Abstract
A fundamental feature of Alzheimer disease (AD) is the accumulation of β-amyloid (Aβ), a peptide generated from the amyloid precursor protein (APP). Emerging evidence suggests that soluble Aβ oligomers adversely affect synaptic function, which leads to cognitive failure associated with AD. The Aβ-induced synaptic dysfunction has been attributed to the synaptic removal of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (AMPARs); however, it is unclear how Aβ induces the loss of AMPARs at the synapses. In this study we have examined the potential involvement of Ca2+/calmodulin-dependent protein kinase II (CaMKII), a signaling molecule critical for AMPAR trafficking and function. We found that the synaptic pool of CaMKII was significantly decreased in cortical neurons from APP transgenic mice, and the density of CaMKII clusters at synapses was significantly reduced by Aβ oligomer treatment. In parallel, the surface expression of GluR1 subunit as well as AMPAR-mediated synaptic response and ionic current was selectively decreased in APP transgenic mice and Aβ-treated cultures. Moreover, the reducing effect of Aβ on AMPAR current density was mimicked and occluded by knockdown of CaMKII and blocked by overexpression of CaMKII. These results suggest that the Aβ-induced change in CaMKII subcellular distribution may underlie the removal of AMPARs from synaptic membrane by Aβ.
The accumulation of β-amyloid (Aβ),2 a peptide generated from the amyloid precursor protein (APP), is one of the hallmarks of Alzheimer disease (AD), a progressive neurodegenerative disorder (1, 2). Although it is still unclear how Aβ contributes to the etiology and pathogenesis of AD, emerging evidence suggests that Aβ causes “synaptic failure” before the formation of senile plaques and the occurrence of neuron death (3). Soluble oligomeric Aβ forms rather than amyloid plaques correlate with the severity of cognitive impairment in AD (4, 5). Application of naturally secreted Aβ oligomers adversely affects glutamatergic synaptic transmission and plasticity (6, 7). Neurons from transgenic mice overexpressing AD-linked mutant APP also show deficits in long term potentiation of synaptic transmission, a synaptic basis of learning and memory (8–11). In addition, decreased expression of synaptic proteins and loss of synapses have been found with elevated Aβ levels (12–16). This Aβ-induced synaptic dysfunction has been attributed to the synaptic removal of AMPA receptors (17–19). However, it is unclear how Aβ induces the loss of AMPARs at the synapses.
The trafficking of AMPA receptors directly controls excitatory synaptic efficacy (20). Several mechanisms have been proposed to regulate the transport of AMPARs to and from the cell surface and lateral diffusion at synaptic and extrasynaptic sites (21), including PDZ domain-mediated interactions between channel and scaffolding proteins (22, 23), clathrin-dependent endocytosis (24, 25), and motor protein-based delivery along microtubule or actin cytoskeletons (26, 27). CaMKII, a multifunctional kinase highly enriched at postsynaptic densities (PSD) of glutamatergic synapses (28), has been found to drive AMPARs into synapses and reduce “silent” synapses devoid of AMPARs (22, 29).
CaMKII achieves the efficacy and specificity of signal transduction via compartmentalized localization (30, 31). Different pools of CaMKII compartmentalized at membrane, postsynaptic densities, nucleus, and cytosol are responsible for regulating distinct substrates ranging from glutamate receptor channels to transcription factors. It has been found that CaMKII dynamically alters its subcellular distribution in response to activity changes (32) or activation of certain G-protein coupled receptors (33). Because CaMKII located in postsynaptic sites plays a crucial role in synaptic plasticity through the regulation of postsynaptic AMPA receptors (22, 34, 35), in this study we have examined the possibility that synaptic CaMKII is reduced by Aβ, which underlies the Aβ-induced decrease of AMPAR delivery to synapses.
EXPERIMENTAL PROCEDURES
An AD Model and Aβ Oligomer Preparation—APP transgenic mice carrying the Swedish mutation (K670N, M671L) (36) were purchased from Taconic (Germantown, NY). Eight-week-old transgenic males (on B6SJLF1 hybrid background) were bred with mature B6SJLF1 females. The genetic background of these mice is the same with this breeding scheme. Genotyping were performed by PCR according to the manufacturer's protocol. Different ages of male transgenic and wild-type littermates were used in the experiments.
The procedure of Aβ oligomer preparation was similar to what was described before (37). In brief, the Aβ-(1–42) peptide (Tocris) was dissolved in hexafluoroisopropanol to 1 mm. Hexafluoroisopropanol was then removed under vacuum. The remaining peptide was then resuspended in DMSO to 5 mm and diluted in H2O to 0.1 mm. The oligomeric Aβ was formed by incubating at 4 °C for 24 h.
Subcellular Fractionation of Proteins—Subcellular fractions were prepared as described previously (38) with modifications. In brief, blocks of frontal cortex were cut out, weighed, and homogenized in ice-cold lysis buffer (10 ml/g, 15 mm Tris, pH 7.6, 0.25 m sucrose, 1 mm phenylmethylsulfonyl fluoride, 2 mm EDTA, 1 mm EGTA, 10 mm Na3VO4, 25 mm NaF, 10 mm sodium pyrophosphate, and protease inhibitor tablet). 50 μl of homogenates were removed as the total protein, and the remaining were subjected to several steps of centrifugation. After centrifugation at 800 × g for 5 min to remove nuclei and large debris, the remaining supernatant was subjected to 10,000 × g centrifugation for 10 min. The supernatant was again centrifuged at 165,000 × g for 30 min to obtain the cytosolic fraction (supernatant (S)). The crude synaptosome fraction (pellet) was suspended in lysis buffer containing 1% Triton X-100 and 300 mm NaCl, homogenized again, and centrifuged at 16,000 × g for 30 min to obtain Triton soluble fraction (supernatant (P1)) and Triton insoluble fraction (pellet (P2)). The P2 fraction was dissolved in 1% SDS. The major component of P1 fraction includes cytosolic proteins from synpatosomes, whereas the P2 fraction mainly includes membrane-associated proteins from synpatosomes.
Western Blotting—Equal amounts of proteins from WT and APP mice were subjected to 7.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk for 1 h at room temperature followed by incubation with various primary antibodies, including CaMKII α subunit (Santa Cruz, 1:2000), CaMKII β subunit (Zymed Laboratories Inc., 1:2000), Thr-286-p-CaMKII α subunit (Santa Cruz, 1:2000), actin (Santa Cruz, 1:2000), PSD-95 (Affinity BioReagents, 1:3000), GluR1 (Santa Cruz, 1:1000), NR1 (Upstate Biotechnology, 1:2000), Ser-831-p-GluR1 (Upstate, 1:500), GluR2 (Chemicon, 1:1000), Ser-880-p-GluR2 (Abcam, 1:1000). The Aβ antibody (Chemicon, 6E10, 1:500) was used for detecting the oligomeric Aβ preparation. After incubation with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences), the blots were exposed to the enhanced chemiluminescence substrate (Amersham Biosciences). Quantitation was obtained from densitometric measurements of immunoreactive bands on films with Image J software.
Biochemical Measurement of Surface Receptors—The surface AMPA or NMDA receptors were detected as described previously (39). Briefly, cortical slices were incubated with artificial cerebrospinal fluid containing 1 mg/ml Sulfo-NHS-LC-Biotin (Pierce) for 20 min on ice. The slices were then rinsed 3 times in Tris-buffered saline to quench the biotin reaction followed by homogenization in 500 μl of modified radioimmune precipitation assay buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mm Na3PO4, 150 mm NaCl, 2 mm EDTA, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, and 1 mg/ml leupeptin). The homogenates were centrifuged at 16,000 × g for 30 min at 4 °C. Supernatant was collected and incubated with neutravidin-agarose (Pierce) for 2 h at 4 °C. Bound proteins were resuspended in SDS sample buffer and boiled. Quantitative Western blots were performed on biotinylated (surface) proteins using antibodies against GluR1 (Santa Cruz, 1:1000, N terminus) or NR1 (Upstate, 1:1000, C terminus).
