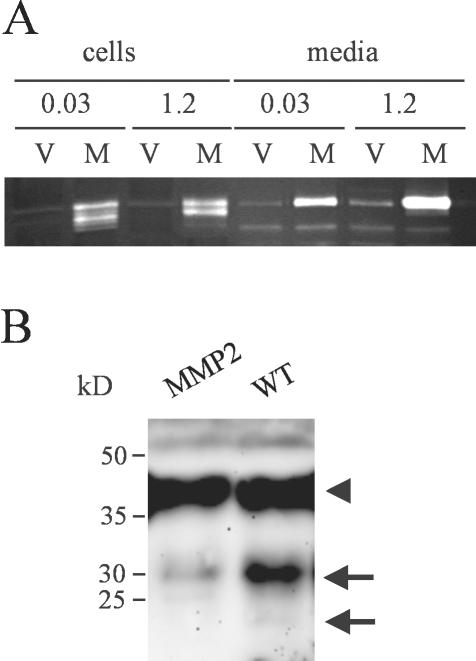
Previous studies have demonstrated that MMPs can regulate cell proliferation by degrading IGFBPs (Manes et al., 1999a). Therefore, we looked for degradation of IGFBP-3, the major IGFBP produced by HaCaT cells, in conditioned media of the stable cell clones. For these experiments, cells were grown in keratinocyte-SFM containing 0.03 mM CaCl2 because this condition favors production of IGFBP-3 (Edmondson et al., 2001). Media of HaCaT-WT2 cells contained an intact 46-kDa form of IGFBP-3 corresponding to the glycosylated molecule and proteolytic fragments of ∼30 and 19 kDa. These fragments were present in significantly reduced amounts in media from HaCaT-pIRES and were almost absent from media of HaCaT-EA2 cells, indicating a dominant-negative effect of the inactive MMP-19 mutant (Figure 3C). We tried to reproduce this effect on IGFBP-3 with HaCaT stably overexpressing MMP-2 (HaCaT-MMP2), which has previously been shown to degrade IGFBP-3 in vitro (Fowlkes et al., 1994). Significant overexpression was obtained in the clone used for this study. However, active forms of MMP-2 were only detected in cell lysates, presumably representing cell surface activated MMP-2. Furthermore, a slight increase in the amount of secreted MMP-2 was observed in 1.2 mM CaCl2 (Figure 4A). In comparison with HaCaT-WT media, only a negligible portion of IGFBP-3 was degraded to a 30-kDa fragment (Figure 4B).
Figure 4.
MMP-2 does not contribute to the degradation of IGFBP-3 in HaCaT keratinocytes. (A) Conditioned media and lysates of HaCaT-MMP2 (M) grown for 3 d in keratinocyte-SFM with 0.03 mM and 1.2 mM calcium were analyzed for overexpression of MMP-2 by comparison with HaCaT carrying the empty vector (V) by using gelatin zymography. Although media contained only the MMP-2 proform, processed MMP-2 was found to be associated with the cells. (B) HaCaT-WT (WT) and HaCaT-MMP2 (MMP2) were grown for 72 h in keratinocyte-SFM. Conditioned media were concentrated 10-fold and analyzed for IGFBP-3 proteolysis by Western blotting. The arrowhead indicates the position of intact IGFBP-3, whereas arrows point to 30- and 19-kDa IGFBP-3 proteolytic fragments. This experiment is representative of three independent experiments.
To confirm that MMP-19 was responsible for IGFBP-3 degradation, we used a cell-free assay in which unglycosylated recombinant human IGFBP-3 was incubated with a purified GST-fusion protein of MMP-19. GST-MMP19 degraded the unglycosylated rhIGFBP-3 in a time-dependent manner into three distinct fragments. A major product was generated with an apparent molecular weight of ∼19 kDa (Figure 3D).
Degradation of IGFBP-3 by MMP-19 Leads to Activation of IGF-IR
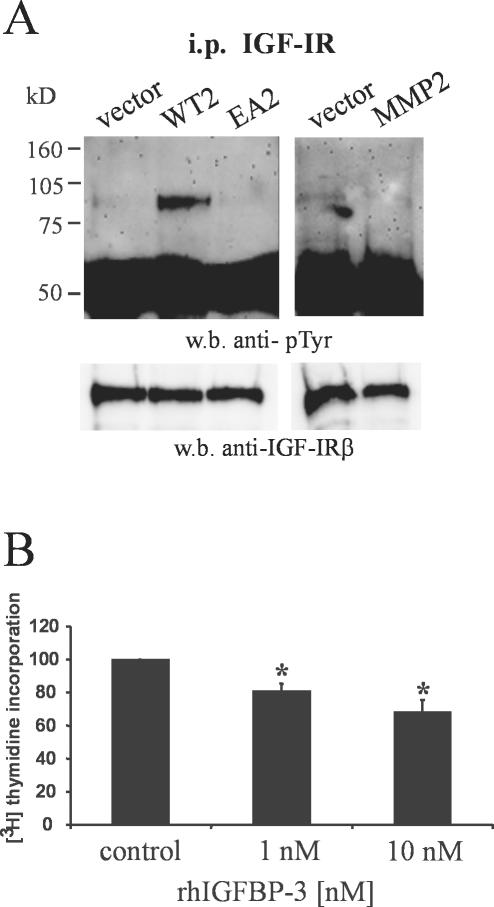
Having identified IGFBP-3 as a novel substrate for MMP-19, we then determined whether its degradation results in increased bioavailability of IGF. Because IGF-IR becomes tyrosine phosphorylated after binding of IGF, we examined steady-state IGF-IR autophosphorylation in MMP-19–transfected HaCaT cells, grown in serum-containing medium. A clear increase in the tyrosine phosphorylation of the 98-kDa IGF-IR β-subunit was observed in HaCaT-WT2, whereas both HaCaT-pIRES and HaCaT-EA2 cells displayed only minimal IGF-IR phosphorylation (Figure 5A). In accordance with the data obtained on IGFBP-3 proteolysis no phosphorylated IGF-IR could be detected in HaCaT-MMP2 cells (Figure 5A)
Figure 5.
