Abstract
Wnt-5a is a non-transforming Wnt protein that is implicated in cell polarity, adhesion, and motility. We have previously shown that low expression of Wnt-5a is a predictor of shorter disease-free survival in human breast cancer. Here, we investigated whether β-catenin/E-cadherin-mediated cell-cell adhesion was affected by loss of Wnt-5a in breast carcinomas, thereby promoting a metastatic behavior of the tumor. We show that Wnt-5a stimulation of human breast epithelial cells leads to an increased Ca2+-dependent cell-cell adhesion. Furthermore, Wnt-5a/casein kinase Iα (CKIα)-specific Ser-45 phosphorylation of β-catenin is associated with an increased complex formation of β-catenin/E-cadherin. Mutation of Ser-45 decreases the β-catenin/E-cadherin association. Also, the inhibitory effect of Wnt-5a on breast epithelial cell invasion is reduced upon mutation of β-catenin-Ser-45. The Wnt-5a-CKIα-induced Ser-45 phosphorylation does not lead to degradation of β-catenin. Finally we show that human breast cancers lacking Wnt-5a protein have a significantly lower level of membrane-associated β-catenin. Down-regulation of Wnt-5a expression and subsequent reduction of membrane-associated β-catenin in invasive breast cancer, can therefore contribute to a decreased cell-cell adhesion and increased motility resulting in a higher probability for metastatic disease.
In a normal mammary gland the ducts are lined with epithelial cells that are both interconnected and connected to the basement membrane. Mutations in genes encoding adhesion molecules have been implicated in the progression of breast cancer (1). E-cadherin is a transmembrane, calcium (Ca2+)-dependent adhesion molecule that connects adjacent epithelial cells. The loss of cell-cell adhesion at the original tumor site has been suggested to be related to the loss of E-cadherin expression or function in many epithelial cancers (2). The E-cadherins are dynamically linked to the cytoskeleton via β-catenin, plakoglobin, p120, and α-catenin (3, 4). Mutations in the E-cadherin gene are frequent in invasive lobular breast cancers, whereas they are absent or might occur at a low frequency in the more common invasive ductal breast carcinomas (5). Despite this, a low membrane expression of β-catenin was recently shown to be associated with a poor outcome in human breast cancer patients (6). The question as to whether Wnt β-catenin signaling can affect E-cadherin-dependent cellular adhesion, or vice versa, has long been debated (7).
β-Catenin plays a role both in the β-catenin/E-cadherin complex and as a transcriptional regulator in the nucleus upon canonical Wnt signaling (reviewed in Ref. 8). There are therefore three different pools of β-catenin; a membrane bound, a cytoplasmic that is constantly being phosphorylated and targeted for ubiquitination and degradation, and a nuclear unphosphorylated form. It has previously been suggested that β-catenin targeted to adhesive or transcriptional complexes have distinct molecular forms that would allow it to coordinate the two processes (9). However, the mechanisms that target β-catenin to adhesive complexes have not yet been described. It is known that the association between β-catenin/E-cadherin is increased when E-cadherin is phosphorylated on serine residues 834, 836, and 842 (8). GSK-3β2 and CK2 have been implicated as potential kinases. In contrast, phosphorylation of serine 846 by CKI and tyrosine phosphorylation of both E-cadherin and β-catenin has been suggested to decrease the association (10, 11), although conflicting data are present (12). Still, there is little evidence concerning β-catenin phosphorylation and E-cadherin association. In a recent review (8) it was suggested that the interaction might be affected by Wnt signals because the CK1, CK2, and GSK-3β kinases are part of this pathway.
Members of the Wnt family are signaling proteins that are involved in many differentiation events (13). They can be divided into three different classes based on experiments performed in C57MG mammary myoepithelial cells (14), the transforming (Wnt-1, Wnt-2, Wnt-3, and Wnt-3a), the weakly transforming (Wnt-6 and Wnt-7a), and the non-transforming group (Wnt-4, Wnt-5a, Wnt-5b, and Wnt-7b). The transforming Wnt proteins signal via the canonical β-catenin pathway and activate TCF/LEF-dependent transcription. In the absence of a Wnt signal, β-catenin is targeted for ubiquitination and degradation by a complex consisting of CKIα, GSK-3β, APC, and Axin (APC-Axin-destruction complex) (15). Upon Wnt signaling this complex is inactivated and β-catenin is accumulated in the cytosol, thereby leading to nuclear redistribution (7). In the present study we will focus on the role of the nontransforming Wnt-5a. Wnt-5a primarily activates distinct noncanonical pathways, including the Wnt/Ca2+ signaling pathway (16). The Wnt-5a protein has been shown to be involved in cell adhesion, motility, and polarity (17, 18), processes that are known to relate to the β-catenin/E-cadherin complex.
We have previously shown that expression of Wnt-5a is a prognostic factor for longer disease-free survival in human breast cancer (19, 20). It was further shown that a low Wnt-5a expression led to a decreased adherence of the cells to collagen and increased motility of ductal breast epithelial cells (21). Together, these data indicate that loss of Wnt-5a protein expression might be involved in the initial de-adhesion events of metastasis in invasive breast carcinomas. The role for Wnt-5a as a prognostic marker has now been confirmed in several other cancers (22-24). However, the present available data concerning how Wnt-5a affects Ca2+-dependent intercellular adhesion (E-cadherin) (25), does not match the clinical findings. In this study we show that in contrast to previous data, but in line with clinical findings, the non-transforming Wnt-5a protein actually increases the Ca2+-dependent intercellular adhesion in human breast epithelial cells by affecting the cellular distribution and function of β-catenin.
EXPERIMENTAL PROCEDURES
Cell Culture—We used the human breast cancer cell line MCF-7 and the non-cancerous mammary epithelial cell line HB2, which is a subclone of the MTSV-7 cell line originating from the laboratory of Dr. J. Taylor-Papadimitriou (ICRF, UK) and 4T1 mouse mammary cancer cells (from ATCC). The following clones, which had previously been produced in our laboratory (21) were used: Wnt-5a, overexpressing cells stably transfected with a pLNCX Wnt-5a-HA vector (Wnt-5ahigh HB2 or MCF-7 cells), Wnt-5a-antisense cells obtained by transfecting with a pLNCX vector containing the Wnt-5a cDNA in the antisense direction (3′-5′; Wnt-5alow HB2 cells).
Chemicals and Reagents—Antibodies directed toward Ser(P)-45 and Ser(P)-33/37/41 β-catenin were from Cell Signaling (Beverly, MA), anti-β-catenin and E-cadherin from BD Transduction Laboratories (Franklin Lakes, NJ), the anti-E-cadherin antibody for immunoprecipitations and immunofluorescence was clone SHE 78-7 from Axxora (Takara, Shiga, Japan), anti-FLAG (Sigma), anti-pan phospho-Ser/Thr (BD Transduction Laboratories) and G410 anti-phosphotyrosine antibody from Upstate Biotech Inc. (Lake Placid, NY). The polyclonal rabbit anti-human Wnt-5a antibody was developed in our laboratory (19). Recombinant Wnt-5a (rWnt-5a) was from R&D Systems (Minneapolis, MN) and was used at a concentration of 1.2 μg/ml for the times indicated on serum-starved cells. The α-E-catenin, CKIα, and Axin antibodies were from Santa Cruz Biotechnologies (CA) as was the casein kinase 1 α/ε inhibitor IC261 that was used at 50 μm 1.5 h after the addition of rWnt-5a (a total of 1 h). The protease inhibitor Z-Leu-Leu-Leu-al also known as MG132 (Sigma) was used at a concentration of 10 μm for 5 h prior to cell lysis, and the protease inhibitor N-ethylmaleimide (Sigma) was used at a concentration of 10 mm in the lysis buffer.
Tumor Samples—Samples were collected from primary tumors that had been removed from 94 consecutive patients with invasive breast carcinoma. Of the 94 tumors, 85 were ductal, four mucinous, two medullar, and three lobular. According to the classification system of the International Union against Cancer, 23 patients had stage I, 56 patients stage II, one patient stage III, and six patients stage IV breast carcinomas. In eight patients, the stage of the disease was not known due to a lack of data on tumor size or axillary node status. None of the patients had received radiation treatment or chemotherapy before surgery. Tissue samples were fixed in formalin, embedded in paraffin, and used for routine morphological examination (grading and immunostaining) and construction of tumor tissue arrays. To ensure that clearly defined areas of malignant tissue were used in the arrays, for each tumor sample, a slide with a fresh tissue section was prepared from the paraffin block and stained with hematoxylin. Areas including representative tumor cells were identified and marked, and two biopsies (Ø 0.6 mm (diameter)) corresponding to the marked areas on the slide were taken from each paraffin block. These biopsies were remounted in a new paraffin block in a tissue array machine according to the manufacturer's instructions (Becher Instruments, MD). At the time of analysis of membrane-associated β-catenin, 24 random tumors were lost from the paraffin block and thus 70 tumors were left to analyze.
