Abstract
Therapeutics based on the actions of the incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), have recently been introduced for the treatment of type 2 diabetes mellitus. The serine/threonine kinase Akt is a major mediator of incretin action on the pancreatic islet, increasing β-cell mass and function and promoting β-cell survival. The mechanisms underlying incretin activation of Akt are thought to involve an essential phosphoinositide 3-kinase-mediated phosphorylation of threonine 308, similar to the prototypical Akt activator, insulin-like growth factor-I (IGF-I). In this study, using activity assays on immunoprecipitated Akt, we discovered that GIP and GLP-1 were capable of stimulating Akt in the INS-1 β-cell line and isolated mouse islets via a mechanism that did not require phosphoinositide 3-kinase or phosphorylation of Thr308 and Ser473, and this pathway involved the production of cAMP. Furthermore, we found that GIP stimulated anti-apoptotic signaling via this alternate mode of Akt activation. We conclude that incretins can activate Akt via a novel noncanonical mechanism that may provide an alternative therapeutic target for the treatment of type 2 diabetes mellitus and have broader implications for Akt physiology in human health and disease.
The incretin hormones, glucagon-like peptide-1 (GLP-1)2 and glucose-dependent insulinotropic polypeptide (GIP), have been targeted as therapeutics for type 2 diabetes mellitus (T2D) (1). This has resulted in the development of long acting GLP-1 receptor agonists such as exendin-4 (Byetta™) and inhibitors of the incretin-degrading enzyme dipeptidyl peptidase IV, Sitagliptin™ and Vildagliptin™ (1, 2). The effects of incretins on pancreatic islets are mediated through interaction with their cognate G protein-coupled receptors, resulting in several beneficial effects, including acute actions on pancreatic β- and α-cell secretion and increased islet growth and survival (2, 3). Stimulation of the β-cell by GLP-1 and GIP involves Akt signaling (4-8). This serine/threonine kinase promotes increases in β-cell number, size, function, and survival (9, 10). Interestingly, diminished signaling via the insulin receptor substrate-2 (IRS2)/phosphatidylinositol 3-kinase (PI3K)/Akt module has been postulated to play a major role in the susceptibility of β-cells to apoptosis in T2D (11). For this reason, understanding the underlying mode of Akt activation by incretins is of significant interest.
The stimulation of Akt by prototypical activators such as insulin-like growth factor-I (IGF-I) involves the activation of PI3K, resulting in the recruitment of Akt and PDK-1 (3-phosphoinositide dependent kinase-1) to the plasma membrane (12, 13). PDK-1 then phosphorylates threonine 308 of Akt (P-Akt308) (14), an event that has been deemed essential for Akt activation (12, 13, 15). Akt is also phosphorylated at a second site that is thought to be necessary to evoke full kinase activity, serine 473 (P-Akt473), in the C-terminal hydrophobic motif (16, 17). Recent evidence indicates that the major kinase responsible for this latter event is the mammalian target of the rapamycinrictor complex (mTORC2) (18). In contrast, activation of Akt by incretins appears to be more complex. Stimulation of the GLP-1 receptor in β-cells increases IRS2 protein levels (19) and activates PI3K via transactivation of the epidermal growth factor receptor (20, 21). Recently, GLP-1 was also shown to activate Akt in a PI3K-independent manner (6). The underlying mechanism by which GIP activates Akt is unclear. Stimulation of INS-1 β-cells with GIP increases Akt activity and levels of P-Akt473 (7, 22) through signaling pathways proposed to involve PI3K (7, 8), CAMK II and protein kinase A (PKA) (8). However, these pathways were identified in cells incubated in high glucose for relatively long periods (7, 8). In view of the powerful insulinotropic actions of GIP on β-cells, it was therefore difficult to discriminate between the direct actions of GIP and the autocrine actions of released insulin. Additionally, the majority of studies on Akt activity in β-cells have used indirect methods of measurement (i.e. phosphorylation of serine 473 and/or threonine 308), which may not necessarily equate with Akt activity. In the current study, using activity assays on immunoprecipitated Akt, we examined the role of PI3K in the direct and rapid activation of Akt by GIP in INS-1 cells and mouse islets. Surprisingly, we found that neither PI3K nor phosphorylation of Akt308 and Akt473 were essential for Akt activation. The pathway responsible appears to involve EPAC (exchange protein activated by cAMP)- but not PKA-mediated signaling. Furthermore, GLP-1 and forskolin but not insulin or high glucose stimulation exerted similar effects. We also showed that stimulation of this alternate mode of Akt activation by GIP promotes anti-apoptotic signaling. Given the therapeutic applications of incretins and the importance of Akt in promoting β-cell survival, this finding has important implications for T2D drug development.
EXPERIMENTAL PROCEDURES
Cell Culture for INS-1 Cells and Mouse Islets—The INS-1 β-cell line (clone 832/13) was kindly provided by Dr. C. B. Newgard (Duke University Medical Center). Cells were maintained in 11 mm glucose/RPMI 1640 medium (Sigma) supplemented with 2 mm glutamine, 50 μm β-mercaptoethanol, 10 mm HEPES, 1 mm sodium pyruvate, 10% fetal bovine serum, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. Mouse islets were isolated by collagenase digestion (23) from male C57BL/6 mice (10-12 weeks old; Jackson Laboratories) and maintained in RPMI 1640 medium supplemented with 5 mm glucose, 0.25% HEPES (pH 7.4), 10% fetal bovine serum, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate. Approximately 12 h prior to experiments, cells received fresh serum-starved medium (replaced with 0.1% bovine serum albumin) that contained low glucose (3 mm). Inhibitors were added 30 min prior to corresponding experiments and were from Calbiochem.
Plasmid Constructs and Transfection—The vector used was pcDNA3. The construct encoding hemagglutinin (HA)-tagged human Akt1 was acquired from William Sellers (24) through the Addgene Repository. Point mutations were generated by QuikChange (Stratagene). Briefly, the oligonucleotides used were ggt gcc acc atg aag gcc ttt tgc ggc aca c and gtg tgc cgc aaa agg cct tca tgg tgg cac c to generate a T308A mutation, ctt ccc cca gtt cgc cta ctc ggc cag and ctg gcc gag tag gcg aac tgg ggg aag to generate a S473A mutation, and ccg cta cta cgc cat gat gat cct caa gaa gga ag and ctt cct tct tga gga tca tca tgg cgt agt agc gg to generate a K179M mutation. To transfect cells, DNA was incubated with Lipofectamine™ LTX (Invitrogen) and PLUS™ reagent (Invitrogen) at a ratio of 1.0 μg of DNA/3.0 μl of Lipofectamine LTX™/1.0 μl of PLUS™ in 200 μl of OPTI-MEM I (Invitrogen) for 30 min at room temperature. This was subsequently added to wells (in a 6-well dish) containing 2 × 106 cells in 1 ml of maintenance medium without antibiotics. Experiments were performed 36 h following transfection.