Wild-type MMP-19 increases IGF-triggered cell signaling in keratinocytes. (A) IGF-IR was immunoprecipitated from HaCaT-WT2 (WT2), HaCaT-EA2 (EA2), HaCaT-MMP2 (MMP2), and HaCaT-pIRES (vector). Precipitates were immunoblotted and membranes were probed sequentially with anti-phosphotyrosine and polyclonal anti-IGF-IR antibodies. (B) HaCaT-WT2 cells were seeded at 15,000 cells/well in microtiter plates and grown for 48 h in standard culture medium in the absence (control) or presence of 1 and 10 nM rhIGFBP-3. Values obtained without rhIGFBP-3 were considered 100%. Given are the means + SEM (n = 3). *, significantly different from control with p < 0.05.
To further confirm that degradation of IGFBP-3 is responsible for increased proliferation in MMP-19–overexpressing cells, we determined [3H]thymidine incorporation in the presence of exogenously added rhIGFBP-3. These experiments revealed that the proliferation of HaCaT-WT2 keratinocytes was dose dependently inhibited by rhIGFBP-3 (Figure 5B). HaCaT-pIRES and HaCaT-EA2 cells were not sensitive in their proliferation to the addition of rhIGFBP-3 (our unpublished data). In these cells, the amount of bioavailable IGFs might not have been further reduced by exogenously added IGFBP-3, which is in accordance with the minimal stimulation of the IGF-IR that was observed in these cells (Figure 5A).
MMP-19 Promotes Wound Healing In Vitro via Increased Bioavailability of IGF
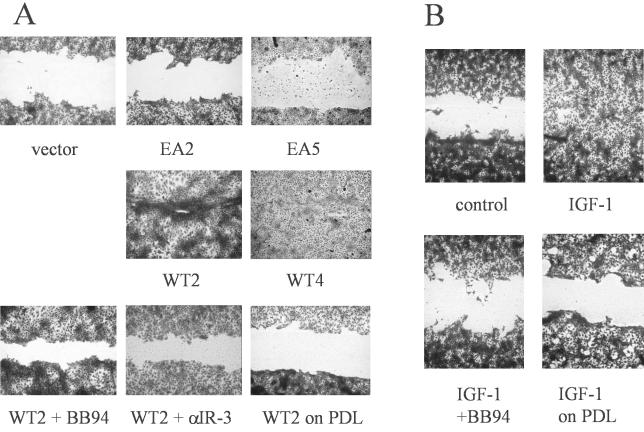
In an effort to determine further cellular functions mediated by enforced expression of MMP-19, we tested stable transfectants in a scratch assay on type I collagen, considered to be an in vitro model for keratinocyte mgration during wound healing. Scratch closure was completed after 48 h in HaCaT-WT clones, whereas HaCaT-EA clones and HaCaT-pIRES cells could not recover the denuded area within this time. Migration by HaCaT overexpressing MMP19WT was inhibited by the broad-spectrum metalloproteinase inhibitor BB94, indicating that the effect was dependent on MMP-19 activity. In addition, scratch closure did not occur on the nonintegrin substrate poly-d-lysine (Figure 6A). Because HaCaT-WT keratinocytes displayed increased IGF-triggered cell signaling, MMP-19 activity in this assay might either be needed for providing free active IGF, which could then trigger migration as has been described for other substrates (Ando and Jensen, 1993; Mira et al., 1999) or for the migratory process itself. The contribution of IGF-mediated proliferation to scratch closure was excluded by the treatment of cells with hydroxyurea.
Figure 6.
MMP-19 regulates IGF-stimulated migration on type I collagen. (A) Independent clones of HaCaT-WT (WT), HaCaT-EA (EA), and HaCaT-pIRES (vector) were seeded on type I collagen. At confluence, a cell-free area was introduced and migration was evaluated after 48 h. Recovery of the denuded area by HaCaT-WT2 was inhibited in the presence of 1 μM BB94 and 1 μg/ml anti-IGF-IR antibody (αIR3), and did not occur when cells were seeded on the nonintegrin substrate poly-d-lysine (PDL). (B) HaCaT cells were seeded on type I collagen. At confluence, a cell-free area was introduced and cells were left untreated (control) or treated with 100 ng/ml IGF-I in the presence or absence of 1 μM BB94. In addition, migration of HaCaT was evaluated on PDL in the presence of 100 ng/ml IGF-I.
We addressed the role of IGF in keratinocyte migration by determining scratch closure by HaCaT-WT2 cells in the presence of an IGF-IR neutralizing antibody (αIR-3). Figure 6A shows that this treatment completely abolished migration on type I collagen, confirming that providing bioavailable IGF is one mechanism by which MMP-19 promotes scratch closure. We next determined whether parental HaCaT cells migrate on type I collagen in response to IGF-I. Scratch closure was completed after 48 h in the presence of IGF-I. The migratory response to IGF-I was, however, inhibited by BB94 and did not occur on the nonintegrin substrate poly-d-lysine (Figure 6B). To determine whether MMP-19 activity is needed for IGF-driven scratch closure, we stained IGF-I–treated and untreated keratinocytes cultured on type I collagen with a mAb against MMP-19. The staining revealed equal expression of MMP-19 in treated and untreated cells. Specifically, no up-regulation was observed in those keratinocytes induced to migrate into the denuded area (our unpublished data).