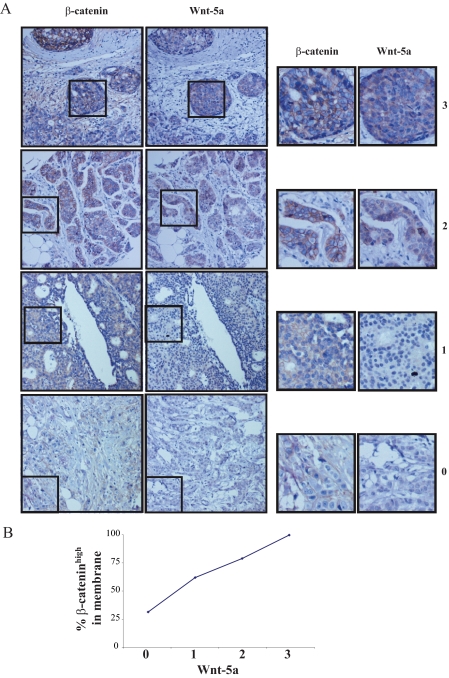
Immunohistochemistry—Immunohistochemistry was performed as previously described (20). The antibodies were Wnt-5a (19) and β-catenin (Sigma). Two independent observers (G. L. and C. M.), with no knowledge of the clinical outcome, evaluated the stained slides. The tumors were graded based on the intensity of the staining: for Wnt-5a (0, 1, 2, and 3) immunoreactivity equivalent to that seen in: 3, normal epithelial cells; 2, immunoreactivity moderately decreased; 1, weak immunoreactivity; 0, no immunoreactivity; and for membrane-associated β-catenin (0, 1, 2, and 3); 3, high immunoreactivity in the membrane; 2, normal immunoreactivity; 1, immunoreactivity moderately decreased; 0, weak immunoreactivity (Table 1 and Fig. 1A). In the histogram (Fig. 1A) the gradings (0 and 1) were viewed as β-cateninlow tumors and gradings (2 and 3) as β-cateninhigh tumors. For statistical analysis a Chi-square test was used and the linear to linear association between Wnt-5a and membrane-bound β-catenin was assessed.
TABLE 1.
Wnt-5a protein levels in relation to membrane-associated β-catenin levels in a total of 70 primary breast cancers
|
β-Catenin in Membrane
|
Wnt-5a
|
Total
|
p
|
|||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| 0 | 4 | 8 | 2 | 0 | 14 | |
| 1 | 5 | 5 | 2 | 0 | 12 | |
| 2 | 3 | 15 | 8 | 3 | 29 | |
| 3 | 1 | 6 | 7 | 1 | 15 | |
| Total | 13 | 34 | 19 | 4 | 70 | 0.004 |
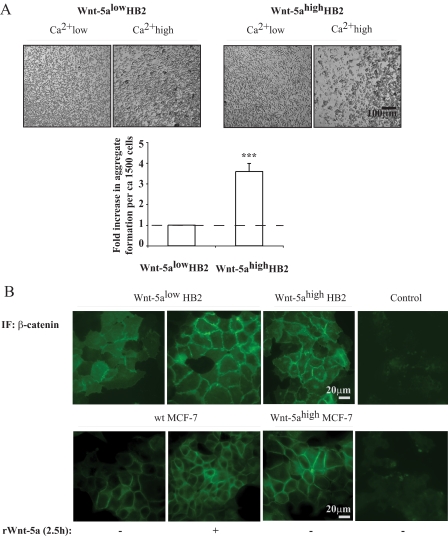
FIGURE 1.
A, cell aggregation assay. Human breast epithelial cells with varying Wnt-5a protein expression levels of HB2 Wnt-5alow and HB2 Wnt-5ahigh cells were treated with Versene, resuspended in Ca2+ high as compared with Ca2+ low medium and allowed to form aggregates for 1 h upon slow rocking. HB2 Wnt-5ahigh cells formed larger cell-cell clusters than HB2 Wnt-5alow cells. The levels of propidium iodide staining were equal. The diagram represents the fold-increase in number of aggregates formed per approximately 1500 cells in HB2 Wnt-5ahigh compared with HB2 Wnt-5alow cells. Error bars indicate S.E., n = 7, ***, p < 0.001. B, immunofluorescence (IF) studies of β-catenin membrane localization in human breast epithelial cells with varying Wnt-5a protein expression levels or treated with recombinant Wnt-5a. HB2 Wnt-5alow, HB2 Wnt-5alow treated with recombinant Wnt-5a and HB2 Wnt-5ahigh (upper); MCF-7, MCF-7 and treated with recombinant Wnt-5a and MCF-7 Wnt-5ahigh (lower)(n = 5).
Membrane Fractionations—Adherent cells were lysed and incubated for 30 min in 350 μl of lysis buffer A containing 20 mm Hepes, pH 8, 2 mm MgCl2 1 mm EDTA, 5 mm Na3VO4, 0.6 mm pefabloc, and 4 μg/ml leupeptin. The cells were further lysed with a homogenizer 25 times and centrifuged for 10 min at 500 × g. The supernatants were collected, centrifuged for 10 min at 10,000 × g, and collected again and centrifuged for 5 min at 10,000 × g, before ultracentrifuged for 1 h at 200,000 × g. The pellets (plasma membrane) were suspended in lysis buffer A and the protein concentrations were measured.
Immunoprecipitation and Western Blotting—Adherent cells were lysed in lysis buffer containing 50 mm Tris, pH 7.4, 1% Nonidet P-40, 5 mm EDTA, 5 mm EGTA, 50 mm NaCl, 5 mm sodium fluoride, 1 mm Na3VO4, 20 μg/ml aprotinin, 1 μg/ml leupeptin, 2.5 mm benzamidine, and 2 mm pefabloc. For immunoprecipitations, Protein A-Sepharose beads (Amersham Biosciences) were added and the lysates were precleared for 30 min. Thereafter specific antibodies were added for 5 min prior to addition of new Protein A-Sepharose beads with incubation at 4 °C for 1 h. The beads were washed six times in Hepes, pH 7.5, wash buffer with 1 mm Na3VO4.
Transient Transfections—Wnt-5alow and Wnt-5ahigh HB2 or MCF-7 cells were transfected with Oligofectamine or Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. For the TOP/FOPflash (Upstate Biotechnology) experiments each transfection was cotransfected with 0.2 μg of cytomegalovirus-controlled Renilla reporter gene. Luciferase assays were performed with a Dual Luciferase™ Reporter Assay System (Promega). For the FLAG immunoprecipitation experiments wild type β-catenin, Ser-45Δβ-catenin, and Ser-33Δβ-catenin, kindly provided by Professor David Rimm, were used. At the end of the transfection the cells were either left untreated or stimulated with rWnt-5a (20 nm; R&D Systems) as indicated, and subsequently immunoprecipitated with an anti-FLAG antibody. The coprecipitated proteins were analyzed by Western blots using E-cadherin and β-catenin-specific antibodies. The kinase-deficient CKIα mutants pCS2-CKIα2-K46A, pCS2-CKIα2-D136N, and pCS2-CKIα2-D136N-K138E were kind gifts from Professor Jiandong Chen (26).
Pulse-Chase—Wnt-5alow HB2 cells were starved overnight in serum-free medium and then washed and incubated in methionine-free DMEM (Invitrogen) supplemented with heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 units/ml streptomycin, 4 mm l-glutamine, 10 mm Hepes, and 20 μCi/ml l-[35S]methionine-stabilized aqueous solution, Easy tag (PerkinElmer Life Sciences) for 2 h. Thereafter cold DMEM plus 2 mm methionine were added. Cells were stimulated with rWnt-5a, LiCl, and harvested after 0 and 2 h. Lysates were subjected to immunoprecipitation using an antibody against β-catenin. Radiolabeled β-catenin was visualized and quantified using a Fuji LAS3000 phosphorimager and Multigauge software (Fujifilm, Tokyo, Japan).
