Abstract
To ascertain the identities of cyclic nucleotide-binding proteins that mediate the insulin secretagogue action of cAMP, the possible contributions of the exchange protein directly activated by cAMP (Epac) and protein kinase A (PKA) were evaluated in a pancreatic beta cell line (rat INS-1 cells). Assays of Rap1 activation, CREB phosphorylation, and PKA-dependent gene expression were performed in combination with live cell imaging and high throughput screening of a fluorescence resonance energy transfer-based cAMP sensor (Epac1-camps) to validate the selectivity with which acetoxymethyl esters (AM-esters) of cAMP analogs preferentially activate Epac or PKA. Selective activation of Epac or PKA was achieved following exposure of INS-1 cells to 8-pCPT-2′-O-Me-cAMP-AM or Bt2cAMP-AM, respectively. Both cAMP analogs exerted dose-dependent and glucose metabolism-dependent actions to stimulate insulin secretion, and when each was co-administered with the other, a supra-additive effect was observed. Because 2.4-fold more insulin was secreted in response to a saturating concentration (10 μm) of Bt2cAMP-AM as compared with 8-pCPT-2′-O-Me-cAMP-AM, and because the action of Bt2cAMP-AM but not 8-pCPT-2′-O-Me-cAMP-AM was nearly abrogated by treatment with 3 μm of the PKA inhibitor H-89, it is concluded that for INS-1 cells, it is PKA that acts as the dominant cAMP-binding protein in support of insulin secretion. Unexpectedly, 10–100 μm of the non-AM-ester of 8-pCPT-2′-O-Me-cAMP failed to stimulate insulin secretion and was a weak activator of Rap1 in INS-1 cells. Moreover, 10 μm of the AM-ester of 8-pCPT-2′-O-Me-cAMP stimulated insulin secretion from mouse islets, whereas the non-AM-ester did not. Thus, the membrane permeability of 8-pCPT-2′-O-Me-cAMP in insulin-secreting cells is so low as to limit its biological activity. It is concluded that prior reports documenting the failure of 8-pCPT-2′-O-Me-cAMP to act in beta cells, or other cell types, need to be re-evaluated through the use of the AM-ester of this cAMP analog.
Epac1 and Epac2 are guanine nucleotide exchange factors activated by adenosine-3′,5′-cyclic monophosphate (cAMP), and which are known to be expressed in numerous mammalian cell types (1, 2). An accumulating body of evidence indicates that the existence of Epac may explain novel protein kinase A (PKA)2 independent actions of cAMP that underlie cellular responsiveness to hormones, neurotransmitters, and pharmacological agents of therapeutic importance (3). Selective activation of Epac may be achieved through the use of 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, also known as 8-pCPT-2′-O-Me-cAMP (4, 5). This cAMP analog, which incorporates a 2′-O-methyl group on the ribose ring of the nucleotide, as well as a 4-chlorophenylthio group on position 8 of the adenine moiety, acts as a “superactivator” of Epac while having a greatly diminished ability to activate PKA (4). Thus, 8-pCPT-2′-O-Me-cAMP is an Epac-selective cAMP analog (ESCA) (6).
8-pCPT-2′-O-Me-cAMP can cross the plasma membrane and is able to alter diverse cellular functions that include Rap1 GTPase activity, PKB, and ERK1/2 protein kinase activity, phospholipase Cε activity, Ca2+ signaling, ion channel activity, exocytosis, cell adhesion, and gene expression (7–9). Although no selective antagonist of Epac activation exists, these effects of 8-pCPT-2′-O-Me-cAMP are believed to be Epac-mediated because they are observed under conditions in which PKA activity is blocked, whereas they are reduced or eliminated when Epac gene expression is down-regulated. Furthermore, such actions of 8-pCPT-2′-O-Me-cAMP are measurable in cells that do not express the cyclic nucleotide-regulated ion channels that constitute an alternative target of cAMP action.
Interestingly, published findings exist in which cAMP-elevating agents were found to exert actions not attributable to their effects at PKA or cyclic nucleotide-regulated ion channels, and which were not replicated upon administration of 8-pCPT-2′-O-Me-cAMP (10–14). Thus, there may exist actions of cAMP that are independent of the known cAMP-binding proteins. Alternatively, it is possible that in some cell types, access of 8-pCPT-2′-O-Me-cAMP to the cytosol is so poor that it limits its ability to activate Epac. If so, prior reports concerning the failure of 8-pCPT-2′-O-Me-cAMP to influence various cellular functions need to be re-evaluated, possibly through use of an acetoxymethyl ester (AM-ester) of 8-pCPT-2′-O-Me-cAMP that was recently synthesized by Vliem and co-workers (15). This ESCA (8-pCPT-2′-O-Me-cAMP-AM) has greater membrane permeability and it is metabolized by intracellular esterases, thereby generating the free biologically active 8-pCPT-2′-O-Me-cAMP.
In the present study we have used a cAMP reporter (Epac1-camps) (16) that exhibits a decrease of fluorescence resonance energy transfer (FRET) when it binds to cAMP (or various cAMP analogs), to demonstrate a surprisingly poor membrane permeability of the non-AM-ester of 8-pCPT-2′-O-Me-cAMP in rat INS-1 cells (an insulin-secreting cell line used as a model system for the study of pancreatic beta cell function) (17). We also report that the AM-ester of 8-pCPT-2′-O-Me-cAMP has improved membrane permeability while retaining Epac selectivity, as demonstrated in assays of INS-1 cell Rap1 activation, CREB phosphorylation, and PKA-dependent gene expression. Furthermore, we report that although 8-pCPT-2′-O-Me-cAMP is known to exhibit low-affinity binding to the regulatory subunits of PKA in vitro (5), treatment of INS-1 cells with the AM-ester of 8-pCPT-2′-O-Me-cAMP does not interfere with the ability of endogenous cAMP to activate PKA-dependent gene expression that is under the control of a cyclic AMP response element (CRE). By comparing the insulin secretagogue action of 8-pCPT-2′-O-Me-cAMP-AM with that of the PKA-selective cAMP analog N6,2′-O-dibutyryl adenosine 3′,5′-cyclic monophosphate-AM (Bt2cAMP-AM), we have established the relative importance of Epac and PKA to the cAMP-dependent stimulation of insulin secretion from INS-1 cells. We conclude that prior reports documenting the failure of 8-pCPT-2′-O-Me-cAMP to act in beta cells, or other cell types, need to be re-evaluated through the use of the AM-ester of this cAMP analog.
EXPERIMENTAL PROCEDURES
Cell Culture—INS-1 cells (passage numbers 70–90) were maintained in a humidified incubator (95% air, 5% CO2) at 37 °C in RPMI 1640 medium containing 10 mm Hepes, 11.1 mm glucose, 10% fetal bovine serum, 100 units ml-1 penicillin G, 100 μgml-1 streptomycin, 2.0 mm l-glutamine, 1.0 mm sodium pyruvate, and 50 μm 2-mercaptoethanol (17). INS-1 cells were passaged by trypsinization and subcultured once a week. Cell culture reagents were obtained from Invitrogen.