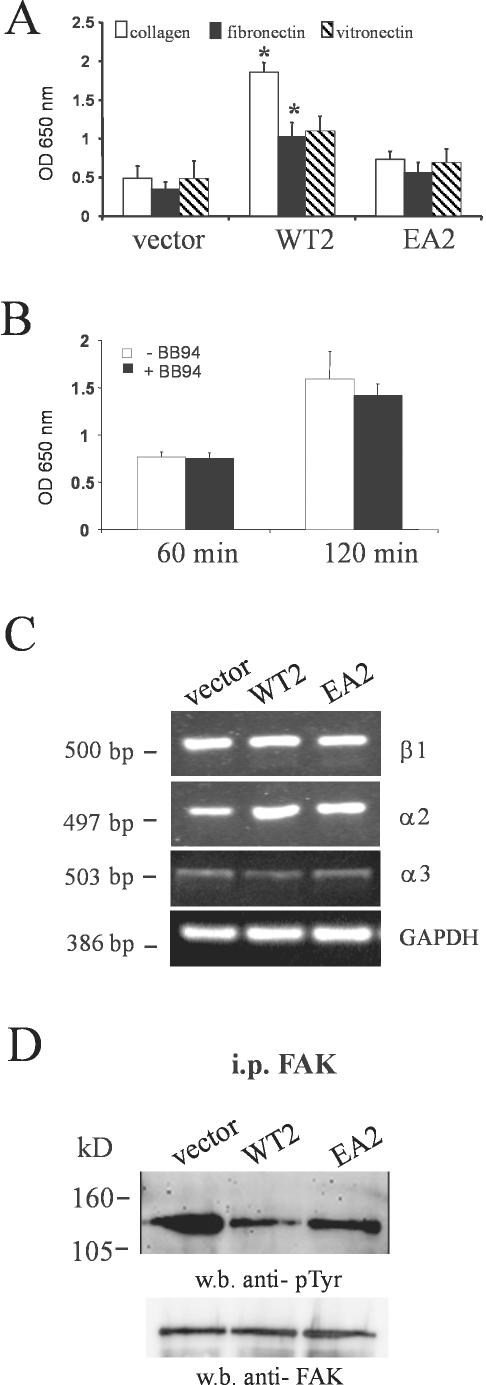
Keratinocytes Overexpressing MMP-19 Display Increased Adhesion on Type I Collagen Due to Dephosphorylation of FAK
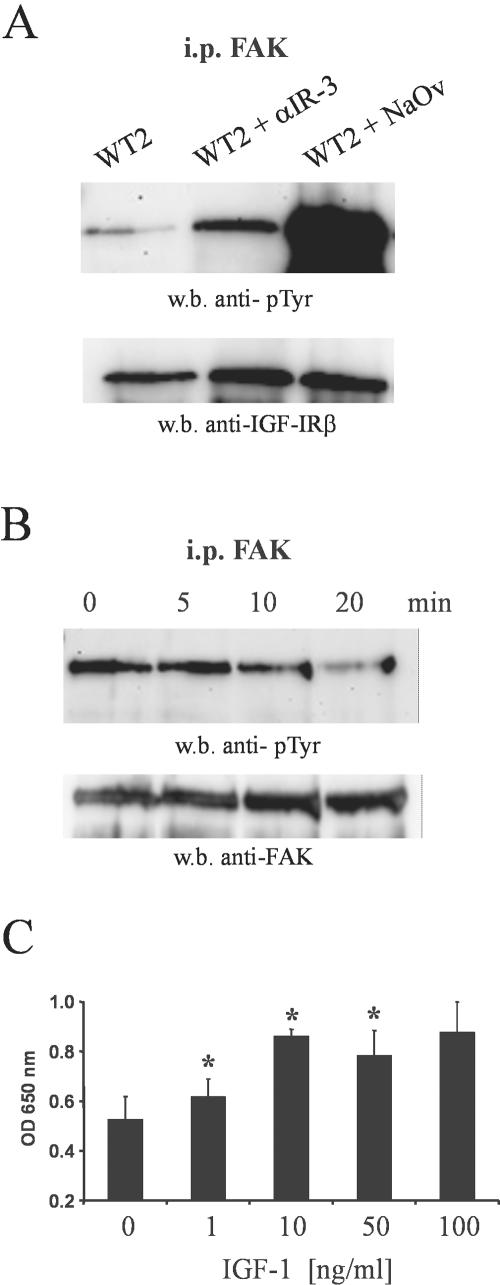
Cell migration is a dynamic interplay between adhesion to the extracellular matrix, leading to organization of the actin cytoskeleton, and deadhesion by inactivating the focal adhesion formed. Therefore, we hypothesized that MMP-19 would also be involved in regulating adhesive properties of keratinocytes. HaCaT cells expressing MMP19WT, MMP19EA, or the empty vector were seeded onto culture dishes coated with type I collagen, fibronectin, and vitronectin. After 2 h, nonadherent cells were washed away and attached cells were quantified. HaCaT-WT2 cells showed an increase in adhesiveness to the collagen matrix compared with HaCaT-EA2 and HaCaT-pIRES cells. They also adhered much stronger to collagen than to fibronectin and vitronectin, whereas no significant difference in adhesiveness to the three substrates was observed for MMP19EA- or vector-transfected cells (Figure 7A). Thus, we concluded that overexpression of MMP-19 specifically augments adhesion to type I collagen. Of note, that increase of adhesion could not be inhibited by pretreating the cells with BB94 (Figure 7B). The adhesion of keratinocytes to type I collagen is mediated by α2β1 and/or α3β1 integrins. However, no differences in integrin expression were found between HaCaT expressing MMP-19WT, MMP-19EA, or the empty vector (Figure 7C). Therefore, we next determined whether increased adhesion of HaCaT-WT cells could be attributed to integrin activation, priming the cells for attachment on the substratum. Activation of integrins involves clustering at focal adhesions and tyrosine phosphorylation of FAK and other cytoplasmic proteins. Immunoprecipitation of cell lysates with anti-FAK, followed by immunoblotting with anti-phosphotyrosine, however, revealed that steady-state tyrosine phosphorylation levels of FAK were decreased in HaCaT-WT2 relative to HaCaT-pIRES and HaCaT-EA2 cells (Figure 7D). FAK has been reported to be a substrate of the IGF-IR (Baron et al., 1998), which, depending on the cell type, is either phosphorylated or dephosphorylated after stimulation of IGF-IR. Therefore, we next analyzed the effect of an IGF-IR neutralizing antibody (αIR-3) on tyrosine phosphorylation of FAK in HaCaT-WT2 cells. Figure 8A shows that this treatment significantly increased steady-state tyrosine phosphorylation levels of FAK. An enhancement of phosphorylation was also observed after treatment of the cells with the phosphatase inhibitor sodium orthovanadate. Because it has been reported that IGF-IR may phosphorylate FAK in nonadhered cells (Baron et al., 1998), we analyzed FAK phosphorylation in pIRES-MMP19WT-, pIRES-MMP19EA-, and vector-transfected HaCaT that were kept in suspension for different time points. FAK phosphorylation was also not increased in MMP-19WT–overexpressing cells under these conditions (our unpublished data). We next determined the effect of IGF-I on the phosphorylation of FAK and adhesion of parental HaCaT on a type I collagen matrix. IGF-I decreased FAK phosphorylation in a time-dependent manner (Figure 8B) and increased adhesion to type I collagen in a dose-dependent manner (Figure 8C).
