Abstract
Liver plays a major role in regulating energy homeostasis in mammals. During feeding conditions, excessive glucose is converted into a preferred storage form of energy sources as triacylglycerol in liver via a collective metabolic pathway termed lipogenesis. Sterol regulatory element-binding protein 1c is a master regulator for this process by activating number of enzyme genes, such as Fasn or Acaca, that are involved in this pathway at the transcriptional level. Here we show that the salt-inducible kinase (SIK) family of proteins regulates the hepatic lipogenesis by modulating SREBP-1c activity. Overexpression of SIK1 inhibits hepatic expression of lipogenic genes, such as Fasn, whereas knockdown of SIK1 in liver greatly enhances their expression. Regulation of the Fasn gene by SIK kinases is mediated at the level of transcription via phosphorylation and inactivation of nuclear SREBP-1c. Among candidate sites for SIK-dependent regulation of SREBP-1c, the serine 329 residue is shown to be a critical regulatory site for SIK-mediated repression of SREBP-1c activity by in vitro kinase assay and reverse transcription-PCR analysis in primary hepatocytes. Finally, reduced hepatic triacylglycerol levels and lipogenic gene expression by adenoviral SIK1 transgenic expression are restored to normal levels by co-infection of mutant SREBP-1c, suggesting that SIK-dependent regulation of hepatic lipogenesis is indeed mediated through the phosphorylation of SREBP-1c in vivo. The process for the development of nonalcoholic fatty liver involves de novo lipogenesis via the activation of SREBP-1c. Modulation of SREBP-1c activity by SIK proteins would provide an attractive means for the regulation of such diseases.
The mammalian liver is a primary organ for regulating energy homeostasis. After the food intake, high levels of glucose are transported to the liver through the bloodstream. Initially, the remnant of glucose not utilized immediately is metabolized into glycogen. For the longer term storage, excess glucose is converted into triglycerides via a collective metabolic pathway termed lipogenesis that includes glycolysis, fatty acid biosynthesis, and triglyceride synthesis and maturation.
The control of lipogenesis during feeding conditions is achieved via the activation of key rate-limiting enzymes both at the enzyme activity and at the level of gene expression (1–4). The latter regulatory mechanism is considered as an adaptive response and, in many cases, is achieved by the enhanced transcription of lipogenic genes, such as Fasn (fatty acid synthase) or Acaca (acetyl-CoA carboxylase α). The control of lipogenic gene expression is coordinately regulated by various hormonal and nutritional cues. Inhibition of such gene expression is achieved by fasting hormone glucagon or its intracellular signal, cAMP-mediated transcriptional repression (5). Expression of genes in this pathway is also down-regulated by polyunsaturated fatty acids, suggesting a mechanism for a feedback inhibition by its end-point product of lipogenesis (6, 7). On the contrary, increased carbohydrate metabolism and insulin actions in liver following food consumption are shown to be responsible for the transcriptional activation of entire lipogenic programs in mammals (8–14).
Sterol regulatory element-binding protein (SREBP)2 is a member of the basic helix loop helix leucine zipper (b/HLH/LZ) transcription factor families that are involved in various pathways of lipid homeostasis (15, 16). SREBP is first synthesized as a precursor form that contains the N-terminal transcription factor domains and C-terminal regulatory domain linked with a central transmembrane domain. Bound in the endoplasmic reticulum via the interaction of SCAP and INSIG proteins, the N-terminal transcription factor is released by sequential proteolytic cleavages in the Golgi apparatus in response to the low sterol conditions (17–20). This process is also shown to be activated during feeding conditions in liver, although the exact mechanism has not been demonstrated to date. Among family members, the SREBP-1c isoform is implicated as a master transcriptional regulator for the hepatic lipogenesis. Insulin is responsible for the transcriptional activation of SREBP-1c as well as its target genes (21, 22). Expression of lipogenic genes, such as Fasn or Acaca, is elevated in liver-specific SREBP-1c transgenic mice, whereas induction of these genes in response to a high carbohydrate diet is impaired in livers of SREBP-1c knock-out mice (23, 24). Recently, nonalcoholic fatty liver disease is shown to be highly associated with metabolic diseases, including diabetes (25, 26). The development of this disease is characterized with increased de novo lipogenesis in part due to the hyperactivation of lipogenic transcription factor SREBP-1c, underscoring the importance of tight regulation of SREBP-1c and hepatic fatty acid synthesis (27, 28).
Although the activity of SREBP-1c is shown to be regulated by protein kinase A or AMP-activated protein kinases (AMPKs), the exact underlying mechanisms have yet to be demonstrated in vivo (29–31). Here we show that SIK1, a member of the AMPK-related kinases, directly regulates SREBP-1c-dependent hepatic lipogenesis. Overexpression of SIK1 reduces lipogenic gene expression, whereas hepatic knockdown of SIK1 leads to the enhanced expression of SREBP-1c target genes. SREBP-1c is shown to be a direct substrate for SIK1 in vitro. Mutation of a major regulatory site serine 329 to alanine on SREBP-1c negates the effects of SIK1 in SREBP-1c target gene expression and hepatic triglyceride levels in mice. These data suggest that SIK1-dependent regulation of SREBP-1c activity would be critical in the modulation of hepatic lipid homeostasis.
EXPERIMENTAL PROCEDURES
Materials—T0901317 was purchased from Cayman Chemical Co.
