Abstract
JDP2 (Jun dimerization protein 2, an AP-1 transcription factor) is involved in the regulation of the differentiation and proliferation of cells. We report here that JDP2-deficient mouse embryonic fibroblasts (Jdp2-/- MEF) are resistant to replicative senescence. In the absence of JDP2, the level of expression of p16Ink4a, which is known to rise as normal fibroblasts age, fell significantly when cells were cultured for more than 2 months. Conversely, the overexpression of JDP2 induced the expression of genes for p16Ink4a and p19Arf. Moreover, at the promoter of the gene for p16Ink4a in Jdp2-/- MEF, the extent of methylation of lysine 27 of histone H3 (H3K27), which is important for gene silencing, increased. Polycomb-repressive complexes (PRC-1 and PRC-2), which are responsible for histone methylation, bound efficiently to the promoter to repress the expression of the gene for p16Ink4a. As a result, JDP2-deficient MEF became resistant to replicative senescence. Our results indicate that JDP2 is involved in the signaling pathway for senescence via epigenetic regulation of the expression of the gene for p16Ink4a.
Cells in primary cultures of untransformed cells stop growing after several weeks and undergo senescence, a phenomenon that is related to so-called “cellular aging. ” Senescence protects normal cells from abnormal growth signals and oncogenic transformation by interrupting the cell cycle. Senescence is induced not only by cellular aging but also by the forced activation of the MAPK3 pathway and by genotoxic stressors, such as peroxide and certain DNA-damaging compounds. There is evidence to suggest that, in mice, two inhibitors of progression of the cell cycle, p16Ink4a and p19Arf (Arf and p14ARF in humans), are the main regulators of senescence. These proteins are encoded by overlapping reading frames at the CDKN2A (MTS1) locus (1-3). The expression of both p16Ink4a and p19Arf is enhanced in rodent cells with aging (1, 2). By contrast, in human cells, senescence is generally associated with the increased expression of p16Ink4a but not of Arf (1-3). Several types of stimuli have been reported to induce the expression of p16Ink4a and/or p19Arf, such as UV irradiation, oxygen radicals, ionizing radiation, chemical agents, and telomere dysfunction (4-11). However, unlike the rapid response of ataxia telangiectasia mutated kinase to activation of the p21Waf1 pathway via ataxia telangiectasia mutated and Rad3-related protein kinase, Chk1 (checkpoint kinase-1), Chk2 (checkpoint kinase-2), and p53, a significant increase in the level of p16Ink4a occurs only 2-4 weeks after exposure of cells to any one of these DNA-damaging stimuli. It therefore seems likely that genotoxic stimuli might be indirect inducers of the expression of p16Ink4a. In addition, oncogenic stimulation of the MAPK pathway by H-Ras induces premature senescence in primary fibroblasts (12, 13). A recent study has shown that p53, whose expression is temporarily switched on 8 days after tumor initiation by γ-irradiation, induces potent tumor-protective activity and that this activity is ARF-dependent (14). It has been proposed that senescence is induced by oncogenic signaling via the accumulation of genetic mutations that are caused by DNA-damaging stimuli (15, 16). The expressed p19Arf stabilizes p53 in an MDM2-dependent manner, and then p53 trans-activates the expression of p21Waf1. The p21Waf1 protein binds to the cyclin-CDK2 complex or the cyclin-CDK4 complex to inhibit the activity of each, and in this way, p21Waf1 functions as a negative regulator of progression of the cell cycle at G1. The p16Ink4a protein controls the activity of cyclin D-CDK4 kinase. In the absence of p16Ink4a, the activity of cyclin D-CDK4 is elevated, and this elevated activity results in phosphorylation of retinoblastoma protein and the consequent release of E2F, which accumulates on the regulatory regions of genes whose products are essential for the G1/S transition, such as cyclin D and cyclin E (17). Several nuclear effectors that act directly to regulate transcription of gene for p16Ink4a and p19Arf have been identified, such as Ets1/2 in the case of the gene for p16Ink4a and DMP1 in the case of the gene for p19Arf (18, 19). In addition, Polycomb repressive complexes (PRC-1 and PRC-2) form at the p16Ink4a/p19Arf locus in young cells and methylate lysine 27 of histone H3 (H3K27). The complexes dissociate from this locus in aged cells, with resultant demethylation of H3K27, which facilitates the expression of p16Ink4a (20, 21). Retinoblastoma protein is known to be involved in a negative feedback loop that establishes a balance between INK4-mediated inhibition and cyclin D-mediated activation of progression though G1. However, the precise molecular mechanisms that govern events at the p16Ink4a/p19Arf locus in response to aging, oxidative stress, and oncogenic signaling remain to be elucidated.
JDP2 (Jun dimerization protein 2) is a DNA-binding protein that can form homodimers or heterodimers with c-Jun, ATF2, and C/EBPγ (22-24). JDP2 is involved the repression and the activation of transcription, depending on cell type (24-31), and it has been implicated in a variety of biological phenomena, such as the proliferation and differentiation of cells and apoptosis (22, 24, 28, 29, 32, 33). For example, the overexpression of JDP2 inhibits the retinoic acid-dependent differentiation of embryonic carcinoma F9 cells (25). Recently, we generated JDP2-deficient (Jdp2-KO) mice and reported that mouse embryonic fibroblasts (MEFs), derived from Jdp2-KO mice (Jdp2-/- MEF), were susceptible to adipocyte differentiation, suggesting that JDP2 might be involved in a signaling pathway for differentiation (34). In subsequent studies of Jdp2-/- MEF, we found that Jdp2-/- MEF were able to proliferate for longer periods of time in culture than wild-type (WT) MEF. Therefore, we investigated the pathway in which JDP2 might be implicated in the regulation of the growth and life span of MEF.
The results of this study suggest that JDP2-deficient MEF might resist replicative senescence by recruiting the Polycomb-repressive complexes PRC-1 and PRC-2 to the promoters at the p16Ink4a locus.
EXPERIMENTAL PROCEDURES
Transgenic Mice and MEF—We generated Jdp2-/- and WT embryos by crossing Jdp2+/- mice and used littermates from day 12.5 of gestation for the isolation of MEF. Each embryo was disaggregated in 0.25% trypsin plus 0.02% EDTA, and fragments were cultured overnight at 37 °C in an atmosphere of 5% CO2 in 20% O2 in Dulbecco's modified Eagle's medium (DMEM; Nissui Pharmaceutical Co., Tokyo, Japan), supplemented with 10% fetal calf serum (FCS; Invitrogen) and 2 mm l-glutamine (Invitrogen) in a 10-cm dish. Cells were re-plated to enrich for fibroblasts. After 2 days, cells were stored frozen as “fresh MEF. ” Genotypes were identified by PCR using a portion of each culture, as described elsewhere (34). For long term growth experiments, cells were cultured in the presence of either 3 or 20% O2 and passaged every 4 days; mean population doublings were calculated from cell numbers. A total of 5 × 105 cells was reseeded at each subculture. At indicated times, total RNA was extracted and subjected to analysis by real time RT-PCR. Immortalized WT MEF were generated by serial passaging in 20% O2 for 80 days. The generation of Jdp2 “knock-out” mice has been described elsewhere (34).
