Abstract
Ectopic bone formation after joint replacement or brain injury in humans is a serious complication that causes immobility of joints and severe pain. However, mechanisms underlying such ectopic bone formation are not fully understood. Bone morphogenetic protein (BMPs) are defined as inducers of ectopic bone formation, and they are regulated by several types of inhibitors. ANA is an antiproliferative molecule that belongs to Tob/BTG family, but its activity in bone metabolism has not been known. Here, we examined the role of ANA on ectopic bone formation activity of BMP. In ANA-deficient and wild-type mice, BMP2 was implanted to induce ectopic bone formation in muscle. ANA deficiency increased mass of newly formed bone in vivo compared with wild-type based on 3D-μCT analyses. ANA mRNA was expressed in bone in vivo as well as in osteoblastic cells in vitro. Such ANA mRNA levels were increased by BMP2 treatment in MC3T3-E1 osteoblastic cells. Overexpression of ANA suppressed BMP-induced expression of luciferase reporter gene linked to BMP response elements in these cells. Conversely, ANA mRNA knockdown by small interference RNA enhanced the BMP-dependent BMP response element reporter expression. It also enhanced BMP-induced osteoblastic differentiation in muscle-derived C2C12 cells. Immunoprecipitation assay indicated that ANA interacts with Smad8. Thus, ANA is a suppressor of ectopic bone formation induced by BMP, and this inhibitory ANA activity is a part of the negative feedback regulation of BMP function.
Ectopic or heterotopic bone formation is one of the major complications associated with joint replacement surgery and brain injury (1, 2). Such pathological ectopic bone formation and heterotopic ossification cause ankylosis of joints or persistent pain and significantly deteriorated quality of life. However, the molecules involved in these ectopic bone formation events have not been fully understood.
Bone morphogenetic proteins (BMPs)3 were first identified as an inducer of ectopic bone formation (3, 4). Although BMP2 is required for fracture healing, skeletal development of the limbs was normal in the absence of BMP2 (5). In contrast, BMP is absolutely required for ectopic bone formation in muscle (3), and no other growth factors could replace such potent ectopic bone formation activity of this molecule. Thus, BMP action on ectopic bone formation is one of the unique features of this molecule.
BMP activity is under the control of several groups of modulators against its signaling pathway. One of these groups of modulators that control BMP2 signaling in osteoblasts includes the Tob/BTG antiproliferative protein family, comprising Btg1, Btg2, Tob, Tob2, Pc3, and ANA. These molecules share a homologous region (BTG/Tob homology domain) in their N-terminal ends (6) and suppress cell proliferation when they are overexpressed in NIH3T3 cells (7–13).
Among the members of Tob/BTG family, Tob was shown to be a BMP antagonist previously (14, 15). However, effects of other members of Tob/BTG family on BMP activity have not yet been fully understood. BTG2 is necessary for vertebral patterning by modulating BMP signaling (16) and Tob2-deficient mice exhibit a decrease in their bone mass in association with an increase in RANKL (receptor activator of NF-κB ligand) expression (17). These observations suggest that each of the molecules in the Tob/BTG family members may possess their distinct functions.
ANA (abundant in neuroepithelial area)/BTG3 is known to inhibit progression of the cell cycle in NIH3T3 cells (18). ANA mRNA is expressed in a variety of adult tissues, including testis, prostate, ovary, thymus, and liver. In embryonic mice, ANA is detected in the ventricular zone of brain, Meckel's cartilage, and rib cartilage (10). In vitro, ANA associates with Caf1/Cnot7 (CCR4 transcription factor-associated protein) (19), which is involved in negative regulation of bone mass (20, 21).
Although ANA has been detected in embryonic cartilage, its effects on BMP-induced bone formation have not been known. Recently, ectopic and heterotopic ossifications have been reported to be in association with high levels of expression of BMP2 (22). Here, we examined whether ANA regulates BMP activities with respect to ectopic bone formation. To investigate the influence of ANA, we implanted BMP into the muscle of either wild-type or ANA-deficient mice and compared the levels of ectopic bone formation. We found that ANA deficiency enhances BMP2-induced ectopic bone formation. We further found that ANA suppresses BMP signaling pathway in osteoblasts at least in part via modulation of transcriptional events.
EXPERIMENTAL PROCEDURES
Generation of ANA-deficient Mice—ANA-deficient mice in C57B6/J background were produced by Yoneda et al. as reported elsewhere (34). Heterozygous F1 mice were crossed to produce homozygous ANA-deficient mice. Wild-type and ANA-deficient mice were maintained under conventional conditions. All the animal experiments were approved by animal welfare committee of our university.
BMP Implantation Assay—Sixteen-week-old either wild-type or ANA-deficient male mice were used as recipients of BMP implantation. The quadriceps muscle was exposed, and an incision was made in the muscle to implant a pellet of cholesterol-bearing pullulan cross-linked by polyethylene glycol (CHP-PEG) containing 2 μg of rhBMP2 (in the right side thigh muscle) or another CHP-PEG pellet containing vehicle alone (in the left side thigh muscle) (23). CHP-PEG was previously used to carry bone anabolic agent such as prostaglandin E2 and has been proven to be effective in bone formation in vivo (24). Muscle and skin were sutured respectively. The mice were sacrificed 2 weeks later.
Bone Marrow Ablation—The left femora of 9-week-old male mice were subjected to bone marrow ablation. Briefly, a drill hole was made using a 26-gauge needle from the intercondyle groove at the distal end of the femur. The hole was gradually expanded by insertion of dental files (0.15–0.6 mm diameter). Kirschner wire with a diameter of 0.6 mm was then inserted into the bone marrow, and x-ray images were taken to confirm that the bone marrow was precisely ablated. Finally the wire was removed and the wound was closed. The bone was harvested 9 days later.