Figure 7.
Increased adhesion of HaCaT-WT cells is not dependent on altered integrin expression but correlates with dephosphorylation of FAK. (A) HaCaT-WT2 (WT2), HaCaT-EA2 (EA2), and HaCaT-pIRES (vector) were seeded on type I collagen, vitronectin, and fibronectin matrices and were allowed to adhere for 2 h. Nonadherent cells were then washed away and attached cells were quantitated as described under MATERIALS AND METHODS. Given are the means + SEM (n = 3). *, significantly different from HaCaT-pIRES with p <0.05. (B) Time course of HaCaT-WT2 adhesion to type I collagen after pretreatment of the cells with BB94 (1 μM). (C) For evaluating the mRNA expression of integrins in HaCaT-WT2 (WT2), HaCaT-EA2 (EA2), and HaCaT-pIRES (vector), total RNA was isolated and analyzed by RT-PCR with primers specific for β1, α2, and α3 integrin. GAPDH was used as an internal control. Results are representative of three experiments. (D) FAK was immunoprecipitated from HaCaT-WT2 (WT2), HaCaT-EA2 (EA2), and HaCaT-pIRES (vector). Precipitates were immunoblotted and membranes were probed sequentially with anti-phosphotyrosine and polyclonal anti-FAK antibodies.
Figure 8.
MMP-19–induced activation of the IGF-IR–mediated dephosphorylation of FAK and increased adhesion to type I collagen. (A) HaCaT-WT2 cells (WT2) were incubated for 24 h with 1 μg/ml anti-IGF-IR antibody (αIR-3) or 100 nM sodium orthovanadate (NaOv). FAK was immunoprecipitated from the lysates and precipitates were immunoblotted. Membranes were probed sequentially with anti-phosphotyrosine and polyclonal anti-FAK antibodies. (B) HaCaT cells were serum starved for 24 h. IGF-I (100 ng/ml) was added and incubated for the indicated time points. FAK was immunoprecipitated from the lysates and analyzed for phosphorylation as described above. (C) HaCaT was incubated with the indicated concentrations of IGF-I for 24 h under serum-free conditions. Cells were then trypsinized and seeded on type I collagen and were allowed to adhere for 2 h. Nonadherent cells were then washed away and attached cells were quantified as described under MATERIALS AND METHODS. Given are the means + SEM (n = 3). *, significantly different from control with p < 0.05.
DISCUSSION
MMP expression in the skin is observed during wound healing, inflammation, and neovascularization. This has led to the hypothesis that a major function of MMPs in the skin is degradation or remodeling of the extracellular matrix to facilitate cell migration of resident or inflammatory cells (Birkedahl-Hansen et al., 1993; Parks and Mecham, 1998; Vu and Werb, 2000). The results reported here reveal a distinct role of MMP-19 in human epidermis because it is constitutively expressed in undifferentiated keratinocytes and influences cell proliferation and migration by increasing the bioavailability of IGF.
Our experiments with the human keratinocyte cell line HaCaT might indicate that MMP-19 is down-regulated in the suprabasal compartment by the increased calcium concentration, that are found in these layers (Bikle and Pillai, 1993). Calcium is able to induce keratinocyte differentiation through receptor binding (Oda et al., 1998) or a functional activation of cadherins (Hodivala and Watt, 1994; Lewis et al., 1994). Because calcium did only suppress MMP-19 at high cell densities, it is unlikely that this calcium effect was mediated by activation of the calcium receptor. This is also supported by the previous observations that calcium does not affect MMP-19 in primary keratinocytes when cultured at low density (Impola et al., 2003; Sadowski et al., 2003). Therefore, we rather suggest that the calcium-dependent formation of E-cadherin–mediated cell-cell contacts is responsible for the observed down-regulation of MMP-19. Regarding the distribution of E-cadherin expression in human epidermis, we further assume, that as keratinocytes move vertically through the spinous and granular layers, increasing calcium concentration promotes E-cadherin–mediated cell-cell contacts that in turn switch off the expression of MMP-19 in suprabasal layers.
E-Cadherins sequester β-catenin at the cell surface, which upon release can interact with the adenomatosis polyposis coli tumor suppressor protein as well as transcription factors of the T cell factor family, inducing the regulation of several genes, e.g., MMP-7 (Brabletz et al., 1999; Crawford et al., 1999). Although intriguing, this might not apply for the regulation of MMP-19, because inhibition of E-cadherin–mediated intercellular adhesion by the antibody DECMA-1 does not lead to a translocation of β-catenin to the cell nucleus (St. Croix et al., 1998).