Cell Lysis and in Vitro Kinase Assays—Cells were lysed in 20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin supplemented with 1 mm phenylmethylsulfonyl fluoride, and 1× proteinase inhibitor mixture set III (Calbiochem catalog no. 539134). Endogenous Akt kinase activity (KA) assays were performed using the Akt kinase assay kit (Nonradioactive 9840) from Cell Signaling Technology (Beverly, MA). Briefly, endogenous Akt was immunoprecipitated with an anti-Akt monoclonal antibody covalently linked with agarose beads (catalog no. 9279). Beads from immunoprecipitates were washed twice in lysis buffer and twice in kinase buffer and subsequently resuspended in 60 μl of kinase buffer containing 200 μm ATP and 0.75 μg of a glutathione S-transferase (GST)-tagged GSK3 fusion peptide (catalog no. 9237) that was used as a substrate for Akt and contained the optimal consensus sequence for Akt phosphorylation (25, 26). Kinase reactions were performed at 30 °C for 30 min. Kinase activity assays for the transfected HA-Akt1 protein were similarly performed except that the protein was immunoprecipitated with protein G beads (Amersham Biosciences) conjugated to anti-HA antibody (antibody 2367; Cell Signaling), and kinase reactions were extended for an additional 30-min time period. Phosphorylation of GST-GSK3 was determined via Western analysis with a phospho-specific antibody unique to the Akt kinase assay kit.
Western Blot—Cell lysates and kinase assays were subjected to 15% SDS-PAGE and electroblotted onto nitrocellulose membranes (Bio-Rad). Membranes were probed accordingly with the following antibodies from Cell Signaling Technology: anti-Akt (antibody 9272), anti-β-actin (antibody 4967), anti-GST (antibody 2622), anti-phospho-Akt (Thr308 (244F9); antibody 4056), anti-phospho-Akt (Ser473; antibody 9271), anti-phospho-Foxo1 (Ser256; antibody 9461), anti-phospho-MDM2 (Ser166; antibody 3521), and anti-phospho-Raf1 (Ser259; antibody 9421). The anti-MDM2 antibody was from Sigma (catalog no. M4308). Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Biosciences) using horseradish peroxidase-conjugated IgG secondary antibodies. For quantification of band density, films were analyzed using densitometric software (Eagle Eye, Stratagene).
Cell Death Assays—INS-1 cell death was induced by adding 100 nm staurosporine (Sigma catalog no. K1890) to the medium. INS-1 cells were treated in medium containing 500 ng/ml propidium iodide (Invitrogen) and 250 ng/ml Hoechst dye (Sigma). Cell death was measured by counting propidium iodide-positive nuclei, and total cell number was measured by counting Hoechst-positive nuclei. Propidium iodide and Hoechst were imaged with a high throughput imaging system (KineticScan, Cellomics Inc., Pittsburgh, PA), and % cell death was calculated as the number of propidium iodide-positive cells/Hoechst-positive cells multiplied by 100.
Statistical Analysis—Data, expressed as mean ± S.E., were analyzed using the nonlinear regression analysis program PRISM (GraphPad, San Diego, CA). Values of n in all cases represent individual experiments. The statistical significance of differences in mean value was tested using analysis of variance with Newman-Keuls post hoc test. A p value of <0.05 was considered significant.
RESULTS
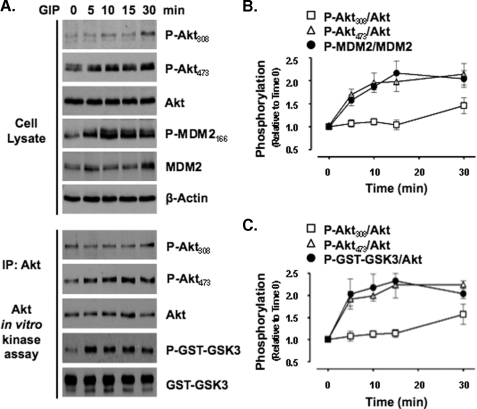
GIP Activates Akt without Increasing Phosphorylation of Threonine 308—To assess whether GIP directly activates Akt in β-cells, INS-1 cells were treated with GIP for 0-30 min in low glucose (3 mm) and serum-starved media, thus negating the secretion of insulin and the activation of Akt by serum growth factors. In response to GIP treatment, Western analysis of cell lysates showed that the levels of P-Akt473 increased as early as 5 min and remained elevated for the full 30-min period of study (Fig. 1, A-C). Similar temporal responses were observed with Akt activity, as demonstrated by the levels of phosphorylated Akt substrate peptide (P-GST-GSK3) in Akt KA assays on immunoprecipitated Akt and those of the endogenous Akt substrate, phosphorylated MDM2 (P-MDM2166), in cell lysates. Increases in levels of the phosphorylated forms of two additional Akt substrates that were also examined in cell lysates, GSK3α/β21/9 and Raf1259, exhibited the same temporal patterns (data not shown). However, surprisingly, levels of P-Akt308 did not change in either cell lysates or Akt KA assays until 30 min of stimulation.
FIGURE 1.
GIP activates Akt without increasing phosphorylation of Akt308. A, INS-1 cells were stimulated with 10 nm GIP for 0, 5, 10, 15, or 30 min, and Western analysis or Akt KA assays were performed on cell lysates with indicated antibodies. IP, immunoprecipitate. B and C, for quantification of P-Akt308, P-Akt473, and phosphorylated Akt substrate peptide (P-GST-GSK3), levels were normalized to total Akt, whereas P-MDM2166 was normalized to total MDM2. Shown are the mean ± S.E. changes in phosphorylated protein relative to time 0 (n ≥ 4) for cell lysates (B) and KA assays (C). Anti-β-actin and anti-GST (GST-GSK3) blots were internal controls.
