Abstract
In this study, we explore the effects of several FOX and mutant FOX transcription factors on adipocyte determination, differentiation, and metabolism. In addition to Foxc2 and Foxo1, we report that Foxf2, Foxp1, and Foxa1 are other members of the Fox family that show regulated expression during adipogenesis. Although enforced expression of FOXC2 inhibits adipogenesis, Foxf2 slightly enhances the rate of differentiation. Constitutively active FOXC2-VP16 inhibits adipogenesis through multiple mechanisms. FOXC2-VP16 impairs the transient induction of C/EBPβ during adipogenesis and induces expression of the transcriptional repressor Hey1 as well as the activator of Wnt/β-catenin signaling, Wnt10b. The constitutive transcriptional repressor, FOXC2-Eng, enhances adipogenesis of preadipocytes and multipotent mesenchymal precursors and determines NIH-3T3 and C2C12 cells to the adipocyte lineage. Although PPARγ ligand or C/EBPα are not necessary for stimulation of adipogenesis by FOXC2-Eng, at least low levels of PPARγ protein are absolutely required. Finally, expression of FOXC2-Eng in adipocytes increases insulin-stimulated glucose uptake, further expanding the profound and pleiotropic effects of FOX transcription factors on adipocyte biology.
FOX (Forkhead box) proteins are a family of transcriptional regulators that are defined by a conserved 100-amino acid DNA-binding domain (DBD)2 and that regulate diverse cellular processes including differentiation, metabolism, development, proliferation, and apoptosis (1-4). There are 17 FOX subfamilies, and at least 41 human genes have been identified (3). Although the shared winged helix DBDs generally recognize and bind similar DNA elements, sequence specificity is achieved through interactions outside the DBD or through interactions with additional proteins (2). The regulated activities are diverse; some FOX proteins are exclusively transcriptional activators, some are transcriptional repressors, and some proteins are reported to have both activities. Foxa proteins serve as “pioneer factors” that modify chromatin access for other tissue-specific factors (1). Nuclear exclusion mediated by phosphorylation appears to be exceedingly important for some subfamilies (e.g. Foxo, Foxa), but transcriptional control can also be achieved through post-translational modifications such as monoubiquitination and acetylation (2, 3). Additional complexity is found through interaction with other transcription factors such as nuclear hormone receptors (2).
The regulation of preadipocyte differentiation has been extensively studied, and a cascade of transcriptional events culminating in expression of PPARγ and C/EBPα has been partially defined (5-9). These master adipogenic transcription factors are under direct or indirect control by many other transcriptional regulators, including members of the FOX family (10-12). For example, expression of Foxo1, 3, and 4 is increased during adipogenesis coincident with expression of PPARγ and C/EBPα, but Foxo1 activation is delayed until the end of clonal expansion (10). Expression of a constitutively active Foxo1 mutant prevents the differentiation of 3T3-L1 preadipocytes, and in adipocytes, Foxo1 physically interacts with C/EBPα to induce expression of adiponectin (13). Expression of a dominant-negative Foxo1 mutant promotes adipogenesis in cultured cells and increases oxygen consumption and expression of brown adipocyte genes in white adipose tissue of transgenic mice (14).
Foxa2 also plays a role as a regulator of adipocyte differentiation and metabolism. Expression of Foxa2 is undetectable in adipose tissue of lean mice but is increased dramatically with diet-induced or genetic obesity (15). In ob/ob mice, Foxa2 is expressed in both the stromal vascular and adipocyte fractions. Enforced expression in preadipocytes blocks adipogenesis through induction of Pref1 (i.e. Dlk1), whereas expression in adipocytes increases Glut4 and other genes involved in glucose metabolism. Mice haploinsufficient for Foxa2 are highly susceptible to diet-induced obesity, and adipocytes from these mice have profound alterations in glucose homeostasis and insulin sensitivity (15).
A third member of the FOX family with well investigated effects on adipocyte biology is Foxc2. Expression of Foxc2 is restricted to white and brown adipose tissue, and increased expression of human FOXC2 in adipose tissues of transgenic mice leads to a lean and insulin-sensitive phenotype (16, 17). In this context FOXC2 decreases white adipose tissue accumulation and dramatically expands the amount of brown adipose tissue by increasing sensitivity of the β-adrenergic cAMP protein kinase A signaling pathway through alteration of protein kinase A holoenzyme composition and expression of phosphodiesterase-4 (17, 18). Expression of Foxc2 is induced in preadipocyte models by tumor necrosis factor α and insulin, and enforced expression of Foxc2 blocks adipogenesis in part by inhibiting induction of a subset of PPARγ targets (12, 19).
To further our understanding of the effects of FOX transcription factors on adipocyte differentiation and metabolism, we created fusion proteins containing the FOX DBD fused to either the herpes simplex virus transcriptional activator, VP16 to create a constitutively active transcription factor, or the Drosophila engrailed protein to make a constitutive transcriptional repressor. This approach circumvents much of the complex regulatory control discussed above and has been widely used for mechanistic analysis of other transcription factors, including FoxD3, retinoid-related orphan receptor γ, p53, Ptf1a, cAMP-responsive element-binding protein, and PPARδ (20-25). In this manuscript we investigate mechanisms by which FOX transcription factors influence adipocyte determination, differentiation, and metabolism.
EXPERIMENTAL PROCEDURES
Reagents—8-Bromo-cAMP (Calbiochem, La Jolla CA) was dissolved in dimethyl sulfoxide. Troglitazone (5 μm; Pfizer Inc., Groton CT) was diluted in dimethyl sulfoxide. PD068235 was a generous gift from Pfizer Inc. Cytochalasin B was purchased from Sigma-Aldrich.
Cell Culture—Maintenance and adipogenesis of 3T3-L1 preadipocytes were as described previously using methylisobutylxanthine, dexamethasone, and insulin (MDI) (26). For adipogenesis, cells that had been confluent for 2 days (Day 0) were treated with 10% fetal calf serum (FCS), 1 μm dexamethasone, 0.5 mm methylisobutylxanthine, 1 μg/ml insulin, and 5 μm troglitazone. On day 2, the cells were fed 1 μg/ml insulin in 10% FCS media, and on day 4 and every 2 days thereafter, the cells were refed with 10% FCS. Lipid accumulation in adipocytes was visualized by staining with Oil Red-O (27).
NIH-3T3 fibroblasts and C2C12 myoblasts were purchased from the American Type Tissue Culture repository. C2C12 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FCS. Marrow-derived ST2 cells were incubated at 37 °C and 5% CO2 in α-minimal essential medium supplemented with 10% FCS (Atlanta Biologicals). Mouse embryonic fibroblasts (MEFs) were maintained in Dulbecco's modified Eagle's medium that contained 10% FCS and 100 units/ml penicillin/streptomycin. C/EBPα-/- MEFs were a kind gift from Gretchen Darlington (Baylor College of Medicine). PPARγ-/- fibroblasts were from Evan Rosen (Harvard University) (28).
