Abstract
Villin is an actin-binding protein localized to intestinal and kidney brush borders. In vitro, villin has been demonstrated to bundle and sever F-actin in a calcium-dependent manner. Although villin is not necessary for the bundling of F-actin in vivo, it is important for the reorganization of the actin cytoskeleton elicited by stress during both physiological and pathological conditions (Ferrary et al., 1999). These data suggest that villin may be involved in actin cytoskeleton remodeling necessary for many processes requiring cellular plasticity. Here, we study the role of villin in hepatocyte growth factor (HGF)-induced epithelial cell motility and morphogenesis. For this purpose, we used primary cultures of enterocytes derived from wild-type and villin knock-out mice and Madin-Darby canine kidney cells, expressing villin in an inducible manner. In vitro, we show that epithelial cell lysates from villin-expressing cells induced dramatic, calcium-dependent severing of actin filaments. In cell culture, we found that villin-expressing cells exhibit enhanced cell motility and morphogenesis upon HGF stimulation. In addition, we show that the ability of villin to potentiate HGF-induced actin reorganization occurs through the HGF-activated phospholipase Cγ signaling pathway. Collectively, these data demonstrate that villin acts as a regulator of HGF-induced actin dynamics.
INTRODUCTION
Villin is a tissue-specific actin-binding protein expressed in the brush border of enterocytes and proximal kidney cells. Villin is a member of the gelsolin family of proteins that contain three to six homologous evolutionarily conserved domains. Villin (Janmey and Matsudaira, 1988), advillin (Marks et al., 1998), and supervillin (Wulfkuhle et al., 1999) all have additional domains and display specialized roles in the organization of actin microfilaments in the restricted cell types in which they are expressed. Villin (Northrop et al., 1986), gelsolin (Kwiatkowski and Yin, 1987), and adseverin (Lueck et al., 1998) have the common property of binding to barbed ends of actin filaments with high affinity and severing actin filaments. CapG binds to barbed ends but does not sever actin filaments (Southwick and DiNubile, 1986; Mishra et al., 1994). Villin is unique in presenting capping, bundling, and severing properties in a single protein. These properties are regulated by the calcium level: at low calcium concentrations, villin bundles F-actin filaments; at high calcium concentrations, it severs F-actin; and at intermediate concentrations, villin caps filaments (Bretscher and Weber, 1980). The bundling activity of villin was first assessed in transfection experiments in cultured cells: villin induced the growth of microvilli on the dorsal face of fibroblastic CV1 cells, including reorganization of the underlying actin cytoskeleton. The villin core domain (lacking the head piece domain) was unable to promote these morphological modifications (Friederich et al., 1989, 1992). Furthermore, using an antisense RNA strategy in human intestinal Caco-2 cells, permanent down-regulation of the endogenous villin mRNA has been shown to reversibly affect brush-border assembly (Costa de Beauregard et al., 1995).
We (Ferrary et al., 1999) and others (Pinson et al., 1998) have shown that the ultrastructural organization of microvilli is not altered in vil-/- mice brush borders, suggesting that the bundling property of villin may be compensated for by another microvillar actin-binding protein. However, when mice were submitted to stress conditions known to modulate the intracellular calcium concentration, we found that the severing of actin filaments did not occur in vil-/- mice, compared with wild-type animals. Our in vivo data suggest a role for villin in cellular plasticity related to cell injury. Thus, villin could play a role in the actin cytoskeleton dynamics triggered by various signals necessary for cell motility and wound repair (Ferrary et al., 1999).
Epithelial cell dispersal during cell motility requires a transition from an epithelial morphology toward a more mesenchymal fibroblastic phenotype, referred to as epithelial-mesenchyme transition (Boyer et al., 1996), modulated by HGF and its receptor, the tyrosine kinase c-met. Several intracellular signaling pathways have been shown to act downstream of the HGF receptor to mediate scattering or tubulogenesis response. For example, c-met was reported to directly interact with signaling proteins such as phosphatidylinositol-3 kinase (Graziani et al., 1991), phospholipase Cγ (PLCγ), and pp60c-src (Ponzetto et al., 1994). After the activation of the hepatocyte growth factor (HGF) receptor, these interactions have been shown to generate phosphoinositides and raise the intracellular calcium concentration (Mine et al., 1991; Gual et al., 2000). The morphogenetic effect of HGF has recently been shown to require sustained PLCγ activation (Gual et al., 2000). Many actin-binding proteins such as gelsolin have been shown to be regulated by phosphoinositides (Kwiatkowski, 1999). Several lines of evidence suggest that villin function can be regulated by these signaling molecules. Villin interacts with several signaling molecules, including PLCγ (Khurana et al., 1997; Papakonstanti et al., 2000; Panebra et al., 2001), phosphatidylinositol 4,5-biphosphate (PIP2) (Janmey et al., 1987), and calcium (Janmey and Matsudaira, 1988). In vitro, phosphorylation of villin tyrosines has been shown to enhance actin severing and to inhibit the actin-bundling property of villin (Zhai et al., 2001). These findings suggest that villin plays an essential role in the actin cytoskeleton dynamics in response to specific physiological stimuli.
The purpose of this work was to test the role of villin in cellular processes that require actin cytoskeletal reorganization and dynamics such as cell motility, cell morphogenesis, and wound repair. Our results demonstrate that villin acts as a regulator of HGF-induced actin dynamics.
MATERIALS AND METHODS
Primary Cultures of vil+/+ and vil-/- Enterocytes
Preparation of primary cultures of enterocytes was performed as described previously (Evans et al., 1992) and modified in Athman et al. (2002b). The small intestines of 4-week-old mice were placed in Hanks' balanced salt solution (HBSS) (Sigma-Aldrich, St. Louis, MO), cut into 2- to 3-mm fragments, washed in HBSS, and incubated in an enzymatic solution (0.1 mg/ml dispase [Roche Diagnostics, Indianapolis, IN] and 300 U/ml collagenase [Sigma-Aldrich], in HBSS) at 25°C for 30 min, 150 rpm. After pipetting and sedimentation steps, the solution was resuspended in DMEM-S (2.5% fetal calf serum and 2% sorbitol) and centrifuged at 300 rpm for 3 min. The pellet was resuspended in DMEM containing epidermal growth factor (10 ng/ml), insulin (0.25 μg/ml), penicillin (100 U/ml), streptomycin (60 μg/ml), nonessential amino-acids (1×), and l-glutamine (2 mM). The cells were then plated on 25-mm glass coverslips.
Villin cDNA Construct
Human villin cDNA (Arpin et al., 1988) containing the complete coding sequence was inserted in a Bluescript-KS plasmid (KS-villin). Villin was tagged by incorporating a sequence coding for the vesicular stomatitis virus glycoprotein G (VSVG), carried by the KS plasmid (KS-tag), at the 5′ end of villin cDNA. To allow villin cDNA ligation to this tag, Eco RI and Bam HI restriction sites were incorporated into the 5′ end of this cDNA, resulting in the amplification of a 600-base pair fragment. The sense primer (5′GGGAATTCCCATGACCAAGCT3′) contained the Eco RI restriction site and the reverse primer (5′CGCGGATCCTCGGATCTCCTTGG3′) contained the Bam HI restriction site. The Eco RI and Bam HI digested sequence was ligated into the the KS-tag plasmid after digestion by these same enzymes (KS-tag-vil 5′). To reconstitute the full-length villin cDNA, the KS-villin plasmid and the KS-tag-vil 5′ were both digested according to Sma I-Xba I restriction sites. The resulting fragment was then ligated with the digested KS-tag-vil 5′ plasmid, leading to the formation of a recombinant plasmid bearing Not I restriction sites at the 5′ end of the tag sequence and at the 3′ end of villin cDNA. To allow inducible expression of villin, the villin construct was digested using Not I and inserted into the pUHD plasmid (BD Biosciences Clontech, Palo Alto, CA) downstream of the tetracycline-responsive element.
Tet Off Madin-Darby Canine Kidney (MDCK) Cells Expressing Villin cDNA
The Tet Off system has been described previously (Gossen and Bujard, 1992). MDCK Tet Off cells stably expressing the TetR/VP16 transactivator protein were purchased from BD Biosciences Clontech. The pUHD-villin construct was cotransfected by the lipofectine procedure (Invitrogen, Carlsbad, CA) with a selection plasmid carrying the hygromycin resistance gene driven by the thymidine kinase promoter. Transfected cells were selected by growing in media containing hygromycin (400 μg/ml), G418 (200 μg/ml), and doxycycline (1 μg/ml). Five clones were obtained, all expressing villin in a doxycycline-dependent manner. One clone was selected and routinely grown in the presence of 0.5 μg/ml doxycycline. For the experiments, cells were separated into vil-/- cells (repressed), grown in presence of 2 μg/ml doxycycline, and vil+/+ cells (induced) expressing the villin transgene, grown in absence of the antibiotic.
Purification of Recombinant Villin
The pGEX2T plasmid containing a GST-villin cDNA construct and purification procedures have been described previously (Friederich et al., 1999). Villin-glutathione S-transferase fusion proteins were purified from the soluble fraction of sonicated Escherichia coli BL21 bacteria by glutathione-Sepharose (Pharmacia, Peapack, NJ) chromatography following standard procedures. The bead-bound fusion protein was digested with thrombin (purified from human plasma; Sigma-Aldrich). Proteins were dialyzed against the appropriate storage buffer.
