Abstract
The entorhinal cortex is closely associated with the consolidation and recall of memories, Alzheimer disease, schizophrenia, and temporal lobe epilepsy. Norepinephrine is a neurotransmitter that plays a significant role in these physiological functions and neurological diseases. Whereas the entorhinal cortex receives profuse noradrenergic innervations from the locus coeruleus of the pons and expresses high densities of adrenergic receptors, the function of norepinephrine in the entorhinal cortex is still elusive. Accordingly, we examined the effects of norepinephrine on neuronal excitability in the entorhinal cortex and explored the underlying cellular and molecular mechanisms. Application of norepinephrine-generated hyperpolarization and decreased the excitability of the neurons in the superficial layers with no effects on neuronal excitability in the deep layers of the entorhinal cortex. Norepinephrine-induced hyperpolarization was mediated by α2A adrenergic receptors and required the functions of Gαi proteins, adenylyl cyclase, and protein kinase A. Norepinephrine-mediated depression on neuronal excitability was mediated by activation of TREK-2, a type of two-pore domain K+ channel, and mutation of the protein kinase A phosphorylation site on TREK-2 channels annulled the effects of norepinephrine. Our results indicate a novel action mode in which norepinephrine depresses neuronal excitability in the entorhinal cortex by disinhibiting protein kinase A-mediated tonic inhibition of TREK-2 channels.
The entorhinal cortex (EC)2 is an essential structure in the limbic system that is closely related to emotional control (1), consolidation and recall of memories (2, 3), Alzheimer disease (4, 5), schizophrenia (6, 7), and temporal lobe epilepsy (8, 9). The physiological and pathological roles of the EC are likely to be determined by its unique position in the brain; the EC serves as the interface to control the flow of information into and out of the hippocampus. Afferents from the olfactory structures, parasubiculum, perirhinal cortex, claustrum, amygdala, and neurons in the deep layers of the EC (layers V-VI) (10, 11) converge onto the superficial layers (layer II/III) of the EC, whereas the axons of principal neurons in layer II form the major component of perforant path that innervates the dentate gyrus and CA3 (12), and those of the pyramidal neurons in layer III form the temporoammonic pathway and synapse onto the distal dendrites of pyramidal neurons in the CA1 and subiculum (12-14). The output from the hippocampus is then projected to the deep layers of the EC that relay information back to the superficial layers (15-18) and to other cortical areas (10).
The EC receives abundant noradrenergic projections from the locus coeruleus in the brain stem (19-21) and expresses α1 (22), α2 (23-25), and β (26) adrenergic receptors (ARs), although the identities of cells expressing these ARs in the EC remain to be determined. Accordingly, application of norepinephrine (NE) in the EC has been shown to inhibit glutamatergic transmission via activation of α2 ARs (27, 28) and facilitate GABAergic transmission via activation of α1 ARs (29). The concerted effects of NE on glutamatergic and GABAergic transmission would result in powerful inhibition in the EC likely contributing to its antiepileptic actions observed in this brain region (22, 30). Whereas application of NE has been shown to generate membrane hyperpolarization in a proportion (∼54%) of principal neurons in layer II of the EC (28) via α2 AR-mediated activation of K+ channels (27), which may partially explain its inhibitory effects on glutamatergic transmission, the following questions still remain unaddressed. First, what subtype(s) of α2 ARs is/are involved in NE-mediated hyperpolarization in the EC, because members of the α2 AR family include the α2A, α2B, and α2C subtypes (31)? Second, does NE have any effects on the excitability of neurons in other layers of the EC, because the superficial layers are the sender and the deep layers are the recipient of hippocampal information? Third, which type(s) of K+ channels is/are involved in NE-mediated hyperpolarization? Finally, what are the signaling mechanisms underlying NE-induced hyperpolarization in the EC? In the present study, we address these questions, and our results demonstrate that NE generates membrane hyperpolarization in the superficial layers, especially layer III, with no effects on neuronal excitability in the deep layers (V/VI) of the EC. NE activates TREK-2, a two-pore domain K+ channel, via α2A AR-mediated inhibition of the protein kinase A (PKA) pathway. Our results provide a novel cellular and molecular mechanism that may at least partially explain the antiepileptic effects of NE in the EC as well as its roles in learning and memory.
EXPERIMENTAL PROCEDURES
Slice Preparation—Horizontal brain slices (400 μm), including the EC, subiculum, and hippocampus, were cut using a vibrating blade microtome (VT1000S, Leica, Wetzlar, Germany) from 13- to 20-day-old Sprague-Dawley rats as described previously (29, 32-35). After being deeply anesthetized with isoflurane, rats were decapitated and their brains were dissected out in ice-cold saline solution that contained (in mm) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2, pH 7.4. Slices were initially incubated in the above solution at 35 °C for 40 min for recovery and then kept at room temperature (∼24 °C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Whole Cell Recordings from the EC Neurons—Whole cell patch clamp recordings using an Axopatch 200B or two Multi-clamp 700B amplifiers (Molecular Devices, Sunnyvale, CA) in current- or voltage-clamp mode were made usually from pyramidal neurons in layer III of the medial EC visually identified with infrared videomicroscopy (Olympus BX51WI) and differential interference contrast optics. Unless stated otherwise, recording electrodes were filled with (in mm) 130 K+-gluconate, 0.5 EGTA, 2 MgCl2, 5 NaCl, 2 ATP2Na, 0.4 GTP-Na, and 10 HEPES, pH 7.4. The extracellular solution comprised (in mm) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.5 MgCl2, and 10 glucose, saturated with 95% O2 and 5% CO2, pH 7.4. Because NE has been shown to increase GABA release (29) and inhibit glutamatergic transmission (27, 28) in the EC, the preceding extracellular solution was routinely supplemented with bicuculline (10 μm) and CGP55845 (1 μm) to block GABAA and GABAB responses, respectively, and 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (10 μm) and dl-2-amino-5-phosphonovaleric acid (50 μm) to block glutamatergic transmission. Under these conditions, the excitability of pyramidal neurons in layer III was not affected by the indirect effects of NE on GABAergic and glutamatergic transmission. Data were filtered at 2 kHz, digitized at 10 kHz, acquired and analyzed using pCLAMP 9 software (Molecular Devices).
Action potential firing was recorded from pyramidal neurons in layer III of the EC. Because dialysis of K+-containing internal solution into cells can change the resting membrane potential and influence action potential firing, we waited for ∼10 min after the formation of whole cell recordings to allow the resting membrane potential to stabilize. Usually, for most of the cells a positive current injection was needed to bring the resting membrane potential to ∼-50 mV to induce action potential firing. NE was applied after the action potential firing had been stable for 5-10 min. To avoid potential desensitization induced by repeated applications of NE, one slice was limited to only one application of NE. Frequency of action potentials was calculated by Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA).
Holding currents at -55 mV were recorded from layer III pyramidal neurons as well as the neurons in layer V/VI in some experiments. The preceding extracellular solution was supplemented with tetrodotoxin (0.5 μm) to block action potential firing. Because gradual dialysis of K+ into cells changed the holding currents, we began our recordings after waiting for ∼10 min from the formation of whole cell configuration unless stated otherwise. Holding currents at -55 mV were recorded every 3 s and then averaged per minute. We subtracted the average of the holding currents recorded for the last minute prior to the application of NE from those recorded at different time points to zero the basal level of holding currents for better comparison.
The voltage-current relationship was obtained by using a ramp protocol from -140 mV to -70 mV at a speed of 0.1 mV/ms. We compared the voltage-current curves recorded before and during the application of NE for ∼3-4 min when the effect of NE was maximal.
Expression of α2A ARs and K2P Channels in HEK293 Cells and Electrophysiological Recordings from the Transfected Cells—A cDNA construct containing the human α2A AR subtype (GenBank™ accession number NM_000681) subcloned into pcDNA3.1 (Clontech, Palo Alto, CA) was purchased from Missouri University Science and Technology cDNA Resource Center. cDNA constructs coding for TREK-2 (NM_021161, subcloned into the pCMV6-XL4 vector), TREK-1 (NM_014217, subcloned into the pCMV6-entry vector), TWIK-1 (NM_002245, subcloned into pCMV6-XL5 vector), or TRESK (NM_181840, subcloned into pCMV6-XL5 vector) were purchased from Origene (Rockville, MD). cDNA constructs coding for TASK-1 (AF_031384) and TASK-3 (AF_391084) subcloned into pcDNA3.1 (35) were generous gifts from Dr. Douglas A. Bayliss (University of Virginia, Charlottesville, VA). In addition, a previously characterized PKA-insensitive TREK-2 mutant (S359A) subcloned into pcDNA3.1 (36) was a generous gift from Dr. Donghee Kim (Rosalind Franklin University of Medicine and Science, Chicago, IL). An empty pEGFP N-3 green fluorescent protein (GFP) fusion protein vector (GenBank™ accession number U57609) was purchased from Clontech. Dulbecco's minimum essential medium and fetal bovine serum were purchased from Atlanta Biologicals (Atlanta, GA). Cell culture grade penicillin and streptomycin was purchased from Mediatech, Inc. (Herndon, VA).
