Abstract
Neuritic extension is the resultant of two vectorial processes: outgrowth and retraction. Whereas myosin IIB is required for neurite outgrowth, retraction is driven by a motor whose identity has remained unknown until now. Preformed neurites in mouse Neuro-2A neuroblastoma cells undergo immediate retraction when exposed to isoform-specific antisense oligonucleotides that suppress myosin IIB expression, ruling out myosin IIB as the retraction motor. When cells were preincubated with antisense oligonucleotides targeting myosin IIA, simultaneous or subsequent addition of myosin IIB antisense oligonucleotides did not elicit neurite retraction, both outgrowth and retraction being curtailed. Even during simultaneous application of antisense oligonucleotides against both myosin isoforms, lamellipodial spreading continued despite the complete inhibition of neurite extension, indicating an uncoupling of lamellipodial dynamics from movement of the neurite. Significantly, lysophosphatidate- or thrombin-induced neurite retraction was blocked not only by the Rho-kinase inhibitor Y27632 but also by antisense oligonucleotides targeting myosin IIA. Control oligonucleotides or antisense oligonucleotides targeting myosin IIB had no effect. In contrast, Y27632 did not inhibit outgrowth, a myosin IIB-dependent process. We conclude that the conventional myosin motor, myosin IIA, drives neurite retraction.
INTRODUCTION
Neuronal growth cone motility is intrinsic to the formation of the mammalian central and peripheral nervous systems during development (Letourneau et al., 1991; Gordon-Weeks, 2000). Growth cone advance occurs through adhesive interactions with the surrounding extracellular matrix in response to external guidance cues; such movement is the result of two alternating processes, outgrowth and retraction, necessary to achieve net protrusive advance (Kater and Guthrie, 1990; Letourneau et al., 1991; Gordon-Weeks, 2000). Elaboration and subsequent disassembly of focal contacts at the leading edge of the growth cone in response to Rac- and Rho-dependent signaling pathways (Hall, 1998; Luo, 2000) brings about changes in trajectory. Such regulatory cascades target the activity of molecular motors and the arrangement of the actin cytoskeleton, which determine growth cone directionality.
The neuronal growth cone comprises two very distinct regions (Bridgman and Dailey, 1989; Lewis and Bridgman, 1992): a relatively massive central domain (C), containing the bulk of the actin cytoskeleton in which actin is arranged as a three-dimensional meshwork, and a thin peripheral region (P), encompassing the lamellipodium and filopodia. Filamentous actin within the P domain originates at the leading edge and is propelled backwards by retrograde actin flow (Lin and Forscher, 1995). Lamellipodial protrusion, as distinct from neuritic process outgrowth, is a dynamic activity localized entirely within the P domain; it is driven by dendritic nucleation under the control of Arp 2/3 and does not necessarily require myosin action (Pantalini et al., 1999; Borisy and Svitkina, 2000), although myosin participation is not excluded (Wang et al., 1996; Diefenbach et al., 2002). However, much greater forces are required to achieve neuritic process dynamics and these forces operate within the C domain and at its periphery with the P domain to move the bulk of the growth cone cytoskeleton. Actin microfilaments constitute the bulk of the cytoskeleton in this region (Chang and Goldman, 1973; Letourneau, 1981; Lewis and Bridgman, 1992), and consequently, actin-based motors are likely to be responsible for fundamental aspects of growth cone movement.
The myosin superfamily comprises at least 18 separate classes of molecule (Berg et al., 2001). Members of the “conventional” class II myosins (Kuczmarski and Rosenbaum, 1978; Cheng et al., 1992; Miller et al., 1992; Li et al., 1994; Rochlin et al., 1995; Ruchhoeft and Harris, 1997) as well as isoforms of “uncoventional” myosins of classes I (Li and Chantler, 1992; Miller et al., 1992; Wagner et al., 1992; Lewis and Bridgman, 1996; Wang et al., 1996), V (Espreafico et al., 1992; Wang et al., 1996; Coling et al., 1997), VI (Suter et al., 2000), and X (Berg and Cheney, 2002) have been found in neuronal growth cones. It would seem likely that these different isoforms perform distinct tasks during the normal functioning of the cell. However, although myosin V (Wang et al., 1996) and myosin 1c (Diefenbach et al., 2002) have been implicated in lamellipodial dynamics, and myosin X in intrafilopodial motility, it would seem that the forces responsible for neurite extension are generated by conventional myosin isoforms (Wylie et al., 1998; Bridgman et al., 2001; Wylie and Chantler, 2001).
At least two different isoforms of conventional myosin, expressed from separate genes (A and B), are found in neuronal cells (Katsuragawa et al., 1989; Kawamoto and Adelstein, 1991; Simons et al., 1991), myosin IIB being the predominate isoform (Katsuragawa et al., 1989; Kawamoto and Adelstein, 1991; Miller et al., 1992; Itoh and Adelstein, 1995; Rochlin et al., 1995). These isoforms exhibit differential localization within the growth cone; myosin IIB, found in the P-domain and adjacent to the C-domain, exhibits a more peripheral distribution than myosin IIA, which is located mainly within the C-domain and does not extend into the marginal zone (Rochlin et al., 1995). Isoform sorting of these conventional myosins may occur by mechanisms intrinsic to their structures (Kolega, 1998). Using antisense knock-down and transgenic knock-out approaches, respectively, we (Wylie et al., 1998) and others (Bridgman et al., 2001) have identified myosin IIB as the molecular motor responsible for neurite outgrowth. We have also shown that myosin IIA, although not directly involved in outgrowth per se, is required for assembly of focal contacts that provide adhesion (Wylie and Chantler, 2001), against which the forward propulsive force acts (Lamoureux et al., 1989). If myosin IIB drives outgrowth and myosin IIA is responsible for tension maintenance during adhesion, it remains to determine what kind of motor is responsible for driving neurite retraction. Here, using an isoform-specific functional knock-down approach combined with pharmacological targeting, we show that myosin IIA, but not myosin IIB, drives neurite retraction.
MATERIALS AND METHODS
Cell Culture and Cytochemistry
Neuro-2A cells were cultured as described previously (Wylie et al., 1998; Wylie and Chantler, 2001). Cytochemistry was preceded by a brief extraction step (Cramer and Mitchison, 1995) in cytoskeletal buffer (10 mM MES pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA) supplemented with 0.32 M sucrose, 0.1% Triton X-100, and 1 μg/ml phalloidin, followed by fixation for 30 min in cytoskeletal buffer supplemented with 0.32 M sucrose and 4% formaldehyde, as described previously (Wylie and Chantler, 2001). Cells were permeabilized for 10 min in PBS plus 0.5% Triton X-100, rinsed in phosphate-buffered saline (PBS), and then immersed in a blocking solution comprising 2% decomplemented horse serum in PBS for 20 min. Incubation with primary and secondary antibodies was performed as described previously (Wylie and Chantler, 2001). Nonmuscle myosin IIA was detected by indirect immunofluorescence using polyclonal rabbit anti-nonmuscle (rat) myosin IIA as primary antibody (Choi et al., 1996) (1:300 dilution) and Alexa-Fluor 633-conjugated goat antirabbit IgG (Molecular Probes, Eugene, OR) (1:100 dilution) as secondary antibody. Paxillin was detected by direct immunofluorescence using a fluorescein isothiocyanate-conjugated monoclonal mouse anti-paxillin IgG (BD Biosciences, San Jose, CA) (1:100 dilution)(Leventhal and Feldman, 1996). Filamentous actin was detected using Texas Red phalloidin (Molecular Probes) added at a concentration of 165 nM. All antibody dilutions were made up in 1% decomplemented horse serum/PBS including 0.1% Triton X-100/1.5 mM sodium azide. Coverslips were mounted using MOWIOL (Calbiochem, San Diego, CA)/glycerol [25% (vol/vol)]/50 mM Tris pH 8.5/1,4-diazabicyclo[2-2-2]octane (DABCO) (Sigma) [2.5% (wt/vol)]/3 mM sodium azide.