Plasmids and Transfections—Full-length human FOXC2 (nucleotides 1192-3289, GenBank™ accession number Y08223) and the DBD of FOXC2 (amino acids 65-182 from NP005242) were subcloned from the pCB6+ vector into a retroviral plasmid pLCNX2. The constitutive FOX repressor, FOXC2-Eng, was created by fusion of the FOX DBD in-frame with the Drosophila engrailed transcriptional repressor domain. The constitutively active FOXC2, FOXC2-VP16, was created by cloning the FOXC2 DBD in-frame with the VP16 transcriptional activation domain. FOXC2-Eng and FOXC2-VP16 were created in pCB6+ and subsequently subcloned into retroviral vectors, pLXSN, pBABE PURO, and pMSCV PURO (Clontech, Mountain View, CA). Full-length Foxf2 cDNA (NP010225) was subcloned into retroviral vector pLXSN. Hey1 was PCR-amplified from clone IMAGE6809680 (primers sense, 5′-ggatccgaccctcctcggagcccac-3′, and antisense, 5′-ttagaaagctccgatctctgtcc-3′) and subcloned into pBABE PURO. pMSCV-PPARγ was provided by Evan Rosen (Harvard Medical School, Boston, MA). pMSCV-C/EBPα was as described by Kang et al. (29). Wnt10a cDNA was subcloned into pNH2 retroviral vector (27) and Wnt10b into modified pTS13.
Retroviral Transduction of Cells—293T cells (10-cm plates) were transfected by calcium phosphate coprecipitation with the viral packaging vectors SVε-E-MLV-env and SVψ-E-MLV in addition to retroviral vectors as indicated in the figure legends (7.5 μg of each). Virus-containing medium was collected 16 h after transfection and passed through a 0.45-μm syringe filter. Polybrene (hexadimethrine bromide; Sigma) was added to a final concentration of 8 μg/ml. This medium was then applied to subconfluent (30% to 50%) cells in 10-cm plates. The infection protocol was repeated every 8-16 h until cells were 80% confluent. The cells were then trypsin-treated and replated in Dulbecco's modified Eagle's medium supplemented with 10% calf serum or FCS and the appropriate antibiotic: 150 μg/ml hygromycin (Invitrogen) for pTS13-based vectors, 400 μg/ml G418 (Invitrogen) for neomycin resistance-based vectors, or 2 μg/ml puromycin (Sigma) for pBABE/pMSCV-based vectors.
Quantitative Reverse Transcription-PCR—One μg of total RNA was transcribed to cDNA using the TaqMan system (Applied Biosystems, Foster City, CA). Quantitative PCR was performed according to the manufacturer's protocol. SYBR green I was used to monitor amplification of DNA on MyiQ real time PCR detection system (Bio-Rad). After amplification, melting curve analysis was performed as described by the manufacturer. Gene expression was normalized to 18 S, cyclophilin, or TATA box-binding protein RNAs, depending on the relative abundance of mRNA. Oligonucleotide primers for amplification of 18 S (29), cyclophilin (29), C/EBPα (29), PPARγ (29), Wnt10b (30), Foxo1 (31), Foxp1 (32), TATA box-binding protein (33), Foxa1 (34), and Foxa2 (34) have been reported. Primers for the following genes were designed using PRIMER EXPRESS software (Applied Biosystems) and validated for quantitative PCR: Wnt6 (forward, 5′-ggatgcgcagcacaagc-3′; reverse, 5′-cgcctcgttgttgtgcagt-3′), Hey1 (forward, 5′-tgagctgagaaggctggtac-3′; reverse, 5′-accccaaactccgatagtcc-3′), Foxc2 (forward, 5′-gaaagcgcccctctctcag-3′; reverse, 5′-tgcggataagttacctgcga-3′), and Foxf2 (forward, 5′-agcagagctacttgcaccagaac-3′; reverse, 5′-agtggagtggtgctggtaacg-3′).
Glucose Uptake Assay—Day 8-11 NIH-3T3 adipocytes were incubated for 3 h in serum- and glucose-free medium. Basal and insulin-induced (100 nm) glucose uptake was measured by rinsing cells and then incubating for 30 min with or without insulin followed by addition of unlabeled 2-deoxy-d-glucose (20 μm) and 1 μCi of radiolabeled 2-deoxy-d-[14C]glucose (Amersham Biosciences/GE Healthcare). The results were corrected for nonspecific binding using controls run in the presence of 20 μm cytochalasin B. After incubation for 5 min at room temperature, the medium was removed, and the cells were washed in cold phosphate buffered saline and lysed in 750 μl of 0.1% SDS. We then added 250 μl to a scintillation vial, and radioactivity was determined in a scintillation counter using 3 ml of Scinti-Verse scintillation liquid. Glucose uptake was normalized to protein content as measured from the remaining cell lysate with the Bio-Rad protein assay.
Western Blots—Immunoblots were performed with antibodies specific for C/EBPβ (M. D. Lane, Johns Hopkins University, Baltimore, MD), C/EBPα (a polyclonal antibody generated against a synthetic polypeptide corresponding to amino acids 253-265 (35)), PPARγ (Santa Cruz Biotechnology), and FABP4 (David Bernlohr, University of Minnesota, Minneapolis, MN). Laminin was purchased from Novus Biologicals (Littleton, CO). Bound horseradish peroxidase-coupled secondary antibody was visualized with Pierce Super Signal or Super Signal Ultra enhanced chemiluminescence substrates (Thermo Fisher).
RESULTS
Regulated Expression of FOX Transcription Factors during Adipocyte Differentiation—To further our understanding of mechanisms by which FOX transcription factors influence adipocyte differentiation, we first evaluated the expression pattern of family members identified by Affymetrix gene profiling as having substantial expression during 3T3-L1 adipogenesis. We observed that Foxc2 is transiently induced with peak expression levels 8-24 h after induction of differentiation, whereas Foxo1 is expressed in adipocytes, with maximal expression levels reached by day 4 (Fig. 1A). In addition, we observed that Foxa1, Foxf2, and Foxp1 are also transiently induced with peak expression observed at 8 h. We did not find significant expression of Foxa2 (cycle threshold of ∼35), which is consistent with the observation that Foxa2 is expressed in adipose tissue only under conditions of obesity (15). Collectively, the regulated expression of at least five members of the FOX family of transcription factors, Foxa1, Foxc2, Foxf2, Foxo1, and Foxp1, suggest complex and potentially overlapping functional roles for these proteins during adipogenesis.
FIGURE 1.
