Abstract
Growth-arrested 3T3-L1 preadipocytes rapidly express CCAAT/enhancer-binding protein-β (C/EBPβ) upon hormonal induction of differentiation. However, the DNA binding activity of C/EBPβ is not activated until the cells synchronously reenter S phase during the mitotic clonal expansion (MCE) phase of differentiation. In this period, C/EBPβ is sequentially phosphorylated by MAPK and glycogen synthase kinase-3β, inducing C/EBPβ DNA binding activity and transcription of its target genes. Because the DNA binding activity of C/EBPβ is further enhanced by oxidation in vitro, we investigated how redox state affects C/EBPβ DNA binding and MCE during adipogenesis. When 3T3-L1 cells were treated with H2O2 and hormonal stimuli, differentiation was accelerated with increased expression of peroxisome proliferator-activated receptor γ. Interestingly, cell cycle progression (S to G2/M phase) was markedly enhanced by H2O2, whereas antioxidants caused an S phase arrest during the MCE. H2O2 treatment resulted in the early appearance of a punctate pattern observed by immunofluorescent staining of C/EBPβ, which is a hallmark for C/EBPβ binding to regulatory elements, whereas a short antioxidant treatment rapidly dispersed the centromeric localization of C/EBPβ. Consistently, reactive oxygen species production was increased during 3T3-L1 differentiation. Our results indicate that redox-induced C/EBPβ DNA binding activity, along with the dual phosphorylation of C/EBPβ, is required for the MCE and terminal differentiation of adipocytes.
Adipocyte differentiation involves an elaborate network of transcription factors that regulate the expression of numerous genes responsible for the phenotype of mature adipocytes (1–3). Several members of the C/EBP2 family as well as PPARγ participate in a transcriptional cascade during adipogenesis (2, 4). Most of our knowledge about transcriptional regulation in adipocytes comes from studies using the murine 3T3-L1 preadipocyte model. The differentiation of growth-arrested 3T3-L1 cells into adipocytes is induced by hormonal stimuli, which immediately causes the expression of C/EBPβ (5–7). C/EBPβ is thought to initiate mitotic clonal expansion of the preadipocytes and to later coordinate the transcription network by turning on two principal adipogenic factors, C/EBPα and PPARγ (1, 8). Although the expression of C/EBPβ reaches its maximal level within 4 h of induction, the binding of C/EBPβ to DNA and the expression of C/EBPα and PPARγ are only observed after a long lag period (4, 5). During this period, cells synchronously enter the cell cycle (6, 9), which is accompanied by the nuclear localization of glycogen synthase kinase 3β (GSK3β) (10).
A recent investigation has revealed that phosphorylation of C/EBPβ by nuclear GSK3β enhances its DNA binding activity (10). Thr188 of C/EBPβ is phosphorylated by MAPK immediately after its expression is induced and then later by GSK3β on Ser184 or Thr179. The priming phosphorylation of C/EBPβ-Thr188 is maintained throughout S phase and mitotic clonal expansion through the activity of Cdk2-cyclin A after MAPK activity is down-regulated (11). Because the translocation of GSK3β into the nucleus is observed 12–14 h after induction, it has been suggested that the dual phosphorylation of C/EBPβ is associated with the activation of its DNA binding function. This finding has been confirmed in our experiment using highly purified recombinant C/EBPβ (12). In that study, when recombinant C/EBPβ protein was exposed to mild oxidizing conditions, only doubly phosphorylated C/EBPβ had enhanced DNA binding activity. This suggests that the dual phosphorylation of C/EBPβ leads to a conformational change that facilitates dimerization through its C-terminal leucine zipper domain, rendering the basic DNA-binding region of C/EBPβ accessible to the C/EBP regulatory element on C/EBPα or PPARγ. In this scenario, under oxidizing conditions the monomer-dimer equilibrium of dual phosphorylated C/EBPβ would be shifted toward dimer formation and the binding of the regulatory element to confer the proper level of transcriptional activation (12).
Although the mechanisms by which redox state actively regulates cellular metabolism are not clear, several reports have proposed that oxidative stress is an important element in metabolic diseases. For example, in obesity, fat accumulation is correlated with systemic oxidative stress in humans and mice (13). Production of reactive oxygen species (ROS) increases selectively in the adipose tissue of obese mice, accompanied by increased expression of NADPH oxidase and decreased expression of antioxidative enzymes. In addition, increased ROS levels are reported to be an important trigger for insulin resistance (14). Consistent with this, it has been shown that oxidative stress impairs glucose uptake in mature adipocytes (15) and decreases insulin secretion from pancreatic β-cells (16). ROS generation during adipogenesis has also been observed, and it has been proposed that insulin and insulin-like growth factor-I are active stimulators of NADPH-dependent hydrogen peroxide generation (17); however, the mechanisms by which ROS affect adipocyte differentiation have not been elucidated.
In this study, we showed how redox state affects mitotic clonal expansion during adipocyte differentiation. H2O2 treatment in conjunction with hormonal stimulation of 3T3-L1 cells caused accelerated cell cycle progression (S to G2/M phase) and the early appearance of punctate patterns observed from the immunofluorescent staining of C/EBPβ. On the other hand, short antioxidant treatment (4 h) rapidly dispersed the centromeric localization of C/EBPβ and arrested the mitotic clonal expansion phase, indicating that ROS production is crucial for the mitotic clonal expansion of preadipocytes. Our findings indicate that, in addition to the dual phosphorylation of C/EBPβ, redox-induced DNA-binding of C/EBPβ is required for the mitotic clonal expansion and terminal differentiation of adipocytes.
EXPERIMENTAL PROCEDURES
Cell Culture and Induction of Differentiation—3T3-L1 and 3T3-F442A preadipocytes were maintained in DMEM containing 10% calf serum. To induce differentiation, 2-day postconfluent 3T3-L1 cells (designated day 0) were incubated in DMEM containing 10% FBS, 0.5 mm 3-isobutyl-1-methylxanthine (IBMX), 1 μm dexamethasone, and 1 μg/ml insulin for 2 days. For 3T3-F442A cells, differentiation was induced by 10% FBS and 1 μg/ml insulin for 2 days. Cells were then fed DMEM containing 10% FBS and insulin for another 2 days, after which they were fed DMEM containing 10% FBS. In some experiments, H2O2 (50 or 100 μm), N-acetyl cysteine (10 mm), genistein (50 μm), or resveratrol (50 μm) was added at the time of differentiation induction and maintained for 2 days unless otherwise stated. Cell numbers were determined on day 2, and oil red O staining (18) was performed on day 8.