Human embryonic kidney (HEK) 293 cells obtained from American Type Culture Collection (Manassas, VA) were maintained in Dulbecco's minimum essential medium containing 10% fetal bovine serum, penicillin (100 units/ml) and streptomycin (100 units/ml). Confluent HEK293 cells were washed in Hanks' balance salt solution, trypsinized, and seeded at the appropriate density in 35-mm dishes to ensure 40-50% cell confluence within 24 h. Transient transfection of the cDNA constructs was performed after 24 h with TransIT-293 transfection reagent according to the manufactures protocol (Mirus Bio Corp., Madison, WI) using a 6-μl reagent per 3-μg cDNA ratio for the transfection mixture. Transfected HEK293 cells were subsequently used for electrophysiological recordings 24-48 h post-transfection.
Holding currents at -55 mV were recorded from the HEK293 cells that showed fluorescence under a fluorescence microscope (Olympus 1X70) by whole cell recordings. The extracellular solution contained (in mm) 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 10 HEPES, and 10 glucose. pH was adjusted to 7.4 using NaOH and HCl. The above K+-gluconate internal solution was used for this experiment. A continuous gravity perfusion system (flow, 5-7 ml/min) was used to change solutions.
Immunocytochemistry—Rats (18 days old) were anesthetized with pentobarbital sodium (50 mg/kg) and then perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS). Brains were rapidly removed and postfixed in the same fixative for an additional 2 h. After postfixation, brains were cryoprotected with 30% sucrose in PBS for 12 h and then cut into 20-μm slices in thickness horizontally in a Leica cryostat (CM 3050 S) at -21 °C. Slices were washed in 0.1 m PBS and then treated with 0.3% hydrogen peroxide (H2O2) to quench endogenous peroxidase activity. After being rinsed in 0.1 m PBS containing 1% Triton X-100 and 1.5% normal donkey serum for 30 min, slices were incubated with the primary antibodies (goat anti-α2A AR polyclonal antibody, sc-31357, or anti-TREK-2 polyclonal antibody, sc-11559, Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:100 at 4 °C for 12 h. Slices were incubated at room temperature initially with biotinylated donkey anti-goat IgG (ABC Staining System, Santa Cruz Biotechnology) for 1 h, and then with avidin-biocytin complex (ABC Staining System) for 30 min. After each incubation, slices were washed three times for a total of 30 min. Diaminobenzidine (ABC Staining System) was used for a color reaction to detect the positive signals. Finally, slices were mounted on slides, dehydrated through an alcohol range, cleared in xylene, and covered with coverslips. Slides were visualized and photographed with a Leica microscope (DM 4000B). We stained 5-6 nonadjacent sections and each staining was repeated by using three rats.
To confirm the specificities of α2A AR and TREK-2 antibodies, we also performed immunostaining of HEK293 cells transfected with GFP alone or GFP plus α2A ARs or GFP plus TREK-2 channels. The transfected cells on sterile glass coverslips were washed briefly with PBS and fixed in methanol at -10 °C for 5 min, then washed three times with PBS. Cells were incubated with normal donkey blocking serum (5%) containing 1% Triton X-100. Cells were then incubated with goat anti-α2AAR (Santa Cruz Biotechnology, sc-31357) or anti-TREK-2 channel (Santa Cruz Biotechnology, sc-11559) primary antibody at a dilution of 1:200 at 4 °C overnight. After washing three times in PBS, cells were incubated with donkey anti-goat IgG-rhodamine (1:200, Santa Cruz Biotechnology, sc-2094) at room temperature. Finally, the coverslips with the stained cells were mounted on slides, viewed, and photographed under an Olympus Fluoview 300 laser scanning confocal microscope. Each staining was repeated three times in three different transfections.
Western Blot—Brain tissues for Western blot experiments were taken from seven rats (18 days old). For each rat, horizontal brain slices were cut initially, and the medial EC region was punched out from the slices under a microscope. The isolated brain region was lysed in tissue protein extraction buffer containing protease inhibitors (Pierce). The lysates were centrifuged at 10,000 × g for 10 min to remove the insoluble materials and protein concentrations in the supernatant were determined (37). An equivalent of 40 μg of total protein was loaded to each lane. Proteins were separated by 12% SDS-PAGE and transferred to the polyvinylidene difluoride (Immobilon-P, Millipore, Billerica, MA) membranes using an electrophoretic transfer system (Bio-Rad). Blots were blocked with 5% powdered milk, and then incubated with either TREK-2 (1:500) or α2AAR (1:500) primary antibody overnight at 4 °C followed by incubation with the secondary antibody (donkey anti-goat IgG-horseradish peroxidase, 1:2000) for 1 h at room temperature. Tris-buffered saline with 1% Tween 20 was used to wash the blots three times (10 min each) after incubation with both primary and secondary antibodies. Immunoreactive bands were visualized by SuperSignal West Pico Chemiluminescent Substrate (Pierce) and detected by a Biospectrum Imagining System (UVP, Upland, CA).
Data Analysis—Data are presented as the means ± S.E. An NE concentration-response curve was fit by using the Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Student's paired or unpaired t test or analysis of variance was used for statistical analysis as appropriate; p values are reported throughout the text and significance was set as p < 0.05. The N number in the text represents the cells examined.
Chemicals—(Rp)-cAMPS, tertiapin, CGP 55845, pertussis toxin, and guanosine-5′-O-(2-thiodiphosphate) (GDP-β-S) were from BIOMOL (Plymouth Meeting, PA). Corynathine, propranolol, yohimbine, UK14,304, BRL44408, imiloxan, and MDL-12,330A were purchased from Tocris (Ellisville, MO). Antibodies to α2A ARs (sc-31357), TWIK-1 (sc-11483), TASK-1 (sc-32067), TASK-3 (sc-11322), TRESK (sc-51240), TREK-1 (sc-11554), and TREK-2 (sc-11559) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Other chemicals were products of Sigma-Aldrich.
RESULTS
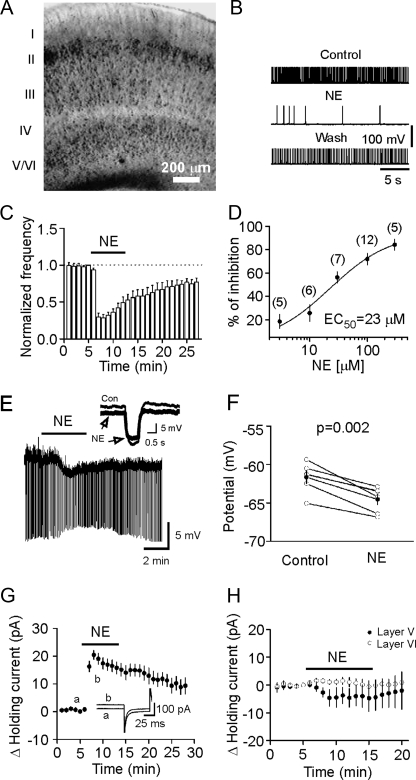
NE Inhibits the Neuronal Excitability in the Superficial Layers with No Effects on the Deep Layers of the EC—The EC can be divided into six layers (layers I-VI, Fig. 1A) (38). Layer I is the molecular layer, which has a scarcity of cells, whereas layer IV is the cell-sparse, fiber-rich narrow layer that constitutes the lamina dissecans. Thus the EC is actually classified as the superficial layers (layers II-III) that provide innervations to the hippocampus and the deep layers (layers V-VI) that receive hippocampal outputs. We initially examined the effects of NE on neuronal excitability in the superficial layers. Because the effects of NE in layer II have been studied previously (27, 28), we focused on layer III. Pyramidal neurons are the principal cells in layer III, and the axons of these neurons provide the primary inputs to CA1 regions (12, 13). The importance of layer III pyramidal neurons is further highlighted by the observations that these neurons are selectively lost in epileptic animals (39, 40). We examined the effects of NE on neuronal excitability by recording action potential firing from the pyramidal neurons in layer III in current-clamp mode. The resting membrane potentials of these neurons were -61.1 ± 1.3 mV (n = 12) immediately after the formation of whole cell configuration, and thus these neurons did not fire spontaneous action potentials. We therefore injected a positive current to bring the membrane potential to ∼-50 mV to induce action potential firing. To prevent potential influences of NE-induced changes in GABA (29) and glutamate (27, 28) release on the excitability of layer III pyramidal neurons, we included in the extracellular solution 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (10 μm) and dl-2-amino-5-phosphonovaleric acid (50 μm) to block glutamatergic, and bicuculline (10 μm) and CGP55845 (1 μm) to block GABAergic transmission. Under these conditions, application of NE (100 μm) significantly reduced the frequency of action potential firing (28 ± 5% of control, p < 0.001, Fig. 1, B and C) in 12 out of 12 pyramidal neurons examined suggesting that NE reduces the excitability of pyramidal neurons in layer III of the EC. The EC50 of NE to reduce pyramidal neuron excitability was calculated to be 23 μm (Fig. 1D).
FIGURE 1.