Antibodies—The monoclonal antibody against Jdp2 was prepared as described elsewhere (26). Antibodies directed against GFP (sc-8334; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), β-galactosidase (55976; Cappel, Rockland, PA), Bmi1 (05-637; Upstate Biotechnology Co., Lake Placid, NY), Ezh2 (07-400; Upstate Biotechnology Co.), histone H3 (26103; Abcam, Cambridge, UK), methyl-H3K27 (6002; Abcam), actin (sc-1616; Santa Cruz Biotechnology, Inc.). and polymerase II (sc-899; Santa Cruz Biotechnology, Inc.) were purchased commercially.
Real Time RT-PCR—Total RNA was extracted with the TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was performed with Superscript™ III (Invitrogen) and reverse primers specific for each gene, as described below. Real time PCR was performed with the Power SYBR® Green Master Mix (Applied Biosystems, Foster City, CA) with the following primers: p16Ink4a, 5′-gagtccgctgcagacagact-3′ and 5′-ccaggcatcgcgcacatcca-3′; p19Arf, 5′-agttcgtgcgatcccggaga-3′ and 5′-ccaggcatcgcgcacatcca-3′; p21waf, 5′-gcccgagaacggtggaactt-3′ and 5′-gacaaggccacgtggtcctc-3′; Bmi1, 5′-tggactgacgaatgctggag-3′ and 5′-ggaagattggtggttagcac-3′; Ezh2, 5′-tgctctgcctcctgaatgtactcca-3′ and 5′-gctggtaacactgtggtccacaa-3′; Jdp2, 5′-cgctgacatccgcaacattg-3′ and 5′-catctggctgcagcgacttt-3′; cyclin E1, 5′-agacccacaccaacagcttg-3′ and 5′-tcattctgtctcctgctcgc-3′; GPDH, 5′-ccacttgaagggtggagcca-3′ and 5′-tcatggatgaccttggccag-3′; and 18 S ribosomal RNA, 5′-gggagcctgagaaacggc-3′ and 5′-gggtcgggagtgggtaattt-3′.
ChIP Assays—ChIP assays were performed as described by Kotake et al. (35) with modification of the washing conditions. The immunoprecipitated protein-DNA complexes were washed twice with binding buffer (10 mm HEPES (pH 7.9), 10 mm Tris-HCl (pH 7.9), 12.5% glycerol, 0.25% Nonidet P-40, 0.5% Triton X-100, 0.24 m NaCl, 0.75 mm MgCl2, 1.1 mm EDTA, and protease inhibitor mixture (Sigma)). Then they were washed twice with Tris/EDTA buffer (10 mm Tris-Cl (pH 7.9), 1 mm EDTA). The protein-DNA complexes were eluted; cross-links were disrupted, and proteins were digested with proteinase K (Sigma) at pH 6.8. DNA was extracted with phenol and chloroform, precipitated in ethanol, and analyzed by real time PCR with the Power SYBR® Green Master Mix and the following primers: p16Ink4a promoter, 5′-gacccactggtcacacgact-3′ and 5′-tacccgactgcagatgggac-3′; and p19Arf promoter, 5′-agttcgtgcgatcccggaga-3′ and 5′-gcagcttcggagggcctttc-3′.
Histone Methylation Assay—The nuclear extracts used for histone methylation assay were prepared by the protocol described by Fiskus et al. (36). 5 × 106 of NIH3T3 cells were transfected either with nonspecific RNA (Am 4635; Ambion, Austin, TX) or siRNA against Ezh2 (L-040882-00; Dharmacon, Lafayette, CO) with Lipofectamine™ 2000 (Invitrogen) for 48 h. Cells were washed twice with phosphate-buffered saline (PBS(-)) and lysed in a hypotonic buffer (20 mm HEPES (pH 7.9), 1 mm EDTA, 1 mm EGTA, 20 mm NaF, 1 mm Na4P2O7, 1 mm dithiothreitol, and proteinase inhibitor mixture (Sigma)). The cell lysates were kept on ice for 10 min and then centrifuged at 2,000 × g for 30 s. Nuclei were resuspended in a hypotonic buffer that contained 0.05% Nonidet P-40 and precipitated by centrifugation at 2,000 × g for 2 min. The nuclei were resuspended again in hypotonic buffer C (hypotonic buffer supplemented with 420 mm NaCl and 20% glycerol), with rotation at 4 °C for 30 min. Finally, nuclear extracts were collected as supernatants after centrifugation at 12,000 × g at 4 °C for 30 min.
The histone methylation assay was performed with the EpiQuick™ histone methyltransferase activity/inhibition assay kit (H3-K27; Epigentek Inc., Brooklyn, NY) according to the manufacturer's protocol. In brief, 50 ng of biotinylated substrate were incubated with 50 ng of recombinant GST-JDP2 for 30 min and then methylated by 10 μg of nuclear extract in the presence of S-adenosylmethionine for 1 h on streptavidin-coated plates. Plates were then incubated with antibody specific for H3K27. The level of methylated H3 was measured with a color development system at 450 nm with horseradish peroxidase-conjugated second antibodies.
GST Pulldown Assay—For the construction of expression vectors for JDP2 and its derivatives M3 in Escherichia coli, we cloned the appropriate PCR-amplified fragments of DNA into the pGEX-4T-1 vector as described elsewhere (27). Purified core histones were purchased from Upstate Biotechnology, Inc. For the protein-binding assay, GST-JDP2 was incubated with core histones in a final volume of 500 μl of binding buffer (50 mm Tris (pH 7.5), 5 mm MgCl2, 100 mm NaCl, 10% glycerol, 0.5 mg/ml of bovine serum albumin, 5 mm β-mercaptoethanol) at 4 °C for 1 h, and then 30 μl of a suspension of glutathione-Sepharose beads (Amersham Biosciences) were added. After incubation for 2 h at 4 °C, samples were extensively washed three times with phosphate-buffered saline, and protein complexes were eluted with a buffer (50 mm Tris (pH 7.5), 5 mm MgCl2, 100 mm NaCl, 10% glycerol, 5 mm β-mercaptoethanol) that contained 10 mm glutathione for analysis by SDS-PAGE; 10% acrylamide.