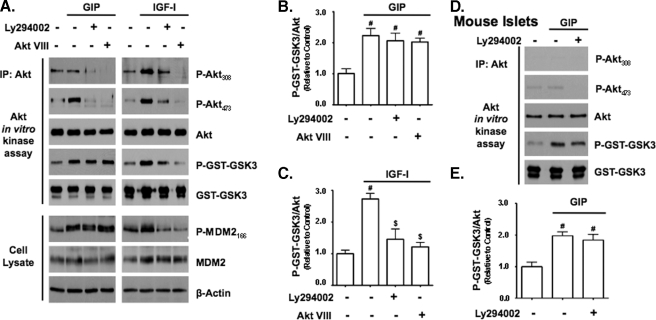
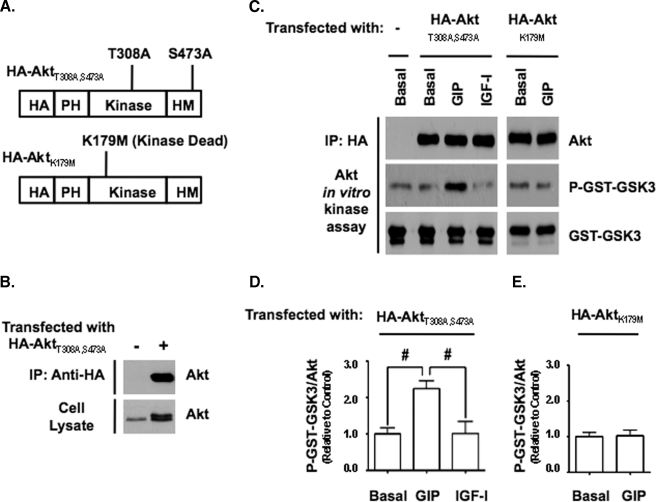
GIP Activates Akt Independently of PI3K and Phosphorylation of Akt308 and Akt473—Because phosphorylation of Akt at threonine 308 is PI3K-dependent, the results in Fig. 1 suggested that PI3K was not involved in the rapid GIP-stimulated activation of Akt. To exclude the involvement of PI3K, INS-1 cells were treated with or without the PI3K inhibitor, LY294002, or an inhibitor that prevents recruitment of Akt to the plasma membrane, Akt VIII (27-29), prior to a 15-min period of GIP stimulation (Fig. 2A). In addition, parallel experiments were performed with IGF-I because PI3K-dependent phosphorylation of Akt308 and Akt473 is a well established component of its Akt activation mechanism (17, 37). In the absence of an inhibitor, GIP increased Akt activity as well as levels of P-Akt473 but not P-Akt308, whereas IGF-I increased Akt activity and phosphorylation of both sites. In cells treated with either LY294002 or Akt VIII, levels of P-Akt308 and P-Akt473 were significantly reduced in both GIP- and IGF-I-treated cells, and this was associated with a significant reduction in Akt activity in IGF-I-treated cells (Fig. 2C), demonstrating that the canonical upstream Akt activation pathway was inhibited. In GIP-treated cells however, the activation of Akt was not inhibited by either LY294002 or Akt VIII (Fig. 2B), indicating that neither PI3K nor phosphorylation of Akt308 and Akt473 were required for GIP-stimulated activation of Akt in INS-1 cells. To validate these findings with primary cells, mouse islets were treated with LY294002 prior to GIP stimulation. As was found with INS-1 cells, GIP stimulated the enzyme activity of Akt irrespective of the presence or absence of LY294002 (Fig. 2, D and E). To definitively exclude a requirement for P-Akt308 and P-Akt473, INS-1 cells were transfected with plasmid DNA (Fig. 3A) encoding either HA-tagged human Akt1 (HA-Akt1) containing alanine point mutations at threonine 308 and serine 473 (HA-Akt1308/473) or a kinase-dead form containing a methionine point mutation at lysine 179 (HA-Akt1179), and responses to GIP or IGF-I stimulation for 15 min were compared with untreated cells by Western analysis (Fig. 3C). Stimulation of INS-1 cells with GIP but not IGF-I resulted in a significant increase in the kinase activity of HA-Akt1308/473 (Fig. 3D) but not HA-Akt1179 (Fig. 3E). To further examine the relationship between phosphorylation status and Akt activity, prior to GIP stimulation for 15 min, INS-1 cells were treated with or without an Akt inhibitor (Akt IV) that was shown to inhibit the activation of Akt by GLP-1 in β-cells, when the PI3K inhibitor LY294002 was ineffective (6). Although treatment of cells with Akt IV did not alter the basal activity of Akt (data not shown) and had no effect on the levels of P-Akt308 and P-Akt473, it ablated the stimulatory effect of GIP on Akt activity when assessed with both P-GST-GSK3 and P-MDM2 assays (Fig. 4, A and B).
FIGURE 2.
Inhibitors of the PI3K/Akt signaling module do not inhibit the activation of Akt by GIP. A, INS-1 cells were pretreated with dimethyl sulfoxide (DMSO; control) or inhibitors of PI3K signaling (15 μm LY294002 or 5 μm Akt VIII) and then treated with ±10 nm GIP or 10 nm IGF-I for 15 min. Western analysis and Akt KA assays were performed on cell lysates with indicated antibodies. IP, immunoprecipitate. B and C, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to DMSO control (n = 4) for GIP- (B) and IGF-I-treated (C) cells. #, p < 0.05 versus DMSO control; $, p < 0.05 versus IGF-I without inhibitor. D, mouse islets were pretreated with DMSO (control) or 15 μm LY294002 and then treated with ±10 nm GIP for 15 min. Western analysis and Akt KA assays were performed with indicated antibodies. E, shown is the mean change ± S.E. in Akt activity relative to DMSO control (n = 3). #, p < 0.05 versus DMSO control. Anti-β-actin and anti-GST (GST-GSK3) blots were internal controls.
FIGURE 3.
Phosphorylation of Akt at threonine 308 or serine 473 are not required for the activation of Akt by GIP. A, illustration of HA-Akt1 constructs and the respective point mutations used for this study. PH, pleckstrin homology domain; HM, hydrophobic motif. B, Western blot showing representative levels of HA-Akt1T308A/S473A protein in transfected cells relative to endogenous Akt in untransfected cells, as well as the purity of Akt1T308A/S473A immunoprecipitates. C, INS-1 cells transfected with HA-Akt1308/473 or HA-Akt1179 and 36 h later treated with ±10 nm GIP or 10 nm IGF-I for 15 min. Western analysis was performed on Akt KA assays with indicated antibodies. D and E, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to Basal (n = 4) for INS-1 cells transfected with HA-Akt1308/473 (D) or HA-Akt1179 (E). #, p < 0.05 versus Basal. Anti-GST (GST-GSK3) blots were internal controls.
FIGURE 4.
Akt IV inhibits the activation of Akt by GIP without affecting the phosphorylation of Akt at threonine 308 or serine 473. A, INS-1 cells were pretreated with DMSO (control) or Akt inhibitor (500 nm Akt IV) for 1 h and then treated with ±10 nm GIP for 15 min, and Western analysis was performed on cell lysates and Akt KA assays with indicated antibodies. IP, immunoprecipitate. B, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to DMSO control (n = 4). #, p < 0.05 versus DMSO control; $, p < 0.05 versus GIP without inhibitor. Anti-β-actin and anti-GST (GST-GSK3) blots were internal controls.