3D-μCT Analyses—Femur and newly formed bone (ossicles) induced by BMP implantation in muscle were fixed in 4% paraformaldehyde overnight and fixed again in 70% ethanol, and then stored at 4 °C for 3D-μCT analyses. Three-dimensional images of femur and newly formed bone after BMP implantation were taken by using Scan-Xmate-E090 (Comscan Tecno Co.), and the bone volume was quantified by using an image analyzer (TRI/3D-BON, Ratoc System Engineering Co.) for the analyses.
LacZ Staining—To examine the expression pattern of ANA in bone, E16.5 heterogeneous mutant embryos carrying LacZ in one allele of the ANA gene locus were subjected to LacZ staining as previously described (20). Briefly mouse embryos were fixed in a phosphate buffer containing 0.2% glutaraldehyde, 5 mm EGTA, 2 mm MgCl2, and 0.8% formaldehyde for 45 min at room temperature, rinsed twice in a buffer containing 2 mm MgCl2, 0.1% sodium deoxycholate, and 0.2% Nonidet P-40 for a half hour each and incubated in X-gal staining solution (1 mg/ml X-gal, 5 mm K4Fe(CN)6, 5 mm K3Fe(CN)6 in rinse buffer) for 1–3 days. After staining, embryos were rinsed in phosphate-buffered saline and refixed in 4% paraformaldehyde at 4 °C overnight. For frozen sections, the spine and lower limb were removed, immersed in 30% sucrose solution overnight, and embedded in Tissue-Tek O.C.T. compound. Frozen sections (5 μm in thickness) were prepared using a microtome (Leica CM 3050 S).
Transfection and Luciferase Assay—MC3T3-E1 cells were seeded in 24-well plates at a density of 1 × 104 cells per well 1 day before transfection. One day later, the cells were transfected with plasmid DNA and/or siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacture's protocol. For overexpression experiments, we transfected human ANA expression vector constructed in a pME-18S mammalian expression vector with insertion of full-length human ANA cDNA or an empty vector in combination with 7× BMP response element (BRE) containing luciferase DNA (7×BRE-luc). After culture for 48 h in the presence or absence of rhBMP2 (200 ng/ml), the cells were lysed in Passive Lysis Buffer (Promega), and the lysates were mixed with luciferin substrates. Bioluminescence was measured by using Lumat LB 9507 analyzer (Berthold Technologies). We also transfected siRNA for ANA (Ambion) or Silencer Negative Control (Ambion) as a reference. After incubation for 4 h in the presence or absence of rhBMP2, the cells were lysed for measurement of luciferase activities.
Immunoprecipitation and Immunoblotting—COS-7 cells were transfected with 6Myc-tagged Smads (14) and FLAG-tagged human ANA expression vector using FuGene6 (Roche Applied Science). After incubation for 48 h cells were lysed with TNE lysis buffer (1% Nonidet P-40, 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm NaF, 1% aprotinin, 50 μm MG132). After precleared with protein G-Sepharose (Amersham Biosciences Bioscience), the lysates were incubated sequentially with monoclonal antibodies to FLAG (M2, Sigma) and protein G-Sepharose. Immunoprecipitates were rinsed five times with lysis buffer followed by immunoblotting with monoclonal antibodies to Myc (9E10, Santa Cruz Biotechnology).
ALP Activity Assay—C2C12 cells were seeded in 96-well plates at a density of 3 × 103 cells per well 1 day before transfection. These cells were transfected with siRNA for ANA or negative control. After incubation for 72 h in the presence or absence of rhBMP2, the cells were lysed with lysis buffer, and the lysates were subjected to examination of alkaline phosphatase (ALP) activity (14).
Primary Cultures of Osteoblast-enriched Cells—Primary cultures of osteoblast-enriched cells were prepared as described previously (25). Briefly, mouse osteoblast-enriched cells were prepared from the calvariae of 3- to 5-day old newborn mice as follows. Calvariae of the newborn mice were taken aseptically and rinsed in phosphate-buffered saline. They were digested in a solution containing 2.5% collagenase (Sigma), 0.25% trypsin, 40 mm EDTA, and 1% antibiotics in phosphate-buffered saline sequentially for six times. The last three fractions out of the six fractions were collected and cultured in α-minimal essential medium supplemented with 10% fetal bovine serum and 1% antibiotics.
Reverse Transcription-Real-time PCR Analyses—Tissue RNAs were obtained from adult mice according to the methods described elsewhere (26). MC3T3-E1 cells and the osteoblast-enriched cells obtained from mouse calvariae were cultured in α-minimal essential medium supplemented with 10% fetal bovine serum with in the presence or absence of rhBMP2 (100 ng/ml). Twenty-four hours later, total RNA was isolated using TRIzol reagent (Invitrogen). Reverse transcription was performed using 1 μg of total RNA, oligo(dT) primers (Invitrogen), and Superscript II transcriptase (Invitrogen) according to the manufacture's protocol. Real-time PCR was carried out using SYBR Green Supermix and iCycler (Bio-Rad). The data were analyzed by using iQ5 data analyzing software. Primer sequences for real-time PCR were as follows: ana, forward (5′-CCA AAG GTC AGG CCT ACA GA-3′) and reverse (5′-CCC AGG TCG CTG TAC AAG AT-3′); gapdh, forward (5′-AGA AGG TGG TGA AGC AGG CAT C-3′) and reverse (5′-CGA AGG TGG AAG AGT GGG AGT TG-3′). All the quantified data were normalized against those for GAPDH.