Cultures of Primary Hepatocytes—Rat primary hepatocytes were prepared from Sprague-Dawley rats, as described (32). After 16 h of adenoviral infection, cells were maintained in serum-free medium 199 (MediaTech) for an additional 24–48 h. Cells were subsequently harvested for reporter assays (Ad-Fasn-Luc) or quantitative PCR analysis.
Transfection Assays—Human hepatoma HepG2 cells were maintained with Ham's F-12 medium (MediaTech) supplemented with 10% fetal bovine serum, 10 units/ml penicillin, and 10 μg/ml streptomycin. Each transfection was performed with 300 ng of luciferase construct, 50 ng of β-galactosidase plasmid, and 2.5–100 ng of expression vector for SIK1, AMPKα2 CA, adiponectin, nSREBP-1c (WT and mutants), or liver X receptor α (LXRα) using Fugene 6 reagent according to the manufacturer's instructions.
Plasmid DNA—pCMV SPORT nSREBP-1c was purchased from Addgene. S265A/S266A, S329A, or S265A/S266A/S329A mutant nSREBP-1c was generated using the QuikChange site-directed mutagenesis kit (Stratagene).
Adenoviruses—Ad-GFP, Ad-SIK1, Ad-US, and Ad-SIK1 RNA interference adenoviruses were described previously (33). Adenoviruses for nSREBP-1c WT, nSREBP-1c S265A/S266A/S329A, and Fasn luciferase were generated as described.
Animal Experiments—Male, 7-week-old C57BL/6 mice were purchased from Charles River Laboratories. Recombinant adenovirus (0.5 × 109 plaque-forming units) was delivered by tail vein injection to mice. All experiments were conducted by the guidelines of Sungkyunkwan University School of Medicine Institutional Animal Care and Use Committee.
Western Blot Analysis—For Western blot, whole cell extracts were prepared using SDS lysis buffer, as described (33). Western blot analysis on 50–100 μg of protein was performed as described. Primary antibodies against SIK1, α-tubulin, and GFP were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibody against ACC was purchased from Cell Signaling Technology. M2 monoclonal antibody (for FLAG) was from Sigma. Monoclonal antibody against FAS was kindly provided by Prof. Kyung-Sup Kim (Yonsei University, Korea).
Quantitative PCR—Total RNA from either primary hepatocytes or liver tissue was extracted using the RNeasy minikit (Qiagen). cDNAs generated by Superscript II enzyme (Invitrogen) were analyzed by quantitative PCR using a SYBR Green PCR kit and TP800 thermal cycler DICE real time system (TAKARA). All data were normalized to ribosomal L32 expression.
In Vitro Kinase Assay—Affinity-purified FLAG-SIK fusion protein was incubated with 1 μg of GST-SREBP-1c protein and 370 kilobecquerels of [γ-32P]ATP in 30 μl of kinase buffer (25 mm HEPES, pH 7.5, 50 mm Tris, 50 mm MgCl2, 5 mm MnCl2, 5 mm dithiothreitol) at 30 °C for 30 min. The kinase reaction was stopped by the addition of SDS-polyacrylamide gel electrophoresis loading buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Immunoprecipitated FLAG-SIK was confirmed by Western blotting with α-FLAG antibody. Radiolabeled proteins were visualized and quantified on BAS (Fuji).
Hepatic Triacylglycerol (TAG) Analysis—Total lipid from liver was extracted by the method of Folch et al. (34) with slight modification. Briefly, mouse liver was homogenized with chloroform/MeOH (2:1, v/v) and centrifuged at room temperature. The supernatant was washed with 0.2 volumes of 0.9% NaCl and centrifuged. After the removal of the upper phase, the remaining solution was evaporated under vacuum. TAG content of hepatic lipid was measured using an enzymatic colorimetric assay kit (Wako Chemicals).
Chromatin Immunoprecipitation Assays—Nuclear isolation, cross-linking and chromatin immunoprecipitation assays using anti-FLAG antibody on rat primary hepatocytes was performed as described previously (35). nSREBP-1c occupancy over the FAS promoter was analyzed by PCR with primers for rat Fasn (forward, ggcggccacgccacatgggctgacagc; reverse, ccccggcgctcctcagtcccagcccca).
Statistical Analyses—Results represent mean ± S.E. The comparison of different groups was carried out using a two-tailed unpaired Student's t test as described (36). Differences were considered statistically significant at p < 0.05.