Immunofluorescence—Wnt-5ahigh or Wnt-5alow cells were seeded on glass coverslips. The cells were fixed in freshly prepared 4% paraformaldehyde for 15 min, permeabilized in phosphate-buffered saline, 0.5% Triton X-100 for 15 min, and then blocked in 3% bovine serum albumin, phosphate-buffered saline for 1 h at room temperature. Staining was done with anti-E-cadherin (SHE 78-7), anti-β-catenin or isotype control antibodies in 1% bovine serum albumin, phosphate-buffered saline and with secondary anti-rabbit Alexa Fluor 488. The coverslips were mounted in fluorescent mounting medium (DAKO A/S), and examined and photographed in a Nikon Eclipse 800 microscope, using a ×60 objective. Images were recorded with a scientific-grade, charge-coupled device (CCD) camera (Hamamatsu, Japan) and analyzed with HazeBuster deconvolution software (VayTek, Inc., Fairland, CT).
Cell Aggregation—Wnt-5ahigh or Wnt-5alow HB2 cells were detached by treatment with Versene. The cells were washed in phosphate-buffered saline and then resuspended in Ca2+ low (10 μm CaCl2; DMEM, 10% fetal calf serum, 4 mm EGTA, 1 mm MgCl2) or Ca2+ high (1.8 mm CaCl2; DMEM, 10% fetal calf serum) medium. Approximately 5 × 106 cells/ml and sample were rocked gently in an Eppendorf tube for 1 h in a normal cell incubator. The cells were then poured into a 12-well plate and viewed under a light microscope. Approximately 1500 cells were counted from each well and the number of aggregates was estimated. 200 μl was taken from each sample and propidium iodide was added for 20 min prior to analysis using a FACS Calibur flow cytometer (n = 6, standard bars = S.E.).
Ca2+-induced Cell-Cell Adhesion—Confluent Wnt-5alow HB2 cells were quickly washed with Versene and subsequently with Ca2+ low medium (10 μm CaCl2; DMEM, 10% fetal calf serum, 4 mm EGTA, 1 mm MgCl2). The cells were viewed under a microscope to assure that the cell-cell contacts were disrupted (∼1 min). Thereafter Ca2+ high (1.8 mm CaCl2; DMEM, 10% fetal calf serum) medium was added to the cells in the presence or absence of rWnt-5a and incubated for 1 h prior to lysis. In the wells containing Ca2+ high medium the cell-cell contacts had clearly been re-established during this time, whereas in the control wells with Ca2+ low medium no cell-cell contacts were present.
Matrigel Invasion Assays—Invasion assays were carried out using Matrigel invasion chambers (BD) with 8.0-μm pore size membranes in 24-well plates. Transiently transfected (48 h) Wnt-5alow HB2 cells were harvested, washed, and resuspended at a concentration of 5 × 105 cells per ml in serum-free culture media. Serum containing medium (10% fetal calf serum) was added to the lower well and 0.5 ml (2.5 × 105 cells) of the cell suspension, containing recombinant Wnt-5a (0.8 μg/ml) where indicated, was added to the invasion chamber, and the cells were allowed to invade for 72 h. Cells that had invaded through the Matrigel were fixed (4% paraformaldehyde), stained with crystal violet (Sigma), and counted. The lower wells were always checked for cells to ensure that the experiments were stopped at the appropriate time. Transfection procedures were as described above, and the transfection efficiency (FLAG-β-catenin) and endogenous levels of β-catenin were evaluated using Western blot of β-catenin on 6% SDS gels.
RESULTS
Wnt-5a Increases the Intercellular Adhesion in Human Breast Epithelial Cells—To experimentally investigate the effects of Wnt-5a on Ca2+-dependent intercellular adhesion in human ductal breast epithelial cells, we initially investigated whether Wnt-5a signaling affected intercellular adhesion of a non-cancerous ductal breast epithelial cell line (HB2, see “Experimental Procedures”) that was either stably transfected with antisense Wnt-5a (HB2 Wnt-5alow) or with Wnt-5a (HB2 Wnt-5ahigh) (21). To this end, we analyzed the fraction of large cell aggregates formed upon slow rocking of single cell suspensions in Ca2+-depleted and Ca2+-containing medium for 1 h (Fig. 1A, Ca2+low and Ca2+high). We found that Wnt-5ahigh HB2 cells formed more aggregates as compared with Wnt-5alow HB2 cells in the presence of extracellular Ca2+ (Fig. 1A) without affecting the amount of dead cells (data not shown).
Wnt-5a Induces β-Catenin Membrane Association—To investigate whether the Wnt-5a-induced increase in intercellular adhesion was mediated by the β-catenin/E-cadherin complex we analyzed the membrane association of β-catenin in human breast epithelial cells using immunofluorescense with β-catenin-specific antibodies. We used two cell lines, the non-cancerous human ductal breast epithelial cell line (HB2) and the MCF-7 ductal breast cancer cell line, that again were either stably transfected with antisense Wnt-5a (HB2 Wnt-5alow) or with Wnt-5a (HB2 Wnt-5ahigh and MCF-7high) (21). All experiments were confirmed by using recombinant Wnt-5a (rWnt-5a). The advantage of using both Wnt-5a overexpressing cells (HB2 Wnt-5ahigh and MCF-7high cells) and stimulating HB2 Wnt-5alow cells with rWnt-5a is that the overexpressing cells have a continuous release of Wnt-5a, whereas a prompt rWnt-5a treatment give us a better opportunity to directly analyze Wnt-5a-specific cell signaling cascades. Interestingly, in HB2 Wnt-5ahigh cells and in HB2 Wnt-5alow cells that were treated with rWnt-5a (2.5 h), the increase in membrane-associated β-catenin was substantial (Fig. 1B). Wnt-5a treatment also led to an altered morphology of the cells with membrane protrusions, suggesting initiation of intercellular contacts (Figs. 1B and 2A). We did not detect significant differences in nuclear β-catenin (Fig. 1B). Also in the breast cancer cell line MCF-7, an increased membrane association of β-catenin was found upon Wnt-5a expression (MCF-7high) or treatment with rWnt-5a (Fig. 1B, lower panel).
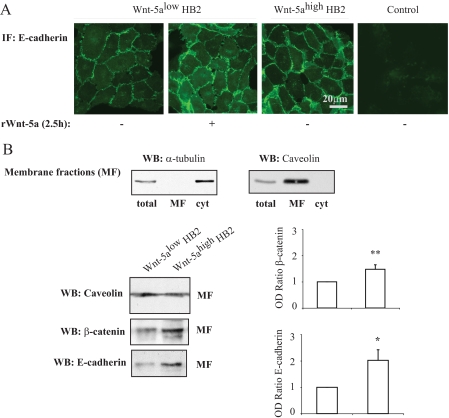
FIGURE 2.
A, immunofluorescence (IF) studies of E-cadherin membrane localization in human breast epithelial cells (HB2) with varying Wnt-5a protein expression levels or in HB2 Wnt-5alow cells treated with recombinant Wnt-5a (rWnt-5a)(n = 3). B, membrane fractionation experiments. The purity of the membrane fractions (total cell lysate (total), membrane fraction (MF), and cytosol (cyt)) was analyzed with α-tubulin and caveolin antibodies (upper panel). Western blot analysis of E-cadherin and β-catenin protein expression levels in membrane fractions from human breast epithelial cells with varying Wnt-5a protein expression levels (lower panels). To ensure equal loading, protein measurements were done using a Coomassie® protein assay reagent (Pierce). The blots shown are representative of several separate experiments and OD measurements of the band intensities were performed to quantify the differences (right panel, histograms). Error bars indicate S.E., n = 16; *, p < 0.05; **, p < 0.01. WB, Western blot.
The same results were obtained using immunofluorescence with E-cadherin-specific antibodies (Fig. 2A). We next performed membrane fractionations using α-tubulin as a control for the cytosolic fractions and caveolin as control for the membrane fractions (Fig. 2B). As shown in Fig. 2B, the levels of β-catenin in the cell membrane were significantly elevated in HB2 Wnt-5ahigh cells compared with HB2 Wnt-5alow cells. When the membrane fractions were analyzed for E-cadherin we found a profound increase in the levels of membrane-associated E-cadherin (Fig. 2B). Neither the protein nor mRNA levels of β-catenin or E-cadherin were affected by a short (2.5 h) stimulation with rWnt-5a in human breast epithelial cells. This indicates that the increased membrane level of β-catenin was not caused by a general increase in β-catenin expression. In line with this, TCF/LEF reporter assays using the TOPflash/FOPflash reporters in human breast epithelial cells indicated that Wnt-5a signaling did not induce canonical β-catenin signaling (supplemental materials Fig. S1A and Ref. 16).