A, regulated expression of FOX transcription factors during adipogenesis. 3T3-L1 cells were induced to differentiate into adipocytes, and RNA was prepared at the indicated times and analyzed by real time PCR for Foxa1, Foxc2, Foxf2, Foxo1, and Foxp1. The data are representative of at least two independent experiments. The data are presented as percentages of maximum expression observed. B, ectopic expression of FOXC2 blocks adipogenesis in vitro. 3T3-L1 preadipocytes were infected with a retrovirus vector alone (pLNCX2) or retrovirus carrying the gene for FOXC2. Two days post-confluence, the cells were induced to differentiate with MDI and were stained with Oil Red-O at day 14. The cells were lysed at the indicated times for immunoblot analyses of C/EBPα, FABP4, and C/EBPβ ((LAP2)). These results are representative of at least three independent experiments. C, C/EBPα and PPARγ partially rescue adipocyte differentiation of FOXC2-expressing cells. Control (pLNCX2) or FOXC2-expressing cells were reinfected with a retrovirus vector alone (pBabe) or retroviruses carrying the genes for C/EBPα or PPARγ and were co-selected with G418 and puromycin. Two days post-confluence, the cells were induced to differentiate with MI. At day 10, the cells were photomicrographed. D, ectopic expression of Foxf2 promotes adipogenesisinvitro.3T3-L1 preadipocytes were infected with a retrovirus vector alone (pLXSN) or retrovirus carrying the gene for Foxf2. Two days post-confluence, the cells were induced to differentiate with MI. The cells were lysed at the indicated times for immunoblot analyses of C/EBPβ (LAP2 and LIP), FABP4, and C/EBPα. These results are representative of at least three independent experiments.
FOXC2 Blocks Adipocyte Differentiation, and Adipogenesis Is Rescued by C/EBPα or PPARγ—Consistent with prior published reports (12), ectopic expression of FOXC2 blocks adipocyte differentiation (Fig. 1B) as demonstrated by decreased neutral lipid accumulation and reduced expression of C/EBPα, C/EBPβ, and FABP4 proteins. To delineate at what level FOXC2 inhibits adipocyte differentiation, we tested whether enforced expression of C/EBPα or PPARγ is sufficient to rescue the inhibition of differentiation caused by FOXC2. To this end, we infected FOXC2-expressing cells with retroviruses carrying the genes for C/EBPα or PPARγ. After selection, the cells were induced to differentiate with MDI. Within 2 weeks, FOXC2-infected cells overexpressing C/EBPα or PPARγ acquired lipid droplets (Fig. 1C). These results suggest that FOXC2 represses differentiation by acting upstream of these adipogenic transcription factors, consistent with temporal expression of Foxc2 prior to C/EBPα and PPARγ (Fig. 1A) but counter to a prior report (12). In contrast, enforced expression of Foxf2 mildly stimulates adipocyte differentiation as observed by earlier acquisition of adipocyte morphology and expression of adipocyte genes (Fig. 1D). When we enforced expression of Foxp1 in 3T3-L1 cells, no change in adipocyte differentiation was observed (data not shown). Thus, members of the FOX family, although sharing similar expression patterns, can have opposing effects on adipogenesis.
Effect of FOXC2-VP16 and FOXC2-Eng on Adipocyte Differentiation—To probe general mechanisms by which FOX transcription factors regulate adipogenesis, we used a strategy in which we evaluated effects of FOX transcription factors with constitutively active or dominant-negative activities. Specifically, we prepared constructs that drive the expression of chimeric proteins in which the DBDs of FOXC2 or Foxf2 are linked to either the VP16 transcriptional activation domain (VP16) or the engrailed repressor domain (Eng). As expected, FOXC2-VP16 activates, whereas FOXC2-Eng represses an artificial forkhead reporter gene (supplemental Fig. S1). Furthermore, both chimeric proteins bind specifically to consensus forkhead binding sites in vitro (supplemental Fig. S1). Finally, fusions between FOXC2-VP16 or FOXC2-Eng with enhanced green fluorescent protein reveal that both chimeric proteins are exclusively nuclear when expressed in COS7 or HEK293 cells (supplemental Fig. S1 and data not shown).
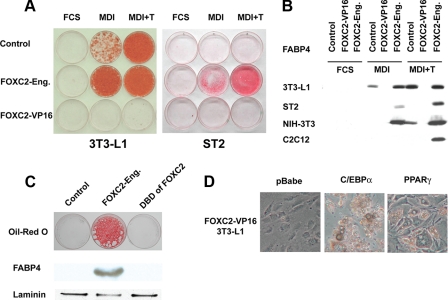
We then enforced expression of FOXC2-Eng and FOXC2-VP16 in cell lines with variable adipogenic potential, including 3T3-L1 preadipocytes, NIH-3T3 fibroblasts, ST2 mesenchymal precursor cells, C2C12 myoblasts, and C57Bl6 MEFs. Although no spontaneous differentiation was observed in the presence of FCS alone, FOXC2-Eng strongly increased adipocyte differentiation in all cell lines tested upon treatment with MDI, as determined by staining of neutral lipid (Fig. 2A and data not shown) and immunoblot analysis for FABP4 (Fig. 2B). Notably, this panel of cells includes C2C12 cells, which are committed to the myocyte lineage. In contrast to this, FOXC2-VP16 completely blocks adipocyte differentiation, even in the pro-adipogenic 3T3-L1 cell line and in the presence of a strong stimulus (i.e. addition of troglitazone to the differentiation mixture (MDI+T)). Similar results were observed with the Foxf2 chimeric constructs in 3T3-L1 cells (data not shown). In summary, we found that a similar basic observation holds true across the cell models. FOXC2-VP16 is a very strong inhibitor of adipocyte differentiation (Fig. 2 and data not shown), whereas FOXC2-Eng stimulates adipocyte differentiation and, in the cells investigated, alters commitment to the adipocyte lineage (Fig. 2).
FIGURE 2.
FOXC2-VP16 represses preadipocyte differentiation and FOXC2-Eng promotes adipogenesis of a variety of precursor cell lines. A, 3T3-L1 and ST2 cells were infected with an empty retroviral vector or a retroviral vector containing FOXC2-VP16 or FOXC2-Eng. At 2 days post-confluence, the cells were induced to differentiate with FCS, MDI, or MDI+T. At day 14, the cells were stained with Oil Red-O and lysed for immunoblot analysis. B, FOXC2-Eng stimulates expression of the adipocyte marker FABP4 in 3T3-L1, ST2, NIH-3T3, and C2C12 cells. C, NIH-3T3 cells were infected with a control retroviral vector or a retroviral vector containing FOXC2-Eng or the DBD of FOXC2 alone. The cells were stained with Oil Red-O and lysed for immunoblot analysis of FABP4 and laminin (loading control) 14 days after induction of adipogenesis with MDI. D, FOXC2-VP16-expressing cells were reinfected with a retrovirus vector alone (pBabe) or retroviruses containing the genes for C/EBPα or PPARγ and were co-selected with G418 and puromycin. Two days post-confluence, the cells were induced to differentiate with MDI, and 10 days later, photomicrographs were taken.
