Abstract
Induction of cell cycle arrest in lymphocytes after exposure to the Aggregatibacter actinomycetemcomitans cytolethal distending toxin (Cdt) is dependent upon the integrity of lipid membrane microdomains. In this study we further demonstrate that the association of Cdt with lymphocyte plasma membranes is dependent upon binding to cholesterol. Depletion of cholesterol resulted in reduced toxin binding, whereas repletion of cholesterol-depleted cells restored binding. We employed fluorescence resonance energy transfer and surface plasmon resonance to demonstrate that toxin association with model membranes is dependent upon the concentration of cholesterol; moreover, these interactions were cholesterol-specific as the toxin failed to interact with model membranes containing stigmasterol, ergosterol, or lanosterol. Further analysis of the toxin indicated that the CdtC subunit contains a cholesterol recognition/interaction amino acid consensus (CRAC) region. Mutation of the CRAC site resulted in decreased binding of the holotoxin to cholesterol-containing model membranes as well as to the surface of Jurkat cells. The mutant toxin also exhibited reduced capacity for intracellular transfer of the active toxin subunit, CdtB, as well as reduced toxicity. Collectively, these observations indicate that membrane cholesterol serves as an essential ligand for Cdt and that this association can be blocked by either depleting membranes of cholesterol or mutation of the CRAC site.
The cytolethal distending toxins (Cdts)2 are a family of heat-labile protein cytotoxins produced by several different bacterial species including diarrheal disease-causing enteropathogens such as some Escherichia coli isolates, Campylobacter jejuni, Shigella species, Haemophilus ducreyi, and Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans (1–7). There is clear evidence that Cdts are encoded by three genes, designated cdtA, cdtB, and cdtC, which are arranged as an apparent operon (7–14). These three genes specify three polypeptides designated CdtA, CdtB, and CdtC with apparent molecular masses of 28, 32 and 20 kDa, respectively, that form a heterotrimeric holotoxin. Cdt toxicity is associated with cell cycle arrest and eventual cell death resulting from activation of the apoptotic cascade (10, 11, 15–17). Although several cell lines and cell types have been shown to be susceptible to the toxic effects of Cdt, we have previously shown that lymphocytes are the most sensitive (18). For this reason we believe that lymphocytes are a likely in vivo target of Cdt and further propose that Cdt represents a novel immunotoxin.
Considerable agreement exists among investigators that regardless of the microbial source of Cdt, the heterotrimeric holotoxin functions as an AB2 toxin where CdtB is the active (A) unit and the complex of CdtA and CdtC comprises the binding (B) unit (12, 19, 20). There is compelling evidence that CdtB must be internalized to induce cell cycle arrest (19, 21, 22). Moreover, we have recently demonstrated that the active Cdt subunit, CdtB, functions as a phosphatidylinositol 3,4,5-triphosphate phosphatase similar to that of the tumor suppressor phosphatases, PTEN and SHIP1 (23–25). We have further demonstrated that Cdt-induced G2 arrest is dependent upon its ability to function as a lipid phosphatase and most likely results from toxin induced perturbations in the Akt signaling pathway.
In previous studies we have shown that binding subunits CdtA and CdtC are not only required for the toxin to associate with cells but are necessary to localize the toxin to lipid membrane microdomains (18, 26). Furthermore, Cdt-mediated toxicity is dependent upon the integrity of these lipid domains. We now report that not only does cholesterol depletion and disruption of lipid membrane microdomains confer resistance to Cdt-induced G2 arrest, but association of the Cdt holotoxin to lymphocytes also involves cholesterol. Specifically, we have utilized both fluorescence resonance energy transfer (FRET) and surface plasmon resonance (SPR) analysis to demonstrate interaction between the CdtC subunit and cholesterol in cholesterol containing liposomes. Moreover, we have identified a cholesterol recognition/interaction amino acid consensus site (CRAC) on CdtC that is required for these interactions (27). Mutation of the CRAC site reduces interaction with lymphocyte plasma membrane and with cholesterol-containing liposomes and also reduces delivery of CdtB to the cell and concomitant G2 arrest.
EXPERIMENTAL PROCEDURES
Cell Lines and Analysis of Cell Cycle—The human leukemic T cell line Jurkat was maintained in RPMI 1640 supplemented with 10% fetal calf serum, 2 mm glutamine, 10 mm HEPES, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were harvested in mid-logarithmic growth phase and plated at 5 × 105 cells/ml or as indicated in 24-well tissue culture plates. The cells were exposed to medium or Cdt and incubated for 18 h. To measure Cdt-induced cell cycle arrest, Jurkat cells were washed and fixed for 60 min with cold 80% ethanol (18). After washing, the cells were stained with 10 μg/ml propidium iodide containing 1 mg/ml RNase (Sigma) for 30 min. Samples were analyzed on a BD Biosciences FacstarPLUS flow cytometer. Propidium iodide fluorescence was excited by an argon laser operating at 488 nm, and fluorescence was measured with a 630/22-nm bandpass filter using linear amplification. A minimum of 15,000 events was collected on each sample; cell cycle analysis was performed using Modfit (Verity Software House; Topsham, ME).
Construction and Expression of CdtABCY71P Mutant—Amino acid substitution was introduced into the cdtC gene by in vitro site-directed mutagenesis using the following oligonucleotide primer pair: (forward) 5′-GGAATTAATTGATCCCAAGGGAAAAGA-3′ and (reverse) 5′-TCTTTTCCCTTGGGATCAATTAATTCC-3′ (bold letters indicate nucleotide substitutions). Site-directed mutagenesis was performed using the QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's directions. Amplification of the mutant plasmid was carried out using PfuUltra HF DNA polymerase (Stratagene) and pUCAacdtABChis as a template; construction and characterization of this plasmid was previously described (28). The mutation was verified by DNA sequencing. Expression of the plasmid and purification of the mutant peptide is described below.