Live Cell Imaging of Epac1-camps—INS-1 cells adherent to glass coverslips were imaged after transient transfection and expression of a cAMP FRET reporter (Epac1-camps) that incorporates the cyclic nucleotide-binding domain of Epac1 flanked at its N terminus by yellow fluorescent protein (YFP) and at its C terminus by cyan fluorescent protein (CFP) (16). The cells were bathed in a standard extracellular saline (SES) solution containing (in mm): 138 NaCl, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 11.1 glucose, and 10 Hepes (295 mosmol, pH 7.4). Imaging of cells was performed using a Nikon TE-300 inverted microscope equipped with a N.A. 1.45 TIRF objective (×60, Nikon), a CARV2 filter changer under computer control (BD Bioscience), an X-CITE light source with a mercury vapor short arc lamp (EXFO), and a Cascade 512b EMCCD camera (Roper Scientific). Ratiometric analysis of the emitted light corresponding to fluorescence originating within a defined region of the cytoplasm was performed using a Chameleon-2 filter set (Chroma Technology Corp.) comprised of a D440/20 excitation filter, a 455DCLP dichroic, and D485/40 (CFP) or D535/30 (YFP) emission filters. Images were acquired and processed using Metafluor version 7.5 software (Molecular Devices). Detailed methods of live cell FRET imaging using this reporter are provided in our prior collaborative reports (18, 19).
High Throughput Assay of Epac1-camps—An INS-1 cell clone (C-10) stably expressing Epac1-camps was generated by G418 selection after transfection of cells with the Epac1-camps coding sequence in pcDNA3.1 (16). Cells were plated at 80% confluence on 96-well clear bottom assay plates (Costar 3904) and maintained in culture medium overnight prior to their use. Assays were performed using a FlexStation 3 microplate reader equipped with excitation and emission monochromators, and controlled using SoftMax Pro software (Molecular Devices). After replacement of the culture medium with 170 μl/well of the SES solution, the excitation light was delivered at 435/9 nm (455 nm cut-off), and the emitted light was detected at 485/15 nm (CFP) or 535/15 nm (YFP). The excitation light source was a Xenon flash lamp, and the emission intensities were the average of 12 excitation flashes for each time point. Test solutions comprised of cAMP analogs dissolved in the SES solution containing 0.1% dimethyl sulfoxide were placed in V-bottom 96-well plates (Greiner) and an automated pipetting procedure was used to transfer 30 μl of each test solution to the assay plate containing the cells. The test solutions were injected into each well at a pipette height that corresponded to a fluid level of 150 μl, and the rate of injection was 31 μl/s. The CFP/YFP emission ratio was calculated for each well, and values for 24–36 wells were averaged. The time course of the change of FRET ratio was then plotted after exporting these data to Origin version 7.5 (OriginLab).
Live Cell Imaging of Epac1-EGFP—INS-1 cells were transfected with a construct directing expression of a protein comprised of human Epac1 fused to EGFP at its C terminus (20). Clones of cells stably expressing this reporter were obtained by G418 selection. Live cell imaging and data analysis was performed using a LSM510 laser-scanning confocal microscope, a ×100 objective, and AxioVision 4.30 software (Zeiss).
Rap1 Activation Assay—Activation of Rap1 by cAMP-elevating agents was assayed by Western blot analysis using lysates derived from INS-1 cells transiently transfected with FLAG epitope-tagged Rap1 and Epac1 using Lipofectamine Plus reagent (Invitrogen). The methods used in this Ral-GDS-RBD-GST pull-down assay employing glutathione-conjugated agarose beads are described elsewhere (1). The activated Rap1-GTP and the total Rap1 were detected using an anti-FLAG M2 monoclonal antibody conjugated to horseradish peroxidase (Sigma, catalog number A8592).
CREB Phosphorylation Assay—The phosphorylated form of CREB was assayed by Western blot analysis using an anti-phospho-Ser133 CREB polyclonal antiserum (Cell Signaling Technology, catalog number 9191). Immunoreactivity corresponding to the phosphorylated and non-phosphorylated forms of CREB (i.e. total CREB) was measured using a monoclonal 48H2 antibody (Cell Signaling Technology, catalog number 9197). An horseradish peroxidase-conjugated secondary antiserum (Sigma, catalog number A6667) was used in combination with these two primary antisera. Immunoreactivity was imaged using a SuperSignal West Pico ECL detection system (Thermo Science) and a ChemiDoc XRS gel documentation system (Bio-Rad).
CRE-Luc Reporter Gene Assay—RIP1-CRE-Luc comprised of a 4-fold multimerized CRE found within the rat insulin I gene promoter, and fused to the coding sequence of firefly luciferase in pLuc-MCS (21), was introduced into INS-1 cells by transient transfection. After overnight incubation in cell culture medium, the cells were exposed for 4 h to serum-free medium containing 0.1% bovine serum albumin and the indicated test substances. Cells were lysed and assayed for luciferase-catalyzed photoemissions using a luciferase assay kit (Promega) and a FlexStation 3 microplate reader in the luminometer mode (Molecular Devices).
Insulin Secretion Assay—INS-1 cells were plated at a density of 100,000 cells/well onto 96-well plates (Costar) after coating the plates with rat tail collagen (Collaborative Biomedical Products). After overnight equilibration in culture medium, the medium was replaced with 100 μl/well Krebs-Ringer buffer (KRBH) containing test substances. After 30 min incubation at 37 °C, the buffer was collected, cellular debris was removed by centrifugation, and the insulin content determined by radioimmunoassay (Millipore, Sensitive Rat Insulin RIA, catalog number SRI-13K) or by an enzyme-linked immunosorbent assay (Mercodia Ultrasensitive Rat Insulin ELISA, catalog number 10-1137-01). The amount of insulin in each sample was determined by comparison with a standard curve generated using calibrated samples of rat insulin provided with these kits. Assays were performed in duplicate.
Analysis of Data—The repeatability of all findings presented here was confirmed by performing each experiment a minimum of two times. Values of fold-stimulation correspond to measurements obtained in a single experiment. Statistical analysis was performed using Student's paired t test and SigmaStat (Systat Software, Inc.). A p value of <0.05 was considered significant.
Sources and Preparation of Reagents—cAMP analogs and phosphate-AM3 were synthesized by BIOLOG Life Science Institute (Germany). All AM-esters were applied under serum-free conditions to avoid extracellular hydrolysis. H-89, forskolin, and isobutylmethylxanthine were from Sigma. Epac1-camps in pcDNA3.1 was from M. Lohse (Germany). FLAG-Rap1 in pCMV2 was from L. Quilliam (United States). RalGDS-RBD-GST in pGEX was from J. L. Bos (Netherlands). Epac1-EGFP in pEGFP-N3 was from X. Cheng (United States). FLAG-Epac1 in pCMV2 was generated in our laboratory using the human Epac1 cDNA (1).