Antisense Treatment Protocols
Sequences of the isoform-specific antisense and sense oligonucleotides used in these studies, together with the scrambled (control) oligonucleotides used, are displayed in Table 1. For myosin IIA, sense oligonucleotides (AQ5) were synthesized to correspond to a region of isoform-specific sequence located toward the 5′ end of the transcript, where it normally codes for the amino acid sequence NPILEA (accession no. U31463; Choi et al., 1996). The antisense oligonucleotide sequence (AQ3) was the inverse complement of AQ5. For myosin IIB, sense oligonucleotides (BQ5) were synthesized to correspond to a region of isoform-specific sequence within the 5′ coding region of the myosin IIB transcript that codes for the amino acid sequence ADPILES (accession no. U15766; Itoh and Adelstein, 1995). The antisense oligonucleotide sequence (BQ3) was the inverse complement of BQ5. Scrambled (control) oligonucleotides corresponded in base composition to those of the antisense oligonucleotides except that the sequence was scrambled and did not match any other entry in the GenBank or European Molecular Biology Laboratory databases.
Table 1.
List of oligonucleotides used during the treatment of Neuro-2A cells in the experiments reported here
| Oligo | Isoform | Sequence | Direction and location | Species | Accession no. |
|---|---|---|---|---|---|
| AQ5 | MHCIIa | 5′-caaccctatcctagaggcct-3′ | Sense, 5′-coding | Rat | U31463 |
| AQ3 | MHCIIa | 5′-aggcctctaggatagggttg-3′ | Antisense, 5′-coding | Rat | U31463 |
| AQ3R | * | 5′-ctaagtgactagtggtgggc-3′ | AQ3 scrambled | * | * |
| BQ5 | MHCIIb | 5′-gcagatccaattctggaatca-3′ | Sense, 5′-coding | Rat | U15766 |
| BQ3 | MHCIIb | 5′-tgattccagaattggatctgc-3′ | Antisense, 5′-coding | Rat | U15766 |
| BQ3R | * | 5′-ggctacgatgacagctatttt-3′ | BQ3 scrambled | * | * |
Shown are the oligonucleotide code name, myosin isoform, oligonucleotide sequence, direction and location of the sequence, the species from which the sequence was derived and the database accession number. *, asterisks signify that the scrambled antisense control does not correspond to any known sequence in the databanks.
Neuro-2A cells in culture were transferred to serum-free media and treated separately, or in combination, with sense, antisense, or scrambled oligonucleotides derived from myosin IIA or myosin IIB sequences. Initial incubations took place with 50 μM oligonucleotides, whereas subsequent additions, every 12 h, were at 25 μM (Wylie et al., 1998). At appropriate times, cells were fixed then examined by differential interference contrast (DIC) microscopy or stained with antibodies before viewing by confocal laser scanning microscopy. Where performed, reverse transcription-polymerase chain reaction was carried out as described previously (Wylie et al., 1998; Wylie and Chantler, 2001).
Confocal Laser Scanning and DIC Microscopy
Confocal fluorescence microscopy was performed, as described previously (Wylie and Chantler, 2001), by using an LSM 510 confocal laser scanning microscope (Carl Zeiss, Jena, Germany) equipped with a 40× Fluar oil immersion objective (numerical aperture 1.3), argon (λex = 488 nm), and He-Ne (λex = 543 nm) lasers. Neurite outgrowth was monitored through DIC microscopy using an Axiovert 135 inverted microscope (Carl Zeiss). Images were digitized before neurite length measurement using Kontron 300 software. Statistical analysis was performed as described previously (Wylie et al., 1998; Wylie and Chantler, 2001).
Application of Lysophosphatidate (LPA), Thrombin, and Y27632: Serial Measures on Individual Neurites
LPA (1 μM; Sigma-Aldrich, St. Louis, MO) and thrombin (5 NIH units [75 μg/ml]; Sigma-Aldrich) were freshly prepared in media from appropriately diluted stock solutions and added as a 20-μl pulse to the cultured cells, in a preselected quadrant of the coverslip under observation (Jalink and Moolenaar, 1992; Suidan et al., 1992), initiating retraction. In those experiments where the Rho-kinase inhibitor Y27632 (50 μM; Calbiochem) was required, it was added 30 min before the addition of either LPA or thrombin.
For serial measures, the same patch of cells was monitored using DIC optics from the start of the timed series, digitized images (Kontron KS-300) being stored at the following intervals: 0, 2, 5, 10, 15, 30, 60, and 90 min. The microscope stage was maintained at 34-39°C throughout these measurements. Cultures were returned to the incubator, as appropriate, between observations. Both the stage micromanipulator (Axiovert 135 inverted microscope; Carl Zeiss), and the Kontron image display, assisted in the accurate localization of the cells at each time point.
RESULTS
Antisense Oligonucleotides Targeting Myosin IIB Elicit Neurite Retraction in Cultured Neuro-2A Cells
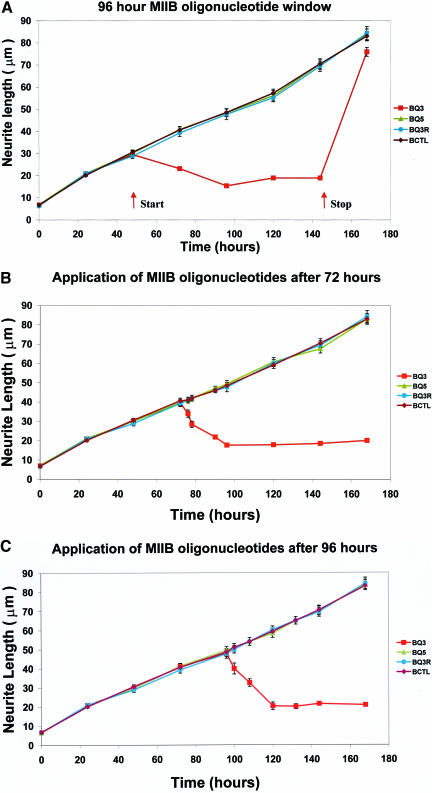
Previously, we had demonstrated that continual treatment of Neuro-2A cells with antisense oligonucleotides targeting myosin IIB transcripts attenuates neurite outgrowth (Wylie et al., 1998). Here, we have applied a 96-h myosin IIB oligonucleotide treatment window to cells that had already been allowed to extend processes for various times up to 120 h. An underlying retractive process was uncovered upon application of the myosin IIB antisense treatment window (Figure 1), whereas sense, scrambled, or untreated controls had no effect. Process retraction eventually reached a minimum length of around 20 μm, whereupon further retraction was halted. The effects of the antisense regime were reversible, evident when the medium was replaced with one lacking oligonucleotides after a 96-h antisense treatment subsequent to unhindered outgrowth for 48 h (Figure 1a). When this underlying retractive process was examined with greater temporal precision by following individual cells and taking measurements at 6-8 hourly intervals after the application of myosin IIB oligonucleotides at either 72 h (Figure 1b) or 96 h (Figure 1c) after initiation of outgrowth, the actual rate of retraction could be measured. It was found to be relatively rapid, 50% of complete retraction ensuing within ∼8 h (i.e., 1-2 μm/h) (Figure 1, b and c).
Figure 1.
Application of myosin IIB oligonucleotide treatment at various times after initiation of neurite outgrowth. These experiments examine the effect of myosin IIB antisense treatment windows on neurite outgrowth at different times of onset after plating: 48 h (a), 72 h (b), and 96 h (c). (a) Application of a 96-h duration myosin IIB oligonucleotide treatment window, beginning at 48 h; oligonucleotides were removed and replaced by oligonucleotide-free media at 144 h. Arrows indicate the start and termination times of the oligonucleotide treatment and illustrate the reversibility of the process by showing that outgrowth can be restored. Measurements are plotted as mean lengths and SEMs for at least 100 neurites per data point (range 163-847 neurites). (b and c) Measurement of the rate of neurite retraction after induction by myosin IIB antisense oligonucleotide treatment at various times after initiation of neurite outgrowth. To measure the initial rate of retraction, myosin IIB antisense oligonucleotides were applied 72 (b) or 96 (c) hours after initiation of neurite outgrowth and measurements of neurite length were followed over time intervals of 6-8 h during the first 24 h after oligonucleotide application. As in a, measurements are plotted as mean lengths and SEMs for at least 100 neurites per data point. Ranges were 308-537 neurites (72 h) and 240-421 neurites (96 h). Symbols for sense (BQ5), antisense (BQ3), and scrambled (BQ3R) treated cells, or untreated (BCTL) control cells, are shown in the side panels. Note the progressive retraction of neuritic process length, regardless of initial neurite length, at the start of each treatment window. Also note that retraction stalls at a minimum value of ∼15-20 μm, below which it does not proceed further.