Primary Culture—Rat prefrontal cortex cultures were prepared as described previously (33). Briefly, prefrontal cortex was dissected from 18-day rat embryos, and cells were dissociated by incubating with 0.25% trypsin-EDTA for 30 min and subsequent trituration through a Pasteur pipette. The neurons were plated on coverslips (coated with poly-l-lysine, put in 24-well plates) in Dulbecco's modified Eagle's medium with 10% fetal calf serum at a density of 1 × 105 cells/cm2 for electrophysiological experiments and at a lower density of 0.2 × 105 cells/cm2 for immunocytochemical staining. When neurons attached to the coverslip within 24 h, the medium was changed to Neurobasal with B27 supplement. Cytosine arabinoside (1.25 μm) was added to the culture media from day 4 to inhibit glia growth. Neurons were maintained for 2–4 weeks.
Immunocytochemistry—After Aβ treatment, neurons cultured on coverslips (DIV 25–28) were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and washed 3 times with PBS. For total protein staining, neurons were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 5 min. For surface protein staining, neurons were not permeabilized. Then neurons were incubated with 5% bovine serum albumin for 1 h to block nonspecific staining. Next, neurons were incubated with primary antibodies at 4 °C overnight, including anti-CaMKII α subunit (Santa Cruz, 1:200), anti-GluR1 (Upstate, 1:100, N-term), and anti-NR1 (Chemicon, 1:100, EC loop). After washing, neurons were incubated with Alexa-488 (green) or Alexa-594 (red)-conjugated secondary antibodies (Molecular Probes, 1:500) for 2 h at room temperature. For the staining of F-actin, neurons were incubated with Alex568-conjugated phalloidin (1 unit/ml, Invitrogen) at room temperature for 20 min. After washing in phosphate-buffered saline for three times, the coverslips were mounted on slides with VECTASHIELD mounting media (Vector Laboratories, Burlingame, CA).
Fluorescent images were obtained using a 100× objective with a cooled CCD camera mounted on a Nikon microscope. All specimens were imaged under identical conditions and analyzed using identical parameters with the Image J software. The clusters for CaMKII, F-actin, GluR1, surface NR1, or surface GluR1 were measured. To define dendritic clusters, a single threshold was chosen manually so that clusters corresponded to puncta of at least 2-fold intensity of the diffuse fluorescence on the dendritic shaft. On each coverslip, the cluster density of 30 neurons (2–3 dendritic segments of 50 μm length per neuron) was measured. Three to four independent experiments for each of the treatments were performed. Quantitative analyses were conducted blindly (without knowledge of experimental treatment).
Transfection—To knockdown or overexpress CaMKII, a small interfering RNA (siRNA) against CaMKII or an enhanced GFP-fused wild-type CaMKII plasmid was transfected into cultured cortical neurons (DIV 14–16) using Lipofectamine 2000 reagent as we described before (39, 33). The siRNA oligonucleotide sequences for CaMKII α subunit were 5′-GGAGUAUGCUGCCAAGAUUtt-3′ (sense) and 5′-AAUCUUGGCAGCAUACUCCtg-3′ (antisense). CaMKII siRNA or a scrambled control siRNA was co-transfected (10 nm) with enhanced GFP (0.2 μg/ml). To test the specificity of CaMKII siRNA, a mutated CaMKII plasmid carrying siRNA-insensitive silent mutations was constructed using the QuikChange multisite-directed mutagenesis kit (Stratagene). The mutated sequence on CaMKII was GAGTATGCAGCAAAGATT (mutated nucleotides are underlined). Cultures were used 3 days after transfection.
Patch Clamp Recordings in Slices and Cultures—The whole-cell voltage clamp technique was used to measure AMPAR-EPSC and NMDAR-EPSC in cortical slices (39). The slice (300 μm) was incubated with artificial cerebrospinal fluid containing bicuculline (15 μm). The internal solution contained 130 mm cesium methanesulfonate, 10 mm CsCl, 4 mm NaCl, 1 mm MgCl2, 10 mm HEPES, 5 mm EGTA, 2.2 mm QX-314 (Sigma), 12 mm phosphocreatine, 5 mm MgATP, 0.5 mm Na2GTP, pH 7.2–7.3, 265–270 mOsm. Neurons were visualized with a 40× water-immersion lens and illuminated with near infrared IR light. All recordings were performed using a Multiclamp 700A amplifier. Tight seals (2–10 gigaohms) were generated by applying negative pressure. Additional suction was applied to disrupt the membrane and obtain the whole-cell configuration. EPSCs were evoked by stimulating the neighboring cortical neurons with a bipolar tungsten electrode (FHC, Inc.) located at a few hundred micrometers away from the neuron under recording. Stimulation pulses (5.5 V, 0.05 ms) were used to evoke AMPAR-EPSC on neurons held at -70 mV. To evoke NMDAR-EPSC, stimulation pulses (6.5 V, 0.5 ms) were used, and neurons (bathed in 10 μm 6,7-dinitroquinoxaline-2,3-dione) were depolarized from -70 to +60 mV for 3 s before stimulation to fully relieve the voltage-dependent Mg2+ block of NMDARs.
Whole-cell recordings of AMPAR and NMDAR channel currents in cultured neurons used standard voltage clamp techniques (39). The internal solution contained 180 mm N-methyl-d-glucamine, 4 mm MgCl2, 40 mm HEPES, 0.5 mm BAPTA, 12 mm phosphocreatine, 3 mm Na2ATP, and 0.5 mm Na2GTP, pH 7.2–7.3, 265–270 mOsm. The external solution for AMPAR currents contained 127 mm NaCl, 20 mm CsCl, 1 mm MgCl2, 10 mm HEPES, 5 mm BaCl2, 12 mm glucose, 0.001 mm tetrodotoxin, pH 7.3–7.4 (300–305 mOsm/liter tetrodotoxin). For recording NMDAR currents, external solution was modified to contain 0 mm MgCl2, 1 mm CaCl2, and 20 μm glycine. Recordings were obtained with an Axon Instruments 200B amplifier that was controlled and monitored by an IBM PC running pClamp 8 with a DigiData 1320 series interface (Axon instruments). After seal rupture, series resistance (4–10 megaohms) was compensated (70–90%). NMDAR- or AMPAR-mediated current was evoked by application of NMDA (100 μm) or glutamate (100 μm) for 2 s every 30 s in neurons held at -60 mV. Drugs were delivered with a “sewer pipe” system. The array of drug capillaries (∼150 μm inner diameter) was positioned a few hundred microns from the cell under examine. Solution changes were controlled by the SF-77B fast-step solution stimulus delivery device (Warner Instruments). Data were analyzed with Clampfit (Axon instruments) and Kaleidagraph (Albeck Software).