Statistical Evaluation—Data were expressed as mean ± S.D. for all values. Results were evaluated using Students' t test or one-way analysis of variance followed by Tukey-Kramers' posthoc test.
RESULTS
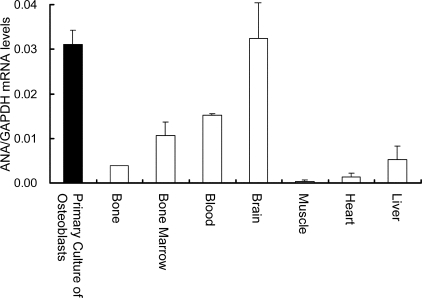
ANA mRNA Is Expressed in Bone Tissue and in Osteoblasts—As an initial step of examination, RNAs from adult mouse tissues were obtained and ANA mRNA expression levels normalized against GAPDH levels were evaluated based on real-time PCR. ANA mRNA was expressed in adult bone tissues in vivo (Fig. 1). ANA mRNA is also expressed in other tissues such as bone marrow, brain, heart, and liver. Further analyses revealed that ANA mRNA was expressed in the primary cultures of osteoblast-enriched cells and the levels of expression in these cells were higher than those in RNAs prepared from bone tissues (Fig. 1).
FIGURE 1.
ANA mRNA expression in bone and primary osteoblast culture. RNAs from bone and indicated tissues were obtained from wild-type adult mice and primary cultures of calvarial osteoblasts. The expression levels of ANA mRNA were examined based on quantitative real-time PCR. All the data were normalized against GAPDH as reference.
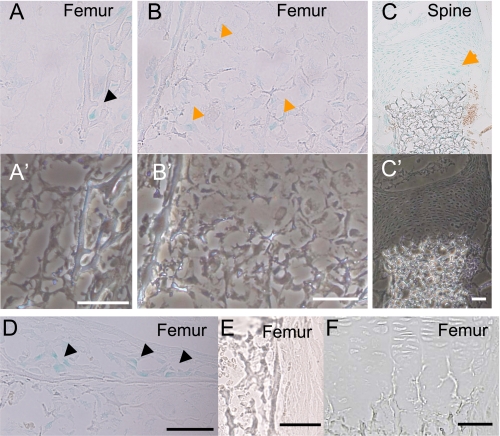
ANA Is Expressed in Developing Bone—To localize ANA-expressing cells in bone in vivo, tibiae, and lumbar vertebrae of the E16.5 homozygote ANA-deficient embryos containing LacZ gene in both of the ANA locus were subjected to LacZ staining. LacZ activity was observed in the cells of the epiphyseal bone revealing the expression in osteoblasts (Fig. 2A). Hypertrophic chondrocytes in the femur (Fig. 2B) and proliferating chondrocytes (Fig. 2C) are also positive for LacZ. Some of the stromal cells were also positive for LacZ staining. Phase-contrast images of the corresponding regions indicated the presence of cells that were not shown in LacZ staining images (Fig. 2, A′–C′). LacZ-positive osteoblasts were also aligned along the bone collar (Fig. 2D). As control, the cells in wild-type embryos were negative for LacZ staining (Fig. 2, E, bone collar, and F, cartilage). These observations indicated that ANA is expressed in the skeletal cells in developing bone.
FIGURE 2.
In vivo localization of ANA expression in bone. LacZ staining of E16.5 embryonic mice revealed that ANA gene is expressed in osteoblasts in the epiphyseal region of femur (A, black arrowhead), hypertrophic chondrocytes in growth plates (B, orange arrowheads), proliferative chondrocytes (C, orange arrow), and osteoblasts in the bone collar (D, black arrowheads). The cells in the femur of wild type were negative for LacZ staining in the periosteum (E), and in the growth plate (F) (scale bar, 50 μm). Lower panels were corresponding images taken with a phase-contrast microscope (A′–C′).
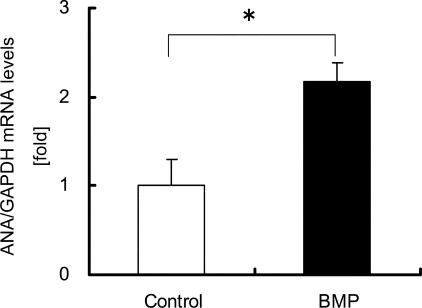
The Link between ANA and BMP Action in Osteoblastic Differentiations—Because ANA is expressed in osteoblasts, we examined the link between ANA and BMP. First, we tested whether ANA is a target gene of BMP action. MC3T3-E1 osteoblastic cells were cultured in the presence or absence of rhBMP2. rhBMP2 treatment enhanced the levels of ANA mRNA expression normalized against GAPDH ∼2-fold compared with vehicle treatment (Fig. 3). Thus, ANA is a novel target of BMP in these cells.
FIGURE 3.
ANA mRNA expression in osteoblasts is enhanced by BMP treatment. ANA mRNA expression levels in MC3T3-E1 cells cultured in the presence or absence of rhBMP2 (100 ng/ml) for 24 h. RNAs extracted and were subjected to real-time PCR analysis. BMP increased ANA mRNA level ∼2-fold. The data were normalized against GAPDH as reference. *, p < 0.01.