Wnt-5a Induces an Increased β-Catenin/E-Cadherin Complex Formation—To investigate the underlying mechanism responsible for the Wnt-5a-induced membrane association of β-catenin, we performed co-immunoprecipitations of β-catenin and E-cadherin. We found an increased association of E-cadherin to β-catenin both in HB2 Wnt-5ahigh cells and in HB2 Wnt-5alow cells treated with rWnt-5a for 2.5 h (Fig. 3, A and B, left panel). The Wnt-5a-induced association occurred already after 1 h of treatment with rWnt-5a (Fig. 3A). This was also seen in the breast cancer cell line MCF-7 either treated with rWnt-5a for 2.5 h, or constitutively overexpressing Wnt-5a (MCF-7 Wnt-5ahigh; Fig. 3B, right panel).
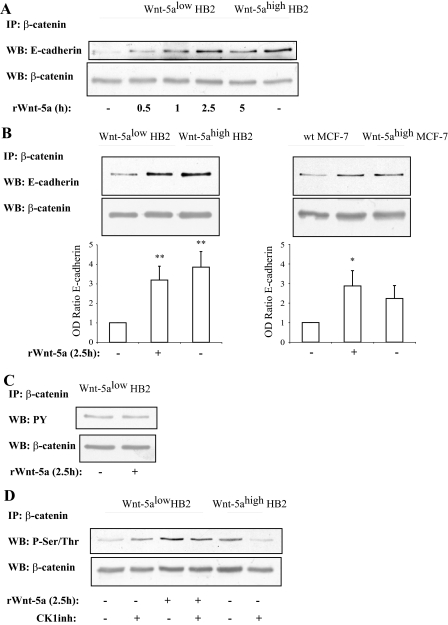
FIGURE 3.
A, time kinetics of Wnt-5a inducedβ-catenin/E-cadherin complex formation indicates that it is a rapid and specific process unrelated to general protein expression levels (n = 3). B, β-catenin/E-cadherin complex formation is potentiated by Wnt-5a signaling as judged by co-immunoprecipitations of β-catenin/E-cadherin complexes from human mammary epithelial cell line HB2 (left) and human breast cancer cell line MCF-7 (right) cells with varying Wnt-5a expression levels or in cells treated with recombinant Wnt-5a. The blot shown is representative of several separate experiments and OD measurements of the band intensities were performed to quantify the differences (lower panel; histograms). Error bars indicate S.E., n = 8, n = 5; *, p < 0.05; **, p < 0.01. C, tyrosine phosphorylation of β-catenin is not affected by Wnt-5a stimulation, whereas serine/threonine phosphorylation is (D) as judged by immunoprecipitation of β-catenin and Western blot (WB) using a total Tyr(P) or Ser/Thr antibodies. Inhibition of the serine/threonine kinase CKI, with the casein kinase 1 α/ε inhibitor IC261, disrupts the Wnt-5a-induced Ser/Thr phosphorylation of β-catenin, whereas cells lacking Wnt-5a remain Ser/Thr phosphorylated on β-catenin (D). The CKI inhibitor was added for 1 h at the end of the incubation with rWnt-5a (n = 5). Wt, wild type.
Wnt-5a Does Not Affect Tyrosine Phosphorylation of β-Catenin—β-Catenin can be tyrosine phosphorylated at two residues, Tyr(P)-142 and Tyr(P)-691. Previous studies suggest that one of the phosphorylated tyrosines leads to dissociation from α-catenin, whereas the other leads to dissociation from the E-cadherin complex (27). To explore whether Wnt-5a signaling can affect the association of β-catenin to E-cadherin by affecting the tyrosine phosphorylations of β-catenin, we performed immunoprecipitation studies of total β-catenin from HB2 Wnt-5alow and Wnt-5alow cells treated with rWnt-5a (Fig. 3C). However, we did not see a reduced tyrosine phosphorylation of β-catenin. The experiment was also performed comparing HB2 Wnt-5alow and HB2 Wnt-5ahigh cells with the same result (data not shown).
Wnt-5a-induced Serine Phosphorylation of β-Catenin Leads to an Increased E-Cadherin Complex Formation—It has been suggested that phosphorylation of specific Ser/Thr residues on β-catenin can increase the binding of β-catenin to E-cadherin (7, 8). To investigate whether β-catenin is serine/threonine phosphorylated upon Wnt-5a treatment we performed new immunoprecipitations of β-catenin and analyzed the phosphoserine/threonine levels (Fig. 3D). We could detect an increased phosphorylation of serine/threonine both in HB2 Wnt-5ahigh cells and Wnt-5alow cells treated with rWnt-5a.
The serine/threonine kinase CKI has previously been shown to phosphorylate β-catenin-Ser-45 in the absence of canonical Wnt signaling (28), but also to phosphorylate E-cadherin on Ser-846, hence leading to a decreased association to β-catenin (11). However, there are conflicting data as to whether CKI activity actually is negative for the β-catenin/E-cadherin interaction (11, 12). We have previously shown that Wnt-5a activates CKIα in human breast epithelial cells (16). Interestingly, upon addition of the selective CKIα/ε inhibitor (IC261), we saw a small reduction in phosphoserine/threonine levels of β-catenin (Fig. 3D). More importantly, this only occurred in cells stimulated with Wnt-5a.
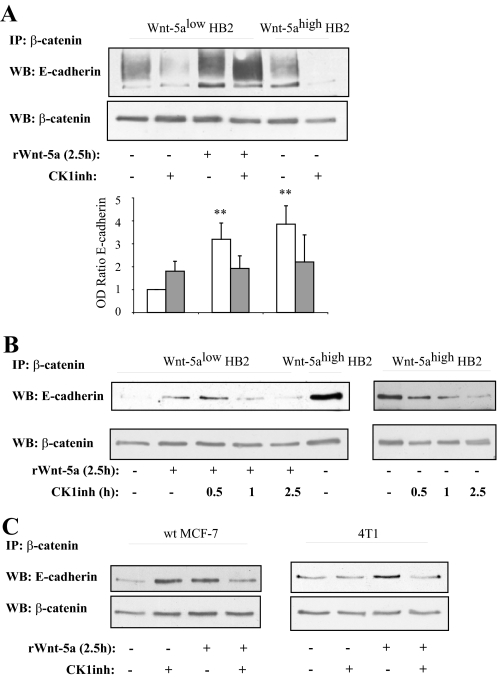
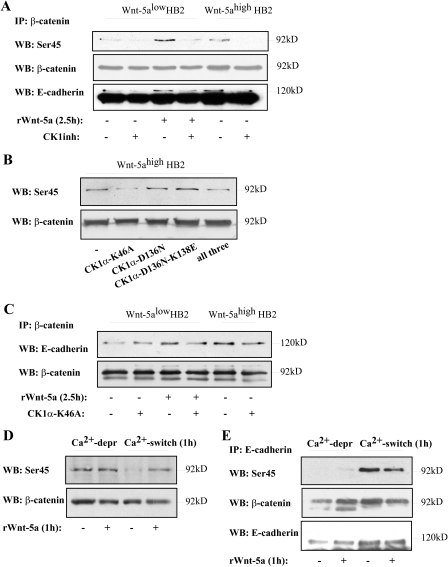
The Wnt-5a-induced β-catenin/E-Cadherin complex formation was also inhibited upon addition of the CKI inhibitor (Fig. 4A). Again, this was Wnt-5a specific because the HB2 Wnt-5alow cells treated with the CKI inhibitor did not show a decreased complex formation (see below). The complex formation was inhibited after a 1-h treatment with CKI inhibitor and had virtually disappeared after 2.5 h. Interestingly, and in line with a recent study mentioned above (11), treatment with the CKI inhibitor in cells lacking Wnt-5a signaling rather led to an increased complex formation of E-cadherin to β-catenin (Fig. 4, A and C, left). In rWnt-5a-stimulated cells where CKI had only been inhibited for 0.5 h (Fig. 4B) we still have an increased β-catenin association to E-cadherin, indicating that the induced β-catenin/E-cadherin complex formation indeed is Wnt-5a specific, and due to its activation of CKI. As seen in supplemental materials Fig. S1B, the overall β-catenin and E-cadherin levels were not reduced upon treatment with the CKI inhibitor at the time points used in the experiments, although at 12 h a reduction of E-cadherin levels could be observed. Finally, we could also show that the Wnt-5a/CKI-induced β-catenin/E-cadherin complex formation was induced in breast cancer cell lines treated with rWnt-5a (Fig. 4C, left, MCF-7; and right, 4T1 mouse mammary cancer cells lacking endogenous Wnt-5a; for statistics see supplemental materials Fig. S2A).