RESULTS
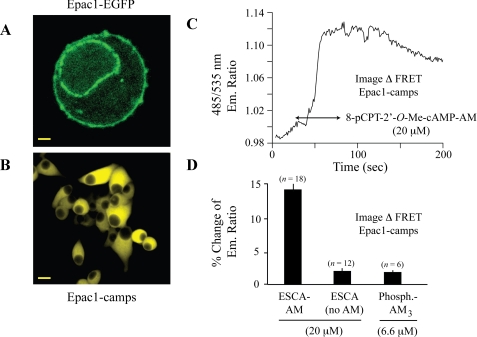
The AM-ester of 8-pCPT-2′-O-Me-cAMP Activates Epac1-camps in INS-1 Cells—The biochemical properties of ESCAs are measurable through the use of a genetically encoded biosensor in which the cyclic nucleotide-binding domain of Epac1 is fused to YFP and CFP. This biosensor, designated as Epac1-camps, exhibits a decrease of FRET when it binds to cAMP or various cAMP analogs (16). Epac1-camps reported global changes of [cAMP] in the cytosol of INS-1 cells because unlike full-length Epac1 that was expressed at the plasma membrane and perinuclear membrane (Fig. 1A), Epac1-camps was homogeneously distributed within the cytoplasm, with nuclear exclusion clearly evident (Fig. 1B). When 8-pCPT-2′-O-Me-cAMP-AM was applied to the extracellular surface of individual INS-1 cells expressing Epac1-camps, resultant activation of the biosensor could be monitored in real time by performing spectrofluorimetry in combination with live cell imaging. Thus, a 50-s application of 8-pCPT-2′-O-Me-cAMP-AM (20 μm) produced a decrease of FRET that was measured as an increase of the CFP/YFP (485/535 nm) emission ratio (Fig. 1, C and D). Remarkably, no such increase of emission ratio was measured using an equivalent concentration of the non-AM-ester of 8-pCPT-2′-O-Me-cAMP (Fig. 1D). Furthermore, application of 6.6 μm phosphate-AM3, a control AM-ester that liberates 3 mol eq of acetic acid and formaldehyde per mol of phosphate when it is hydrolyzed by intracellular esterases, failed to produce a change of FRET (Fig. 1D). Such findings indicate that the AM-ester substitution of 8-pCPT-2′-O-Me-cAMP dramatically improved its membrane permeability, thereby allowing it to activate Epac1-camps in INS-1 cells.
FIGURE 1.
8-pCPT-2′-O-Me-cAMP-AM activates the cAMP reporter Epac1-camps. A, expression of a fusion protein of Epac1 and EGFP in stably transfected INS-1 cells (calibration bar, 0.8 μm). B, stable expression of Epac1-camps in INS-1 cells (calibration bar, 3 μm). C, FRET-based live cell imaging of Epac1-camps in INS-1 cells exposed to 8-pCPT-2′-O-Me-cAMP-AM (20 μm; arrows indicate duration of exposure). D, single cell population study summarizing the action of 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM; 20 μm) to activate Epac1-camps in INS-1 cells. Note that both the non-AM-ester of 8-pCPT-2′-O-Me-cAMP (ESCA, no AM;20 μm) and acetoxymethyl ester phosphate (Phosphate-AM3; 6.6 μm) were without significant effect. All solutions were administered in SES containing 0.1% dimethyl sulfoxide. Values above each histogram bar indicate the numbers of cells (n values) assayed for each experimental condition. For panels C and D, the experiment was repeated 3 times with similar results.
The dose dependence with which 8-pCPT-2′-O-Me-cAMP-AM activates Epac1-camps may be determined using a high throughput assay in which INS-1 cells stably expressing the biosensor are monitored using a plate-reading spectrofluorimeter equipped with single excitation and dual emission wavelength monochromators. Under these conditions, application of 0.3 μm 8-pCPT-2′-O-Me-cAMP-AM to an INS-1 cell monolayer produced a significant increase of the 485/535 nm emission ratio within 100 s following its application (Fig. 2A). Higher concentrations of 8-pCPT-2′-O-Me-cAMP-AM increased both the magnitude and rate of onset of this response (Fig. 2A). The non-AM-ester of 8-pCPT-2′-O-Me-cAMP was considerably less potent in this assay because no increase of emission ratio was measured in response to 10 μm 8-pCPT-2′-O-Me-cAMP. However, a dose-dependent action of 8-pCPT-2′-O-Me-cAMP was apparent when it was tested at 30 and 100 μm (Fig. 2B). Despite this fact, the change of emission ratio measured in response to 100 μm 8-pCPT-2′-O-Me-cAMP was considerably smaller than that which was measured in response to 1.0 μm 8-pCPT-2′-O-Me-cAMP-AM (Fig. 2B). Although not illustrated, the activation of Epac1-camps by 8-pCPT-2′-O-Me-cAMP-AM was unaffected by treatment of INS-1 cells with 10 μm H-89, an isoquinolinesulfonamide that is a moderately selective inhibitor of PKA (22).
FIGURE 2.
High throughput assay for activation of Epac1-camps by cAMP analogs. A, concentration-response relationship for the activation of Epac1-camps by 0.3–3.0 μm 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM). The vehicle solution was comprised of SES containing 0.1% dimethyl sulfoxide. A vertical arrow indicates the time at which the test solutions were injected. An increase of 485/535 nm emission ratio signifies the activation of Epac1-camps. B, differential potencies of 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM, blue line and symbols) and 8-pCPT-2′-O-Me-cAMP (ESCA-No-AM, red line and symbols) to activate Epac1-camps. For panels A and B, each data point corresponds to the mean of 24–36 wells, and each experiment was repeated 3 times with similar results.
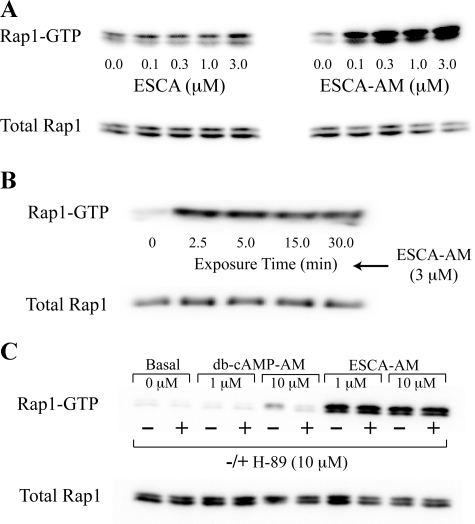
Activation of Rap1 by 8-pCPT-2′-O-Me-cAMP-AM Is Dose- and Time-dependent—The dose and time dependence with which an ESCA activates Epac may also be indirectly ascertained through the use of a Rap1 activation assay (1). In INS-1 cells expressing recombinant human Epac1 and FLAG epitope-tagged Rap1, a 30-min treatment with the non-AM-ester of 8-pCPT-2′-O-Me-cAMP (0.1–3.0 μm) produced a weak activation of Rap1 (Fig. 3A, left panel). However, a stronger and dose-dependent activation of Rap1 was measured in response to equivalent concentrations of 8-pCPT-2′-O-Me-cAMP-AM (Fig. 3A, right panel). This effect of 8-pCPT-2′-O-Me-cAMP-AM was measured within 2.5 min following exposure of INS-1 cells to the ESCA (Fig. 3B).
FIGURE 3.
Differential Rap1 activation properties of cAMP analogs. A, Western blot analysis demonstrating the weak effect of 8-pCPT-2′-O-Me-cAMP (ESCA, left panel) and the strong effect of 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM, right panel) to activate Rap1 in lysates prepared from monolayers of INS-1 cells transfected with FLAG-Rap1 and Eapc1. Active Rap1 is labeled as Rap1-GTP and the quantity of active Rap1 was assessed by densitometry. For the left panel, treatment with 3.0 μm 8-pCPT-2′-O-Me-cAMP increased the amount of active Rap1 by 1.41-fold relative to the untreated control value. For the right panel, treatment with 3.0 μm 8-pCPT-2′-O-Me-cAMP-AM increased the amount of active Rap1 by 4.79-fold relative to the untreated control value. B, time course of Rap1 activation by 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM). The exposure time corresponds to the time in which INS-1 cells were exposed to the test solution. C, contrasting actions of PKA-selective Bt2cAMP-AM (db-cAMP-AM) and Epac-selective 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM) to activate Rap1 under conditions in which INS-1 cells were or were not pretreated with the PKA inhibitor H-89 (10 μm). For panels A-C, each experiment was repeated 3 times with similar results.