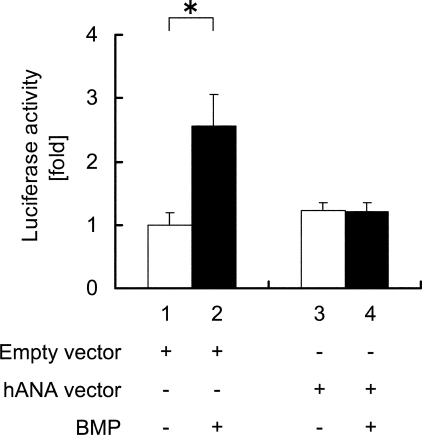
Next, we examined the function of ANA on BMP signaling. For this purpose, human ANA expression vector or an empty vector and a luciferase reporter plasmid containing BRE were co-transfected into MC3T3-E1 cells, and these cells were subjected to BMP2 treatment. BMP treatment enhanced the BRE reporter activity in the presence of empty vector (Fig. 4, lanes 2 versus 1). In contrast, overexpression of human ANA suppressed BMP-induced BRE activity (Fig. 4, lanes 4 versus 3).
FIGURE 4.
ANA overexpression suppresses BMP-induced reporter activity. Relative luciferase activities in MC3T3-E1 cells were obtained based on luciferin and Renilla ratios. The cells were transfected with either empty vector or ANA expression vector and cultured in the presence or absence of rhBMP2 (200 ng/ml). *, p < 0.05.
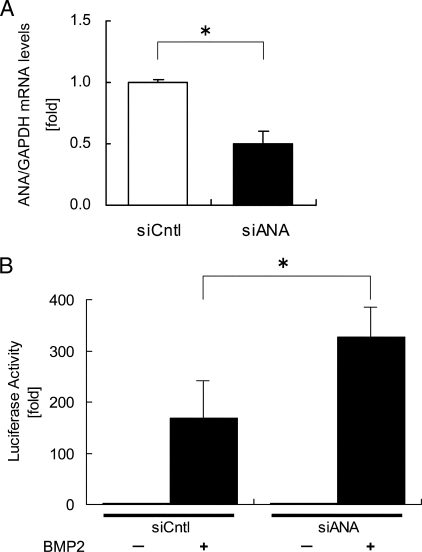
To carry out reverse experiments to examine the function of ANA on BMP activity, siRNAs were used. Transfection of siRNA for ANA reduced ANA mRNA expression levels by ∼50% compared with the cells treated with control siRNA (Fig. 5A). Under this condition, siRNAs were co-transfected with BRE-luc reporter, and these MC3T3-E1 cells were subjected to BMP treatment. Transfection of siRNA targeting ANA enhanced the BMP2-induced reporter activities ∼2-fold compared with control siRNA (Fig. 5B). These data indicated that ANA is inhibitory against BMP-induced transcriptional events.
FIGURE 5.
Down-regulation of ANA enhances BMP action on BRE reporter expression. A, MC3T3-E1 cells were transfected with siRNA for either control (siCntl) or ANA (siANA). ANA mRNA expression levels were evaluated by real-time PCR after 24 h. The data were normalized against GAPDH as reference. *, p < 0.01. B, relative luciferase activities with siRNA for either ANA (siANA) or control (siCntl) in the presence or absence of rhBMP2 (200 ng/ml) in MC3T3-E1 cells. *, p < 0.05.
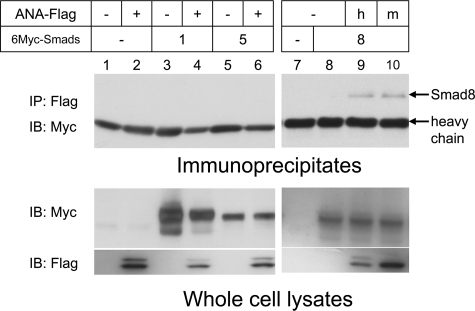
We then asked how ANA interferes BMP signaling in the cells. Because ANA does not have a nuclear localizing signal, it is a cytoplasmic protein. Because Smads mediate BMP signaling events in the cytoplasmic pathway from the receptor to the nuclei, we tested whether ANA interacts with Smads. 6Myc-tagged Smads and FLAG-tagged ANA were transfected into COS-7 cells, and the interactions of ANA and Smads were tested based on immunoprecipitation followed by immunoblotting. Although BMP signaling is mediated by Smad1 and -5, ANA did not interact with Smad1 or -5 (Fig. 6, lanes 4 and 6). However, ANA specifically interacts with Smad8 (Fig. 6, lanes 9). Both human and mouse ANA were co-precipitated with Smad8 (Fig. 6, lanes 9 and 10). Thus, ANA-Smad8 interaction can be observed regardless of different species.
FIGURE 6.
ANA interacts with Smad8. COS-7 cells were transfected with indicated plasmids (FLAG-tagged human-ANA, lanes 2, 4, 6, and 9; FLAG-tagged mouse-ANA, lane 10; 6Myc-Smad1, lanes 3 and 4; 6Myc-Smad5, lanes 5 and 6; 6Myc-Smad8, lanes 8–10). FLAG-tagged ANA was immunoprecipitated (IP), and co-precipitated Smads were immunoblotted (IB). Smad8 was co-precipitated with both human and mouse ANA (lane 9 and 10, respectively). Lower panels indicate immunoblots prepared by using anti-Myc or anti-FLAG antibody of the whole cell lysates.