Antisense Oligonucleotides Targeting Both Myosin IIA and Myosin IIB Prevent Neurite Outgrowth and Retraction in Cultured Neuro-2A Cells
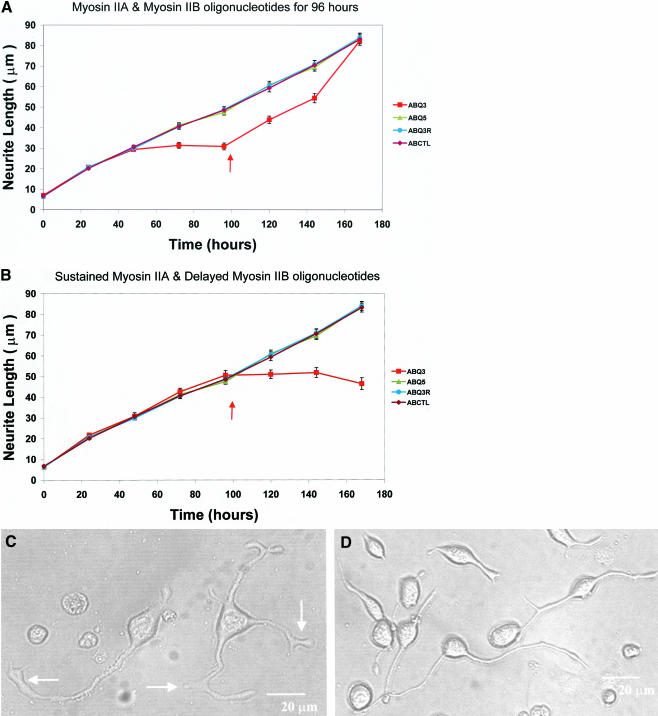
After an initial delay, required for the antisense treatment to take full effect, simultaneous application of both myosin IIA and myosin IIB antisense oligonucleotides to Neuro-2A cells at the time of plating was found not only to suppress neurite extension as expected but also to prevent retraction (Figure 2a). The recovery phase subsequent to oligonucleotide removal was significantly delayed relative to that of cells treated with myosin IIB antisense alone (Wylie et al., 1998). If myosin IIA antisense oligonucleotides were provided continuously from the time of plating and myosin IIB antisense oligonucleotides were added only 96 h later, neurite retraction in the remaining adherent cells (Wylie and Chantler, 2001) was virtually abolished (Figure 2b). This contrasts with the behavior of cells given myosin IIB antisense treatment alone (Figure 1).
Figure 2.
Combined application of oligonucleotides targeting both myosin IIA and myosin IIB to cultured Neuro-2A cells. These experiments examine the effect of a combined treatment of myosin IIA and myosin IIB oligonucleotides on neurite outgrowth. (a) Myosin IIA and myosin IIB oligonucleotides were provided together for 96 h from the time of plating. (b) Myosin IIA oligonucleotides alone were provided for 96 h from the time of plating and then myosin IIB and myosin IIA oligonucleotides were provided simultaneously during the subsequent 96 h. Mean lengths and SEMs are plotted using at least 100 neurites per individual data point (range 100-453 neurites). Arrows indicate either initiation of the recovery phase through replenishment with oligonucleotide-free media (a) or the addition of myosin IIB oligonucleotide treatment (b). Symbols denoting the use of combined myosin IIA and myosin IIB sense (ABQ5), antisense (ABQ3), and scrambled (ABQ3R) treated cells, or untreated (ABCTL) control cells, are defined in the side panels. Note that simultaneous exposure to double antisense oligonucleotides from time zero to 96 h (a) suppresses neurite outgrowth to a similar extent to that observed with IIB antisense alone (Wylie et al., 1998); however, the recovery phase after cessation of treatment is prolonged. When cells are first exposed to myosin IIA antisense for 96 h before myosin IIB antisense exposure (b), both neurite outgrowth and retraction are attenuated. DIC images of Neuro-2A cells treated for 96 h with antisense oligonucleotides targeting both myosin IIA and myosin IIB (c) are shown alongside untreated control cells observed in parallel wells (d). Note that although both neurite outgrowth and retraction are inhibited (a and b) in the cells shown in c, lamellipodial outgrowth continues to occur giving rise to aberrant, extended growth cones. This emphasizes that local lamellipodial dynamics are mechanistically different from the overall mass dynamics of neurite extension. Arrows (c) indicate abnormal club-like lamellipodia. Bars, 20 μm.
Note that there is an alteration in phenotype of the double antisense-treated cells compared with their untreated controls (Figure 2, c and d). Whereas both neurite outgrowth and retraction were attenuated in these cells, lamellipodial outgrowth continued, leading to aberrant, expansive, and veil-like processes. This underlines the fact that, to a first approximation, lamellipodial dynamics and neurite outgrowth operate by different mechanisms.
Effect of Antisense Oligonucleotides Targeting Myosin IIA on the Neuro-2A Cytoskeleton
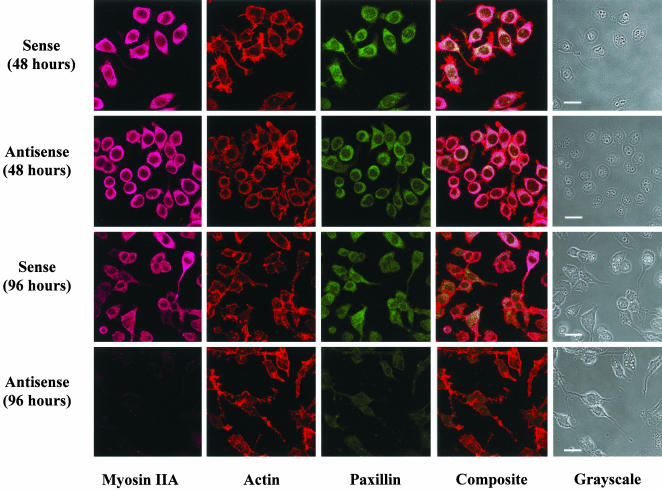
Because our previous results had shown that suppression of myosin IIA for 96 h could be disruptive to the actin cytoskeleton (Wylie and Chantler, 2001), we were concerned that the effects of myosin IIA antisense on retraction might be secondary to such induced disarray. We therefore decided to generate retractive responses to agonists applied after a relatively brief incubation period with myosin IIA oligonucleotides. To observe whether a 48-h myosin IIA antisense pretreatment was disruptive to the cell cytoskeleton, we compared the actin cytoskeleton of sense-treated and antisense-treated cells after 48 or 96 h by using rhodaminephalloidin staining (Figure 3). No differences could be discerned between the staining patterns of sense-treated cells at either 24 h (our unpublished data) or 48 (Figure 3) h with those of antisense-treated cells at similar times. Disruption of the actin cytoskeleton and attenuation of myosin IIA and paxillin immunofluorescence were observed at 96 h, consistent with previous observations (Wylie and Chantler, 2001).
Figure 3.
Brief treatment of Neuro-2A cells with antisense oligonucleotides targeting myosin IIA does not bring about extensive disruption of the actin cytoskeleton. Neuro-2A cells were treated for either 48 or 96 h with sense or antisense oligonucleotides targeting myosin IIA, as indicated. Disruption to the actin cytoskeleton, observed at 96 h simultaneous with the ablation of myosin IIA and paxillin expression, was not discernible after 48 h treatment with myosin IIA antisense oligonucleotides. Images obtained by confocal microscopy. Filamentous actin detected by rhodamine phalloidin staining. Myosin IIA detected by indirect immunofluorescence (Alexa-Fluor 633). Paxillin detected by direct immunofluorescence (fluorescein isothiocyanate). Bar, 20 μm.