Characterization of FOXC2-VP16 or FOXC2-Eng Chimeras—To test whether the DBD had effects independent of the transcriptionally repressive engrailed domain, we also enforced expression of the FOXC2 DBD alone in NIH-3T3 cells and found that it did not promote adipocyte differentiation (Fig. 2C). To exclude that the effects on adipogenesis were due to the engrailed repressor domain or the VP16 activation domain, we also overexpressed fusion proteins of engrailed or VP16 with the Gal4 DBD and found no alterations in adipocyte differentiation compared with control cells (data not shown).
To establish whether FOXC2 and FOXC2-VP16 inhibit differentiation at a similar point, 3T3-L1 preadipocytes were sequentially and stably infected with FOXC2-VP16 and then with either pBabe, C/EBPα, or PPARγ and induced to differentiate. As observed for full-length FOXC2 (Fig. 1C), differentiation is completely blocked in preadipocytes that express FOXC2-VP16; again, stable overexpression of C/EBPα or PPARγ rescues this phenotype and leads to a high frequency of differentiation into adipocytes (Fig. 2D). These data are consistent with the notion that FOXC2 and FOXC2-VP16 exert their effects by a similar mechanism.
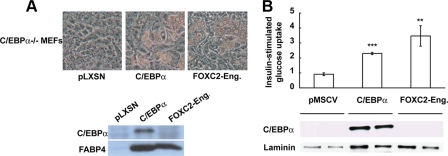
Ectopic Expression of FOXC2-Eng Stimulates Adipogenesis of C/EBPα-/- MEFs—As described above, sustained expression of FOXC2 prevents adipogenesis upstream of C/EBPα. We therefore sought to clarify whether the pro-adipogenic effect of the chimeric transcriptional repressor FOXC2-Eng is dependent on C/EBPα. To this end we retrovirally infected C/EBPα-/- MEFs with control vector (pLXSN) or vectors containing C/EBPα or FOXC2-Eng. These cells were induced to differentiate into adipocytes with MDI+T. As expected (36), C/EBPα-/- MEFs infected with control vector did not differentiate, whereas cells infected with C/EBPα acquired lipid droplets within 10 days (Fig. 3A). Importantly, FOXC2-Eng also robustly stimulated adipogenesis of C/EBPα-/- MEFs as assessed by phase contrast microscopy or immunoblot analysis for FABP4 (Fig. 3A). Because C/EBPα is not expressed in adipocytes ectopically expressing FOXC2-Eng (Fig. 3A), FOXC2-Eng bypasses or perhaps replaces C/EBPα to stimulate expression of adipocyte genes.
FIGURE 3.
FOXC2-Eng rescues adipocyte differentiation and insulin-stimulated glucose uptake in C/EBPα-/- MEFs and NIH-3T3 cells. A, C/EBPα-/- MEFs were infected with control retrovirus (pLXSN) or a retrovirus that expresses C/EBPα or FOXC2-Eng. At confluence, the cells were induced to differentiate in presence of MDI+T. Nine days after induction of adipogenesis, cell morphology was documented with phase-contrast photomicroscopy (top panel), and expression of C/EBPα and FABP4 was determined by immunoblot analysis (bottom panel). B, NIH-3T3 adipocytes infected with control or retroviruses carrying the gene for C/EBPα or FOXC2-Eng were serum-starved for 3 h; glucose uptake in response to 100 nm insulin was assessed using 2-deoxy-d-[14C]glucose (top panel); and expression of C/EBPα and laminin (loading control) was determined by immunoblot analysis (bottom panel). Glucose uptake is expressed as the mean ± S.D. relative to control cells (n = 3), and statistical significance was evaluated with Student's t test: **, p < 0.01; ***, p < 0.001. These results are representative of at least three independent experiments.
FOXC2-Eng Promotes Insulin-stimulated Glucose Uptake—NIH-3T3 fibroblasts were infected with control retroviral vector (pMSCV) or retroviruses containing C/EBPα or FOXC2-Eng. Between days 9 and 11 after induction of differentiation by MDI, insulin-stimulated glucose uptake was measured. As previously demonstrated (36, 37), expression of C/EBPα in NIH-3T3 cells confers the acquisition of insulin-stimulated glucose uptake. Furthermore, adipocytes that express FOXC2-Eng were also insulin-sensitive (Fig. 3B) despite the fact that endogenous C/EBPα was not expressed (Fig. 3B). These data provide further evidence that FOXC2-Eng functionally replaces C/EBPα and mediates not only the differentiation process into adipocytes but also the acquisition of glucose uptake in response to insulin.
FOXC2-Eng Stimulates Adipogenesis in the Presence of a PPARγ Inhibitor but Not in the Complete Absence of PPARγ—To understand further the mechanisms through which FOXC2-Eng stimulates adipogenesis, we treated control and FOXC2-Eng 3T3-L1 preadipocytes with a PPARγ inhibitor (PD068235) and evaluated effects on adipocyte differentiation. As expected, the control cells treated with the PPARγ inhibitor did not differentiate (Fig. 4A), but surprisingly, the cells expressing FOXC2-Eng underwent differentiation (Fig. 4A). Western blot analyses for C/EBPα, PPARγ, or FABP4 show that adipocyte markers are indeed highly expressed in the presence of FOXC2-Eng plus the PPARγ inhibitor (Fig. 4A).
FIGURE 4.
FOXC2-Eng stimulates adipogenesis in the presence of a PPARγ antagonist but FOXC2-Eng cannot overcome the complete lack of PPARγ. A, 3T3-L1 preadipocytes were infected with either a control retrovirus (pLXSN) or a retrovirus containing FOXC2-Eng. Two days after confluence, the cells were treated with inducers of adipogenesis (MDI). A PPARγ antagonist (PD068235; 50 μm) was added to the media at day 0 and 2 of differentiation as indicated. At day 10, cell morphology was documented by photomicroscopy. After protein transfer, the membrane was cut into three pieces; appropriate pieces were exposed to antisera for C/EBPα, PPARγ, and FABP4; and expression was determined by immunoblot analysis. B, PPARγ-/- fibroblasts were infected with a retroviral vector (pMSCV-PURO) carrying the gene for PPARγ or FOXC2-Eng. At confluence, the cells were induced to differentiate with MDI+T. Photomicrographs were taken at day 10.