In accordance with its expression in suprabasal layers of the epidermis, MMP-9 was induced in keratinocytes after the treatment with high calcium concentrations (Kobayashi et al., 1998). During the analysis of HaCaT-MMP2, we also found that high calcium slightly increased levels of MMP-2 in the media. In contrast to recently published data on calcium regulation of MMP-2 in a squamous carcinoma cell line (Munshi et al., 2002), we could not detect any differences in processing of MMP-2 in low and high calcium concentrations. Unlike squamous carcinoma cell, HaCaT are considered nonmalignant, and the activation cascade proposed by Munshi et al. (2002) might therefore only occur in the process of malignant transformation and presumably does not represent a feature of untransformed keratinocytes.
A major finding of this study is that MMP-19 is a potent IGFBP-3 proteinase, because moderate MMP-19 overexpression by stable transfection of HaCaT was sufficient to induce strong degradation of cell-derived IGFBP-3. From previous studies and our own work (Wraight et al., 1997; our unpublished data), we knew that IGFBP-3 expression localizes to the same epidermal compartment as MMP-19. Because proteolytic cleavage of IGFBP-3 in HaCaT-WT cells released IGFs, which then exerted mitogenic effects through IGF-IR, we propose, that MMP-19 takes over similar functions in the stratum basale, thereby maintaining proliferation of basal keratinocytes. In contrast, the growth-promoting effect of MMP-9 by degradation of IGFBP-3, as has been demonstrated in a prostate carcinoma cell line (Manes et al., 1999a), is hard to reconcile with an expression of MMP-9 in the differentiated compartment of human epidermis and the described growth inhibitory effects on keratinocytes, both in vitro and in vivo (Buisson-Legendre et al., 1999; Mohan et al., 2002). Similarly, degradation of IGFBP-3 by MMP-1, -2, and -3, which has originally been found in dermal fibroblasts (Fowlkes et al., 1994), possibly is not important for proliferation of basal keratinocytes, because these MMPs are not expressed in normal, healthy skin. Thus, although IGFBP-3 is not a specific substrate of MMP-19, the spatial distribution of the two molecules in the skin makes MMP-19 a very likely candidate to be the major IGFBP-3 degrading MMP in the quiescent epidermis. We recently reported on the dysregulated expression of MMP-19 in cutaneous diseases exhibiting aberrant epidermal proliferation, such as psoriasis, eczema, and tinea. Under these conditions MMP-19 was found in suprabasal and spinous epidermal layers. Moreover, the processed, activated form of MMP-19 predominates in psoriatic lesion (Sadowski et al., 2003). Because it has been shown that IGFBP-3 as well as the IGF-R are up-regulated in psoriasis (Krane et al., 1992; Wraight et al., 1997), overexpression and increased activation of MMP-19 might be involved in propagation of aberrant proliferation as observed for psoriasis.
MMPs have traditionally been implicated in cell motility and invasion by virtue of their ability to degrade ECM components. Thus, a role in keratinocyte migration on type I collagen has been proposed for MMP-1, which by cleaving its principal substrate might facilitate keratinocyte movement (Pilcher et al., 1997). Conversely, our data demonstrate, that MMP-19, which cannot degrade fibrillar collagen (Stracke et al., 2000), nonetheless can induce migration on type I collagen by providing bioavailable IGF. Interestingly, the motility enhancing effect of IGF on parental HaCaT was inhibited by BB94 and did not occur on the nonintegrin substrate poly-d-lysine. Therefore, in this experimental system it seems that cooperation between IGF, integrins, and a metalloproteinase activity, which, however, was different from MMP-19, was necessary for migration to occur. Although similar phenomena have been previously observed in vitro (Doerr and Jones, 1996; Mira et al., 1999), our data show that in this interplay MMPs can take over a central role by either providing growth factors and/or cleaving ECM substrates. In this respect, it is of interest that MMP-19 expression is up-regulated in HaCaT keratinocytes upon adhesion to type I collagen (Sadowski et al., 2003). From this, we assume an autocrine loop in which after contact of keratinocytes to collagen an increase in MMP-19 activity would provide free IGF, which then could trigger migration by the activation of other MMP(s).
Cell migration in a two-dimensional plane is a dynamic interplay between adhesion to the extracellular matrix, leading to organization of the actin cytoskeleton, and deadhesion by inactivating the focal adhesion formed. It has been proposed that an intermediate level of cell adhesiveness is necessary for motility as otherwise the detachment step would be impaired (Palecek et al., 1997). Phosphorylation of FAK contributes to the stabilization of focal contacts (Schwartz et al., 1995), whereas inhibition of FAK phosphorylation reduces cell adhesion (Miyamoto et al., 1995). Conversely, FAK phosphorylation is crucial for β5-mediated cell migration (Brooks et al., 1997) and dephosphorylation of FAK by PTEN has been reported to inhibit cell migration (Tamura et al., 1998). Furthermore, FAK-deficient fibroblasts show an increased number of focal adhesions, strong adhesiveness, and impaired migration (Ilic et al., 1995). From these observations, it has been concluded that FAK might be involved in the turnover of focal adhesions during cell migration rather than in the formation of focal contacts (Ilic et al., 1995). Our results are in agreement with this view, because we found that decreased levels of FAK phosphorylation in HaCaT-WT cells correlated with increased adhesiveness. Furthermore, our data corroborate previous reports that FAK is a substrate of the IGF-IR (Baron et al., 1998), and add the new finding that it is dephosphorylated in HaCaT keratinocytes. Dephosphorylation of FAK has been shown to be mediated by recruitment of the tyrosine phosphatase SHP-2 to the IGF-IR after stimulation (Manes et al., 1999b). Nevertheless, the question remains, how the effects of IGF-I on adhesion can be reconciled with its ability to promote migration on the same substrate. Manes et al. (1999b), who observed very similar effects of IGF on the adhesion and migration of MCF-7 cells on vitronectin have proposed a two-step model. The activated IGF-IR initially promotes cell adhesion to the substratum by phosphorylating FAK, which is subsequently weakened by the recruitment of SHP-2 leading to FAK dephosphorylation. Indeed, there are other examples showing a physical interaction of IGF-IR and integrins (Shakibaei et al., 1999; Hermanto et al., 2002), which might play a role in the formation of focal contacts. However, these models have in common that FAK dephosphorylation should lead to enhanced motility and thus contradict our and previous results (Brooks et al., 1997; Tamura et al., 1998). We therefore suggest an alternative model, in which dephosphorylation of FAK by the activated IGF-IR stabilizes focal contacts due to impaired turnover. Detachment then is mediated by the activation of proteinases such as MMPs, which cleave the substrate and thereby relieve the cell from its “docked” state. On encountering uncleaved substrate the cell will again adhere until the substrate is cleaved. In agreement with this idea, inhibition of MMPs by BB94 readily inhibited migration on type I collagen, whereas it had no effect on adhesion. Further support is lend by a recent study in which the inhibition of MMP activity increased adhesion of fibroblasts through stabilization of focal adhesion contacts (Ho et al., 2001).