Cell Motility and Chemotaxis Analysis
MDCK cells (25,000) were plated on 25-mm-diameter glass coverslips and starved overnight before the experiment. Primary enterocytes were used after 6 days of primary culture when they form small epithelial islets surrounded by fibroblasts. They were starved only 3 h before the experiment to avoid cell damage. For cell scattering assays, HGF-induced cell motility experiments were performed. HGF (10 IU/ml) from MRC-5 human lung diploid cells culture supernatant (ATCC 171-CCL) was added to the serum-depleted medium, and cell scattering was followed by video microscopy during 24 h. MDCK cell motility was also analyzed after cell pretreatment with U73122 (10 μM), a specific inhibitor of PLCγ, during 1 h.
Chemotaxis experiments were performed on MDCK cells in the presence of small pieces of solidified agar (2%) containing HGF (100 IU/ml) during 24 h. Cell directionality toward the agar pieces was measured as cell persistence, which is the ability of the cell to keep a constant direction during motility. This is evaluated as the number of turns the cells make, expressed in degrees per minute.
Wound-healing experiments were performed on both primary cultures of enterocytes from vil-/- and vil+/+ mice and confluent vil-/- and vil+/+ MDCK cells. After 6 d of primary culture, microscopic lesions were performed on individual islets of enterocytes by using a thin sterile needle under the videomicroscope. On MDCK monolayers, a wound was performed using a 1-ml tip. In both cases, wound closure was followed by videomicroscopy and evaluated by measuring the size of the wound area over time using the MetaMorph software (Universal Imaging, Downingtown, PA).
Videomicroscopy
Cells grown on coverslips were placed in a videomicroscopy chamber (DUTSCHER SA 32Dx7H) containing 1.5 ml of DMEM and the appropriate HGF concentration at 37°C and 5% CO2. One acquisition was taken every 4 min for cell scattering and chemotaxis and every 20 min for wound-healing experiments during 24 h. Cells were tracked using MetaMorph software.
Lamellipod Extension Assay
Lamellipod extension was measured as the increase of the total cell area during HGF-induced cell motility on the time-lapse phase contrast images. The mean relative area was plotted as a function of time to illustrate the kinetics of the protrusive activity after cell stimulation.
Tubulogenesis Assay
This assay was performed on MDCK cells according to the protocol described in Gautreau et al., (1999). The cells were grown in type I rat tail collagen gel (5 mg/ml; BD Biosciences, San Jose, CA) in presence of 100 IU/ml HGF. After 12 d, immunofluorescence analysis was performed on the residual gels by confocal microscopy.
Immunofluorescence Microscopy
Primary enterocytes or MDCK cells were fixed with 3% paraformaldehyde (PFA), permeabilized with 0.2% Triton X-100, and incubated with specific antibodies. F-Actin was stained with tetramethylrhodamine B isothiocyanate (TRITC)-phalloidin (1:350, Sigma-Aldrich), villin with a mouse monoclonal antibody (mAb) ID2C3 (1:500, Dudouet et al., 1987), and keratin with an anti-cytokeratin mAb (A45-B/B3) (1:200; ChromaVision Medical Systems). E-Cadherin staining was performed using a rat antibody (1:500, DECMA; Sigma-Aldrich). To analyze tubule polarization, a rabbit polyclonal antibody against laminin was used (1:2000; gift from Michèle Kedinger, INSERM U381, Strasbourg, France). Nuclei were visualized using Hoechst-33258 (10 μg/ml) in phophate-buffered saline (PBS). Cells or collagen gels were mounted with PBS/glycerol (50%) and viewed using fluorescence microscopy (Leica Microsystems, Deerfield, IL) and confocal microscopy (Leica Microsystems). Images were processed using Photoshop software (Adobe Systems, Mountain View, CA).
Cell Fractionation
To analyze whether HGF stimulation induced villin redistribution, we performed cell fractionation assays on untreated and HGF-treated MDCK cells (10 IU/ml). Cells were grown on 10-cm-diameter plates to 80% confluence, washed with cold PBS, and incubated for 2 min in a cytoskeleton buffer (pH 6.4) containing 50 mM MES, 3 mM EGTA, 5 mM MgCl2, and 0.5% Triton X-100. The plate was then scrapped with Laemmli buffer (1×) to obtain the remaining insoluble pool. Villin was detected by immunoblotting, by using ID2C3 antibody (1:10,000).
Cytosol/membrane fractionation assays were also performed in the same experimental conditions as described previously (Gautreau et al., 2000). To inhibit PLCγ activity, cells were pretreated with 10 μM U73122 (BIOMOL Research Laboratories, Plymouth Meeting, PA) for 1 h before HGF addition.
Coimmunoprecipitation Experiments
To analyze whether HGF stimulation modulates villin association to PLCγ1, coimmunoprecipitation experiments were performed on membrane fractions. For immunoprecipitation procedures, membrane fractions, prepared as described above, were incubated with 3 μg of anti-villin or 1.6 μg of anti-PLCγ antibodies during 1 h at 4°C. After incubation with 100 μl of 50% protein G-Sepharose in PBS (Amersham Biosciences, Piscataway, NJ) for 1 h at 4°C, Sepharose/lysate mixtures were centrifuged, and the beads were washed four times (3 min, 4000 rpm) to eliminate nonspecific binding interactions. Beads were then resuspended in Laemmli buffer (2×). Villin phosphorylation was analyzed using PY20 anti-phosphotyrosine antibody (1:5000; ICN Biomedicals, Costa Mesa, CA).
Western Blot Analysis
Proteins were separated on 7% SDS-PAGE and transferred to nitrocellulose membrane (0.2 μm; Shleicher & Schuell, Keene, NH). After incubation with the primary antibody, the membrane was washed in 0.5% PBS/Tween 20 and probed with horseradish peroxidase secondary antibody. The blots were developed with an enhanced chemiluminescence kit (Roche Diagnostics).
F-Actin Content Measurements in Adherent Cells
This experiment was conducted as described previously (Chan et al., 1998). Cells were grown on 10-cm-diameter plates to 80% confluence in serum-deprived medium overnight and stimulated with HGF (10 UI/ml) for >2 h. After fixation in 3.7% formaldehyde in F buffer, permeabilization with 0.5% Triton X-100 and incubation with 1 μM TRITC-phalloidin for 1 h, the bound phalloidin was extracted using 100% methanol at 4°C for 90 min. Fluorescence was recorded at 540-nm excitation and 570-nm emission, slit 5.
G-Actin Labeling with Fluorescent DNase I
Alexa Fluor 488 DNase I conjugate was purchased from Molecular Probes (Eugene, OR). MDCK cells were grown on 25-mm coverslips as islets and were stimulated with HGF (10 UI/ml) for 2 or 6 h. Cells were fixed in 3% PFA in PBS for 15 min and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Coverslips were then stained by incubation in a 9 μg/ml solution of DNase I for 20 min.
Barbed End Measurements
Nucleation sites were localized as described previously (Chan et al., 1998). After permeabilization for 1 min in the presence of 0.45 μM rhodamine G-actin in buffer G, cells were fixed in PFA. Free nucleation sites were evaluated by quantification of the fluorescence at the leading edge of the cells.
Preparation of vil+/+ and vil-/- Enterocyte Lysates
Lysate preparation was performed as described previously (Weiser, 1973). The removed intestine was rinsed with 0.15 M NaCl and 1 mM dithiothreitol (DTT), ligatured, and filled with PBS for 15 min at 37°C. The solution was replaced with PBS and 15 mM EDTA for 30 min at 37°C. After washing (3000 rpm for 5 min), the cells were counted and lysed in the appropriate lysis buffer.
Direct Observation of Actin Filament Severing by Cell Lysates
F-Actin was prepared from rabbit muscle G-actin rhodamine, purchased from Molecular Probes (5.3 mg/ml). G-Actin (126 μM) was added to F buffer (5 mM Tris, pH 7.5, 100 mM KCl, 0.2 mM ATP 0.5 mM DTT, 0.2 mM CaCl2, 1 mM MgCl2). The preparation was incubated for 3 h at 4°C to allow actin polymerization. The F-actin was resuspended at a concentration of 1 μM in bundling buffer (5 mM KPO4, pH 7, 1 mM EGTA, 1 mM DTT, 40 mM KCl, 1 mM MgCl2), with 1 mM unlabeled phalloidin (Sigma-Aldrich) and incubated overnight at 4°C. The preparation was centrifuged at 60,000 rpm for 5 min. The pellet was resuspended in bundling buffer and the filaments were stored at 4°C (Chabrillat, M., unpublished data).
Direct observation of actin severing was performed as described previously (Chan et al., 2000). The severing of actin filaments by enterocyte or MDCK lysates was observed in a flow chamber coated with 0.1% nitrocellulose in amylacetate. The flow chamber was filled with 0.1 μM F-actin in bundling buffer for 15 min. Unbound F-actin was washed away with antibleaching buffer (10 mM β-mercaptoethanol, 20 μg/ml catalase, 2.5 mg/ml glucose, 0.1 mg/ml glucose oxydase in bundling buffer). Severing was initiated by replacement of the flow solution with total cell lysate prepared as described below. The lysis buffer contained 20 mM Tris-HCl, pH 7.5, 5 mM EGTA, 0.5 mM MgCl2, 0.5% Triton X-100, 0.5 mM ATP, 5 mg/ml bovine serum albumin, 0.036 mg/ml catalase, 0.02 mg/ml glucose oxidase, 6 mg/ml glucose, 1 mM DTT, and protease inhibitors. Enterocytes as well as MDCK cells treated with HGF (10 IU/ml) for 6 h were resuspended in the lysis buffer. Actin severing was directly observed under fluorescent microscopy.