To determine whether FOXC2-Eng stimulates differentiation of cells in the absence of PPARγ, we infected PPARγ-/- fibroblasts with PPARγ or FOXC2-Eng and induced them to differentiate in the presence of MDI+T. As previously described (28), PPARγ-/- fibroblasts do not differentiate on their own but accumulate lipid and express adipocyte genes in response to ectopic expression of PPARγ (Fig. 4B). However, in contrast to the strong adipogenesis observed with FOXC2-Eng in the absence of C/EBPα or in the presence of a PPARγ antagonist, FOXC2-Eng is not sufficient to cause differentiation of PPARγ-/- fibroblasts (Fig. 4B). These data are consistent with a model where ligand availability regulates the dynamics of PPARγ activity, but even in the presence of an antagonist, PPARγ protein or basal activity is indispensible for adipogenesis.
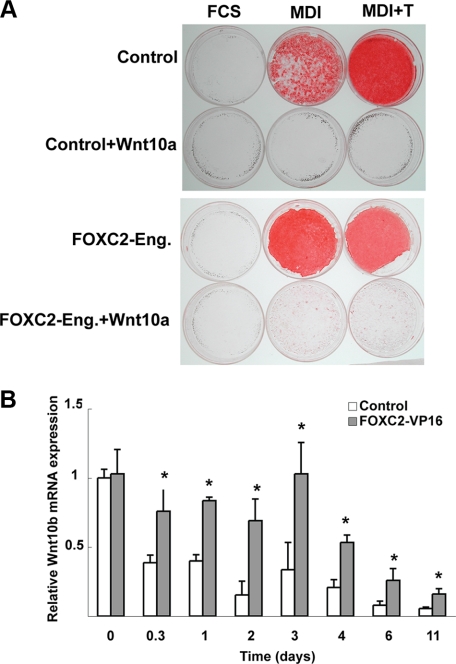
Does Wnt Signaling Play a Role in the Effects of FOXC2-Eng or FOXC2-VP16 on Adipogenesis?—To explore further the interplay between FOXC2-Eng and pathways known to regulate adipogenesis, we ectopically expressed FOXC2-Eng in 3T3-L1 cells already expressing Wnt10a. Expression of Wnt10b (38) or Wnt10a (Fig. 5A) in 3T3-L1 cells blocks adipocyte differentiation. In this context, FOXC2-Eng is not able to overcome the block caused by activation of Wnt/β-catenin signaling to rescue adipocyte differentiation (Fig. 5A). Similarly, expression of FOXC2-Eng is not sufficient to overcome the inhibition of adipogenesis caused by tumor necrosis factor α (data not shown). To ensure that prior activation of Wnt/β-catenin signaling did not alter the development of 3T3-L1 cells such that they could not respond to FOXC2-Eng, we sequentially infected preadipocytes with Wnt10b and then FOXC2-Eng or, alternatively, with FOXC2-Eng and then Wnt10b. In neither case could FOXC2-Eng rescue the differentiation caused by Wnt/β-catenin signaling (data not shown).
FIGURE 5.
A, FOXC2-Eng cannot overcome the block to differentiation caused by Wnt10a. Control (pBabe) or FOXC2-Eng 3T3-L1 cells were reinfected with a retrovirus carrying the gene for Wnt10a (or control empty vector) and were co-selected with puromycin and G418. Two days after confluence, the cells were induced to differentiate in presence of FCS, MDI, or MDI+T. Neutral lipids were stained with Oil Red-O on day 14. All of the experiments are representative of at least three independent experiments. B, FOXC2-VP16 induces expression of Wnt10b mRNA during 3T3-L1 adipogenesis. 3T3-L1 cells were infected with an empty retroviral vector (pBabe) or a retroviral vector containing FOXC2-VP16. Two days post-confluence the cells were induced to differentiate with MDI. RNA was harvested at the indicated times, and expression of Wnt10b was analyzed by quantitative PCR. The data are presented as the means ± S.D. (n = 3). Differences between control and FOXC2-VP16 were evaluated with Student's t test. *, p < 0.05.
Although FOXC2-Eng was not able to overcome the Wnt-induced block to adipogenesis, the possibility remained that FOXC2-VP16 might inhibit differentiation through up-regulation of Wnt signaling. To address this possibility we first measured Wnt10b mRNA levels during adipogenesis in control and FOXC2-VP16 expressing 3T3-L1 adipocytes (Fig. 5B). Interestingly, Wnt10b mRNA expression are increased by FOXC2-VP16 by ∼50% 8 h after induction, and elevated expression is observed throughout the time course. However, Wnt10a mRNA expression is not significantly higher in those same cells (data not shown). To address whether FOXC2-VP16 inhibits adipogenesis by activating Wnt signaling, we expressed a dominant-negative Tcf7l2, which strongly promotes adipogenesis (38) and observed that this factor was insufficient to rescue adipogenesis (data not shown). Thus, whereas Wnt10b is induced by FOX transcription factors, it appears that increased Wnt signaling alone is not sufficient to explain the mechanism whereby FOX transcription factors repress adipogenesis.
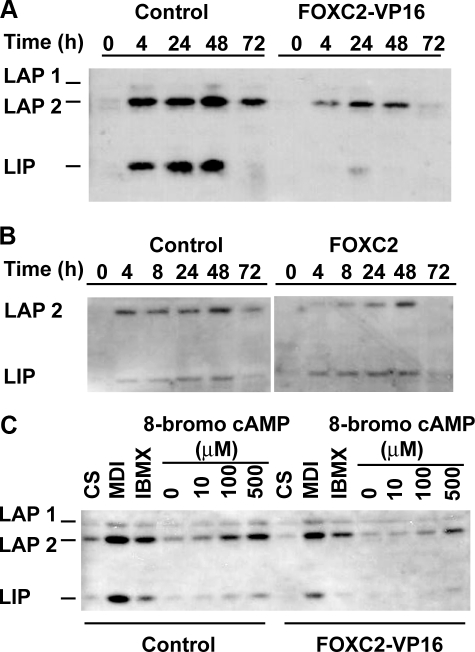
Impaired cAMP Signaling to C/EBPβ with FOXC2-VP16—To understand the mechanisms by which Foxc2 acts during adipogenesis, we performed Affymetrix microarray analyses and profiled changes in gene expression, comparing the effects of FOXC2-VP16 and FOXC2-Eng in confluent 3T3-L1 and ST2 cells. One of the transcription factors involved in adipogenesis and identified as differentially expressed was C/EBPβ, which is down-regulated by ∼50% in FOXC2-VP16 cells compared with FOXC2-Eng (data not shown). Immunoblot analysis confirmed a strong reduction in the expression of C/EBPβ between control and 3T3-L1 cells expressing either FOXC2-VP16 (Fig. 6A) or FOXC2 (Fig. 6B). It is well established that elevated cAMP is the primary inducer of C/EBPβ during adipogenesis. Thus, we incubated control and FOXC2-VP16 3T3-L1 preadipocytes with MDI, isobutylmethylxanthine, or the indicated concentrations of 8-bromo-cAMP, a cell-permeable cAMP analogue, which is more resistant to phosphodiesterases than cAMP. Evaluation of C/EBPβ protein levels by immunoblot analysis indicates that FOXC2-VP16 cells are impaired in their sensitivity to cAMP by about 10-fold (Fig. 6C).