Adenoviral Vectors and Infection—The Adeno-Jdp2 vector was constructed by inserting JDP2 cDNA into pAxCAwt (37), and infectious viral particles were produced and purified as described elsewhere (38). Fresh Jdp2-/- MEF were cultured in 12-well plates at 2 × 105 cells/well and infected with Ad-GFP, Ad-Jdp2, or Ad-LacZ at an m.o.i. of 3 in the presence of DMEM plus 0.05% FCS. Two days after infection, the medium was replaced with DMEM plus 10% FCS, and the cells were harvested after further incubation for 2 days for extraction of RNA or 4 days for quantitation of cells.
Senescence-associated Staining of β-Galactosidase—Senescence-associated staining of β-galactosidase was performed with a senescent cells histochemical staining kit (Sigma) according to the manufacturer's instructions.
Analysis of DNA Methylation—Genomic DNA was extracted from Jdp2-/- MEF after culture for 3, 14, and 80 days, as well as from WT MEF and transformed WT MEF that had been cultured for 3 days. Senescent WT MEF were obtained by passaging at regular intervals for 80 days; cells that began re-growing were used as “immortalized WT MEF.” The conversion of unmethylated cytosine (C) to uracil (U) by the bisulfite method and the detection of methylated and unmethylated alleles by a PCR-based method were performed as described elsewhere (39, 40).
siRNA—siRNA specific for JDP2 (Jdp2-i) was synthesized by Nippon EGT (Toyama, Japan) according to the Gene Swatter siRNA program. The sequence of Jdp2-i (JDP2-inhibitory) siRNA is available from the authors upon request. Nonspecific double-stranded RNA was obtained from Ambion (Austin, TX).
RESULTS AND DISCUSSION
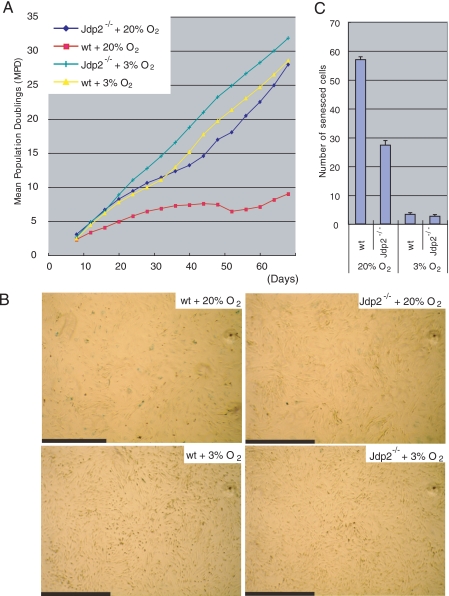
To examine the aging-dependent proliferation of cells, we cultured fresh Jdp2-/- and WT MEF by subculturing aliquots of 5 × 105 cells in 10-cm dishes at 4-day intervals in the presence of environmental (20%) or a low level of (3%) oxygen, and we counted the cells at regular intervals. In 20% O2, Jdp2-/- MEF continued to divide even after 6 weeks, and by this time, WT MEF had almost ceased to proliferate and had become senescent (Fig. 1A). When MEF were cultured in the presence of lower oxidative stress (3% O2), neither WT MEF nor Jdp2-/- MEF succumbed to replicative senescence (Fig. 1A). Senescence-associated staining of β-galactosidase showed that Jdp2-/- MEF was less susceptible to oxidative stress-induced senescence than were WT MEF (Fig. 1, B and C). These data indicate that MEFs that did not express JDP2 were able to escape from irreversible growth-arrest because of environmental oxygen stress.
FIGURE 1.
Analysis of the long term proliferation of cells. A, fresh Jdp2-/- and WT MEF were cultured in the presence of 20 or 3% O2. The cells were passaged, counted, and spread at 5 × 105 cells per dish in 10-cm dishes at 4-day intervals. Mean population doublings (MPD) were calculated from numbers of cells and plotted as shown. B, senescence-associated staining of β-galactosidase in Jdp2-/- and WT MEF that had been cultured for 16 days in the presence of 20% or 3% O2. Scale bars, 1.0 mm. C, numbers of senescent cells. The senescent cells in a 5.9-mm2 field were counted, and results shown are means ± S.E. of results from three independent experiments.
Because the level of p16Ink4a is known to rise as cells age, we examined the expression of this protein in young and aged Jdp2-/- and WT MEF. We analyzed total RNA extracted from MEF on days 0, 3, and 40 by real time RT-PCR (Fig. 2A). After incubation of cells in the presence of 20% oxygen for 40 days, the expression of p16Ink4a was lower in Jdp2-/- MEF than in WT MEF, in which there was an aging-dependent increase in the level of the protein. On day 40, WT MEF that had been cultured in 3% oxygen expressed lower levels of p16Ink4a than did WT MEF that had been cultured in 20% oxygen, whereas there was no significant difference in terms of the level of expression of p16Ink4a between Jdp2-/- MEF cultured in 20 and 3% oxygen (Fig. 2B).
FIGURE 2.
Expression of cell cycle-related genes. A, schematic diagram showing details of cell culture and extraction of RNA. Fresh Jdp2-/- and WT MEF were cultured as indicated in Fig. 1. B-G, total RNA was extracted on days 0, 3, and 40 and subjected to real time RT-PCR for quantitation of transcripts specific for p16Ink4a (B), p19Arf (C), p21Waf (D), Bmi1 (E), Ezh2 (F), and JDP2 (G). Levels of expression were normalized by reference to that of transcripts of the gene for GPDH.