LPA-induced Retraction of Neuritic Processes in Cultured Neuro-2A Cells Is Inhibited by Antisense Oligonucleotides Targeting Myosin IIA but Not Those Targeting Myosin IIB
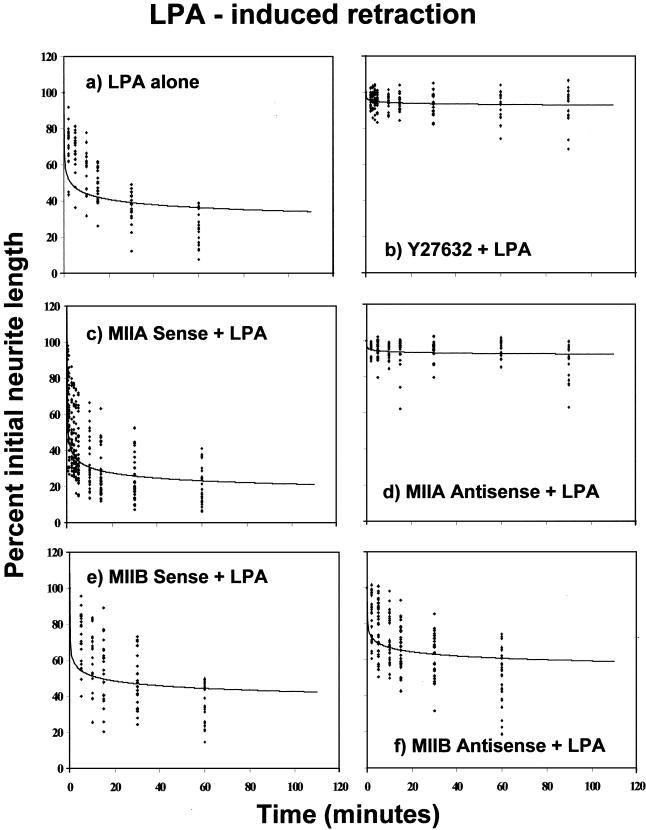
To examine whether LPA was able to induce neurite retraction in Neuro-2A cells, as has been observed in other cells of neuronal origin (Tigyi and Miledi, 1992; Jalink et al., 1993), Neuro-2A cells were grown in culture for 72 h before addition of LPA (1 μM). This pulse of LPA induced rapid process retraction that was virtually complete within 30 min (Figure 4a). This effect could be blocked entirely by prior addition of the Rho-kinase inhibitor Y27632 (25 μM), 30 min before LPA application (Figure 4b).
Figure 4.
Induction of Neuro-2A cell neurite retraction by LPA, after pretreatment with the Rho-kinase inhibitor Y27632 or with oligonucleotides targeting either myosin IIA or myosin IIB. Neuro-2A cells, cultured for 72-120 h in serum-free medium to induce significant neurite outgrowth, were treated with a pulse of LPA (1 μM) either alone (a), or preceded by 30-min incubation with Y27632 (25 μM) (b), 48-h incubation with sense (c) or antisense (d) oligonucleotides directed against myosin IIA sequence, or 18-h incubation with sense (e) or antisense (f) oligonucleotides directed against myosin IIB sequence. In the case of the myosin IIB oligonucleotides treatments, exposure was kept to the minimum necessary to ensure an effect, so that myosin IIB antisense-induced retraction did not eliminate neurites before LPA treatment. At each time point, taken over a 60-min period after LPA addition, the lengths of the same 20 neurites were measured and plotted. For illustrative purposes, data points were fitted using a polynomial equation of the form y = Ax-B; curve-fitting to a polynomial provided a better fit by eye than single- or multiexponential fits. Values for A and B, respectively, were 56.451, 0.1094 (a); 95.847, 0.0074 (b); 44.130, 0.1593 (c); 95.431, 0.0072 (d); 61.688, 0.0818 (e); and 75.235, 0.0529 (f).
LPA-induced retraction was also blocked through preincubation, for 48 h, with antisense oligonucleotides targeting myosin IIA (Figure 4d). Pretreatment with control sense (Figure 4c) or scrambled (our unpublished data) oligonucleotides targeting myosin IIA had no effect on LPA-induced retraction. In contrast, pretreatment with antisense oligonucleotides directed against myosin IIB sequence for a period of 18 h before agonist addition (sufficient time to ensure that at least 60% of the initial neurite length remained in place for LPA challenge) had very little inhibitory effect on LPA-induced retraction (Figure 4f). Here, process withdrawal was ∼80% of that seen in the presence of myosin IIB sense (Figure 4e) or scrambled (our unpublished data) oligonucleotides.
Antisense Oligonucleotides Targeting Myosin IIA but Not Those Targeting Myosin IIB Inhibit Thrombin-induced Retraction of Neuritic Processes in Cultured Neuro-2A Cells
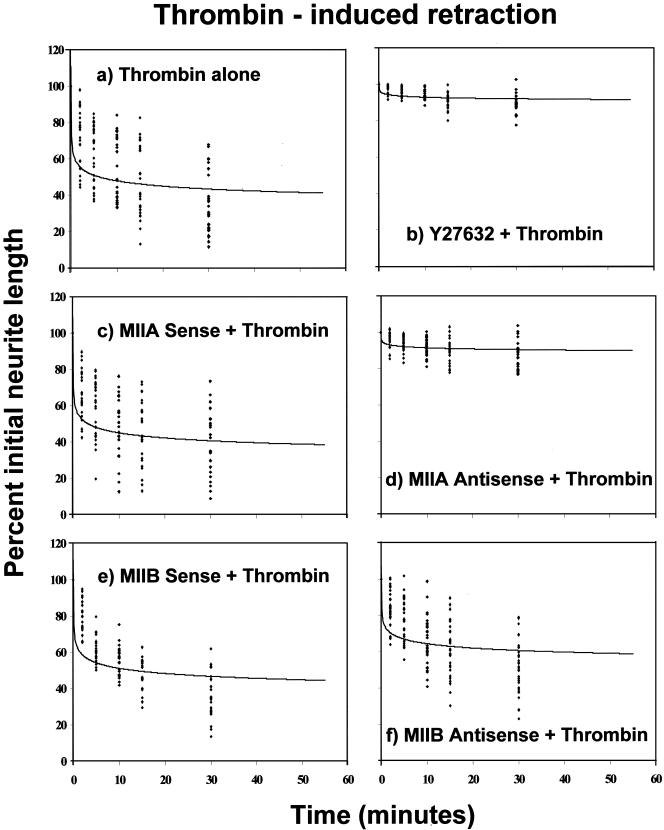
We also determined that thrombin could induce neurite retraction in Neuro-2A cells, as has been observed in other cells of neuronal origin (Jalink and Moolenaar, 1992; Suidan et al., 1992). Thrombin (pulse optimized at a concentration of 5.0 NIH units/ml, as determined from a dose-response curve) caused immediate retraction of preformed neurites (Figure 5a). This could be blocked completely by Y27632 (25 μM) (Figure 5b).
Figure 5.
Induction of Neuro-2A cell neurite retraction by thrombin addition, after pretreatment with the Rho-kinase inhibitor Y27632, or with oligonucleotides targeting either myosin IIA or myosin IIB. Neuro-2A cells, cultured for 72-120 h in serum-free medium to induce significant neurite outgrowth, were treated with a pulse of thrombin (5 NIH units) either alone (a) or preceded by 30-min incubation with Y27632 (25 μM) (b); 48-h incubation with sense (c) or antisense (d) oligonucleotides directed against myosin IIA sequence; or 18-h incubation with sense (e) or antisense (f) oligonucleotides directed against myosin IIB sequence. Comments provided in the legend for the LPA experiments are also pertinent here. Data points were fitted using a polynomial equation of the form y = Ax-B. Values for A and B, respectively, were 58.830, 0.0917 (a); 94.776, 0.0096 (b); 55.926, 0.0962 (c); 93.731, 0.0113 (d); 61.957, 0.0847 (e); and 73.032, 0.0558 (f).
Whereas pretreatment with sense (Figure 5c) or scrambled (our unpublished data) oligonucleotides derived from myosin IIA sequences had no effect, a 48-h pretreatment with antisense oligonucleotides targeting myosin IIA again suppressed thrombin-induced retraction (Figure 5d) similar to results obtained with LPA. In contrast, antisense oligonucleotides targeting myosin IIB sequence had only a minimal effect on thrombin-induced retraction (Figure 5f), process withdrawal reaching ∼80% of that observed in the presence of sense (Figure 5e) or scrambled (our unpublished data) control oligonucleotide levels.