RESULTS
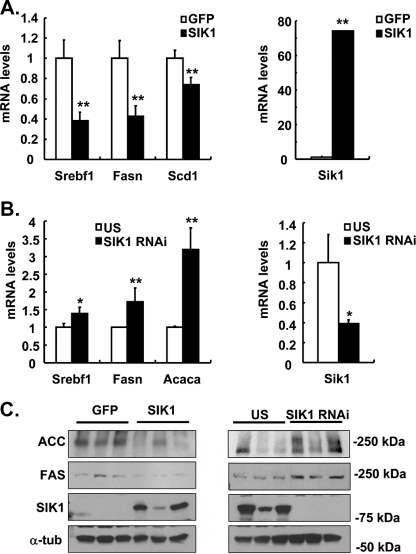
Expression of Lipogenic Genes Are Regulated by SIK in Liver—Previously, we have shown that expression of SIKs inhibits hepatic gluconeogenesis via phosphorylation of the serine 171 residue of CRTC2 (CREB-regulated transcription coactivator 2) (also known as TORC2) (33). To gain further insight into the nature of potential signaling cascades that are regulated by SIK kinases, we performed two sets of microarray analysis using RNAs from mouse liver infected with adenoviruses, one with either Ad-GFP or Ad-SIK1 adenoviruses and the other with either Ad-US control or Ad-SIK1 RNA interference adenoviruses. As shown in Table 1, known target genes in the gluconeogenesis, such as G6pc (glucose-6-phosphatase, catalytic subunit) and Pcx (pyruvate carboxylase) are regulated by changes in SIK1 expression, as reported previously (33). Surprisingly, genes involved in the lipogenesis, such as Fasn, Acaca, and Gpd1 (glycerol-3-phosphate dehydrogenase 1) are up-regulated by SIK1 knockdown, whereas expression levels of such genes are generally shown to be lower with SIK1 overexpression, suggesting that SIK1 could also regulate lipogenic gene transcription. Expression of Srebf1, a gene encoding a major transcriptional regulator SREBP-1c for this process, or Thrsp (thyroid hormone-responsive SPOT14 homolog) is also regulated by SIK similarly to other lipogenic genes. Interestingly, genes involved in the cholesterol biosynthesis or uptake are generally unaffected (aminoacylase 1, Fdps (farnesyl diphosphate synthetase), Ggdps (geranylgeranyl diphosphate synthase 1), Ldlr (low density lipoprotein receptor), and Pmvk (phosphomevalonate kinase)) by either SIK1 overexpression or knockdown. Interestingly, Hmgcs1 (3-hydroxy-3-methylglutaryl-coenzyme A synthase 1) expression is rather increased in SIK1-infected mouse liver, showing a lack of general regulatory mechanisms on cholesterol synthetic enzyme gene expression by SIK. Indeed, changes in expression levels of selected lipogenic genes are confirmed by quantitative reverse transcription-PCR (Fig. 1, A and B) and Western blot analysis (Fig. 1C), showing that SIK could regulate lipogenic gene expression in an in vivo setting.
TABLE 1.
Microarray analysis showing hepatic expression patterns of metabolic genes by SIK1 overexpression or knockdown
Microarray analysis was performed using RNAs from mouse liver infected with Ad-SIK1 or Ad-GFP and with Ad-SIK1 RNAi or Ad-US control RNAi adenoviruses. Genes involved in each metabolic pathway are listed. Lipogenic genes are as follows: Srebf1, sterol regulatory element-binding transcription factor 1; Acaca, acetyl-CoA carboxylase α; Fasn, fatty acid synthase; Thrsp, thyroid hormone-responsive SPOT14 homolog; Gpd1, glycerol-3-phosphate dehydrogenase 1. Gluconeogenic genes are as follows: G6pc, glucose-6-phosphatase, catalytic subunit; Pcx, pyruvate carboxylase. Genes involved in the cholesterol synthesis or uptake are as follows: Pmvk, phosphomevalonate kinase; Hmgcs1, 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1; Fdps, farnesyl diphosphate synthetase; Ldlr, low density lipoprotein receptor; Acy1, aminoacylase 1; Ggps1, geranylgeranyl diphosphate synthase 1.
|
Gene
|
-Fold induction
|
|
|---|---|---|
| SIK1 versus GFP | SIKI RNAi versus US | |
| Srebf1 | 0.571 | 1.347 |
| Acaca | 0.991 | 1.764 |
| Fasn | 0.652 | 1.416 |
| Thrsp | 0.680 | 7.360 |
| Gpd1 | 0.622 | 1.502 |
| G6pc | 0.819 | 1.399 |
| Pcx | 0.853 | 1.257 |
| Pmvk | 0.952 | 0.852 |
| Hmgcs1 | 1.337 | 0.683 |
| Fdps | 0.741 | 0.832 |
| Ldlr | 1.125 | 1.080 |
| Acy1 | 0.959 | 1.051 |
| Ggps1 | 1.234 | 1.15 |
FIGURE 1.
SIK reduces lipogenic genes expression. A, effects of Ad-SIK1 or control (Ad-GFP) on lipogenic gene expression (Srebf1, Fasn, and Scd1) in mouse liver. B, effects of Ad-SIK1 RNA interference or control (Ad-US) on lipogenic gene expression (Srebf1, Fasn, and Acaca) in mouse liver. C, Western blot analysis showing protein levels for SIK1, ACC, FAS, and α-tubulin from mouse liver extracts as in A and B. Data in A and B represent mean ± S.D. (n = 3) (**, p < 0.01; *, p < 0.05, t test).
SIK Regulates SREBP-1c-mediated Transcription in Primary Hepatocytes—To rule out potential secondary effects of adenovirus-mediated SIK overexpression or knockdown on lipogenic gene expression in the liver, we transduced primary hepatocytes with Ad-SIK1 adenovirus or Ad-GFP control viruses. Indeed, adenoviral overexpression of SIK1 reduces mRNA levels of Fasn in primary hepatocytes, showing that the effects of SIK1 adenoviral infection in mouse could be replicated by SIK1 expression in primary hepatocytes (Fig. 2A). As shown previously, AMPK or adiponectin could also reduce Fasn expression, suggesting that SIK may regulate lipogenic gene expression in a similar manner as does AMPK (37). Recently, LXR-mediated SREBP-1c expression was shown to be regulated by AMPK activity in hepatic cells (38). Indeed, we were able to show that LXR ligand T0901317-mediated induction of mRNAs for Fasn or Srebf1 are effectively blocked by adenoviral SIK overexpression in primary hepatocytes (Fig. 2B). LXR-dependent induction of Srebf1 promoter activity is also reduced by SIK1 expression (Fig. 2C). However, SREBP-1c-mediated enhancement of Fasn promoter activity are also reduced by SIK1 overexpression in hepatocytes (Fig. 2D), suggesting that SIK1 could not only regulate SREBP-1c expression but also could regulate its activity to modulate hepatic lipogenic gene expression.