Statistical Analysis
Statistical comparisons were performed using unpaired Student's t tests, by using SigmaStat software, with a probability value <0.05 taken to indicate significance.
RESULTS
Cellular Models
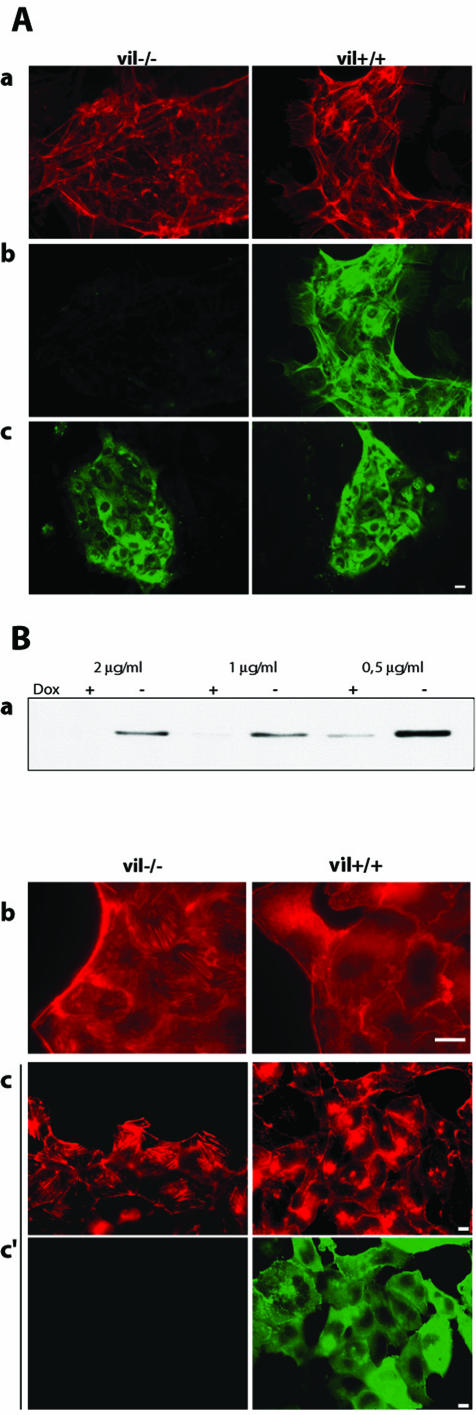
Primary Culture of Enterocytes After 6 days of primary culture, actin labeling showed that enterocytes grow as small, easily detectable islets from surrounding fibroblasts (Figure 1A, a). Villin and cytokeratin labeling performed on vil-/- and vil+/+ primary enterocytes confirmed the lack of villin in vil-/- cells and the epithelial origin of the cells in the islets and showed that they displayed the distinguishing features of enterocyte cytoskeleton (Figure 1A, b and c).
Figure 1.
Cellular models. (A) Primary enterocytes from vil+/+ and vil-/- mice were cultured for 6 d. Epithelial islets were characterized using F-actin (red) (a) and villin (green) (b) double staining. Cytokeratin (green) labeling was used to check the epithelial origin of enterocytes islets (c). (B) Tightly controlled expression of villin by doxycycline (dox) treatment in MDCK cells. Cells grown in presence of the indicated doxycycline concentrations were separated into vil+/+ cells, grown in absence of the antibiotic, and vil-/- cells, grown in presence of doxycycline. Villin expression repression and induction was evaluated by Western blotting. This figure shows that villin induction is efficient when the cells are routinely grown in presence of 0.5 μg/μl doxyxycline (a). However, to avoid leaky expression during repression, the cells are shifted to 2 μg/μl doxycycline. Control of villin expression was also tested by immunofluorescence. Actin was labeled with phalloidin TRITC (b) and villin by using a mAb (c). Bar, 10 μm.
Control of Villin Expression in MDCK Cells Figure 1B, a shows that villin can be switched on and off according to the doxycycline concentration. Immunofluorescence analysis shows that villin expression leads to a decrease in the stress fibers content, as shown by the actin labeling (Figure 1B, b and c). Analysis of villin expression indicated a homogeneous villin localization in vil+/+ MDCK cells, whereas no villin was expressed in vil-/- doxycycline-treated cells (Figure 1B, c).
Villin Enhances HGF-induced Epithelial Cell Motility Because we have previously shown that villin is involved in cell plasticity related to cell injury (Ferrary et al., 1999), we tested the ability of primary enterocytes to remodel the actin cytoskeleton that occurs during the cell motility process.
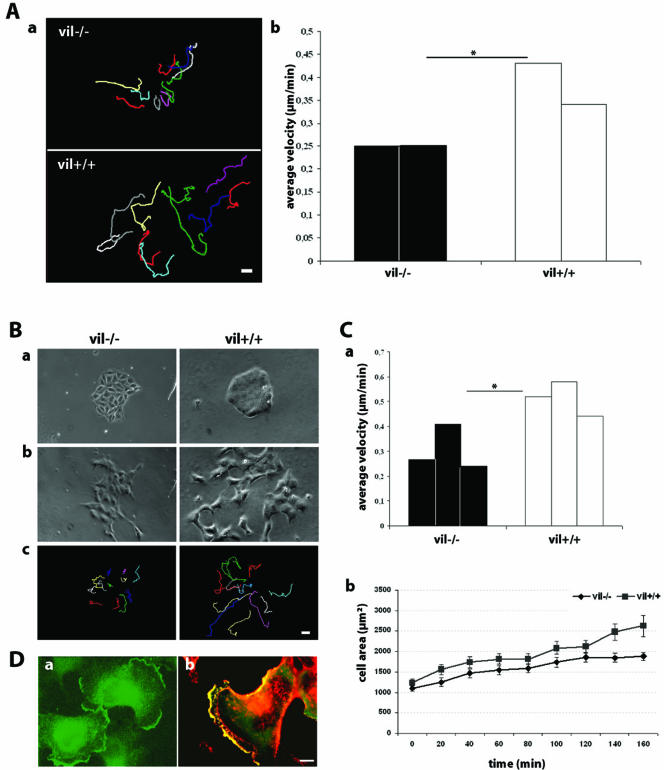
HGF-induced cell scattering experiments were performed using vil+/+ and vil-/- enterocytes. Cell tracking analysis (Figure 2A, a) showed that vil+/+ enterocytes were able to scatter within the epithelial islets, showing individual movements. In contrast, vil-/- enterocytes had limited scattering and moved at a lower velocity (0.25 μm/min) than vil+/+ cells (0.38 μm/min) (n = 12 cells for each experiment) (Figure 2A, b).
Figure 2.
Villin enhances HGF-induced cell motility. (A) Primary enterocytes from vil+/+ and vil-/- mice were cultured for 6 d. Cells were stimulated with 10 IU/ml HGF to allow cell scattering. Individual cell trajectories were followed for 13 h after HGF stimulation (a) and analyzed using MetaMorph software to determine cell velocity data in micrometers per minute (b) (two independent experiments; *p < 0.05). (B) Vil-/- and vil+/+ MDCK cells before (a) and after9hofHGF(10 IU/ml) stimulation (b). c illustrates cell tracking after9hof treatment. (C) a, vil-/- and vil+/+ MDCK cell velocities upon HGF stimulation (three independent experiments; *p < 0.05). b, quantitation of lamellipodia extension as a function of time. The cell area was measured using MetaMorph software. (p = 0.011 at 140 min; p = 0.007 at 160 min). D. MDCK cells grown as small islets were stimulated with HGF (10 IU/ml) for 6 h. a, villin labeling in HGF-stimulated MDCK cells. b, villin colocalization with actin upon HGF stimulation. Cells were fixed and stained for villin (green) and actin (red). a and b, upon HGF stimulation, villin is redistributed to the leading edge of migrating cells and colocalizes with actin in this specialized region. Bar, 10 μm.
We then tested whether villin could be involved in actin dynamics in MDCK cells expressing villin, taking advantage of the well established Tet Off system. The average velocity of control Tet-Off nontransfected cells grown in absence of doxycycline was first tested (0.20 μm/min, n = 12) to make sure that this treatment does not affect cell velocity. We performed cell scattering experiments in vil-/- and vil+/+ MDCK cells. Figure 2B presents the cells before (Figure 2B, a) and after 9 h of HGF treatment (Figure 2B, b) and the tracking showing the trajectories of individual cells (Figure 2B, c). The ability of vil+/+ cells to scatter upon HGF stimulation was significantly higher than that of vil-/- cells as shown by their twofold increased velocity (Figure 2C, a), (n = 12 cells for each experiment).
Using a lamellipodia extension assay, we show that the total cell area was significantly greater in villin-expressing cells compared with vil-/- cells after 2 h of HGF stimulation (Figure 2C, b). These data show that in the presence of villin, the cells are able to form larger lamellipodia, suggesting a higher dynamics after HGF treatment. We therefore determined whether HGF treatment affected villin localization in crawling cells. In colonies of resting vil+/+ MDCK cells, villin was distributed throughout the cytoplasm (Figure 1B, c). After 6 h of HGF stimulation, villin redistributed to the leading edge of HGF-induced lamellipodia (Figure 2D, a), where it colocalized with F-actin (Figure 2D, b).