Y27632 Does Not Inhibit Neurite Outgrowth
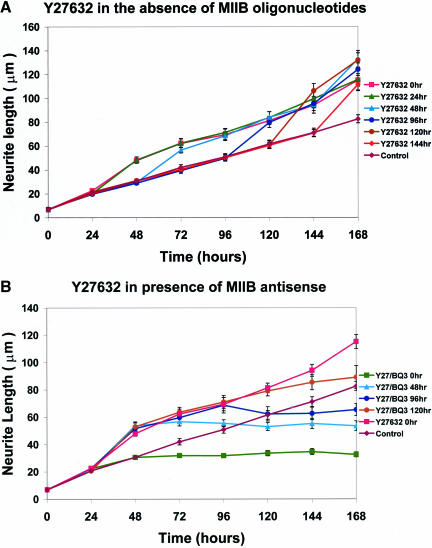
Having used Y27632 to inhibit neurite retraction (Figures 4 and 5), we also examined whether this Rho kinase inhibitor had any effect on neurite outgrowth. Application of Y27632 to Neuro-2A cells at various times after plating demonstrated no inhibition of neurite outgrowth (Figure 6a). Indeed, the rate of outgrowth was somewhat enhanced. In a separate series of experiments, Y27632 was applied continuously from the time of plating then antisense oligonucleotides directed against myosin IIB were added at later times (Figure 6b). Under these conditions, myosin IIB antisense oligonucleotides remained effective at halting outgrowth, irrespective of the time of application. These results further suggest that Y27632 and myosin IIB antisense oligonucleotides interfere with separate pathways and thereby imply that retraction and outgrowth, respectively, involve distinctive molecular motors regulated by separate pathways.
Figure 6.
Y27632 neither inhibits neurite outgrowth from Neuro-2A cells nor antagonizes attenuation of outgrowth by antisense oligonucleotides that target myosin IIB. (a) Neuro-2A cells were treated with Y27632 at various times (0, 24, 48, 96, 120, and 144 h) after plating. Note that no inhibition of outgrowth is observed irrespective of the time of Y27632 administration; rather, a small but significant increase in the rate of outgrowth is seen during times subsequent to Y27632 application. (b) Neuro-2A cells were treated with Y27632 continuously from the time of plating. At various times (0, 48, 96, and 120 h), antisense oligonucleotides targeting myosin IIB were administered and continuously applied. Note that attenuation of neurite outgrowth occurred subsequent to application of the myosin IIB oligonucleotides, and this followed a familiar time course (Figure 2) even in the continued presence of Y27632. Mean neurite lengths and SEMs are plotted for at least 100 neurites per data point (ranges 100-165 neurites in a and 100-218 neurites in b). Symbols used to illustrate the various treatments are defined in the side panels.
DISCUSSION
The ability of neuronal cells to project long processes, with lengths up to several thousand times the diameter of a cell body, has engaged the interests of cell biologists since the first observations by Ramón y Cajal at the end of the nineteenth century. Growth cones located at the tips of neuritic processes control the extension of these protrusions in vivo, which lengthen in response to a variety of external cues, integrating activity-dependent growth with attractive or inhibitory signals that arise from the extracellular matrix and synaptic targets (Kater and Guthrie, 1990; Letourneau et al., 1991; Gordon-Weeks, 2000; Luo, 2002). Net growth evolves from a balance of forces, the mechanism involving alternation between periods of outgrowth and retraction, attachment and detachment. Each of these operational modes is presumably determined by the actions of one or more molecular motors, the activities of which must be coordinated to generate a resultant displacement in the required direction. We have shown previously that the molecular motors, myosin IIB and myosin IIA, undertake different roles in this process: myosin IIB drives outgrowth (Wylie et al., 1998), whereas myosin IIA maintains tensile adhesion (Wylie and Chantler, 2001). Here, we show that myosin IIA also drives neurite retraction.
By introducing isoform-specific myosin IIB oligonucleotides at different times after initiation of neurite outgrowth rather than continuously from the time of plating, as described previously (Wylie et al., 1998), we were able to observe an underlying process of continual neurite retraction (Figure 1). This retraction is, in turn, inhibited by antisense nucleotides directed against myosin IIA, which is thus the agent of the retraction process. Most striking, our observations are consistent with a constitutive retraction process that requires myosin IIA but is independent of myosin IIB. It is of interest to note that retraction is never complete, but ceases at neurite lengths ∼10-20 μm from the cell body (Figure 1). This limit may represent a differentiative boundary below which committed neurites can no longer shorten, an interpretation consistent with different roles for upstream regulators during neurite initiation compared with neurite elongation (Sebok et al., 1999).
Initial outgrowth up to ∼20 μm occurs in the presence of myosin IIB antisense oligonucleotides (Wylie et al., 1998), even when antisense oligonucleotides against both myosin IIA and myosin IIB are present from the time of plating (Figure 2a). Once this minimal length is attained, however, both antisense actions become effective so that outgrowth and retraction are equally constrained. Similarly, continued outgrowth of adherent cells in the chronic presence of antisense oligonucleotides targeting myosin IIA is curtailed upon addition of myosin IIB antisense oligonucleotides (Figure 2b), but retraction does not supervene. These results (Figure 2, a and b), together with data from the myosin IIB antisense window experiments (Figure 1), suggest that myosin IIA is important for neurite retraction. Despite the complete cessation of neurite outgrowth and retraction that accompanies treatment with both antisense reagents, lamellipodial expansion continued, yielding aberrant club-like structures (Figure 2, c and d). Thus, the dynamics of neurite extension (Wylie et al., 1998; Bridgman et al., 2001; Wylie and Chantler, 2001) and lamellipodial protrusion (Rochlin et al., 1995; Wang et al., 1996; Diefenbach et al., 2002) are, by implication, controlled by separate mechanisms.
To exclude the possibility that myosin IIA antisense effects arise from disruption of the actin cytoskeleton, we used LPA or thrombin to induce rapid neurite retraction in Neuro-2A cells that had been incubated with oligonucleotides for too brief a time to cause cytoskeletal disruption (Figures 3, 4, 5). Observation of the actin cytoskeleton by confocal microscopy did not identify any disturbance of the cytoarchitecture as a consequence of the relatively brief (48-h) incubation with myosin IIA antisense oligonucleotides (Figure 3). LPA and thrombin bind to different cognate heterotrimeric Ras family G-protein-coupled receptors; in either case binding engenders Rho activation, growth cone collapse and neurite retraction (Jalink et al., 1994; Tigyi et al., 1996; Katoh et al., 1998b). LPA-induced retraction of neurites from N1E-115 cells has been shown to be inhibited by Y27632 (Hirose et al., 1998), itself a specific inhibitor of Rho-kinase (also known as p160ROCK or ROKα). Rho-kinase is also known to induce neurite retraction (Hirose et al., 1998; Katoh et al., 1998a) and its action involves myosin II activation (Amano et al., 1998). Rho kinase, activated by Rho, phosphorylates and thus inhibits myosin light chain phosphatase, leading to the persistence of myosin II-actin interaction. Y27632 inhibits the action of Rho-kinase; phosphatase activity is consequently preserved and the myosin regulatory light-chain is dephosphorylated, thereby terminating cross-bridge cycling.
Our data show that both LPA- and thrombin-induced Neuro-2A cell neurite retraction, which reaches completion within 30 min of addition of agonist, can be inhibited by prior exposure to antisense oligonuclotides directed against myosin IIA transcripts (Figures 4 and 5). Inhibition is essentially complete (Figures 4d and 5d), as also seen when Y27632 alone is added (Figures 4b and 5b). Most importantly, antisense-dependent inhibition is isoform-specific: oligonucleotides that suppress myosin IIB transcripts have a minimal, albeit finite, effect on neurite retraction irrespective of whether growth cone collapse is induced by LPA (Figure 4f) or by thrombin (Figure 5f).