FIGURE 4.
A, the Wnt-5a-induced β-catenin/E-cadherin complex formation is specifically inhibited by the CKI inhibitor only in cells treated with, or expressing, Wnt-5a. The blot shown is representative of several separate experiments and OD measurements of the band intensities were performed to quantify the differences (lower panel, histograms). Error bars indicate S.E., n = 8; *, p < 0.05; **, p < 0.01. B, time kinetics of treatment with the CKI inhibitor indicates that Wnt-5a is upstream of CKI in induction of β-catenin/E-cadherin complex formation (n = 3). C, the Wnt-5a/CKI induced β-catenin/E-cadherin complex formation is also seen in two different breast cancer cell lines: MCF-7 (left) and 4T1 (right). The blot shown is representative of several separate experiments and OD measurements of the band intensities were performed to quantify the differences (supplemental Fig. S2A). WB, Western blot; IP, immunoprecipitate.
Wnt-5a/CKI-induced β-Catenin Ser-45 Phosphorylation Correlates with an Increased β-Catenin/E-Cadherin Complex Formation—It is well established that CKIα induces phosphorylation of Ser-45 on β-catenin (28, 29). However, it has to our knowledge not been shown that a non-canonical Wnt can induce this Ser-45 phosphorylation. We have previously shown that Wnt-5a activates CKIα in human breast epithelial cells (16), an observation that was recently confirmed in dopaminergic neurons (30). Upon stimulation with Wnt-5a we observed a distinct increase in Ser-45 phosphorylation in Wnt-5alow cells treated with rWnt-5a and a higher level of Ser-45 phosphorylation in HB2 Wnt-5ahigh cells (Fig. 5A). The elevated levels were abolished upon addition of the CKI inhibitor. More importantly, the levels of co-precipitated E-cadherin correlated well with Ser-45 phosphorylation of β-catenin (Fig. 5A). We also performed reverse co-immunoprecipitations with an antibody toward E-cadherin (supplemental materials Fig. S1C) but the levels of coprecipitated β-catenin were constant. However, the level of Ser-45 phosphorylation varied in accordance with previous results. We do not know the reason for this observation, but we suspect that the only antibody available for immunoprecipitation of E-cadherin (SHE 78-7, recognizing its ectodomain) only detects E-cadherin bound to β-catenin, because unbound E-cadherin is degraded (31). To confirm that the Wnt-5a-induced Ser-45 phosphorylation of β-catenin was caused by CKIα we transfected Wnt-5ahigh cells with three different kinase-deficient mutants of CKIα (pCS2-CKIα2-K46A, pCS2-CKIα2-D136N, and pCS2-CKIα2-D136N-K138E). These behave differently as it was previously shown that CKIα2-K46A had lost the ability to not only phosphorylate but also to bind its target protein (26). As shown in Fig. 5B, only overexpression of the kinase-deficient pCS2-CKIα2-K46A plasmid inhibited Ser-45 phosphorylation of β-catenin by Wnt-5a. In support of this, the Wnt-5a-induced increase in β-catenin/E-cadherin complex formation also was inhibited upon expression of pCS2-CKIα2-K46A (Fig. 5C).
FIGURE 5.
A, Wnt-5a/CKI specifically induces Ser-45 phosphorylation of β-catenin. This correlates with increased levels of co-immunoprecipitated E-cadherin (n = 3). The lower band in the E-cadherin reblot is β-catenin. B, transfection of Wnt-5ahigh HB2 cells with three different variants of dominant negative kinase-deficient mutants of CKIα (26): K46A, D136N, and D136N-K138E or all three. Overexpression of CKIα-K46A disrupts the Wnt-5a-induced Ser-45 phosphorylation of β-catenin. C, overexpression of CKIα-K46A also disrupts the Wnt-5a-induced increase in β-catenin/E-cadherin complex formation. D, initiation of cell-cell adhesion per se does not induce Ser-45 phosphorylation of β-catenin. Ca2+-dependent cell-cell adhesion was initiated by performing a Ca2+-switch experiment (see “Experimental Procedures”). Wnt-5alow HB2 cells were allowed to re-adhere for 1 h after the Ca2+ switch in the absence or presence of rWnt-5a. Cells where cell-cell contacts had recently been disrupted and cells treated with rWnt-5a show β-catenin Ser-45 phosphorylation (also see C). In sharp contrast, initiation of de novo cell-cell contacts per se does not lead to Ser-45 phosphorylation, whereas addition of rWnt-5a does (n = 3). E, the pool of Ser-45-phosphorylated β-catenin seen after cell-cell contact disruption in B is not associated to E-cadherin complexes as shown by co-immunoprecipitations of E-cadherin after Ca2+ deprivation. In sharp contrast, the E-cadherin-associated β-catenin after a Ca2+ switch (re-adherence) is Ser-45 phosphorylated. As described in the text it is not possible to compare the amount of coprecipitated β-catenin using the (SHE 78-7) E-cadherin ectodomain antibody and this might explain why we do not see an increased Ser-45 phosphorylation after Wnt-5a stimulation compared with unstimulated cells in this particular set of experiments. The lower band in the E-cadherin reblot is β-catenin. WB, Western blot; IP, immunoprecipitate.
An Increased Intercellular Adhesion Does Not Induce β-Catenin Ser-45 Phosphorylation per se—Because it is known that Wnt-5a increases cell-extracellular adhesion by various mechanisms it was important to analyze whether an increased cell-cell adhesion per se could induce β-catenin Ser-45 phosphorylation on its own. We therefore performed Ca2+-switch experiments to experimentally induce Ca2+-dependent E-cadherin/E-cadherin interactions and analyzed the grade of β-catenin Ser-45 phosphorylation in the absence or presence of rWnt-5a (1 h). Interestingly, Ca2+-deprived cells that just recently (5 min) lost cell-cell contacts show a clear phosphorylation of β-catenin Ser-45 (Fig. 5D). However, reintroduction of Ca2+-dependent cell-cell contacts led to a decreased β-catenin Ser-45 phosphorylation (1 h) in the absence of rWnt-5a (Fig. 5D). Importantly, the addition of Wnt-5a still led to a clear β-catenin Ser-45 phosphorylation indicating that Wnt-5a indeed phosphorylates β-catenin on Ser-45 (Fig. 5D). In an attempt to understand why β-catenin became Ser-45 phosphorylated upon Ca2+ deprivation, we next performed co-immunoprecipitations of E-cadherin in Ca2+-deprived cells, or cells where Ca2+-dependent cell-cell contacts had been reintroduced, with or without rWnt-5a stimulation. As seen in Fig. 5E, the β-catenin that associated with E-cadherin was not Ser-45 phosphorylated after Ca2+ deprivation, indicating that the Ser-45-phosphorylated β-catenin in Ca2+-deprived cells was cytosolic. In contrast, in cells where Ca2+-dependent cell-cell contacts had been reintroduced the E-cadherin-associated β-catenin was Ser-45 phosphorylated. As previously mentioned, it is not possible to compare the levels of coprecipitated β-catenin using the (SHE 78-7) E-cadherin ectodomain antibody. Nevertheless, we conclude that the Ser-45-phosphorylated β-catenin in Ca2+-deprived cells is most likely due to an increased level of cytosolic, degrading β-catenin as a result of disruption of Ca2+-dependent cell-cell contacts. Thus, the increase in β-catenin Ser-45 phosphorylation seen in Fig. 5D is indeed induced by Wnt-5a.