Western Blotting—At each time indicated, cells were washed once with cold phosphate-buffered saline (PBS) and then scraped into lysis buffer containing 1% SDS and 60 mm Tris-HCl, pH 6.8. Lysates were boiled for 10 min and verified by centrifugation, and then equal amounts of protein were subjected to SDS-PAGE and immunoblotted with antibodies to C/EBPβ (5), PPARγ, cyclin A, or SIRT1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Small Interfering RNA (siRNA)—3T3-L1 preadipocytes were plated with DMEM containing 10% calf serum at a density of 6 × 105 cells/dish in 60-mm dishes. Twenty-four hours later, medium was changed to OPTI-MEM (Invitrogen), and the cells (about 70% confluent) were transfected with siRNA using Lipofectamine RNAi/MAX reagent (Invitrogen), according to the manufacturer's protocol. The next day, medium was replaced with fresh DMEM containing 10% calf serum for 24 h before the induction of differentiation. The modified synthetic CEBPβ siRNA (stealth siRNA) and SIRT1 siRNA were from Invitrogen. The sequences are as follows: siCEBPβ,5′-AGUAG AAGUU GGCCA CUUCC AUGGG-3′; siSIRT1, 5′-GAUGA AGUUG ACCUC CUCAT T-3′.
FACS Analysis—3T3-L1 preadipocytes were induced to differentiate, as described above. At the times indicated, cells were trypsinized, washed with PBS, and fixed with 90% cold methanol. Fixed cells were washed with PBS and incubated in the dark for 30 min with propidium iodide staining solution containing 50 μg/ml propidium iodide and 100 μg/ml RNase A in PBS. Labeled cells were analyzed using a FACSCalibur flow cytometry system (BD Biosciences). Fluorescence was measured at 650 nm for propidium iodide, and data were analyzed using CellQuest software (BD Bioscience).
Immunohistochemistry—3T3-L1 cells were differentiated on coverslips in 6-well plates at the same density as usual, as described above. At the times indicated, cells were washed with PBS, fixed in 4% formaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 20 min on ice, and then blocked in 3% bovine serum albumin in PBS for 1 h. Cells were then incubated in blocking solution containing C/EBPβ antibody (5) (1:250 dilution) for 1 h, followed by secondary antibody (anti-rabbit IgG-fluorescein isothiocyanate) for 1 h. The cells were mounted in 4′,6-diamidino-2-phenylindole.
Chromatin Immunoprecipitation (ChIP)—ChIP analysis was performed following the protocol of the ChIP assay kit (Upstate Biotechnology, Inc., Lake Placid, NY). DNA-protein complexes were immunoprecipitated with antibodies against C/EBPβ for 4 h and then collected with protein A-agarose for 3 h. The beads were washed, and chromatin complexes were eluted from the beads. After reversal of the cross-linking, DNA was purified, and input control or ChIP samples were used as a template in PCR using the following primers to amplify the PPARγ2 promoter containing the C/EBP binding site: sense, 5′-TGCTA TCCAA ATTCT ATAAA-3′, antisense 5′-ATTTA AATTT TACTA GCCTT-3′.
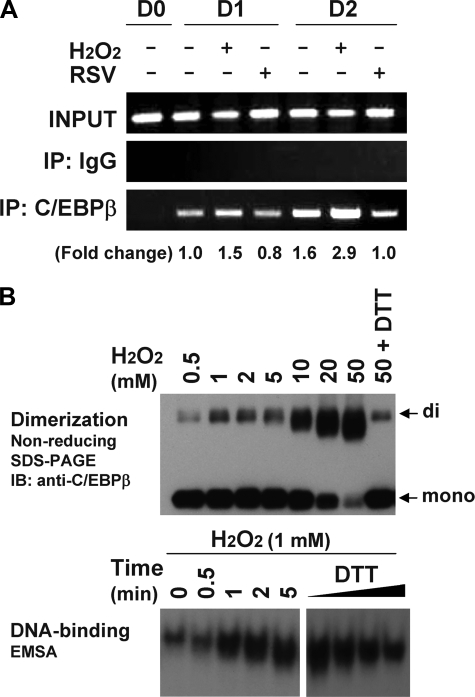
In Vitro Phosphorylation and Oxidation of Recombinant C/EBPβ, EMSA, and Nonreducing SDS-PAGE—Recombinant C/EBPβ protein was prepared and phosphorylated by MAPK and GSK3β, as described (12). For phosphorylation, 500 ng of recombinant protein was incubated with activated MAPK (Calbiochem) and GSK3β (Upstate Biotechnology) in 50 mm Hepes (pH 7.6), 10 mm MgCl2, 0.5 mm ATP, and 1 mm DTT at 30 °C for 30 min. After phosphorylation, C/EBPβ was treated with various concentrations of H2O2 for 30 min, followed by 20 mm iodoacetamide. Samples (40 ng of protein) were run in 10% NuPAGE (Invitrogen) without reducing agent, transferred, and subjected to immunoblotting with anti-C/EBPβ antibody. EMSA was performed, essentially as described (12). In oxidation-induced binding experiments, DTT was omitted unless indicated. The C/EBP-binding sequence in the C/EBPα promoter is 5′-GGCGT TGCGC CACGA TCTCT CT-3′.
Measurement of ROS—ROS were detected with the peroxide-sensitive fluorophore 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) (Invitrogen). At the times indicated, DCF-DA dissolved in DMSO was added to cells at 10 μm (final concentration). After incubation for 1 h at 37 °C under an atmosphere including 5% CO2, 3T3-L1 cells were washed with PBS and resuspended in PBS. Cells were analyzed by the FAC-SCalibur flow cytometry system (BD Biosciences). A 488-nm argon laser beam was used for excitation. The arithmetic mean fluorescence channel was derived by CellQuest software.