NE generates hyperpolarization in layer III pyramidal neurons with no effects in layer V/VI of the EC. A, a picture taken under an infrared microscope (Olympus BX51WI) with differential interference contrast optics in low magnification showing different layers of the medial EC in slices. B, action potentials recorded from a layer III pyramidal neuron prior to, during, and after application of NE (100 μm). Note that NE reversibly inhibited the firing frequency of action potentials. C, summarized time course of action potential firing frequency recorded from 12 layer III pyramidal neurons. The number of action potentials at each minute was normalized to that recorded at 1 min prior to the application of NE. D, concentration-response curve of NE-induced inhibition of action potential firing frequency. Numbers in parenthesis are numbers of cells recorded at each concentration. E, application of NE (100 μm)-generated membrane hyperpolarization and reduced input resistance in a layer III pyramidal neuron of the EC. Resting membrane potential was recorded in current-clamp mode and a hyperpolarizing current (-50 pA, 500 ms) was injected every 5 s to measure the input resistance. Note that NE generated hyperpolarization and reduced input resistance. Inset, voltage traces taken before (thin) and during (thick) the application of NE. F, summarized data for NE-induced changes in resting membrane potentials. G, application of NE (100 μm) induced an outward holding current (n = 10). Neurons were held at -55 mV, and the holding currents were recorded every 3 s in the extracellular solution supplemented with bicuculline (10 μm), CGP55845 (1 μm), 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (10 μm), dl-2-amino-5-phosphonovaleric acid (50 μm), and tetrodotoxin (0.5 μm). The holding currents were averaged per min, and NE-induced changes in holding currents were calculated by subtracting the averaged holding currents at each min from the holding current just prior to application of NE. Inset, holding currents prior to (a) and during (b) the application of NE. H, application of NE (100 μm) failed to change the holding currents recorded at -55 mV from the principal neurons in layer V (n = 15) and layer VI (n = 10).
To test whether the inhibitory effect of NE on action potential firing frequency was mediated by NE-induced hyperpolarization, we recorded NE-induced changes in resting membrane potential in the presence of bicuculline (10 μm), CGP55845 (1 μm), 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (10 μm), dl-2-amino-5-phosphonovaleric acid (50 μm), and tetrodotoxin (0.5 μm) to block potential indirect effects from synaptic transmission. A negative current (-50 pA for 500 ms) was injected every 5 s to assess the changes of input resistance induced by NE (Fig. 1E). Under these circumstances, application of NE (100 μm) generated membrane hyperpolarization (control: -61.6 ± 0.8 mV; NE: -64.5 ± 0.7 mV, n = 6, p = 0.002, Fig. 1, E and F) and reduced the input resistance (control: 280.4 ± 5.2 MΩ, NE: 188.1 ± 7.9 MΩ; n = 6, p < 0.001, Fig. 1E) suggesting that NE increases membrane conductance. We then used voltage-clamp mode and recorded the holding currents at -55 mV, a potential close to the resting membrane potential of these neurons. Under these conditions, application of NE (100 μm) generated an outward holding current (maximal effect at the third minute after the beginning of NE application, 20.4 ± 1.6 pA, n = 10, p < 0.001, Fig. 1G). Together, these results indicate that NE inhibits the excitability of layer III pyramidal neurons by generating membrane hyperpolarization in the EC.
About 54% of the neurons in layer II of the EC are responsive to NE (27, 28). Our results demonstrated that every pyramidal neuron examined in layer III of the EC was responsive to NE. These results together indicate that NE inhibits the excitability of neurons in the superficial layers of the EC. However, the effects of NE on the deep layer neurons have not been examined. We accordingly studied the effects of NE on neuronal excitability in the deep layers by recording the holding currents at -55 mV from neurons in layer V and layer VI. Larger pyramidal neurons are the principal cells in layer V whereas small, densely packed neurons are the major cell type in layer VI. However, application of NE (100 μm) failed to change the holding currents recorded from layer V (-4.5 ± 3.6 pA, n = 15, p = 0.23, Fig. 1H) and layer VI (1.1 ± 1.0 pA, n = 10, p = 0.31, Fig. 1H) neurons. These results suggest that NE selectively inhibits neuronal excitability in the superficial layers of the EC with no effects on the deep layers. Because the maximal response ratio was observed in layer III pyramidal neurons, we recorded from this layer for the rest of the experiments to further explore the mechanisms underlying NE-mediated inhibition in the EC.
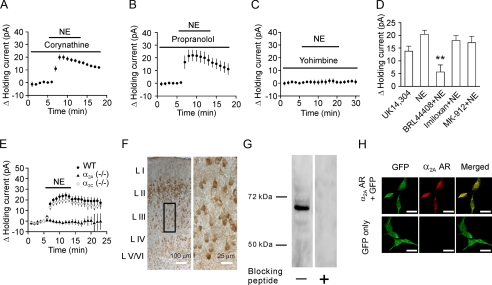
NE Depresses Neuronal Excitability via Activation of α2A ARs—NE possesses high potency for α1, α2, and β1 ARs but has weak activity on β2 ARs. Activation of these receptors has been shown to generate opposite effects in basolateral amygdala neurons (41). We next examined the roles of these receptors in NE-induced inhibition of neuronal excitability. Application of corynathine (100 μm), a selective α1 AR blocker, failed to alter NE-induced increases in outward holding currents (19.9 ± 2.1 pA, n = 5, p < 0.001, Fig. 2A). Neither did application of propranolol (100 μm), a β AR antagonist, significantly change NE-induced outward holding currents (21.9 ± 4.3 pA, n = 5, p = 0.007, Fig. 2B). However, application of yohimbine (50 μm), an α2 AR antagonist, completely prevented NE-induced changes in outward holding currents (0.7 ± 1.8 pA, n = 5, p = 0.73, Fig. 2C). Furthermore, application of UK14,304 (50 μm), a selective α2 AR agonist, induced an outward holding current (13.8 ± 1.9 pA, n = 5, p = 0.002, Fig. 2D). Collectively, these results suggest that NE inhibits neuronal excitability via activation of α2 ARs in the EC.
FIGURE 2.
NE-induced hyperpolarization is mediated by activation of α2A ARs. A, pretreatment of slices with and continuous bath application of corynathine (100 μm), an α1 AR inhibitor, did not block NE-induced increases in outward holding currents (n = 5). B, pretreatment of slices with and continuous bath application of propranolol (100 μm), a β AR inhibitor, did not block NE-induced increases in outward holding currents (n = 5). C, pretreatment of slices with and continuous bath application of yohimbine (50 μm), a α2 AR inhibitor, completely blocked NE-induced increases in outward holding currents (n = 5). D, Bath application of UK14,304 (50 μm), an α2 AR agonist, induced an outward holding current (n = 5). Pretreatment of slices with and continuous bath application of BRL44408 (3 μm), an α2A AR antagonist, significantly reduced NE-induced increases in outward holding currents (n = 10), whereas application of imiloxan (3 μm, n = 6), an α2B AR antagonist, or MK-912 (3 μm, n = 7), an α2C AR antagonist, failed to alter NE-induced increases in outward holding currents. E, application of NE (100 μm) did not change the holding currents significantly in 16 slices cut from 3 α2A KO mice, whereas NE-induced outward holding currents were still observed in 12 slices cut from 3 wild-type mice and 13 slices cut from 3 α2C KO mice. F, immunoreactivity of α2A ARs was detected in the EC. The right panel is the enlargement of the selected region in the left panel. G, Western blot demonstrated the expression of α2A ARs in the EC. Lysates of the tissues taken from the medial EC were loaded to the gel and blotted against the α2A AR antibody. A band of ∼67 kDa was detected, whereas no band was observed when the α2A AR antibody was pretreated with the corresponding blocking peptide. H, immunoreactivity to α2A ARs was observed only in HEK293 cells transfected with both GFP and α2A ARs (upper panel), but not in HEK293 cells transfected with GFP alone (lower panel) corroborating the specificity of α2A AR antibody. Scale bars equal 20 μm.
α2 AR subtypes are classified as α2A, α2B, and α2C (31). We next identified the roles of these three subtypes of α2 ARs in NE-induced hyperpolarization by combining pharmacological approaches and knock-out (KO) animal models. Pretreatment of slices with and continuous bath application of BRL44408 (3 μm), a selective α2A AR blocker, significantly reduced NE-induced increases in outward holding currents (5.6 ± 2.8 pA, n = 10, p < 0.001 versus NE alone, Fig. 2D), whereas NE-induced increases in outward holding currents were not significantly altered when slices were treated in the same fashion with imiloxan (3 μm, 17.9 ± 1.9 pA, n = 6, p = 0.36 versus NE alone, Fig. 2D), a selective α2B AR antagonist, or MK-912 (3 μm, 17.1 ± 2.5 pA, n = 7, p = 0.27 versus NE alone, Fig. 2D), a selective α2C AR antagonist. In accordance with these pharmacological results, application of NE (100 μm) failed to alter the holding currents significantly in 16 slices cut from 3 α2A KO mice (42) (0.6 ± 0.8 pA, n = 16 slices, p = 0.42, Fig. 2E), whereas NE-induced outward holding currents were still observed in α2C KO mice (42) (18.6 ± 2.2 pA, n = 13 slices from 3 mice, p < 0.001, Fig. 2E) and wild-type mice (21.4 ± 2.4 pA, n = 12 slices from 3 wild-type mice, p < 0.001, Fig. 2E). Together, these results indicate that NE inhibits the excitability of pyramidal neurons by activating α2A ARs.