Two possible interpretations arise to explain why myosin IIB antisense oligonucleotides have a small effect (Figures 4f and 5f), rather than none at all. Either myosin IIB could act, in part, as a retraction motor or, alternatively, the action of myosin IIA requires normal myosin IIB functionality. The observation that myosin IIA antisense oligonucleotides alone inhibit retraction completely (Figures 4d and 5d), similar to Y27632 (Figures 4b and 5b), argues against the former, because if true, one would not expect the effect of myosin IIA antisense alone to be maximal. Even though the LPA (Figure 4a) and thrombin (Figure 5a) stimuli for retraction used in these experiments are themselves maximal, when in combination with the action of antisense oligonucleotides targeting myosin IIB suboptimal retraction is observed (Figures 4f and 5f). Consequently, it is likely that optimal IIA action is conditional upon normal IIB activity during retraction. This conditional linkage is not reciprocal in its action and is so far revealed only in the context of retraction. For example, suboptimal neurite outgrowth (requiring the myosin IIB motor) is not observed when cells are subject to myosin IIA antisense (Wylie and Chantler, 2001) or Y27632 (Figure 6). If the two motors were linked in a reciprocal manner, then suboptimal outgrowth would be predicted. Y27632 has no inhibitory effect on neurite outgrowth (Figure 6a) and does not antagonize attenuation of outgrowth by antisense oligonucleotides targeting myosin IIB (Figure 6b). The small increase in the rate of neurite outgrowth that accompanies Y27632 addition (Figure 6, a and b) is likely due to a rearrangement of the actin cytoskeleton arising as a consequence of myosin IIA inhibition downstream of the Rho-kinase inhibitor. Thus, the vectorial balance of forces will be altered, facilitating an increased rate of outgrowth powered by myosin IIB.
These results again reveal the separate roles performed by the distinct yet closely homologous isoforms, myosins IIA and IIB (Wylie and Chantler, 2001). They show, for the first time, that myosin IIA but not myosin IIB drives growth cone collapse and neurite retraction. Our data, together with the work of others (Amano et al., 1998; Hirose et al., 1998; Katoh et al., 1998a), place the regulation of myosin IIA downstream of Rho and Rho-kinase activity and imply that a different pathway regulates the reciprocal functions of myosin IIB. It is likely that this latter control pathway is regulated upstream by Rac, the overexpression of which is known to induce neurite outgrowth (Albertinazzi et al., 1998) and which has been shown to regulate the phosphorylation state of the myosin heavy chain (van Leeuwen et al., 1999). We infer that the target for heavy chain phosphorylation is myosin IIB, the isoform that has previously been shown (Wylie et al., 1998; Bridgman et al., 2001) to be required for neurite outgrowth.
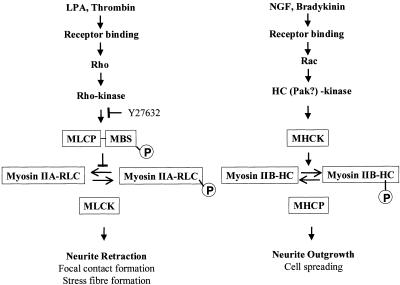
The above-mentioned results allow us to construct an overall scheme (Figure 7) for the separate regulation of myosin IIA and myosin IIB functions, one which also incorporates results from several other laboratories (Ridley, 1996; Sanders et al., 1999; van Leeuwen et al., 1999; Bishop and Hall, 2000). Myosin IIB activity, responding to external agonists, such as bradykinin or nerve growth factor, is necessary for neurite outgrowth (Wylie et al., 1998; Bridgman et al., 2001) and cell spreading (Diefenbach et al., 2002). Myosin IIA activity, responding to a variety of external agonists, including LPA and thrombin, is required for neurite retraction (this study) and focal contact formation (Wei and Adelstein, 2000; Wylie and Chantler, 2001) and is likely to be the isoform involved in stress fiber formation (Chrzanowska-Wodnicka and Burridge, 1996). Communications between pathways have been found that hint at reciprocal upstream control of myosin II isoform activity. These include mutual antagonistic effects of Rho relative to both Rac and Cdc42 (Hirose et al., 1998; Rottner et al., 1999; Sander et al., 1999; Sanders et al., 1999; Bishop and Hall, 2000; Yuan et al., 2003), reciprocity of activation of neurite outgrowth and inhibition of neurite retraction or cell rounding by cAMP (Gunning et al., 1981; Tigyi et al., 1996a; Hirose et al., 1998), or PAK (Sanders et al., 1999; van Leeuwen et al., 1999). The two pathways are likely to respond differently to extracellular cues during axonal pathfinding.
Figure 7.
Scheme to illustrate the separate functions and regulation of conventional nonsarcomeric myosin IIA and myosin IIB. We propose separate pathways to regulate the distinctive functions of nonsarcomeric, conventional myosin isoforms, in particular with regard to the dynamic process of neurite extension. We have shown (this study) that myosin IIA is the motor involved in neurite retraction, a process that can be triggered by a number of effectors, including LPA (Tigyi and Miledi, 1992; Jalink et al., 1993, 1994; Tigyi et al., 1996b) and thrombin (Jalink and Moolenaar, 1992; Suidan et al., 1992; Jalink et al., 1994). A direct pathway relating cause and effect is shown (LHS) and involves Rho activation, which, in turn, activates Rho-kinase (Matsui et al., 1996; Ridley, 1996; Bishop and Hall, 2000). When the myosin binding subunit (MBS) of myosin light chain phosphatase (MLCP) is phosphorylated by activated Rho-kinase (Kimura et al., 1996; Ridley, 1996), dephosphorylation of the regulatory light chain (RLC) of myosin is inhibited, allowing myosin IIA to maintain a level of activity determined by the level of phosphorylation at Ser19 on the RLC (Bresnick, 1999). The activity of Rho-kinase is inhibited by Y27632 (Uehata et al., 1997; Davies et al., 2000), leading to activation of MLCP, dephosphorylation of myosin IIA and a decline in cross-bridge cycling, preventing retraction. Interestingly, in vitro studies have demonstrated that the heavy chain of myosin IIA can act as a substrate for protein kinase C (Murakami et al., 1995) and metastasis-associated protein (Mts 1) (Murakami et al., 2000) so dual, or even triple, regulation at the level of the myosin molecule may also be possible. Myosin IIA is also known to be involved in focal contact and stress fiber formation (Chrzanowska-Wodnicka and Burridge, 1996; Wei and Adelstein, 2000; Wylie and Chantler, 2001), processes that can be also be inhibited by Y27632 (Uehata et al., 1997). It is likely that there are other intermediates in the pathway shown as indicated by a requirement for two different tyrosine kinases (Aoki et al., 1999).
We have shown previously that myosin IIB is involved in neurite outgrowth (Wylie et al., 1998), a process that can be initiated in many neuronal cells by nerve growth factor (Gundersen, 1985) or bradykinin (van Leeuwen et al., 1999) (although not required to stimulate outgrowth in the case of Neuro-2A cells). A proposed chain of command is shown (RHS) in which agonist binding activates Rac, leading ultimately to activation of a myosin heavy chain kinase (MHCK) (van Leeuwen et al., 1999). Phosphorylation of the myosin heavy chain has been shown to correlate with cell spreading (van Leeuwen et al., 1999). We propose that myosin IIB is also activated by MHCK in Neuro-2A cells, leading to neurite outgrowth. Casein kinase II and protein kinase C have been implicated as possible candidates for MHCK by in vitro experiments (Murakami et al., 1998). PAK-kinase may also be involved (Sanders et al., 1999; van Leeuwen et al., 1999) and has been demonstrated to phosphorylate the RLC (Chew et al., 1998) in vitro. Once again, the results suggest that some form of dual regulation at the level of the myosin molecule may occur. Putative cross talk between the pathways has not been included in the interest of clarity but may involve reciprocal actions of cAMP (Hirose et al., 1998), PAK (van Leeuwen et al., 1999), and further actions of the small GTPase protein family (Ridley, 1996; Sander et al., 1999; Bishop and Hall, 2000; Yuan et al., 2003).