RESULTS
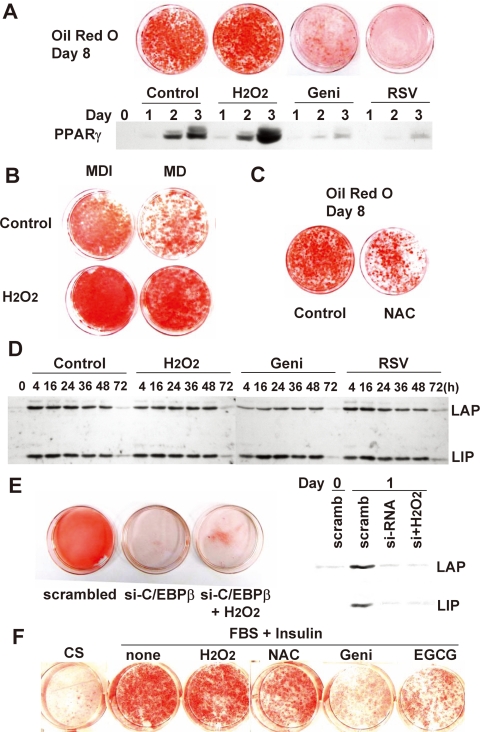
ROS Accelerates the Differentiation of 3T3-L1 Preadipocytes—Postconfluent, growth-arrested 3T3-L1 preadipocytes were induced to differentiate by hormonal stimulation. To investigate whether ROS affects adipocyte differentiation, H2O2 was added for the first 2 days (day 0 to day 2) of the standard differentiation protocol. As shown in Fig. 1A, 2-day H2O2 treatment caused enhanced differentiation, as detected by oil red O staining, which is correlated with PPARγ expression. The H2O2 effect was more pronounced when a partial hormonal stimulus (by removing insulin from the standard regimen) was used. The 3T3-L1 cell differentiation was diminished to ∼50% of control levels in the absence of insulin, whereas the addition of H2O2 markedly enhanced the differentiation (Fig. 1B) even in the absence of insulin. The effect of H2O2 treatment was strongly reproducible, dose-dependent, and observed only in differentiating preadipocytes, whereas preconfluent proliferating cells did not survive the relatively high concentration (100 μm) of H2O2 (not shown). When differentiating preadipocytes were exposed to antioxidants, such as genistein or resveratrol, PPARγ expression was nearly abolished, and cells did not differentiate, as shown by oil red O staining (Fig. 1A). Treatment with the general antioxidant N-acetyl cysteine (NAC) had the same effect on oil red O staining (Fig. 1C).
FIGURE 1.
The effects of ROS on differentiation of 3T3-L1 preadipocytes. Two-day postconfluent 3T3-L1 preadipocytes were induced to differentiate with the standard hormonal regimen (IBMX, dexamethasone, and insulin) in the presence or absence of H2O2 (100 μm), genistein (50 μm), resveratrol (50 μm) (A), or N-acetyl cysteine (10 mm) (B). After 2 days, all groups were fed with FBS, DMEM, and insulin for another 2 days and then with FBS + DMEM. On day 8, cells were fixed, and cytoplasmic triglyceride was stained with oil red O. In addition, whole cell extracts from day 1, 2, and 3 adipocytes were prepared and subjected to immunoblotting with antibody against PPARγ. C, cells were differentiated under the standard regimen (IBMX, dexamethasone, and insulin; MDI) or an incomplete regimen, lacking insulin (MD). D, cell lysates prepared from differentiating preadipocytes were subjected to SDS-PAGE and immunoblotted with antibody against C/EBPβ. LAP and LIP, 38-kDa liver-activating protein and 18-kDa liver-inhibiting protein, respectively. E, 3T3-L1 cells were treated with C/EBPβ siRNA at about 70% confluence using Lipofectamine RNAi/MAX reagent. After 24 h, medium was replaced with fresh DMEM containing 10% calf serum for 24 h before the induction of differentiation. Knockdown of C/EBPβ was verified by immunoblotting, and cells were differentiated with or without H2O2 (2-day treatment) and subjected to oil red O staining at day 8. F, 3T3-F442A cells were differentiated in the presence or absence of H2O2 (10 μm), genistein (50 μm), epigallocatechin 3-gallate (EGCG; 50 μm), or N-acetyl cysteine (10 mm).
In an attempt to identify the mechanism by which H2O2 affects 3T3-L1 cell differentiation, we first investigated the expression of C/EBPβ, a key regulator of C/EBPα and PPARγ expression. Although PPARγ expression and lipid accumulation were affected by H2O2 or antioxidant treatment, the expression of C/EBPβ was essentially unchanged (Fig. 1D). It had been previously shown that mouse embryonic fibroblast cells from C/EBPβ(-/-) mice undergo neither clonal amplification nor terminal differentiation, indicating that C/EBPβ is required for mitotic clonal expansion (6). Consistently, RNA interference of C/EBPβ expression prevented the differentiation of 3T3-L1 cells (Fig. 1E). Importantly, H2O2 treatment did not result in any significant differentiation when the expression of C/EBPβ was reduced by siRNA (Fig. 1E), confirming that C/EBPβ is absolutely required for adipogenesis and also suggesting that the ROS-dependent effect requires the presence of C/EBPβ protein. We also attempted to confirm these findings with the other preadipocyte precursor cell line, 3T3-F442A. This cell line is reported to undergo clonal expansion accompanied by DNA synthesis and increased cell numbers (19, 20), which means that the differentiation of 3T3-F442A would be also affected by ROS. A similar result was observed with 3T3-F442A (Fig. 1F), indicating that the ROS effect is not confined to 3T3-L1 cells. These findings indicate that ROS may accelerate the differentiation process by enhancing the activation of C/EBPβ.