Expression and Purification of Cdt Holotoxin—Construction and expression of the plasmid containing the cdt genes for the holotoxin (pUCAacdtABChis) has previously been reported (28), and CdtABCY71P is described above. The plasmids were constructed so that the cdt genes were under control of the lac promotor and transformed into E. coli DH5α. Cultures of transformed E. coli were grown in 1 liter of LB broth and induced with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside for 2 h; bacterial cells were harvested, washed, and resuspended in 50 mm Tris (pH 8.0). The cells were frozen overnight, thawed, and sonicated. The histidine-tagged peptide holotoxin was isolated by nickel affinity chromatography as previously described (10).
Preparation of Lipid Vesicles and Analysis of Cdt-Liposome Binding—Phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, sphingomyelin, and cholesterol were obtained from Avanti Polar Lipids; lipids were stored in chloroform at -20 °C with desiccation. Briefly, large unilamellar vesicles (LUVs) were prepared with a lipid ratio of phosphatidylcholine/sphingomyelin/phosphatidylethanolamine = 50:13:37 (mol %) or as indicated in the presence of varying amounts of cholesterol as described by Hekman et al. (29). For some experiments LUV cholesterol was substituted with ergosterol, stigmasterol, or lanosterol as described in the figure legends. Lipids were co-solubilized in chloroform and dried under N2, and trace amounts of residual solvent were removed under high vacuum. The lipid mixtures were re-hydrated at a concentration of 8 mm (total phospholipid) in 20 mm Tris-HCl (pH 7.4) containing 50 mm NaCl for 1 h; the suspensions were then vortexed and freeze-thawed in liquid nitrogen, and LUVs were prepared by extrusion, 11 passes through a 100-nm membrane (Mini Extruder; Avanti Polar Lipids).
All SPR experiments were carried out on a Biacore X (Biacore AB; Upsala, Sweden) with active temperature control at 25 °C. The running buffer for the experiments was 20 mm HEPES (pH 7.4) containing 50 mm NaCl. LUVs (0.8 mm) in running buffer equivalent to 5000 RU were injected onto a LI sensor chip (Biacore). Routinely, cholesterol-containing vesicles were coupled to flow cell 1 (Fc1) and control vesicles containing no cholesterol to flow cell 2 (Fc2). Cdt holotoxin (or mutant) was diluted in the running buffer and flowed across the immobilized liposomes for 3 min at a flow rate of 5 μl/min (association), sample was replaced with buffer, and disassociation of bound toxin was followed for 10 min. The chip surface was regenerated by injecting a 1-min pulse of 30 mm n-octyl-β-d-glucopyranoside. Sensorgrams were corrected for nonspecific binding by subtracting the control sensorgram (Fc2) from the experimental surface sensorgram (Fc1).
FRET Analysis—Vesicle solutions were prepared using the rapid solvent exchange technique (30). Briefly, stock solutions of phospholipid, either 1,2-dimyristoyl-sn-glycero-3-phosphocholine or 1,2-dioleoyl-sn-glycero-3-phosphocholine, sphingomyelin (SM), cholesterol, and 1-myristoyl-2-[12-[(5-dimethylamino-1-naphthalenesulfonyl)amino]-dodecanoyl]-sn-glycero-3-phosphocholine (DAN-PC) dissolved in chloroform were added to 20-ml flat-bottom vials in the required proportions. Three ml of aqueous buffer at 60 °C was added, and the solution was vortexed under continuous vacuum at 25 inches of Hg for 1 min. The lipid mixtures were diluted with 0.15 m NaCl, 5 mm CaCl2, 5 mm HEPES, and 3 mm NaN3 (pH 7.4) to a final lipid concentration of 0.5 mm. Samples contained 10, 25, or 40 mol % cholesterol, 10 or 40 mol % SM, and 1,2-dioleoyl-sn-glycero-3-phosphocholine. For each lipid composition, one sample was labeled with 3% DAN-PC, and one sample was unlabeled as indicated in the figure legends.
FRET provides a measure of the average distance between an array of donor and acceptor molecules. Toxin binding was detected by an increase in the efficiency of energy transfer as the average distance between tryptophan on the protein and DAN-PC in the vesicle increases upon binding. 63.75 μg of toxin was added to 15 ml of each vesicle solution and incubated at room temperature for 1 h. The solution was then separated into 5 aliquots of 3 ml each for the emission scans. The extent of energy transfer for each series of vesicle preparation was determined upon excitation (ex285) of tryptophan and emission from em325 to 550 nm. All emission scans were conducted at 30 °C. The emission profiles were smoothed using a Savitsky and Golay protocol (31). FRET efficiency was calculated from the measured steady-state fluorescence intensity of the donor (tryptophan) at its maximal emission wavelength (360 nm) in the presence and absence of acceptor (DAN-PC) using the equation E(%) = 1 - FDA/FD, where FDA and FD are the donor emission intensities in the presence and absence of acceptor, respectively (32). Fluorescence measurements were obtained using a steady-state fluorescence spectrometer (Photon Technology International, Ontario, Canada) with a circulating water bath to maintain the sample temperature to +0.5 °C. The temperature was read on a cuvette thermometer (Fisher).
Immunofluorescence and Flow Cytometry—Jurkat cells (2 × 106) were incubated for 2 h in the presence of medium or 2 μg/ml of CdtABCwt or CdtABCY71P at 5 °C. Cells were washed, exposed to normal mouse IgG (Zymed Laboratories Inc.; San Franscisco, CA) and then stained (30 min) for cell surface CdtC peptides with anti-Cdt subunit monoclonal antibody conjugated to Alexafluor 488 (Molecular Probes; Eugene, OR) according to the manufacturer's directions. After washing, the cells were fixed in 2% paraformaldehyde and analyzed by flow cytometry or confocal microscopy as previously described (26). Intracellular CdtB was detected after exposure of Jurkat cells to 2 μg/ml concentrations of either CdtABCwt or CdtABCY71P for 60 min at 37 °C. Cells were fixed with 2% formaldehyde for 30 min, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate, and stained with anti-CdtB monoclonal antibody conjugated to Alexafluor 488 (Molecular Probes).