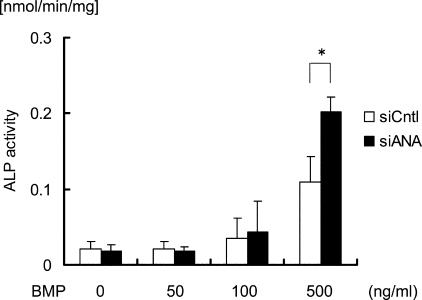
We further examined whether observations on the above intracellular events are translated into ANA regulation of cellular function. Because ALP activity is a phenotypic marker of osteoblastic differentiation, we measured ALP activities in C2C12 cells transfected with siRNA targeting ANA or control siRNA and cultured in the presence or absence of rhBMP2. C2C12 cells are a muscular cell line, and BMP treatment converts these cells to become osteoblasts. This in vitro phenomenon mimics the ectopic bone formation activity of BMP in vivo. BMP treatment enhanced ALP activity in a dose-dependent manner (Fig. 7, white bar). Transfection of siRNA for ANA, which suppressed ANA expression by ∼50% compared with control (data not shown), enhanced ALP activity in the cells treated with higher concentration of rhBMP2 (Fig. 7). These results indicated that ANA suppresses BMP actions on the expression of osteoblastic differentiation marker in muscle lineage cells.
FIGURE 7.
Knockdown of ANA enhances BMP-induced osteoblastic differentiation from the cells of muscle lineage in vitro. Alkaline phosphatase (ALP) activities in muscle-derived C2C12 cells transfected with siRNA for either ANA (siANA) or negative control (siCntl) were measured in the presence or absence of rhBMP2 (50, 100, and 500 ng/ml). *, p < 0.05.
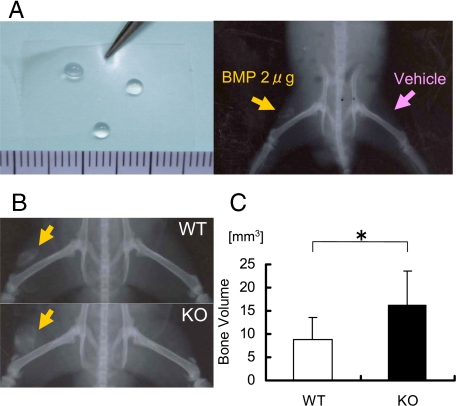
ANA Deficiency Enhances the Levels of Ectopic Bone Formation Induced by BMP2 Implantation in Muscle—To examine the effects of ANA deficiency on ectopic bone formation, we implanted BMP in muscles. It is notable that original definition of BMP activity is to induce ectopic bone formation in vivo. rhBMP2 or vehicle was transplanted into the femoral quadriceps muscles of the right side or the left side, respectively, in either wild-type or ANA-deficient mice using CHP-PEG Nanogel as a carrier (23, 24) (Fig. 8A). We implanted 2 μg of BMP based on our previous experiments that indicated that this dose is optimal for ectopic bone formation in mice (data not shown). Plain x-ray images indicated that ANA deficiency enhanced the size of radiopaque shadow in quadriceps (Fig. 8B). The ossicles in muscle were excised from quadriceps and were subjected to 3D-μCT analyses. Quantification of the three-dimensional images indicated that ANA deficiency increased the bone volume of the ossicles in this implantation assay ∼2-fold compared with wild type (Fig. 8C). Thus, ANA suppresses in vivo bona fide BMP activity to induce ectopic bone formation in muscle.
FIGURE 8.
ANA deficiency enhances bone formation after BMP implantation in muscles. A: left panel, shape and size of cholesterol-bearing pullulan cross-linked by poly ethylene glycol (CHP-PEG); right panel, CHP-PEG containing BMP2 was implanted into the quadriceps of the right side (orange arrow) and CHP-PEG-containing vehicle was implanted into the left side (pink arrow). B, x-ray images of newly formed bone after implantation of BMP in CHP-PEG in quadriceps of wild-type (top) and ANA-deficient mice (bottom). Orange arrows indicate radiopaque signal of ectopically formed bone induced by BMP. Contralateral side (CHP-PEG with vehicle alone) of either wild-type or ANA-deficient mice did not indicate any bone formation. C, three-dimensional bone volume of the ectopically formed bone (ossicle) after implantation in both wild-type and ANA-deficient mice. *, p < 0.05.
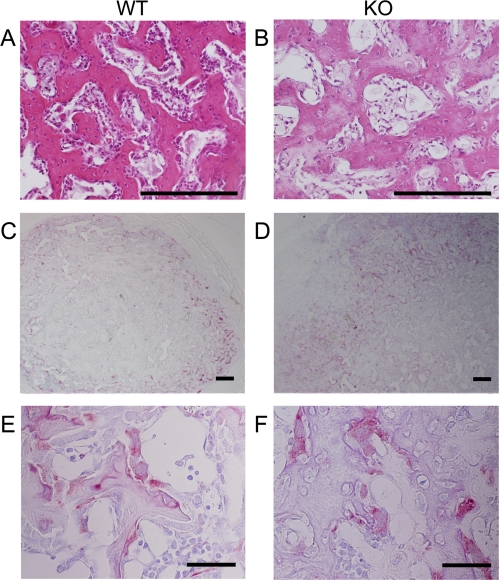
Quality of ectopically formed bone in ANA-deficient mice was examined morphologically. Histological inspection showed that the ossicles formed in ANA-deficient mice contained woven bones similar to those formed in wild-type mice (Fig. 9, A and B). In some part of the section, occasional chondrogenic tissues were observed in the ossicles of both wild-type and ANA-deficient mice (data not shown). Osteoclasts were also observed in these ossicles, and their distribution and shape were similar in wild-type and ANA-deficient groups (Fig. 9, C–F). Thus, morphological properties of the ossicles (bone) induced in ANA-deficient mice were similar to those of the ossicles (bone) in wild type.
FIGURE 9.
Histological features of the ectopically formed bone are comparable between wild-type and ANA-deficient mice. Ectopically formed ossicles (bone) contain woven bone (hematoxylin and eosin stain; A and B). tartrate-resistant acid phosphatase-positive multinucleated cells were observed in the ossicle (C–F) (scale bars: A–D, 200 μm; E and F, 50 μm). These morphological structures of bone and cells were similar between the two genotypes.