Keratinocyte migration is needed for reepithelialization after tissue injury. Proteolysis of IGFBP-3 could be important in this situation, because otherwise the increased amounts of IGF-I that are supplied by platelet degranulation and fibroblasts might not become available to keratinocytes. However, various IGFBP-3 proteases are found in the wound, such as, for example, the neutrophil enzymes cathepsin G and elastase as well as several MMPs (for review, see Pilcher et al., 1999). Their precise temporal and spatial expression indicates distinct functions in the wound healing process. Thus, MMP-1 and MMP-10 were found at the leading edge of migrating cells, with MMP-1 being necessary and sufficient for migration over collagen. Conversely, MMP-19 colocalizes with MMP-3 and MMP-28 to hyperproliferative keratinocytes behind the migrating front (Impola et al., 2003). Because MMP-1 and MMP-3 have been reported to cleave IGFBP-3, the specific contribution of MMP-19 to proteoloysis of IGFBP-3 remains to be established. Conversely, the colocalization of IGFBP-3 and MMP-19 in the quiescent epidermis where other MMPs, except for the recently cloned MMP-28 (Lohi et al., 2001; Saarialho-Kere et al., 2002), are absent, suggests that this enzyme is a likely candidate to be the major IGFBP-3 degrading MMP in the quiescent epidermis. This activity might have widespread consequences for the behavior of epidermal keratinocytes.
Acknowledgments
We thank Prof. E. Proksch and Prof. T. Braulke for helpful discussions concerning skin pathology and IGFBP-3, respectively. We thank Prof. N. Fusenig of German Cancer Research Center (Heidelberg, Germany) for HaCaT cells, Elsbeth Schultz for excellent technical assistance, Vance Matthews for critical reading of the manuscript, and Prof. S. Rose-John for constructive comments throughout this work. This work was supported by grants from the Deutsche Forschungsgemeinschaft given to R.S. (SE 878/2-1 and SFB 617/A8).
References
- Ando, Y., and Jensen, P.J. (1993). Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J. Investig. Dermatol. 100, 633-639. [DOI] [PubMed] [Google Scholar]
- Baron, V., Calleja, V., Ferrari, P., Alengrin, F., and van Obberghen, E. (1998). p125FAK focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tryrosine kinase receptors. J. Biol. Chem. 273, 7162-7168. [DOI] [PubMed] [Google Scholar]
- Bikle, D.D., and Pillai, S. (1993). Vitamin D, calcium, and epidermal differentiation. Endocr. Rev. 14, 3-19. [DOI] [PubMed] [Google Scholar]
- Birkedahl-Hansen, H., Moore, W.G., Bodden, M.-K., Windsor, L.J., Birkedahl-Hansen, B., DeCarlo, A., and Engler, J.-A. (1993). Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. 4, 197. [DOI] [PubMed] [Google Scholar]
- Brabletz, T., Jung, A., Dag, S., Hlubek, F., and Kirchner, T. (1999). beta-Catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 155, 1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, P.C., Klemke, R.L., Schoen, S., Lewis, J.M., Schwartz, M.A., and Cheresh, D.A. (1997). Insulin-like growth factor receptor cooperates with integrin αvβ5 to promote tumor cell dissemination in vivo. J. Clin. Investig. 99, 1390-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson-Legendre, N. Bernard, P., Bobichon, H., Emonard, H., Schneider, C., Maquart, F.X., Haye, B., and Hornebeck, W. (1999). Involvement of the 92-kDa gelatinase (matrix metalloproteinase-9) in the ceramide-mediated inhibition of human keratinocyte growth. Biochem. Biophys. Res. Commun. 260, 634-640. [DOI] [PubMed] [Google Scholar]
- Cohen, P., Graves, H.C., Peehl, D.M., Kamarei, M., Guidice, L.C., and Rosenfeld, R.G. (1992). Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J. Clin. Endocrinol. Metab. 75, 1046-1053. [DOI] [PubMed] [Google Scholar]
- Conover, C.A., and De Leon, D.D. (1994). Acid-activated insulin-like growth factor-binding protein-3 proteolysis in normal and transformed cells. Role of cathepsin D. J. Biol. Chem. 269, 7076-7080. [PubMed] [Google Scholar]
- Crawford, H.C., Fingleton, B.M., Rudolph-Owen, L.A., Goss, K.J., Rubinfeld, B., Polakis, P., and Matrisian, L.M. (1999). The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene 18, 2883-2891. [DOI] [PubMed] [Google Scholar]
- Doerr, M.E., and Jones, J.I. (1996). The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J. Biol. Chem. 271, 2443-2447. [DOI] [PubMed] [Google Scholar]
- Edmondson, S.R., Werther, G.A., and Wraight, C.J. (2001). Calcium regulates the expression of insulin-like growth factor binding protein-3 by the human keratinocyte cell line HaCaT. J. Investig. Dermatol. 116, 491-497. [DOI] [PubMed] [Google Scholar]
- Fowlkes, J.L., Enghild, J.J., Suzuki, K., and Nagase, H. (1994). Matrix metalloproteinases degrade insulin-like growth factor binding protein-3 in dermal fibroblast cultures. J. Biol. Chem. 269, 25742-25746. [PubMed] [Google Scholar]
- Hangauer, D.G., Monzingo, A.F., and Matthews, B.W. (1984). An interactive computer graphics study of thermolysin-catalyzed peptide cleavage and inhibition by N-carboxymethyl dipeptides. Biochemistry 23, 5730-5741. [DOI] [PubMed] [Google Scholar]