RESULTS
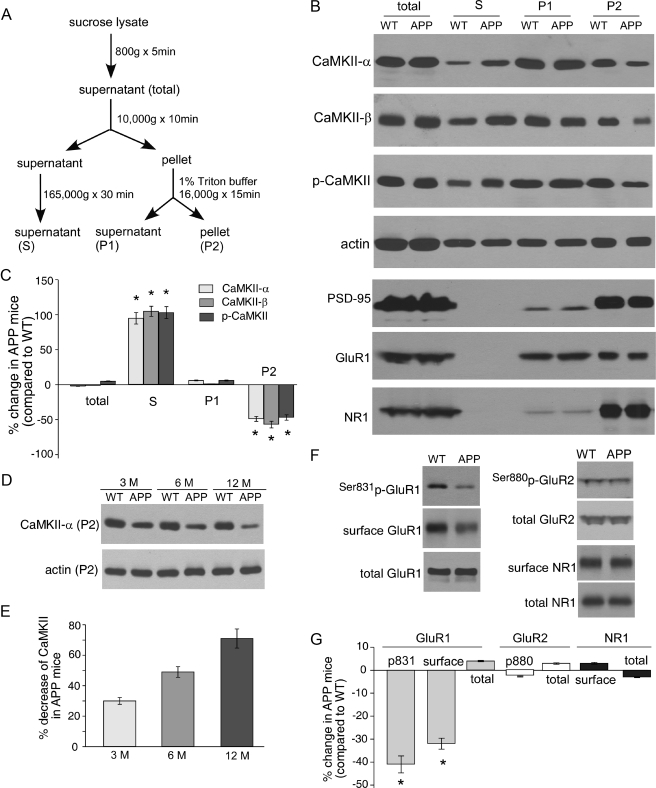
CaMKII Subcellular Distribution and GluR1 Surface Expression Are Altered in APP Transgenic Mice—To detect the potential change of CaMKII by Aβ, we compared the expression of CaMKII in frontal cortex from WT versus APP mice. As shown in Fig. 1, A–C, the total amount of CaMKII α subunit was not altered in 6-month-old APP mice compared with the age-matched WT littermates (2 ± 0.2% decrease, n = 8); however, the subcellular distribution of α-CaMKII was remarkably changed in APP mice, with a significant decrease in the Triton-insoluble synaptosome (P2) fraction (49 ± 3.5% decrease, n = 8, p < 0.01, ANOVA), a significant increase in cytosolic (S) fraction (95 ± 8.3% increase, n = 8, p < 0.01, ANOVA), and virtually no change in Triton-soluble synaptosome (P1) fraction (6 ± 0.5% increase, n = 8).
FIGURE 1.
Aberrant CaMKII subcellular distribution and GluR1 surface expression in APP transgenic mice. A, diagram showing the procedure of subcellular fractionation of proteins. S, the cytosolic fraction; P1, Triton-soluble fraction in the crude synaptosome fraction, which mainly includes cytosolic proteins in synapses; P2, Triton-insoluble fraction in the crude synaptosome fraction, which mainly includes membrane-associated proteins in synapses. B, Western blots showing the expression of CaMKII (α subunit, β subunit, and autophosphorylated), actin, PSD-95, GluR1, and NR1 in different subcellular fractions in the frontal cortex from wild-type versus APP mice. C, cumulative data (mean ± S.E.) showing the percentage change of CaMKII (α subunit, β subunits, and autophosphorylated) in different fractions from APP mice, compared with wild-type mice. *, p < 0.01, ANOVA. D and E, Western blots and quantifications showing the expression of CaMKII α subunit in Triton-insoluble synaptosome (P2) fraction from different ages (3, 6, 12 months old) of APP mice compared with age-matched wild-type mice. F and G, Western blots and quantifications showing the expression of Ser-831-phosphorylated GluR1, surface and total GluR1, Ser-880-phosphorylated GluR2, total GluR2, and surface and total NR1 levels in the frontal cortex of APP mice compared with wild-type mice. *, p < 0.01, ANOVA.
Because CaMKII β subunit functions as an F-actin targeting module that localizes CaMKII holoenzyme to dendritic spines (40), we also examined the impact of Aβ on β-CaMKII distribution. Similar to α-CaMKII, β-CaMKII in APP mice was not altered in the total level but was significantly decreased in P2 fraction (57 ± 4.8% decrease, n = 8, p < 0.01, ANOVA) and increased in S fraction (105 ± 7.2% increase, n = 8, p < 0.01, ANOVA).
CaMKII is autophosphorylated at Thr-286 when the enzyme is activated (41), therefore, prolonging the function of the enzyme beyond the transient rise of [Ca2+]i signal. Thus, we also examined whether Aβ altered the distribution of activated CaMKII (Thr-286-phosphorylated α-CaMKII) in APP mice. As shown in Fig. 1, A–C, the total amount of p-CaMKII was not changed in APP mice (5 ± 0.4% increase, n = 8); however, p-CaMKII exhibited a similar alteration pattern in its subcellular distribution as CaMKII α subunit (47 ± 3.9% decrease in P2 fraction and 103 ± 8.5% increase in S fraction, n = 8, p < 0.01, ANOVA). Actin was not changed in all of the separated cellular fractions including the supernatant (mainly G-actin) and pellets (mainly F-actin). These data suggest that CaMKII (α subunit, β subunit, or activated form) is redistributed from postsynaptic sites to cytosol in APP transgenic mice.
Next, we examined the potential change of several other synaptic proteins by Aβ, including the scaffolding protein PSD-95, AMPA receptor GluR1 subunit, and NMDA receptor NR1 subunit. As shown in Fig. 1B, all of these synaptic proteins, which were present in Triton-soluble (P1) or insoluble (P2) synaptosome fractions, were not altered in APP mice. It suggests that APP transgenic mice, which do not have a significant loss of synapses at this stage, have selectively lost synaptic CaMKII.
We further examined the change of CaMKII in APP transgenic mice at different time points. As shown in Fig. 1, D and E, the CaMKII α subunit in P2 fraction was reduced to a greater extent in older APP mice (3 month: 30 ± 2.2% decrease, n = 8; 6 month: 49 ± 3.5% decrease, n = 8; 12 month: 71 ± 6.3% decrease, n = 8), suggesting a progressive loss of synaptic CaMKII by Aβ.
Previous studies have shown that activated CaMKII can potentiate synaptic transmission by enhancing AMPAR channel conductance via GluR1 phosphorylation at Ser-831 (42, 43) and by delivering new AMPA receptors to the synapse (22, 29). Thus, we examined whether the reduced CaMKII at synaptic sites in APP transgenic mice might result in changes in the AMPAR phosphorylation and surface expression. As shown in Fig. 1, F and G, APP mice exhibited a significant decrease in Ser-831-phosphorylated GluR1 (41 ± 3.7% decrease, n = 8, p < 0.01, ANOVA) and the level of surface GluR1 (32 ± 2.4% decrease, n = 8, p < 0.01, ANOVA). No significant change was observed on the level of Ser-880-phosphorylated GluR2, a target of protein kinase C, in WT and APP mice (Fig. 1, F and G). Unlike GluR1, the level of surface or total NMDAR NR1 subunit was not significantly altered (3 ± 0.4% increase, n = 8). It suggests that the decreased synaptic CaMKII in APP transgenic mice preferably disrupts AMPAR localization at the synaptic membrane.
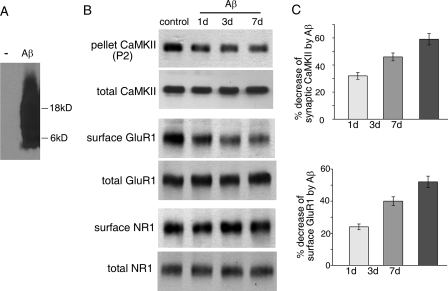
Synaptic CaMKII and Surface GluR1 Clusters Are Reduced after Aβ Exposure—To determine the direct impact of Aβ on CaMKII, we examined the effect of Aβ exposure on CaMKII subcellular redistribution in cultured cortical neurons. We first detected the oligomeric Aβ preparation using an Aβ antibody. As shown in the Western blot (Fig. 2A), there was a smear band of signals between 4–22 kDa, suggesting the presence of Aβ monomers, dimers, and trimers.
FIGURE 2.
Reduction of synaptic membrane-associated CaMKII and surface GluR1 in cultured cortical neurons after Aβ exposure. A, Western blots showing the oligomeric Aβ. B, Western blots showing the expression of synaptic membrane-associated CaMKII α subunit (in P2 fraction) and surface and total GluR1 and NR1 in rat cortical cultures after exposure to Aβ oligomer (1 μm) for 1, 3, and 7 days. C, cumulative data (mean ± S.E.) showing the percentage change of synaptic membrane-associated CaMKII α subunit and surface-expressed GluR1 in cortical cultures by Aβ exposure for different durations.