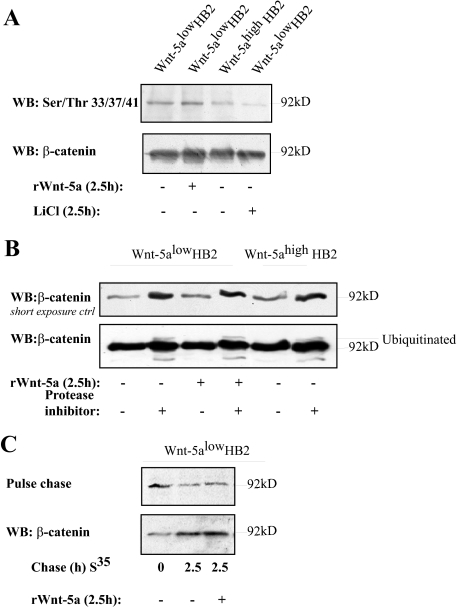
Wnt-5a Signaling Does Not Lead to β-Catenin Degradation in Human Breast Epithelial Cells—Because we found that β-catenin is phosphorylated on Ser-45 by Wnt-5a/CKIα signaling in human mammary epithelial cells and that it is known since before that Wnt-5a could inhibit canonical Wnt β-catenin signaling through β-catenin degradation (32), it was important to control whether β-catenin is targeted for degradation upon Wnt-5a signaling. Initially, we performed Western blots using Ser(P)-33/Ser(P)-37/Thr(P)-41 β-catenin-specific antibodies of cell lysates from Wnt-5alow cells stimulated or not with rWnt-5a for 2.5 h and Wnt-5ahigh HB2 cells. We did not see an increased phosphorylation of β-catenin Ser-33/Ser-37/Thr-41 (Fig. 6A). We next performed co-immunoprecipitations of Axin to investigate whether the Wnt-5a/CKIα-induced β-catenin Ser-45 phosphorylation could occur in the classical Wnt destruction complex (supplemental materials Fig. S1D). The association of both CKIα and β-catenin with Axin were slightly induced upon Wnt-5a signaling supporting that the Wnt-5a-induced Ser-45 phosphorylation of β-catenin occurs in the conventional APC-Axin-destruction complex in the cytosol. We next analyzed the effects of Wnt-5a on β-catenin ubiquitination, using protease inhibitors (Fig. 6B, upper panel, loading control). In line with the previous results we could not detect an increased level of ubiquitinated β-catenin upon Wnt-5a treatment (lower panel). Finally we performed pulse-chase experiments to verify whether Wnt-5a affects degradation of β-catenin in human breast epithelial cells or not. As shown in Fig. 6C degradation of β-catenin in human breast epithelial cells was similar in untreated and Wnt-5a-treated cells at the time points used in this study (2.5 h). The experiment was also performed using longer chase periods with similar results (data not shown). This is in agreement with the finding that we observed similar levels of β-catenin protein in resting compared with Wnt-5a-stimulated cells. Together this indicates that Wnt-5a induces Ser-45 phosphorylation of β-catenin in human breast epithelial cells, but neither induces a GSK-3β nor Siah-2-specific degradation of β-catenin (32-34).
FIGURE 6.
A, Wnt-5a signaling does not induce Ser-33/Ser-37/Thr-41 phosphorylation in human breast epithelial cells. LiCl (10 μm) was used as a control. Note that the level of total β-catenin is slightly higher in the second lane of this particular experiment. B, analysis of β-catenin ubiquitination. HB2 Wnt-5alow cells and HB2 Wnt-5alow cells stimulated with rWnt-5a (2.5 h) or HB2 Wnt-5ahigh cells were treated with the protease inhibitor MG132 for 5 h prior to cell lysis (lysis buffer contained the protease inhibitor N-ethylmaleimide) and levels of ubiquitinated β-catenin were analyzed (n = 3). C, pulse-chase experiments were performed to analyze the rate of β-catenin degradation. Wnt-5a does not induce β-catenin degradation in human breast epithelial cells as compared with untreated cells. Longer chase periods gave similar results (data not shown). The level of total β-catenin was controlled by Western blot (WB) (lower panel).
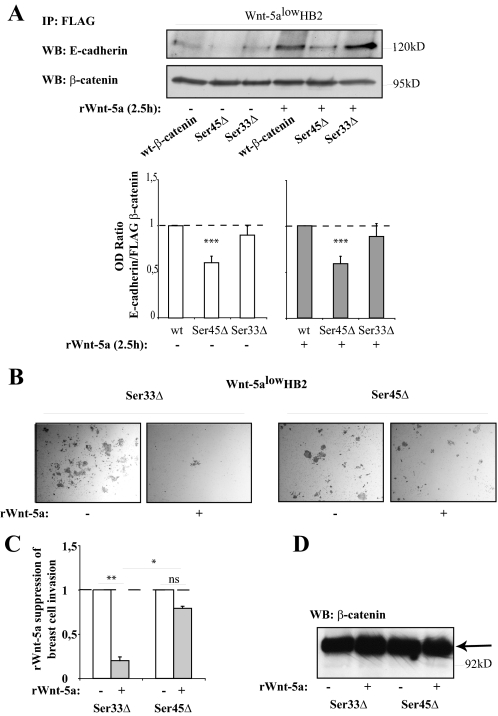
Mutation of β-Catenin Ser-45 Leads to a Decreased β-Catenin/E-Cadherin Association—To verify that Ser-45 phosphorylation of β-catenin affects the association between β-catenin and E-cadherin we performed co-immunoprecipitations of transfected FLAG-tagged β-catenin mutants (wild type or Ser-45Δ and Ser-33Δ mutants (35)) and blotted for endogenous E-cadherin from cells stimulated or not with Wnt-5a. The amounts of immunoprecipitated FLAG β-catenin in each sample added to the gel were set to equal levels, and OD measurements of co-immunoprecipitated E-cadherin versus immunoprecipitated FLAG-β-catenin were performed (ratio E-cadherin/FLAG β-catenin). In line with our previous observations, a mutation at Ser-45 led to a significantly decreased association between β-catenin and E-cadherin as compared with wild type β-catenin, both under non-stimulating (white bars) and Wnt-5a stimulating (gray bars) conditions. A Ser-33Δ mutated form of β-catenin where Ser-45 still is phosphorylated did not have this profound effect on the association between E-cadherin and β-catenin (Fig. 7A), especially not under Wnt-5a stimulating conditions. This supports the fact that a Wnt-5a-induced Ser-45 phosphorylation of β-catenin is essential for an increased association between β-catenin and E-cadherin.
FIGURE 7.
A, co-immunoprecipitation of E-cadherin with different forms of FLAG-tagged β-catenin (35). HB2 Wnt-5alow cells were transfected with FLAG-tagged wild type (wt) β-catenin, Ser-45Δ-mutated β-catenin, and Ser-33Δ-mutated β-catenin. Immunoprecipitation was performed using anti-FLAG antibodies. Due to a decreased degradation of β-catenin harboring the Ser-45Δ and Ser-33Δ mutations, FLAG β-catenin had to be set to equal levels in the blots by a pre-Western blot (WB) analysis after which the level of coprecipitated E-cadherin compared with FLAG β-catenin in the final blot could be measured using OD measurements. The histogram thus shows the relative levels (OD E-cadherin/OD FLAG-β-catenin) of bound E-cadherin to FLAG-tagged wild type β-catenin as compared with FLAG-tagged Ser-45Δ and Ser-33Δ β-catenin, under normal (left) or Wnt-5a stimulating (right) conditions (***, p < 0.001 compared with wild type, S.E., n = 7). B, Matrigel-invasion assay. HB2 Wnt-5alow cells were transfected with FLAG-tagged Ser-33Δ β-catenin or Ser-45Δ β-catenin. After a 48-h transfection period the cells were detached, counted, and 25,000 cells/chamber were allowed to invade Matrigel invasion chambers in the absence or presence of rWnt-5a in the upper chamber. C, the histogram (representing B) shows the fold-reduction in number of invaded cells upon rWnt-5a treatment for both Ser-33Δ β-catenin- and Ser-45Δ β-catenin-transfected cells (**, p < 0.01; *, p < 0.05; S.E.; n = 3). D, the levels of FLAG-tagged β-catenin protein were similar between the samples in B and C.
The Wnt-5a-specific Inhibition of Human Breast Epithelial Cell Matrigel Invasion Is Reduced upon Mutation of β-Catenin Ser-45—We also tested whether the Wnt-5a-specific inhibition of human breast epithelial cell Matrigel invasion (16) would be affected by mutation of β-catenin Ser-45. To assure that the mutation-induced accumulation of β-catenin was equal in all samples, and therefore not a parameter that would affect the outcome of the assay, we transfected HB2 Wnt-5alow cells with either β-catenin Ser-33Δ (control samples) or β-catenin Ser-45Δ (Fig. 7, B and C). After a 48-h transfection period we transferred the cells to Matrigel invasion chambers (see “Experimental Procedures”) and either left the cells untreated, or treated them with rWnt-5a. As shown in Fig. 7, B and C, we could still see the Wnt-5a specific reduction of invasive capacity in β-catenin Ser-33Δ expressing cells, whereas cells expressing β-catenin Ser-45Δ, where the β-catenin/E-cadherin complexes are unresponsive to Wnt-5a stimulation, invaded the Matrigel also after addition of Wnt-5a. The levels of transfected FLAG β-catenin were similar in each experiment and the FLAG β-catenin protein levels exceeded the endogenous β-catenin levels (Fig. 7D). Interestingly, and for unknown reasons, as shown in Fig. 7B, the basic invasive capacity of FLAG β-catenin Ser-45Δ-transfected cells was lower than that for FLAG β-catenin Ser-33Δ-transfected cells.