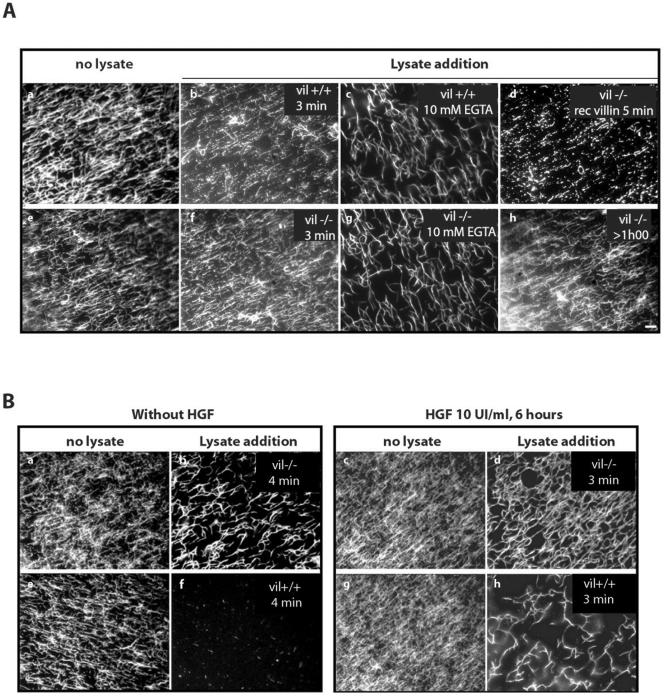
Villin Induces F-Actin Reorganization In Vitro To compare the ability of vil+/+ and vil-/- enterocytes to remodel the actin cytoskeleton, we used an in vitro assay to visualize directly the effects of enterocytes lysates on F-actin microfilament organization (Chan et al., 2000). Figure 3A, a and e, shows that before addition of cell lysates, actin filaments look like a network of actin filaments. The addition of vil-/- lysate to these actin filaments induced their reorganization into a network of interconnected actin filaments (Figure 3A, f). This was also the case after lysate treatment with 10 mM EGTA that induces calcium chelation (Figure 3A, g). On the contrary, lysates from vil+/+ enterocytes induced the severing of actin filaments within 3 min, resulting in the formation of numerous fragmented actin filaments (Figure 3A, b). The severing capacity of vil+/+ lysates was inhibited after treatment with 10 mM EGTA (Figure 3A, c). One should note that EGTA treatment alone induce, after both vil-/- and vil+/+ lysate addition, a reinforcement of the actin filaments, suggesting a villin-independent bundling effect. Actin severing was also observed by adding 1 mg/ml recombinant villin to actin filaments previously reorganized by vil-/- lysate (Figure 3A, d). In all cases, the actin network induced by vil-/- lysate remained the same for >1 h (Figure 3A, h). This demonstrates that the observed effects were only due to lysate or villin additions and not to actin filament alteration over time.
Figure 3.
Villin induces F-actin reorganization in vitro. Rhodamine-labeled actin filaments were prepared as described in MATERIALS AND METHODS. They were allowed to attach to a nitrocellulose matrix on a glass coverslip mounted as a flow chamber during 15 min on ice. After washing with an antibleaching buffer, 12 μl of cell lysate (10 mg/ml) was perfused and the analysis was directly performed under fluorescent microscopy. (A) Enterocyte lysates. a and e, rhodamine-labeled F-actin filaments before lysate perfusion as internal controls. Note that in absence of any perfusion, the actin filaments correspond to disorganized actin network. B, 3 min after vil+/+ lysate addition. c, 5 min after EGTA treated vil+/+ lysate addition. f, 3 min after vil-/- lysate addition. g, 5 min after EGTA-treated vil-/- lysate addition. d, perfusion of vil-/- lysate followed by addition of 1 μg/μl recombinant villin during 5 min. h, actin filaments >1 h after vil-/- lysate perfusion. (B) MDCK cells lysates. a, c, e, and g, actin filaments before lysate perfusion. In absence of HGF, vil-/- (b) and vil+/+ (f) lysates. After 6 h of HGF stimulation, vil-/- (d) and vil+/+ (h) lysates. Bar, 10 μm.
To better understand how villin induces actin cytoskeleton reorganization upon HGF stimulation, we analyzed the ability of cell lysates from MDCK cells, stimulated or not with HGF for 6 h, to reorganize F-actin filaments in vitro. Using the assay previously described, we showed that, in the absence of any stimulation, lysates from villin-expressing cells induced F-actin severing as shown by the almost complete disappearance of filaments in the numerous microscopic fields analyzed (Figure 3B, f). In contrast, vil-/- cell lysates led to the thickening of actin filaments (Figure 3B, b). We next analyzed the ability of cell lysates from MDCK cells, stimulated with HGF during 6 h, to reorganize F-actin filaments in vitro. Figure 3B, c and g, show the actin filaments before lysate perfusion as controls. Perfusion of cell lysates from vil-/- cells, after HGF stimulation, induce the organization of actin into a dense network of thick actin filaments (Figure 3B, d). In contrast, lysates from HGF-stimulated villin expressing cells lead to the formation of thick and short hooked actin filaments (Figure 3B, h). Thus, in both cell models, villin-expressing lysates lead to dramatic actin microfilaments fragmentation. However, pretreatment of MDCK cell cultures expressing villin with HGF induces the formation of a thick hooked actin filaments network (compare Figure 3, d and h). This may be due to other proteins of the cell lysates that participate in addition to villin to this reorganization and remain to be investigated.
Together, severing of actin filaments occurred only after exposure to vil+/+ lysate or villin perfusion because no spontaneous actin filament alteration was observed. Moreover, the actin microfilament fragmentation is sensitive to villin and dependent of Ca2+. These observations suggest that villin enhanced HGF-induced motility occurs through actin filaments dynamics at the leading edge of lamellipodia in motile cells.
Villin Expression Enhances HGF-induced Chemotaxis and Wound Repair To further analyze the role of villin in actin-mediated cell functions, we examined the involvement of villin in HGF-induced cell motility during chemotaxis and wound repair.
Having shown that villin potentiates HGF-induced motility, we also tested whether it participated in the chemotaxis process. Assays with MDCK cells by using pieces of solidified agar (2%) containing HGF (100 IU/ml) showed that the scattering was much more efficient in villin-expressing cells (0.49 μm/min), compared with vil-/- cells (0.16 μm/min), thus corroborating the scattering experiments described above. Moreover, villin-expressing cells exhibited persistence in their movement toward the HGF-containing agar piece, thus showing directionality in their movements. The obtained values, in micrometers per minute × degree, were 7.59 in vil-/- cells versus 15.11 in vil+/+ cells (p < 0.001).
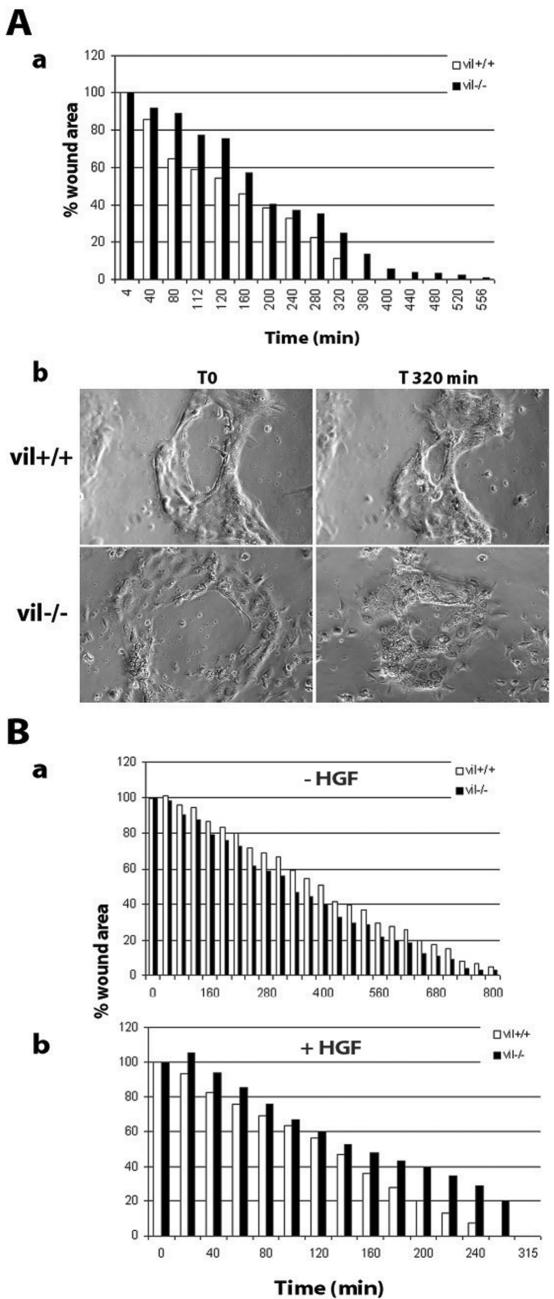
Wound-healing assays performed on primary cultures of enterocytes showed that in vil+/+ cells wound repair was complete after 6 h, whereas wound closure was delayed in vil-/- enterocytes, because 100% of the lesion area was closed after 9 h (Figure 4A, a). Figure 4 antibody presents the epithelial islets of enterocytes before (T0) and 5 h after the beginning of wound closure. Wound healing was also evaluated in HGF-stimulated and nonstimulated, vil-/-, and vil+/+ MDCK cells. In the absence of any stimulation, vil-/- and vil+/+ cells were able to perform efficient wound repair as shown by complete wound closure within 13 h after wounding (Figure 4B, a). However, after HGF stimulation, wound repair was enhanced in both cases (threefold), but wound closure was faster in villin-expressing cells. When wound closure was completed in vil+/+ cells, 20% of the wound area remained to be repaired in vil-/- cells, which completed the wound 60 min later (Figure 4B, b). Cells move first as sheets and then scatter at the end of the wound closure process.