FIGURE 2.
Regulation of lipogenic gene transcription in primary hepatocytes. A, left, effects of Ad-SIK1 or control (Ad-GFP) on mRNA levels for Fasn in rat primary hepatocytes. Right, effects of Ad-adiponectin, Ad-AMPK T172D constitutively active mutant (AMPK CA), or control (Ad-GFP) on insulin/glucose-induced mRNA levels for Fasn in rat primary hepatocytes. B, effects of Ad-SIK1 on LXR ligand (T0901317)-induced lipogenic gene expression (Srebf1 and Fasn) in rat primary hepatocytes. Ad-GFP or Ad-SIK1 adenovirus was infected for 16 h and then exposed to 10μm T0901317 for 18 h. C, effects of SIK on LXRα-induced SREBP-1c promoter activity in HepG2 cells. SREBP-1c (–570/+43) luciferase was transfected with pcDNA empty vector, 10 ng of pcDNA-LXRα, or pcDNA-SIK expression vector together with RSV β-galactosidase plasmid for 48 h before being harvested for the luciferase assay. D, effects of SIK on Fasn promoter activity in rat primary hepatocytes. Ad Fasn (–150/–43) pyruvate kinase luciferase adenovirus was infected with either Ad-GFP, Ad-nSREBP-1c WT and/or Ad-SIK1 adenoviruses for 16 h and then cultured for an additional 24–48 h before being harvested for the luciferase assay. Data in A–D represent mean ± S.D. (n = 3) (**, p < 0.01; *, p < 0.05, t test).
SIK Directly Modifies SREBP-1c at Three Serine Residues within the b/HLH/LZ Domain—As members of the AMPK-related kinases, SIK proteins phosphorylate serine or threonine residues that are a fixed distance from basic and hydrophobic amino acids (HBXX(S/T)XXH, where H represents a hydrophobic amino acid, B is a basic amino acid, X is any amino acid, and S/T is serine/threonine) (35). Close investigation of amino acid sequences of SREBP-1c revealed three potential serine residues at amino acid positions 265, 266, and 329 that are conserved among mouse, rat, and human species and could be phosphorylated by SIK kinases (Fig. 3, A and C). Consequently, we performed an in vitro kinase assay using immunoprecipitated FLAG-SIK1 together with four purified GST-fused nSREBP-1c proteins bearing wild type sequences (WT) or sequences containing alanine substitutions at serines 265 and 266 (S265/266A), alanine substitution at serine 329 (S329A), or alanine substitutions at all three serine residues depicted above (3A). An in vitro kinase assay revealed that SIK1 could indeed phosphorylate wild type nSREBP-1c. Although mutations at serines 265 and 266 affect the phosphorylation of SREBP-1c by SIK1 modestly, S329A mutant is strongly refractory to SIK-dependent phosphorylation, suggesting that the serine 329 residue is one of major target sites for SIK-mediated phosphorylation of SREBP-1c (Fig. 3B). Mutations at all three serine residues almost completely block SIK-mediated phosphorylation of nSREBP-1c, suggesting that the three sites represent major regulatory targets for SIK kinases.
FIGURE 3.
Schematics of putative SIK-mediated phosphorylation sites on mouse nSREBP-1c sequences. A, putative phosphorylation sites by SIK kinases on mouse SREBP-1c nuclear form are shown. Each mutant construct is designed as an alanine substitution for the serine residue indicated. B, in vitro kinase assay was performed with purified GST-fused nSREBP-1c proteins and immunoprecipitated FLAG-tagged SIK1. Effects of specific alanine substitution mutations on SREBP-1c for SIK-induced phosphorylation were shown. C, comparison of amino acid sequences for nSREBP-1c within the SIK target region (serine 265/266 and serine 329) among humans, mice, and rats. 3A, S265A/S266A/S329A.