Statistical Analyses—Data were analyzed using Sigma Stat Version 3.1. Concentration-dependent binding of toxin to sterol or sphingomyelin-containing vesicles were analyzed by Student t test using a 95% confidence interval. One-way analysis of variance was used to determine whether there was a statistically significant trend in binding of toxin to liposomes or energy transfer between toxin and liposomes of various compositions.
RESULTS
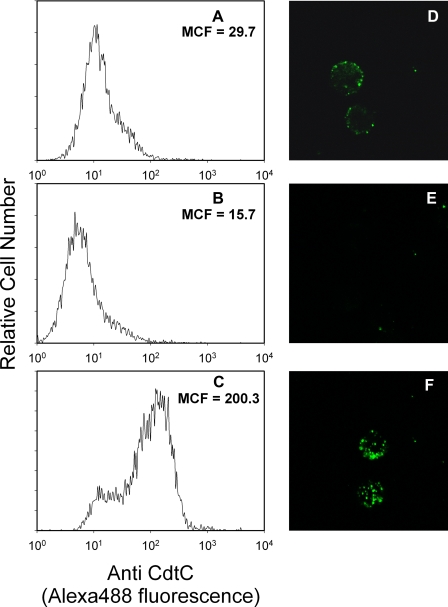
It is generally accepted that the CdtA and CdtC subunits are required for interaction of the Cdt holotoxin with target cell membranes leading to intracellular delivery of the active subunit, CdtB, and subsequent G2 arrest. We have previously shown that after exposure to Cdt holotoxin, the individual subunits can be detected associated with Jurkat cell membranes and, in particular, with membrane lipid rafts. We have also demonstrated that Cdt association with lymphocytes is dependent upon the integrity of lipid membrane microdomains (26). Furthermore, we now demonstrate that the association of Cdt holotoxin with lymphocytes is cholesterol-dependent. Jurkat cell membranes were depleted of cholesterol with 5 mm methyl β-cyclodextrin (MβCD) for 30 min and then exposed to Cdt holotoxin for 1 h. Toxin association with cells was monitored by assessing immunofluorescence of the CdtC subunit with monoclonal antibody. As shown in Fig. 1, panels A, B, D, and E), cholesterol depletion resulted in a significant reduction in the level of Cdt associated with Jurkat cells; mean channel fluorescence (MCF) was reduced from 29.7 in control (untreated) cells to 15.7 in MβCD-treated cells. Furthermore, cholesterol repletion with cholesterol-saturated MβCD restored toxin binding (MCF = 200.3) to levels greater than that observed in control cells (Fig. 1, C and F). Alterations in toxin binding paralleled changes in levels of cholesterol extracted from similarly treated cells: 11.8 (control cells), 0.37 (MβCD-treated cells), and 20.7 μg of cholesterol/107 cells (MβCD-saturated cholesterol-repleted cells).
FIGURE 1.
Cdt holotoxin association with Jurkat cells is dependent upon the presence of cholesterol. Jurkat cells were exposed to medium (panels A and D) or 5 mm MβCD (panels B and E (Sigma)) for 30 min. Cells were washed, and some of the MβCD-treated cells were incubated with cholesterol-saturated MβCD (0.5 mm) for 30 min (panels C and F). Cells were incubated with Cdt holotoxin (2 μg/ml) for 1 h, washed, and treated with control murine IgG (data not shown) or anti-CdtC monoclonal antibody conjugated to Alexafluor 488. Jurkat cells were then analyzed by flow cytometry (panels A–C), or images of cells were taken on a Bio-Rad Radiance 2100 Confocal Microscope (Bio-Rad); representative images are shown in panels D–F. Results are representative of three experiments. The relative level of cholesterol in 107 Jurkat cells was compared by semiquantitative TLC. Cholesterol was identified based on calculated Rf values and color after detection by charring with sulfuric acid/EtOH (1:1 vol:vol). The intensity of cholesterol for each sample was compared with the intensity of known standards using digital densitometry; values were 11.8, 0.37, and 20.7 μg cholesterol/107 cells for control, MβCD-treated, and cholesterol-saturated MβCD-treated cells, respectively.
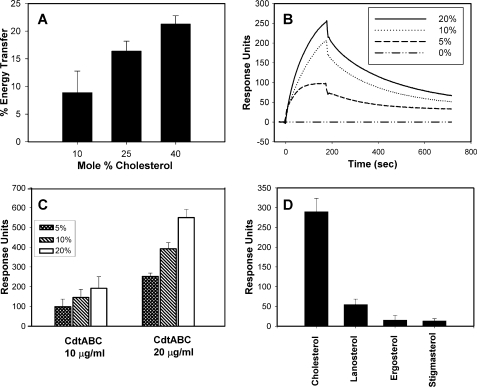
In the next series of experiments we utilized model membranes to determine whether Cdt binding was the result of direct interaction of the toxin with membrane cholesterol. FRET was first employed to assess Cdt association with model membranes composed of varying amounts of cholesterol. For these experiments, DAN-PC was incorporated into LUVs, and energy transfer to tryptophan residues intrinsic to Cdt was measured as a function of increasing cholesterol (Fig. 2A). Energy transfer increased from 8.9% in the presence of 10% cholesterol to 21.3% in the presence of 40% cholesterol. Direct binding of Cdt to cholesterol was also measured in real-time using SPR. Cholesterol-containing LUVs were immobilized on L1 sensor chips; LUVs prepared in the absence of cholesterol were also immobilized and used as a control. Cdt holotoxin was injected, and the association of toxin with LUV was followed for 3 min. The sample was then replaced with buffer, and the dissociation of the complex was followed for another 10 min. Fig. 2B shows a group of sensorgram overlays for the binding of Cdt holotoxin to immobilized LUVs containing varying amounts of cholesterol (0–20%). Increases in binding were observed as the percentage of cholesterol in the LUV increased from 46 RU in the presence of 2.5% cholesterol (not shown) to 267 RU in the presence of 20% cholesterol. Furthermore, we observed that the amount of Cdt binding is dependent upon the concentration of toxin (Fig. 2C); significantly more toxin binding was observed in the presence of 20 μg/ml Cdt than with 10 μg/ml Cdt with LUVs containing 5–20% cholesterol. Cholesterol specificity was tested by replacing cholesterol in the LUVs with stigmasterol, ergosterol, or lanosterol. Cdt binding to the LUVs was significantly reduced when cholesterol was replaced with each of these sterols (Fig. 2D); we observed maximum RU of 937 in the presence of cholesterol compared with 179, 92, and 32 RU with lanosterol, ergosterol, and stigmasterol, respectively. These results suggest that binding is specific for cholesterol as there was no difference in the relative amount of each sterol extracted from the liposomes (see the Fig. 2 legend).