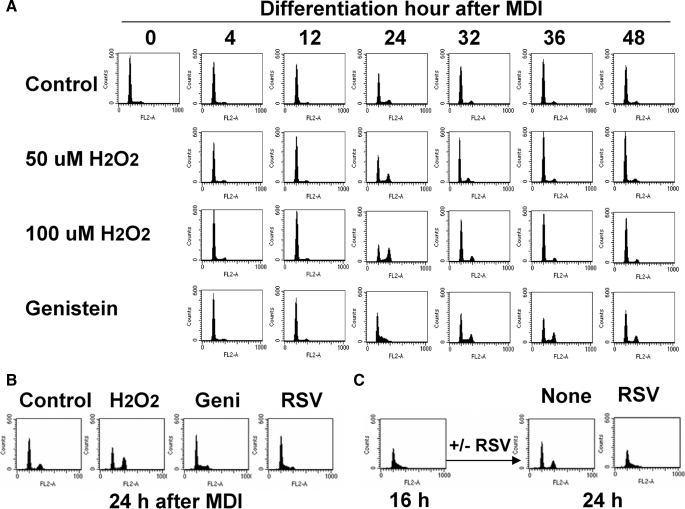
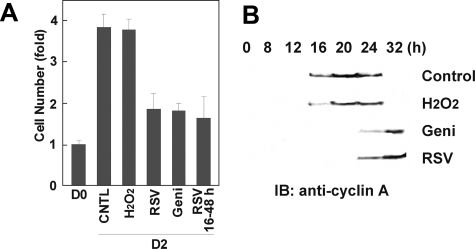
ROS Facilitates Cell Cycle Progression into G2/M Phase during Mitotic Clonal Expansion—Next, we examined how cell cycle events during mitotic clonal expansion are affected by ROS production. Upon hormonal stimulation, growth-arrested 3T3-L1 cells synchronously reenter the cell cycle, resulting in the detection of dividing cells (G2/M) by FACS analysis after ∼24 h of induction (Fig. 2A). After exiting M phase, these cells either undergo a second round of division or begin the terminal differentiation process; therefore, induced cells are no longer synchronous beyond 24 h of hormone stimulation (1). Interestingly, H2O2 treatment caused an increase in the G2/M population during adipocyte differentiation, in a dose-dependent manner (Fig. 2A). In contrast, genistein or resveratrol treatment significantly delayed the progression of the cell cycle, suggesting that ROS production is required for clonal expansion during adipogenesis. Importantly, preadipocytes treated with antioxidants were still in S phase after 24 h (Fig. 2B), indicating that ROS production is specifically required for progression of these cells from S to G2/M. Consistently, short term antioxidant treatment after 16 h of induction (after cells have already entered S phase) completely blocked the progression of cells into G2/M phase by 24 h (Fig. 2C). These findings indicate that ROS production is critical for the S to G2/M transition during the mitotic clonal expansion of the adipocyte differentiation process. Arresting or delaying cell cycle progression resulted in decreased cell numbers at 2 days of induction compared with control group (Fig. 3A). The cell cycle delay was also confirmed by assaying the expression of cyclin A, as shown in Fig. 3B. We also determined that expression of MAPK-p, MAPK, GSK3β, p27, p21, cyclin E, and Cdk2 were not significantly changed by these treatments (data not shown). It should be noted that although it resulted in accelerated cell cycle progression, H2O2 treatment did not lead to an increase in cell number after clonal amplification, suggesting that there may be spatial constraints regulating the termination of clonal expansion. Nevertheless, our observations during the first 24-h period of mitotic clonal expansion, during which cells are highly synchronous, indicate that ROS production is important for the progression of mitotic clonal expansion during adipocyte differentiation.
FIGURE 2.
FACS analysis during MCE of adipocyte differentiation. Cells were induced to differentiate in the presence or absence of H2O2, genistein (50 μm), or resveratrol (50 μm), and at the times indicated, cells were trypsinized and fixed. Changes in DNA content were analyzed by FACS using a FACSCalibur flow cytometry system, and data were analyzed using CellQuest software. FACS analysis was performed during 0–72 h of differentiation induction (A) and after 24 h of induction (B). C, cells were differentiated with the standard protocol for 16 h, after which resveratrol (50 μm) was added to the medium. FACS analysis was done at 24 h after induction of differentiation.
FIGURE 3.
Effects of ROS on cell number and expression of cyclin A during MCE. A, on day 2 of differentiation, cells were trypsinized and subjected to cell counting. Cells were treated with H2O2 (100 μm), resveratrol (50 μm), or genistein (50 μm) for 48 or 32 h, as indicated. B, cell lysates were prepared at the times indicated and subjected to SDS-PAGE and immunoblotting (IB) to measure the expression of cyclin A.
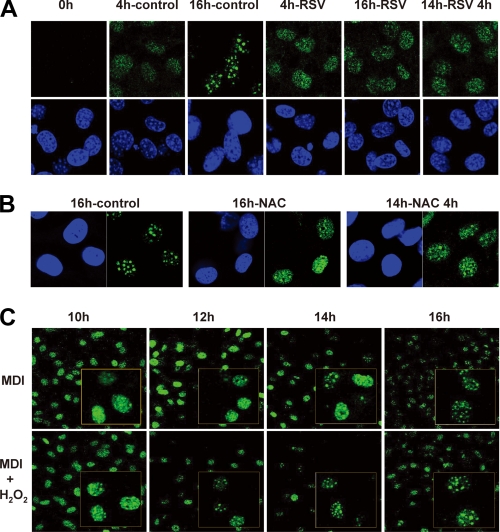
ROS Production Is Associated with the Activation of C/EBPβ—Previous investigations have shown that C/EBPβ is required first for initiating mitotic clonal expansion and later for activating the expression of pleiotropic transcription factor, C/EBPα, and PPARγ (6). Despite the early appearance of C/EBPβ protein after the induction of differentiation, it does not bind to target sites on DNA until cells enter S phase. As C/EBPβ acquires its DNA binding activity, it becomes localized to centromeres, resulting in a characteristic “punctate” pattern in immunofluorescence studies (Fig. 4A) (5). This pattern is observed from 12 to 16 h postinduction (i.e. S phase) when C/EBPβ is subjected to a second phosphorylation event by nuclear GSK3β (10). Since we have previously reported that the binding activity of C/EBPβ is further enhanced in an oxidative environment, in vitro (12), we assumed that C/EBPβ binding to centromeres would be altered by changes in ROS production during adipogenesis. As shown in Fig. 4A, the immunofluorescent staining pattern of C/EBPβ remained diffuse at the 16 h time point when differentiating cells were treated with resveratrol. The same effect was observed when the general antioxidant NAC was used (Fig. 4B). More importantly, the addition of resveratrol or NAC after 14 h, at which time C/EBPβ is already localized to centromeres, rapidly converted the normal punctate pattern to a diffuse nuclear staining pattern (Fig. 4, A and B). This indicates that in the presence of appropriate oxidative stress, the equilibrium of C/EBPβ binding is shifted toward tight DNA binding, giving rise to a punctate pattern. In other words, when oxidative stress is removed with antioxidants, C/EBPβ does not readily bind to DNA despite having been activated by dual phosphorylation. To verify the ROS effect directly, we performed immunofluorescent stainings of C/EBPβ after the addition of H2O2 in 2-h time intervals between 10 and 16 h postinduction. In the standard differentiation protocol, cells began to have punctate patterns of C/EBPβ between 14 and 16 h postinduction, whereas H2O2 treatment caused the early appearance of the punctate pattern at around 12 h (Fig. 4C). This strongly suggests that in the presence of proper ROS production, after dual phosphorylation, C/EBPβ protein binds to DNA well enough to allow its centromeric localization.
FIGURE 4.