FIGURE 6.
Expression of FOXC2-VP16 or FOXC2 impairs induction of C/EBPβ during adipocyte differentiation or in response to cAMP. A and B, FOXC2-VP16 and FOXC2-infected 3T3-L1 cells, respectively, were induced to differentiate in presence of MDI. At the indicated times, cells were lysed, and C/EBPβ (LAP1, LAP2, and LIP) expression was determined by immunoblot analysis. C, at confluence, control or FOXC2-VP16 cells were treated for four h with 10% calf serum (CS), MDI, isobutylmethylxanthine (IBMX), or increasing concentrations of 8-bromo-cAMP in Me2SO as indicated. The cells were lysed, and expression of C/EBPβ proteins was evaluated. The experiments are representative of three independent experiments.
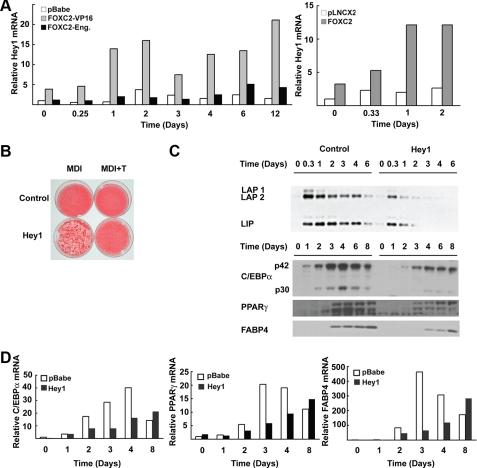
Effects of FOXC2 on Adipogenesis Are Mediated in Part through Hey1—Gene profiling identified Hey1 as one of the most highly up-regulated genes between cells expressing FOXC2-VP16 and FOXC2-Eng. Hey1 is elevated by FOXC2-VP16 by 1.6 and 3.5-fold in 3T3-L1 and ST2 cells, respectively, compared with FOXC2-Eng (data not shown). These data were confirmed and extended by real time PCR on RNA extracted from 3T3-L1 cells expressing an empty retroviral vector (pBabe) or vectors containing FOXC2-VP16 or FOXC2-Eng at different times during adipocyte differentiation (Fig. 7A). In confluent preadipocytes (Day 0), FOXC2-VP16 induces Hey1 by about 4-fold. Furthermore, FOXC2-VP16 and MDI interact to induce the expression of Hey1 by ∼15-fold at day 2, and elevated levels of expression are maintained through at least day 12 of differentiation. Similar results were observed with ectopic expression of FOXC2 in 3T3-L1 cells (Fig. 7A, right panel). Expression of Hey1 was not substantially altered by FOXC2-Eng compared with the control.
FIGURE 7.
Role for Hey1 in suppression of adipogenesis by FOXC2. A, 3T3-L1 preadipocytes were infected with a retrovirus vector alone (pBabe or pLNCX2) or retroviruses carrying the genes for FOXC2-VP16, FOXC2-Eng, or FOXC2 as indicated. Two days post-confluence, the cells were induced to differentiate with MDI. RNA was purified at the indicated times and expression of Hey1 analyzed by quantitative PCR. B, 3T3-L1 preadipocytes were infected with a retrovirus vector alone (pBabe) or a vector carrying the gene for Hey1. Two days after confluence, the cells were induced to differentiate in presence of MDI or MDI+T. The cells were stained with Oil Red-O on day 14. C, on the indicated days of differentiation, the cells were lysed, and expression of C/EBPβ, C/EBPα, PPARγ, and FABP4 was analyzed by immunoblot analysis. D, RNA was purified from cells described in C and analyzed by quantitative PCR. The experiments are representative of at least two independent experiments.
To establish whether elevated Hey1 contributes to the inhibitory effects of FOXC2-VP16 on differentiation, we enforced the expression of Hey1 in 3T3-L1 cells using retroviral infection and observed that Hey1 impairs adipogenesis as assessed by accumulation of neutral lipid (Fig. 7B). Hey1 also decreased and/or delayed the expression of C/EBPβ, C/EBPα, PPARγ, and FABP4 proteins (Fig. 7C) and mRNAs (Fig. 7D). These data are consistent with Hey1 mediating, in part, the repression of adipogenesis caused by FOXC2.
DISCUSSION
Although the inhibitory effects of Foxc2, Foxo1, and Foxa2 on adipogenesis have been explored previously (10, 12, 15, 17), during the course of this investigation, we discovered a number of additional FOX transcription factors that show regulated expression during adipogenesis and thus potentially influence adipocyte differentiation or metabolism. We confirmed prior reports that ectopic expression of Foxc2 blocks adipogenesis (Fig. 1B), but we were surprised to find that expression of Foxf2 does not share this activity and that Foxf2 slightly enhances the differentiation process. To explore general effects of FOX transcription factors on adipocyte differentiation and metabolism and to bypass the complex transcriptional and post-translational regulation of FOX family members, we used the modular design of transcription factors to create relatively simple FOX activators or FOX repressors to investigate common mechanisms by which this family influences adipocyte biology. The generality of our findings will require further investigation, as we do not know, for example, whether FOXC2 or Foxf2 DBDs bind to all forkhead sites or only a subset under these experimental conditions. For instance, we did not identify Pref1 as a target of FOXC2-VP16, suggesting that our experimental system may not be appropriate for analysis of Foxa2 (15).
Constitutively active VP16 chimeras with the FOXC2 or Foxf2 DBD repressed adipogenesis of all cell types tested (Fig. 2 and data not shown). As was observed with FOXC2 (Fig. 1B), expression of FOXC2-VP16 impaired induction of C/EBPβ after exposure of preadipocytes to differentiation inducers or in response to 8-bromo cAMP (Fig. 6). Expression of FOXC2 in adipose tissue of transgenic mice increases cAMP signaling through increased expression of the protein kinase A regulatory subunit R1α (17). We also observed a ∼2-fold increase in R1α in preadipocytes expressing FOXC2 or FOXC2-VP16 (data not shown), suggesting that cAMP signaling may be increased in these cells. However, the fact that induction of C/EBPβ by cAMP is blunted by FOXC2 or FOXC2-VP16 indicates that these transcription factors must work through a different mechanism. In any case, it is likely that reduced expression of C/EBPβ is part of the mechanism by which FOXC2 inhibits adipogenesis.