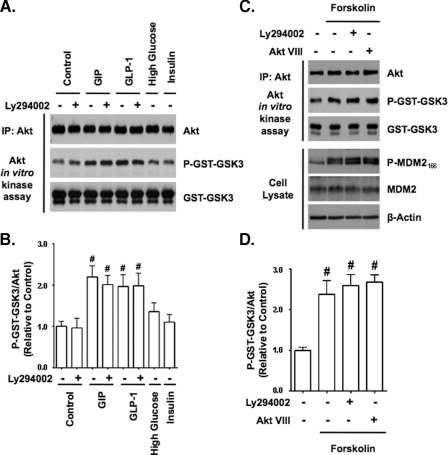
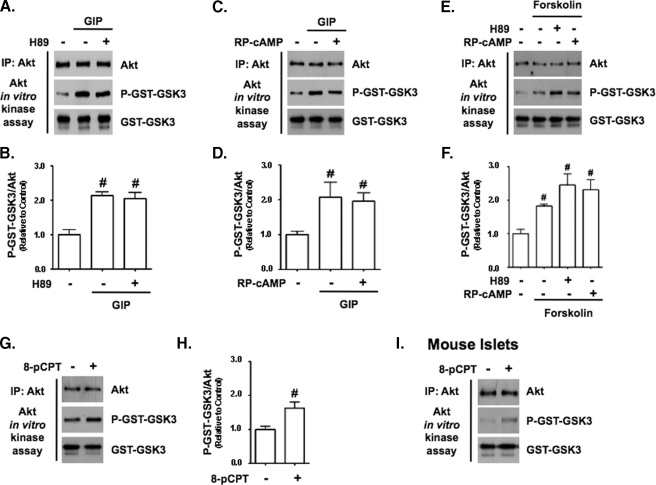
GLP-1 and Adenylate Cyclase Activation Mimic Akt Kinase Stimulatory Effects of GIP—Because both GIP and GLP-1 generally promote similar actions in β-cells through the stimulation of Gαs (30), we evaluated whether GLP-1 and forskolin also stimulated Akt in a PI3K-independent manner (Fig. 5A). In the presence of 3 mm glucose, GLP-1 activated Akt in a manner equivalent to GIP irrespective of the presence of LY294002, whereas neither high glucose nor insulin (500 pm) stimulated Akt activity (Fig. 5B), showing that the secreted insulin was unlikely to have contributed to incretin-induced activation. Forskolin (1 μm) also activated Akt in INS-1 cells, whether treated with or without LY294002 and Akt VIII (Fig. 5, C and D), indicating that cAMP production was likely involved in the underlying mode of Akt activation by GIP. Because GIP had been shown previously to activate PKA in INS-1 cells, its involvement in Akt activation was examined by treatment with the PKA inhibitor H89 or (Rp)-cAMP prior to a 15-min stimulation with GIP (Fig. 6, A-D) or forskolin (Fig. 6, E and F). It was found that neither inhibitor diminished the ability of GIP or forskolin to activate Akt. GIP has also been shown to activate the cAMP-responsive protein, EPAC, in β-cells (31). To examine a role for EPAC in Akt activation, INS-1 cells were treated with 100 μm 8-cpt-cAMP, an EPAC-selective agonist. This resulted in a significant increase in Akt activity (Fig. 6, G and H). To validate this finding with primary cells, mouse islets were similarly treated, and this also resulted in increased Akt activity (Fig. 6I).
FIGURE 5.
GLP-1 and forskolin but not high glucose nor insulin mimics the Akt kinase stimulatory effects of GIP. A, INS-1 cells were pretreated with DMSO (control) or 15 μm LY294002 for 30 min and then treated ± 10 nm GIP, 10 nm GLP-1, or 500 pm insulin (all with 3 mm glucose) or 16 mm glucose for 15 min, and Western analysis was performed on Akt KA assays with indicated antibodies. IP, immunoprecipitate. B, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to DMSO (n = 4). #, p < 0.05 versus control without LY294002. C, INS-1 cells were pretreated with DMSO (control) or PI3K/Akt signaling inhibitors (15 μm LY294002 or 5 μm Akt VIII) for 1 h and then treated with ±1 μm forskolin for 15 min, and Western analysis was performed on cell lysates or Akt KA assays with indicated antibodies. D, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to DMSO control (n = 4). #, p < 0.05 versus DMSO control. Anti-β-actin and anti-GST (GST-GSK3) blots were internal controls.
FIGURE 6.
Stimulation of Akt by GIP involves EPAC signaling but not PKA. A, C, and E, INS-1 cells were pretreated with DMSO (control) or PKA inhibitor, 10 μm H89 (A and E) or 250 μm (Rp)-cAMP (C and E) for 30 min and then treated with ±10 nm GIP (A and C) or 1 μm forskolin (E) for 15 min, and Western analysis was performed on Akt KA assays with indicated antibodies. IP, immunoprecipitate. B, D, and F, shown is the mean change ± S.E. in Akt activity (P-GSK3sub/Akt) relative to DMSO control (n = 4) in GIP-treated (B and D) or forskolin-treated (F) cells. #, p < 0.05 versus DMSO control. G, INS-1 cells were treated with ±100 μm of the EPAC agonist, 8-cpt-2′-O-Me-cAMP (8-cpt-cAMP), for 15 min, and Western analysis was performed on Akt KA assays with the indicated antibodies. H, shown is the mean change ± S.E. in Akt activity relative to control (n = 4). I, mouse islets were treated with ± 100 μm 8-cpt-cAMP for 15 min, and Western analysis was performed on Akt KA assays with the indicated antibodies (n = 1). Anti-GST (GST-GSK3) blots were internal controls.