Effects of ROS on intracellular localization of C/EBPβ during MCE. 3T3-L1 preadipocytes were induced to differentiate with the standard protocol in the presence or absence of H2O2 (100 μm), resveratrol (50 μm), or N-acetyl cysteine (10 mm), as indicated. Cells were fixed and subjected to immunofluorescence analysis with antibody against C/EBPβ and 4′,6-diamidino-2-phenylindole. Fluorescence images were obtained by confocal microscopy. Resveratrol (A) and N-acetyl cysteine (B) prevented the characteristic punctate pattern at 16 h of induction and also rapidly dispersed the centromeric localization after short term treatment (14–18 h of induction). C, H2O2 caused the early appearance of a punctate pattern in immunofluorescent stainings.
C/EBPβ is responsible both for mitotic clonal expansion and the activation of the promoters of specific genes, such as C/EBPα and PPARγ. To assay C/EBPβ binding to specific promoters, we next performed the ChIP assays in the absence or presence of H2O2 treatment. As illustrated in Fig. 5A, H2O2 treatment caused increased binding of C/EBPβ to the PPARγ2 promoter (1.5- and 2.9-fold at day 1 and 2, respectively), whereas resveratrol treatment decreased C/EBPβ binding, demonstrating that C/EBPβ binding to its target sites is enhanced by ROS production. The enhanced DNA binding activity of C/EBPβ in response to H2O2 treatment was also assessed in vitro in EMSAs using recombinant C/EBPβ protein. We had previously produced highly purified recombinant C/EBPβ protein using the GST fusion method and ion exchange chromatography and had confirmed the dual phosphorylation of the recombinant C/EBPβ protein by MAPK and GSK3β (12). As had previously been shown using oxidized glutathione (12), H2O2 treatment caused the dimerization of dually phosphorylated recombinant C/EBPβ protein, as detected by nonreducing SDS-PAGE. H2O2 treatment also resulted in increased C/EBPβ DNA binding activity in the EMSAs (Fig. 5B). These results provide evidence that C/EBPβ binding to its regulatory element is enhanced by ROS, which might occur during normal differentiation of 3T3-L1 cells, in vivo.
FIGURE 5.
DNA-binding of C/EBPβ protein, in vivo and in vitro. A, chromatin immunoprecipitation (IP) analysis using the PPARγ promoter. At the indicated times after induction of differentiation with or without H2O2 (100 μm) or resveratrol (50 μm), cells were cross-linked with formaldehyde, the DNA was fragmented, and the chromatin-associated DNA was immunoprecipitated with IgG or antibodies against C/EBPβ. PCR amplification of the DNA fragments was conducted with specific primers corresponding to the PPARγ2 promoter sequence. Relative -fold changes of binding were calculated using a densitometer. B, EMSA of recombinant C/EBPβ protein treated by H2O2. Recombinant protein doubly phosphorylated by MAPK and GSK3β was treated with the indicated concentrations of H2O2 and/or 5 mm DTT and subjected to nonreducing SDS-PAGE, without DTT, and immunoblotting (IB) with anti-C/EBPβ antibody (top). The same reaction using 1 mm H2O2 was used for EMSA, except the reaction was terminated with 20 mm iodoacetamide at the indicated times, and 5 ng of protein was used for the binding reaction with the C/EBP site (bottom). DTT was added as marked (0–5 mm).
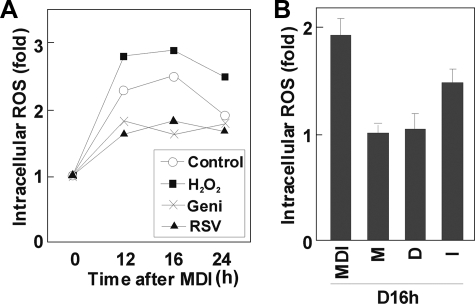
ROS Production Is Increased during Mitotic Clonal Expansion—Because oxidative stress has been highlighted in many pathologic conditions, there have been several attempts to measure ROS production in mature adipocytes (13, 17). However, ROS production has not specifically been measured during the mitotic clonal expansion of differentiating adipocytes. The results shown above suggest that ROS are required for the proper differentiation of preadipocytes and predict that ROS production should be a normal part of the differentiation program. Since the FACS data showed that the ROS effect was particularly important between 12 and 24 h after induction (Fig. 2), we measured intracellular ROS levels with DCF-DA using the FACSCalibur flow cytometry system during this time window. As shown in Fig. 6A, ROS production was significantly elevated during mitotic clonal expansion in adipogenesis. Also, intracellular ROS was suppressed by the addition of antioxidants, such as genistein, resveratrol (Fig. 6A), or NAC (data not shown). It is noteworthy that ROS production peaks during the 12–16 h period, then decreases. We also observed that ROS production increases in almost a linear fashion between 0 and 12 h (data not shown), suggesting that ROS production is a direct response to hormonal stimulation and reaches a threshold, after which C/EBPβ binds to DNA effectively. We next examined which component of the standard hormonal regimen for differentiation is responsible for elevating ROS levels. As shown in Fig. 6B, although it appears that all of the components (IBMX, dexamethasone, and insulin) are required for full elevation of ROS in adipocytes, insulin may be particularly important.
FIGURE 6.
ROS production during MCE in adipogenesis. ROS were detected with the peroxide-sensitive fluorophore DCF-DA. At the times indicated, cells were incubated with DCF-DA (10 μm) for 1 h and analyzed by the FACSCalibur flow cytometry system; data are presented as relative intracellular ROS levels. A, ROS levels during MCE in the presence or absence of H2O2 (100 μm), genistein (50 μm), or resveratrol (50 μm); B, 3T3-L1 preadipocytes were treated with IBMX (M), dexamethasone (D), or insulin (I), respectively; ROS production was measured, as described above.