We explored whether FOX transcription factors might be mediating their effects through the Wnt signaling pathway. In fact, FOXC2-VP16 increases expression of Wnt10b, which inhibits adipogenesis (Fig. 5A and Ref. 38). However, neither of these genes is repressed by FOXC2-Eng (data not shown), thus indicating that effects of FOXC2-Eng are independent of these specific Wnts. The complexity of the interactions between FOX and Wnt signaling is underscored by the observations that dominant-negative Tcf7l2 cannot overcome the block of adipogenesis by FOXC2-VP16 and FOXC2-Eng cannot rescue inhibition of adipogenesis caused by ectopic expression of Wnt10a (Fig. 5A) or Wnt10b (data not shown). Thus, the dependence between FOX transcription factors and Wnt signaling is unclear because the repressive adipogenic signals dominate over the positive ones.
Gene set enrichment analysis was not particularly helpful in that it only identified a single cluster of adipocyte genes as differentially expressed (ADIP_VS_FIBRO_UP); however, gene profiling with Affymetrix chips of ST2 or 3T3-L1 cells expressing FOXC2-VP16 or FOXC2-Eng did identify Hey1 as differentially expressed. We then used quantitative PCR to confirm that Hey1 is expressed during adipogenesis (maximum cycle threshold of 28) and dramatically up-regulated by FOXC2-VP16 and FOXC2 (Fig. 7, A and B). Hey1 is a basic helix-loop-helix protein and a member of the HES-related repressor protein family. Although Hey1 is most widely studied as a notch target gene and as a heterodimer of HES1, enforced expression of Hey1 inhibits myogenesis (39) and plays a positive intermediary role in BMP9-induced osteogenesis (40). Our results demonstrate that enforced expression of Hey1 in 3T3-L1 cells impairs adipocyte differentiation and induction of C/EBPβ, raising the possibility that Hey1 binds to E boxes present in the C/EBPβ promoter/enhancer and mediates the inhibition of C/EBPβ by FOXC2. Again, similar to the situation with Wnt10a and Wnt10b, FOXC2-Eng does not repress Hey1 expression, further supporting the notion that the pro-adipogenic mechanism of action of this chimeric transcriptional repressor is more complex than simply inhibiting genes that are activated by FOXC2.
The vast potential for combinatorial complexity and functional redundancy of FOX transcription factors make identification of the specific FOX target or targets responsible for the adipogenic effects of FOXC2-Eng challenging. Nevertheless, several aspects of FOXC2-Eng action warrant further thought. First of all, engrailed fusion proteins with FOXC2 and Foxf2 DBDs profoundly stimulate both determination to the adipocyte lineage and differentiation of preadipocytes. These observations are consistent with the idea that effects of FOXC2-Eng are not restricted to inhibition of endogenous Foxc2 function but extend to the activities of other family members. This is in line with the observation that antiadipogenic target genes of FOXC2 action like Wnt10a, Wnt10b, or Hey1 are not repressed by FOXC2-Eng. Second, it is notable that the DBDs alone have no effect on adipogenesis (Fig. 2C), indicating that active repression of transcription, and not displacement of endogenous FOX factors from their DNA binding sites, is a critical part of the mechanism. Third, the repressive FOX transcription factor, FOXC2-Eng, bypasses the adipogenic requirement for C/EBPα, a characteristic shared with PPARγ (28), Krüppel-like factor 15 (41), and the Ebf (O/E) family of helix-loop-helix transcription factors (42). Finally, stimulation of adipogenesis by FOXC2-Eng results in cells that are insulin-responsive and express adiponectin, even in the absence of C/EBPα, a characteristic that is not shared with PPARγ and that has been previously thought to be absolutely dependent on C/EBPα (12, 43). Taken together, these data further our understanding of mechanisms used by FOX transcription factors to broadly influence adipocyte biology, including effects on determination, differentiation, and metabolism.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants DK51563 and DK62876 (to O. A. M.). This work was also supported by the Belgian Fonds National de la Recherche Scientifique and the Center for Organogenesis (to I. G.), the Michigan Metabolomics and Obesity Center, European Commission Contract LSHMCT-2005-018734 under Project HEPADIP, Swedish Research Council Grant K2005-32BI-15324-01A (to S. E.), European Union Grants QLK3-CT-2002-02149 and LSHM-CT-2003-503041, the Arne and Inga Britt Foundation, the Söderberg Foundation, and the Swedish Foundation for Strategic Research through the Center for Cardiovascular and Metabolic Research.
The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Fig. S1.
Footnotes
The abbreviations used are: DBD, DNA-binding domain; FCS, fetal calf serum; C/EBP, CCAAT/enhancer-binding protein; PPAR, peroxisome proliferator-activated receptor; FABP4, fatty acid binding protein 4; MEF, mouse embryonic fibroblast; MDI, methylisobutylxanthine, dexamethasone, and insulin; MDI+T, MDI with troglitazone.
References
- 1.Friedman, J. R., and Kaestner, K. H. (2006) Cell Mol. Life Sci. 63 2317-2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijchers, P. J., Hoekman, M. F., Burbach, J. P., and Smidt, M. P. (2006) Brain Res. 1068 23-33 [DOI] [PubMed] [Google Scholar]
- 3.Myatt, S. S., and Lam, E. W. (2007) Nature Rev. 7 847-859 [DOI] [PubMed] [Google Scholar]
- 4.Arden, K. C. (2008) Oncogene 27 2345-2350 [DOI] [PubMed] [Google Scholar]
- 5.Farmer, S. R. (2006) Cell Metab. 4 263-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregoire, F. M., Smas, C. M., and Sul, H. S. (1998) Physiol. Rev. 78 783-809 [DOI] [PubMed] [Google Scholar]
- 7.Rangwala, S. M., and Lazar, M. A. (2000) Annu. Rev. Nutr. 20 535-559 [DOI] [PubMed] [Google Scholar]
- 8.Rosen, E. D., and Spiegelman, B. M. (2000) Annu. Rev. Cell Dev. Biol. 16 145-171 [DOI] [PubMed] [Google Scholar]