Given that prior studies of other cell types have linked increased PKA activity to Rap1 activation (23), we sought to determine whether the Rap1 activation function of 8-pCPT-2′-O-Me-cAMP-AM described here for INS-1 cells was reproduced by the AM-ester of N6,2′-O-dibutyryl-cAMP (Bt2cAMP-AM), which exhibits PKA selectivity as a consequence of its substitution at position 6 on the adenine moiety of cAMP (24–26). We found that treatment of INS-1 cells with Bt2cAMP-AM resulted in a weaker activation of Rap1, even when the concentration of Bt2cAMP-AM was raised to 10 μm (Fig. 3C). This effect of Bt2cAMP-AM was mediated by PKA rather than by Epac because Bt2cAMP-AM failed to activate Rap1 when INS-1 cells were pretreated with the PKA inhibitor H-89 (Fig. 3C). In contrast, the ability of 8-pCPT-2′-O-Me-cAMP-AM to activate Rap1 was unaffected by H-89 (Fig. 3C). Thus, 8-pCPT-2′-O-Me-cAMP-AM exerted a potent dose- and time-dependent action to activate Rap1, an effect that was mediated by Epac. However, Bt2cAMP-AM acted through PKA rather than Epac to activate Rap1.
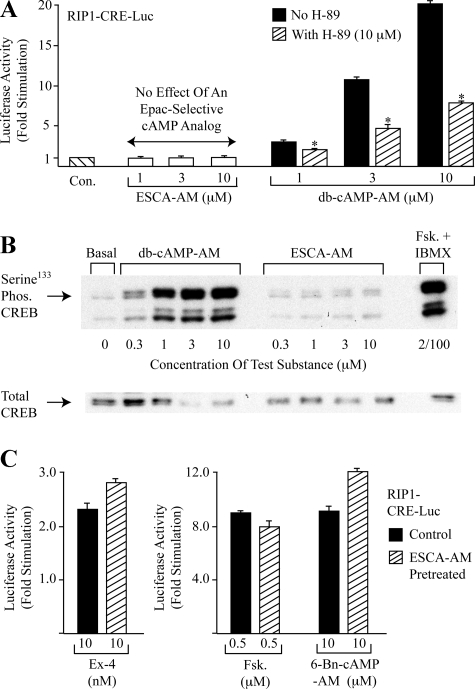
8-pCPT-2′-O-Me-cAMP-AM Fails to Stimulate PKA-dependent Gene Expression—Although prior in vitro studies demonstrated the selectivity with which the non-AM-ester of 8-pCPT-2′-O-Me-cAMP activates Epac (4, 5, 26), concern exists that this cAMP analog might have the unwanted side effect to indirectly activate PKA in living cells. This concern is prompted by the finding that 8-pCPT-2′-O-Me-cAMP acts in vitro to inhibit the activity of certain isoforms of cyclic nucleotide phosphodiesterases (PDEs) (5, 27). Thus, under conditions in which PDEs bind 8-pCPT-2′-O-Me-cAMP, the hydrolysis of endogenous cAMP in living cells might be slowed, thereby leading to increased levels of cAMP with accompanying activation of PKA. For INS-1 cells, such a confounding effect does not appear to be of major importance because when these cells were transfected with a PKA-activated luciferase reporter (RIP1-CRE-Luc) in which luciferase expression was placed under the control of a multimerized CRE present within the rat insulin I gene promoter (RIP1) (21), 8-pCPT-2′-O-Me-cAMP-AM failed to stimulate luciferase expression (Fig. 4A). In these same cells the PKA-selective cAMP analog Bt2cAMP-AM (1–10 μm) stimulated luciferase expression up to 20-fold, and this effect was reduced by the PKA inhibitor H-89 (Fig. 4A).
FIGURE 4.
cAMP analogs differentially regulate RIP-CRE-Luc and CREB. A, 8-pCPT-2′-O-Me-cAMP-AM (ESCA-AM) failed to stimulate luciferase activity in lysates prepared from INS-1 cells expressing RIP1-CRE-Luc. Equivalent concentrations of Bt2cAMP-AM (db-cAMP-AM) stimulated luciferase activity, and this effect was antagonized by 10 μm of the PKA inhibitor H-89 (*, p < 0.05). Results are the average of three experiments and each data point within a single experiment was the average of two determinations. B, Western blot analysis of untransfected INS-1 cells demonstrated increased Ser133 CREB phosphorylation after treatment of the cells for 30 min with Bt2cAMP-AM (db-cAMP-AM) or a combination of forskolin (Fsk., 2 μm) and isobutylmethylxanthine (IBMX, 100 μm). No such effect was observed in response to 8-pCPT-2′-O-Me-cAMP-AM. Similar findings were obtained in 4 experiments. C, pretreatment of INS-1 cells with 8-pCPT-2′-O-Me-cAMP-AM did not reduce the stimulatory actions of exendin-4 (Ex-4), forskolin (Fsk.), or a PKA-selective cAMP analog (6-Bn-cAMP) in assays of RIP1-CRE-Luc-dependent luciferase expression in INS-1 cells. Results are the average of three experiments and each data point within a single experiment was the mean of two determinations.
Because it is the PKA-phosphorylated form of transcription factor CREB that binds to the CRE and which transactivates gene expression (28), the differential efficacies of Bt2cAMP-AM and 8-pCPT-2′-O-Me-cAMP-AM in these assays of RIP1-CRE-Luc activity might be explained by an ability, or lack of ability, of the cAMP analogs to promote CREB phosphorylation. This was the case because we found that in INS-1 cells, Bt2cAMP-AM (0.3–10 μm) promoted Ser133 phosphorylation of CREB, whereas no such effect was observed using 8-pCPT-2′-O-Me-cAMP-AM (Fig. 4B, Ser133 is the residue at which PKA phosphorylates CREB) (28). As expected, the phosphorylation of CREB was also increased by the adenylyl cyclase activator forskolin administered with the PDE inhibitor isobutylmethylxanthine (Fig. 4B).
8-pCPT-2′-O-Me-cAMP-AM Does Not Prevent PKA Activation by Endogenous cAMP—Despite the selectivity with which 8-pCPT-2′-O-Me-cAMP-AM activates Epac, it is possible that this ESCA might also exhibit low affinity binding to the regulatory subunits of PKA (26), thereby acting as an antagonist to prevent activation of PKA by endogenous cAMP. This was not the case for INS-1 cells because a 15-min pretreatment with 8-pCPT-2′-O-Me-cAMP-AM (10 μm) failed to reduce RIP1-CRE-Luc activation by the glucagon-like peptide-1 receptor agonist exendin-4 (Fig. 4C, left panel). Similarly, pretreatment with 8-pCPT-2′-O-Me-cAMP-AM failed to reduce RIP1-CRE-Luc activation by forskolin or N6-benzyl-cAMP-AM (Fig. 4C, right panel). Exendin-4 and forskolin are stimulators of cAMP production in INS-1 cells (29–32), whereas 6-benzyl-cAMP-AM is the AM-ester of a PKA-selective cAMP analog (33).