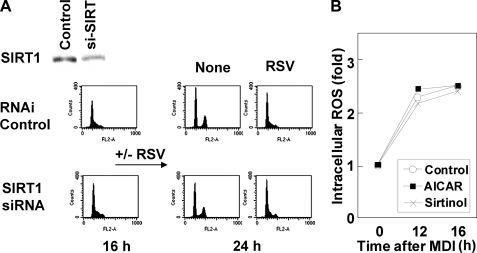
Affect of Resveratrol on Mitotic Clonal Expansion Is Independent of the SIRT Pathway—The results illustrated in Fig. 2C show that resveratrol blocked cell cycle progression of induced preadipocytes at the S to G2/M transition. Apart from its antioxidant activity, resveratrol has been implicated as a SIRT1 activator in regulating glucose metabolism, lipid metabolism, and the longevity of organisms (21, 22). Although we had confirmed that most of our data using resveratrol can be reproduced using a general antioxidant NAC, we could not rule out the possible involvement of the SIRT1 pathway. To assess whether SIRT1 is involved in the modulation of the cell cycle by ROS, we carried out FACS analysis with or without RNA interference to reduce SIRT1 expression. As shown in Fig. 7A, the blocking of the S to G2/M transition by resveratrol was not influenced by SIRT1 knockdown. Moreover, sirtinol, an inhibitor of SIRT1 (23), neither affected the progression of clonal expansion, as assessed by FACS (data not shown), nor modulated ROS production during mitotic clonal expansion (Fig. 7B), suggesting that resveratrol affects the cell cycle of differentiating adipocytes in a SIRT1-independent manner, at least during the mitotic clonal expansion phase of adipogenesis. Meanwhile, some investigators have suggested that AMP-dependent kinase is a downstream target of resveratrol (22, 24, 25). It has also been suggested that persistent activation of AMP-dependent kinase inhibits adipocyte differentiation (26, 27). In our experiments, aminoimidazole carboxamide ribonucleotide treatment did not change the level of ROS production in 3T3-L1 cells (Fig. 7B), indicating that AMP-dependent kinase is not involved in ROS production during adipogenesis. This does not rule out the possibility that increased ROS production during mitotic clonal expansion down-regulates AMP-dependent kinase activity, as has been proposed by another group (26); however, it does confirm that resveratrol inhibits mitotic clonal expansion by modulating ROS production, independent of the SIRT1 and AMP-dependent kinase pathways.
FIGURE 7.
Resveratrol effects are independent of SIRT1 pathway. A, 3T3-L1 preadipocytes were subjected to RNA interference against siSIRT1. A ∼50% reduction of SIRT1 expression was confirmed by Western blotting, and FACS showed that SIRT1 knockdown did not affect the S phase accumulation of cells driven by resveratrol. B, ROS measurement during MCE from cells treated with aminoimidazole carboxamide ribonucleotide (AICAR) (1 mm) or sirtinol (20 μm).
DISCUSSION
The differentiation of 3T3-L1 preadipocytes is triggered by hormonal agents and carried out by the coordination and precise control of transcriptional cascades. The first stage of adipocyte differentiation is growth arrest at a confluent state (28), and proliferating cells will not accumulate lipid droplets in their cytoplasms, even in the presence of hormonal stimulation, until they become confluent. Upon induction of differentiation, growth-arrested cells undergo additional rounds of cell division, known as mitotic clonal expansion (1). Other preadipocyte lineages, such as 3T3-F442A and Ob17 cells, like 3T3-L1 cells, are also believed to undergo a clonal amplification process, since inhibition of DNA synthesis prevents the differentiation of these cells (29). On the other hand, primary preadipocytes derived from human adipose tissue have been reported to differentiate even in the presence of alkylating agents that inhibit mitosis (30). It is uncertain whether primary preadipocytes prepared from human fat pads have already undergone mitotic clonal expansion in vivo (28, 29). It is worth pointing out that the mitotic clonal expansion after the confluent stage differs from the cell divisions that occur during the proliferation stage (5), especially in regard to the use of activating genes, such as PPARγ and C/EBPα, after growth arrest, suggesting that critical events may take place between mitotic clonal expansion and terminal differentiation. Hence, it is important to investigate how the molecular events during mitotic clonal expansion, whether or not they are related to cell cycle, are associated with terminal differentiation as well as with the expression of C/EBPα and PPARγ.
C/EBPβ is one of the critical transcription factors expressed during the mitotic clonal expansion period (4). During adipocyte differentiation, C/EBPβ is immediately expressed upon hormonal stimulation and sequentially phosphorylated by MAPK and GSK3β (5, 10). C/EBPβ lacks DNA binding activity until the protein is phosphorylated by GSK3β at 12–16 h after induction (10). At this time, C/EBPβ becomes localized to centromeres as preadipocytes synchronously enter S phase at the onset of mitotic clonal expansion (5). Because the localization to centromeres occurs through C/EBP consensus-binding sites in centromeric satellite DNA, it is thought that the C/EBPβ becomes active only after dual phosphorylation by MAPK and GSK3β, although the maximal expression of C/EBPβ is much earlier (4 h after induction). Previously, we were able to assess how C/EBPβ becomes activated by dual phosphorylation using highly purified recombinant C/EBPβ protein (12). A model was proposed in which dual phosphorylation of C/EBPβ caused a conformational change that facilitates S–S bond formation and dimerization, rendering the basic region accessible to the C/EBP regulatory element. In this point of view, we hypothesized that differentiating cells are exposed to an oxidative environment, which enhances C/EBPβ function during adipogenesis, in vivo. In the present study, we show that adipogenesis is accelerated by increasing ROS levels, which are related to C/EBPβ DNA binding activity. ROS production is increased during adipogenesis induced by the standard protocol, particularly within 12–16 h after differentiation induction (Fig. 6). This period corresponds to the S phase of the mitotic clonal expansion, during which C/EBPβ localizes to centromeres. Interestingly, reducing ROS levels by antioxidant treatment prevented not only the localization of C/EBPβ but also cell cycle progression into G2/M phase. Although this is not absolute proof that C/EBPβ is directly involved in the S to G2/M transition, it should be noted that short term antioxidant treatment dispersed the centromeric localization of C/EBPβ, in addition to blocking S to G2/M progression, showing that the activation of C/EBPβ is at least associated with cell cycle progression. It should be noted that although C/EBPβ is reported to be crucial for adipogenesis in 3T3-L1 cells and mouse embryonic fibroblasts from C/EBPβ(-/-) mice (6), its absolute requirement was argued by other experiments (31). Also, C/EBPβ-deficient mice have some adiposity, probably due to abnormal lipogenesis (31). In this regard, it was suggested that C/EBPδ may compensate for the loss of C/EBPβ, with some redundancy in the early steps of adipogenesis in vivo (2, 32). It remains to be clarified whether the ROS effect on C/EBPβ is related to adipogenesis in vivo, which is also associated with controversy about the requirement of mitotic clonal expansion in vivo (28, 29).