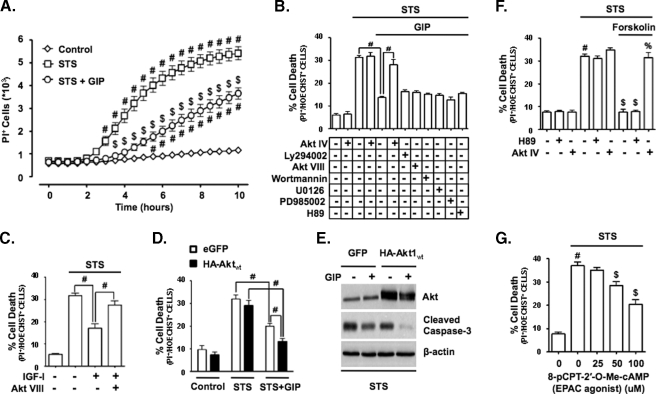
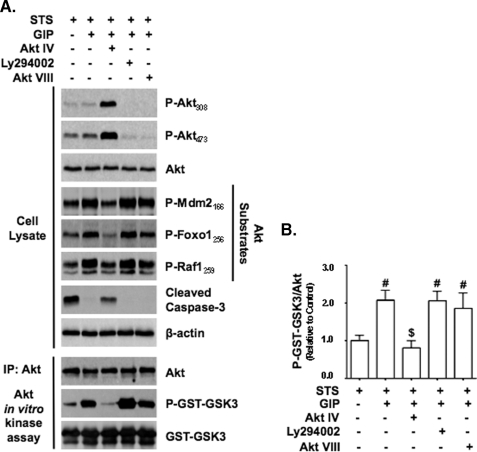
Alternative Mode of Akt Activation by GIP Promotes INS-1 Survival—A major effect of Akt signaling is to promote cell survival. To assess whether the alternative mode of Akt activation by GIP was functionally important, we examined whether GIP could promote INS-1 cell survival via Akt signaling. Staurosporine (STS), a well characterized apoptosis-inducing agent (32), rapidly induced INS-1 cell death, and GIP potently suppressed this effect (Fig. 7A). To establish that GIP-mediated survival involved the activation of Akt in a PI3K-independent manner, several approaches were used. First, the pro-survival effects of GIP were determined in the absence or presence of an Akt inhibitor (Akt IV), PI3K/Akt pathway inhibitors (LY294002, Akt VIII, or wortmannin), or inhibitors of other kinases shown to be involved in GIP action: Erk1/2 (UO126 or PD985002) or PKA (H89). GIP strongly suppressed STS-induced cell death, and only Akt IV reversed this effect (Fig. 7B). By comparison, IGF-I promotion of INS-1 survival under STS treatment was blocked by inhibiting PI3K/Akt signaling with Akt VIII (Fig. 7C). Second, INS-1 cells were transfected with wild-type HA-Akt1 or enhanced green fluorescent protein and then treated with STS ± GIP. The % reduction and absolute reduction in cell death resulting from GIP treatment were both greater in cells transfected with HA-tagged wild-type Akt1 than those transfected with enhanced green fluorescent protein (Fig. 7D), as was the reduction in levels of cleaved caspase-3 (Fig. 7E). To validate that this survival pathway was mediated via cAMP production but not PKA signaling, forskolin was added to STS-treated INS-1 cells in the presence or absence of the PKA inhibitor H89 or the Akt inhibitor Akt IV (Fig. 7F). Forskolin stimulation promoted INS-1 survival, and this was completely ablated by Akt IV, but H89 was without effect. To determine whether EPAC signaling was involved in GIP-mediated Akt activation and associated with cell survival, STS-treated INS-1 cells were stimulated with 8-cpt-cAMP. The EPAC activator promoted cell survival in a dose-dependent manner (Fig. 7G). Lastly, it was assessed whether GIP stimulated Akt signaling in STS-treated INS-1 cells. Cells were treated with or without Akt IV, LY294002, or Akt VIII prior to the addition of STS ± GIP. It was found that GIP stimulated the activation of Akt, as shown by the increased phosphorylation levels of three Akt substrates, Foxo1, Raf1, and MDM2, in cell lysates and GST-GSK3 in Akt KA assays, despite the loss of Akt phosphorylation in LY294002- and Akt VIII-treated cells (Fig. 8, A and B). Conversely, despite a robust increase in Akt phosphorylation in Akt IV-treated cells, the activation of Akt by GIP was completely ablated, and this was associated with increased levels of cleaved caspase-3. Together, these studies therefore strongly support the existence of a GIP-mediated Akt activation mechanism that is independent of the canonical pathway involving phosphorylation of threonine 308 and serine 473 and indicate that this alternate pathway is central to the anti-apoptotic effects of the incretin hormones.
FIGURE 7.
GIP stimulation of survival in STS-treated INS-1 cells involves Akt. A, INS-1 cells were treated without or with 100 nm STS and with or without 10 nm GIP for 10 h, and onset of cell death (defined as propidium iodide-positive (PI+) incorporation into cell nuclei) was measured in real time every 30 min. The mean ± S.E. of dead cells (n = 6) is shown. #, p < 0.05 versus DMSO control; $, p < 0.05 versus STS without GIP. B, cell death (%) in INS-1 cells treated for 6 h with STS (100 nm), with or without 10 nm GIP, and without or with an inhibitor of Akt (500 nm Akt IV), PI3K signaling (15 μm LY294002, 5 μm Akt VIII, or 200 nm wortmannin), Erk1/2 signaling (5 μm U0126 or 50 μm PD985002), or PKA signaling (10 μm H89). Mean ± S.E. of dead cells (n = 6). #, p < 0.05 (comparisons are shown). C, shown is the cell death (%) in INS-1 cells treated for 6 h with STS ± 10 nm IGF-I ± PI3K signaling inhibitor, 5 μm Akt VIII. Mean ± S.E. of dead cells (n = 4); #, p < 0.05 (comparisons are shown). D, shown is the cell death (%) in INS-1 cells transfected with enhanced green fluorescent protein (eGFP) or HA-tagged wild-type Akt1 and treated for 6 h with STS ± GIP. Mean ± S.E. of % cell death (n = 4). #, p < 0.05 (comparisons are shown). E, INS-1 cells were transfected with green fluorescent protein or HA-tagged wild-type Akt and treated for 4 h with STS ± GIP, and Western analysis was performed with indicated antibodies. F, shown is the cell death (%) in INS-1 cells treated for 6 h with STS (100 nm), without or with 1 μm forskolin, and without or with inhibitors of Akt signaling (500 nm Akt IV) or PKA signaling (10 μm H89). Mean ± S.E. of dead cells (n = 6); #, p < 0.05 versus DMSO control; $, p < 0.05 versus STS without inhibitor; %, p < 0.05 versus STS + forskolin. G, shown is the cell death (%) in INS-1 cells treated for 6 h with STS (100 nm) + increasing concentrations of the EPAC agonist, 8-cpt-cAMP. Mean ± S.E. of dead cells (n = 6). #, p < 0.05 versus DMSO control; $, p < 0.05 versus STS without 8-cpt-cAMP. Anti-β-actin blots were internal controls.
FIGURE 8.
GIP stimulates Akt signaling in STS-treated INS-1 cells. A, INS-1 cells were treated with STS without or with GIP for 4 h without or with Akt inhibitor, 500 nm Akt IV, or PI3K signaling inhibitors (15 μm LY294002 or 5 μm Akt VIII), and Western analysis was performed on cell lysates and Akt KA assays with indicated antibodies. IP, immunoprecipitate. B, shown is the mean change ± S.E. in Akt activity (P-GST-GSK3/Akt) relative to control (n = 4). #, p < 0.05 versus control; $, p < 0.05 versus GIP without inhibitor. Anti-β-actin and anti-GST (GST-GSK3) blots were internal controls.
DISCUSSION
The major physiological action of incretin hormones is considered to be the potentiation of glucose-stimulated insulin secretion from the β-cell during a meal (30). However, additional important effects of the incretins on β-cells have been described, including the stimulation of insulin biosynthesis as well as β-cell proliferation (33) and survival (2, 3). Because diminished β-cell function (34) and survival (35) are central to the pathogenesis of T2D, enhancing the actions of incretins has been a major focus for the treatment of diabetes (1, 36), and such therapies have shown very promising results (2, 37, 38).