We also analyzed the expression of p19Arf and its downstream target p21Waf1. The patterns of expression of both p19Arf and p21Waf1 were similar to those of p16Ink4a, even though relative differences in levels of the former mRNA were lower (Fig. 2, C and D). Moreover, analysis of levels of p16Ink4a and p19Arf by Western blotting demonstrated that the expression of p19Arf was not dramatically different in Jdp2-/- and WT MEFs, probably being due to small biological differences or technical reasons, whereas the level of p16Ink4a was clearly elevated in WT MEF cultured in 20% oxygen (supplemental Fig. S1). These observations indicated that the aging-associated expression of p16Ink4a and p19Arf was dependent on oxygenic stress and that JDP2 might control the expression of both p16Ink4a and p19Arf. However, because the difference between Jdp2-/- and WT MEF in terms of the level of expression of p16Ink4a was more significant than that of p19Arf, it seemed that suppression of expression of p16Ink4a in Jdp2-/- MEF might have been the main contributor to the escape of senescence. We also analyzed the expression of the Polycomb ring finger oncoprotein Bmi1 and that of the enhancer of zeste homolog 2, Ezh2, which are known to be upstream repressors of the expression of p16Ink4a and p19Arf (20, 21). We did not find any dramatic suppression of the expression of Bmi1 or of Ezh2 in the absence of JDP2, although the levels of each transcript were variable (Fig. 2, E and F, and supplemental Fig. S1). Thus, it seems likely that JDP2 does not directly regulate the levels of transcripts of these two proteins. We observed, however, that expression of JDP2 in WT MEF increased significantly in the presence of 20% oxygen but not of 3% oxygen (Fig. 2G). This observation suggests that the expression of JDP2 might depend on oxygenic stress and that accumulated JDP2 might play a role in activation of the transcription of the genes for p16Ink4a and p19Arf. The possibility that JDP2 might be a positive regulator of the expression of both p16Ink4a and p19Arf was supported by the observation that “knockdown” of the expression of JDP2 by siRNA in aged WT MEF decreased the levels of expression of p16Ink4a and p19Arf (Fig. 3).
FIGURE 3.
Effects of knockdown of JDP2 by siRNA on the expression of p16Ink4a, p19Arf, and JDP2. Aged (day 30) WT MEF (5 × 105 cells) were transfected with 30 pmol of siRNA specific for JDP2 (JDP2-i). The same amount of nonspecific double-stranded RNA was used as a negative control (NC). After transfection for 3 days, cells were harvested and total RNA was subjected to real time RT-PCR with primers specific for p16Ink4a, p19Arf and JDP2. Levels of expression were normalized to that of GPDH. Data are shown as percentages relative to results for the negative control.
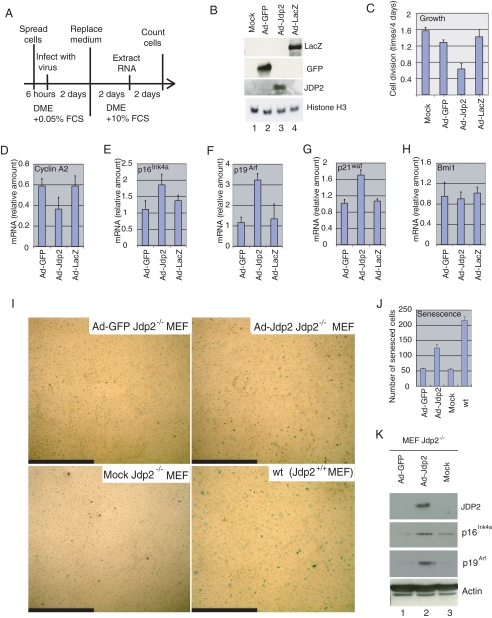
To determine whether JDP2 is an inhibitor of progression of the cell cycle, we infected serum-starved Jdp2-/- MEF with an adenoviral vector that encoded either JDP2 (Ad-Jdp2) or a negative control protein, either GFP (Ad-GFP) or β-galactosidase (Ad-lacZ) (m.o.i. of 3; see Fig. 4A). After infection for 2 days, we replaced the medium with DMEM supplemented with 10% FCS and then harvested cells 2 days later for analysis of RNA and 4 days later for quantitation of cells (Fig. 4A). We confirmed the expression of each protein by Western blotting with specific antibodies (Fig. 4B).
FIGURE 4.
JDP2 inhibits proliferation of MEF and promotes the expression of inhibitors of progression of the cell cycle. A, schematic diagram of the experiment. Fresh Jdp2-/- MEF were infected with Ad-GFP, Ad-Jdp2, or Ad-LacZ at an m.o.i. of 3 in the presence of DMEM plus 0.05% FCS. Two days after infection, the medium was replaced with DMEM plus 10% FCS, and the cells were harvested after further incubation for 2 days for extraction of RNA or for 4 days for quantification of cells. B, expression of GFP, JDP2, β-galactosidase (LacZ), and histone H3 in virus-infected cells. Extracts from 5 × 104 cells were analyzed by Western blotting with the relevant antibodies. C, effects of JDP2 on cell proliferation. Divisions per day of mock-, Ad-GFP-, Ad-Jdp2- and Ad-LacZ-infected cells are shown. D-H, expression of cyclin A2 (D), p16Ink4a (E), p19Arf (F), p21waf (G), and Bmi1 (H) was analyzed by real time RT-PCR with the appropriate primers. Levels of expression were normalized by reference to that of 18 S ribosomal RNA. I, induction of senescence by overexpression of JDP2. Jdp2-/- MEF, maintained in the presence of 3% O2, were infected with Ad-GFP or Ad-JDP2 at an m.o.i. of 3 and cultured in the presence of 20% O2 for 4 days. Then cells were subjected to senescence-associated staining of β-galactosidase. Mock-infected Jdp2-/- and WT MEF served as negative and positive controls, respectively. Scale bars, 1.0 mm. J, numbers of senescent cells. Senescent cells in a 6.2-mm2 field were counted, and results shown are the means ± S.E. of results from three independent experiments. K, expression of JDP2, p16Ink4a, p19Arf, and actin was examined in virus-infected cells. Extracts from 5 × 104 cells (20 μg of protein) were analyzed by Western blotting with the indicated antibodies.
Assays of cell growth demonstrated that the proliferation of Ad-Jdp2-infected cells was significantly inhibited as compared with that of the other lines of cells (Fig. 4C). The expression of cyclin A2, which is expressed during the G1-S transition and is a marker of progression of the cell cycle (17), was inhibited in the Ad-Jdp2-infected cells (Fig. 4D). By contrast, levels of expression of mRNA for p16Ink4a, p19Arf, and p21Waf1 were elevated upon overexpression of JDP2 (Fig. 4, E-G), whereas that of Bmi1 remained unchanged (Fig. 4H). The enhanced expression of p16Ink4a and p19Arf in the presence of JDP2 was confirmed by Western blotting (Fig. 4K). Taken together, the results suggested that JDP2 affected the expression of proteins related to regulation of the cell cycle, such as p16Ink4a and p19Arf, but not that of Bmi1.
To examine whether expression of JDP2 induces cellular senescence, we infected Jdp2-/- MEF with the adenoviral vector Ad-Jdp2 or Ad-GFP, at an m.o.i. of 3, cultured the cells for 4 days, and then subjected them to senescence-associated β-galactosidase staining. After 4 days the number of senescent Jdp2-/- MEF that had been infected with Ad-Jdp2 was approximately twice that of senescent Jdp2-/- MEF infected with Ad-GFP and of mock-infected cells (Fig. 4, I and J). These data are consistent with the hypothesis that JDP2 is involved in the induction of senescence via positive regulation of the expression of p16Ink4a and p19Arf.