FIGURE 2.
Cdt holotoxin preferentially binds to LUVs containing cholesterol. The interaction of Cdt holotoxin with LUVs containing varying amounts of cholesterol was analyzed by FRET and SPR. Panel A shows FRET analysis of Cdt with LUVs containing varying amounts (10–40 mol %) cholesterol. Values are the mean ± S.D. (n = 3), expressed as relative % energy transfer. Results are statistically significant (p < 0.05; multivariant analysis of variance with post-hoc Scheffe test) for differences in energy transfer as cholesterol concentration is increased. Panel B shows the SPR results of Cdt interaction with LUVs. An overlay of sensorgrams shows the interaction of Cdt (10 μg/ml) with immobilized LUVs containing decreasing concentrations of cholesterol; data points were collected every 0.2 s. Data are plotted as response units versus time and are representative of three experiments. Panel C shows the results of SPR analysis for the interaction of two concentrations of Cdt (10 and 20 μg/ml) with immobilized LUVs containing 5, 10, and 20% cholesterol; data are the mean ± S.D. of three experiments and are plotted as the number of response units obtained from each sensorgram after 3 min post-injection. Results are statistically significant (p < 0.29; multivariant analysis of variance) for differences in response units as toxin concentration is increased. Panel D shows the results of SPR analysis of Cdt (20 μg/ml) with immobilized LUVs containing 20% of either cholesterol, lanosterol, ergosterol, or stigmasterol. The mean ± S.D. of the maximum response is plotted for three experiments. Results are statistically significant for differences between cholesterol and lanosterol (p < 0.001), cholesterol and ergosterol (p = 0.029), and cholesterol and stigmasterol (p = 0.029). To verify that liposomes contained comparable levels of sterol, aliquots of liposomes were extracted, and the amount of sterol was determined as described in Fig. 1; extraction yields were 7.9 μg (lanosterol), 8.6 μg (ergosterol), 7.6 μg (stigmasterol), and 7.8 μg (cholesterol).
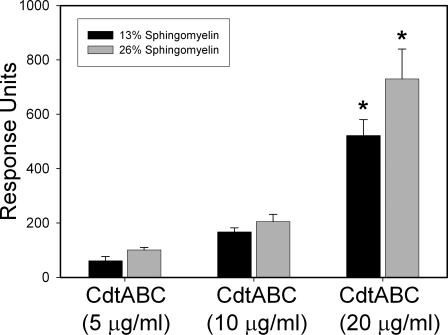
To mimic the association of toxin with lipid rafts, LUVs were prepared with lipid compositions favoring raft formation by increasing SM content from 13 to 26% in the presence of 10% cholesterol. As shown in Fig. 3, Cdt binding to LUV was measured by SPR and found to be dose-dependent; maximum RU for LUV containing 13% SM increased from 61 RU in the presence of 5 μg/ml Cdt to 522 RU in the presence of 20 μg/ml Cdt. There was an increase in toxin binding at all toxin concentrations to LUVs containing 26% SM; at the highest toxin concentration, 20 μg/ml Cdt, we observed a 30% increase in Cdt binding, which was statistically significant. Toxin association with membrane rafts was further investigated using FRET. Energy transfer between DAN-PC and toxin increased as the mole fractions of raft-associated cholesterol increased; that is, 15.3 ± 2.2% energy transfer at 0.56 mol fraction cholesterol versus 6.2 ± 2.4% energy transfer at 0.11 mol fraction cholesterol. The SPR and FRET studies suggest that the Cdt holotoxin favors association with membrane raft-associated cholesterol.
FIGURE 3.
CdtABC preferentially associates with cholesterol and lipid rafts. LUVs were prepared containing either 13% (black bars) or 26% (gray bars) SM along with 20% cholesterol. The LUVs were assessed in real time for binding to immobilized Cdt. The maximum response units (mean ± S.D. of five experiments) is plotted versus Cdt concentration. The asterisk denotes a statistically significant difference between 13 and 26% sphingomyelin (p = 0.040); no significant difference was detected at the lower toxin concentrations.