Figure 4.
Villin expression enhances HGF-induced wound repair. (A) Wound-healing experiments performed on primary cultures of enterocytes. The wound has been performed using a thin needle on islets of enterocytes. a, measurement of the wound area over time. b, phase contrast image of enterocytes at T0 and 320 min after wound. (B) Wound-healing experiments on confluent monolayers of MDCK cells. The wound has been performed using a tip. a and b, measurement of the wound area over time in the absence of HGF (a) and in presence of HGF (10 UI/ml) (b). In both cases, wound repair was expressed as a percentage of the initial wound area.
Using two independent cell models and three separate ways to monitor cell motility, we have shown that villin enhances HGF-induced cell motility by increasing the actin cytoskeleton dynamics at the leading edge of lamellipodia. Together, these observations support the hypothesis that villin can act as a potentiator of HGF-stimulated cell motility.
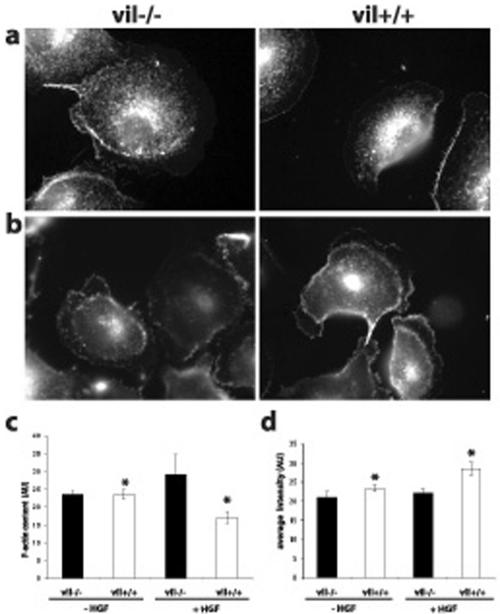
HGF Treatment Decreases F-Actin Content and Promotes the Formation of Villin-induced Barbed Ends To analyze how HGF stimulation affected the actin cytoskeleton, we measured the F-actin content by fluorometry analysis before and after 3 h of HGF stimulation by using TRITC-labeled phalloidin. As shown in Figure 5c, in the absence of stimulation, the F-actin content is the same in the presence or in absence of villin. However, after 3 h of HGF stimulation, the F-actin content is increased in vil-/- cells, indicating a net polymerization process, whereas it is significantly decreased in villin-expressing cells (p = 0.03). This suggests that depending on the presence or absence of villin, HGF stimulation involves different actin dynamic processes affecting actin equilibrium. The severing/capping properties of villin can favor the formation of short actin filaments, thus increasing actin dynamics at the leading edge of motile cells.
Figure 5.
Villin-expressing cells present a decrease of the F-actin content and a higher level of barbed ends at the leading edge of MDCK cells upon HGF stimulation. Free barbed ends were visualized by rhodamine G-actin incorporation before (a) and after 6 h of HGF treatment (b). The amount of free barbed ends at the leading edge of the cells was evaluated by quantitation of the average fluorescence intensity using MetaMorph software (d). Eighteen vil-/- and 24 vil+/+ unstimulated cells were analyzed. Twentyseven vil-/- and 30 vil+/+ cells after 6 h of HGF treatment were analyzed (*p = 0.004). The F-actin content was evaluated by measuring the fluorescence intensity of TRITC-palloidin–labeled actin filaments in MDCK cells before and after 3 h of HGF treatment (c). The amount of total protein was the same in vil-/- and vil+/+ cells; n = 3 experiments.
To evaluate whether villin redistribution to the leading edge of motile cells affected actin organization at this site, we analyzed the presence of free barbed ends, an evidence for active cytoskeleton dynamics. Previous studies have shown the requirement for free barbed ends in the control of actin dynamics at the leading edge (Handel et al., 1990). Free barbed end visualization was performed before (Figure 5a) and after 6 h of HGF stimulation (Figure 5b) and was quantified at the leading edge of individual cells (Figure 5d). The quantification of the average fluorescence intensity shows that the level of free barbed ends is slightly increased (33%) after villin induction. However, HGF treatment induced a significant increase of the newly formed barbed ends at the leading edge of villin-expressing cells (p = 0.004). This indicates that villin by itself stimulates the formation of short actin filaments at the leading edge of the cells and that this process is enhanced after HGF stimulation.
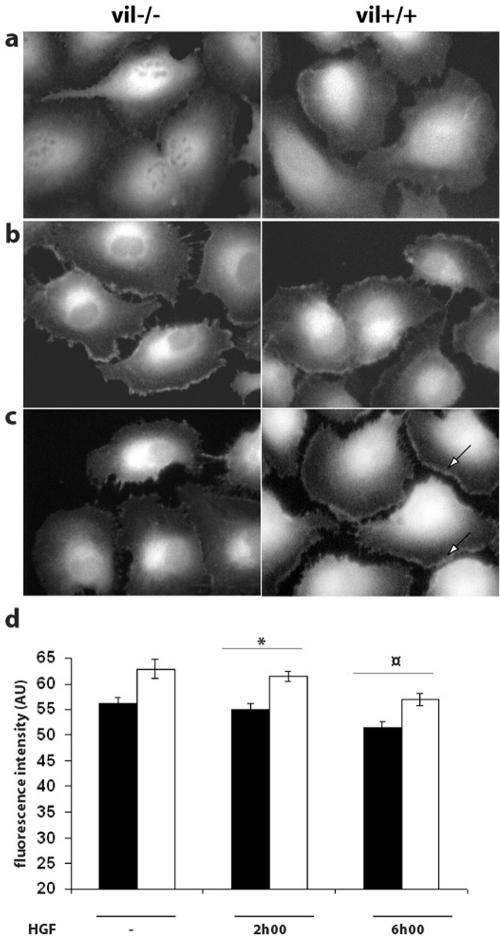
Because actin dynamics involves the rapid turnover of G-actin monomers, we tested whether villin expression affected the amount of monomers. We analyzed the amount of G-actin in the lamellipodia of vil+/+ and vil-/- MDCK cells before (Figure 6a) and after HGF treatment during 2 h (Figure 6b) and 6 h (Figure 6c). The level of fluorescence was increased at the leading edge of the cells in both vil+/+ and vil-/- cells after 2 h of treatment. However, after 6 h of treatment, the amount of available G-actin was diffuse in the lamellipodia of vil-/- cells, whereas the staining remained concentrated in the leading edge of vil+/+ cells. This pool of actin monomers available at the leading edge of motile cells can explain the sustained motility effect observed in vil+/+ cells. The level of G-actin quantified by DNase I staining in the lamellipodia of vil+/+ and vil-/- moving cells slightly decreased after 2 and 6 h of HGF treatment (Figure 6d), suggesting the recruitment of the available G actin pool during the motility process. However, after HGF stimulation, the amount of G-actin remains significantly higher in villin-expressing cells compared with vil-/- cells. Together, in the presence of villin, the decrease of the F-actin content (Figure 5c) associated with an increase of both the amount of free barbed ends (Figure 5d) and the level of the available G-actin pool (Figure 6d) strongly supports the view that, upon HGF stimulation, villin could play an active role in the actin polymerization/depolymerization cycle that is a feature that is required in the lamellipodia of moving cells.
Figure 6.
Actin dynamics of villin-expressing cells relies on a higher available pool of actin monomers than in repressed cells. The G-actin pool was visualized using fluorescent DNase I, which binds to actin monomers with a high affinity. Cells were either unstimulated (a) or stimulated with HGF (10 UI/ml) for 2 h (b) and 6 h (c). Arrows show maintenance of G-actin labeling in the leading edge of induced cells after 6 h of HGF treatment. Fluorescence intensity in the lamellipodia was quantified using MetaMorph software (d). Among the induced cells, 26 nonstimulated cells, 31 cells treated with HGF for 2 h, and 30 cells treated with HGF for 6 h were analyzed. Among the repressed cells, 31 nonstimulated cells, 36 cells treated with HGF for 2 h, and 30 cells treated with HGF for 6 h were analyzed. (p = 0.026 at 2 h; p = 0.001 at 6 h).
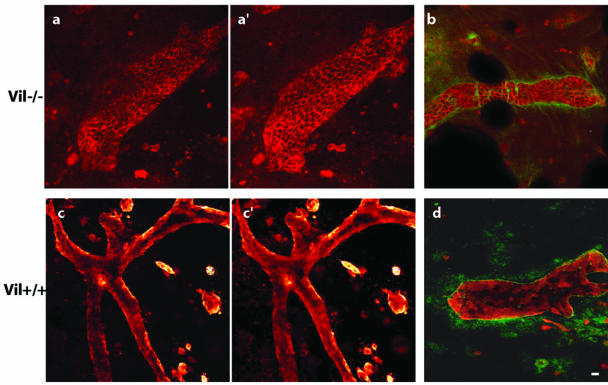
Villin Enhances HGF-induced Cell Morphogenesis To evaluate the role of villin in actin cytoskeleton remodeling, we also performed tubulogenesis assays. MDCK cells were grown in a collagen gel in the presence of 100 IU/ml HGF to promote cell tubulogenesis. After 12 d in a collagen matrix, tubulogenesis was complete in vil-/- cells. Differentiated tubules, forming a lumen (Figure 7, a and a′) and surrounded by a laminin matrix, could be observed (Figure 7b). However, numerous apoptotic figures were noticed at this stage of tubulogenesis. In contrast, vil+/+ cells showed an active tubulogenesis process as evidenced by the massive gel retraction. At 12 d, some cells were still in the process of tubulogenesis, whereas others developed large branched tubular structures with a well defined lumen (Figure 7, c and c′) and a laminin containing matrix (Figure 7d). These data show that villin-expressing cells are able to induce a very potent morphogenesis. Tubulogenesis is thus more efficient than in control cells lacking villin, as shown by the highly developed branched structures lasting >12 d. This demonstrates the ability of villin-expressing cells to reorganize the actin cytoskeleton, probably through efficient cell-to-cell and cell-to-matrix interactions acting upstream and downstream of the HGF signaling pathway.