To confirm the electrophysiological data further, we used immunocytochemistry and Western blot to detect the expression of α2A ARs in the EC. Immunoreactivity of α2A ARs was detected in both the superficial and deep layers (Fig. 2F). A band of ∼67 kDa was detected from the lysates of the EC by Western blot (Fig. 2G), consistent with the reported molecular mass of α2A ARs (43). The specificity of α2A AR antibody was further confirmed by the result showing that the immunofluorescence of α2A ARs was detected only in HEK293 cells co-expressing α2A ARs and GFP but not in HEK293 cells expressing GFP alone (Fig. 2H).
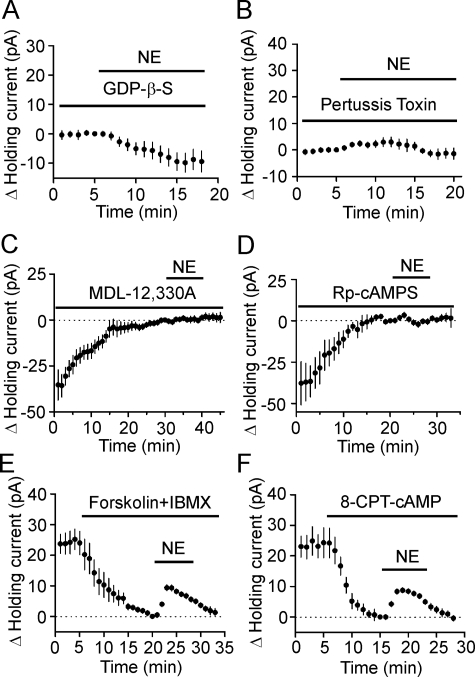
NE-induced Hyperpolarization Is Dependent on Gαi Proteins and Requires PKA Activity—α2A ARs are coupled to Gαi proteins resulting in inhibition of cAMP production and PKA activity (31). We next examined the roles of this intracellular pathway in NE-induced inhibition of neuronal excitability in the EC. We replaced the intracellular GTP with GDP-β-S (4 mm), a G-protein inactivator, and recorded NE-induced changes in holding currents at -55 mV. In the presence of GDP-β-S, NE did not significantly change the holding currents (-9.4 ± 3.6 pA, n = 5, p = 0.06, Fig. 3A) suggesting that NE-induced hyperpolarization is G-protein-dependent. We then identified the roles of Gαi proteins by using pertussis toxin (PTX), an inhibitor of Gαi/o proteins. Slices were pretreated with PTX (500 ng/ml) in the extracellular solution continuously oxygenated with 95% O2 and 5% CO2 for ∼8 h. Bath application of NE (100 μm) failed to significantly increase the outward holding currents in slices pretreated with PTX (2.4 ± 1.1 pA, n = 5, p < 0.001, Fig. 3B), whereas treatment of slices for the same period in the same condition without PTX failed to change NE-induced increases in outward holding currents (24.5 ± 3.6 pA, n = 5, p = 0.003, data not shown) suggesting that the PTX-sensitive G-proteins are involved in NE-induced inhibition of neuronal excitability in the EC.
FIGURE 3.
NE-mediated hyperpolarization in layer III pyramidal neurons of the EC requires the functions of inhibitory G-proteins (Gαi), AC, and PKA. A, intracellular application of GDP-β-S (4 mm) via the recording pipettes blocked NE-induced increases in outward holding currents (n = 5). NE was applied after the formation of whole cell configuration for >20 min to allow the diffusion of GDP-β-S into cells. B, application of NE (100 μm) failed to induce an outward holding current in slices pretreated with pertussis toxin for ∼8 h (n = 5). C, intracellular dialysis of MDL-12,330A (2 mm) via the recording pipettes generated an outward holding current per se, and subsequent application of NE (100 μm) in the presence of MDL-12,330A did not significantly alter the holding currents (n = 8). Holding currents were recorded immediately after the formation of whole cell configuration and zeroed by subtracting the holding current at 1 min prior to the application of NE. D, intracellular dialysis of (Rp)-cAMPS (1 mm) via the recording pipettes generated an outward holding current per se and blocked NE-induced increases in outward holding currents (n = 6). Holding currents were recorded immediately after the formation of whole cell configuration and zeroed by subtracting the holding current at 1 min prior to the application of NE. E, bath application of forskolin (20 μm) and 3-isobutyl-1-methylxanthine (IBMX, 1 mm) to increase intracellular cAMP concentration induced an inward holding current, and subsequent application of NE (100 μm) in the presence of forskolin and 3-isobutyl-1-methylxanthine induced a smaller outward holding current (n = 5). Holding currents were zeroed by subtracting the current recorded at 1 min before application of NE. F, bath application of 8-CPT-cAMP (500 μm), a membrane-permeable cAMP analog, induced an inward holding current and reduced NE-induced increases in outward holding current (n = 6).
The primary function of α2A ARs is to inhibit adenylyl cyclase (AC) activity and to reduce the levels of cAMP thereby resulting in an inhibition of PKA (31). We next determined the roles of AC and PKA in NE-induced hyperpolarization. Intracellular dialysis of MDL-12,330A (2 mm), an AC inhibitor, via the recording pipettes for ∼30 min induced an outward holding current (35.4 ± 8.3 pA, n = 8, p = 0.004, Fig. 3C). Application of NE (100 μm) in the continuous presence of MDL-12,330A failed to further increase the outward holding currents significantly (0.3 ± 0.9 pA, n = 8, p = 0.75, Fig. 3C) suggesting that the activity of AC is required for NE-induced hyperpolarization. Likewise, intracellular application of (Rp)-cAMPS (1 mm), a specific PKA inhibitor, via the recording pipettes for ∼20 min after the formation of whole cell configuration induced an outward holding current (37.8 ± 13.1 pA, n = 6, p = 0.03, Fig. 3D). Subsequent application of NE (100 μm) did not significantly increase the outward holding currents (0.9 ± 1.6 pA, n = 6, p = 0.6, Fig. 3D).
The above results support the notion that AC-cAMP-PKA pathway has a tonic control over the holding currents and NE-induced hyperpolarization is mediated by inhibiting this pathway. If this is the case, elevation of intracellular cAMP level should generate depolarization and alleviate the hyperpolarizing effect of NE. We therefore bath-applied forskolin (20 μm, an AC activator) and 3-isobutyl-1-methylxanthine (1 mm, a phosphodiesterase inhibitor) to increase intracellular cAMP concentration. This treatment generated an inward holding current (-23.9 ± 3.8 pA, n = 5, p = 0.003, Fig. 3E) suggesting that increases in intracellular cAMP concentration produce depolarization. In the presence of forskolin and 3-isobutyl-1-methylxanthine, application of NE (100 μm) generated a much smaller increase in outward holding currents (9.3 ± 1.0 pA, n = 5) compared with control (20.4 ± 1.6 pA, n = 10, p < 0.001, Fig. 3E). Similarly, bath application of 8-CPT-cAMP (500 μm), a membrane-permeant cAMP analog, induced an inward holding current (-24.3 ± 4.9 pA, n = 6, p = 0.004, Fig. 3F) and significantly reduced NE-induced increases in outward holding currents (control: 20.4 ± 1.6 pA, n = 10, 8-CPT-cAMP: 8.7 ± 0.3 pA, n = 6, p < 0.001, Fig. 3F). Together, these results indicate that activation of α2A ARs activates Gαi resulting in an inhibition of the AC-cAMP-PKA pathway to decrease neuronal excitability in the EC.
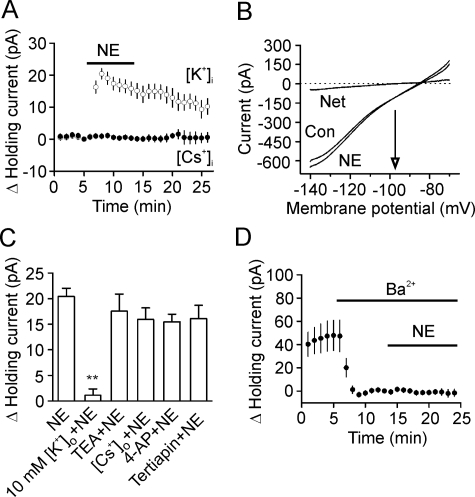
NE-induced Hyperpolarization Is Mediated by Activation of Two-pore Domain K+ Channels—We then tested the hypothesis that NE generates membrane hyperpolarization by activating a background K+ conductance. First, we replaced the intracellular K+ with the same concentration of Cs+ on the basis that, if K+ channels are involved, replacement of intracellular K+ with Cs+ should block NE-induced increases in outward holding currents, because K+ channels are not permeable to intracellular Cs+. Application of NE (100 μm) did not significantly change the holding currents (0.4 ± 0.6 pA, n = 8, p = 0.49, Fig. 4A) when intracellular K+ was replaced by Cs+. Second, if NE generates hyperpolarization via activation of K+ channels, the NE-induced currents should have a reversal potential that is close to the reversal potential of K+. We measured the reversal potential of the NE-induced currents using a ramp protocol (from -140 mV to -70 mV) prior to and during the application of NE. Application of NE (100 μm) in the presence of 3.5 mm K+ induced a net current that had a reversal potential of -97.7 ± 1.6 mV (n = 6, Fig. 4B), close to the theoretical K+ reversal potential calculated by the Nernst equation (-92.2 mV) suggesting that NE produces membrane hyperpolarization by activating a background K+ conductance. Third, if NE-induced hyperpolarization was mediated by activation of K+ channels, increases in extracellular K+ concentration should reduce K+ concentration gradient and diminish NE-induced increases in outward holding currents. Elevation of extracellular K+ concentration to 10 mm significantly reduced NE-induced enhancement of outward holding currents (3.5 mm K+: 20.4 ± 1.6 pA, n = 10, 10 mm K+: 1.1 ± 1.3 pA, n = 6, p < 0.001, Fig. 4C). Together, these results indicate that NE generates membrane hyperpolarization by activating a background K+ conductance.