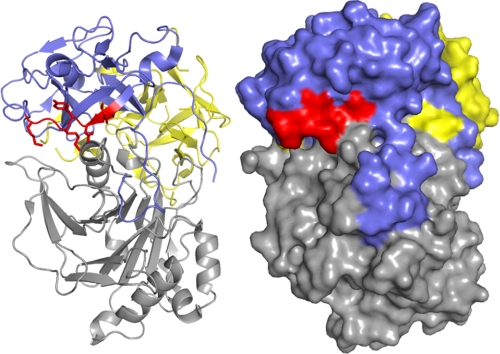
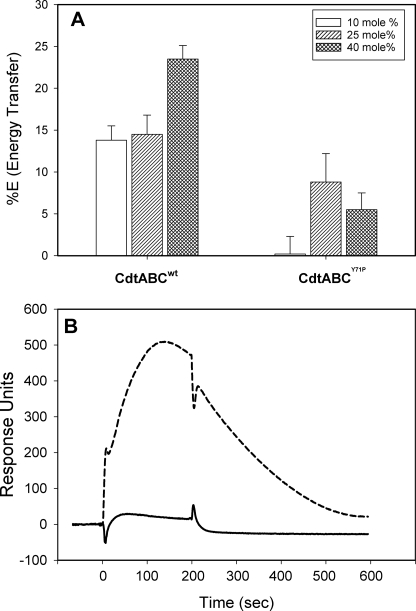
Collectively, our results strongly support the notion that Cdt holotoxin interaction with membranes and cells is dependent upon cholesterol. Therefore, we extended our studies to consider the possibility that binding subunits, CdtA and/or CdtC, contain a cholesterol recognition motif. In this regard it has been reported that several proteins that interact with cholesterol contain an amino acid sequence known as the CRAC region. The CRAC site conforms to the pattern (L/V)X1–5-YX1–5(R/K) in which X1–5 represents between one and five residues of any amino acid (27). Motif analysis of Cdt identified a CRAC site within the CdtC subunit, 68LIDYKGK74. Location of the CRAC site in CdtC is shown in Fig. 4; this site is at the surface of the molecule and, theoretically, accessible to the membrane. To determine whether the CRAC region was required for cell surface interaction and the downstream toxic effects of Cdt, we generated a single-point mutant, CdtABCY71P. It should be noted that the tyrosine residue in this position has been shown by mutational analysis to be critical for cholesterol binding in other proteins (33). We initially employed FRET to determine whether the CdtABCY71P mutant toxin was capable of interacting with cholesterol in model membranes. As shown in Fig. 5A, CdtABCY71P compared with the wild type toxin (CdtABCwt) exhibited a significant reduction in energy transfer between DAN-PC and tryptophan residues intrinsic to the toxin at all concentrations of cholesterol employed. Although energy transfer with CdtABCwt exhibited cholesterol dose dependence, CdtABCY71P did not and is consistent with the reduced binding of the mutant toxin resulting from nonspecific interactions. Furthermore, SPR analysis indicated that the mutant toxin was unable to bind to immobilized LUVs containing cholesterol as compared with CdtABCwt (Fig. 5B); the maximum RU for CdtABCwt was 508 compared with 28 RU for the CRAC mutant.
FIGURE 4.
Localization of the CRAC site on CdtC. Ribbon representation (left) of A. actinomycetemcomitans Cdt holotoxin (PDB accession number 2F2F). CdtC is represented in blue with residues Leu-68—Lys-74 of the CRAC site colored red. CdtA is shown in yellow, and CdtB is shown in gray. Shown is a surface representation (right) of the holotoxin indicating the accessibility of the CRAC site.
FIGURE 5.
Cdt holotoxin containing a CRAC mutation (CdtABCY71P) exhibits reduced ability to associate with cholesterol. Panel A shows the results of the FRET analysis of the ability of CdtABCWT versus that of CdtABCY71P to bind to LUVs containing 10–40 mol % cholesterol. Results are plotted as the percentage of energy transfer versus cholesterol concentration and represent the mean ± S.D. of three experiments. Panel B, SPR analysis of the ability of immobilized CdtABCWT (broken line) and CdtABCY71P (solid line) to bind LUVs containing 20% cholesterol; results are representative of three experiments. Results are statistically significant (p < 0.035) for the differences between CdtABCwt and CdtABCY71P at all concentrations of cholesterol.
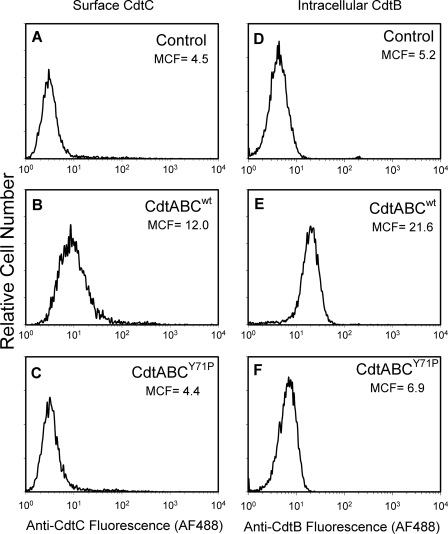
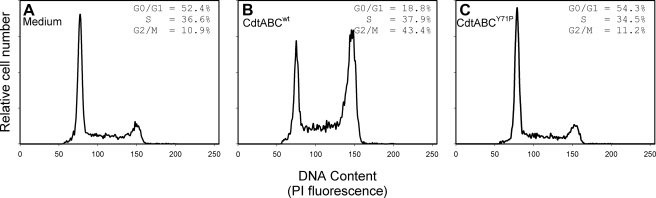
In addition to interaction with model membranes, we employed immunofluorescence and flow cytometry to assess CdtABCY71P for its ability to interact with Jurkat cells. In Fig. 6, A–C, association of the mutant Cdt holotoxin was compared with the wild type toxin by assessing anti-CdtC fluorescence. The MCF for cells treated with CdtABCwt was 12 compared with 4.5 for control cells exposed to medium alone. The CRAC mutant exhibited a significant reduction in its ability to associate with Jurkat cells; cells treated with the CdtABCY71P exhibited a MCF of 4.4. Because it was possible that the decreased immunofluorescence resulted from reduced availability of the epitope as opposed to decreased toxin binding, we also assessed the relative ability of the CdtABCwt and CdtABCY71P to deliver CdtB intracellularly. As shown in Figs. 6, D–F, and as we have previously reported, CdtABCwt was effective in delivering CdtB intracellularly in Jurkat cells; 1 h after exposure to 2 μg/ml toxin, MCF increased from 5.2 in control cells to 21.6. By comparison, the MCF was 6.9 in cells exposed to 2 μg/ml CdtABCY71P. Finally, we assessed the CRAC mutant for its ability to induce G2 arrest in Jurkat cells (Fig. 7). Sixteen hours after exposure to CdtABCwt, we observed an increase in the percentage of G2 cells from 10.9% (control) to 43.4%; this compares to 11.2% in cells similarly treated with CdtABCY71P.
FIGURE 6.