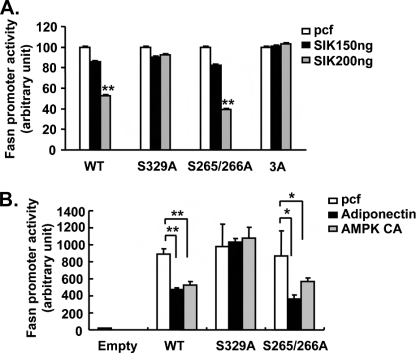
Triple Mutant SREBP-1c Is Refractory to SIK-dependent Inhibition—To further confirm the importance of serine residues of SREBP-1c on SIK-mediated inhibition, we generated expression vectors for nSREBP-1c wild type, S265A/S266A, S329A, and S265A/S266A/S329A and performed co-transfection experiments with a Fasn Luc reporter plasmid. As shown in Fig. 4A, SIK1 overexpression effectively reduces Fasn Luc activity driven by WT or S265A/S266A nSREBP-1c expression. However, S329A is largely refractory to SIK1-mediated inhibition, and S265A/S266A/S329A mutant nSREBP-1c is almost completely unaffected by SIK1 expression. These data suggest that indeed serine 329 of SREBP-1c represents a major target for SIK-mediated regulation of lipogenic gene expression, with a contribution from other serine residues, such as serine 265 and 266, within the b/HLH/LZ domain. Similar results were obtained in experiments using either adiponectin or constitutively active AMPK expression vector, suggesting that these two pathways indeed share a similar regulatory mechanism (Fig. 4B). To further verify the importance of these three serine residues of SREBP-1c, we generated adenoviruses for nSREBP-1c WT and S265A/S266A/S329A and tested in primary hepatocytes. As shown in Fig. 5, A and B, SIK1-mediated inhibition of either Ad Fasn Luc reporter activity or endogenous Fasn gene expression is blocked by S265A/S266A/S329A expression but not by WT expression, confirming that SIK-dependent regulation of lipogenesis could be achieved via phosphorylation of three major serine residues of SREBP-1c identified. To understand the molecular mechanism by which SIK-mediated serine phosphorylation affects SREBP-1c transcriptional activity, we performed a chromatin immunoprecipitation assay. As shown in Fig. 5C, binding of WT nSREBP-1c on the Fasn promoter is partially blocked by SIK1 overexpression in primary hepatocytes, suggesting that the phosphorylation at three serine residues within the b/HLH/LZ domain by SIK1 may inhibit the DNA binding and/or dimerization potential of SREBP-1c. In accordance with this idea, S265A/S266A/S329A mutant nSREBP-1c remains on the Fasn promoter even with SIK1 overexpression. Interestingly, SIK1 does not alter the stability or the cellular localization of nSREBP-1c in primary hepatocytes (data not shown), suggesting that the phosphorylation status of serines 265, 266, and 329 of SREBP-1c may only affect its DNA binding activity.
FIGURE 4.
SIK and AMPK regulates hepatic lipogenesis by modulating serine 329 phosphorylation of SREBP-1c. A, luciferase assay showing effects of SIK1 on SREBP-1c-induced Fasn promoter activity in HepG2 cells co-transfected with either wild type or mutant nSREBP-1c. B, luciferase assay showing effects of adiponectin or AMPK CA on SREBP-1c-induced Fasn promoter activity in HepG2 cells co-transfected with either wild type or mutant nSREBP-1c. Data in A and B represent mean ± S.D. (n = 3) (**, p < 0.01; *, p < 0.05, t test). 3A, S265A/S266A/S329A.
FIGURE 5.
Triple mutant SREBP-1c is refractory to SIK-mediated regulation of hepatic lipogenesis. A, effects of SIK on SREBP1c WT and S265A/S266A/S329A mutant (3A)-induced Fasn gene expression in rat primary hepatocytes. B, luciferase assay showing effects of SIK1 on SREBP-1c-induced Fasn promoter activity in rat primary hepatocytes expressing either wild type or mutant nSREBP-1c. C, effects of Ad-SIK1 on nSREBP-1c occupancy over the Fasn gene by a chromatin immunoprecipitation (IP) assay in primary hepatocytes. Data in A–C represent mean ± S.D. (n = 3) (**, p < 0.01; *, p < 0.05, t test).
SIK Regulates Hepatic TAG Levels by Targeting the Serine Residues of SREBP-1c within the b/HLH/LZ Domain—Having seen the direct inhibitory effects of SIK on SREBP-1c in the cell culture or in vitro, we wanted to test whether SIK-mediated repression of the lipogenic gene results in decreased hepatic TAG levels in vivo. We thus injected adenovirus for GFP alone, SIK1 alone, or SIK1 plus S265A/S266A/S329A mutant SREBP-1c into the tail veins of mice. Indeed, overexpression of SIK1 greatly reduces blood glucose levels, as reported previously (data not shown) (33). Furthermore, SIK1-injected mice display significant reduction in hepatic TAG levels and lipogenic gene expression compared with GFP control mice, showing that indeed SIK could regulate hepatic lipogenesis in vivo. The effect of SIK1 overexpression is almost completely blocked by co-infection of S265A/S266A/S329A mutant SREBP-1c. Hepatic expression of Fasn, Scd1 (stearoyl-coenzyme A desaturase 1), or Thrsp is significantly higher with Ad-S265A/S266A/S329A plus Ad-SIK-injected mice compared with mice injected with SIK1 adenovirus alone (Fig. 6A). Furthermore, SIK-mediated reduction of FAS protein levels is partially restored by Ad-S265A/S266A/S329A nSREBP-1c co-infection, and reduction in hepatic TAG levels is also restored with co-injection of S265A/S266A/S329A and SIK1 adenoviruses (Fig. 6, B and C). These data clearly indicate that SIK-mediated regulation of hepatic lipogenesis is indeed targeted via the phosphorylation of serine 329 and/or 265/266 of SREBP-1c. As reported previously, SIK expression greatly reduces gluconeogenic gene expression and plasma glucose level via the regulation of CRTC2 phosphorylation (33). Although the expression of S265A/S266A/S329A mutant nSREBP-1c restores SIK-mediated repression of hepatic lipogenesis, plasma glucose levels remains significantly lower with Ad-SIK plus S265A/S266A/S329A-injected mice compared with GFP control mice (data not shown). Rather, S265A/S266A/S329A co-expression with SIK1 further reduces hepatic mRNAs for cytosolic Pck1 (phosphoenolpyruvate carboxykinase 1) or G6pc (Fig. 6D), in accordance with a notion that SREBP-1c might be involved in the transcriptional inhibition of gluconeogenic genes (39). These data further suggest that SIK-mediated regulation of SREBP-1c and lipogenesis is distinct from that of CRTC2 and hepatic gluconeogenic gene expression.