The integrated mechanism by which both myosin IIA and myosin IIB contribute to neurite elongation will be discussed elsewhere (Chantler and Wylie, 2003). However, it is worth noting that the differential localization of myosin IIB and myosin IIA (Rochlin et al., 1995; Kolega, 1998), together with the distinct spatial organization of actin cytoarchitecture with the growth cone (Bridgman and Dailey, 1989; Lewis and Bridgman, 1992), are critical to their actions. The separate but linked functions of myosin IIA and myosin IIB, together with their distinct localization, lead one to speculate that an additional tier of their control may reside in compartmentalization of key regulatory signals. For example, myosin IIA is required for both neurite retraction and for focal contact formation; whereas retraction must involve extensive breakdown of existing contacts, outgrowth, while dependent on myosin IIB, can only occur with participation of adhesive forces. It is possible, therefore, that activation of myosin IIA is spatially restricted, focal contact formation being stimulated by local signaling through the matrix binding sites.
Although many details remain to be clarified, we conclude from our work that two highly conserved conventional myosin motors exhibit separate and complementary functions, acting in response to distinct regulatory pathways: myosin IIB powering neurite outgrowth, and myosin IIA driving neurite retraction and facilitating adhesion. Their actions combine to generate the vectorial forces required for neurite extension.
Acknowledgments
We are grateful to Drs. Bob Adelstein (National Institutes of Health) and Primal de Lanerolle (University of Illinois, Chicago, IL) for gifts of antibodies. This work was supported by a project grant from the Biotechnology and Biological Sciences Research Council and an instrumentation grant from the Wellcome Trust (to P.D.C.).
References
- Albertinazzi, C., Gilardelli, D., Paris, S., Longhi, R., and de Curtis, I. (1998). Overexpression of a neural-specific Rho family GTPase, cRac1B, selectively induces enhanced neuritogenesis and neurite branching in primary neurons. J. Cell Biol. 142, 815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano, M., Chihara, K., Nakamura, N., Fukata, Y., Yano, T., Shibata, M., Ikebe, M., and Kaibuchi, K. (1998). Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells 3, 177-188. [DOI] [PubMed] [Google Scholar]
- Aoki, J., Katoh, H., Yasui, H., Yamaguchi, Y., Nakamura, K., Hasegawa, H., Ichikawa, A., and Negishi, M. (1999). Signal transduction pathway regulating prostaglandin EP3 receptor-induced neurite retraction: requirement for two different tyrosine kinases. Biochem. J. 340, 365-369. [PMC free article] [PubMed] [Google Scholar]
- Berg, J.S., and Cheney, R.E. (2002). Myosin X is an unconventional myosin that undergoes intrafilopodial motility. Nat. Cell Biol. 4, 246-250. [DOI] [PubMed] [Google Scholar]
- Berg, J.S., Powell, B.C., and Cheney, R.E. (2001). A millennial myosin census. Mol. Biol. Cell 12, 780-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A.L., and Hall, A. (2000). Rho GTPase and their effector proteins. Biochem. J. 348, 241-255. [PMC free article] [PubMed] [Google Scholar]
- Borisy, G.G., and Svitkina, T.M. (2000). Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 12, 104-112. [DOI] [PubMed] [Google Scholar]
- Bresnick, A.R. (1999). Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26-33. [DOI] [PubMed] [Google Scholar]
- Bridgman, P.C., and Dailey, M.E. (1989). The organization of myosin and actin in rapid frozen nerve growth cones. J. Cell Biol. 108, 95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman, P., Dave, S., Asnes, C.F., Tullio, A.N., and Adelstein, R.S. (2001). Myosin IIB is required for growth cone motility. J. Neurosci. 21, 6159-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.M. and Goldman, R.D. (1973). The localization of actin-like fibers in cultured neuroblastoma cells as revealed by heavy meromyosin labelling. J. Cell Biol. 57, 867-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler, P.D., and Wylie, S.R. (2003). Elucidation of the separate roles of myosins IIA and IIB during neurite outgrowth, adhesion and retraction. Nanobiotech. (in press). [DOI] [PubMed]
- Cheng, T.P.O., Murakami, N., and Elzinga, M. (1992). Localization of myosin IIB at the leading edge of growth cones from rat dorsal root ganglion cells. FEBS Lett. 311, 91-94. [DOI] [PubMed] [Google Scholar]
- Chew, T.L., Masaracchia, R.A., Goeckeler, Z.M., and Wysolmerski, R.B. (1998). Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (γ-PAK). J. Mus. Res. Cell Mot. 19, 839-854. [DOI] [PubMed] [Google Scholar]
- Choi, O.H., Park, C.S., Itoh, K., Adelstein, R.S., and Beaven, M.A. (1996). Cloning of the cDNA encoding rat myosin heavy chain-A and evidence for the absence of myosin heavy chain-B in cultured rat mast (RBL-2H3) cells. J. Mus. Res. Cell Mot. 17, 69-77. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., and Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coling, D.E., Espreafico, E.M., and Kachar, B. (1997). Cellular distribution of myosin-V in the guinea pig cochlea. J. Neurocytol. 26, 113-120. [DOI] [PubMed] [Google Scholar]
- Cramer, L.P., and Mitchison, T.J. (1995). Myosin is involved in postmitotic cell spreading. J. Cell Biol. 131, 179-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S.P., Reddy, H., Caivano, M., and Cohen, P. (2000). Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach, T.J., Latham, V.M., Yimlamai, D., Liu, C.A., Herman, I.M., and Jay, D.G. (2002). Myosin 1c and myosin IIB serve opposing roles in lamellipodial dynamics of the neuronal growth cone. J. Cell Biol. 158, 1207-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espreafico, E.M., Cheney, R.E., Matteoli, M., Nascimento, A.A.C., De Camilli, P.V., Larson, R.E., and Mooseker, M.S. (1992). Primary structure and localization of chicken brain myosin V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks, P.R. (2000). Neuronal Growth Cones, Cambridge, United Kingdom: Cambridge University Press.
- Gundersen, R.W. (1985). Sensory neurite growth cone guidance by substrate absorbed nerve growth factor. J. Neurosci. Res. 13, 199-212. [DOI] [PubMed] [Google Scholar]
- Gunning, P.W., Landerth, G.E., Bothwell, M.A., and Shooter, E.M. (1981). Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J. Cell Biol. 89, 240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- Hirose, M., Ishizaki, T., Watanabe, N., Uehata, M., Kranenburg, O., Moolenaar, W.H., Matsumura, F., Maekawa, M., Bito, H., and Narumiya, S. (1998). Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 141, 1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K., and Adelstein, R.S. (1995). Neuronal cell expression of inserted isoforms of vertebrate nonmuscle myosin heavy chain II-B J. Biol. Chem. 270, 14533-14540. [DOI] [PubMed] [Google Scholar]
- Jalink, K., and Moolenaar, W.H. (1992). Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J. Cell Biol. 118, 411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink, K., Eichholtz, T., Postma, F.R., van Corven, E.J., and Moolenaar, W.H. (1993). Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Diff. 4, 247-255. [PubMed] [Google Scholar]
- Jalink, K., van Corven, E., Hengeveld, T., Morii, N., Narumiya, S., and Moolenaar, W. (1994). Inhibition of lysophosphatidate and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126, 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater, S.B. and Guthrie, P.B. (1990). Neuronal growth cone as an integrator of complex environmental information. In: Cold Spring Harbor Symposia on Quantitative Biology, vol. LV, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 359-370. [DOI] [PubMed] [Google Scholar]
- Katoh, H., Aoki, J., Ichikawa, A., and Negishi, M. (1998a). p160 RhoA-binding kinase ROKa induces neurite retraction. J. Biol. Chem. 273, 2489-2492. [DOI] [PubMed] [Google Scholar]
- Katoh, H., Aoki, J., Yamaguchi, Y., Kitano, Y., Ichikawa, A., and Negishi, M. (1998b). Constitutively active Gα12, Gα13 and Gαq induce Rho-dependent neurite retraction through different signaling pathways. J. Biol. Chem. 273, 28700-28707. [DOI] [PubMed] [Google Scholar]
- Katsuragawa, Y., Yanagisawa, Y., Inoue, A., and Masaki, T. (1989). Two distinct nonmuscle myosin heavy chain mRNAs are differentially expressed in various chicken tissues. Eur. J. Biochem. 184, 611-616. [DOI] [PubMed] [Google Scholar]
- Kawamoto, S., and Adelstein, R.S. (1991). Chicken nonmuscle myosin heavy chains: differential expression of two mRNAs and evidence for two different polypeptides. J. Cell Biol. 112, 915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K., et al. (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 273, 245-248. [DOI] [PubMed] [Google Scholar]
- Kolega, J. (1998). Cytoplasmic dynamics of myosin IIA and IIB: Spatial 'sorting' of isoforms in locomoting cells. J. Cell Sci. 111, 2085-2095. [DOI] [PubMed] [Google Scholar]
- Kuczmarski, E., and Rosenbaum, J.L. (1978). Studies on the organization and localization of myosin in neurons. J. Cell Biol. 80, 356-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux, P., Buxbaum, R.E., and Heidemann, S.R. (1989). Direct evidence that growth cones pull. Nature 340, 159-162. [DOI] [PubMed] [Google Scholar]
- Letourneau, P.C. (1981). Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell-substratum adhesions. Dev. Biol. 85, 113-122. [DOI] [PubMed] [Google Scholar]
- Letourneau, P.C., Kater, S.B. and Macagno, E.R. (ed.) (1991). The Nerve Growth Cone, New York: Raven Press.