CdtABCY71P exhibits a reduction in association with Jurkat cells as well as in its ability to deliver CdtB. Jurkat cells were exposed to medium (panels A and D), CdtABCWT (panels B and E), or CdtABCY71P (panels C and F) for 1 h and then analyzed by immunofluorescence for the presence of surface CdtC (panels A–C) and intracellular CdtB (panels D–F). Alexafluor fluorescence is plotted versus relative cell number. Numbers represent the mean channel fluorescence; at least 10,000 cells were analyzed per sample. Results are representative of three experiments.
FIGURE 7.
CdtABCY71P exhibits reduced ability to induce cell cycle arrest. Jurkat cells were incubated with medium alone (panel A), 50 pg/ml CdtABCWT (panel B), or 50 pg/ml CdtABCY71P (panel C) for 18 h, stained with propidium iodide, and analyzed for cell cycle distribution by flow cytometry as described under “Experimental Procedures.” Numbers represent the percentage of cells in the G0/G1, S, and G2/M phases of the cell cycle. Results are representative of three experiments; 15,000 cells were analyzed for each sample. PI, propidium iodide.
DISCUSSION
A. actinomycetemcomitans Cdt holotoxin is composed of a heterotrimeric complex consisting of three distinct peptides, designated CdtA, CdtB, and CdtC. Several investigators have demonstrated that CdtB is the active subunit (18, 34–36); our own studies indicate that CdtB exhibits a unique lipid phosphatase activity whereby it de-phosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-triphosphate (23). Although the role for CdtA and CdtC has been elusive, there is compelling evidence that these subunits are involved in forming a stable holotoxin complex and for recognition of cell associated receptors (12, 14, 37, 38). Indeed, our observations as well as those of other investigators support the notion that not only are CdtA and CdtC required for maximum toxin activity but also that they are involved in toxin-cell interaction (18, 26). These observations are also supported by analysis of the crystal structure of both A. actinomycetemcomitans and H. ducreyi Cdt. Nesic et al. (12) showed direct contact between CdtA-CdtB, CdtA-CdtC, and CdtB-CdtC and further that the H. ducreyi tripartite complex shows no signs of oligomerization. Further analysis suggests the CdtA and CdtC are both lectin-like structures, analogous to the B-chain repeats of ricin. CdtA and CdtC together form two surface elements, an aromatic cluster and a deep groove. The Cdt holotoxin structure has been confirmed for A. actinomycetemcomitans Cdt (37), where it has been suggested that CdtA and CdtC form the cap of a “fat stem mushroom.” These investigators further report that CdtA and CdtC have structural features similar to the lectin repeats in ricin, although they cannot be superimposed as a rigid unit over these repeats. It should also be noted that McSweeney and Dreyfus (38) have shown that E. coli Cdt binding to HeLa cells can be blocked by glycoproteins and fucose specific lectins but not simple sugars; these investigators proposed that Cdt binds to HeLa cells via an N-linked fucose-containing structure. These studies, however, could not discriminate between specific inhibition of binding to fucose versus steric hindrance of a distinct receptor located in the proximity of lectin and glycoprotein binding.
Our previous studies clearly indicate that the Cdt holotoxin interacts with the Jurkat cell surface and specifically associates with lipid microdomains where the Cdt subunits initially colocalize with the ganglioside, GM1. We further demonstrated that cholesterol depletion using MβCD protects cells from toxin-induced G2 arrest; thus, lipid raft integrity is necessary for the action of Cdt on target cells. In this study we also provide evidence that cholesterol depletion reduces the ability of Cdt to associate with Jurkat cells. Moreover, cholesterol repletion of MβCD-treated cells restored toxin binding to Jurkat cells to levels greater than that observed in control cells; this increase is consistent with the levels of cholesterol extracted from similarly treated cells. These results suggest that the toxin binding subunits, CdtA and/or CdtC, recognize the sterol, perhaps, in the context of lipid rafts. The possibility that Cdt interacts with cholesterol is further demonstrated by our studies in which we employed model membranes. Clearly, FRET and SPR analysis of Cdt interaction with LUVs was dependent upon the presence and concentration of cholesterol; moreover, toxin recognition of sterols was specific to cholesterol, whereas the toxin failed to bind to LUVs containing lanosterol, ergosterol, and stigmasterol. In other experiments LUVs were prepared in which the SM levels were increased from 13 to 26% to mimic a lipid composition that favors lipid raft formation (29). These experiments demonstrated an increase in Cdt binding as detected by SPR in the presence of 20% cholesterol and further suggest that Cdt binds to cholesterol under conditions where lipid raft formation is favored. These observations clearly establish that not only are lipid rafts involved in Cdt holotoxin-Jurkat cell interactions but also that toxin binding to both cell and model membranes directly involves cholesterol.
Several proteins have been shown to bind cholesterol; these include the benzodiazepine receptor, the human immunodeficiency virus transmembrane protein gp41, and caveolin (27, 33, 39, 40). Each of these cholesterol-binding proteins contain the cholesterol recognition amino acid consensus sequence (CRAC), (L/V)X1–5YX1–5(R/K), where X1–5 represents one to five residues of any amino acid. Based upon the results from our experiments along with the effects of cholesterol depletion of lymphocytes on Cdt binding and toxicity, we analyzed both CdtA and CdtC to determine whether they also contain a CRAC motif. Motif analysis of these subunits identified a CRAC site within the CdtC subunit, 68LIDYKGK74; furthermore, structural analysis of CdtC in the context of the holotoxin indicates that this site is at the surface of the molecule and, theoretically, is accessible to the membrane. Mutation of the tyrosine residue within this motif has resulted in significant reduction in the ability of the holotoxin to interact with LUVs. Moreover, the mutant toxin exhibited reduced binding to Jurkat cells along with a reduced intracellular transfer of CdtB and a concomitant reduction in toxicity. These observations are consistent with those of Jamin et al. (33) who mutated the same residue of the CRAC motif within the benzodiazepine receptor, which also resulted in a loss of cholesterol binding. Collectively, our results provide strong support for the notion that not only is Cdt association with target cells dependent upon the integrity of membrane lipid rafts but, furthermore, that the CdtC subunit binds to cholesterol.