In the present study, we evaluated the role of PI3K in Akt activation by GIP in the INS-1 β-cell. Consistent with previous reports (7, 8, 22), GIP treatment increased Akt activity in association with increases in P-Akt473 levels (Fig. 1). However, GIP-mediated stimulation of Akt activity was not found to require PI3K or phosphorylation of Akt308, and even the phosphorylation of Akt473 was not essential for the stimulation of enzyme activity in INS-1 cells or mouse islets (Fig. 2, A, B, D, and E; and Fig. 3, C and D). This was in contrast to IGF-I, which was unable to activate Akt in the presence of PI3K/Akt pathway inhibitors (Fig. 2, A and C) or activate transfected HA-Akt1 protein that contained alanine point mutations at threonine 308 and serine 473 (Fig. 3, C and D), consistent with previous studies (12, 13). One caveat of using inhibitors is that they often lack absolute target specificity and, indeed, LY294002 (39) and Akt VIII (27) have been shown to inhibit off-target proteins. However, because LY294002 and Akt VIII are potent inhibitors of PI3K/Akt signaling, yet did not affect GIP-stimulated activation of Akt, such off-target effects of either inhibitor did not contribute to the responses obtained. On the basis of these results, we conclude that GIP stimulated Akt via a noncanonical pathway. Although the mechanism of action of Akt IV has not been clarified because it blocked the activation of Akt by GIP (Fig. 4), we propose that it interferes somehow with this alternative mode of Akt activation. Similar to GIP, GLP-1 also stimulated Akt in a PI3K-independent manner (Fig. 5, A and B), a finding that is consistent with the observations of Liu and Habener (6), who demonstrated that Akt IV but not LY294002 prevented Akt-mediated activation of the Wnt signaling pathway under GLP-1-stimulated conditions, although they did not assess the underlying mechanism of Akt activation. Furthermore, neither high glucose nor insulin promoted Akt activation under the applied experimental conditions. This finding is consistent with studies by Rhodes and colleagues, who have shown that neither insulin (40) nor high glucose (41) has any notable effect on Akt in β-cells during short term stimulation. Thus, we conclude that the effects on Akt in the present study were a result of direct incretin stimulation and not autocrine effects of secreted insulin. Moreover, incretins stimulate Gαs (30) and cAMP production (42-44) in β-cells, and forskolin was also capable of stimulating Akt activity in a PI3K-independent manner (Fig. 5, C and D), indicating a likely role for cAMP in incretin-mediated stimulation of Akt activity. Because a role for PKA was not supported in our study (Fig. 6, A-F), we proposed that this alternative mode of Akt activation could involve EPAC proteins, which have been described to be activated by both GIP and GLP-1 in β-cells (31). Indeed, we showed that the stimulation of INS-1 cells with 8-cpt-cAMP could enhance Akt activity (Fig. 6, G-I). The mechanism by which EPAC mediates this response is currently unknown.
There has been a widely held view that incretins activate Akt via the canonical PI3K-mediated pathway (1, 3, 45, 46). This proposal was based mainly on the fact that PI3K inhibitors prevented the phosphorylation of Akt at serine 473. However, the current studies demonstrate that GIP and GLP-1 are capable of activating Akt independently of PI3K, indicating that the role of Akt in GLP-1- and GIP-mediated actions needs to be re-evaluated. For example, in the recent study by Yusta et al. (47) it was reasoned that Akt was not essential for GLP-1-mediated survival of β-cells under conditions of endoplasmic reticulum stress. This conclusion was based on the finding that LY294002 blocked Akt phosphorylation but did not influence GLP-1-mediated effects on eIF2α, ATF-4, or CHOP in cells exposed to thapsigargin. However, because LY294002 does not appear to suppress GLP-1-mediated activation of Akt activity, a role for Akt cannot be excluded. The determination of the phosphorylation state, rather than enzyme activity, may also explain the disparate results regarding the mode of action of GLP-1 reported in the literature (5, 48). Wang et al. (5) showed that GLP-1 acutely stimulated phosphorylation of Akt at serine 473 in a PI3K-dependent manner and concluded that GLP-1 rapidly increased Akt enzyme activity. On the other hand, Tews et al. (48), using the same cell line, reported that phosphorylation of serine 473 did not occur until after 24 h and concluded that GLP-1 activated Akt indirectly through elevations in IRS2 protein levels. Previous studies on the GIP-mediated activation of Akt in β-cells using PI3K inhibitors, including our own, have examined only the phosphorylation of Akt at serine 473 (8) or applied an inhibitor for a prolonged period of time (6 h) (7). To our knowledge, the functional relationships between the phosphorylation status of threonine 308 and serine 473 and early GIP stimulation of Akt kinase activity and the phosphorylation of endogenous Akt substrates (e.g. MDM2) have not been studied previously in β-cells. On the basis of the current results, we conclude that their phosphorylation is not required for Akt activation.
The serine/threonine kinase Akt plays a central role in glucose homeostasis (49) and the promotion of β-cell survival (9, 10), including incretin-mediated responses (4-8). Mice expressing constitutively active Akt in β-cells develop larger islet mass and have improved glucose tolerance and resistance to diabetes (50, 51), and transplanted human islets expressing constitutively active Akt improve glucose tolerance more effectively in diabetic mice (52). Conversely, transgenic mice expressing kinase-dead Akt in β-cells develop diabetes due to defects in insulin secretion (53). It has been argued by Rhodes (11) that diminished signaling via the IRS2/PI3K/Akt module plays a major role in the susceptibility of β-cells to apoptosis in T2D, and enhancing β-cell Akt signaling has been proposed as an alternative therapy for T2D, with a view to maintaining or increasing functional β-cell mass (9, 10). In light of the powerful pro-survival effects exerted by Akt (15), we examined the underlying mechanisms by which GIP promotes β-cell survival using INS-1 cells treated with the well characterized apoptosis-inducing agent STS. In the presence of STS, GIP promotion of INS-1 survival (Fig. 7A) was only blocked by the Akt inhibitor, Akt IV (Fig. 7B) and not by the inhibitors of PI3K, Erk1/2, or PKA signaling. Akt IV also blocked the survival effects of forskolin, whereas the inhibition of PKA was without effect (Fig. 7F). These results indicated that EPAC-mediated activation of Akt promotes GIP-mediated survival of STS-treated INS-1 cells. And, indeed, EPAC activation promoted cell survival (Fig. 7G). By comparison, although IGF-I also promoted INS-1 cell survival, inhibition of Akt signaling with Akt VIII blocked this effect (Fig. 7C). Interestingly, STS+GIP-treated INS-1 cells exhibited a large increase in P-Akt308 and P-Akt473 levels in the presence of Akt IV, despite a complete ablation of Akt signaling (Fig. 8). As with kinase activation, these differential effects of Akt inhibitors demonstrate that the promotion of cell survival involves an alternate mode of Akt activation.