Wnt-5a Expression Correlates with β-Catenin Membrane Expression in Human Breast Tumors—We have previously shown that human breast cancers with a low Wnt-5a protein expression are more prone to metastasize and hence Wnt-5a expression is a predictor of longer disease-free survival (19, 20). Furthermore, it was previously shown that a low membrane expression of β-catenin correlated with a poor outcome in human breast cancer patients (6). To further investigate this, and to compare the findings in this study to clinical parameters, we analyzed if changes in Wnt-5a protein expression in human breast tumors relates to an altered membrane distribution of β-catenin. We analyzed the membrane expression levels of β-catenin and the expression levels of Wnt-5a in a human breast tumor tissue microarray consisting of 70 primary breast tumors. The breast tissue tumor arrays were stained with antibodies against β-catenin and Wnt-5a and were categorized according to the expression levels of membrane-bound β-catenin (0, 1, 2, 3) and total Wnt-5a ((0, 1, 2, 3), see “Experimental Procedures”). Interestingly, as shown in Table 1 and Fig. 8, A and B, we found a significant correlation between low membrane expression of β-catenin and low levels of Wnt-5a protein expression (linear by linear association, p = 0.004). In line with the experiments in this study, we did not see a significant difference in cytoplasmic or nuclear β-catenin protein levels in Wnt-5a expressing or non-expressing tumors (data not shown).
FIGURE 8.
A, summarizing presentation of immunostainings of Wnt-5a and membrane-associated β-catenin in representative sections of human invasive breast carcinomas. Staining was done with a polyclonal rabbit antibody against human Wnt-5a (19) and an antibody directed against β-catenin. The tumors in the figure represents gradings 0 (lower) to 3 (upper) (see “Experimental Procedures” for description) for membrane-associated β-catenin and Wnt-5a (left) with magnifications representing the squared fields (right). B, the diagram represents the percentage of β-cateninhigh tumors (grading 2 and 3) in the different Wnt-5a groups (0, 1, 2, 3). The statistical Chi-square test was used and the linear to linear association between Wnt-5a and membrane-bound β-catenin was p = 0.004.
DISCUSSION
In this study we show that Wnt-5a can increase the Ca2+-dependent intercellular adhesion in human epithelial breast cells. This is in line with previous clinical observations where loss of Wnt-5a has been associated with an increased risk of metastasis and a worse prognosis (19, 22-24). We explain this fact by the ability of Wnt-5a to specifically induce β-catenin membrane localization through association with the adhesion molecule E-cadherin and an increased E-cadherin-specific intercellular adhesion. We also show that the mechanism behind it is an active process where Wnt-5a-induced activation of CKIα leads to Ser-45 phosphorylation of β-catenin and thereby an increased complex formation of β-catenin/E-cadherin and subsequently increased membrane localization. Finally, we present a clinical correlation where low expression of Wnt-5a in human breast tumors strongly correlates with a reduced membrane association of β-catenin and an increased potential to metastasize.
It is known that the association between β-catenin and E-cadherin is regulated through phosphorylation of a serine-rich stretch of E-cadherin that comprises the β-catenin binding domain (36). Only recently was the specific serine residues that regulates this interaction identified (8). It has also become evident that it is not only phosphorylation of E-cadherin that regulates the β-catenin/E-cadherin interaction. In fact, phosphorylation of β-catenin itself can regulate the binding to E-cadherin (Tyr(P)-654) (8, 37). It is interesting to note that most β-catenin binding partners (cadherins, Axin/APC, and TCF/LEF) bind to the same positively charged groove of the central part of β-catenin. Just as for Axin/APC and TCF/LEF it would be logical that phosphorylation at the NH2-terminal end (Ser-45) of β-catenin could affect the binding of E-cadherin, perhaps indirectly. Indeed, it has been shown that the NH2-terminal end of β-catenin is a prerequisite for binding to E-cadherin (9). In this study we show that Wnt-5a/CKIα signaling can increase the complex formation between β-catenin/E-cadherin. We also show that a Ser-45Δ mutated form of β-catenin binds significantly less E-cadherin.
It has previously been suggested that the β-catenin/E-cadherin association is formed in the endoplasmic reticulum and that E-cadherin is brought up to the surface by β-catenin (38). Whether α-catenin associates with β-catenin before or after joining E-cadherin is not fully understood (9, 39, 40). However, it is known that the binding of α-catenin to β-catenin facilitates the association of β-catenin to E-cadherin (9, 39). Indeed, we were able to show that Wnt-5a signaling increased the amount of both β-catenin and E-cadherin in α-catenin co-immunoprecipitates (supplemental materials Fig. S1E). Also, through its association with either β-catenin/E-cadherin or actin filaments, α-catenin acts as a molecular switch, regulating actin filament assembly at cell-cell contacts (3, 4). We do not know whether α-catenin promotes the β-catenin/E-cadherin assembly, or if the increased binding of α-catenin to β-catenin is a result of increased β-catenin/E-cadherin complex formation at cell-cell contacts. It is interesting to note that Wnt-5a induces actin stress fiber formation in mammary epithelial cells (41) and, as shown in this study, that Wnt-5a treatment leads to cell protrusions resembling a nascent cell-cell contact with lamellipodia formations indicating that the increased binding of α-catenin to β-catenin could lead to a local re-modulation of the actin network (3). However, the half-life of E-cadherin at cell contacts is short (42). Because the membrane protein levels of both β-catenin and E-cadherin are increased upon short time Wnt-5a signaling (<2.5 h), without affecting the total protein levels of the respective proteins at this time point, we propose that Wnt-5a indeed promotes the formation of the β-catenin/E-cadherin complex in the endoplasmic reticulum as a consequence of β-catenin Ser-45 phosphorylation, perhaps facilitated by α-catenin. Indeed, E-cadherin-associated β-catenin Ser-45 phosphorylation was not a secondary effect caused by an increased intercellular adhesion per se, but was clearly induced by Wnt-5a/CKIα signaling.
The Wnt-5a/CKIα-induced Ser-45 phosphorylation of β-catenin does not lead to an increased degradation of β-catenin, ruling out a possible role for both GSK-3β and Siah-2 (32-34). This again points out the differences between varying cell types concerning Wnt induced effects. Our data clearly indicates that Wnt-5a can affect β-catenin in a novel fashion mediating membrane localization without affecting the nuclear distribution or degradation of β-catenin. A study suggested that β-catenin targeted to adhesive or transcriptional complexes have distinct molecular forms that would allow it to coordinate the two processes (9). Indeed, we found that upon mutation of β-catenin-Ser-45, the Wnt-5a-induced β-catenin/E-cadherin association was decreased. In line with these results, expression of the mutated β-catenin-Ser-45 in invasion assays with breast epithelial cells lead to a non-responsiveness to Wnt-5a in contrast to expression of the mutated β-catenin-Ser-33. It is an interesting observation that the NH2-terminal of β-catenin is phosphorylated in the β-catenin/E-cadherin complex in our system, in contrast to what was previously suggested (9). However, it is in agreement with the observation that β-catenin with a truncated NH2 terminus cannot bind E-cadherin (9). Furthermore, it is interesting to note that we see an association of α-catenin with β-catenin under Wnt-5a stimulating conditions, indicating that β-catenin does not preferentially participate in transcriptional activation (9), as supported by our luciferase TCF/LEF transcription experiments (16). Last, the data from the breast tumor tissue array also supports the fact that neither an increased nor a lowered level of membrane-associated β-catenin per se induced a nuclear accumulation of β-catenin.
The observation that cancers lacking E-cadherin mutations, such as ductal breast cancer, might still have an altered E-cadherin intercellular adhesion property due to a secondary mechanism such as loss of Wnt-5a protein expression and signaling is interesting. The effect of Wnt-5a on β-catenin membrane localization presented in this study is a novel mechanism ascribed to Wnt proteins that is of importance for the analysis of metastatic cancers lacking E-cadherin mutations. It also strengthens the role of Wnt-5a as a tumor suppressor in breast cancer, now pointing to a role not only in cell-extracellular adhesion (20, 21, 43) but also in Ca2+-dependent intercellular adhesion.
Supplementary Material
Acknowledgments
We thank Professor D. Rimm for the FLAG β-catenin plasmids and Professor J. Chen for the kinase-deficient CKIα plasmids. We also thank Elise Nilsson for histological stainings, Lena Axelsson for technical assistance, Dr. R. Massoumi for scientific advice regarding the ubiquitination protocol, Professor L. Rönnstrand for professional advice concerning the pulse-chase assays, and Prof. T Leanderson for critical reading of the manuscript.