There are several possible mechanisms to explain how JDP2 might regulate the transcription of genes for p16Ink4a and p19Arf. For example, JDP2 might bind strongly to AP-1-like sites in the promoters of the genes for p16Ink4a and p19Arf upon exposure of cells to oxidative stress or aging. There are a few reports that some members of the AP-1 family control the expression of genes for p16Ink4a and p19Arf. For example, AP-1 dimers of members of a basic zipper family similar to the c-Jun/Fra-1 heterodimer were reported to activate the transcription of genes for p14ARF and p19Arf (41), and JunB is known to suppress cell proliferation by transcriptional activation of the gene for p16Ink4a (42). Alternatively, the extent of DNA damage might increase in WT MEF but not in JDP2-/- MEF during oxidative stress or aging. It has been reported that c-Jun-/- MEF sustain greater DNA damage than normal fibroblasts at higher concentrations of oxygen, an observation that implies that senescence might result from the chronic accumulation of spontaneously damaged DNA, as demonstrated by the recruitment of a derivative of histone H2A, γH2AX, and ataxia telangiectasia mutated to the p16Ink4a locus (43). Finally, JDP2 might affect the modification of chromatin or DNA at the p16Ink4a/p19Arf locus. In this study, we focused on this final possibility and on the participation of JDP2 in the response to oxidative stress and aging.
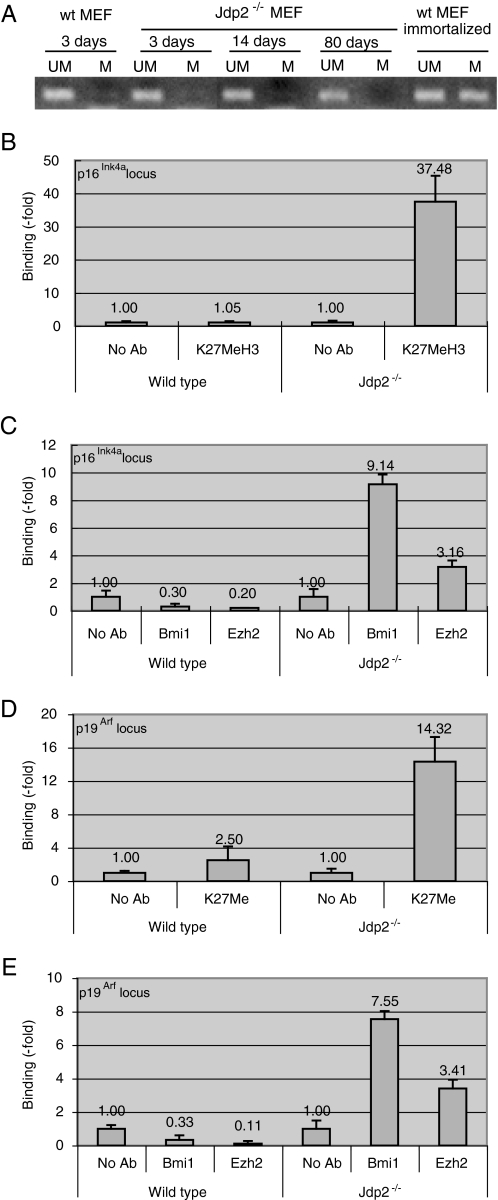
In tumor cells, the CpG islands at the p16Ink4a locus are prone to hypermethylation, which results in the epigenetic silencing of the expression of p16Ink4a (40, 44). Therefore, we analyzed the methylation status of the promoter region of the gene for p16Ink4a in Jdp2-/- MEF. We extracted genomic DNA from Jdp2-/- MEF that had been cultured for 3, 14, and 80 days and also from WT and transformed MEF that had been cultured for 3 days (methylated and nonmethylated controls, respectively). We analyzed genomic DNA by the bisulfite method and amplified sequences by PCR with primers specific for the methylated and unmethylated sequences that flanked the site of initiation of translation of the gene for p16Ink4a. The results of our PCR-based DNA methylation assay revealed that the promoter of the gene for p16Ink4a in Jdp2-/- MEF was unmethylated, even after 80 days of cell culture, whereas this promoter was methylated in transformed WT MEF (Fig. 5A). These observations indicated that the weak expression of p16Ink4a and the resistance to irreversible growth arrest of Jdp2-/- MEF were not because of the hypermethylation of the CpG island at the p16Ink4a locus. By contrast, the CpG island is hypermethylated in tumor cells and in transformed cells, which express p16Ink4a only weakly. Recent studies have demonstrated that the methylation of lysine 27 (Lys-27) of histone H3 (H3K27), when this histone is bound to the p16Ink4a/p19Arf locus, epigenetically silences the expression of the two genes at this locus in primary cultures of young proliferating cells. By contrast, H3K27 is demethylated, and the genes become transcriptionally active in aged and senescent cells (20, 21, 35). Therefore, we examined the methylation status of H3K27 at the p16Ink4a/p19Arf locus in Jdp2-/- MEF. Using extracts of Jdp2-/- and WT MEF that had been cultured for 40 days in the presence of 20% O2, we performed ChIP assays with an antibody specific for methylated H3K27 (H3K27Me). We analyzed the immunoprecipitated DNA by real time PCR with primers specific for the promoter of the gene for p16Ink4a. The results revealed a low level of methylation of H3K27 in WT MEF cultured in 20% O2 and also a higher level of methylation of H3K27 in Jdp2-/- MEF than in WT MEF (Fig. 5B). Next, we examined the binding of PRC-1 and PRC-2, which are known to govern the methylation of H3K27 on promoters, by ChIP assays with antibodies against Bmi1 and Ezh2, which are components of PRC-1 and PRC-2, respectively. We found that PRC-1-associated Bmi1 and PRC-2-associated Ezh2 bound more efficiently to the promoter of the gene for p16Ink4a in Jdp2-/- MEF than in WT MEF (Fig. 5C). We obtained similar results for the promoter of the gene for p19Arf. The level of methylation of Lys-27 of H3 at the p19Arf locus was higher (Fig. 5D), and in addition, larger amounts of PRC-1 and PRC-2 were recruited to the promoter in Jdp2-/- MEF than in WT MEF (Fig. 5E).
FIGURE 5.