Alternative PI3K-independent pathways for Akt activation have been reported that involve the stimulation of cAMP production (54) and PKA (55), the activation of calcium/calmodulin-dependent kinase kinase (56), as well as other undefined pathways (57), but to our knowledge, this is the first demonstration of a hormonal activation of Akt that does not depend on the phosphorylation of Akt308. This finding is contrary to the current consensus that the phosphorylation of threonine 308 is essential for Akt activation (12, 13, 15, 16). The main reason that such an alternative pathway has been previously overlooked is probably because the focus of the majority of studies on the mechanism of Akt activation has been via receptor tyrosine kinases (e.g. the IGF-I receptor), rather than G protein-coupled receptors such as those for the incretins. In support of this proposal, β-adrenergic agonists were found to activate Akt in a wortmannin-insensitive manner in rat adipocytes (57), but an in-depth analysis of the Akt phosphorylation status was not performed because phosphorylation-specific antibodies were not yet available. Because forskolin stimulation led to Akt activation in INS-1 cells, similar to GIP stimulation, it is quite likely that the activation of several G protein-coupled receptor proteins that are present on β-cells (e.g. PACAP and glucagon) may also promote a similar response. The biological significance of this will be an interesting avenue of future research.
Three major questions arise from this study. 1) What is the signaling mechanism by which EPAC mediates Akt activation by incretins? 2) Can Akt be activated in a similar manner in vivo, and if so, can this be exploited as a therapy for diabetes? 3) Is Akt activated in a similar manner in other tissues by other hormones or growth factors? Because, in addition to its importance in β-cell survival, Akt is also crucial for insulin-mediated glucose disposal (49) and the coordination of a wide range of additional biological actions (15), the answers to these questions may uncover further aspects of Akt physiology that can be targeted for therapeutics.
Acknowledgments
We thank Dr. C. B. Newgard (Duke University Medical Center, Durham, NC) for kindly providing us with INS-1 cells (clone 832/13) and Dr. William Sellers (the Addgene Repository) for providing the HA-Akt1 construct.
This work was supported by the Canadian Institutes of Health Research and the Canadian Foundation for Innovation (to C. H. S. M.) and scholarships from the Natural Sciences and Engineering Research Council of Canada and the Michael Smith Foundation for Health Research (to S. W.).
Footnotes
The abbreviations used are: GLP-1, glucagon-like peptide-1; T2D, type 2 diabetes mellitus; HA, hemagglutinin; KA, kinase activity; PKA, protein kinase A; GST, glutathione S-transferase; PI3K, phosphatidylinositol 3-kinase; DMSO, dimethyl sulfoxide; 8-cpt-cAMP, 8-(4-chlorophenylthio)-2′-O-methyl-cAMP; GIP, glucose-dependent insulinotropic polypeptide; IGF-I, insulin-like growth factor-I; STS, staurosporine; IRS2, insulin receptor substrate-2.
References
- 1.Salehi, M., Aulinger, B. A., and D'Alessio, D. A. (2008) Endocr. Rev. 29 367-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntosh, C. H. (2008) Front Biosci. 13 1753-1773 [DOI] [PubMed] [Google Scholar]
- 3.Baggio, L. L., and Drucker, D. J. (2007) Gastroenterology 132 2131-2157 [DOI] [PubMed] [Google Scholar]
- 4.Lingohr, M. K., Buettner, R., and Rhodes, C. J. (2002) Trends Mol. Med. 8 375-384 [DOI] [PubMed] [Google Scholar]
- 5.Wang, Q., Li, L., Xu, E., Wong, V., Rhodes, C., and Brubaker, P. L. (2004) Diabetologia 47 478-487 [DOI] [PubMed] [Google Scholar]
- 6.Liu, Z., and Habener, J. F. (2008) J. Biol. Chem. 283 8723-8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, S. J., Winter, K., Nian, C., Tsuneoka, M., Koda, Y., and McIntosh, C. H. (2005) J. Biol. Chem. 280 22297-22307 [DOI] [PubMed] [Google Scholar]
- 8.Trumper, A., Trumper, K., and Horsch, D. (2002) J. Endocrinol. 174 233-246 [DOI] [PubMed] [Google Scholar]
- 9.Elghazi, L., Balcazar, N., and Bernal-Mizrachi, E. (2006) Int. J. Biochem. Cell Biol. 38 157-163 [DOI] [PubMed] [Google Scholar]
- 10.Dickson, L. M., and Rhodes, C. J. (2004) Am. J. Physiol. 287 E192-E198 [DOI] [PubMed] [Google Scholar]
- 11.Rhodes, C. J. (2005) Science 307 380-384 [DOI] [PubMed] [Google Scholar]
- 12.Hanada, M., Feng, J., and Hemmings, B. A. (2004) Biochim. Biophys. Acta 1697 3-16 [DOI] [PubMed] [Google Scholar]
- 13.Alessi, D. R., Andjelkovic, M., Caudwell, B., Cron, P., Morrice, N., Cohen, P., and Hemmings, B. A. (1996) EMBO J. 15 6541-6551 [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens, L., Anderson, K., Stokoe, D., Erdjument-Bromage, H., Painter, G. F., Holmes, A. B., Gaffney, P. R., Reese, C. B., McCormick, F., Tempst, P., Coadwell, J., and Hawkins, P. T. (1998) Science 279 710-714 [DOI] [PubMed] [Google Scholar]
- 15.Manning, B. D., and Cantley, L. C. (2007) Cell 129 1261-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, J., Cron, P., Good, V. M., Thompson, V., Hemmings, B. A., and Barford, D. (2002) Nat. Struct. Biol. 9 940-944 [DOI] [PubMed] [Google Scholar]
- 17.Yang, J., Cron, P., Thompson, V., Good, V. M., Hess, D., Hemmings, B. A., and Barford, D. (2002) Mol. Cell 9 1227-1240 [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005) Science 307 1098-1101 [DOI] [PubMed] [Google Scholar]
- 19.Park, S., Dong, X., Fisher, T. L., Dunn, S., Omer, A. K., Weir, G., and White, M. F. (2006) J. Biol. Chem. 281 1159-1168 [DOI] [PubMed] [Google Scholar]
- 20.Buteau, J., Roduit, R., Susini, S., and Prentki, M. (1999) Diabetologia 42 856-864 [DOI] [PubMed] [Google Scholar]