Figure 7.
Tubulogenesis is enhanced in villin expressing MDCK cells. Immunofluorescence on collagen gels. a and a′, two confocal sections of E-cadherin labeled vil-/- tubules. b, double labeling of E cadherin and laminin. c and c′, two confocal sections of villin labeled vil+/+ tubules. d, double villin and laminin labeling. Laminin labeling is used to check for the differentiation status of the tubules. Bar, 40 μm.
How Is Villin Involved in the HGF Signaling Pathway? Because villin has been reported to interact with PLCγ upon carbachol treatment, we investigated whether HGF treatment could modulate this interaction.
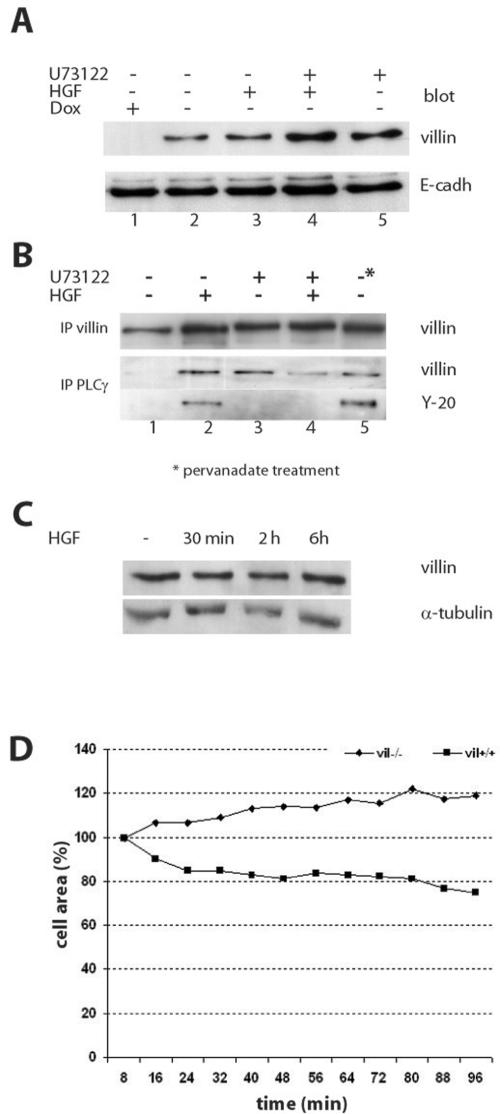
We found that HGF stimulation modulates villin distribution in Triton X-100–soluble and–insoluble fractions, leading to a 10% increase of villin in the Triton-soluble pool (our unpublished data). This observation led us to analyze whether this treatment affected villin association with the plasma membrane. We therefore studied villin distribution in membrane fractions after HGF stimulation. As shown in Figure 8A, a small pool of villin (10% of the cytosoluble pool) was present in the membrane fraction in the absence of HGF stimulation (Figure 8A, lane 2). The amount of villin was quantified by comparison with E-cadherin, used here as a membrane marker (equal protein loading, four experiments). HGF treatment for 2 h results in a 1.3-fold increase of the villin pool to the membrane fraction (Figure 8A, lane 3). PLCγ inhibition performed by U73122 treatment led to a greater increase in the amount of villin in the membrane fraction (twofold compared with 1.3-fold; Figure 8A, lane 5). Double treatment with HGF and U73122 showed the same pattern (twofold increase) (Figure 8A, lane 4). To test whether this pool of villin associated with the membrane was bound to PLCγ, we performed a coimmunoprecipitation experiment with proteins solubilized from the membrane fraction (Figure 8B). After HGF treatment, the amount of immunoprecipitated villin was increased, as shown in Figure 8B (lane 2, top). Moreover, U73122 treatment led to an accumulation of villin in the membrane fraction (lane 3, top) and that was also the case after double treatment with HGF and U73122 (lane 4, top). Using PLCγ immunoprecipitation, we observed that HGF treatment allowed PLCγ to coimmunoprecipitate with villin (Figure 8B, lane 2), suggesting that HGF promotes the association of villin with this signaling protein. Double treatment with U73122 and HGF reduced the amount of villin bound to PLCγ (lane 4), suggesting a competition for villin between PIP2, in excess at the membrane after U73122 treatment, and PLCγ. Pervanadate treatment led to an increase in the amount of villin bound to PLCγ (lane 5). Using a specific antibody against tyrosine phosphorylated proteins (anti-Y-20), we showed that the villin pool found to be associated to PLCγ after HGF stimulation was tyrosine phosphorylated (Figure 8B, lane 2, blot Y-20). The pool of villin found associated to PLCγ after U73122 treatment (Figure 8B, lanes 3 and 4) was not tyrosine phosphorylated, attesting for the specific effect of HGF stimulation. In addition, pervanadate treatment favors the binding of tyrosine-phosphorylated villin to PLCγ (Figure 8B, lane 5). These experiments show that HGF treatment promotes the association of tyrosine-phosphorylated villin to PLCγ at the plasma membrane. This interaction has been shown to activate the PLCγ pathway, leading to the hydrolysis of PIP2 and an increase in the intracellular calcium concentration that could activate the F-actin–severing activity of villin. Thus, the increase of villin in the Triton X-100–soluble pool and in the membrane fraction after PLCγ inhibition could be due to the sequestration of villin in the membrane fraction by an increased amount of PIP2.
Figure 8.
HGF stimulation induces villin association to the plasma membrane and increases its association to PLCg. (A) Villin redistribution in the membrane fraction upon HGF stimulation. This figure presents villin and E-cadherin blotting, shown as a control for equal loading. Cells were either unstimulated (lane 2), stimulated with HGF for 2 h (lane 3), treated with U73122 (10 μM) to inhibit PLCγ (lane 5), or double-treated with HGF and U73122 (lane 4). (B) Coimmunoprecipitation of villin and PLCγ in the membrane fraction. The first panel presents villin immunoprecipitation, blotted for villin. The second panel presents PLCγ immunoprecipitation blotted either for villin or for tyrosine phosphorylated proteins. The tyrosine-phosphorylated protein detected was at the molecular weight of villin. For all panels, the lanes are the following: lane 1, unstimulated cells; lane 2, after 2 h of HGF stimulation; lane 3, after U73122 (10 μM) treatment, lane 4, double treated with HGF and U73122; and lane 5, unstimulated cells but treated with pervanadate to induce protein phosphorylation. (C) Villin and α-tubulin blotting in total cell lysates before and after HGF treatment. (D) Lamellipod extension assays in presence of HGF in vil-/- and vil+/+ cells pretreated with U73122 (10 μM). Data are represented as the percentage of the initial cell area (T0).
The total amount of villin expressed was evaluated in unstimulated cells and HGF-treated cells during 30 min, 2 h, and 6 h. As shown in Figure 8C, HGF treatment does not increase the amount of total villin content with time, the amount of α-tubulin being used as a loading control. Thus, the increase of villin in the membrane fractions observed in Figure 8, A and B, is the consequence of villin redistribution rather than stimulation of protein synthesis.
We also examined whether PLCγ inhibition by U73122 affected HGF-induced cell scattering. For this purpose, vil-/- and vil+/+ MDCK cells were pretreated with U73122 for 1 h. To go further, we evaluated lamellipod extension by measuring the total cell area of MDCK cells pretreated with this inhibitor before HGF-induced scattering. Figure 8D shows that U73122 pretreatment leads to the inhibition of the villin-dependent HGF-induced cell surface extension. These data support the fact that villin induced cell motility is dependent on the PLCγ signaling pathway.
Collectively, these data suggest that villin-induced cell motility is dependent on the HGF-activated PLCγ pathway.
DISCUSSION
In this study, we examined the role of the actin-binding protein villin in the reorganization of the actin cytoskeleton elicited by extracellular signals such as HGF stimulation. We show that villin, usually described as a structural protein, is able to participate in the actin microfilament dynamics elicited by ligand-cell surface receptor interactions.
Actin filaments are a major component of eucaryotic cells. A family of related actin-binding proteins seems to be choice candidates for the control of actin-based motility processes in response to signaling (Moon and Drubin, 1995). The molecular processes required for cell motility have been characterized using simple models such as Listeria monocytogenes, and these studies have given us clues as to the minimum machinery needed for cell movement and morphogenesis (Friederich et al., 1995). Although cellular movement is a natural process in fibroblastic cells or macrophages, this complex process is not naturally performed in epithelial cells. In these specialized cells, cell motility requires an epithelium-mesenchymal transition in response to extracellular signals. This transition involves the reorganization of the actin cytoskeleton taking place downstream of activated signaling pathways. We propose that the signaling events leading to actin dynamics are mediated by proteins that are specific to the epithelial lineage considered and are therefore necessary to accomplish dynamic processes, thus increasing the efficiency of the cellular response. This adaptive process is necessary for epithelial cells to make a rapid and efficient response to extracellular signals during physiological or pathological situations without de novo protein synthesis.