- Hermanto, U., Zong, C.S., Li, W., and Wang, L.H. (2002). RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol. Cell. Biol. 22, 2345-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, A.T., Voura, E.B., Soloway, P.D., Watson, K.L.M., and Khokha, R. (2001). MMP inhibitors augment fibroblast adhesion through stabilization of focal adhesion contacts and up-regulation of cadherin function. J. Biol. Chem. 276, 40215-40224. [DOI] [PubMed] [Google Scholar]
- Hodivala, K.J., and Watt, F.M. (1994). Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 124, 589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M., and Yamamoto, T. (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539-544. [DOI] [PubMed] [Google Scholar]
- Impola, U., Toriseva, M., Suomela, S., Jeskanen, L., Hieta, N., Jahkola, T., Grenman, R., Kahari, V.M., and Saarialho-Kere, U. (2003). Matrix metalloproteinase-19 is expressed by proliferating epithelium but disappears with neoplastic dedifferentiation. Int. J. Cancer 103, 709-716. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., Onoda, N., Takagi, T., Hori, H., Hattori, S., Nagai, Y., Tajima, S., and Nishikawa, T. (1996). Immunolocalizations of human gelatinase (type IV collagenase, MMP-9) and TIMP (tissue inhibitor of metalloproteinases) in normal epidermis and some epidermal tumors. Arch. Dermatol. Res. 288, 239-244. [DOI] [PubMed] [Google Scholar]
- Koshizuka, S., Kanazawa, K., Kobayashi, N., Takazawa, I., Waki, Y., Shibusawa, H., and Shumiya, S. (1997). The beneficial effects of recombinant human insulin-like growth factor-I (IGF-I) on wound healing in severely wounded senescent mice. Surg. Today 27, 946-952. [DOI] [PubMed] [Google Scholar]
- Krane, J.F., Gottlieb, A.B., Carter, D.M., and Krueger, J.G. (1992). The insulin-like growth factor I receptor is overexpressed in psoriatic epidermis, but is differentially regulated from the epidermal growth factor receptor. J. Exp. Med. 175, 1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J.E., Jensen, P.J., and Wheelock, M.J. (1994). Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J. Investig. Dermatol. 102, 870-877. [DOI] [PubMed] [Google Scholar]
- Lohi, J., Wilson, C.L., Roby, J.D., and Parks, W.C. (2001). Epilysin. A novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. J. Biol. Chem. 276, 10134-10144. [DOI] [PubMed] [Google Scholar]
- Liu, J.P., Baker, J., Perkins, A.S., Robertson, E.J., and Efstratiadis, A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59-72. [PubMed] [Google Scholar]
- Lund, L.R., Rømer, J. Bugge, T.H., Nielsen, B.S., Frandsen, T.L., Degen, J.L., Stephens, R.W., and Danø, K. (1999). Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 18, 4645-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes, S., Llorente, M., Lacalle, R.A., Gomez-Mouton, C., Kremer, L., Mira, E., and Martinez-A., C. (1999a). The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in D.U-145 carcinoma cells. J. Biol. Chem. 274, 6935-6945. [DOI] [PubMed] [Google Scholar]
- Manes, S., Mira, E., Gomez-Mouton, C., Zhao, Z.J., Lacalle, R.A., and Martinez-A. C. (1999b). Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 19, 3125-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch, S., Kolb, C., Kolb, B., Sadowski, T., and Sedlacek, R. (2002). Matrix metalloproteinase-19 is expressed in myeloid cells in an adhesion-dependent manner and associates with the cell surface. J. Immunol. 168, 1244-1251. [DOI] [PubMed] [Google Scholar]
- McCusker, R.H., Busby, W.H., Dehoff, M.H., Camacho-Hubner, C., and Clemmons, D.R. (1991). Insulin-like growth factor (IGF) binding to cell monolayers is directly modulated by the addition of IGF-binding proteins. Endocrinology 129, 939-949. [DOI] [PubMed] [Google Scholar]
- Mira, E., Manes, S., Lacalle, R.A., Marquez, G., and Martinez-A, C. (1999). Insulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9. Endocrinology 140, 1657-1664. [DOI] [PubMed] [Google Scholar]
- Miyamoto, S., Teramoto, H., Coso, O., Gutkind, J., Burbelo, P., Akiyama, S., and Yamada, K. (1995). Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131, 791-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan, R. et al. (2002). Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 277, 2065-2072. [DOI] [PubMed] [Google Scholar]
- Morgunova, E., Tuuttila, A., Bergmann, U., Isupov, M., Lindqvist, Y., Schneider, G., and Tryggvason, K. (1999). Structure of human pro-matrix metalloproteinase-2, activation mechanism revealed. Science 284, 1667-1670. [DOI] [PubMed] [Google Scholar]
- Mueller, M.S., Mauch, S., Kolb, C., Kusch, J., Sadowski, T., and Sedlacek, R. (2000). The murine ortholog of matrix metalloproteinase 19, its cloning, gene organization, and expression. Gene 256, 101-111. [DOI] [PubMed] [Google Scholar]
- Munshi, H.G., Wu, Y.I., Ariztia, E.V., and Stack, M.S. (2002). Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J. Biol. Chem. 277, 41480-41488. [DOI] [PubMed] [Google Scholar]