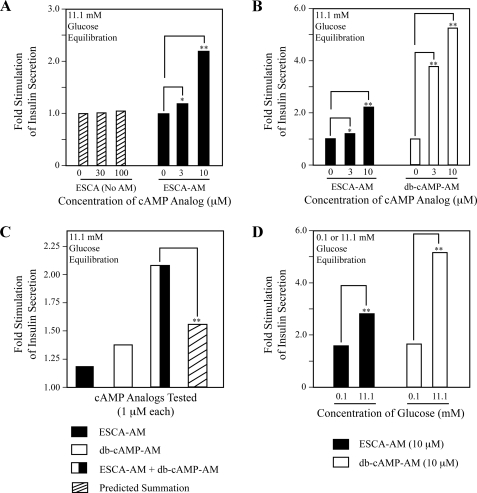
Insulin Secretagogue Properties of Epac- and PKA-selective cAMP Analogs—Under conditions in which INS-1 cells were equilibrated in KRBH containing the metabolizable sugar d-glucose, it was possible to study the relative contributions of Epac and PKA to the cAMP-dependent potentiation of glucose-stimulated insulin secretion (34, 35). In the present study, and contrary to prior reports (36, 37), we found that the non-AM-ester of 8-pCPT-2′-O-Me-cAMP failed to stimulate insulin secretion from INS-1 cells equilibrated in buffer containing 11.1 mm glucose (Fig. 5A). However, a dose-dependent stimulation of insulin secretion was observed using 1–10 μm 8-pCPT-2′-O-Me-cAMP-AM (Fig. 5A). Increasing the concentration of 8-pCPT-2′-O-Me-cAMP-AM to 20 μm did not produce an additional effect, and phosphate-AM3 was without effect when administered at a concentration of 3.3 μm (data not shown).
FIGURE 5.
Secretagogue properties of cAMP analogs in INS-1 cells. A, differential actions of 8-pCPT-2′-O-Me-cAMP and 8-pCPT-2′-O-Me-cAMP-AM under conditions in which cells were equilibrated for 30 min in buffer containing 11.1 mm glucose. B, differential actions of Bt2cAMP-AM (db-cAMP-AM) and 8-pCPT-2′-O-Me-cAMP-AM. Note that the data for 8-pCPT-2′-O-Me-cAMP-AM presented in A were re-scaled on the y axis of panel B. C, synergistic interaction of Bt2cAMP-AM (db-cAMP-AM) and 8-pCPT-2′-O-Me-cAMP-AM (1 μm each) to stimulate insulin secretion. D, the actions of 8-pCPT-2′-O-Me-cAMP-AM and Bt2cAMP-AM (db-cAMP-AM) were glucose-dependent. For panels A-C, a y axis value of 1.0 corresponds to basal insulin secretion (15 ng/ml/30 min) occurring in the presence of 11.1 mm glucose and no cAMP analog. For panel D, a y axis value of 1.0 corresponds to insulin secretion that occurred during 30 min in the presence of 0.1 mm glucose (6 ng/ml/min) or 11.1 mm glucose (15 ng/ml/30 min), but in the absence of added cAMP analogs. For panels A–D, *, p < 0.05; **, p < 0.005; and each determination is the mean of two determinations. These results summarize findings of a single experiment and this experiment was repeated 3 times with similar outcomes.
The PKA-selective cAMP analog Bt2cAMP-AM also exerted a potent insulin secretagogue action under conditions in which INS-1 cells were equilibrated in KRBH containing 11.1 mm glucose. The effect of Bt2cAMP-AM was dose-dependent, and when the analog was tested at a saturating concentration of 10 μm, the magnitude of the response was 2.4-fold larger than that which was observed using the equivalent concentration of 8-pCPT-2′-O-Me-cAMP-AM (Fig. 5B). It is noteworthy that there existed a supra-additive interaction of 8-pCPT-2′-O-Me-cAMP-AM and Bt2cAMP-AM to stimulate insulin secretion when these two cAMP analogs were co-administered at a concentration of 1 μm each (Fig. 5C). Furthermore, lowering the concentration of glucose in the assay buffer to 0.1 mm attenuated the actions of both 8-pCPT-2′-O-Me-cAMP-AM and Bt2cAMP-AM (Fig. 5D). Taken as a whole, these findings indicate that for INS-1 cells, PKA and Epac interact synergistically to potentiate glucose-stimulated insulin secretion, and that it is PKA that plays a quantitatively more important role in this process under conditions of 11.1 mm glucose equilibration.
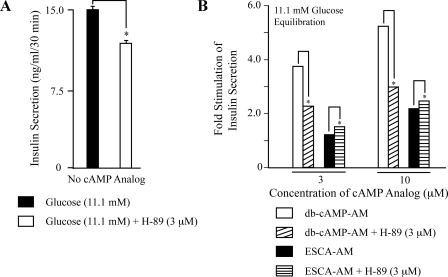
H-89 Disrupts PKA- but Not Epac-regulated Insulin Secretion—When INS-1 cells were treated with 3 μm of the PKA inhibitor H-89, three experimental outcomes were measurable. First, in the absence of added cAMP analogs, H-89 reduced 11.1 mm glucose-stimulated insulin secretion by 21% (Fig. 6A). Second, when cells were equilibrated in KRBH containing 11.1 mm glucose and H-89, the ability of Bt2cAMP-AM to potentiate insulin secretion was greatly diminished (Fig. 6B). Third, the action of 8-pCPT-2′-O-Me-cAMP-AM to potentiate insulin secretion was increased slightly by H-89 (Fig. 6B). This augmentation of ESCA action reflected the ability of H-89 to reduce the underlying 11.1 mm glucose-stimulated insulin secretion that was measurable in the absence of cAMP analog, and which we define here as “basal” insulin secretion (Fig. 6A). Thus, when calculating the fold-stimulation of insulin secretion relative to basal levels, the secretagogue action of 8-pCPT-2′-O-Me-cAMP-AM was larger in the presence of H-89 than in its absence (Fig. 6B). These findings indicate that under the experimental conditions defined here, and contrary to one prior report (36), the Epac-mediated action of cAMP to stimulate insulin secretion does not necessarily require concomitant activation of PKA.
FIGURE 6.
Contrasting actions of protein kinase inhibitor H-89 on insulin secretion. A, pretreatment of INS-1 cells with H-89 reduced 11.1 mm glucose-stimulated insulin secretion. B, the secretagogue action of Bt2cAMP-AM (db-cAMP-AM) was antagonized by H-89, whereas the action of 8-pCPT-2′-O-Me-cAMP-AM was slightly augmented. For A and B, the cultures were incubated in 11.1 mm glucose KRBH with or without H-89 for 15 min prior to a 30-min exposure to test solutions containing 11.1 mm glucose and the indicated test substances. For B, a y axis value of 1.0 corresponds to insulin secretion occurring in the presence of 11.1 mm glucose, either with H-89 (11.7 ng/ml/30min) or without H-89 (15 ng/ml/min). For panels A and B, *, p < 0.05 and each determination is the mean of two determinations. These results summarize findings of a single experiment and this experiment was repeated 3 times with similar outcomes.