FIGURE 4.
NE-mediated hyperpolarization is mediated by activation of a background K+ channel. A, replacing K+ in the intracellular solution with the same concentration of Cs+ blocked NE-induced increases in outward holding currents (n = 8). B, voltage-current relationship induced by a ramp protocol from -140 mV to -70 mV at a speed of 0.1 mV/ms prior to and during the application of NE (100 μm). Subtraction of the current prior to from the current during the application of NE generated a net current. The traces were averages from six cells. Note that the reversal potential was -97.7 ± 1.6 mV (n = 6), close to the calculated K+ reversal potential (-92.2 mV). C, NE-induced increases in outward holding currents were insensitive to extracellular application of the classic K+ channel blockers (TEA, 10 mm, n = 5, p = 0.4; Cs+, 3 mm, n = 6, p = 0.12; 4-aminopyridine (4-AP), 2 mm, n = 5, p = 0.1; tertiapin, 50 nm, n = 5, p = 0.2, versus application of NE alone) but significantly reduced when the extracellular K+ concentration was increased to 10 mm (n = 6, p < 0.001). D, inclusion of Ba2+ (3 mm) in the extracellular solution induced an inward holding current per se but prevented NE-induced increases in outward holding currents (n = 6).
We then determined the properties of the K+ channels involved in NE-induced hyperpolarization by applying the classic K+ channel blockers. NE-induced increases in outward holding currents were not significantly altered by including, in the extracellular solution, tetraethylammonium (TEA, 10 mm, 17.5 ± 3.3 pA, n = 5, p = 0.4, Fig. 4C), Cs+ (3 mm, 15.9 ± 2.3 pA, n = 6, p = 0.12, Fig. 4C), and 4-aminopyridine (2 mm, 15.5 ± 1.5 pA, n = 5, p = 0.1, Fig. 4C) suggesting that NE-activated K+ channels are insensitive to the classic K+ channel blockers. Whereas inward rectifier K+ channels are involved in controlling resting membrane potential, they are unlikely to be the channels activated by NE, because these channels are also sensitive to TEA and Cs+. Moreover, application of the inward rectifier K+ channel blocker, tertiapin (50 nm), failed to block the effect of NE (16.1 ± 2.6 pA, n = 5, p = 0.2, versus NE alone, Fig. 4C).
The two-pore domain K+ channels (K2P) are involved in controlling resting membrane potentials, and they are insensitive to the classic K+ channel blockers (TEA, 4-aminopyridine, and Cs+). We next examined the roles of K2P in NE-induced membrane hyperpolarization. K2P channels are grouped according to sequence and functional similarities into six subfamilies: TWIK, THIK, TREK, TASK, TALK, and TRESK (44, 45), some of which are sensitive to Ba2+. We therefore tested the roles of Ba2+ in NE-induced membrane hyperpolarization. Inclusion of Ba2+ (3 mm) in the extracellular solution per se generated an inward holding current (-47.8 ± 13.0 pA, n = 6, p = 0.02, Fig. 4D) suggesting that Ba2+-sensitive K2P channels contribute substantially to controlling resting membrane potentials in layer III pyramidal neurons. In the presence of Ba2+, application of NE (100 μm) failed to change the holding currents significantly (-1.6 ± 3.1 pA, n = 6, p = 0.6, Fig. 4D). This result suggests that NE-induced membrane hyperpolarization is mediated by a Ba2+-sensitive K2P channel.
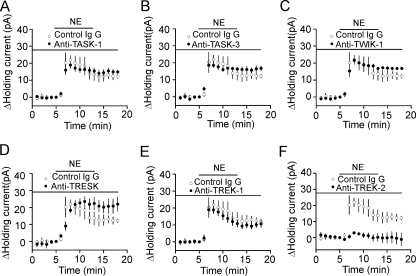
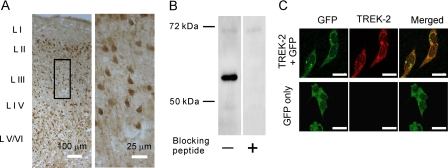
TREK-2 Channels Are Required for NE-induced Hyperpolarization—We took the advantage that the sensitivities of K2P channels to Ba2+ have been well documented to further identify the involved type(s) of K2P channels. Among the K2P channels, TASK-1 (46), TASK-3 (46, 47), TREK-1 (48), TREK-2 (46), TWIK-1 (49), and TRESK (50) are sensitive to Ba2+. Because our results showed that NE-mediated hyperpolarization was sensitive to Ba2+, it was reasonable to speculate that the effects of NE were mediated via activation of one or more of these K2P channels. Because specific blockers for these K2P channels are not available, we tried to identify the roles of these K2P channels in NE-induced hyperpolarization by applying, via the recording pipettes, their specific antibodies. The rationale for this experiment was that the epitopes for all these antibodies are located to the intracellular C or N terminus of the K2P channels (51), and the specific bindings of the large IgG molecules to the channels would interfere with the functions of the channels and block or at least reduce the effects of NE. To ensure complete intracellular dialysis of these antibodies because they are large IgG molecules, we waited for ∼65 min after the formation of whole cell recordings. We initially tested whether long-time infusion of antibody had any nonspecific effects on NE-induced increases in outward holding currents. Application of control IgG (40 μg/ml) via the recording pipettes did not significantly change NE-induced outward holding currents (Control: 20.4 ± 1.6 pA, n = 10, IgG: 21.6 ± 2.1 pA, n = 5, p = 0.67, Student's unpaired t test) suggesting that intracellular infusion of IgG had no nonspecific effects on NE-induced increases in outward holding currents. Intracellular application of antibodies to TASK-1 (n = 5, p = 0.37, Fig. 5A), TASK-3 (n = 5, p = 0.25, Fig. 5B), TWIK-1 (n = 5, p = 0.99, Fig. 5C), TRESK (n = 5, p = 0.6, Fig. 5D), and TREK-1 (n = 5, p = 0.32, Fig. 5E) failed to change NE-induced increases in outward holding currents significantly compared with the effect of NE when control IgG was applied via the recording pipettes. However, application of antibody to TREK-2 significantly reduced NE-induced increases in outward holding currents (2.9 ± 0.9 pA, n = 6, p < 0.001 versus control IgG, Fig. 5F). These results indicate that NE inhibits neuronal excitability selectively by activating TREK-2 channels in the EC. We further corroborated the expression of TREK-2 channels in the EC by immunocytochemistry and Western blot. Immunoreactivity of TREK-2 channels was detected in each layer of the EC (Fig. 6A). The expression of TREK-2 channel proteins in the EC was confirmed by Western blot. A band that had a molecular mass of ∼59 kDa was detected in the lysates of the EC, whereas pretreatment of the TREK-2 antibody with the corresponding blocking peptide prevented the detection of the band (Fig. 6B). The measured molecular weight for TREK-2 was close to the reported molecular weight of TREK-2 (52, 53). To test the specificity of the TREK-2 antibody further, we transfected HEK293 cells with GFP alone or GFP plus TREK-2 channels. Immunoreactivity of TREK-2 channels was detected only in HEK293 cells transfected with both GFP and TREK-2 channels but not in HEK293 cells transfected with GFP alone (Fig. 6C).
FIGURE 5.
TREK-2 channels are required for NE-mediated hyperpolarization in layer III pyramidal neurons of the EC. A-E, intracellular infusion of normal IgG (n = 5) or antibodies to TASK-1 (A, n = 5), TASK-3 (B, n = 5), TWIK-1 (C, n = 5), TRESK (D, n = 5), and TREK-1 (E, n = 5) at 40 μg/ml failed to significantly change NE-induced increases in outward holding current. F, intracellular infusion of antibody to TREK-2 (40 μg/ml, n = 6) prevented NE-induced increases in outward holding currents.
FIGURE 6.
The EC expresses high density of TREK-2 channels. A, immunoreactivity to TREK-2 channels was detected in each layer of the EC. The right panel is the enlargement of the selected region in the left panel. B, Western blot detected a band that had a molecular mass of ∼59 kDa in the lysate of the EC. Preincubation of TREK-2 antibody with the corresponding blocking peptide prevented the detection of the band. C, the specificity of the TREK-2 antibody was confirmed by expressing TREK-2 channels in HEK293 cells. Immunoreactivity to TREK-2 channels was observed only in HEK293 cells transfected with both GFP and TREK-2 channels (upper panel), but not in HEK293 cells transfected with GFP alone (lower panel). Scale bars, 20 μm.