Lipid rafts represent liquid-ordered microdomains that are distributed in the plasma membrane and whose lipid composition and high cholesterol content differs from the rest of the membrane. Thus, a protein such as a bacterial toxin that recognizes cholesterol would be expected to co-localize with lipid rafts; this is consistent with our observations for Cdt (26). Furthermore, association with lipid microdomains provides several advantages for proteins in general and bacterial toxins in particular because these membrane regions serve several functions including triggering internalization and transport of extracellular proteins as well as signaling platforms (41, 42). Moreover, it has become increasingly evident that several pathogens and microbial-derived toxins interact with their target cell via membrane rafts (43, 44). For instance, rafts may provide a mechanism by which receptors are concentrated and thereby promote ligand or pathogen binding. One such example is cholera toxin, which is pentameric and binds to targets cells via the ganglioside GM1. It is likely that cholera toxin simultaneously binds with high affinity to multiple receptors as result of receptor concentration within the raft (44, 45). Likewise, pore-forming toxins such as aerolysin from Aeromonas hydrophila, which binds to glycosylphosphatidylinositol-anchored proteins, utilize the concentrating properties of rafts to facilitate oligomerization, a requisite for channel formation (44, 46). Membrane rafts may also facilitate signaling after the binding of bacterial toxins to raft-associated receptors. In this regard, bacterial lipopolysaccharide (LPS) interacts with rafts via CD14, a glycosylphosphatidylinositol-anchored receptor; LPS binding results in mitogen-activated protein kinase activation and eventually cytokine production. Finally, membrane rafts may serve as entry sites for pathogens; in this regard several pathogens enter host cells in a cholesterol-dependent manner (43, 44). For example, the uptake of E. coli strains which express FimH have been shown to involve cholesterol-rich rafts. Similarly, Shigella invades cells via interaction between the invasin, IpaB, and the raft-associated receptor, CD44 (47). Several enveloped and non-enveloped viruses (for example, SV40, human immunodeficiency virus, and herpes simplex virus) also require lipid rafts for binding or entry by endocytosis (48, 49).
In conclusion, we propose that binding of cholesterol by the CRAC region contained in the CdtC subunit results in the association of the Cdt holotoxin with membrane lipid rafts. It is likely that lipid raft association is critical for the internalization of the active subunit, CdtB, leading to cell cycle arrest and eventual cell death. These studies predict that cholesterol disposition within the membrane influences binding of CdtC to the cell surface; therefore, we propose that CdtC favors raft-associated cholesterol, resulting in localized toxin-rich regions. This association may also be critical to the mode of action of the toxin, thereby allowing it to hijack lipid raft-associated signaling platform(s) and perhaps provide access to pools of inositol 1,4,5-triphosphate. Although these studies do not exclude the possibility of the existence of a second receptor that might be recognized by CdtA, they do clearly demonstrate that disruption of cholesterol binding by either cholesterol depletion or mutation of the CRAC region is sufficient to block Cdt-mediated toxicity in lymphocytes.
Acknowledgments
We thank the School of Dental Medicine Flow Cytometry Core Facility for technical expertise and support of this study.
This work was supported, in whole or in part, by National Institutes of Health Grant DE06014 from the USPHS.
Footnotes
The abbreviations used are: Cdt, cytolethal distending toxin; FRET, fluorescence resonance energy transfer; SPR, surface plasmon resonance; CRAC, cholesterol recognition/interaction amino acid consensus site; LUV, large unilamellar vesicle; DAN-PC, 1-myristoyl-2-[12-[(5-dimethylamino-1-naphthalenesulfonyl)amino]dodecanoyl]-sn-glycero-3-phosphocholine; MβCD, methyl β-cyclodextrin; MCF, mean channel fluorescence; SM, sphingomyelin; RU, response units; Fc, flow cell; GM1, monosialotetrahexosylganglioside.
References
- 1.Comayras, C., Tasca, C., Peres, S. Y., Ducommun, B., Oswald, E., and De Rycke, J. (1997) Infect. Immun. 65 5088-5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuda, J., Fukumoto, M., Takeda, Y., and Nishibuchi, M. (1997) Infect. Immun. 65 428-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuda, J., Kurazono, H., and Takeda, Y. (1995) Microb. Pathog. 18 167-172 [DOI] [PubMed] [Google Scholar]
- 4.Scott, D. A., and Kaper, J. B. (1994) Infect. Immun. 62 244-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickett, C. L., Cottle, D. L., Pesci, E. C., and Bikah, G. (1994) Infect. Immun. 62 1046-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer, M., Bueno, L., Hansen, E., and DiRienzo, J. M. (1999) Infect. Immun. 67 1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickett, C. L., and Whitehouse, C. A. (1999) Trends Microbiol. 7 292-297 [DOI] [PubMed] [Google Scholar]
- 8.Shenker, B. J., and Gray, I. (1976) J. Immunol. Methods 13 161-166 [DOI] [PubMed] [Google Scholar]