DISCUSSION
Pancreatic beta cells located within the islets of Langerhans are unique in that they secrete insulin in response to an elevation of extracellular glucose concentration (35). This glucose-stimulated insulin secretion is potentiated by cAMP-elevating agents such as the incretin hormone glucagon-like peptide-1, or the structurally related incretin mimetic exendin-4 (38–41). Because it is uncertain whether it is Epac or PKA that mediates the cAMP-dependent potentiation of glucose-stimulated insulin secretion (34), a primary goal of the present study was to compare the insulin secretagogue properties of cAMP analogs under experimental conditions in which the selectivity of the analogs for Epac or PKA were validated in assays of Epac1-camps activation, Rap1 activation, CREB phosphorylation, and PKA-dependent gene expression. We now report that under conditions of 11.1 mm glucose equilibration, 8-pCPT-2′-O-Me-cAMP-AM exerted a potent insulin secretagogue action in INS-1 cells. The action of 8-pCPT-2′-O-Me-cAMP-AM was most likely mediated by Epac because it was not diminished by a PKA inhibitor (H-89), was accompanied by Epac-dependent activation of Rap1, and was not associated with increased phosphorylation or transactivation function of the PKA substrate CREB. Importantly, the magnitude of the secretory response measured after exposure of INS-1 cells to a saturating concentration of 8-pCPT-2′-O-Me-cAMP-AM was only 43% of that measured in response to the PKA-selective cAMP analog Bt2cAMP-AM. Therefore, whereas Epac activation promotes exocytosis of insulin in INS-1 cells, it appears that for this cell type, it is PKA that plays a more dominant role as a determinant of secretory function. The importance of PKA to the cAMP-dependent potentiation of glucose-stimulated insulin secretion is emphasized by prior electrophysiological and imaging studies of beta cells (42–48).
Vliem and co-workers (15) recently reported the synthesis and initial characterization of 8-pCPT-2′-O-Me-cAMP-AM. This acetoxymethyl group of the analog masks the charged polar phosphate, thus rendering the molecule highly membrane permeant. After entering a cell, the AM-ester is hydrolyzed by intracellular esterases to liberate free and biologically active 8-pCPT-2′-O-Me-cAMP. In studies of non-endocrine cell types treated with this analog, it was demonstrated that the potency of 8-pCPT-2′-O-Me-cAMP-AM was 100 to 1,000-fold greater than that of 8-pCPT-2′-O-Me-cAMP in assays of Rap1 activation and cell adhesion (15). Such prior findings suggested to us the possible usefulness of 8-pCPT-2′-O-Me-cAMP-AM as a tool with which to investigate a novel cAMP signal transduction mechanism that stimulates insulin secretion, and which is proposed to involve the Epac-mediated activation of Rap1 (49–53). Using INS-1 cells, we now confirm that 8-pCPT-2′-O-Me-cAMP-AM is a remarkably potent activator of Rap1, an effect that was measured at concentrations less than 1 μm, and which appeared within 2.5 min following its application. Similarly, 8-pCPT-2′-O-Me-cAMP-AM was also a potent activator of Epac1-camps. In fact, in a FRET-based assay of Epac1-camps activation, the action of 1 μm 8-pCPT-2′-O-Me-cAMP-AM exceeded that of 100 μm 8-pCPT-2′-O-Me-cAMP. Because 8-pCPT-2′-O-Me-cAMP was also a poor activator of Rap1 in these same cells, the available evidence indicates that for INS-1 cells, the access of 8-pCPT-2′-O-Me-cAMP to the cytosol is a major limiting factor governing its activity. With these points in mind, prior negative findings obtained in studies of beta cells, or other cell types, should now be re-evaluated. Specifically, in the absence of control experiments in which the access of 8-pCPT-2′-O-Me-cAMP to the cytosol is confirmed, its failure to produce an effect must not be misinterpreted as evidence ruling out a role for Epac in a biological process.
In view of the limited access of 8-pCPT-2′-O-Me-cAMP to the cytosol of INS-1 cells, it is understandable that the non-AM-ester of this ESCA exerted little or no insulin secretagogue action when tested at 10–100 μm. What is surprising is that in two prior studies of INS-1 cells, and one report concerning mouse islets, secretagogue actions of 8-pCPT-2′-O-Me-cAMP were measurable when the ESCA was tested at 50–100 μm (36, 37, 53). Although the basis for this discrepancy remains unresolved, we point out that a prior study of isolated mouse islets demonstrated that 250 μm 8-pCPT-2′-O-Me-cAMP failed to stimulate insulin secretion when it was administered extracellularly (54). In contrast, a stimulation of insulin secretion was observed when 100 μm 8-pCPT-2′-O-Me-cAMP was administered to rat islets treated with α-toxin to permeabilize the plasma membrane and to allow access of the ESCA to the cytosol (55). We too have evaluated the action of 8-pCPT-2′-O-Me-cAMP in mouse islets and find that it is an ineffective stimulator of insulin secretion when tested at 10 μm, whereas this same concentration of the AM-ester of 8-pCPT-2′-O-Me-cAMP exerts a potent insulin secretagogue effect (supplementary data Fig. S1). Thus, there is good reason to believe that it is the AM-ester of 8-pCPT-2′-O-Me-cAMP rather than the non-AM-ester that will be of special value in future studies of Epac-regulated insulin secretion.
When evaluating prior published findings concerning potential Epac-mediated actions of 8-pCPT-2′-O-Me-cAMP, the concentration dependence with which this ESCA acts must be considered. In the present study, 8-pCPT-2′-O-Me-cAMP had a small but measurable stimulatory action at Epac1-camps when it was applied extracellularly at concentrations of 30–100 μm. These concentrations are well within the range of concentrations that were used in prior studies examining effects of this ESCA on [Ca2+] and KATP channel activity in INS-1 cells (56–59). Thus, the quantities of 8-pCPT-2′-O-Me-cAMP that gain entry to the cytosol of INS-1 cells during its extracellular application are sufficient to produce detectable changes of [Ca2+] and KATP channel activity, whereas they seem to be too low to induce much insulin secretion, at least under the experimental conditions defined here.
An additional question addressed in this study relates to prior reports demonstrating an ability of 8-pCPT-2′-O-Me-cAMP to exert a small inhibitory effect on cyclic nucleotide PDE activity (5, 27), an effect that would be expected to raise levels of endogenous cAMP and to activate PKA. Because multiple pools of PKA exist within a cell, some associated with PDEs and some not, it could be that in some cell types, 8-pCPT-2′-O-Me-cAMP will inhibit PDEs, raise levels of cAMP, and promote PKA-dependent phosphorylation of a restricted subset of substrate proteins (those associated with PDEs), although failing to influence others. Although we did not formally address this possibility when considering how 8-pCPT-2′-O-Me-cAMP-AM influences insulin secretion, we found that a moderately selective PKA inhibitor (H-89) failed to diminish the secretagogue action of 8-pCPT-2′-O-Me-cAMP-AM, whereas it did reduce the action of Bt2cAMP-AM. Thus, available information indicates that the stimulation of insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM reported here resulted from Epac activation rather than PDE inhibition and the indirect activation of PKA.