One may argue that the above negative results obtained by applying the antibodies to TASK-1, TASK-3, TREK-1, TWIK-1, and TRESK via the recording pipettes were due to the inability of these antibodies to recognize the native conformation of these channels. We addressed this issue by applying, via the recording pipettes, the antibodies of individual K2P channels to the HEK293 cells expressing the corresponding K2P channels and recorded the changes of the holding currents at -55 mV. The rationale for this experiment was that, if the antibodies of the K2P channels were effective at recognizing their corresponding channels, intracellular dialysis of these antibodies would lead to an inhibition of the currents generated by these channels. In agreement with our expectation, intracellular dialysis of these antibodies generated an inhibition of the channel function by producing an inward holding current whereas intracellular application of irrelevant antibodies failed to change the holding currents significantly (supplemental Fig. S1). These data indicate that the ineffectiveness of the antibodies to TASK-1, TASK-3, TREK-1, TWIK-1, and TRESK to block the effect of NE was unlikely due to the inability of these antibodies to recognize the native channels.
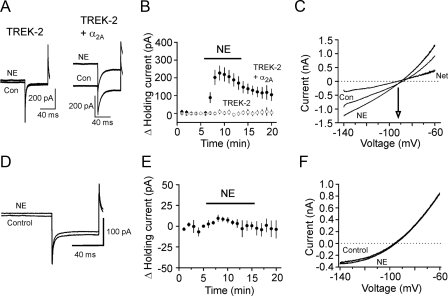
To further confirm the roles of TREK-2 channels in NE-mediated hyperpolarization, we co-transfected α2A ARs with TREK-2 channels in HEK293 cells and recorded the holding currents at -55 mV from the transfected cells. Application of NE (100 μm) generated an outward holding current in the cells co-transfected with α2A ARs and TREK-2 channels (229.3 ± 39.9 pA, n = 9, p < 0.001, Fig. 7, A and B), whereas application of the same concentration of NE failed to significantly change the holding currents in HEK293 cells transfected with TREK-2 channels alone (9.3 ± 12.4 pA, n = 5, p = 0.49, Fig. 7, A and B). To test whether the effects of NE on the holding currents in the transfected cells were mediated by K+ channels, we applied a ramp protocol (from -140 mV to -60 mV at a rate of 0.1 mV/ms) before and during the application of NE. The reversal potential for the NE-induced currents was -93.8 ± 1.4 mV (n = 9) in cells co-transfected with α2A ARs and TREK-2 (Fig. 7C). The measured reversal potential was close to the calculated K+ reversal potential (-96.1 mV) in our recording conditions suggesting that the effects of NE were mediated by inhibition of TREK-2 channels in the transfected cells.
FIGURE 7.
Serine 359, the PKA phosphorylation site on TREK-2 channels, is required for NE-mediated activation of TREK-2 channels in HEK293 cells co-expressing α2A ARs and TREK-2 channels. A, holding currents recorded at -55 mV from a HEK293 cell expressing TREK-2 channels alone (left) and from a HEK293 cell expressing TREK-2 channels together with α2A ARs (right) before and during the application of NE (100 μm). Note that application of NE had little effects on the holding currents in the HEK293 cell expressing TREK-2 channels alone but induced an outward current in the HEK293 cell co-expressing TREK-2 channels and α2A ARs. B, pooled data from nine HEK293 cells co-expressing α2A ARs and TREK-2 channels and five HEK293 cells expressing TREK-2 channels alone. C, the NE-induced outward currents had a reversal potential of ∼93 mV that was close to the reversal potential of K+ (-96.1 mV) suggesting that the effect of NE was mediated by activation of TREK-2 channels. D, current traces recorded from a HEK293 cell co-expressing α2A ARs and the mutated TREK-2 channels in which serine 359 was replaced with alanine (S359A) before and during the application of NE (100 μm). Note that application of NE failed to change the holding currents in this condition. E, summarized data from nine HEK293 cells co-expressing α2A ARs and TREK-2 S359A mutant. F, application of NE (100 μm) did not induce noticeable difference for the voltage-current relationship in HEK293 cells co-expressing α2A ARs and TREK-2 S359A mutant.
NE-mediated Hyperpolarization Is Dependent on PKA-mediated Phosphorylation on Serine 359 of TREK-2 Channels—Up to now, our results support an action mode in which activation of α2A ARs by NE activates Gαi proteins resulting in decreased activity of AC-cAMP-PKA pathway and increased function of TREK-2 channels to generate hyperpolarization in the EC. Consistent with our results, both TREK-1 (48, 54) and TREK-2 (55, 56) channels are inhibited by PKA-mediated phosphorylation. TREK-2 channels are phosphorylated by PKA on serine 359 of the C terminus (56). Accordingly, we tested the role of this phosphorylation site in NE-mediated hyperpolarization. We co-expressed in HEK239 cells α2A ARs with a mutant TREK-2 channel in which serine 359 was mutated to alanine (S359A) (36) to nullify PKA-mediated phosphorylation of TREK-2 channels. Alanine substitution of this site did not prevent the expression and functions of TREK-2 channels in HEK293 cells (36). However, application of NE (100 μm) failed to change the holding currents significantly in HEK293 cells co-expressing S359A mutant and α2A ARs (4.5 ± 2.7 pA, n = 9, p = 0.14, Fig. 7, D and E), and there was little change in the voltage-current relationship when NE was applied (Fig. 7F). Together, these results indicate that serine 359, the PKA phosphorylation site on TREK-2 channels, is necessary for NE-mediated hyperpolarization.
DISCUSSION
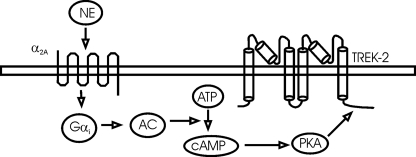
In the present study, we have addressed four questions that are essential for understanding NE-induced inhibition of neuronal excitability in the EC. First, we systematically examined the effects of NE on neuronal excitability in each layer of the EC, and our results reveal that NE only inhibits neuronal excitability in the superficial layers with the highest response ratio in layer III pyramidal neurons. NE does not alter the excitabilities of neurons located in the deep layers (layer V/VI) suggesting that NE inhibits the inflow of excitatory information from the EC to the hippocampus. Second, using both pharmacological methods and knockout animal models we have identified that α2A ARs are required for NE-mediated hyperpolarization in the EC. Third, we have found that TREK-2 channels are the types of K+ channels that are involved in NE-induced hyperpolarization in the EC. Finally, we have determined that NE-mediated inhibition of entorhinal neurons requires the functions of Gαi proteins and AC-cAMP-PKA pathway. As schematically illustrated in Fig. 8, our results support an action mode in which activation of α2A ARs by NE down-regulates AC-cAMP-PKA pathway and disinhibits the tonic inhibition of TREK-2 channels by this pathway resulting in a depression of EC neuronal excitability.
FIGURE 8.
Schematic diagram illustrating the signal transduction pathway involved in NE-mediated activation of TREK-2 channels. NE activates α2A ARs resulting in activation of the inhibitory G-proteins (Gαi). Activation of Gαi inhibits the activity of adenylyl cyclase (AC) leading to a reduction of cAMP from ATP and an inhibition of PKA activity. Normally, PKA exerts a tonic inhibition on TREK-2 channels by phosphorylating serine 359 on TREK-2 channels. NE-mediated inhibition of PKA annuls PKA-mediated tonic inhibition of TREK-2 channels, and thus, increases the function of TREK-2 channels. The ultimate result is the inhibition of neuronal excitability in the EC.
Ionic Mechanisms underlying NE-mediated Inhibition of Neuronal Excitability in the EC—Background K+ channels are the major determinants of neuronal membrane potential, and their functional modification is one of the principal mechanisms by which neurotransmitters modulate neuronal excitability. K2P channels are the major K+ channels that are responsible for controlling resting membrane potentials. K2P channels can be grouped according to sequence and functional similarities into six subfamilies: TWIK, THIK, TREK, TASK, TALK, and TRESK (44). Our results for the first time reveal that NE inhibits the excitability of entorhinal neurons, particularly the layer III pyramidal neurons, by activating TREK-2, a type of K2P channels based on the following bodies of evidence. First, TREK-2 channels are K2P channels that are sensitive to Ba2+ (46), and our results showed that NE-mediated hyperpolarization was blocked by bath application of Ba2+. Second, intracellular infusion of antibody specific for TREK-2 channels blocked NE-induced hyperpolarization. Third, application of NE induced membrane hyperpolarization in HEK293 cells co-expressing α2A ARs with TREK-2 channels suggesting that activation of α2A ARs up-regulates TREK-2 channels. Fourth, application of NE failed to generate membrane hyperpolarization in HEK293 cells co-expressing α2A ARs with the mutant TREK-2 channels in which the PKA-mediated phosphorylation site, serine 359, was mutated to alanine.