As shown in Fig. 2, B and C, the CaMKII α subunit at Triton-insoluble synaptosome (P2) fraction was significantly (p < 0.01, ANOVA) decreased after being exposed to Aβ oligomer (1 μm) for 1, 3, or 7 days (1 day: 32 ± 2.5% decrease, n = 8; 3 days: 46 ± 2.9% decrease, n = 8; 7 days: 59 ± 4.2% decrease, n = 8), although the total amount of CaMKII was not changed. In parallel, the surface level of GluR1 subunit was significantly (p < 0.01, ANOVA) decreased after Aβ exposure (1 day: 24 ± 1.8% decrease, n = 8; 3 days: 40 ± 2.7% decrease, n = 8; 7 days: 52 ± 3.6% decrease, n = 8), whereas the surface level of NR1 subunit was not significantly changed (2–3% change, n = 8). The total amount of either GluR1 or NR1 was not altered by Aβ treatment. These data suggest that Aβ oligomers induce a selective loss of CaMKII from synapses and removal of AMPARs from synaptic membrane.
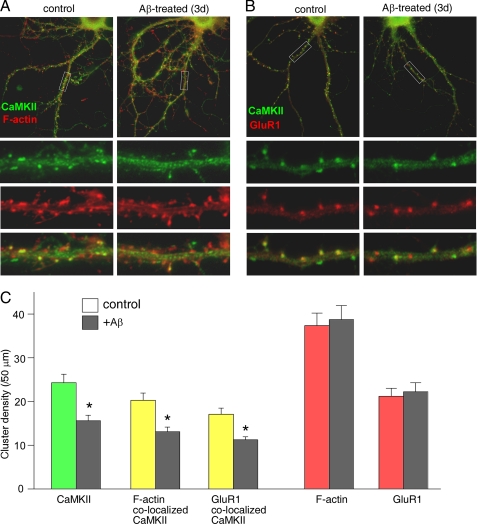
Next, we performed immunocytochemical experiments in neuronal cultures to test the impact of Aβ on CaMKII synaptic localization. Because F-actin or GluR1 is highly enriched at synapses, the synaptically localized CaMKII was revealed as that co-localizing with F-actin or GluR1. As shown in Fig. 3, A and B, in untreated control cultures most of CaMKII clusters (green) were co-localized with F-actin or GluR1 clusters (red) at dendritic spines, the major sites of synapse formation, as indicated by numerous yellow puncta. Aβ exposure (1 μm, 3 days) significantly decreased CaMKII clusters, but not F-actin or GluR1 clusters, which led to less yellow puncta (co-localized clusters) along dendrites. Quantitative analysis (Fig. 3C) indicated that Aβ significantly (p < 0.01, ANOVA) reduced the cluster density (number of clusters/50-μm dendrite) of CaMKII (control, 24.3 ± 1.9; Aβ, 15.6 ± 1.2, n = 30), CaMKII co-localized with F-actin (control, 20.3 ± 1.6; Aβ, 13.1 ± 1.0, n = 30), and CaMKII co-localized with GluR1 (control, 17.1 ± 1.4; Aβ: 11.2 ± 0.7 n = 30). No significant change was observed in the cluster density of F-actin (control, 37.4 ± 2.8; Aβ, 38.8 ± 3.2) or GluR1 (control, 21.2 ± 1.8; Aβ, 22.3 ± 2.0), suggesting the lack of changes on spine density by Aβ. Neuronal viability assays (44) by co-staining of MAP2 (to label survival neurons) and propidium iodide (to label apoptotic neurons) revealed no toxicity with the Aβ treatment of cortical cultures (data not shown). These data indicate that Aβ selectively decreases the pool of CaMKII located at synapses.
FIGURE 3.
Reduction of synaptic CaMKII clusters in cultured cortical neurons after Aβ exposure. A and B, immunocytochemical images showing the co-staining of CaMKII α subunit with F-actin (A) or GluR1 (B) in cortical cultures (DIV 28) without or with Aβ (1 μm) exposure for 3 days. C, cumulative data (mean ± S.E.) showing the density of CaMKII clusters, CaMKII clusters co-localized with F-actin, CaMKII clusters co-localized with GluR1, F-actin clusters, and GluR1 clusters in control versus Aβ-treated cultures. *, p < 0.01, ANOVA.
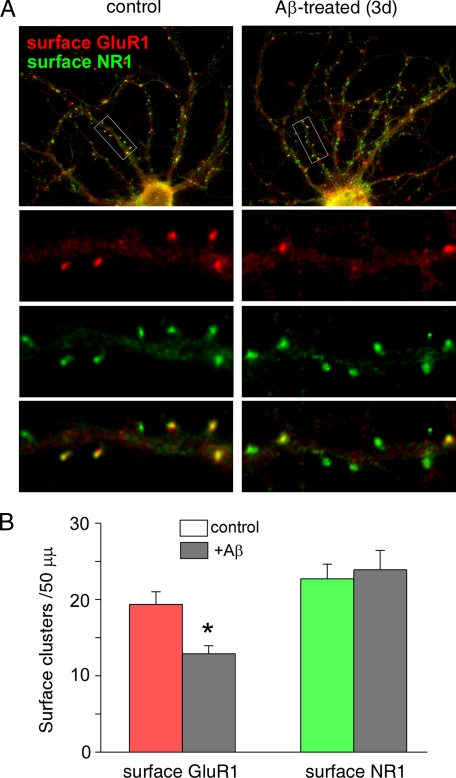
Because biochemical assays showed that the level of surface GluR1 was selectively reduced in APP transgenic mice and in Aβ-treated cultures, we performed immunocytochemical experiments to test the impact of Aβ on GluR1 located at synaptic membrane. The surface expression of AMPA or NMDA receptors was measured with antibodies against the extracellular domain of GluR1 or NR1 in non-permeabilized cultures. As shown in Fig. 4A, in untreated control cultures most of surface GluR1 clusters (red) were co-localized with surface NR1 clusters (green) at dendritic spines, as indicated by numerous yellow puncta. Aβ exposure (1 μm, 3 days) significantly decreased GluR1 clusters, but not NR1 clusters, which led to more green puncta (NR1 only) along dendrites. Quantitative analysis (Fig. 4B) indicated that Aβ significantly reduced the density (number of clusters/50-μm dendrite) of surface GluR1 clusters (control, 19.4 ± 1.6; Aβ, 12.9 ± 1.0, n = 30, p < 0.01, ANOVA), whereas the surface NR1 cluster density was not significantly changed (control, 22.7 ± 2.0; Aβ, 23.9 ± 2.6, n = 30). These data show that Aβ indeed decreases the number of AMPARs at synaptic membranes.
FIGURE 4.
Reduction of surface GluR1 clusters in cultured cortical neurons after Aβ exposure. A, immunocytochemical images showing the co-staining of surface GluR1 and surface NR1 in cortical cultures (DIV 28) without or with Aβ (1 μm) exposure for 3 days. B, cumulative data (mean ± S.E.) showing the density of surface GluR1 clusters and surface NR1 clusters in control versus Aβ-treated cultures. *, p < 0.01, ANOVA.
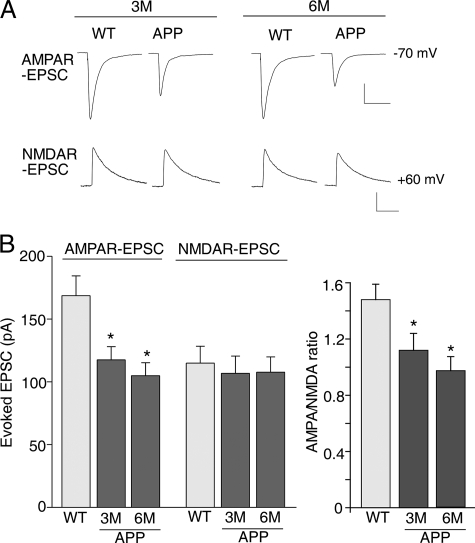
AMPAR-mediated Synaptic Transmission and Ionic Current Are Impaired by Aβ—Given the decreased level of surface GluR1 in APP mice, we investigated whether AMPAR-mediated synaptic currents are impaired in APP mice. As shown in Fig. 5, the amplitude of AMPAR-EPSC evoked in frontal cortical slices was significantly (p < 0.01, ANOVA) decreased in APP mice compared with age-matched wild-type mice (WT, 168.6 ± 15.8 pA, n = 30; 3-month-old APP mice, 117.5 ± 10.4, n = 15; 6-month-old APP mice, 105.0 ± 10.2, n = 15). In contrast, the amplitude of NMDAR-EPSC was not significantly changed (WT, 114.9 ± 13.5 pA, n = 30; 3-month-old APP mice, 106.8 ± 13.8, n = 15; 6-month-old APP mice, 107.7 ± 12.2, n = 15). Thus, the ratio of AMPAR-EPSC to NMDAR-EPSC was significantly (p < 0.01, ANOVA) smaller in cortical pyramidal neurons from APP mice (Fig. 5B).