It is interesting to note that 8-pCPT-2′-O-Me-cAMP can bind with low affinity to PKA (5, 26), and although it has a very limited ability to activate PKA in vitro (4), it might still act as an antagonist to disrupt activation of this kinase by endogenous cAMP in living cells. If this were to be the case, studies of the action of 8-pCPT-2′-O-Me-cAMP would be complicated by the undesired side effect of reduced substrate phosphorylation by PKA. To our knowledge, the present report is the first to demonstrate that such a confounding effect does not occur, at least when considering the potential of 8-pCPT-2′-O-Me-cAMP-AM to antagonize PKA-regulated gene expression. Thus, we found that pretreatment of INS-1 cells with 8-pCPT-2′-O-Me-cAMP-AM was an ineffective means by which to antagonize the stimulatory actions of cAMP-elevating agents (exendin-4 and forskolin) at RIP1-CRE-Luc.
In conclusion, we have used INS-1 cells to document the selectivity with which 8-pCPT-2′-O-Me-cAMP-AM and Bt2cAMP-AM activate Epac and PKA, respectively. By monitoring Epac1-camps activation, Rap1 activation, CREB phosphorylation, and PKA-dependent gene expression, the suitability of these two cyclic nucleotide analogs as molecular probes for the analysis of cAMP-regulated signal transduction in INS-1 cells has been established. We demonstrate that for INS-1 cells, it is PKA that serves as the dominant cAMP-binding protein in support of insulin secretion. Nevertheless, we do find that Epac activation stimulates insulin secretion, although this effect is quantitatively smaller than that attributable to PKA. Taken as a whole, such findings are understandable if the recruitment, docking, priming, and Ca2+-dependent exocytosis of insulin-containing secretory granules are processes of stimulus-secretion coupling that are regulated by both Epac and PKA. It will be particularly interesting in future studies to evaluate potential cross-talk between these two limbs of the cAMP signaling pathway. For INS-1 cells, we found an apparent synergistic interaction between Epac- and PKA-selective cAMP analogs to stimulate insulin secretion, yet we also found that the Epac activator 8-pCPT-2′-O-Me-cAMP-AM retained its secretagogue action under conditions in which cells were treated with a concentration of H-89 (3 μm) that inhibits PKA and which minimizes non-selective inhibitory effects of this compound at serine/threonine protein kinases unrelated to PKA (22). Thus, it would seem that as is the case for presynaptic nerve endings (60, 61), Epac acts in INS-1 cells to facilitate exocytosis independently of PKA, in addition to synergizing with PKA. Determining how Epac and PKA interact to regulate insulin secretion remains a question of considerable interest, and additional future studies using isolated islets of Langerhans will undoubtedly address this issue.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants DK045817 and DK069575 (to G. G. H.). This work was also supported by a American Diabetes Association Research Award (to C. A. L.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: PKA, protein kinase A; AM-ester, acetoxymethyl ester; 8-pCPT-2′-O-Me-cAMP-AM, 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate acetoxymethyl ester; CRE, cyclic AMP response element; CREB, cyclic AMP response element-binding protein; Bt2cAMP-AM, N6-2′-O-dibutyryl adenosine 3′,5′-cyclic monophosphate acetoxymethyl ester; ESCA, Epac-selective cAMP analog; FRET, fluorescence resonance energy transfer; KRBH, Krebs-Ringer buffer containing sodium bicarbonate and HEPES; H-89, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride; Luc, luciferase; PDE, cyclic nucleotide phosphodiesterase; YFP, yellow fluorescent protein; CFP, cyan fluorescent protein; SES, standard extracellular saline; RIP1, rat insulin 1 gene promoter; Epac, exchange protein directly activated by cAMP.
References
- 1.de Rooij, J., Zwartkruis, F. J. T., Verheijen, M. H. G., Cool, R. H., Nijman, S. M. B., Wittinghofer, A., and Bos, J. L. (1998) Nature 396 474-477 [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki, H., Springett, G. M., Mochizuki, N., Toki, S., Nakaya, M., Matsuda, M., Housman, D. E., and Graybiel, A. M. (1998) Science 282 2275-2279 [DOI] [PubMed] [Google Scholar]
- 3.Bos, J. L. (2006) Trends Biochem. Sci. 31 680-686 [DOI] [PubMed] [Google Scholar]
- 4.Enserink, J. M., Christensen, A. E., de Rooij, J., Triest, M. V., Schwede, F., Genieser, H. G., Døskeland, S. O., Blank, J. L., and Bos, J. L. (2002) Nat. Cell Biol. 4 901-906 [DOI] [PubMed] [Google Scholar]
- 5.Poppe, H., Rybalkin, S. D., Rehmann, H., Hinds, T. R., Tang, X. B., Christensen, A. E., Schwede, F., Genieser, H. G., Bos, J. L., Doskeland, S. O., Beavo, J. A., and Butt, E. (2008) Nat. Methods 5 277-288 [DOI] [PubMed] [Google Scholar]
- 6.Holz, G. G., Chepurny, O. G., and Schwede, F. (2008) Cell. Signal. 20 10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seino, S., and Shibasaki, T. (2005) Physiol. Rev. 85 1303-1342 [DOI] [PubMed] [Google Scholar]
- 8.Holz, G. G., Kang, G., Harbeck, M., Roe, M. W., and Chepurny, O. G. (2006) J. Physiol. 577 5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roscioni, S. S., Elzinga, C. R., and Schmidt, M. (2008) Naunyn-Schmiedeberg's Arch. Pharmacol. 377 345-357 [DOI] [PubMed] [Google Scholar]
- 10.Gambaryan, S., Butt, E., Tas, P., Smolenski, A., Allolio, B., and Walter, U. (2006) Am. J. Physiol. 290 E423-E433 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Iglesias, A. E., Jiang, Y., Tomić, M., Kretschmannova, K., Andric, S. A., Zemkova, H., and Stojilkovic, S. S. (2006) Mol. Endocrinol. 20 2231-2246 [DOI] [PubMed] [Google Scholar]
- 12.Zhao, C., Lai, J. S., Warsh, J. J., and Li, P. P. (2006) J. Neurosci. Res. 84 389-397 [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, R., Biswas, R., Brazie, M., Steplock, D., Shenolikar, S., and Weinman, E. J. (2009) Am. J. Physiol. Renal. Physiol. 296 F355-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faour, W. H., Gomi, K., and Kennedy, C. R. (2008) Cell. Signal. 20 2156-2164 [DOI] [PubMed] [Google Scholar]
- 15.Vliem, M. J., Ponsioen, B., Schwede, F., Pannekoek, W. J., Riedl, J., Kooistra, M. R., Jalink, K., Genieser, H. G., Bos, J. L., and Rehmann, H. (2008) ChemBioChem 9 2052-2054 [DOI] [PubMed] [Google Scholar]
- 16.Nikolaev, V. O., Bünemann, M., Hein, L., Hannawacker, A., and Lohse, M. J. (2004) J. Biol. Chem. 279 37215-37218 [DOI] [PubMed] [Google Scholar]
- 17.Asfari, M., Janjic, D., Meda, P., Li, G., Halban, P. A., and Wollheim, C. B. (1992) Endocrinology 130 167-178 [DOI] [PubMed] [Google Scholar]