Signal Transduction Mechanisms Whereby NE Inhibits Neuronal Excitability in the EC—G-protein-coupled receptors are the major modulators of K2P channels. Among the K2P channels, TASK-1 (57), TASK-3 (57), TREK-1 (54), TREK-2 (36, 55), and TWIK-1 (35) are reported to be modulated by G-protein-coupled receptors, including the Gαq-, Gαs-, and Gαi-coupled receptors. Activation of Gαq- and Gαs-coupled receptors usually inhibits (36, 55, 57), whereas stimulation of Gαi-coupled receptors facilitates (35, 55), K2P channels. Gαq-induced inhibition of K2P channels could be mediated via intracellular singling molecules (58-60) or direct G-protein coupling (32, 57), whereas Gαi-induced activation may require intracellular signals, because Gαi does not directly interact with K2P channels (57). Among the K2P channels, TREK-1 (48, 54) and TREK-2 (55) channels contain the phosphorylation site for PKA. Serine 333 in TREK-1 (54) and serine 359 in TREK-2 (56) are the putative PKA phosphorylation sites, and activation of PKA down-regulates channel activities. α2 ARs are coupled to Gαi proteins resulting in inhibition of AC and subsequent reduction in intracellular cAMP production and inhibition of PKA (31). Our results demonstrate that this pathway is required for NE-mediated inhibition of entorhinal neurons based on the following arrays of evidence. First, intracellular application of GDP-β-S blocked NE-induced hyperpolarization suggesting that the function of G-proteins is required for the effects of NE. Second, the type of G-protein is identified to be Gαi because pretreatment of slices with PTX blocked NE-induced hyperpolarization. Third, application of MDL-12,330A, an AC inhibitor, blocked NE-induced increases in outward holding currents. Fourth, intracellular application of (Rp)-cAMPS, a specific PKA inhibitor, blocked NE-induced increases in outward holding currents. Finally, application of NE failed to induce membrane hyperpolarization in HEK293 cells co-expressing α2A ARs with the mutated TREK-2 channels in which the PKA phosphorylation site, serine 359, was mutated to alanine. Together, our results support a scenario in which α2A AR-mediated activation of Gαi leads to a down-regulation of PKA activity resulting in a reduction in the tonic inhibition of TREK-2 channels mediated by PKA. The ultimate result is an up-regulation of TREK-2 channels and hyperpolarization of entorhinal neurons (Fig. 8).
Physiological and Pathological Significances—Neurons in the superficial and deep layers of the EC have distinct functions. Whereas neurons in the superficial layers convey information to the hippocampus, those in the deep layers are the recipients of hippocampal output, and they project the information back to the superficial layers or to other cortices. Our results show that NE inhibits neuronal excitability in the superficial layers with no effects on the deep layers suggesting that NE inhibits the inflow of excitatory inputs from the EC to the hippocampus. Although the reasons that NE failed to modulate the excitability of neurons in the deep layers are unknown, it seems unlikely that lack of α2A ARs and TREK-2 channels in the deep layer neurons is responsible for the negative results of NE, because these neurons express α2A ARs (Fig. 2F) and TREK-2 channels (Fig. 6A). Because NE-mediated depression of neuronal excitability requires collaborative efforts of other signals, including G-proteins, AC, cAMP, and PKA, it is conceivable to speculate that one or more of these molecules may be missing in the deep layers of the EC. Because there are numerous isoforms for these intracellular signals, one explanation for the negative results of NE in the deep layers is that the specific isoform of these molecules essential for NE-induced hyperpolarization is not expressed by the deep layer neurons. Further experiments will be performed to address this issue in the future. Whereas NE generates membrane hyperpolarization in ∼54% of the neurons in layer II (28), our results demonstrate that NE-mediated hyperpolarization is observable in each of the pyramidal neurons examined in layer III suggesting that the major effect of NE is on layer III pyramidal neurons. Because the axons of the neurons in layers II and III form two parallel pathways to the hippocampus and exert distinct functions in spatial learning (61), the uneven effects of NE on these two layer neurons may underlie separate roles of NE in different patterns of memories. Furthermore, layer III pyramidal neurons are selectively lost during epilepsy (39, 40), the high response ratio to NE observed in this layer enlightens an important role of NE in epilepsy as well. Whereas the resting membrane potentials of the layer III neurons are close to -60 mV which prevents spontaneous firing of these neurons, NE remarkably inhibits the firing frequency of action potentials once they are evoked. The fact that epilepsy is a disorder in which neuronal excitability is significantly enhanced further highlights the antiepileptic role of NE.
NE exerts potent depression in the EC via at least three avenues. First, our previous results have revealed that NE facilitates GABAergic transmission via α1 ARs (29). Second, it has been shown that NE inhibits neuronal excitability in a proportion (∼54%) of layer II neurons (28), and our present results indicate that the excitabilities of each of the layer III pyramidal neurons examined are inhibited by NE via α2A ARs. Third, NE depresses glutamatergic transmission in the superficial layers of the EC (27). The synergistic interactions of these mechanisms result in powerful inhibition in the EC, which likely underscores NE-induced antiepileptic actions (22, 30). Furthermore, NE has been implicated in the modulation of learning and memory. Whereas activation of α2A ARs is believed to impair memory (62-64), the underlying cellular and molecular mechanisms remain unexplored. Our results could explain the detrimental effects of α2A AR activation on memory, because inhibition of the excitability of the neurons in the superficial layers of the EC likely limits the inflow of excitatory inputs to the hippocampus and depresses cognitive functions. In conclusion, our results provide a novel cellular and signaling mechanism to explain at least the roles of NE in some physiological functions such as learning and memory and pathological diseases such as epilepsy.
Supplementary Material
Acknowledgments
We greatly appreciate the generous gift of cDNA for the mutant TREK-2 channels (S359A) from Dr. Donghee Kim (Rosalind Franklin University of Medicine and Science, Chicago, IL) and cDNA for TASK-1 and TASK-3 from Dr. Douglas A. Bayliss (University of Virginia, Charlottesville, VA).
This work was supported, in whole or in part, by National Institutes of Health Grants R01MH082881 (to S. L.) and 5P20RR017699 (to S. L.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: EC, entorhinal cortex; NE, norepinephrine; AC, adenylyl cyclase; PKA, protein kinase A; TTX, tetrodotoxin; AR, adrenergic receptor; GFP, green fluorescent protein; HEK, Human embryonic kidney; PBS, phosphate-buffered saline; KO, knockout; PTX, pertussis toxin; AC, adenylyl cyclase; TEA, tetraethylammonium; K2P, two-pore domain K+ channels; 8-CPT-cAMP, 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate; (Rp)-cAMPS, (Rp)-adenosine 3′,5′-cyclic monophosphorothioate; GABAergic, γ-aminobutyric acid-mediated; GDP-β-S, guanosine 5′-O-(2-thiodiphosphate).