FIGURE 5.
Decreased AMPAR synaptic responses in cortical pyramidal neurons from APP mice. A, representative AMPAR-EPSC and NMDAR-EPSC traces evoked by electrical stimulation of frontal cortical slices from wild-type versus APP transgenic mice (3-month-old). Scale bar, 50 pA, 50 ms (AMPAR-EPSC) or 200 ms (NMDAR-EPSC). B, cumulative data (mean ± S.E.) showing AMPAR-EPSC amplitude, NMDAR-EPSC amplitude, and AMPAR-EPSC/NMDAR-EPSC ratio in wild-type versus APP mice (3 or 6 months old). *, p < 0.01, ANOVA.
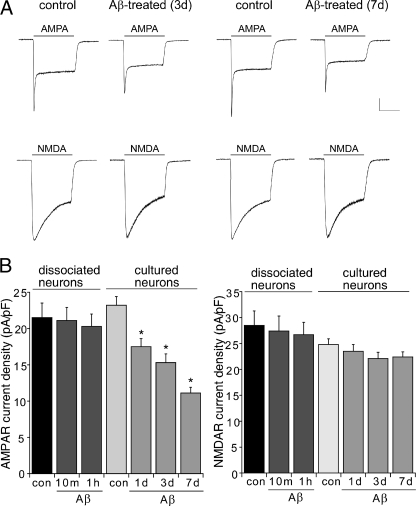
We further examined the direct effect of Aβ exposure on AMPAR-mediated ionic currents in cultured cortical neurons. As shown in Fig. 6, Aβ (1 μm, 1–7 days) exposure significantly (p < 0.01, ANOVA) decreased AMPAR current density (control, 23.2 ± 1.2 pA/pF, n = 15; 1-day Aβ, 17.5 ± 1.1 pA/pF, n = 15; 3-day Aβ, 15.3 ± 1.2 pA/pF, n = 15; 7-day Aβ, 11.1 ± 0.8 pA/pF, n = 15) but not NMDAR current density (control, 24.8 ± 1.1 pA/pF, n = 15; 1-day Aβ, 23.5 ± 1.3 pA/pF, n = 15; 3-day Aβ, 22.1 ± 1.2 pA/pF, n = 15; 7-day Aβ, 22.4 ± 1.0 pA/pF, n = 15). A short (10 min to 1 h) application of Aβ failed to alter AMPAR or NMDAR current density (Fig. 6B). Taken together, these data suggest that AMPAR function is selectively impaired by Aβ, which is consistent with our biochemical and immunocytochemical results.
FIGURE 6.
Decreased AMPAR ionic currents in cultured cortical pyramidal neurons after Aβ exposure. A, representative whole-cell AMPAR- or NMDAR-mediated ionic current traces in cultured cortical neurons without or with Aβ (1 μm) exposure for 3 or 7 days. Scale bar, 0.2 nA, 1 s. B, cumulative data (mean ± S.E.) showing the density of AMPAR and NMDAR currents in acutely dissociated or cultured cortical neurons untreated (control) or treated with Aβ oligomers for different durations. *, p < 0.01, ANOVA.
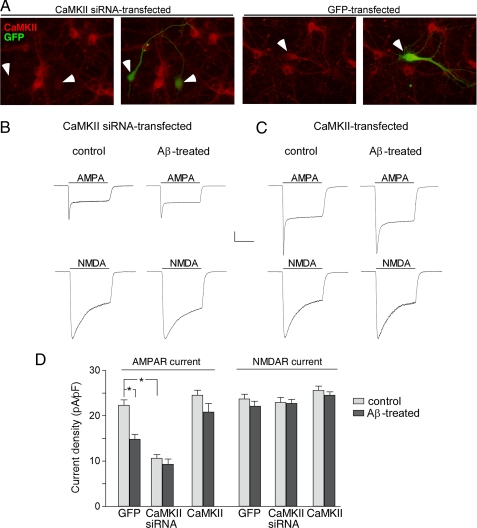
CaMKII Is Causally Involved in Aβ Reduction of AMPAR Current—Because CaMKII plays a key role in regulating AMPAR trafficking and function (22, 29), we would like to know whether the Aβ-induced decrease of AMPAR surface expression and channel currents is caused by Aβ-induced reduction of CaMKII synaptic distribution. To do so, we manipulated CaMKII expression in cultured cortical neurons and examined the impact of Aβ on AMPAR currents. CaMKII was down-regulated by transfecting with a CaMKII siRNA and up-regulated by overexpressing wild-type CaMKII. The CaMKII siRNA-induced specific suppression of CaMKII expression in transfected (GFP-positive) neurons is illustrated in Fig. 7A. As shown in Fig. 7, B and D, knocking down CaMKII significantly decreased AMPAR current density (control, 22.3 ± 1.2 pA/pF; CaMKII siRNA, 10.6 ± 0.8 pA/pF, n = 15, p < 0.01, ANOVA) and prevented Aβ from reducing AMPAR current density further (9.3 ± 1.2 pA/pF, n = 15). On the other hand, CaMKII overexpression slightly increased AMPAR current density and blocked the reducing effect of Aβ on AMPAR current density (Fig. 7C, control, 24.5 ± 1.1 pA/pF, n = 15; Aβ, 20.8 ± 1.9 pA/pF, n = 15, Fig. 7D). In contrast, the NMDAR current density was not affected by CaMKII siRNA (control, 23.7 ± 1.1 pA/pF; CaMKII siRNA, 22.9 ± 1.1 pA/pF, n = 15, Fig. 7D), and Aβ had little effect on NMDAR current density in neurons with CaMKII down-regulated (Fig. 7B, 22.7 ± 0.9 pA/pF, n = 15, Fig. 7D) or up-regulated (Fig. 7C, 24.5 ± 0.8 pA/pF, n = 15, Fig. 7D).
FIGURE 7.
Involvement of CaMKII in Aβ reduction of AMPAR currents. A, immunocytochemical staining of CaMKII (red) in cultured cortical neurons transfected with CaMKII siRNA (co-transfected with GFP) or GFP alone. Arrowheads point to GFP+ neurons. B and C, representative traces of whole-cell AMPAR and NMDAR currents in cortical neurons transfected with CaMKII siRNA (B) or wild-type CaMKII (C) without or with Aβ treatment (1 μm, 3 days). Scale bar, 0.2 nA, 1 s. D, cumulative data (mean ± S.E.) showing the density of AMPAR or NMDAR currents in neurons transfected with GFP, CaMKII siRNA, or wild-type CaMKII without or with Aβ treatment.