Modification of the chromatin at the p16Ink4a and p19Arf promoters by JDP2. A, methylation of DNA at the p16Ink4a locus. Genomic DNA was extracted from Jdp2-/- MEF that had been cultured for 3, 14, and 80 days and also from WT MEF and transformed WT MEF that had been cultured for 3 days. Then 2 μg of each preparation of genomic DNA was modified by the bisulfite method, as described in the text. The promoter of the gene for p16Ink4a was analyzed by PCR with primers specific for methylated (M) and unmethylated (UM) sequences. B, methylation of histone H3 at the p16Ink4a locus. Extracts from 2 × 106 Jdp2-/- and WT MEF that had been cultured for 40 days in the presence of 20 or 3% O2 were subjected to ChIP assays. Extracts were precipitated with the antibody (Ab) specific for methylated H3K27, and DNA was analyzed by real time PCR with primers specific for the p16Ink4a/p19Arf promoter. Levels were normalized by reference to the input DNA. C, binding of Bmi1 and Ezh2 to the p16Ink4a promoter. ChIP assays were performed with Bmi1- and Ezh2-specific antibodies, as described in the text. D and E, methylation of histone H3 at the p19Arf locus and binding of Bmi1 and Ezh2 to the promoter of the gene for p19Arf. ChIP assays were performed with antibodies specific for methylated H3K27 (D), Bmi1, and Ezh2 (E). Precipitated DNA, as described in legends to B and C, was analyzed with primers specific for p19Arf. Levels were normalized by reference to input DNA. Results shown are means ± S.E. of results from three independent experiments.
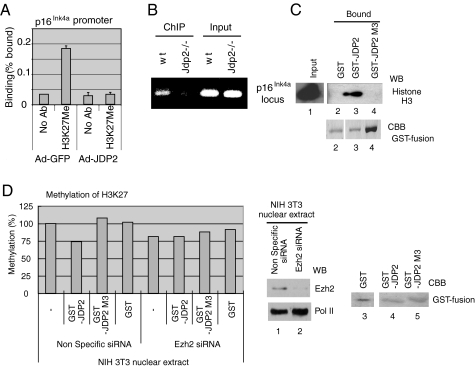
We infected Jdp2-/- MEF with Ad-Jdp2 and with Ad-GFP to determine whether the overexpression of JDP2 might affect the extent of methylation of histone H3K27. The results of a ChIP assay with an antibody specific for H3K27Me demonstrated that methylated histone H3K27 was not recruited to the p16Ink4a locus in the presence of Ad-Jdp2, indicating that JDP2 might depress the extent of methylation in vivo (Fig. 6A). As shown in Fig. 6B, the ChIP assays with JDP2-specific antibody and an extract of WT MEF, but not of Jdp2-/- MEF, clearly revealed the binding of endogenous JDP2 at the p16Ink4a locus in vivo (Fig. 6B). We also examined the ability of JDP2 to bind to the core histone in a “GST-pulldown” assay and evaluated the results by Western blotting with antibodies specific for histone H3 (45). The results of the GST-pulldown assays with recombinant JDP2 fused to GST, prepared from E. coli, revealed that GST-JDP2 was able to bind core histone. However, both GST itself and the mutant derivative of GST-JDP2 designated M3 (GST-JDP2 M3), which is unable to bind the core histone (45), did not associate with core histone (Fig. 6C, lanes 1-4). As reported previously by our group, JDP2 has histone binding activity and also inhibits histone acetylation, acting as an INHAT (inhibitor of histone acetyltransferase (45)). Therefore, we postulated that JDP2 might also protect histones from methylation via direct interaction with them. To examine this hypothesis, we incubated biotinylated recombinant histones with GST-JDP2, GST-JDP2 M3, or GST alone and then methylated them using a nuclear extract of NIH3T3 cells that had been transfected with nonspecific control RNA or with siRNA specific for Ezh2 (Fig. 6D). We determined the extent of histone methylation by an enzyme-linked immunosorbent assay using avidin-coated wells and an antibody against H3K27Me. The results of the histone methylation assays demonstrated that the methylation of histone H3K27 was indeed inhibited by GST-JDP2 but not by GST-JDP2 M3 or by GST (Fig. 6D). Moreover, no inhibition of methylation of H3K27 was evident when we used a nuclear extract of NIH3T3 cells, as source of enzymes, in which expression of Ezh2 had been suppressed by the specific siRNA. The nuclear extract from NIH3T3 cells transfected with the control siRNA gave the converse results (Fig. 6D, lanes 1 and 2). Thus, it appeared that JDP2 might inhibit specifically the methylation of H3K27 by PRC-2 (Fig. 6D). Such inhibition by JDP2 might be due to a masking effect of JDP2, via binding to the core histone that interferes with access by the H3K27 methylase. We did not determine whether JDP2 has stronger affinity for the nonmethylated histone, specifically nonmethylated at H3K27, than for the methylated core histone. Details of the histone-binding region of JDP2 that prevents the methylation of H3K27 remain to be clarified.
FIGURE 6.
Methylation of histone H3K27 at the p16Ink4a locus. A, methylation of histone H3 at the p16Ink4a locus in JDP2-overexpressing cells. Jdp2-/- MEF were infected with Ad-JDP2 at an m.o.i. of 3. An extract of 1 × 106 cells was precipitated with antibody specific for methylated H3K27, and DNA was analyzed by real time PCR with primers specific for the p16Ink4a promoter. Levels were normalized by reference to the input DNA. B, binding of JDP2 to the p16Ink4a promoter. Extracts from 5 × 106 Jdp2-/- and WT MEF were subjected to ChIP assays with antibody specific for JDP2. Precipitated DNA and input DNA were analyzed by PCR with primers specific for the p16Ink4a promoter. C, binding of JDP2 to histone H3. Recombinant GST (500 ng) or GST-JDP2 (250 ng) or GST-JDP2 M3 (250 ng) was incubated with chicken core histones (1 μg), and then reaction mixtures were supplemented with glutathione-Sepharose beads (Amersham Biosciences). Proteins eluted from the beads and input proteins were analyzed by Western blotting (WB) with the antibody specific for histone H3. Amounts of GST fusion proteins were assessed by staining with Coomassie Brilliant Blue (CBB) R-250. D, inhibition of histone methylation. Biotinylated histones (50 ng) were incubated with 50 ng of GST-JDP2, GST-JDP2 M3, or GST, in wells of streptavidin-coated plates, and methylated by addition of 10 μg (protein) of nuclear extracts of NIH3T3 cells that had been transfected either with nonspecific RNA or with siRNA directed against Ezh2. Methylated H3K27 was detected with antibodies against methyl-H3K27 and horseradish peroxidase-conjugated second antibodies as indicated. Levels of methylation were determined colorimetrically from absorbance at 450 nm.