- 9.Rosen, E. D., and MacDougald, O. A. (2006) Nat. Rev. Mol. Cell Biol. 7 885-896 [DOI] [PubMed] [Google Scholar]
- 10.Nakae, J., Kitamura, T., Kitamura, Y., Biggs, W. H., III, Arden, K. C., and Accili, D. (2003) Dev. Cell 4 119-129 [DOI] [PubMed] [Google Scholar]
- 11.Armoni, M., Harel, C., Karni, S., Chen, H., Bar-Yoseph, F., Ver, M. R., Quon, M. J., and Karnieli, E. (2006) J. Biol. Chem. 281 19881-19891 [DOI] [PubMed] [Google Scholar]
- 12.Davis, K. E., Moldes, M., and Farmer, S. R. (2004) J. Biol. Chem. 279 42453-42461 [DOI] [PubMed] [Google Scholar]
- 13.Qiao, L., and Shao, J. (2006) J. Biol. Chem. 281 39915-39924 [DOI] [PubMed] [Google Scholar]
- 14.Nakae, J., Cao, Y., Oki, M., Orba, Y., Sawa, H., Kiyonari, H., Iskandar, K., Suga, K., Lombes, M., and Hayashi, Y. (2008) Diabetes 57 563-576 [DOI] [PubMed] [Google Scholar]
- 15.Wolfrum, C., Shih, D. Q., Kuwajima, S., Norris, A. W., Kahn, C. R., and Stoffel, M. (2003) J. Clin. Investig. 112 345-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, J. K., Kim, H. J., Park, S. Y., Cederberg, A., Westergren, R., Nilsson, D., Higashimori, T., Cho, Y. R., Liu, Z. X., Dong, J., Cline, G. W., Enerback, S., and Shulman, G. I. (2005) Diabetes 54 1657-1663 [DOI] [PubMed] [Google Scholar]
- 17.Cederberg, A., Gronning, L. M., Ahren, B., Tasken, K., Carlsson, P., and Enerback, S. (2001) Cell 106 563-573 [DOI] [PubMed] [Google Scholar]
- 18.Gronning, L. M., Baillie, G. S., Cederberg, A., Lynch, M. J., Houslay, M. D., Enerback, S., and Tasken, K. (2006) FEBS Lett. 580 4126-4130 [DOI] [PubMed] [Google Scholar]
- 19.Gronning, L. M., Cederberg, A., Miura, N., Enerback, S., and Tasken, K. (2002) Mol. Endocrinol. 16 873-883 [DOI] [PubMed] [Google Scholar]
- 20.Steiner, A. B., Engleka, M. J., Lu, Q., Piwarzyk, E. C., Yaklichkin, S., Lefebvre, J. L., Walters, J. W., Pineda-Salgado, L., Labosky, P. A., and Kessler, D. S. (2006) Development (Camb.) 133 4827-4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raichur, S., Lau, P., Staels, B., and Muscat, G. E. (2007) J. Mol. Endocrinol. 39 29-44 [DOI] [PubMed] [Google Scholar]
- 22.Johnson, T. M., Meade, K., Pathak, N., Marques, M. R., and Attardi, L. D. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 1215-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarikji, Z. H., Vanamala, S., Beck, C. W., Wright, C. V., Leach, S. D., and Horb, M. E. (2007) Dev. Biol. 304 786-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reusch, J. E., and Klemm, D. J. (2002) J. Biol. Chem. 277 1426-1432 [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y. X., Lee, C. H., Tiep, S., Yu, R. T., Ham, J., Kang, H., and Evans, R. M. (2003) Cell 113 159-170 [DOI] [PubMed] [Google Scholar]
- 26.Hemati, N., Ross, S. E., Erickson, R. L., Groblewski, G. E., and MacDougald, O. A. (1997) J. Biol. Chem. 272 25913-25919 [DOI] [PubMed] [Google Scholar]
- 27.Erickson, R. L., Hemati, N., Ross, S. E., and MacDougald, O. A. (2001) J. Biol. Chem. 276 16348-16355 [DOI] [PubMed] [Google Scholar]
- 28.Rosen, E. D., Hsu, C. H., Wang, X., Sakai, S., Freeman, M. W., Gonzalez, F. J., and Spiegelman, B. M. (2002) Genes Dev. 16 22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang, S., Bennett, C. N., Gerin, I., Rapp, L. A., Hankenson, K. D., and Macdougald, O. A. (2007) J. Biol. Chem. 282 14515-14524 [DOI] [PubMed] [Google Scholar]
- 30.Chavey, C., Mari, B., Monthouel, M. N., Bonnafous, S., Anglard, P., Van Obberghen, E., and Tartare-Deckert, S. (2003) J. Biol. Chem. 278 11888-11896 [DOI] [PubMed] [Google Scholar]
- 31.Jing, E., Gesta, S., and Kahn, C. R. (2007) Cell Metab. 6 105-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jepsen, K., Gleiberman, A. S., Shi, C., Simon, D. I., and Rosenfeld, M. G. (2008) Genes Dev. 22 740-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St-Pierre, J., Drori, S., Uldry, M., Silvaggi, J. M., Rhee, J., Jager, S., Handschin, C., Zheng, K., Lin, J., Yang, W., Simon, D. K., Bachoo, R., and Spiegelman, B. M. (2006) Cell 127 397-408 [DOI] [PubMed] [Google Scholar]
- 34.Zhang, L., Rubins, N. E., Ahima, R. S., Greenbaum, L. E., and Kaestner, K. H. (2005) Cell Metab. 2 141-148 [DOI] [PubMed] [Google Scholar]
- 35.Lin, F. T., MacDougald, O. A., Diehl, A. M., and Lane, M. D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 9606-9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu, Z., Rosen, E. D., Brun, R., Hauser, S., Adelmant, G., Troy, A. E., McKeon, C., Darlington, G. J., and Spiegelman, B. M. (1999) Mol. Cell 3 151-158 [DOI] [PubMed] [Google Scholar]
- 37.El-Jack, A. K., Hamm, J. K., Pilch, P. F., and Farmer, S. R. (1999) J. Biol. Chem. 274 7946-7951 [DOI] [PubMed] [Google Scholar]
- 38.Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L., and MacDougald, O. A. (2000) Sciences (N. Y.) 289 950-953 [DOI] [PubMed] [Google Scholar]
- 39.Buas, M. F., Kabak, S., and Kadesch, T. (2009) J. Cell. Physiol. 218 84-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharff, K. A., Song, W. X., Luo, X., Tang, N., Luo, J., Chen, J., Bi, Y., He, B. C., Huang, J., Li, X., Jiang, W., Zhu, G. H., Su, Y., He, Y., Shen, J., Wang, Y., Chen, L., Zuo, G. W., Liu, B., Pan, X., Reid, R. R., Luu, H. H., Haydon, R. C., and He, T. C. (2008) J. Biol. Chem. 284 649-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori, T., Sakaue, H., Iguchi, H., Gomi, H., Okada, Y., Takashima, Y., Nakamura, K., Nakamura, T., Yamauchi, T., Kubota, N., Kadowaki, T., Matsuki, Y., Ogawa, W., Hiramatsu, R., and Kasuga, M. (2005) J. Biol. Chem. 280 12867-12875 [DOI] [PubMed] [Google Scholar]
- 42.Jimenez, M. A., Akerblad, P., Sigvardsson, M., and Rosen, E. D. (2007) Mol. Cell. Biol. 27 743-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park, B. H., Qiang, L., and Farmer, S. R. (2004) Mol. Cell. Biol. 24 8671-8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buratowski, S., and Chodosh, L. (2003) Current Protocols in Molecular Biology, pp. 1221-1227, John Wiley and Son, New York, NY
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.