FIGURE 6.
SIK regulates hepatic lipogenesis by controlling SREBP-1c in vivo. A, effects of Ad-SIK1, Ad-GFP, and/or Ad-SREBP 1c S265A/S266A/S329A mutant on lipogenic gene expression (Srebf1, Fasn, Scd1, and Thrsp) in mouse liver. B, left, Western blot analysis from mouse liver showing expression of SIK1 or nuclear SREBP-1c. GFP levels were shown to ensure equal adenovirus infection in each mouse. Right, quantitation of FAS protein levels as in B. C, hepatic TAG levels were analyzed from mouse liver tissue infected with adenoviruses as in A (n = 4). D, mRNA levels of gluconeogenic genes in mouse liver (n = 3). Effects of SIK1 and SREBP-1c S265A/S266A/S329A (3A) mutant are shown. Data in A, C, and D represent mean ± S.D. (n = 3) (**, p < 0.01; *, p < 0.05, t test).
DISCUSSION
SREBPs are the major regulators for lipid homeostasis in mammals (17–20). Among family members, SREBP-2 is known to regulate cholesterol uptake or biosynthesis in liver by controlling expression of genes involved in the pathway such as Ldlr, Hmgcs1, Hmgcr (3-hydroxy-3-methylglutaryl-coenzyme A reductase), and Fdps, whereas SREBP-1c selectively activates transcription of genes such as Fasn, Acaca, or Scd1 in the fatty acid biosynthetic program. The regulatory mechanisms for these two factors show considerable differences. Although SREBP-2 expression is not regulated by sterols, its processing is tightly regulated by intracellular levels of sterols, showing a case of end product-mediated inhibition. On the other hand, SREBP-1c is mainly regulated at the level of transcription by insulin, although it was suggested that its activity is also enhanced in the presence of insulin in some cell types. Interestingly, oxysterol-sensing transcription factor LXR is shown to be involved in the transcriptional activation of SREBP-1c, suggesting that two SREBP factors might not be active simultaneously in some physiological conditions. Here we suggest another layer of regulatory mechanisms for differential modulation of two SREBPs by SIK kinases, by showing that SIKs can only specifically shut down fatty acid biosynthetic pathways over cholesterol metabolism via the phosphorylation of SREBP-1c.
Recent studies revealed potential involvement of various kinase pathways to regulate SREBP-1c activity. In a study conducted in HepG2 hepatoma cell lines, protein kinase A was shown to affect DNA binding ability of SREBP-1a by its phosphorylation of serine 338, which corresponds to serine 265 of SREBP-1c (31), although modification of this site alone by SIK might not be critical in the regulation of SREBP proteins based on our results. According to a report by Bengoechea and Ericsson (40), another serine/threonine kinase glycogen synthase kinase was also shown to be involved in the down-regulation of SREBP-1 activity. Unlike the regulatory mechanisms by protein kinase A or SIK, GSK3 targets sequences that are C terminal to the b/HLH/LZ domain and promotes ubiquitin ligase Fbw7-dependent degradation of SREBP-1 proteins. The implication of multiple kinases for the regulation of SREBP-1 would underscore its importance in the regulation of hepatic lipogenesis.
AMPK has long been implicated in the regulation of hepatic fatty acid biosynthetic pathway via transcriptional mechanisms. Although not shown unswervingly, it is thought to target SREBP-1c directly to modulate entire lipogenic programs. As AMPK-related kinases, SIKs share similar substrate recognition sequences with AMPK as in the case of serine 171 phosphorylation of CRTC2. In our hands, among three potential AMPK/SIK target sequences in SREBP-1c, serine 329, together with serine 265/266 within the b/HLH/LZ domain, is shown to be critical in the SIK-mediated regulation. Indeed, the inhibitory effects of AMPK or adiponectin on SREBP-mediated Fasn promoter activity are also blocked by S329A mutation of SREBP-1c (Fig. 4B), suggesting that two kinases would similarly target specific sequences of SREBP-1c to regulate hepatic fatty acid biosynthesis. As shown previously, effects of adiponectin may selectively activate the AMPK pathway, since SIK1 or SIK2 knockdown in primary hepatocytes did not alter adiponectin-dependent down-regulation of lipogenic gene expression (data not shown).
What could be the logical explanation for the presence of multiple kinases to regulate the same pathway? One would suspect that the existence of seemingly redundant kinase functions for regulating hepatic lipogenesis in mammals might accentuate the importance of tight regulation of lipid homeostasis. Alternatively, previously identified roles of AMPK in the regulation of hepatic energy metabolism might be indeed assumed in part by other related kinases, including SIK proteins, since most conclusions were drawn from studies utilizing the pharmacological activation of AMPK, systemic knock-out of AMPK α subunits in mice, or transgenic expression of constitutively active AMPK in the liver (for reviews, see Refs. 41 and 42). Furthermore, AMPK would function to regulate various metabolic pathways not only in peripheral organs but also in the hypothalamus in the brain, making it even harder to gauge the hepatocyte-specific role of AMPK. In our current studies, we were able to observe increased hepatic lipogenic gene expression with SIK1 knockdown (Fig. 1B), suggesting that indeed SIK1 is critical in the regulation of endogenous fatty acid synthetic gene expression.