Our observations indicate that, in the absence of JDP2, H3K27 is methylated by PRC-1 and PRC-2, although increased expression of JDP2 stimulates the release of PRC-1 and PRC-2 from the p16Ink4a/p19Arf locus, thereby decreasing the extent of methylation of H3K27. It was reported recently that the role of PRC-1 is to recognize methylated H3K27 and to maintain repression via formation of a stable complex with DNA, PRC-2, histones, and other DNA-binding factors (46). By contrast, PRC-2 contains intrinsic histone modifying activities to form a trimethylation mark on H3K27 for recruitment of PRC-1 (46).
Our data demonstrate that JDP2 plays an important role in the regulation of cellular senescence. The absence of JDP2 allows MEF to escape senescence, and conversely, overexpression of JDP2 induces arrest of the cell cycle. The absence of JDP2 decreases the expression of both p16Ink4a and p19Arf, which inhibits progression of the cell cycle. We propose the following model as a possible explanation for our results. The cumulative results of oxidative stress and/or of other damaging environmental stimuli during aging stimulate the expression of JDP2 in primary untransformed cells. When the expression of JDP2 is enhanced, JDP2 is able to associate tightly with histones and to protect them from modifications, such as methylation and acetylation. Upon masking of histones by JDP2, PRC-2 is unable to methylate H3K27. As a result, PRC-1 can no longer form a repressive complex, and both PRC-1 and PRC-2 are released from the locus, with resultant enhancement of the expression of p16Ink4a and p19Arf and the onset of senescence (Fig. 7). However, we cannot rule out other possibilities, as described above. Further studies of the role of JDP2 in mediating the effects of DNA damage and epigenetic changes, as well as the control of promoter activity by JDP2, are in progress in our laboratory.
FIGURE 7.
Proposed model of the epigenetic regulation of the expression of genes for p16Ink4a and p19Arf by JDP2. Exposure of young primary cultures of cells to aging stress leads to the accumulation of JDP2. JDP2 binds to histones and inhibits methylation of H3K27 at the p16Ink4a/p19Arf locus. As a result, PRC-1 and PRC-2 fail to form stable repressive complexes and are released from the locus. The consequent expression of p16Ink4a and p19Arf in the aged cells leads to senescence.
There is some evidence that JDP2 acts as a tumor suppressor. For example, JDP2 inhibits the Ras-dependent transformation of NIH3T3 cells (33), and disruption of the gene for JDP2 is often found in lymphomas that have been induced by insertional mutagenesis with Moloney murine leukemia virus in MYC/Runx2 transgenic mice (47). We propose that JDP2 not only inhibits the transformation of cells but also plays a role in the induction of senescence. Both functions of JDP2 might be important in its role as an inhibitor of tumor formation. Our findings shed new light on the molecular mechanisms by which senescence is induced in the context of the epigenetic regulation of the expression of the two genes at the p16Ink4a/p19Arf locus.
Supplementary Material
Acknowledgments
We thank Drs. Eiji Hara, Naoko Ohtani, Keiichi Itakura, Chunyuan Jin, and Gabriel Gachelin for careful reading of and comments on the original manuscript and Dr. Yuichi Obata for encouragement. We also thank Kumiko Inabe, Megumi Hirose, and Miyuki Yamamoto for their skilled technical assistance.
This work was supported by grants from the RIKEN Bioresource Project (to K. K. Y.) and from the Ministry of Education, Culture, Sport, Science and Technology of Japan 18770185 and 20570193 (to K. N.) and 19041069 (to K. K. Y.).
The on-line version of this article (available at http://www.jbc.org) contains Fig. S1.
Footnotes
The abbreviations used are: MAPK, mitogen-activated protein kinase; ChIP, chromatin immunoprecipitation; DMEM, Dulbecco's modified Eagle's medium; FCS, fetal calf serum; GPDH, glycerol 3-phosphate dehydrogenase; MEF, mouse embryonic fibroblasts; PRC-1 (-2), Polycomb repressive complex-1 (-2); RT, reverse transcription; WT, wild type; siRNA, small interfering RNA; ChIP, chromatin immunoprecipitation; GST, glutathione S-transferase; GFP, green fluorescent protein; m.o.i., multiplicity of infection.
References
- 1.Krishnamurthy, J., Torrice, C., Ramsey, M. R., Kovalev, G. I., Al-Regaiey, K., Su, L., and Sharpless, N. E. (2004) J. Clin. Investig. 114 1299-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zindy, F., Quelle, D. E., Roussel, M. F., and Sherr, C. J. (1997) Oncogene 15 203-211 [DOI] [PubMed] [Google Scholar]
- 3.Melk, A., Schmidt, B. M., Takeuchi, O., Sawitzki, B., Rayner, D. C., and Halloran, P. F. (2004) Kidney Int. 65 510-520 [DOI] [PubMed] [Google Scholar]
- 4.Wang, Y., Schulte, B. A., LaRue, A. C., Ogawa, M., and Zhou, D. (2006) Blood 107 358-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robles, S. J., and Adami, G. R. (1998) Oncogene 16 1113-1123 [DOI] [PubMed] [Google Scholar]
- 6.Pavey, S., Conroy, S., Russell, T., and Gabrielli, B. (1999) Cancer Res. 59 4185-4189 [PubMed] [Google Scholar]
- 7.Meng, A., Wang, Y., Van Zant, G., and Zhou, D. (2003) Cancer Res. 63 5414-5419 [PubMed] [Google Scholar]
- 8.Ito, K., Hirao, A., Arai, F., Matsuoka, S., Takubo, K., Hamaguchi, I., Nomiyama, K., Hosokawa, K., Sakurada, K., Nakagata, N., Ikeda, Y., Mak, T. W., and Suda, T. (2004) Nature 431 997-1002 [DOI] [PubMed] [Google Scholar]
- 9.Ito, K., Hirao, A., Arai, F., Takubo, K., Matsuoka, S., Miyamoto, K., Ohmura, M., Naka, K., Hosokawa, K., Ikeda, Y., and Suda, T. (2006) Nat. Med. 12 446-451 [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. H., Stoeber, K., Kingsbury, S., Ozanne, S. E., Williams, G. H., and Hales, C. N. (2004) J. Biol. Chem. 279 49439-49446 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs, J. J., and de Lange, T. (2004) Curr. Biol. 14 2302-2308 [DOI] [PubMed] [Google Scholar]