This work was supported in part by grants from the Swedish Cancer Foundation (to G. L., T. A., and K. L.), the Universitetssjukhuset-Malmö Allmänna Sjukhus Research Foundations (to T. A. and K. L.), Universitetssjukhuset-Malmö Allmänna Sjukhus Cancer Foundation (to T. A. and K. L.), The Swedish Research Council (to T. A.), Gunnar Nilsson's Cancer Foundation (to T. A. and K. L.), the Crafoord Foundation (to K. L.), and the Royal Physiographic Society in Lund (to K. L.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: GSK-3β, glycogen synthase kinase-3β; CK, casein kinase; APC, adenomatous polyposis coli; DMEM, Dulbecco's modified Eagle's medium; rWnt-5a, recombinant Wnt-5a.
References
- 1.Osborne, M. P. (2000) Breast Anatomy and Development, 2nd Ed., Philadelphia, PA
- 2.Berx, G., Nollet, F., and van Roy, F. (1998) Cell Adhes. Commun. 6 171-184 [DOI] [PubMed] [Google Scholar]
- 3.Drees, F., Pokutta, S., Yamada, S., Nelson, W. J., and Weis, W. I. (2005) Cell 123 903-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada, S., Pokutta, S., Drees, F., Weis, W. I., and Nelson, W. J. (2005) Cell 123 889-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei, H., Sjoberg-Margolin, S., Salahshor, S., Werelius, B., Jandakova, E., Hemminki, K., Lindblom, A., and Vorechovsky, I. (2002) Int. J. Cancer 98 199-204 [DOI] [PubMed] [Google Scholar]
- 6.Dolled-Filhart, M., McCabe, A., Giltnane, J., Cregger, M., Camp, R. L., and Rimm, D. L. (2006) Cancer Res. 66 5487-5494 [DOI] [PubMed] [Google Scholar]
- 7.Nelson, W. J., and Nusse, R. (2004) Science 303 1483-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugherty, R. L., and Gottardi, C. J. (2007) Physiol. (Bethesda) 22 303-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottardi, C. J., and Gumbiner, B. M. (2004) J. Cell Biol. 167 339-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu, P., O'Keefe, E. J., and Rubenstein, D. S. (2001) J. Investig. Dermatol. 117 1059-1067 [DOI] [PubMed] [Google Scholar]
- 11.Dupre-Crochet, S., Figueroa, A., Hogan, C., Ferber, E. C., Bialucha, C. U., Adams, J., Richardson, E. C., and Fujita, Y. (2007) Mol. Cell. Biol. 27 3804-3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catimel, B., Layton, M., Church, N., Ross, J., Condron, M., Faux, M., Simpson, R. J., Burgess, A. W., and Nice, E. C. (2006) Anal. Biochem. 357 277-288 [DOI] [PubMed] [Google Scholar]
- 13.Bienz, M. (2005) Curr. Biol. 15 R64-R67 [DOI] [PubMed] [Google Scholar]
- 14.Shimizu, H., Julius, M. A., Giarre, M., Zheng, Z., Brown, A. M., and Kitajewski, J. (1997) Cell Growth & Differ. 8 1349-1358 [PubMed] [Google Scholar]
- 15.Kimelman, D., and Xu, W. (2006) Oncogene 25 7482-7491 [DOI] [PubMed] [Google Scholar]
- 16.Dejmek, J., Safholm, A., Kamp Nielsen, C., Andersson, T., and Leandersson, K. (2006) Mol. Cell. Biol. 26 6024-6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon, R. T., Campbell, R. M., Christian, J. L., McGrew, L. L., Shih, J., and Fraser, S. (1993) Development 119 97-111 [DOI] [PubMed] [Google Scholar]
- 18.Pandur, P., Maurus, D., and Kuhl, M. (2002) Bioessays 24 881-884 [DOI] [PubMed] [Google Scholar]
- 19.Jonsson, M., Dejmek, J., Bendahl, P. O., and Andersson, T. (2002) Cancer Res. 62 409-416 [PubMed] [Google Scholar]
- 20.Dejmek, J., Leandersson, K., Manjer, J., Bjartell, A., Emdin, S. O., Vogel, W. F., Landberg, G., and Andersson, T. (2005) Clin. Cancer Res. 11 520-528 [PubMed] [Google Scholar]
- 21.Jonsson, M., and Andersson, T. (2001) J. Cell Sci. 114 2043-2053 [DOI] [PubMed] [Google Scholar]
- 22.Dejmek, J., Dejmek, A., Safholm, A., Sjolander, A., and Andersson, T. (2005) Cancer Res. 65 9142-9146 [DOI] [PubMed] [Google Scholar]
- 23.Blanc, E., Roux, G. L., Benard, J., and Raguenez, G. (2005) Oncogene 24 1277-1283 [DOI] [PubMed] [Google Scholar]
- 24.Kremenevskaja, N., von Wasielewski, R., Rao, A. S., Schofl, C., Andersson, T., and Brabant, G. (2005) Oncogene 24 2144-2154 [DOI] [PubMed] [Google Scholar]
- 25.Torres, M. A., Yang-Snyder, J. A., Purcell, S. M., DeMarais, A. A., McGrew, L. L., and Moon, R. T. (1996) J. Cell Biol. 133 1123-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, L., Li, C., Pan, Y., and Chen, J. (2005) Mol. Cell. Biol. 25 6509-6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brembeck, F. H., Rosario, M., and Birchmeier, W. (2006) Curr. Opin. Genet. Dev. 16 51-59 [DOI] [PubMed] [Google Scholar]
- 28.Liu, C., Li, Y., Semenov, M., Han, C., Baeg, G. H., Tan, Y., Zhang, Z., Lin, X., and He, X. (2002) Cell 108 837-847 [DOI] [PubMed] [Google Scholar]
- 29.Sakanaka, C. (2002) J. Biochem. (Tokyo) 132 697-703 [DOI] [PubMed] [Google Scholar]
- 30.Bryja, V., Schulte, G., Rawal, N., Grahn, A., and Arenas, E. (2007) J. Cell Sci. 120 586-595 [DOI] [PubMed] [Google Scholar]
- 31.Huber, A. H., Stewart, D. B., Laurents, D. V., Nelson, W. J., and Weis, W. I. (2001) J. Biol. Chem. 276 12301-12309 [DOI] [PubMed] [Google Scholar]
- 32.Topol, L., Jiang, X., Choi, H., Garrett-Beal, L., Carolan, P. J., and Yang, Y. (2003) J. Cell Biol. 162 899-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, J., Stevens, J., Rote, C. A., Yost, H. J., Hu, Y., Neufeld, K. L., White, R. L., and Matsunami, N. (2001) Mol. Cell 7 927-936 [DOI] [PubMed] [Google Scholar]
- 34.Matsuzawa, S. I., and Reed, J. C. (2001) Mol. Cell. 7 915-926 [DOI] [PubMed] [Google Scholar]
- 35.Provost, E., Yamamoto, Y., Lizardi, I., Stern, J., D'Aquila, T. G., Gaynor, R. B., and Rimm, D. L. (2003) J. Biol. Chem. 278 31781-31789 [DOI] [PubMed] [Google Scholar]
- 36.Stappert, J., and Kemler, R. (1994) Cell Adhes. Commun. 2 319-327 [DOI] [PubMed] [Google Scholar]
- 37.Huber, A. H., and Weis, W. I. (2001) Cell 105 391-402 [DOI] [PubMed] [Google Scholar]
- 38.Chen, Y. T., Stewart, D. B., and Nelson, W. J. (1999) J. Cell Biol. 144 687-699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castano, J., Raurell, I., Piedra, J. A., Miravet, S., Dunach, M., and Garcia de Herreros, A. (2002) J. Biol. Chem. 277 31541-31550 [DOI] [PubMed] [Google Scholar]
- 40.Hinck, L., Nathke, I. S., Papkoff, J., and Nelson, W. J. (1994) J. Cell Biol. 125 1327-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safholm, A., Leandersson, K., Dejmek, J., Nielsen, C. K., Villoutreix, B. O., and Andersson, T. (2006) J. Biol. Chem. 281 2740-2749 [DOI] [PubMed] [Google Scholar]
- 42.Shore, E. M., and Nelson, W. J. (1991) J. Biol. Chem. 266 19672-19680 [PubMed] [Google Scholar]
- 43.Dejmek, J., Dib, K., Jonsson, M., and Andersson, T. (2003) Int. J. Cancer 103 344-351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.