- Oda, Y., Tu, C.L., Pillai, S., and Bikle, D.D. (1998). The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J. Biol. Chem. 273, 23344-23352. [DOI] [PubMed] [Google Scholar]
- Oh, Y. (1997). IGFBPs and neoplastic models. New concepts for roles of IGFBPs in regulation of cancer cell growth. Endocrine 7, 111-113. [DOI] [PubMed] [Google Scholar]
- Oliver, M.H., Harrison, N.K., Bishop, J.E., Cole, P.J., and Laurent, G.J. (1989). A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J. Cell Sci. 92, 513-518. [DOI] [PubMed] [Google Scholar]
- Palecek, S.P., Loftus, J.C., Ginsberg, M.H., Lauffenburger, D.A., and Horwitz, A.F. (1997). Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385, 537-540. [DOI] [PubMed] [Google Scholar]
- Paramio, J.M., and Jorcano, J.L. (1997). Role of protein kinases in the in vitro differentiation of human epidermal HaCaT cells. Br. J. Dermatol. 137, 44-50. [PubMed] [Google Scholar]
- Parks, W.C., and Mecham, R.P. (eds) (1998) Matrix Metalloproteinases, San Diego: Academic Press.
- Pece, S., and Gutkind, J.S. (2000). Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 275, 41227-41233. [DOI] [PubMed] [Google Scholar]
- Pilcher, B.K., Dumin, J.A., Sudbek, B.D., Krane, S.M., Welgus, H.G., and Parks, W.C. (1997). The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J. Cell Biol. 137, 1445-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher, B.K., Wang, M., Qin, X.J., Parks, W.C., Senior, R.M., and Welgus, H.G. (1999). Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N.Y. Acad. Sci. 878, 12-24. [DOI] [PubMed] [Google Scholar]
- Rudman, S.M., Philpott, M.P., Thomas, G.A., and Kealey, T. (1997). The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J. Investig. Dermatol. 109, 770-777. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere, U.K., Kovacs, S.O., Pentland, A.P., Olerud, J.E., Welgus, H.G., and Parks, W.C. (1993). Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J. Clin. Investig. 92, 2858-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere, U.K., Pentland, A.P., Birkedal-Hansen, H., Parks, W.C., and Welgus, H.G. (1994). Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Investig. 94, 79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere, U.K., Kerkelä, E., Jahkola, T., Suomela, S., Keski-Oja, J., and Lohi, J. (2002). Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J. Investig. Dermatol. 199, 14-21. [DOI] [PubMed] [Google Scholar]
- Sadowski, T., Dietrich, S., Mueller, M., Havlickova, B., Schunck, M., Proksch, E., Mueller, M.S., and Sedlacek, R. (2003) MMP-19 expression in normal and diseased skin: dysregulation by epidermal proliferation. J. Investig. Dermatol. (in press). [DOI] [PubMed]
- Schwartz, M., Schaller, M., and Ginsberg, M. (1995). Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell. Dev. Biol. 11, 549-599. [DOI] [PubMed] [Google Scholar]
- Shakibaei, M., John, T., De Souza, P., Rahmanzadeh, R., and Merker, H.J. (1999). Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem. J. 342 Pt 3, 615-623. [PMC free article] [PubMed] [Google Scholar]
- Shimasaki, S., and Ling, N. (1991). Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog. Growth Factor Res. 3, 243-266. [DOI] [PubMed] [Google Scholar]
- Suomela, S., Kariniemi, A., Impola, U., Karvonen, S.L., Snellman, E., Uurasmaa, T., Peltonen, J., and Saarialho-Kere, U. (2003). Matrix Metalloproteinase-19 is expressed by keratinocytes in psoriasis. Acta Derm. Venereol. 83, 108-114. [DOI] [PubMed] [Google Scholar]
- St. Croix, B., Sheehan, C., Rak, J.W., Flørenes, V.A., Slingerland, J.M., and Kerbel, R.S. (1998). E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J. Cell Biol. 142, 557-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke, J.O., Hutton, M., Stewart, M., Pendás, A.M., Smith, B., López-Otín, C., Murphy, G., Knäuper, V. (2000).). Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J. Biol. Chem. 275, 14809-14816. [DOI] [PubMed] [Google Scholar]
- Tamura, M., Gu, J., Matsumoto, K., Aota, S., Parsons, R., and Yamada, K.M. (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614-1617. [DOI] [PubMed] [Google Scholar]
- Tavakkol, A., Elder, J.T., Griffiths, C.E.M., Cooper, K.D., Talwar, H., Fisher, G.J., Keane, K.M., Foltin, S.K., and Voorhees, J.J. (1992). Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J. Invest. Dermatol. 99, 343-349. [DOI] [PubMed] [Google Scholar]
- Todd, C., and Reynolds, N.J. (1998). Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am. J. Pathol. 153, 39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu, T.H., and Werb, Z. (2000). Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 14, 2123. [DOI] [PubMed] [Google Scholar]
- Wraight, C.J., Edmondson, S.R., Fortune, D.W., Varigos, G., and Werther, G.A. (1997). Expression of insulin-like growth factor binding protein-3 (IGFBP-3) in the psoriatic lesion. J. Investig. Dermatol. 108, 452-456. [DOI] [PubMed] [Google Scholar]