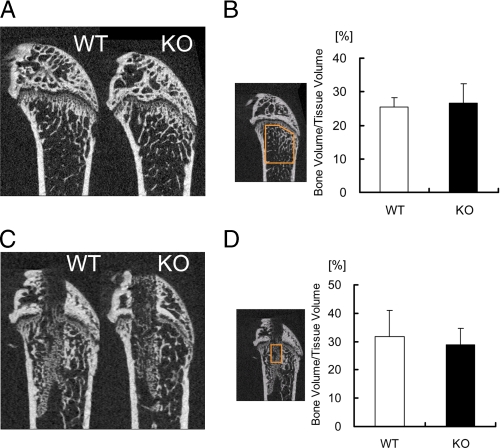
To examine whether the effects of ANA deficiency on bone formation is specific to BMP-induced ectopic bone formation or they are also seen in another bone formation model in vivo, bone marrow ablation was conducted. Interestingly, although ANA suppresses BMP action in ectopic bone formation assay, basal levels of bone volume in cancellous bone in ANA-deficient mice were similar to those in wild-type mice (Fig. 10, A and B). Cortical envelope sizes were comparable (data not shown). The levels of newly formed bone after bone marrow ablation were also similar in both genotypes (Fig. 10, C and D). Thus, enhancing effects of ANA deficiency on bone formation were specific to BMP-induced ectopic bone formation and not a general one in any type of bone formation.
FIGURE 10.
New bone formation levels after bone marrow ablation are similar between ANA deficient and wild-type mice. A, intact side femora of the mice. 2D-μCT images of the distal ends of femora were taken as described under “Experimental Procedures.” B, bone volume/tissue volume were quantified in the indicated epiphyseal area that was set between 0.2 and 2.2 mm away from the growth plate. C, new bone formation 10 days after surgery in the ablated side femora of the mice. 2D-μCT images of the distal end of femora were taken. D, bone volume/tissue volume of the newly formed bone were quantified after ablation in the indicated area of the femur in wild-type and ANA-deficient mice.
DISCUSSION
We have found that ANA deficiency enhanced BMP-induced ectopic bone formation in vivo. To our knowledge, this is the first report on the molecule that specifically suppresses the levels of ectopic bone formation induced by BMP without affecting basal bone mass levels. Our observations point to the fact that ANA is a unique modulator of BMP-induced ectopic bone formation activity. ANA differs in many ways from previously reported molecules. For instance, deletion of Noggin deteriorates embryonic joint development severely and results in embryonic lethality (28). Deficiency of Tob and Caf1 is not lethal but enhances bone volume in the cancellous bone envelope in adult mice (15, 21, 29). In contrast, ANA deficiency specifically suppresses the level of BMP-induced ectopic bone formation, but it does not affect the levels of bone mass systemically, post-injury recovery of bone formation, or morphology of the joints and skeleton.
In the light of contemplating strategies to suppress ectopic bone formation, low molecular weight chemicals mimicking ANA action is desirable. Vice versa, to enhance BMP2 actions and thus apply our observations on the function of this molecule for the treatment of bone defects or fracture healing, which involve the osteoblastic differentiation from soft tissue cells surrounding skeleton, ANA is a possible target to modulate specifically the bone formation in such BMP-induced events in the local environment. It would be advantageous if we could develop inhibitors of ANA that can be administered orally or systemically for the patients who need bone formation induced by BMP at fracture sites or at other sites such as spinal fusion or bone defects
Recently, the causes for fibrodysplasia ossificans progressiva have been reported to be due to active mutations of the ALK, a BMP receptor family member (30). In fibrodysplasia ossificans progressiva, bone formation occurs everywhere in non-skeletal tissues, including muscle, to lead to the death of the patients due to immobilization of thorax and joints. These observations suggest that BMP activities should be properly suppressed in several ways in many places in such non-skeletal tissues as receptors for BMP could be expressed ubiquitously. BMP plays a role during the whole period of life. It is required for the induction of mesoderm in the early embryos, formation of bone and joint morphogenesis in the later embryonic stages, and the growth of bone during childhood and adolescence, and it also plays a role in many non-skeletal tissues, including skin, gut, and heart (27, 31). In fact, BMP receptors are expressed in most of the tissues. Consequently, it is necessary to properly control such BMP activities to establish appropriate balance for the expression of its target genes or proper levels of homeostasis of the activity of these molecules. Identification of ANA, to specifically suppress ectopic bone formation in muscle induced by BMP without affecting the basal levels of steady-state bone metabolism and bone turnover, suggests that ANA plays a pivotal role in this particular pathological condition. It is still to be elucidated whether ANA is related to ectopic or heterotopic bone formation in patients after arthroplasty and brain injury.
During fracture heeling, ∼5% of the patients suffer from non-union. Exact mechanisms or molecules responsible for such non-union have not yet been clearly understood. These patients are healthy in terms of the other skeletal or non-skeletal systems. Therefore, particular action of BMP during the healing of the fracture may be impaired at least in some of the fraction of the patients. Recently, BMP2 molecule (but not the other BMPs) has been implicated specifically in fracture healing (5). However, it is still to be determined whether BMP actions could be regulated during the repair of bone at the local site specifically by some groups of molecules, which may only act in the local milieu but not in the whole body. To this end, if molecules like ANA are involved in such pathological situation, they could be possible targets for the manipulation of the locally applied BMP activities.