- Leventhal, P.S., and Feldman, E.L. (1996). Tyrosine phosphorylation and enhanced expression of paxillin during neuronal differentiation in vitro. J. Biol. Chem. 271, 5957-5960. [DOI] [PubMed] [Google Scholar]
- Lewis, A.K., and Bridgman, P.C. (1992). Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J. Cell Biol. 119, 1219-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, A.K., and Bridgman, P.C. (1996). Mammalian myosin Iα is concentrated near the plasma membrane of nerve growth cones. Cell Motil. Cytoskeleton 33, 139-150. [DOI] [PubMed] [Google Scholar]
- Li, D., and Chantler, P.D. (1992). Evidence for a new member of the myosin I family from mammalian brain. J. Neurochem. 59, 1344-1351. [DOI] [PubMed] [Google Scholar]
- Li, D., Miller, M., and Chantler, P.D. (1994). Association of a cellular myosin II with anionic phospholipids and the neuronal plasma membrane. Proc. Natl. Acad. Sci. USA 91, 853-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C., and Forscher, P. (1995). Growth cone advance is inversely proportional to retrograde actin flow. Neuron 14, 763-771. [DOI] [PubMed] [Google Scholar]
- Luo, L. (2000). Rho GTPases in neuronal morphogenesis. Nat. Rev. 1, 173-180. [DOI] [PubMed] [Google Scholar]
- Luo, L. (2002). Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 18, 601-635. [DOI] [PubMed] [Google Scholar]
- Matsui, T., Amano, M., Yamamoto, T., Chihara, K., Nakafuku, M., Ito, M., Nakano, T., Okawa, K., Iwamatsu, A., and Kaibuchi, K. (1996). Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208-2216. [PMC free article] [PubMed] [Google Scholar]
- Miller, M., Bower, E., Levitt, P., Li, D., and Chantler, P.D. (1992). Myosin II distribution in neurons is consistent with a role in growth cone motility but not synaptic vesicle mobilization. Neuron 8, 25-44. [DOI] [PubMed] [Google Scholar]
- Murakami, N., Chauhan, V.P.S., and Elzinga, M. (1998). Two nonmuscle myosin heavy chain isoforms expressed in rabbit brains: filament forming properties, the effects of phosphorylation by protein kinase C and casein kinase II, and the location of the phosphorylation sites. Biochemistry 37, 1989-2003. [DOI] [PubMed] [Google Scholar]
- Murakami, N., Kotula, L., and Hwang, Y.W. (2000). Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry 39, 11441-11451. [DOI] [PubMed] [Google Scholar]
- Murakami, N., Singh, S.S., Chauhan, V.P.S., and Elzinga, M. (1995). Phospholipid binding, phosphorylation by protein kinase C, and filament assembly of the COOH terminal heavy chain fragments of nonmuscle myosin II isoforms MIIA and MIIB. Biochemistry 34, 16046-16055. [DOI] [PubMed] [Google Scholar]
- Pantaloni, D., Le Clainche, C., and Carlier, M.F. (1999). Mechanism of actin-based motility. Science 292, 1502-1506. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J. (1996). Rho: theme and variations. Curr. Biol. 6, 1256-1264. [DOI] [PubMed] [Google Scholar]
- Rochlin, M., Itoh, K., Adelstein, R.S., and Bridgman, P.C. (1995). Localization of myosin II A and B isoforms in cultured neurons. J. Cell Sci. 108, 3661-3670. [DOI] [PubMed] [Google Scholar]
- Rottner, K., Hall, A., and Small, J.V. (1999). Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640-648. [DOI] [PubMed] [Google Scholar]
- Ruchhoeft, M.L., and Harris, W.A. (1997). Myosin functions in Xenopus retinal ganglion cell growth cone motility in vivo. J. Neurobiol. 32, 567-578. [PubMed] [Google Scholar]
- Sander, E.E., ten Klooster, J.P., van Delft, S., van der Kammen, R., and Collard, J.G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behaviour. J. Cell Biol. 147, 1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, L.C., Matsumura, F., Bokoch, G.M., and de Lanerolle, P. (1999). Inhibition of myosin light chain kinase by p21-activated kinase. Science 283, 2083-2085. [DOI] [PubMed] [Google Scholar]
- Sebok, A., Nusser, N., Debreceni, B., Guo, Z., Santos, M.F., Szeberenyi, J., and Tigyi, G. (1999). Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J. Neurochem. 73, 949-960. [DOI] [PubMed] [Google Scholar]
- Simons, M., Wang, M., McBride, O., Kawamoto, S., Gdula, D., Adelstein, R.S., and Weir, L. (1991). Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ. Res. 69, 530-539. [DOI] [PubMed] [Google Scholar]
- Suidan, H.S., Stone, S.R., Hemmings, B.A., and Monard, D. (1992). Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron 8, 363-375. [DOI] [PubMed] [Google Scholar]
- Suter, D.M., Espindola, F.S., Lin, C.L., Forscher, P., and Mooseker, M.S. (2000). Localization of unconventional myosins V and VI in neuronal growth cones. J. Neurobiol. 42, 370-382. [PubMed] [Google Scholar]
- Tigyi, G., Fischer, D.J., Sebok, A., Marshall, F., Dyer, D.L., and Miledi, R. (1996a). Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-positive effects of cyclic AMP signalling. J. Neurochem. 66, 549-558. [DOI] [PubMed] [Google Scholar]
- Tigyi, G., Fischer, D.J., Sebok, A., Yang, C., Dyer, D.L., and Miledi, R. (1996b). Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and rho. J. Neurochem. 66, 537-548. [DOI] [PubMed] [Google Scholar]
- Tigyi, G., and Miledi, R. (1992). Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 267, 21360-21367. [PubMed] [Google Scholar]
- Uehata, M., et al. (1997). Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990-994. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, F.N., van Delft, S., Kain, H.E., van der Kammen, R.A., and Collard, J.G. (1999). Rac regulates phosphorylation of the myosin II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1, 242-248. [DOI] [PubMed] [Google Scholar]
- Wagner, M.C., Barylko, B., and Albanesi, J.P. (1992). Tissue distribution and subcellular localization of mammalian myosin I. J. Cell Biol. 119, 163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Wolenski, J., Cheney, R., Mooseker, M., and Jay, D. (1996). Function of myosin V in filopodial extension of neural growth cone. Science 273, 660-663. [DOI] [PubMed] [Google Scholar]
- Wei, Q., and Adelstein, R.S. (2000). Conditional expression of a truncated fragment of nonmuscle myosin II-A alters cell shape but not cytokinesis in HeLa cells. Mol. Biol. Cell 11, 3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie, S., and Chantler, P.D. (2001). Separate but linked functions of conventional myosins modulate adhesion and neurite outgrowth. Nat. Cell Biol. 3, 88-92. [DOI] [PubMed] [Google Scholar]
- Wylie, S.R., Wu, P., Patel, H., and Chantler, P.D. (1998). A conventional myosin motor drives neurite outgrowth. Proc. Natl. Acad. Sci. USA 95, 12967-12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X.B., Jin, M., Xu, X., Song, Y.Q., Wu, C.P., Poo, M.M., and Duan, S. (2003). Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 5, 1-8. [DOI] [PubMed] [Google Scholar]