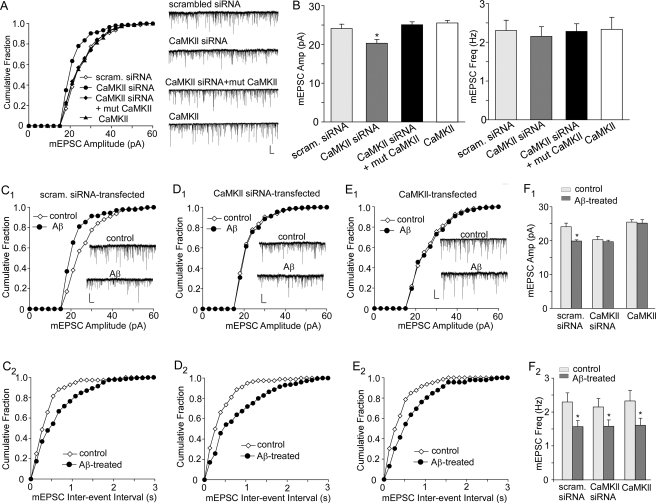
To further examine the role of CaMKII in Aβ regulation of synaptic AMPAR activity, we measured miniature EPSC (mEPSC) in neurons transfected with CaMKII-siRNA or wild-type CaMKII. The mEPSC represents the postsynaptic response to release of individual glutamate vesicles, and a significant change in mEPSC amplitude will suggest a modification of postsynaptic AMPA receptors. As shown in Fig. 8, A and B, knocking down CaMKII caused a significant decrease of mEPSC amplitude, as indicated by a leftward shift in the distribution (scrambled siRNA, 24.1 ± 1.1 pA, n = 20; CaMKII siRNA, 20.3 ± 0.9 pA, n = 15, p < 0.01, ANOVA). Co-transfecting a siRNA-insensitive silent mutant of CaMKII rescued the effect of CaMKII knockdown on mEPSC amplitude (25.1 ± 0.8 pA, n = 15), suggesting the specificity of CaMKII siRNA. No significant effect was seen with wild-type CaMKII transfection (control, 25.5 ± 0.7 pA, n = 17). The frequency of mEPSC was not changed by manipulating CaMKII.
FIGURE 8.
Role of CaMKII in Aβ regulation of synaptic AMPAR activity. A, cumulative plots of the distribution of mEPSC amplitudes in cultured cortical pyramidal neurons (GFP+) transfected with a scrambled siRNA, CaMKII siRNA, CaMKII siRNA + mutant CaMKII, or wild-type CaMKII. Inset, representative mEPSC traces. Scale bars, 20 pA, 1 s. B, bar graphs (mean ± S.E.) showing the mEPSC amplitude and frequency in neurons transfected with different constructs. *, p < 0.01, ANOVA. C–E, cumulative plots of the distribution of mEPSC amplitudes and inter-event interval in untreated (control) or Aβ-treated neurons (GFP+) transfected with a scrambled siRNA (C1, C2), CaMKII siRNA (D1, D2), or wild-type CaMKII (E1, E2). Inset, representative mEPSC traces. Scale bars, 20 pA, 1 s. F, bar graphs (mean ± S.E.) showing the mEPSC amplitude (F1) and frequency (F2) in control or Aβ-treated neurons transfected with different constructs. *, p < 0.01, ANOVA.
In neurons transfected with a scrambled siRNA, Aβ treatment (1 μm, 3 days) significantly (p < 0.01, ANOVA) reduced mEPSC amplitude (Fig. 8C1, control, 24.1 ± 1.1 pA, n = 20; Aβ, 19.9 ± 0.3 pA, n = 15, Fig. 8F1); however, Aβ lost the capability to decrease mEPSC amplitude in CaMKII siRNA-transfected neurons (Fig. 8D1, control: 20.3 ± 0.9 pA, n = 15; Aβ, 19.7 ± 0.4 pA, n = 16, Fig. 8F1). Conversely, Aβ failed to reduce mEPSC amplitude in neurons overexpressing CaMKII (Fig. 8E1, control, 25.5 ± 0.7 pA, n = 17; Aβ, 25.2 ± 1.0 pA, n = 15, Fig. 8F1). Taken together, these data suggest that Aβ decreases ionic and synaptic AMPAR currents via a CaMKII-dependent mechanism. Interestingly, the Aβ-induced reduction of mEPSC frequency was not altered by CaMKII manipulations (Fig. 8, C2–F2), suggesting that Aβ also has some presynaptic effects on glutamatergic synapses that are independent of CaMKII.
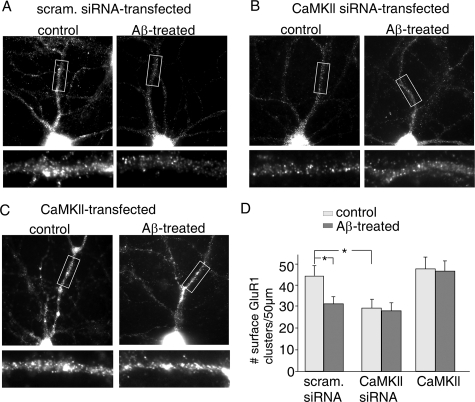
Finally, we performed immunocytochemical experiments to examine the role of CaMKII in Aβ regulation of AMPAR surface expression. Because older cultures are not easy to be transfected, we stained younger cultures (DIV 18). Compared with older cultures (e.g. DIV 28), younger cultures had more and smaller surface GluR1 clusters, probably because of the less maturity of spine development. As shown in Fig. 9A, in neurons transfected with a scrambled siRNA, Aβ exposure (1 μm, 3 days) significantly decreased surface GluR1 cluster density (number of clusters/50-μm dendrite) (Fig. 9D, control, 44.3 ± 4.5, n = 18; Aβ, 31.2 ± 3.5, n = 17, p < 0.01, ANOVA). In CaMKII siRNA-transfected neurons, surface GluR1 cluster density was significantly reduced, and Aβ treatment failed to induce further reduction (Fig. 9B, control, 29.2 ± 4.2, n = 15; Aβ, 28.1 ± 3.8, n = 17, Fig. 9D). On the other hand, in neurons overexpressing CaMKII, surface GluR1 cluster density was slightly increased, and the reducing effect of Aβ was blocked (Fig. 9C, control, 47.6 ± 5.8, n = 15; Aβ, 46.4 ± 5.2, n = 18, Fig. 9D). These data suggest that Aβ decreases the number of surface AMPARs via a CaMKII-dependent mechanism.
FIGURE 9.
Role of CaMKII in Aβ regulation of surface GluR1 clusters. A–C, immunocytochemical images of surface GluR1 staining in cultured cortical pyramidal neurons (DIV 18) transfected (GFP+) with a scrambled siRNA (A), CaMKII siRNA (B), or wild-type CaMKII (C) without (control) or with Aβ (1 μm) exposure for 3 days. D, cumulative data (mean ± S.E.) showing the density of surface GluR1 clusters in control or Aβ-treated neurons transfected with different constructs. *, p < 0.01, ANOVA.
DISCUSSION
Mounting evidence suggests that the effects of soluble Aβ on synapses might be directly linked to the learning and memory deficits in AD (45–47). Thus, the current “Aβ hypothesis” proposes that the gradual accumulation of soluble Aβ oligomers initiates a slow but deadly cascade that adversely affects synaptic function, which leads to cognitive failure associated with AD (48, 49). In this study we have demonstrated that CaMKII, a molecule playing a key role in AMPAR trafficking and synaptic plasticity (20, 22, 29), is reduced at the synapses by Aβ, which leads to the loss of synaptic AMPA receptors and impairment of glutamatergic transmission.
The function of CaMKII is shaped by its autoregulation and subcellular localization (31). After activation, CaMKII undergoes autophosphorylation at Thr-286, which converts the enzyme to a sustained, Ca2+-independent activated state (41), therefore enabling CaMKII to act as a molecular memory device to detect synaptic activity and to coordinate Ca2+ signal transduction (50). CaMKII phosphorylates dozens of substrates throughout the whole cell (51). The response specificity of CaMKII is achieved by its compartmentalized localization (30). Activation of the kinase is limited by the frequency and amplitude of the Ca2+ signal because of the relatively low affinity of CaMKII to Ca2+/CaM. Because Ca2+ signals are highly localized, only CaMKII located closest to the source of Ca2+ is activated. CaMKII can alter its range of substrates by dynamically regulating its subcellular distribution (32). Autophosphorylated CaMKII binds tightly to the NMDA receptors (52–54); thus, it may form additional sites for anchoring AMPA receptors at synapses through a supramolecular linkage (35).