This is the first report showing that villin, known to play a key role in microvillar actin assembly, is also able to play a role in the in vivo actin cycle turnover. Accordingly, villin-enhanced actin dynamics enables epithelial cells to increase actin cytoskeleton dynamics in response to extracellular stimuli such as HGF. The HGF/c-met pathway plays an important role in the mesenchymal-epithelial transition during physiological and pathological processes, such as embryogenesis (Sonnenberg et al., 1993), angiogenesis (Grant et al., 1993) and neoplasia (Bhargava et al., 1993). Treatment with HGF is known to induce cell migration in many types of cultured cells, including MDCK cells (Stoker and Gherardi, 1991), and to promote tubulogenesis (Saelman et al., 1995). Moreover, HGF-induced lamellipodia required for cell displacement are the result of important actin remodeling (Ridley et al., 1995). The signaling molecules (GTPases, phosphoinositides, and kinases) that influence the actin cytoskeleton dynamics upon HGF treatment are well known (Ridley et al., 1995; Potempa and Ridley, 1998; Royal et al., 2000). However, the role of actin-binding proteins that influence the actin dynamics remains to be investigated in cellular processes that require cell plasticity.
In our study, several cell motility assays performed in two different cellular models allowed us to show that villin-expressing cells have a twofold higher cell velocity upon HGF stimulation in comparison with cells that do not express villin. Morphogenesis experiments showed that villin-expressing cells are able to perform greater spatial and temporal tubulogenesis than cells without villin. Villin, a cell-specific, multifunctional actin-binding protein is thus recruited to promote cell plasticity through its various activities on actin filaments to enhance the basal, ubiquitous actin cytoskeleton dynamics. Our data show that villin acts as a potentiator of the HGF signaling pathway, mainly through its actin-severing property. Indeed, HGF-induced cell motility requires different features of actin dynamics in villin-expressing cells compared with cells without villin. Motility is believed to be dependent on actin polymerization at the leading edge of lamellipodia (Chan et al., 1998). However, the events that control this process remain to be specified. Polymerization requires the presence of newly created free ends that are the fast growing ends of the filaments. The generation of free barbed ends can be the result of many mechanisms: uncapping of preexisting filaments and severing or de novo nucleation of new filaments (Eddy et al., 1997). The actin dynamics is followed by further assembly and disassembly of actin monomers. We show here that villin-expressing cells exhibit a decreased F-actin content associated with an increased amount of free barbed ends at the leading edges of motile cells. This observation is consistent with the view that actin filament severing results in the generation of numerous fast growing ends, thus allowing further polymerization. Moreover, we trust that villin capping property is involved in this enhanced actin dynamics but this was not directly analyzed in this study. Capping of preexisting barbed ends can increase the amount of available free fast growing ends. Cells lacking villin show increased F-actin content upon HGF treatment without a significant increase of free barbed ends. Thus, in the absence of villin, cell motility occurs according to the treadmilling model in which the filaments polymerize at the leading edge by insertion of actin monomers. Recent studies have shown that migrating cells present a lower G-/F-actin ratio in the lamellipodia compared with nonmigrating cells (Cramer et al., 2002). This suggests that limited G-actin is available in this region and that G-actin availability is the result of filament disassembly. This is consistent with what occurs in vil-/- MDCK cells after HGF treatment and supports the idea that actin dynamics is dependent on the treadmilling process in these cells. However, in vil+/+ cells, the higher level of available G-actin is most likely to be the consequence of actin severing resulting in rapid depolymerization at the newly created pointed ends (for review, see Athman et al., 2002a).
We also investigated how villin was associated with the HGF signaling pathway. Several intracellular pathways have been described to act downstream of the HGF receptor to mediate the scatter or tubulogenesis responses. For instance, it is well known that c-met is able to directly interact with signaling proteins, including PLCγ. Activated PLCγ initiates rapid turnover of polyphosphoinositides that affect the distribution and activities of actin-modulating proteins such as capG, profilin, and gelsolin (Goldschmidt-Clermont et al., 1991; Janmey et al., 1992). Through its enzymatic activity, PLCγ cleaves PIP2 into diacylglycerol and inositol triphosphate, thus modulating the amount of PIP2 at the plasma membrane. It is now well documented that PIP2 is a regulator of many actin-binding proteins such as gelsolin. Thus, activated PLCγ initiates rapid turnover of phosphoinositides that affect the distribution of actin-modulating proteins. Khurana and collaborators first reported a clear interaction of tyrosine phoshorylated villin with PLCγ in the microvillus membrane fraction of rabbit ileum upon hormonal stimulation (Khurana et al., 1997). Papakonstanti and collaborators have shown that up-regulation of Na+/Pi cotransport stimulates PLCγ and phosphatidylinositol-3 kinase activation and the association of tyrosine phosphorylated villin to PLCγ, leading to an increase of villin actin-severing activity (Papakonstanti et al., 2000). In two recent reports, it has been demonstrated that nonphosphorylated villin binds to PIP2, leading to PLCγ activity inhibition and that phosphorylated villin binds to and activates PLCγ. Moreover, these studies have shown that in its phosphorylated form, villin-severing property is activated, whereas its bundling property is inhibited (Panebra et al., 2001; Zhai et al., 2001). These in vitro experiments suggest that PIP2 can regulate villin activities in vivo by binding to nonphosphorylated villin at the plasma membrane, thus affecting its binding to PLCγ.
To test how HGF stimulation affects villin interaction with PLCγ, we analyzed villin distribution after detergent extraction and cell fractionation. In cell cultures, upon HGF stimulation, a small pool of villin is redistributed to the Triton X-100–soluble fraction and its amount in the membrane fraction is increased. Treatment with U73122, a specific inhibitor of PLCγ leading to an increase of PIP2 at the plasma membrane, induces a greater increase of the villin pool in the membrane and Triton X-100–soluble fractions. Coimmunoprecipitation experiments on membrane fractions show that villin is associated with PLCγ and that this binding is increased after HGF stimulation. Moreover, we show that the pool of villin that is associated with PLCγ after HGF stimulation is tyrosine phosphorylated.
Together, these data allow us to propose the following model: in the absence of stimulation, a pool of villin is able to bind to PIP2 at the plasma membrane, thus inhibiting the capacity of villin to sever actin filaments. On HGF stimulation, a small pool of phosphorylated villin binds to activated PLCγ, thus increasing its PIP2-hydrolyzing activity and leading to inositol triphosphate and diacylglycerol production. The villin pool previously bound to PIP2 is released, and the villin-severing property is promoted by intracellular signals triggered by PLCγ activation, including the increase in intracellular calcium concentration. Activated villin can thus enhance the actin cytoskeleton dynamics by generating new barbed ends at the plasma membrane at the leading edge of growing lamellipodia. This model is supported by the fact that inhibition of PLCγ by U73122 treatment result in the reduction of the scattering process of villin-expressing cells.
This work provides new insights on the ability of villin to act on the actin cytoskeleton dynamics and helps our understanding of the phenotype observed when vil-/- mice are submitted to intestinal injury. Indeed, the ability of villin to reorganize actin filaments may be involved in enterocyte plasticity during stress situations such as bacterial infection. In this context, we have shown that villin plays an important role in the distinct steps of Shigella infection through its ability to remodel the actin cytoskeleton elicited during the invasion process (Athman, R., Fernandez, M-I., Gounon, P., Sansonetti, P., Louvard, D., Philpott, D., and Robine, S., unpublished data).
Acknowledgments
We are grateful to Dr. Evelyne Friederich for providing us with the PGEX-2T villin plasmid and to Véronique Collin who prepared the pUHD villin plasmid used in this study. We thank Dr. Marie-Neige Cordonnier and Jean-Baptiste Sibarita for advice in videomicroscopy; Dr. Monique Arpin for providing HGF/SF; and Drs. P. Pujuguet, J. Plastino, H. Fsihi, and K.P. Janssen for helpful comments. We also thank Prof. Manuel Buchwald for critical reading of the manuscript. This work has been supported by grants from Association dela Recherche contre le Cancer and Action Concertée Incitative.
Abbreviations used: BSA, bovine serum albumin; HBSS, Hanks' balanced salt solution; HGF, hepatocyte growth factor; MDCK, Madin-Darby canine kidney; PIP2, phosphatidylinositol 4,5-biphosphate; PI3K, phosphatidylinositol-3 kinase; PLCγ, phospholipase Cγ.