Nonalcoholic fatty liver disease is now regarded as a critical pathogenic condition that leads to severe metabolic diseases, including diabetes or nonalcoholic steatohepatitis (25, 26). Hepatic de novo lipogenesis, together with expression of genes in the pathway, was shown to be enhanced in nonalcoholic fatty liver disease patients (27, 28, 43). As a master regulator of fatty acid biosynthesis in liver, SREBP-1c was suggested to be involved in the development of this disease by contributing to the onset of fatty liver phenotypes. In our current study, we were able to identify the molecular mechanism by which SIK kinases can regulate hepatic TAG levels via the phosphorylation of serine 329 and serine 265/266 of SREBP-1c. Our results propose that modulation of SREBP-1c activity via the SIK pathway could be an attractive approach to relieve such symptoms. Pharmacological activation of SIK activity might be beneficial to deter the development of severe hepatic diseases.
Acknowledgments
We thank Prof. Kyung-Sup Kim for FAS antibody and Sun-Myeong Park for technical assistance.
This work was supported by Research Program for New Drug Target Discovery Grant M10648000089-08N4800-08910, Korea Science and Engineering Foundation Grant R01-2008-000-11935-0, and Korea Research Foundation Grant 2006-E00037 from the Ministry of Education, Science and Technology and a grant from the Marine Biotechnology Program funded by the Ministry of Land, Transport, and Maritime Affairs, Republic of Korea.
Footnotes
The abbreviations used are: SREBP, sterol regulatory element-binding protein; AMPK, AMP-activated protein kinase; TAG, triacylglycerol; nSREBP-1c, nuclear SREBP-1c; b/HLH/LZ, basic helix loop helix leucine zipper; WT, wild type; Ad, adenovirus; GFP, green fluorescent protein; GST, glutathione S-transferase; LXR, liver X receptor; CA, constitutively active; SIK, salt-inducible kinase; US, unspecific shRNA.
References
- 1.Towle, H. C., Kaytor, E. N., and Shih, H. M. (1997) Annu. Rev. Nutr. 17 405–433 [DOI] [PubMed] [Google Scholar]
- 2.Vaulont, S., and Kahn, A. (1994) FASEB J. 8 28–35 [DOI] [PubMed] [Google Scholar]
- 3.Girard, J., Ferre, P., and Foufelle, F. (1997) Annu. Rev. Nutr. 17 325–352 [DOI] [PubMed] [Google Scholar]
- 4.Hillgartner, F. B., Salati, L. M., and Goodridge, A. G. (1995) Physiol. Rev. 75 47–76 [DOI] [PubMed] [Google Scholar]
- 5.Lemaigre, F. P., and Rousseau, G. G. (1994) Biochem. J. 303 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, S. D., and Jump, D. B. (1996) J. Nutr. 126 1105S–1109S [DOI] [PubMed] [Google Scholar]
- 7.Clarke, S. D., and Jump, D. B. (1994) Annu. Rev. Nutr. 14 83–98 [DOI] [PubMed] [Google Scholar]
- 8.Iynedjian, P. B., Ucla, C., and Mach, B. (1987) J. Biol. Chem. 262 6032–6038 [PubMed] [Google Scholar]
- 9.Minderop, R. H., Hoeppner, W., and Seitz, H. J. (1987) Eur. J. Biochem. 164 181–187 [DOI] [PubMed] [Google Scholar]
- 10.Vaulont, S., Munnich, A., Decaux, J. F., and Kahn, A. (1986) J. Biol. Chem. 261 7621–7625 [PubMed] [Google Scholar]
- 11.Cuif, M. H., Cognet, M., Boquet, D., Tremp, G., Kahn, A., and Vaulont, S. (1992) Mol. Cell. Biol. 12 4852–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, S. D., Armstrong, M. K., and Jump, D. B. (1990) J. Nutr. 120 218–224 [DOI] [PubMed] [Google Scholar]
- 13.Katsurada, A., Iritani, N., Fukuda, H., Matsumura, Y., Nishimoto, N., Noguchi, T., and Tanaka, T. (1990) Eur. J. Biochem. 190 427–433 [DOI] [PubMed] [Google Scholar]
- 14.Paulauskis, J. D., and Sul, H. S. (1989) J. Biol. Chem. 264 574–577 [PubMed] [Google Scholar]
- 15.Horton, J. D., Goldstein, J. L., and Brown, M. S. (2002) J. Clin. Invest. 109 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghow, R., Yellaturu, C., Deng, X., Park, E. A., and Elam, M. B. (2008) Trends Endocrinol. Metab. 19 65–73 [DOI] [PubMed] [Google Scholar]
- 17.Sun, L. P., Seemann, J., Goldstein, J. L., and Brown, M. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 6519–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown, A. J., Sun, L., Feramisco, J. D., Brown, M. S., and Goldstein, J. L. (2002) Mol. Cell 10 237–245 [DOI] [PubMed] [Google Scholar]