Our biochemical fractionation experiments show that the synaptic pool of CaMKII is significantly decreased, whereas the cytosolic pool of CaMKII is increased in cortical neurons from APP transgenic mice or in cortical cultures treated with Aβ oligomers. Immunocytochemical experiments further demonstrate that the density of CaMKII clusters at synapses is reduced by Aβ treatment. How does extracellular application of Aβ affect intracellular distribution of CaMKII? Literature has demonstrated numerous intraneuronal events triggered by extracellular application of Aβ. Some studies also show that synaptic dysfunction and cognitive deficits are caused by intracellular Aβ (8, 10). It is possible that extracellular Aβ is transported intracellularly through an unknown mechanism. Alternatively, extracellular Aβ may bind to an unknown receptor on the cell surface and then trigger intracellular signaling. Previous studies have shown that CaMKII synaptic translocation is dependent on mechanisms involving Ca2+/CaM and F-actin (32, 40, 33). Thus, it is likely that Aβ affects synaptic distribution of CaMKII by altering intracellular calcium signaling and/or actin cytoskeleton dynamics.
Because CaMKII compartmentalization at PSD is critical for the regulation of its synaptic substrates such as AMPA receptors (30), this Aβ-induced loss of synaptic CaMKII could have significant impact on synaptic functions. Consistent with this notion, we have found that Aβ induces a significant loss of AMPAR GluR1 subunits at the synaptosomal fraction and GluR1 phosphorylation at the CaMKII site. Although surface GluR1 levels and surface GluR1 clusters are substantially reduced by Aβ oligomer treatment (3 or 7 days), we have not found a significant change in other synaptic proteins, including the surface NMDAR NR1 subunit, PSD-95, actin, and F-actin clusters. In agreement with this, electrophysiological studies have shown that the synaptic response mediated by AMPARs, but not NMDARs, is selectively impaired in APP mice. Similarly, the density of functional AMPARs, but not NMDARs, at the neuronal surface is selectively reduced by Aβ treatment.
Some of previous reports have shown that Aβ induces synapse loss, alters NMDAR trafficking, and degrades PSD-95 (13, 15, 16). The discrepancy may be because of the different biological properties of exogenous Aβ peptides or overexpressed mutant APP in different preparations. Our finding on the lack of effect of Aβ on NMDAR-EPSC and surface NR1 expression is different from the finding in Ref. 15. However, in this paper, only in “fewer than half the neurons tested” Aβ-(1–42) (1 μm) evoked an inward current (40–800 pA), and only in these neurons was NMDAR current reduced after Aβ application (5 min), whereas in cells that had no Aβ-induced current, NMDAR current was not altered. Moreover, such effects were only observed in some lots of Aβ peptide from BIOSOURCE, whereas no effect was observed with the Aβ peptide from Tocris.3 We have used Aβ from four sources (Tocris, BIOSOURCE, AnaSpec or Sigma) and have almost never found cortical neurons with Aβ-induced inward currents.
In our cortical cultures treatment with Aβ oligomers (1 μm, 3 days) did not seem to affect the spine density (as indicated by the lack of changes on F-actin or GluR1 clusters), which is different from what was found in Shankar et al. (55). In this paper (55), a different neuronal preparation (organotypic hippocampal slices) and a different Aβ (natural oligo Aβ) were used, which could be the reason for the discrepancy. However, as shown in Hsieh et al. (19), Aβ treatment (1 μm, 7 days) did not decrease the spine density in cultured hippocampal slices too much (0.6) compared with untreated control neurons (0.7), and only APP overexpression significantly reduced the spine density (0.4). Consistent with our results, it has been shown that AMPAR current, but not NMDAR current, was reduced in APPswe/PS1dE9 mice, and the density of dendritic spines was not affected by co-expression of the APPswe and PS1dE9 variants (56). It is likely that the loss of synaptic AMPARs precedes other synaptic changes. Indeed, it has been shown that synaptic loss of AMPA receptors is necessary and sufficient to produce loss of dendritic spines and synaptic NMDA responses (19).
Our experiments with manipulated CaMKII expression have provided more direct evidence showing that the Aβ-induced alteration of CaMKII causally links to the loss of synaptic AMPARs. Knockdown of CaMKII significantly reduces AMPAR current density and occludes the reducing effect of Aβ, whereas overexpression of CaMKII prevents Aβ from decreasing AMPAR current density. These results suggest that CaMKII is critical for the surface delivery of AMPA receptors, and Aβ-induced reduction of surface AMPARs is likely through a CaMKII-dependent mechanism.
Acknowledgments
We thank Xiaoqing Chen for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants AG21923 and MH84233. This work was also supported by National Science Foundation of China Grant NSFC 30528009 (to Z. Y.).
Footnotes
The abbreviations used are: Aβ, β-amyloid; AD, Alzheimer disease; APP, amyloid precursor protein; CaMKII, Ca2+/calmodulin-dependent protein kinase II; NMDA, N-methyl-d-aspartate; NMDAR, N-methyl-d-aspartate receptor; AMPAR, AMPA receptor; PSD, postsynaptic density; WT, wild type; p-, phosphorylated; DIV, days in vitro; siRNA, small interfering RNA; EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; ANOVA, analysis of variance; pF, picofarad; GFP, green fluorescent protein; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid.
Z. Gu, W. Liu, and Z. Yan, personal communication.
References
- 1.Tanzi, R. E., and Bertram, L. (2001) Neuron 32 181-184 [DOI] [PubMed] [Google Scholar]
- 2.Selkoe, D. J., and Schenk, D. (2003) Annu. Rev. Pharmacol. Toxicol. 43 545-584 [DOI] [PubMed] [Google Scholar]
- 3.Selkoe, D. J. (2002) Science 298 789-791 [DOI] [PubMed] [Google Scholar]
- 4.Lue, L. F., Kuo, Y. M., Roher, A. E., Brachova, L., Shen, Y., Sue, L., Beach, T., Kurth, J. H., Rydel, R. E., and Rogers, J. (1999) Am. J. Pathol. 155 853-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Beyreuther, K., Bush, A. I., and Masters, C. L. (1999) Ann. Neurol. 46 860-866 [DOI] [PubMed] [Google Scholar]
- 6.Cleary, J. P., Walsh, D. M., Hofmeister, J. J., Shankar, G. M., Kuskowski, M. A., Selkoe, D. J., and Ashe, K. H. (2005) Nat. Neurosci. 8 79-84 [DOI] [PubMed] [Google Scholar]
- 7.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J., and Selkoe, D. J. (2002) Nature 416 535-539 [DOI] [PubMed] [Google Scholar]
- 8.Billings, L. M., Oddo, S., Green, K. N., McGaugh, J. L., and Laferla, F. M. (2005) Neuron 45 675-688 [DOI] [PubMed] [Google Scholar]
- 9.Chapman, P. F., White, G. L., Jones, M. W., Cooper-Blacketer, D., Marshall, V. J., Irizarry, M., Younkin, L., Good, M. A., Bliss, T. V., Hyman, B. T., Younkin, S. G., and Hsiao, K. K. (1999) Nat. Neurosci. 2 271-276 [DOI] [PubMed] [Google Scholar]