References
- 1.Majak, K., and Pitkanen, A. (2003) Eur. J. Neurosci. 18 1652-1659 [DOI] [PubMed] [Google Scholar]
- 2.Dolcos, F., LaBar, K. S., and Cabeza, R. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2626-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffenach, H. A., Witter, M., Moser, M. B., and Moser, E. I. (2005) Neuron 45 301-313 [DOI] [PubMed] [Google Scholar]
- 4.Hyman, B. T., Van Hoesen, G. W., Damasio, A. R., and Barnes, C. L. (1984) Science 225 1168-1170 [DOI] [PubMed] [Google Scholar]
- 5.Kotzbauer, P. T., Trojanowsk, J. Q., and Lee, V. M. (2001) J. Mol. Neurosci. 17 225-232 [DOI] [PubMed] [Google Scholar]
- 6.Joyal, C. C., Laakso, M. P., Tiihonen, J., Syvalahti, E., Vilkman, H., Laakso, A., Alakare, B., Rakkolainen, V., Salokangas, R. K., and Hietala, J. (2002) Biol. Psychiatry 51 1005-1007 [DOI] [PubMed] [Google Scholar]
- 7.Prasad, K. M., Patel, A. R., Muddasani, S., Sweeney, J., and Keshavan, M. S. (2004) Am. J. Psychiatry 161 1612-1619 [DOI] [PubMed] [Google Scholar]
- 8.Spencer, S. S., and Spencer, D. D. (1994) Epilepsia 35 721-727 [DOI] [PubMed] [Google Scholar]
- 9.Avoli, M., D'Antuono, M., Louvel, J., Kohling, R., Biagini, G., Pumain, R., D'Arcangelo, G., and Tancredi, V. (2002) Prog. Neurobiol. 68 167-207 [DOI] [PubMed] [Google Scholar]
- 10.Witter, M. P., Groenewegen, H. J., Lopes da Silva, F. H., and Lohman, A. H. (1989) Prog. Neurobiol. 33 161-253 [DOI] [PubMed] [Google Scholar]
- 11.Burwell, R. D. (2000) Ann. N. Y. Acad. Sci. 911 25-42 [DOI] [PubMed] [Google Scholar]
- 12.Steward, O., and Scoville, S. A. (1976) J. Comp. Neurol. 169 347-370 [DOI] [PubMed] [Google Scholar]
- 13.Witter, M. P., Naber, P. A., van Haeften, T., Machielsen, W. C., Rombouts, S. A., Barkhof, F., Scheltens, P., and Lopes da Silva, F. H. (2000) Hippocampus 10 398-410 [DOI] [PubMed] [Google Scholar]
- 14.Witter, M. P., Wouterlood, F. G., Naber, P. A., and Van Haeften, T. (2000) Ann. N. Y. Acad. Sci. 911 1-24 [DOI] [PubMed] [Google Scholar]
- 15.Kohler, C. (1986) J. Comp. Neurol. 246 149-169 [DOI] [PubMed] [Google Scholar]
- 16.Dolorfo, C. L., and Amaral, D. G. (1998) J. Comp. Neurol. 398 49-82 [DOI] [PubMed] [Google Scholar]
- 17.Dolorfo, C. L., and Amaral, D. G. (1998) J. Comp. Neurol. 398 25-48 [PubMed] [Google Scholar]
- 18.van Haeften, T., Baks-te-Bulte, L., Goede, P. H., Wouterlood, F. G., and Witter, M. P. (2003) Hippocampus 13 943-952 [DOI] [PubMed] [Google Scholar]
- 19.Fallon, J. H., Koziell, D. A., and Moore, R. Y. (1978) J. Comp. Neurol. 180 509-532 [DOI] [PubMed] [Google Scholar]
- 20.Palkovits, M., Zaborszky, L., Brownstein, M. J., Fekete, M. I., Herman, J. P., and Kanyicska, B. (1979) Brain Res. Bull. 4 593-601 [DOI] [PubMed] [Google Scholar]
- 21.Wilcox, B. J., and Unnerstall, J. R. (1990) Synapse 6 284-291 [DOI] [PubMed] [Google Scholar]
- 22.Stanton, P. K., Jones, R. S., Mody, I., and Heinemann, U. (1987) Epilepsy Res. 1 53-62 [DOI] [PubMed] [Google Scholar]
- 23.Unnerstall, J. R., Kopajtic, T. A., and Kuhar, M. J. (1984) Brain Res. 319 69-101 [DOI] [PubMed] [Google Scholar]
- 24.Unnerstall, J. R., Fernandez, I., and Orensanz, L. M. (1985) Pharmacol. Biochem. Behav. 22 859-874 [DOI] [PubMed] [Google Scholar]
- 25.Boyajian, C. L., Loughlin, S. E., and Leslie, F. M. (1987) J. Pharmacol. Exp. Ther. 241 1079-1091 [PubMed] [Google Scholar]
- 26.Booze, R. M., Crisostomo, E. A., and Davis, J. N. (1993) Synapse 13 206-214 [DOI] [PubMed] [Google Scholar]
- 27.Pralong, E., and Magistretti, P. J. (1995) Eur. J. Neurosci. 7 2370-2378 [DOI] [PubMed] [Google Scholar]
- 28.Pralong, E., and Magistretti, P. J. (1994) Neurosci. Lett. 179 145-148 [DOI] [PubMed] [Google Scholar]
- 29.Lei, S., Deng, P. Y., Porter, J. E., and Shin, H. S. (2007) J. Neurophysiol. 98 2868-2877 [DOI] [PubMed] [Google Scholar]
- 30.Stoop, R., Epiney, S., Meier, E., and Pralong, E. (2000) Neurosci. Lett. 287 5-8 [DOI] [PubMed] [Google Scholar]
- 31.Hein, L. (2006) Cell Tissue Res. 326 541-551 [DOI] [PubMed] [Google Scholar]
- 32.Deng, P. Y., Porter, J. E., Shin, H. S., and Lei, S. (2006) J. Physiol. 57.7 497-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng, P. Y., and Lei, S. (2006) J. Physiol. 572 425-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng, P. Y., and Lei, S. (2007) J. Neurophysiol. 97 727-737 [DOI] [PubMed] [Google Scholar]
- 35.Deng, P. Y., Poudel, S. K., Rojanathammanee, L., Porter, J. E., and Lei, S. (2007) Mol. Pharmacol. 72 208-218 [DOI] [PubMed] [Google Scholar]
- 36.Kang, D., Choe, C., Cavanaugh, E., and Kim, D. (2007) J. Physiol. 583 57-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradford, M. M. (1976) Anal. Biochem. 72 248-254 [DOI] [PubMed] [Google Scholar]
- 38.Mulders, W. H., West, M. J., and Slomianka, L. (1997) J. Comp. Neurol. 385 83-94 [PubMed] [Google Scholar]
- 39.Du, F., and Schwarcz, R. (1992) Neurosci. Lett. 147 185-188 [DOI] [PubMed] [Google Scholar]
- 40.Du, F., Whetsell, W. O., Jr., Abou-Khalil, B., Blumenkopf, B., Lothman, E. W., and Schwarcz, R. (1993) Epilepsy Res. 16 223-233 [DOI] [PubMed] [Google Scholar]
- 41.Buffalari, D. M., and Grace, A. A. (2007) J. Neurosci. 27 12358-12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szot, P., Lester, M., Laughlin, M. L., Palmiter, R. D., Liles, L. C., and Weinshenker, D. (2004) Neuroscience 126 795-803 [DOI] [PubMed] [Google Scholar]
- 43.Small, K. M., Schwarb, M. R., Glinka, C., Theiss, C. T., Brown, K. M., Seman, C. A., and Liggett, S. B. (2006) Biochemistry 45 4760-4767 [DOI] [PubMed] [Google Scholar]
- 44.Bayliss, D. A., Sirois, J. E., and Talley, E. M. (2003) Mol. Interv. 3 205-219 [DOI] [PubMed] [Google Scholar]
- 45.Lesage, F. (2003) Neuropharmacology 44 1-7 [DOI] [PubMed] [Google Scholar]
- 46.Han, J., Truell, J., Gnatenco, C., and Kim, D. (2002) J. Physiol. 542 431-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim, Y., Bang, H., and Kim, D. (2000) J. Biol. Chem. 275 9340-9347 [DOI] [PubMed] [Google Scholar]
- 48.Fink, M., Duprat, F., Lesage, F., Reyes, R., Romey, G., Heurteaux, C., and Lazdunski, M. (1996) EMBO J. 15 6854-6862 [PMC free article] [PubMed] [Google Scholar]
- 49.Lesage, F., Guillemare, E., Fink, M., Duprat, F., Lazdunski, M., Romey, G., and Barhanin, J. (1996) EMBO J. 15 1004-1011 [PMC free article] [PubMed] [Google Scholar]
- 50.Sano, Y., Inamura, K., Miyake, A., Mochizuki, S., Kitada, C., Yokoi, H., Nozawa, K., Okada, H., Matsushime, H., and Furuichi, K. (2003) J. Biol. Chem. 278 27406-27412 [DOI] [PubMed] [Google Scholar]
- 51.Goldstein, S. A., Bockenhauer, D., O'Kelly, I., and Zilberberg, N. (2001) Nat. Rev. Neurosci. 2 175-184 [DOI] [PubMed] [Google Scholar]
- 52.Kang, D., Kim, S. H., Hwang, E. M., Kwon, O. S., Yang, H. Y., Kim, E. S., Choi, T. H., Park, J. Y., Hong, S. G., and Han, J. (2007) Exp. Dermatol. 16 1016-1022 [DOI] [PubMed] [Google Scholar]
- 53.Simkin, D., Cavanaugh, E. J., and Kim, D. (2008) J. Physiol. 586 5651-5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murbartian, J., Lei, Q., Sando, J. J., and Bayliss, D. A. (2005) J. Biol. Chem. 280 30175-30184 [DOI] [PubMed] [Google Scholar]
- 55.Lesage, F., Terrenoire, C., Romey, G., and Lazdunski, M. (2000) J. Biol. Chem. 275 28398-28405 [DOI] [PubMed] [Google Scholar]
- 56.Bang, H., Kim, Y., and Kim, D. (2000) J. Biol. Chem. 275 17412-17419 [DOI] [PubMed] [Google Scholar]
- 57.Chen, X., Talley, E. M., Patel, N., Gomis, A., McIntire, W. E., Dong, B., Viana, F., Garrison, J. C., and Bayliss, D. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 3422-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veale, E. L., Kennard, L. E., Sutton, G. L., MacKenzie, G., Sandu, C., and Mathie, A. (2007) Mol. Pharmacol. 71 1666-1675 [DOI] [PubMed] [Google Scholar]
- 59.Mathie, A. (2007) J. Physiol. 578 377-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chemin, J., Girard, C., Duprat, F., Lesage, F., Romey, G., and Lazdunski, M. (2003) EMBO J. 22 5403-5411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakashiba, T., Young, J. Z., McHugh, T. J., Buhl, D. L., and Tonegawa, S. (2008) Science 319 1260-1264 [DOI] [PubMed] [Google Scholar]
- 62.Galeotti, N., Bartolini, A., and Ghelardini, C. (2004) Behav. Brain Res. 153 409-417 [DOI] [PubMed] [Google Scholar]
- 63.Galeotti, N., Bartolini, A., and Ghelardini, C. (2004) Neuroscience 126 451-460 [DOI] [PubMed] [Google Scholar]
- 64.Haapalinna, A., Sirvio, J., and Lammintausta, R. (1998) Eur. J. Pharmacol. 347 29-40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.