- 9.Shenker, B. J., McKay, T. L., Datar, S., Miller, M., Chowhan, R., and Demuth, D. R. (1999) J. Immunol. 162 4773-4780 [PubMed] [Google Scholar]
- 10.Shenker, B. J., Hoffmaster, R. H., McKay, T. L., and Demuth, D. R. (2000) J. Immunol. 165 2612-2618 [DOI] [PubMed] [Google Scholar]
- 11.Shenker, B. J., Hoffmaster, R. H., Zekavat, A., Yamguchi, N., Lally, E. T., and Demuth, D. R. (2001) J. Immunol. 167 435-441 [DOI] [PubMed] [Google Scholar]
- 12.Nesic, D., Hsu, Y., and Stebbins, C. E. (2004) Nature 429 429-433 [DOI] [PubMed] [Google Scholar]
- 13.De Rycke, J., and Oswald, E. (2001) FEMS Microbiol. Lett. 203 141-148 [DOI] [PubMed] [Google Scholar]
- 14.Thelastam, M., and Frisan, T. (2004) Rev. Physiol. Biochem. Pharmacol. 152 111-133 [DOI] [PubMed] [Google Scholar]
- 15.Shenker, B. J., Demuth, D. R., and Zekavat, A. (2006) Infect. Immun. 74 2080-2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielaszewska, M., Sinha, B., Kuczius, T., and Karch, H. (2005) Infect. Immun. 73 552-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohara, M., Hayashi, T., Kusonoki, Y., Miyauchi, M., Takata, T., and Sugai, M. (2004) Infect. Immun. 72 871-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shenker, B. J., Besack, D., McKay, T. L., Pankoski, L., Zekavat, A., and Demuth, D. R. (2005) J. Immunol. 174 2228-2234 [DOI] [PubMed] [Google Scholar]
- 19.Lara-Tejero, M., and Galan, J. E. (2001) Infect. Immun. 69 4358-4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elwell, C. A., Chao, K., Patel, K., and Dreyfus, L. A. (2001) Infect. Immun. 69 3418-3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes-Bratti, X., Chaves-Olarte, E., Lagergard, T., and Thelastam, M. (2000) Infect. Immun. 68 6903-6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McSweeney, L., and Dreyfus, L. A. (2004) Cell. Microbiol. 6 447-458 [DOI] [PubMed] [Google Scholar]
- 23.Shenker, B. J., Dlakic, M., Walker, L., Besack, D., Jaffe, E., Labelle, E., and Boesze-Battaglia, K. (2007) J. Immunol. 178 5099-5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlakic, M. (2001) Science 291 547. [DOI] [PubMed] [Google Scholar]
- 25.Dlakic, M. (2000) Trends Biochem. Sci. 25 272-273 [DOI] [PubMed] [Google Scholar]
- 26.Boesze-Battaglia, K., Besack, D., McKay, T. L., Zekavat, A., Otis, L., Jordan-Sciutto, K., and Shenker, B. J. (2006) Cell. Microbiol. 8 823-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, H., and Papadopoulos, V. (1998) Endocrinology 139 4991-4997 [DOI] [PubMed] [Google Scholar]
- 28.Shenker, B. J., Besack, D., McKay, T. L., Pankoski, L., Zekavat, A., and Demuth, D. R. (2004) J. Immunol. 172 410-417 [DOI] [PubMed] [Google Scholar]
- 29.Hekman, M., Hamm, H., Villar, A., Bader, B., Kuhlmann, J., Nickel, J., and Rapp, U. R. (2002) J. Biol. Chem. 277 24090-24102 [DOI] [PubMed] [Google Scholar]
- 30.Buboltz, J., and Feigenson, G. (1999) Biochem. Biophys. Res. Commun. 1417 232-245 [DOI] [PubMed] [Google Scholar]
- 31.Savitsky, A., and Golay, J. (1964) Anal. Chem. 36 1627-1639 [Google Scholar]
- 32.Lafkowicz, J. (1999) Principles of Fluorescence Spectroscopy, pp. 345-384, Plenum Press, New York
- 33.Jamin, N., Neumann, J., Ostuni, M., Vu, T., Yao, Z., Murail, S., Robert, J., Fiatzakis, C., Papadopoulos, V., and Lacapere, J. (2005) Mol. Endocrinol. 19 588-594 [DOI] [PubMed] [Google Scholar]
- 34.Elwell, C. A., and Dreyfus, L. A. (2000) Mol. Microbiol. 37 952-963 [DOI] [PubMed] [Google Scholar]
- 35.Lara-Tejero, M., and Galan, J. E. (2000) Science 290 354-357 [DOI] [PubMed] [Google Scholar]
- 36.Guerra, L., Teter, K., Lilley, B., Stenerlow, B., Holmes, R., Ploegh, H., Sandvik, J. A., Thelastam, M., and Frisan, T. (2005) Cell. Microbiol. 7 921-934 [DOI] [PubMed] [Google Scholar]
- 37.Yamada, T., Komoto, J., Saiki, K., Konishi, K., and Takusagawa, F. (2006) Protein Sci. 15 362-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McSweeney, L., and Dreyfus, L. A. (2005) Infect. Immun. 73 2051-2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epand, R., Sayer, B., and Epand, R. (2005) J. Mol. Biol. 345 339-350 [DOI] [PubMed] [Google Scholar]
- 40.Vincent, N., Genin, C., and Malvoisin, E. (2002) Biochim. Biophys. Acta 1567 157-164 [DOI] [PubMed] [Google Scholar]
- 41.Dykstra, M., Cherukuri, A., Sohn, H., Tzeng, S., and Pierce, S. (2003) Annu. Rev. Immunol. 21 457-481 [DOI] [PubMed] [Google Scholar]
- 42.Cherukuri, A., Dykstra, M., and Pierce, S. (2001) Immunity 14 657-660 [DOI] [PubMed] [Google Scholar]
- 43.Lencer, W. (2001) Am. J. Physiol. Gastrointest. Liver Physiol. 280 781-786 [DOI] [PubMed] [Google Scholar]
- 44.van der Goot, F. G., and Harder, T. (2001) Semin. Immunol. 13 89-97 [DOI] [PubMed] [Google Scholar]
- 45.Montecucco, C., Papini, E., and Schiavo, G. (1994) FEBS Lett. 3466 92-98 [DOI] [PubMed] [Google Scholar]
- 46.Abrami, L., and van der Goot, F. G. (1999) J. Cell Biol. 147 175-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafont, F., Tran Van Nhieu, G., Hanada, K., Sansonetti, P., and van der Goot, F. G. (2002) EMBO J. 21 4449-4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender, F. C., Whitbeck, J. C., Ponce de Leon, M., Lou, H., Eisenberg, R. J., and Cohen, G. (2003) J. Virol. 77 9542-9552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chazal, N., and Gerlier, D. (2003) Microbiol. Mol. Biol. Rev. 67 226-237 [DOI] [PMC free article] [PubMed] [Google Scholar]