- 19.Yang, T., Espenshade, P. J., Wright, M. E., Yabe, D., Gong, Y., Aebersold, R., Goldstein, J. L., and Brown, M. S. (2002) Cell 110 489–500 [DOI] [PubMed] [Google Scholar]
- 20.DeBose-Boyd, R. A., Brown, M. S., Li, W. P., Nohturfft, A., Goldstein, J. L., and Espenshade, P. J. (1999) Cell 99 703–712 [DOI] [PubMed] [Google Scholar]
- 21.Horton, J. D., Bashmakov, Y., Shimomura, I., and Shimano, H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 5987–5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foretz, M., Pacot, C., Dugail, I., Lemarchand, P., Guichard, C., Le Liepvre, X., Berthelier-Lubrano, C., Spiegelman, B., Kim, J. B., Ferre, P., and Foufelle, F. (1999) Mol. Cell. Biol. 19 3760–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimano, H., Shimomura, I., Hammer, R. E., Herz, J., Goldstein, J. L., Brown, M. S., and Horton, J. D. (1997) J. Clin. Invest. 100 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimano, H., Yahagi, N., Amemiya-Kudo, M., Hasty, A. H., Osuga, J., Tamura, Y., Shionoiri, F., Iizuka, Y., Ohashi, K., Harada, K., Gotoda, T., Ishibashi, S., and Yamada, N. (1999) J. Biol. Chem. 274 35832–35839 [DOI] [PubMed] [Google Scholar]
- 25.Angulo, P. (2002) N. Engl. J. Med. 346 1221–1231 [DOI] [PubMed] [Google Scholar]
- 26.Browning, J. D., and Horton, J. D. (2004) J. Clin. Invest. 114 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohjima, M., Higuchi, N., Kato, M., Kotoh, K., Yoshimoto, T., Fujino, T., Yada, M., Yada, R., Harada, N., Enjoji, M., Takayanagi, R., and Nakamuta, M. (2008) Int. J. Mol. Med. 21 507–511 [PubMed] [Google Scholar]
- 28.Nakamuta, M., Kohjima, M., Morizono, S., Kotoh, K., Yoshimoto, T., Miyagi, I., and Enjoji, M. (2005) Int. J. Mol. Med. 16 631–635 [PubMed] [Google Scholar]
- 29.Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., Musi, N., Hirshman, M. F., Goodyear, L. J., and Moller, D. E. (2001) J. Clin. Invest. 108 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, K. G., Min, A. K., Koh, E. H., Kim, H. S., Kim, M. O., Park, H. S., Kim, Y. D., Yoon, T. S., Jang, B. K., Hwang, J. S., Kim, J. B., Choi, H. S., Park, J. Y., Lee, I. K., and Lee, K. U. (2008) Hepatology 48 1477–1486 [DOI] [PubMed] [Google Scholar]
- 31.Lu, M., and Shyy, J. Y. (2006) Am. J. Physiol. 290 C1477–C1486 [DOI] [PubMed] [Google Scholar]
- 32.Koo, S. H., Satoh, H., Herzig, S., Lee, C. H., Hedrick, S., Kulkarni, R., Evans, R. M., Olefsky, J., and Montminy, M. (2004) Nat. Med. 10 530–534 [DOI] [PubMed] [Google Scholar]
- 33.Koo, S. H., Flechner, L., Qi, L., Zhang, X., Screaton, R. A., Jeffries, S., Hedrick, S., Xu, W., Boussouar, F., Brindle, P., Takemori, H., and Montminy, M. (2005) Nature 437 1109–1111 [DOI] [PubMed] [Google Scholar]
- 34.Folch, J., Lees, M., and Sloane Stanley, G. H. (1957) J. Biol. Chem. 226 497–509 [PubMed] [Google Scholar]
- 35.Screaton, R. A., Conkright, M. D., Katoh, Y., Best, J. L., Canettieri, G., Jeffries, S., Guzman, E., Niessen, S., Yates, J. R., III, Takemori, H., Okamoto, M., and Montminy, M. (2004) Cell 119 61–74 [DOI] [PubMed] [Google Scholar]
- 36.Dentin, R., Liu, Y., Koo, S. H., Hedrick, S., Vargas, T., Heredia, J., Yates, J., III, and Montminy, M. (2007) Nature 449 366–369 [DOI] [PubMed] [Google Scholar]
- 37.Da Silva Morais, A., Lebrun, V., Abarca-Quinones, J., Brichard, S., Hue, L., Guigas, B., Viollet, B., and Leclercq, I. A. (2009) J. Hepatol. 50 489–500 [DOI] [PubMed] [Google Scholar]
- 38.Yang, J., Craddock, L., Hong, S., and Liu, Z. M. (2009) J. Cell. Biochem. 106 414–426 [DOI] [PubMed] [Google Scholar]
- 39.Chakravarty, K., Leahy, P., Becard, D., Hakimi, P., Foretz, M., Ferre, P., Foufelle, F., and Hanson, R. W. (2001) J. Biol. Chem. 276 34816–34823 [DOI] [PubMed] [Google Scholar]
- 40.Bengoechea-Alonso, M. T., and Ericsson, J. (2009) J. Biol. Chem. 284 5885–5895 [DOI] [PubMed] [Google Scholar]
- 41.Kahn, B. B., Alquier, T., Carling, D., and Hardie, D. G. (2005) Cell Metab. 1 15–25 [DOI] [PubMed] [Google Scholar]
- 42.Xue, B., and Kahn, B. B. (2006) J. Physiol. (Lond.) 574 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi, N., Kato, M., Shundo, Y., Tajiri, H., Tanaka, M., Yamashita, N., Kohjima, M., Kotoh, K., Nakamuta, M., Takayanagi, R., and Enjoji, M. (2008) Hepatol. Res. 38 1122–1129 [DOI] [PubMed] [Google Scholar]