- 18.Landa, L. R., Jr., Harbeck, M., Kaihara, K., Chepurny, O., Kitiphongspattana, K., Graf, O., Nikolaev, V. O., Lohse, M. J., Holz, G. G., and Roe, M. W. (2005) J. Biol. Chem. 280 31294-31302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbeck, M. C., Chepurny, O., Nikolaev, V. O., Lohse, M. J., Holz, G. G., and Roe, M. W. (2006) Sci. STKE 2006 pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao, J., Mei, F. C., Popov, V. L., Vergara, L. A., and Cheng, X. (2002) J. Biol. Chem. 277 26581-26586 [DOI] [PubMed] [Google Scholar]
- 21.Chepurny, O. G., and Holz, G. G. (2007) J. Biomol. Screen. 12 740-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies, S. P., Reddy, H., Caivano, M., and Cohen, P. (2000) Biochem. J. 351 95-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vossler, M. R., Yao, H., York, R. D., Pan, M. G., Rim, C. S., and Stork, P. J. (1997) Cell 89 73-82 [DOI] [PubMed] [Google Scholar]
- 24.Schultz, C., Vajanaphanich, M., Harootunian, A. T., Sammak, P. J., Barrett, K. E., and Tsien, R. Y. (1993) J. Biol. Chem. 268 6316-6322 [PubMed] [Google Scholar]
- 25.Bartsch, M., Zorn-Kruppa, M., Kühl, N., Genieser, H. G., Schwede, F., and Jastorff, B. (2003) Biol. Chem. 384 1321-1326 [DOI] [PubMed] [Google Scholar]
- 26.Christensen, A. E., Selheim, F., de Rooij, J., Dremier, S., Schwede, F., Dao, K. K., Martinez, A., Maenhaut, C., Bos, J. L., Genieser, H. G., and Døskeland, S. O. (2003) J. Biol. Chem. 278 35394-35402 [DOI] [PubMed] [Google Scholar]
- 27.Laxman, S., Riechers, A., Sadilek, M., Schwede, F., and Beavo, J. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 19194-19199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montminy, M. (1997) Annu. Rev. Biochem. 66 807-822 [DOI] [PubMed] [Google Scholar]
- 29.Holz, G. G. (2004) Horm. Metab. Res. 36 787-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoglund, G., Hussain, M. A., and Holz, G. G. (2000) Diabetes 49 1156-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chepurny, O. G., Hussain, M. A., and Holz, G. G. (2002) Endocrinology 143 2303-2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chepurny, O. G., and Holz, G. G. (2002) Cell Tissue Res. 307 191-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepe, S., Tortora, G., Noguchi, P. D., Marti, G. E., Washington, G. C., and Cho-Chung, Y. S. (1991) Cancer Res. 51 6263-6267 [PubMed] [Google Scholar]
- 34.Holz, G. G. (2004) Diabetes 53 5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henquin, J. C. (2000) Diabetes 49 1751-1760 [DOI] [PubMed] [Google Scholar]
- 36.Liu, G., Jacobo, S. M., Hilliard, N., and Hockerman, G. H. (2006) J. Pharmacol. Exp. Ther. 318 152-160 [DOI] [PubMed] [Google Scholar]
- 37.Islam, D., Zhang, N., Wang, P., Li, H., Brubaker, P. L., Gaisano, H., Wang, Q., and Jin, T. (2009) Am. J. Physiol. Endocrinol. Metab. 296 E174-181 [DOI] [PubMed] [Google Scholar]
- 38.Holz, G. G., and Habener, J. F. (1992) Trends Biochem. Sci. 17 388-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holz, G. G., Kuhtreiber, W. M., and Habener, J. F. (1993) Nature 361 362-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holz, G. G., and Chepurny, O. G. (2003) Curr. Med. Chem. 10 2471-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holz, G. G., and Chepurny, O. G. (2005) Sci. STKE 2005 pe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammälä, C., Ashcroft, F. M., and Rorsman, P. (1993) Nature 363 356-358 [DOI] [PubMed] [Google Scholar]
- 43.Renström, E., Eliasson, L., and Rorsman, P. (1997) J. Physiol. 502 105-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan, Q. F., Dong, Y., Yang, H., Lou, X., Ding, J., and Xu, T. (2004) J. Gen. Physiol. 124 653-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, Y., and Gillis, K. D. (2004) J. Gen. Physiol. 124 641-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatakeyama, H., Kishimoto, T., Nemoto, T., Kasai, H., and Takahashi, N. (2006) J. Physiol. 570 271-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatakeyama, H., Takahashi, N., Kishimoto, T., Nemoto, T., and Kasai, H. (2007) J. Physiol. 582 1087-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrins, M. J., and Stuenkel, E. L. (2008) J. Physiol. 586 5367-5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozaki, N., Shibasaki, T., Kashima, Y., Miki, T., Takahashi, K., Ueno, H., Sunaga, Y., Yano, H., Matsuura, Y., Iwanaga, T., Takai, Y., and Seino, S. (2000) Nat. Cell Biol. 11 805-811 [DOI] [PubMed] [Google Scholar]
- 50.Kashima, Y., Miki, T., Shibasaki, T., Ozaki, N., Miyazaki, M., Yano, H., and Seino, S. (2001) J. Biol. Chem. 276 46046-46053 [DOI] [PubMed] [Google Scholar]
- 51.Fujimoto, K., Shibasaki, T., Yokoi, N., Kashima, Y., Matsumoto, M., Sasaki, T., Tajima, N., Iwanaga, T., and Seino, S. (2002) J. Biol. Chem. 277 50497-50502 [DOI] [PubMed] [Google Scholar]
- 52.Shibasaki, T., Sunaga, Y., Fujimoto, K., Kashima, Y., and Seino, S. (2004) J. Biol. Chem. 279 7956-796161 [DOI] [PubMed] [Google Scholar]
- 53.Shibasaki, T., Takahashi, H., Miki, T., Sunaga, Y., Matsumura, K., Yamanaka, M., Zhang, C., Tamamoto, A., Satoh, T., Miyazaki, J., and Seino, S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 19333-19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thams, P., Anwar, M. R., and Capito, K. (2005) Eur. J. Endocrinol. 152 671-677 [DOI] [PubMed] [Google Scholar]
- 55.Hashiguchi, H., Nakazaki, M., Koriyama, N., Fukudome, M., Aso, K., and Tei, C. (2006) Diabetes Metab. Res. Rev. 22 64-71 [DOI] [PubMed] [Google Scholar]
- 56.Kang, G., Joseph, J. W., Chepurny, O. G., Monaco, M., Wheeler, M. B., Bos, J. L., Schwede, F., Genieser, H. G., and Holz, G. G. (2003) J. Biol. Chem. 278 8279-8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang, G., Chepurny, O. G., Rindler, M. J., Collis, L., Chepurny, Z., Li, W. H., Harbeck, M., Roe, M. W., and Holz, G. G. (2005) J. Physiol. 566 173-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang, G., Chepurny, O. G., Malester, B., Rindler, M. J., Rehmann, H., Bos, J. L., Schwede, F., Coetzee, W. A., and Holz, G. G. (2006) J. Physiol. 573 595-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang, G., Leech, C. A., Chepurny, O. G., Coetzee, W. A., and Holz, G. G. (2008) J. Physiol. 586 1307-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakaba, T., and Neher, E. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 331-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaneko, M., and Takahashi, T. (2004) J. Neurosci. 24 5202-5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.