References
- Arpin, M., Pringault, E., Finidori, J., Garcia, A., Jeltsch, J.M., Vandekerckhove, J., and Louvard, D. (1988). Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J. Cell Biol. 107, 1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athman, R., Louvard, D., and Robine, S. (2002a). The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am. J. Physiol. 283, G496-G502. [DOI] [PubMed] [Google Scholar]
- Athman, R., Niewohner, J., Louvard, D., and Robine, S. (2002b). Epithelial cells: establishment of primary cultures and immortalization. In: Molecular Cellular Microbiology, vol. 31, ed. P.S.a.A. Zychlinsky, New York: Academic Press, 93-113. [Google Scholar]
- Bhargava, M.M., Li, Y., Joseph, A., Jin, L., Rosen, E.M., and Goldberg, I.D. (1993). HGF-SF: effects on motility and morphology of normal and tumor cells. EXS 65, 341-349. [PubMed] [Google Scholar]
- Boyer, B., Valles, A.M., and Thiery, J.P. (1996). Model systems of epitheliummesenchyme transitions. Acta Anat. 156, 227-239. [DOI] [PubMed] [Google Scholar]
- Bretscher, A., and Weber, K. (1980). Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20, 839-847. [DOI] [PubMed] [Google Scholar]
- Chan, A.Y., Bailly, M., Zebda, N., Segall, J.E., and Condeelis, J.S. (2000). Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J. Cell Biol. 148, 531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, A.Y., Raft, S., Bailly, M., Wyckoff, J.B., Segall, J.E., and Condeelis, J.S. (1998). EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell Sci. 111, 199-211. [DOI] [PubMed] [Google Scholar]
- Costa de Beauregard, M.A., Pringault, E., Robine, S., and Louvard, D. (1995). Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 14, 409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, L.P., Briggs, L.J., and Dawe, H.R. (2002). Use of fluorescently labelled deoxyribonuclease I to spatially measure G-actin levels in migrating and nonmigrating cells. Cell Motil. Cytoskeleton. 51, 27-38. [DOI] [PubMed] [Google Scholar]
- Dudouet, B., Robine, S., Huet, C., Sahuquillo-Merino, C., Blair, L., Coudrier, E., and Louvard, D. (1987). Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29–18 clones. J. Cell Biol. 105, 359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy, R.J., Han, J., and Condeelis, J.S. (1997). Capping protein terminates but does not initiate chemoattractant-induced actin assembly in Dictyostelium. J. Cell Biol. 139, 1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, G.S., Flint, N., Somers, A.S., Eyden, B., and Potten, C.S. (1992). The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 101, 219-231. [DOI] [PubMed] [Google Scholar]
- Ferrary, E., et al. (1999). In vivo, villin is required for Ca2+ dependent F-actin disruption in intestinal brush-borders. J. Cell Biol. 146, 819-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, E., Gouin, E., Hellio, R., Kocks, C., Cossart, P., and Louvard, D. (1995). Targeting of Listeria monocytogenes ActA protein to the plasma membrane as a tool to dissect both actin-based cell morphogenesis and ActA function. EMBO J. 14, 2731-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, E., Huet, C., Arpin, M., and Louvard, D. (1989). Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell 59, 461-475. [DOI] [PubMed] [Google Scholar]
- Friederich, E., Vancompernolle, K., Huet, C., Goethals, M., Finidori, J., Vandekerckhove, J., and Louvard, D. (1992). An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell 70, 81-92. [DOI] [PubMed] [Google Scholar]
- Friederich, E., Vancompernolle, K., Louvard, D., and Vandekerckhove, J. (1999). Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro. J. Biol. Chem. 274, 26751-26760. [DOI] [PubMed] [Google Scholar]
- Gautreau, A., Poullet, P., Louvard, D., and Arpin, M. (1999). Ezrin, a plasma membrane-microfilamentlinker,signalscellsurvivalthroughthephosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96, 7300-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, P.J., Kim, J.W., Machesky, L.M., Rhee, S.G., and Pollard, T.D. (1991). Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science 251, 1231-1233. [DOI] [PubMed] [Google Scholar]
- Gossen, M., and Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89, 5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, D.S., Kleinman, H.K., Goldberg, I.D., Bhargava, M.M., Nickoloff, B.J., Kinsella, J.L., Polverini, P., and Rosen, E.M. (1993). Scatter factor induces blood vessel formation in vivo. Proc. Natl. Acad. Sci. USA 90, 1937-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani, A., Gramaglia, D., Cantley, L.C., and Comoglio, P.M. (1991). The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J. Biol. Chem. 266, 22087-22090. [PubMed] [Google Scholar]
- Gual, P., Giordano, S., Williams, T.A., Rocchi, S., Van Obberghen, E., and Comoglio, P.M. (2000). Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene 19, 1509-1518. [DOI] [PubMed] [Google Scholar]
- Handel, S.E., Hendry, K.A., and Sheterline, P. (1990). Microinjection of covalently cross-linked actin oligomers causes disruption of existing actin filament architecture in PtK2 cells. J. Cell Sci. 97, 325-333. [DOI] [PubMed] [Google Scholar]
- Janmey, P.A., Iida, K., Yin, H.L., and Stossel, T.P. (1987). Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J. Biol. Chem. 262, 12228-12236. [PubMed] [Google Scholar]
- Janmey, P.A., Lamb, J., Allen, P.G., and Matsudaira, P.T. (1992). Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J. Biol. Chem. 267, 11818-11823. [PubMed] [Google Scholar]
- Janmey, P.A., and Matsudaira, P.T. (1988). Functional comparison of villin and gelsolin. Effects of Ca2+, KCl, and polyphosphoinositides. J. Biol. Chem. 263, 16738-16743. [PubMed] [Google Scholar]
- Khurana, S., M. Arpin, R. Patterson, and M. Donowitz. (1997). Ileal microvillar protein villin is tyrosine-phosphorylated and associates with PLC-gamma1. Role of cytoskeletal rearrangement in the carbachol-induced inhibition of ileal NaCl absorption. J. Biol. Chem. 272, 30115-30121. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski, D.J. (1999). Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr. Opin. Cell Biol. 11, 103-108. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski, D.J., and Yin, H.L. (1987). Molecular biology of gelsolin, a calcium-regulated actin filament severing protein. Biorheology 24, 643-647. [DOI] [PubMed] [Google Scholar]
- Lueck, A., Brown, D., and Kwiatkowski, D.J. (1998). The actin-binding proteins adseverin and gelsolin are both highly expressed but differentially localized in kidney and intestine. J. Cell Sci. 111, 3633-3643. [DOI] [PubMed] [Google Scholar]
- Marks, P.W., Arai, M., Bandura, J.L., and Kwiatkowski, D.J. (1998). Advillin (p92): a new member of the gelsolin/villin family of actin regulatory proteins. J. Cell Sci. 111, 2129-2136. [DOI] [PubMed] [Google Scholar]
- Mine, T., Kojima, I., Ogata, E., and Nakamura, T. (1991). Comparison of effects of HGF and EGF on cellular calcium in rat hepatocytes. Biochem. Biophys. Res. Commun. 181, 1173-1180. [DOI] [PubMed] [Google Scholar]
- Moon, A., and Drubin, D.G. (1995). The ADF/cofilin proteins: stimulus-responsive modulators of actin dynamics. Mol. Biol. Cell. 6, 1423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop, J., Weber, A., Mooseker, M.S., Franzini-Armstrong, C., Bishop, M.F., Dubyak, G.R., Tucker, M., and Walsh, T.P. (1986). Different calcium dependence of the capping and cutting activities of villin. J. Biol. Chem. 261, 9274-9281. [PubMed] [Google Scholar]
- Panebra, A., Ma, S.X., Zhai, L.W., Wang, X.T., Rhee, S.G., and Khurana, S. (2001). Regulation of phospholipase C-gamma(1) by the actin-regulatory protein villin. Am. J. Physiol. 281, C1046-C1058. [DOI] [PubMed] [Google Scholar]
- Papakonstanti, E.A., Emmanouel, D.S., Gravanis, A., and Stournaras, C. (2000). PLC-gamma1 signaling pathway and villin activation are involved in actin cytoskeleton reorganization induced by Na+/Pi cotransport up-regulation. Mol. Med. 6, 303-318. [PMC free article] [PubMed] [Google Scholar]
- Pinson, K.I., Dunbar, L., Samuelson, L., and Gumucio, D.L. (1998). Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev. Dyn. 211, 109-121. [DOI] [PubMed] [Google Scholar]
- Ponzetto, C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G., and Comoglio, P.M. (1994). A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261-271. [DOI] [PubMed] [Google Scholar]
- Potempa, S., and Ridley, A.J. (1998). Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol. Biol. Cell. 9, 2185-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., Comoglio, P.M., and Hall, A. (1995). Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15, 1110-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal, I., Lamarche-Vane, N., Lamorte, L., Kaibuchi, K., and Park, M. (2000). Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell. 11, 1709-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelman, E.U., Keely, P.J., and Santoro, S.A. (1995). Loss of MDCK cell alpha-2beta 1 integrin expression results in reduced cyst formation, failure of hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J. Cell Sci. 108, 3531-3540. [DOI] [PubMed] [Google Scholar]
- Sonnenberg, E., Meyer, D., Weidner, K.M., and Birchmeier, C. (1993). Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 123, 223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick, F.S., and DiNubile, M.J. (1986). Rabbit alveolar macrophages contain a Ca2+-sensitive, 41, 000-dalton protein which reversibly blocks the “barbed” ends of actin filaments but does not sever them. J. Biol. Chem. 261, 14191-14195. [PubMed] [Google Scholar]
- Stoker, M., and Gherardi, E. (1991). Regulation of cell movement: the motogenic cytokines. Biochim. Biophys. Acta 1072, 81-102. [DOI] [PubMed] [Google Scholar]
- Weiser, M.M. (1973). Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J. Biol. Chem. 248, 2536-2541. [PubMed] [Google Scholar]
- Wulfkuhle, J.D., Donina, I.E., Stark, N.H., Pope, R.K., Pestonjamasp, K.N., Niswonger, M.L., and Luna, E.J. (1999). Domain analysis of supervillin, an F-actin bundling plasma membrane protein with functional nuclear localization signals. J. Cell Sci. 112, 2125-2136. [DOI] [PubMed] [Google Scholar]
- Zhai, L., Zhao, P., Panebra, A., Guerrerio, A.L., and Khurana, S. (2001). Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J. Biol. Chem. 276, 36163-36167. [DOI] [PubMed] [Google Scholar]