Oxidative stress has been implicated in various diseases, including insulin resistance, obesity, cardiovascular disease, and type 2 diabetes (13–16). Furukawa et al. (13) have reported that increased oxidative stress in accumulated fat is an important pathogenic mechanism of obesity-associated metabolic syndrome. ROS were also reported to have a causal role in multiple forms of insulin resistance (14). On the other hand, glucose 6-phosphate dehydrogenase is proposed to enhance prooxidative enzymes, including iNOS and NADPH oxidase, thus inducing oxidative stress in mature adipocytes (33). Our data show that ROS are also important in regulating mitotic clonal expansion during adipogenesis, supported by the experiments in which H2O2 or antioxidant treatment was added only for the first 2 days of the differentiation protocol. Given that in obese animals mature adipocytes produce higher levels of ROS compared with other tissues, including liver, skeletal muscle, and aorta (13), it is possible that mature adipocytes could be major sources of ROS, accelerating the differentiation of adjacent preadipocytes. Therefore, obesity could be aggravated by the vicious cycle of “adipocyte hypertrophy → increased local ROS → adipocyte hyperplasia.”
Although we did not identify the intracellular source of ROS during MCE, we did show that insulin may be important for the elevation of ROS levels (Fig. 6B). Because preadipocytes do not express insulin receptor until ∼3 days of induction (34, 35), insulin-like growth factor-I signaling may be responsible for increased ROS production. In this regard, it should be noted that removing insulin from the standard differentiation regimen (FBS + IBMX, dexamethasone, and insulin) caused S phase arrest of the mesenchymal stem cell line, C3H10T1/2 (36). Consistently, our experiments also show that the reduction in differentiation seen when incomplete hormonal stimulation (IBMX + dexamethasone) was used was largely compensated by H2O2 treatment (Fig. 1C). This strongly suggests that preadipocytes are stimulated to produce ROS by insulin-like growth factor-I signaling, resulting in the full DNA-binding capacity of C/EBPβ to complete the clonal expansion. Recently, Friedman and colleagues (37) reported that xanthine oxidoreductase is a regulator of adipogenesis and that xanthine oxidoreductase functions downstream of C/EBPβ and upstream of PPARγ. Since exogenous xanthine oxidase is commonly used to induce oxidative damage by increasing superoxide in experimental models, it would be interesting to investigate how xanthine oxidoreductase is regulated by insulin-like growth factor-I signaling as well as how xanthine oxidoreductase affects cell cycle progression in 3T3-L1 cells.
In another study, we have shown that in vitro oxidation of C/EBPβ protein markedly enhances the DNA binding activity by S–S bond formation and conformational change (12). Although any direct disulfide bond formation is evident in vivo, there is increasing evidence for redox regulation of transcription factors. For example, cysteine 179 of the inhibitory κB kinase β subunit is reported to be a target of oxidative inactivation by means of S-glutathionylation (38). In addition, the c-Jun transcription factor is regulated by redox change, which is specifically targeted to the cysteine residue located in the DNA binding site of the protein (39). More interesting data have been reported for the Stat3 transcription factor (40, 41). This protein is activated by a single tyrosine phosphorylation in response to extracellular ligand, resulting in dimerization, DNA binding, and transactivation of specific target genes. Interestingly, substitution of two cysteine residues within the C-terminal region of Stat3 resulted in the spontaneous activation of the transcription factor, creating a constitutive active form of the protein (40). This means that cysteine residues in each molecule, when in close proximity, help bind to the partner molecule tightly. Still, it is doubtful whether disulfide bonds form in the relatively reducing milieu of nuclei; however, it has been suggested that dynamic cysteine-cysteine interactions are possible, as evidenced by the Pax transcription factor (42). In the present study, the DNA binding activity of C/EBPβ is enhanced in the presence of H2O2, as evidenced by immunofluorescent staining (Fig. 4C) as well as EMSA and ChIP assays (Fig. 5), providing an example of a transcription factor that may be regulated by redox changes in vivo.
C/EBPβ is required for the mitotic clonal expansion during adipocyte differentiation (4) and for liver regeneration (43), inflammation processes or the acute phase reaction (44), and cellular proliferation (45). Thus, it will be interesting to investigate whether C/EBPβ activation in these physiological processes is caused by subtle changes of cellular redox state. Explaining how C/EBP family proteins interact together in different combinations in various situations will be important for the understanding of these physiological processes.
Acknowledgments
We are grateful to Dr. M. Daniel Lane and Dr. Qi-Qun Tang for helpful discussions.
This work was supported by Korea Science and Engineering Foundation Grants R13-2002-054-04000-0 and R01-2007-000-10641-0 funded by the Korean government.
Footnotes
The abbreviations used are: C/EBP, CCAAT/enhancer-binding protein; MCE, mitotic clonal expansion; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; RSV, resveratrol; MAPK, mitogen-activated protein kinase; GSK, glycogen synthase kinase; DMEM, Dulbecco's modified Eagle's medium; IBMX, 3-isobutyl-1-methylxanthine; FBS, fetal bovine serum; PBS, phosphate-buffered saline; siRNA, small interfering RNA; FACS, fluorescence-activated cell sorting; ChIP, chromatin immunoprecipitation; DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; NAC, N-acetyl cysteine; EMSA, electrophoretic mobility shift assay.