It is intriguing that several members of the antiproliferative gene family have been shown to exert their effects as BMP modulators. Because BMPs are known to be involved in malignancy such as colon cancer (32), antiproliferative actions as well as anti-BMP actions may be in concert to suppress the oncogenicity. ANA deficiency has been also shown to correlate with lung adenocarcinoma (34). However, so far it has not been known how these two properties of this family member coordinate to play a role in suppression of the oncogenesis. If the reciprocal relationship between proliferation and differentiation could exist in the regulation of the bone development, antiproliferative action of the molecules may lead to the differentiation of the cell. If this is the case, antiproliferative genes may on one hand suppress proliferation to lead to promote differentiation while they may also suppress differentiation to balance the levels of homeostasis for the positive and negative forces for differentiation.
Our observations indicated that ANA is expressed in skeletal tissues where BMP molecules as well as their receptors are also expressed. ANA can be regarded as a part of negative feedback loop to balance the action of BMP signaling. Interestingly, not only ANA but also Tob and Caf1 expression is under the control of BMP (14, 21). BMP is also known to stimulate the expression of Noggin and other BMP antagonists (33). Thus, many BMP inhibitors are under the control of BMP by itself. These observations revealed that the potency of BMP to induce bone formation should be tightly controlled in the normal situation by the action of BMP itself. These inhibitory actions are necessary for the body to survive, because uncontrolled formation of bone in joints and muscle severely deteriorates locomotor function and breathing. The discovery of ANA as one of the molecules involved in this negative feedback system further suggests the presence of “fine” tuning of the potency of BMP in the physiology of the body.
Tob was reported to interact with Smad1, -5, and -8, and its deficiency enhances basal bone mass in mice. Interestingly, ANA does not interact with Smad1 or -5, but instead it interacts with Smad8. Whether this feature may link to the specific effects of ANA deficiency on the enhancement of ectopic bone formation without affecting basal bone mass levels or not remains to be further elucidated.
In conclusion, we identified ANA as a unique and novel BMP antagonist that specifically suppresses ectopic bone formation activity of BMP, a definitive character of this molecule.
This work was supported by grants-in-aid from the Japanese Ministry of Education (Global Center of Excellence Program, International Research Center for Molecular Science in Tooth and Bone Diseases, Grants 18109011, 18659438, 18123456, and 20013014), grants from the Japan Space forum, Japan Aerospace Exploration Agency, and Advanced Bone and Joint Science Strategic Research Networks Projects, and the Japan Society for Promotion of Science Core to Core Program, Research for the Future Program, Genome Science.
Footnotes
The abbreviations used are: BMP, bone morphogenetic protein; CHP-PEG, cholesterol-bearing pullulan cross-linked by polyethylene glycol; siRNA, small interference RNA; BRE, BMP response element; ALP, alkaline phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ANA, abundant in neuroepitherial area; X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
References
- 1.Shaffer, B. (1989) Bull. Hosp. Joint Dis. Orthop. Ins. 49 55-74 [PubMed] [Google Scholar]
- 2.Schaeffer, M. A., and Sosner, J. (1995) Arch. Phys. Med. Rehab. 76 284-286 [DOI] [PubMed] [Google Scholar]
- 3.Urist, M. R. (1965) Science 150 893-899 [DOI] [PubMed] [Google Scholar]
- 4.Sampath, T. K., and Reddi, A. H. (1981) Proc. Natl. Acad. Sci. U. S. A. 78 7599-7603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji, K., Bandyopadhyay, A., Harfe, B. D., Cox, K., Kakar, S., Gerstenfeld, L., Einhorn, T., Tabin, C. J., and Rosen, V. (2006) Nat. Genet. 38 1424-1429 [DOI] [PubMed] [Google Scholar]
- 6.Matsuda, S., Rouault, J. P., Magaud, J. P., and Berthet, C. (2001) FEBS Lett. 497 67-72 [DOI] [PubMed] [Google Scholar]
- 7.Matsuda, S., KawamuraTsuzuku, J., Ohsugi, M., Yoshida, M., Emi, M., Nakamura, Y., Onda, M., Yoshida, Y., Nishiyama, A., and Yamamoto, T. (1996) Oncogene 12 705-713 [PubMed] [Google Scholar]
- 8.Montagnoli, A., Guardavaccaro, D., Starace, G., and Tirone, F. (1996) Cell Growth & Differ. 7 1327-1336 [PubMed] [Google Scholar]