- 12.Lin, A. W., Barradas, M., Stone, J. C., van Aelst, L., Serrano, M., and Lowe, S. W. (1998) Genes Dev. 12 3008-3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D., and Lowe, S. W. (1997) Cell 88 593-602 [DOI] [PubMed] [Google Scholar]
- 14.Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B., and Evan, G. I. (2006) Nature 443 214-217 [DOI] [PubMed] [Google Scholar]
- 15.Efeyan, A., and Serrano, M. (2007) Cell Cycle 6 1006-1010 [DOI] [PubMed] [Google Scholar]
- 16.Finkel, T., Serrano, M., and Blasco, M. A. (2007) Nature 48 767-774 [DOI] [PubMed] [Google Scholar]
- 17.Sharpless, N. E. (2005) Mutat. Res. 576 22-38 [DOI] [PubMed] [Google Scholar]
- 18.Sreeramaneni, R., Chaudhry, A., McMahon, M., Sherr, C. J., and Inoue, K. (2005) Mol. Cell. Biol. 25 220-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtani, N., Zebedee, Z., Huot, T. J., Stinson, J. A., Sugimoto, M., Ohashi, Y., Sharrocks, A. D., Peters, G., and Hara, E. (2001) Nature 409 1067-1070 [DOI] [PubMed] [Google Scholar]
- 20.Bracken, A. P., Dietrich, N., Pasini, D., Hansen, K. H., and Helin, K. (2006) Genes Dev. 20 1123-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracken, A. P., Kleine-Kohlbrecher, D., Dietrich, N., Pasini, D., Gargiulo, G., Beekman, C., Theilgaard-Monch, K., Minucci, S., Porse, B. T., Marine, J. C., Hansen, K. H., and Helin, K. (2007) Genes Dev. 21 525-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronheim, A., Zandi, E., Hennemann, H., Elledge, S. J., and Karin, M. (1997) Mol. Cell. Biol. 17 3094-3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrovsky, O., Bengal, E., and Aronheim, A. (2002) J. Biol. Chem. 277 40043-40054 [DOI] [PubMed] [Google Scholar]
- 24.Piu, F., Aronheim, A., Katz, S., and Karin, M. (2001) Mol. Cell. Biol. 1 3012-3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, C., Li, H., Murata, T., Sun, K., Horikoshi, M., Chiu, R., and Yokoyama, K. K. (2002) Mol. Cell. Biol. 22 4815-4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin, C., Li, H., Ugai, H., Murata, T., and Yokoyama, K. K. (2002) Nucleic Acids Res. 2 S97-S98 [DOI] [PubMed] [Google Scholar]
- 27.Jin, C., Ugai, H., Song, J., Murata, T., Nili, F., Sun, K., Horikoshi, M., and Yokoyama, K. K. (2001) FEBS Lett. 489 34-41 [DOI] [PubMed] [Google Scholar]
- 28.Hwang, H. C., Martins, C. P., Bronkhorst, Y., Randel, E., Berns, A., Fero, M., and Clurman, B. E. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 11293-11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broder, Y. C., Katz, S., and Aronheim, A. (1998) Curr. Biol. 8 1121-1124 [DOI] [PubMed] [Google Scholar]
- 30.Cherasse, Y., Chaveroux, C., Jousse, C., Maurin, A. C., Carraro, V., Parry, L., Fafournoux, P., and Bruhat, A. (2008) FEBS Lett. 582 1537-1541 [DOI] [PubMed] [Google Scholar]
- 31.Weidenfeld-Baranboim, K., Bitton-Worms, K., and Aronheim, A. (2008) Nucleic Acids Res. 36 3608-3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blazek, E., Wasmer, S., Kruse, U., Aronheim, A., Aoki, M., and Vogt, P. K. (2003) Oncogene 22 2151-2159 [DOI] [PubMed] [Google Scholar]
- 33.Heinrich, R., Livne, E., Ben-Izhak, O., and Aronheim, A. (2004) J. Biol. Chem. 279 5708-5715 [DOI] [PubMed] [Google Scholar]
- 34.Nakade, K., Pan, J., Yoshiki, A., Ugai, H., Kimura, M., Liu, B., Li, H., Obata, Y., Iwama, M., Itohara, S., Murata, T., and Yokoyama, K. K. (2007) Cell Death Differ. 14 1398-1405 [DOI] [PubMed] [Google Scholar]
- 35.Kotake, Y., Cao, R., Viatour, P., Sage, J., Zhang, Y., and Xiong, Y. (2007) Genes Dev. 21 49-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiskus, W., Pranpat, M., Balasis, M., Herger, B., Rao, R., Chinnaiyan, A., Atadja, P., and Bhalla, K. (2006) Mol. Cancer Ther. 5 3096-3104 [DOI] [PubMed] [Google Scholar]
- 37.Kanegae, Y., Lee, G., Sato, Y., Tanaka, M., Nakai, M., Sakaki, T., Sugano, S., and Saito, I. (1995) Nucleic Acids Res. 23 3816-3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ugai, H., Yamasaki, T., Hirose, M., Inabe, K., Kujime, Y., Terashima, M., Liu, B., Tang, H., Zhao, M., Murata, T., Kimura, M., Pan, J., Obata, Y., Hamada, H., and Yokoyama, K. K. (2005) Biochem. Biophys. Res. Commun. 331 1053-1060 [DOI] [PubMed] [Google Scholar]
- 39.Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D., and Baylin, S. B. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharpless, N. E., Bardeesy, N., Lee, K. H., Carrasco, D., Castrillon, D. H., Aguirre, A. J., Wu, E. A., Horner, J. W., and DePinho, R. A. (2001) Nature 413 86-91 [DOI] [PubMed] [Google Scholar]
- 41.Ameyar-Zazoua, M., Wisniewska, M. B., Bakiri, L., Wagner, E. F., Yaniv, M., and Weitzman, J. B. (2005) Oncogene 24 2298-2306 [DOI] [PubMed] [Google Scholar]
- 42.Passegue, E., and Wagner, E. F. (2000) EMBO J. 19 2969-2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacLaren, A., Black, E. J., Clark, W., and Gillespie, D. A. (2004) Mol. Cell. Biol. 24 9006-9018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman, J. G., Merlo, A., Mao, L., Lapidus, R. G., Issa, J. P., Davidson, N. E., Sidransky, D., and Baylin, S. B. (1995) Cancer Res. 55 4525-4530 [PubMed] [Google Scholar]
- 45.Jin, C., Kato, K., Chimura, T., Yamasaki, T., Nakade, K., Murata, T., Li, H., Pan, J., Zhao, M., Sun, K., Chiu, R., Ito, T., Nagata, K., Horikoshi, M., and Yokoyama, K. K. (2006) Nat. Struct. Mol. Biol. 13 331-338 [DOI] [PubMed] [Google Scholar]
- 46.Rajasekhar, V. K., and Begemann, M. (2007) Stem Cells 25 2498-2510 [DOI] [PubMed] [Google Scholar]
- 47.Stewart, M., Mackay, N., Hanlon, L., Blyth, K., Scobie, L., Cameron, E., and Neil, J. C. (2007) Cancer Res. 67 5126-5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.