References
- 1.MacDougald, O. A., and Lane, M. D. (1995) Annu. Rev. Biochem. 64 345-373 [DOI] [PubMed] [Google Scholar]
- 2.Farmer, S. R. (2006) Cell Metab. 4 263-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen, E. D., Walkey, C. J., Puigserver, P., and Spiegelman, B. M. (2000) Genes Dev. 14 1293-1307 [PubMed] [Google Scholar]
- 4.Lane, M. D., Tang, Q. Q., and Jiang, M. S. (1999) Biochem. Biophys. Res. Commun. 266 677-683 [DOI] [PubMed] [Google Scholar]
- 5.Tang, Q. Q., and Lane, M. D. (1999) Genes Dev. 13 2231-2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang, Q. Q., Otto, T. C., and Lane, M. D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 850-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandrup, S., and Lane, M. D. (1997) J. Biol. Chem. 272 5367-5370 [DOI] [PubMed] [Google Scholar]
- 8.Christy, R. J., Kaestner, K. H., Geiman, D. E., and Lane, M. D. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 2593-2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang, Q. Q., Otto, T. C., and Lane, M. D. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 44-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang, Q. Q., Gronborg, M., Huang, H., Kim, J. W., Otto, T. C., Pandey, A., and Lane, M. D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 9766-9771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, X., Kim, J. W., Gronborg, M., Urlaub, H., Lane, M. D., and Tang, Q. Q. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11597-11602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, J. W., Tang, Q. Q., Li, X., and Lane, M. D. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 1800-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., Nakayama, O., Makishima, M., Matsuda, M., and Shimomura, I. (2004) J. Clin. Invest. 114 1752-1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houstis, N., Rosen, E. D., and Lander, E. S. (2006) Nature 440 944-948 [DOI] [PubMed] [Google Scholar]
- 15.Rudich, A., Tirosh, A., Potashnik, R., Hemi, R., Kanety, H., and Bashan, N. (1998) Diabetes 47 1562-1569 [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka, T., Kajimoto, Y., Watada, H., Kaneto, H., Kishimoto, M., Umayahara, Y., Fujitani, Y., Kamada, T., Kawamori, R., and Yamasaki, Y. (1997) J. Clin. Invest. 99 144-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger-Brauer, H. I., and Kather, H. (1995) Biochem. J. 307 549-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, J. W., Tang, Q. Q., Vinson, C., and Lane, M. D. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 43-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amri, E. Z., Dani, C., Doglio, A., Grimaldi, P., and Ailhaud, G. (1986) Biochem. Biophys. Res. Commun. 137 903-910 [DOI] [PubMed] [Google Scholar]
- 20.Castro-Munozledo, F., Beltran-Langarica, A., and Kuri-Harcuch, W. (2003) Exp. Cell Res. 284 163-172 [DOI] [PubMed] [Google Scholar]
- 21.Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., Messadeq, N., Milne, J., Lambert, P., Elliott, P., Geny, B., Laakso, M., Puigserver, P., and Auwerx, J. (2006) Cell 127 1109-1122 [DOI] [PubMed] [Google Scholar]
- 22.Baur, J. A., Pearson, K. J., Price, N. L., Jamieson, H. A., Lerin, C., Kalra, A., Prabhu, V. V., Allard, J. S., Lopez-Lluch, G., Lewis, K., Pistell, P. J., Poosala, S., Becker, K. G., Boss, O., Gwinn, D., Wang, M., Ramaswamy, S., Fishbein, K. W., Spencer, R. G., Lakatta, E. G., Le Couteur, D., Shaw, R. J., Navas, P., Puigserver, P., Ingram, D. K., de Cabo, R., and Sinclair, D. A. (2006) Nature 444 337-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grozinger, C. M., Chao, E. D., Blackwell, H. E., Moazed, D., and Schreiber, S. L. (2001) J. Biol. Chem. 276 38837-38843 [DOI] [PubMed] [Google Scholar]
- 24.Zang, M., Xu, S., Maitland-Toolan, K. A., Zuccollo, A., Hou, X., Jiang, B., Wierzbicki, M., Verbeuren, T. J., and Cohen, R. A. (2006) Diabetes 55 2180-2191 [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta, B., and Milbrandt, J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 7217-7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang, J. T., Park, I. J., Shin, J. I., Lee, Y. K., Lee, S. K., Baik, H. W., Ha, J., and Park, O. J. (2005) Biochem. Biophys. Res. Commun. 338 694-699 [DOI] [PubMed] [Google Scholar]
- 27.Villena, J. A., Viollet, B., Andreelli, F., Kahn, A., Vaulont, S., and Sul, H. S. (2004) Diabetes 53 2242-2249 [DOI] [PubMed] [Google Scholar]
- 28.Rosen, E. D., and Spiegelman, B. M. (2000) Annu. Rev. Cell Dev. Biol. 16 145-171 [DOI] [PubMed] [Google Scholar]
- 29.Gregoire, F. M., Smas, C. M., and Sul, H. S. (1998) Physiol. Rev. 78 783-809 [DOI] [PubMed] [Google Scholar]
- 30.Entenmann, G., and Hauner, H. (1996) Am. J. Physiol. 270 C1011-C1016 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka, T., Yoshida, N., Kishimoto, T., and Akira, S. (1997) EMBO J. 16 7432-7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen, E. D., and MacDougald, O. A. (2006) Nat. Rev. Mol. Cell Biol. 7 885-896 [DOI] [PubMed] [Google Scholar]
- 33.Park, J., Choe, S. S., Choi, A. H., Kim, K. H., Yoon, M. J., Suganami, T., Ogawa, Y., and Kim, J. B. (2006) Diabetes 55 2939-2949 [DOI] [PubMed] [Google Scholar]
- 34.Lane, M. D., Reed, B. C., and Clements, P. R. (1981) Prog. Clin. Biol. Res. 66 523-542 [PubMed] [Google Scholar]
- 35.Reed, B. C., Kaufmann, S. H., Mackall, J. C., Student, A. K., and Lane, M. D. (1977) Proc. Natl. Acad. Sci. U. S. A. 74 4876-4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho, Y. C., and Jefcoate, C. R. (2004) J. Cell Biochem. 91 336-353 [DOI] [PubMed] [Google Scholar]
- 37.Cheung, K. J., Tzameli, I., Pissios, P., Rovira, I., Gavrilova, O., Ohtsubo, T., Chen, Z., Finkel, T., Flier, J. S., and Friedman, J. M. (2007) Cell Metab. 5 115-128 [DOI] [PubMed] [Google Scholar]
- 38.Reynaert, N. L., van der Vliet, A., Guala, A. S., McGovern, T., Hristova, M., Pantano, C., Heintz, N. H., Heim, J., Ho, Y. S., Matthews, D. E., Wouters, E. F., and Janssen-Heininger, Y. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 13086-13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klatt, P., Molina, E. P., De Lacoba, M. G., Padilla, C. A., Martinez-Galesteo, E., Barcena, J. A., and Lamas, S. (1999) FASEB J. 13 1481-1490 [DOI] [PubMed] [Google Scholar]
- 40.Bromberg, J. F., Wrzeszczynska, M. H., Devgan, G., Zhao, Y., Pestell, R. G., Albanese, C., and Darnell, J. E., Jr. (1999) Cell 98 295-303 [DOI] [PubMed] [Google Scholar]
- 41.Darnell, J. E., Jr. (1997) Science 277 1630-1635 [DOI] [PubMed] [Google Scholar]
- 42.Tell, G., Scaloni, A., Pellizzari, L., Formisano, S., Pucillo, C., and Damante, G. (1998) J. Biol. Chem. 273 25062-25072 [DOI] [PubMed] [Google Scholar]
- 43.Greenbaum, L. E., Li, W., Cressman, D. E., Peng, Y., Ciliberto, G., Poli, V., and Taub, R. (1998) J. Clin. Invest. 102 996-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akira, S., and Kishimoto, T. (1992) Immunol. Rev. 127 25-50 [DOI] [PubMed] [Google Scholar]
- 45.Umek, R. M., Friedman, A. D., and McKnight, S. L. (1991) Science 251 288-292 [DOI] [PubMed] [Google Scholar]