- 21.Buteau, J., Foisy, S., Joly, E., and Prentki, M. (2003) Diabetes 52 124-132 [DOI] [PubMed] [Google Scholar]
- 22.Trumper, A., Trumper, K., Trusheim, H., Arnold, R., Goke, B., and Horsch, D. (2001) Mol. Endocrinol. 15 1559-1570 [DOI] [PubMed] [Google Scholar]
- 23.Salvalaggio, P. R., Deng, S., Ariyan, C. E., Millet, I., Zawalich, W. S., Basadonna, G. P., and Rothstein, D. M. (2002) Transplantation 74 877-879 [DOI] [PubMed] [Google Scholar]
- 24.Hsieh, A. C., Bo, R., Manola, J., Vazquez, F., Bare, O., Khvorova, A., Scaringe, S., and Sellers, W. R. (2004) Nucleic Acids Res. 32 893-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alessi, D. R., Caudwell, F. B., Andjelkovic, M., Hemmings, B. A., and Cohen, P. (1996) FEBS Lett. 399 333-338 [DOI] [PubMed] [Google Scholar]
- 26.Obata, T., Yaffe, M. B., Leparc, G. G., Piro, E. T., Maegawa, H., Kashiwagi, A., Kikkawa, R., and Cantley, L. C. (2000) J. Biol. Chem. 275 36108-36115 [DOI] [PubMed] [Google Scholar]
- 27.Logie, L., Ruiz-Alcaraz, A. J., Keane, M., Woods, Y. L., Bain, J., Marquez, R., Alessi, D. R., and Sutherland, C. (2007) Diabetes 56 2218-2227 [DOI] [PubMed] [Google Scholar]
- 28.Barnett, S. F., Defeo-Jones, D., Fu, S., Hancock, P. J., Haskell, K. M., Jones, R. E., Kahana, J. A., Kral, A. M., Leander, K., Lee, L. L., Malinowski, J., McAvoy, E. M., Nahas, D. D., Robinson, R. G., and Huber, H. E. (2005) Biochem. J. 385 399-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green, C. J., Goransson, O., Kular, G. S., Leslie, N. R., Gray, A., Alessi, D. R., Sakamoto, K., and Hundal, H. S. (2008) J. Biol. Chem. 283 27653-27667 [DOI] [PubMed] [Google Scholar]
- 30.Drucker, D. J. (2006) Cell Metab. 3 153-165 [DOI] [PubMed] [Google Scholar]
- 31.Kashima, Y., Miki, T., Shibasaki, T., Ozaki, N., Miyazaki, M., Yano, H., and Seino, S. (2001) J. Biol. Chem. 276 46046-46053 [DOI] [PubMed] [Google Scholar]
- 32.Seleznev, K., Zhao, C., Zhang, X. H., Song, K., and Ma, Z. A. (2006) J. Biol. Chem. 281 22275-22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedrichsen, B. N., Neubauer, N., Lee, Y. C., Gram, V. K., Blume, N., Petersen, J. S., Nielsen, J. H., and Moldrup, A. (2006) J. Endocrinol. 188 481-492 [DOI] [PubMed] [Google Scholar]
- 34.Kahn, S. E. (2003) Diabetologia 46 3-19 [DOI] [PubMed] [Google Scholar]
- 35.Butler, A. E., Janson, J., Bonner-Weir, S., Ritzel, R., Rizza, R. A., and Butler, P. C. (2003) Diabetes 52 102-110 [DOI] [PubMed] [Google Scholar]
- 36.Wajchenberg, B. L. (2007) Endocr. Rev. 28 187-218 [DOI] [PubMed] [Google Scholar]
- 37.Balas, B., Baig, M. R., Watson, C., Dunning, B. E., Ligueros-Saylan, M., Wang, Y., He, Y. L., Darland, C., Holst, J. J., Deacon, C. F., Cusi, K., Mari, A., Foley, J. E., and DeFronzo, R. A. (2007) J. Clin. Endocrinol. Metab. 92 1249-1255 [DOI] [PubMed] [Google Scholar]
- 38.DeFronzo, R. A., Ratner, R. E., Han, J., Kim, D. D., Fineman, M. S., and Baron, A. D. (2005) Diabetes Care 28 1092-1100 [DOI] [PubMed] [Google Scholar]
- 39.Davies, S. P., Reddy, H., Caivano, M., and Cohen, P. (2000) Biochem. J. 351 95-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wicksteed, B., Alarcon, C., Briaud, I., Lingohr, M. K., and Rhodes, C. J. (2003) J. Biol. Chem. 278 42080-42090 [DOI] [PubMed] [Google Scholar]
- 41.Dickson, L. M., Lingohr, M. K., McCuaig, J., Hugl, S. R., Snow, L., Kahn, B. B., Myers, M. G., Jr., and Rhodes, C. J. (2001) J. Biol. Chem. 276 21110-21120 [DOI] [PubMed] [Google Scholar]
- 42.Ehses, J. A., Casilla, V. R., Doty, T., Pospisilik, J. A., Winter, K. D., Demuth, H. U., Pederson, R. A., and McIntosh, C. H. (2003) Endocrinology 144 4433-4445 [DOI] [PubMed] [Google Scholar]
- 43.Siegel, E. G., and Creutzfeldt, W. (1985) Diabetologia 28 857-861 [DOI] [PubMed] [Google Scholar]
- 44.Leech, C. A., Holz, G. G., and Habener, J. F. (1996) Ann. N. Y. Acad. Sci. 805 81-92; Discussion 92-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holz, G. G. (2004) Diabetes 53 5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, W., and Egan, J. M. (2008) Pharmacol. Rev. 60 470-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yusta, B., Baggio, L. L., Estall, J. L., Koehler, J. A., Holland, D. P., Li, H., Pipeleers, D., Ling, Z., and Drucker, D. J. (2006) Cell Metab. 4 391-406 [DOI] [PubMed] [Google Scholar]
- 48.Tews, D., Werner, U., and Eckel, J. (2008) Horm. Metab. Res. 40 172-180 [DOI] [PubMed] [Google Scholar]
- 49.Whiteman, E. L., Cho, H., and Birnbaum, M. J. (2002) Trends Endocrinol. Metab. 13 444-451 [DOI] [PubMed] [Google Scholar]
- 50.Tuttle, R. L., Gill, N. S., Pugh, W., Lee, J. P., Koeberlein, B., Furth, E. E., Polonsky, K. S., Naji, A., and Birnbaum, M. J. (2001) Nat. Med. 7 1133-1137 [DOI] [PubMed] [Google Scholar]
- 51.Bernal-Mizrachi, E., Wen, W., Stahlhut, S., Welling, C. M., and Permutt, M. A. (2001) J. Clin. Investig. 108 1631-1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao, P., Roccisana, J., Takane, K. K., Bottino, R., Zhao, A., Trucco, M., and Garcia-Ocana, A. (2005) Diabetes 54 1664-1675 [DOI] [PubMed] [Google Scholar]
- 53.Bernal-Mizrachi, E., Fatrai, S., Johnson, J. D., Ohsugi, M., Otani, K., Han, Z., Polonsky, K. S., and Permutt, M. A. (2004) J. Clin. Investig. 114 928-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sable, C. L., Filippa, N., Hemmings, B., and Van Obberghen, E. (1997) FEBS Lett. 409 253-257 [DOI] [PubMed] [Google Scholar]
- 55.Filippa, N., Sable, C. L., Filloux, C., Hemmings, B., and Van Obberghen, E. (1999) Mol. Cell. Biol. 19 4989-5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano, S., Tokumitsu, H., and Soderling, T. R. (1998) Nature 396 584-587 [DOI] [PubMed] [Google Scholar]
- 57.Moule, S. K., Welsh, G. I., Edgell, N. J., Foulstone, E. J., Proud, C. G., and Denton, R. M. (1997) J. Biol. Chem. 272 7713-7719 [DOI] [PubMed] [Google Scholar]