- 10.Oddo, S., Caccamo, A., Shepherd, J. D., Murphy, M. P., Golde, T. E., Kayed, R., Metherate, R., Mattson, M. P., Akbari, Y., and LaFerla, F. M. (2003) Neuron 39 409-421 [DOI] [PubMed] [Google Scholar]
- 11.Stern, E. A., Bacskai, B. J., Hickey, G. A., Attenello, F. J., Lombardo, J. A., and Hyman, B. T. (2004) J. Neurosci. 24 4535-4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsia, A., Masliah, E., McConlogue, L., Yu, G., Tatsuno, G., Hu, K., Kholodenko, D., Malenka, R., Ricoll, R., and Mucke, L. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3228-3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanz, T. A., Carter, D. B., and Merchant, K. M. (2003) Neurobiol. Dis. 13 246-253 [DOI] [PubMed] [Google Scholar]
- 14.Spires, T. L., Meyer-Luehmann, M., Stern, E. A., McLean, P. J., Skoch, J., Nguyen, P. T., Bacskai, B. J., and Hyman, B. T. (2005) J. Neurosci. 25 7278-7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder, E. M., Nong, Y., Almeida, C. G., Paul, S., Moran, T., Choi, E. Y., Nairn, A. C., Salter, M. W., Lombroso, P. J., Gouras, G. K., and Greengard, P. (2005) Nat. Neurosci. 8 1051-1058 [DOI] [PubMed] [Google Scholar]
- 16.Roselli, F., Tirard, M., Lu, J., Hutzler, P., Lamberti, P., Livrea, P., Morabito, M., and Almeida, O. F. (2005) J. Neurosci. 25 11061-11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamenetz, F., Tomita, T., Hsieh, H., Seabrook, G., Borchelt, D., Iwatsubo, T., Sisodia, S., and Malinow, R. (2003) Neuron 37 925-937 [DOI] [PubMed] [Google Scholar]
- 18.Almeida, C. G., Tampellini, D., Takahashi, R. H., Greengard, P., Lin, M. T., Snyder, E. M., and Gouras, G. K. (2005) Neurobiol. Dis. 20 187-198 [DOI] [PubMed] [Google Scholar]
- 19.Hsieh, H., Boehm, J., Sato, C., Iwatsubo, T., Tomita, T., Sisodia, S., and Malinow, R. (2006) Neuron 52 831-843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derkach, V. A., Oh, M. C., Guire, E. S., and Soderling, T. R. (2007) Nat. Rev. Neurosci. 8 101-113 [DOI] [PubMed] [Google Scholar]
- 21.Malinow, R., and Malenka, R. C. (2002) Annu. Rev. Neurosci. 25 103-126 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, Y., Shi, S. H., Esteban, J. A., Piccini, A., Poncer, J. C., and Malinow, R. (2000) Science 287 2262-2267 [DOI] [PubMed] [Google Scholar]
- 23.Elias, G. M., Funke, L., Stein, V., Grant, S. G., Bredt, D. S., and Nicoll, R. A. (2006) Neuron 52 307-320 [DOI] [PubMed] [Google Scholar]
- 24.Carroll, R. C., Lissin, D. V., von Zastrow, M., Nicoll, R. A., and Malenka, R. C. (1999) Nat. Neurosci. 2 454-460 [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., Liu, L., Wang, Y. T., and Sheng, M. (2002) Neuron 36 661-674 [DOI] [PubMed] [Google Scholar]
- 26.Setou, M., Seog, D. H., Tanaka, Y., Kanai, Y., Takei, Y., Kawagishi, M., and Hirokawa, N. (2002) Nature 417 83-87 [DOI] [PubMed] [Google Scholar]
- 27.Correia, S. S., Bassani, S., Brown, T. C., Lisé, M. F., Backos, D. S., El-Husseini, A., Passafaro, M., and Esteban, J. A. (2008) Nat. Neurosci. 11 457-466 [DOI] [PubMed] [Google Scholar]
- 28.Kennedy, M. B., Bennett, M. K., and Erondu, N. E. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 7357-7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poncer, J. C., Esteban, J. A., and Malinow, R. (2002) J. Neurosci. 22 4406-4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy, M. B. (2000) Science 290 750-754 [DOI] [PubMed] [Google Scholar]
- 31.Hudmon, A., and Schulman, H. (2002) Annu. Rev. Biochem. 71 473-510 [DOI] [PubMed] [Google Scholar]
- 32.Shen, K., and Meyer, T. (1999) Science 284 162-166 [DOI] [PubMed] [Google Scholar]
- 33.Gu, Z., Jiang, Q., Yuen, E. Y., and Yan, Z. (2006) Mol. Pharmacol. 69 813-822 [DOI] [PubMed] [Google Scholar]
- 34.Soderling, T. R., Chang, B., and Brickey, D. (2001) J. Biol. Chem. 276 3719-3722 [DOI] [PubMed] [Google Scholar]
- 35.Lisman, J. E., and Zhabotinsky, A. M. (2001) Neuron 31 191-201 [DOI] [PubMed] [Google Scholar]
- 36.Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang, F., and Cole, G. (1996) Science 274 99-103 [DOI] [PubMed] [Google Scholar]
- 37.Dahlgren, K. N., Manelli, A. M., Stine, W. B., Jr., Baker, L. K., Krafft, G. A., and LaDu, M. J. (2002). J. Biol. Chem. 277 32046-32053 [DOI] [PubMed] [Google Scholar]
- 38.Tang, K., Liu, C., Kuluz, J., and Hu, B. (2004) J. Neurochem. 91 429-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu, Z., Jiang, Q., Fu, A. K., Ip, N. Y., and Yan, Z. (2005) J. Neurosci. 25 4974-4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen, K., Teruel, M. N., Subramanian, K., and Meyer, T. (1998) Neuron. 21 593-606 [DOI] [PubMed] [Google Scholar]
- 41.Miller, S. G., and Kennedy, M. B. (1986) Cell 44 861-870 [DOI] [PubMed] [Google Scholar]
- 42.McGlade-McCulloh, E., Yamamoto, H., Tan, S. E., Brickey, D. A., and Soderling, T. R. (1993) Nature 362 640-642 [DOI] [PubMed] [Google Scholar]
- 43.Barria, A., Muller, D., Derkach, V., Griffith, L. C., and Soderling, T. R. (1997) Science 276 2042-2045 [DOI] [PubMed] [Google Scholar]
- 44.Yuen, E. Y., Ren, Y., and Yan, Z. (2008) Mol. Pharmacol. 74 360-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., Hansen, L. A., and Katzman, R. (1991) Ann. Neurol. 30 572-580 [DOI] [PubMed] [Google Scholar]
- 46.Masliah, E., Mallory, M., Alford, M., DeTeresa, R., Hansen, L. A., McKeel, D. W., Jr., and Morris, J. C. (2001) Neurology 56 127-129 [DOI] [PubMed] [Google Scholar]
- 47.Carter, T. L., Rissman, R. A., Mishizen-Eberz, A. J., Wolfe, B. B., Hamilton, R. L., Gandy, S., and Armstrong, D. M. (2004) Exp. Neurol. 187 299-309 [DOI] [PubMed] [Google Scholar]
- 48.Hardy, J. A., and Higgins, G. A. (1992) Science 256 184-185 [DOI] [PubMed] [Google Scholar]
- 49.Haass, C., and Selkoe, D. J. (2007) Nat. Rev. Mol. Cell Biol. 8 101-112 [DOI] [PubMed] [Google Scholar]
- 50.Frankland, P. W., O'Brien, C., Ohno, M., Kirkwood, A., and Silva, A. J. (2001) Nature 411 309-313 [DOI] [PubMed] [Google Scholar]
- 51.Braun, A. P., and Schulman, H. (1995) Annu. Rev. Physiol. 57 417-445 [DOI] [PubMed] [Google Scholar]
- 52.Strack, S., and Colbran, R. J. (1998) J. Biol. Chem. 273 20689-20692 [DOI] [PubMed] [Google Scholar]
- 53.Leonard, A. S., Lim, I. A., Hemsworth, D. E., Horne, M. C., and Hell, J. W. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3239-3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer, K. U., De Koninck, P., Leonard, A. S., Hell, J. W., and Schulman, H. (2001) Nature 411 801-805 [DOI] [PubMed] [Google Scholar]
- 55.Shankar, G. M., Bloodgood, B. L., Townsend, M., Walsh, D. M., Selkoe, D. J., and Sabatini, B. L. (2007) J. Neurosci. 27 2866-2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shemer, I., Holmgren, C., Min, R., Fülöp, L., Zilberter, M., Sousa, K. M., Farkas, T., Härtig, W., Penke, B., Burnashev, N., Tanila, H., Zilberter, Y., and Harkany, T. (2006) Eur. J. Neurosci. 23 2035-2047 [DOI] [PubMed] [Google Scholar]