- 9.Rouault, J. P., Falette, N., Guehenneux, F., Guillot, C., Rimokh, R., Wang, Q., Berthet, C., MoyretLalle, C., Savatier, P., Pain, B., Shaw, P., Berger, R., Samarut, J., Magaud, J. P., Ozturk, M., Samarut, C., and Puisieux, A. (1996) Nat. Genet. 14 482-486 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida, Y., Matsuda, S., Ikematsu, N., Kawamura-Tsuzuku, J., Inazawa, J., Umemori, H., and Yamamoto, T. (1998) Oncogene 16 2687-2693 [DOI] [PubMed] [Google Scholar]
- 11.Ikematsu, N., Yoshida, Y., Kawamura-Tsuzuku, J., Ohsugi, M., Onda, M., Hirai, M., Fujimoto, J., and Yamamoto, T. (1999) Oncogene 18 7432-7441 [DOI] [PubMed] [Google Scholar]
- 12.Buanne, P., Corrente, G., Micheli, L., Palena, A., Lavia, P., Spadafora, C., Lakshmana, M. K., Rinaldi, A., Banfi, S., Quarto, M., Bulfone, A., and Tirone, F. (2000) Genomics 68 253-263 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki, T., K-Tsuzuku, J., Ajima, R., Nakamura, T., Yoshida, Y., and Yamamoto, T. (2002) Genes Dev. 16 1356-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida, Y., Tanaka, S., Umemori, H., Minowa, O., Usui, M., Ikematsu, N., Hosoda, E., Imamura, T., Kuno, J., Yamashita, T., Miyazono, K., Noda, M., Noda, T., and Yamamoto, T. (2000) Cell 103 1085-1097 [DOI] [PubMed] [Google Scholar]
- 15.Usui, M., Yoshida, Y., Tsuji, K., Oikawa, K., Miyazono, K., Ishikawa, I., Yamamoto, T., Nifuji, A., and Noda, M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 6653-6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park, S., Lee, Y. J., Lee, H. J., Seki, T., Hong, K. H., Park, J., Beppu, H., Lim, I. K., Yoon, J. W., Li, E., Kim, S. J., and Oh, S. P. (2004) Mol. Cell. Biol. 24 10256-10262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajima, R., Akiyama, T., Usui, M., Yoneda, M., Yoshida, Y., Nakamura, T., Minowa, O., Noda, M., Tanaka, S., Noda, T., and Yamamoto, T. (2008) FEBS Lett. 582 1313-1318 [DOI] [PubMed] [Google Scholar]
- 18.Guehenneux, F., Duret, L., Callanan, M., Bouhas, R., Hayette, S., Berthet, C., Samarut, C., Rimokh, R., Birot, A. M., Wang, Q., Magaud, J. P., and Rouault, J. P. (1997) Leukemia 11 370-375 [DOI] [PubMed] [Google Scholar]
- 19.Yoshida, Y., Hosoda, E., Nakamura, T., and Yamamoto, T. (2001) Jpn. J. Cancer Res. 92 592-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washio-Oikawa, K., Nakamura, T., Usui, M., Yoneda, M., Ezura, Y., Ishikawa, I., Nakashima, K., Yamamoto, T., and Noda, M. (2006) J. Cell. Biochem. 99 538-544 [DOI] [PubMed] [Google Scholar]
- 21.Washio-Oikawa, K., Nakamura, T., Usui, M., Yoneda, M., Ezura, Y., Ishikawa, I., Nakashima, K., Noda, T., Yamamoto, T., and Noda, M. (2007) J. Bone Miner. Res. 22 1217-1223 [DOI] [PubMed] [Google Scholar]
- 22.Toom, A., Arend, A., Gunnarsson, D., Ulfsparre, R., Suutre, S., Haviko, T., and Selstam, G. (2007) Calcif. Tissue Int. 80 259-267 [DOI] [PubMed] [Google Scholar]
- 23.Akiyoshi, K., Kobayashi, S., Shichibe, S., Mix, D., Baudys, M., Kim, S. W., and Sunamoto, J. (1998) J. Controlled Release 54 313-320 [DOI] [PubMed] [Google Scholar]
- 24.Kato, N., Hasegawa, U., Morimoto, N., Saita, Y., Nakashima, K., Ezura, Y., Kurosawa, H., Akiyosh, K., and Noda, M. (2007) J. Cell. Biochem. 101 1063-1070 [DOI] [PubMed] [Google Scholar]
- 25.Bellows, C. G., Ciaccia, A., and Heersche, J. N. M. (1998) Bone 23 119-125 [DOI] [PubMed] [Google Scholar]
- 26.Mirams, M., Robinson, B. G., Mason, R. S., and Nelson, A. E. (2004) Bone 35 1192-1199 [DOI] [PubMed] [Google Scholar]
- 27.Canalis, E., Economides, A. N., and Gazzerro, E. (2003) Endocr. Rev. 24 218-235 [DOI] [PubMed] [Google Scholar]
- 28.Brunet, L. J., McMahon, J. A., McMahon, A. P., and Harland, R. M. (1998) Science 280 1455-1457 [DOI] [PubMed] [Google Scholar]
- 29.Usui, M., Yoshida, Y., Yamashita, T., Nifuji, A., Ishikawa, I., Yamamoto, T., and Noda, M. (2000) J. Bone Miner. Res. 15 S190. [DOI] [PubMed] [Google Scholar]
- 30.Shore, E. M., Xu, M., Feldman, G. J., Fenstermacher, D. A., Cho, T. J., Choi, I. H., Connor, J. M., Delai, P., Glaser, D. L., LeMerrer, M., Morhart, R., Rogers, J. G., Smith, R., Triffitt, J. T., Urtizberea, J. A., Zasloff, M., Brown, M. A., and Kaplan, F. S. (2006) Nat. Genet. 38 525-527 [DOI] [PubMed] [Google Scholar]
- 31.Hogan, B. L. M. (1996) Genes Dev. 10 1580-1594 [DOI] [PubMed] [Google Scholar]
- 32.Kodach, L. L., Bleurning, S. A., Musler, A. R., Peppelenbosch, M. R., Hommes, D. W., van Den Brink, G. R., van Noesel, C. J. M., Offerhaus, G. J. A., and Hardwick, J. C. H. (2008) Cancer 112 300-306 [DOI] [PubMed] [Google Scholar]
- 33.Gazzerro, E., Gangji, V., and Canalis, E. (1998) J. Clin. Investig. 102 2106-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneda, M., Suzuki, T., Nakamura, T., Ajima, R., Yoshida, Y., Kakuta, S., Sudo, K., Iwakura, Y., Shibutani, M., Mitsumori, K., Yokota, J., and Yamamoto, T. (2008) Cancer Sci. 100 225-232 [DOI] [PMC free article] [PubMed] [Google Scholar]