Abstract
The effect of an acute bout of moderate treadmill walking on behavioral and neuroelectric indices of the cognitive control of attention and applied aspects of cognition involved in school-based academic performance were assessed. A within-subjects design included twenty preadolescent participants (Age = 9.5 ± 0.5 years; 8 female) to assess exercise-induced changes in performance during a modified flanker task and the Wide Range Achievement Test 3. The resting session consisted of cognitive testing followed by a cardiorespiratory fitness assessment to determine aerobic fitness. The exercise session consisted of 20 minutes of walking on a motor-driven treadmill at 60% of estimated maximum heart rate followed by cognitive testing once heart rate returned to within 10% of pre-exercise levels. Results indicated an improvement in response accuracy, larger P3 amplitude, and better performance on the academic achievement test following aerobic exercise relative to the resting session. Collectively, these findings indicate that single, acute bouts of moderately-intense aerobic exercise (i.e., walking) may improve the cognitive control of attention in preadolescent children, and further supports the use of moderate acute exercise as a contributing factor for increasing attention and academic performance. These data suggest that single bouts of exercise affect specific underlying processes that support cognitive health and may be necessary for effective functioning across the lifespan.
Keywords: Walking, Cardiorespiratory Exercise, Fitness, Event-Related Potentials, P3, Executive Control
The effect of physical activity on brain and cognition has grown in interest in recent years with an increasing number of reports indicating that chronic participation and single, acute bouts of exercise benefit a host of cognitive processes (see Hillman, Erickson, and Kramer, 2008 for review). Interestingly, the majority of research has focused on adult populations, with a more recent focus turning toward child populations. Reviews of early studies testing this relationship suggests that school age children also may derive cognitive benefits from physical activity participation (Sibley and Etnier, 2003; Tomporowski, 2003b). Preadolescent children provide a unique population in which to study physical activity effects on cognition, as researchers are afforded the opportunity to link basic cognitive functions, often measured in the laboratory, with applied aspects of cognition, routinely measured in the schools.
One such aspect of applied cognitive performance is academic achievement. Recent federal mandates have placed an increased importance upon students’ performance on academic achievement tests. Thus, school districts have begun altering their curricula to better provide students with the necessary means to improve their test performance. These curricula changes have come at the cost of non-academic subject matters including reduced instruction time in physical education, arts, and music.
However, Castelli, Hillman, Buck, and Erwin (2007) observed a positive relation between fitness and standardized achievement test performance on mathematics and reading in third and fifth grade children. The results indicated that aerobic fitness was positively related, body mass index was negatively related, and muscle strength and flexibility fitness were unrelated with achievement test performance, suggesting a differential relation between the various aspects of fitness and scholastic performance. Further, Coe and her colleagues (Coe, Pivarnik, Womack, Reeves, and Malina, 2006) administered a 3-day physical activity recall questionnaire to sixth grade children and observed academic performance in four core classes (i.e., mathematics, science, English, world studies) and Terra Nova standardize test scores. They found increased performance in core academic classes for those children who reported vigorous physical activity outside of school relative to those who reported no physical activity (Coe et al., 2006). Several other studies have appeared in the literature as well, indicating positive relations between physical activity and aspects of academic performance (Fields, Diego, and Sanders, 2001;Kim et al., 2003; Lindner, 2002). Although several studies have also observed no relation between physical activity and academic performance (Tomporowski, Davis, Miller, and Naglieri, 2008), no published reports exist suggesting a negative relationship between these factors, indicating that, at the very least, time spent performing physical activity does not hinder academic performance and may lead to improved physical and mental health. To date, few reports are available (e.g., Mahar et al., 2006) on the relation between single, acute bouts of physical activity and performance on academic achievement tests.
Even less is known about the underlying aspects of cognition that are influenced by acute bouts of activity and their relation to applied academic achievement. A recent study indicated that attention and concentration are enhanced following acute bouts of either coordinative exercise or normal sport lessons provided in physical education class in adolescent children (Budde, Voelcker-Rehage, Pietraßyk-Kendziorra, Ribeiro, and Tidow, 2008). However, other research examining a different aspect of cognition (i.e., cognitive flexibility) failed to obtain a beneficial effect of acute aerobic exercise (Tomporowski, Davis, Lambourne, Gregoski, and Tkacz, 2008). Research examining chronic physical activity participation has suggested improved cognitive performance on attentionally demanding tasks in preadolescent children (Buck, Hillman, and Castelli, 2008; Castelli et al., 2007; Davis et al., 2007; Hillman, Castelli, and Buck, 2005; Hillman, Buck, Themanson, Pontifex, and Castelli, 2009).
Several reports have also observed positive effects of acute exercise on adult cognition (see Tomporowski, 2003a for review). In a subset of these reports, event-related brain potential (ERPs) measures have been collected to assess neuroelectric changes that may underlie cognitive performance. This approach offers the requisite temporal precision to gain insight into covert cognitive operations that occur between stimulus engagement and response selection, which may be more sensitive to these processes than overt behavioral measures of task performance. ERPs refer to patterns of neuroelectric activation that occur in response to, or in preparation for, a stimulus or response. Previous studies have examined the P3 component of the stimulus-locked ERP to understand alterations in stimulus engagement. That is, the amplitude of the P3 is believed to index the allocation of attentional resources during stimulus engagement, with greater amplitude indicative of greater resource allocation (Polich, 1987). The latency of the P3 is thought to index stimulus classification and evaluation speed with increased latency reflecting longer processing time (Duncan-Johnson, 1981). Prior studies examining acute exercise effects on the P3 have observed increased amplitude (Kamijo et al., 2004) and shorter latency (Hillman, Snook, and Jerome, 2003) following single, acute bouts of moderately-intense exercise relative to a basal state. Accordingly, the findings have been interpreted to suggest an exercise-induced facilitation of attentional resource allocation and stimulus evaluation speed during tasks requiring the cognitive control of inhibition.
Cognitive control is a term used to describe a subset of goal-directed, self regulatory operations involved in the selection, scheduling, and coordination of computational processes underlying perception, memory, and action. Core cognitive processes collectively termed ‘cognitive control’ or ‘executive control’ include inhibition, working memory, and cognitive flexibility (Diamond, 2006). Of these core processes, inhibition has been most studied in the acute exercise literature (e.g., Hillman et al., 2003; Kamijo et al., 2004) and relates to the ability to act on the basis of choice rather than impulse (Davidson, Amso, Anderson, and Diamond, 2006). That is, inhibitory control often requires one to override a strong internal or external pull to appropriately perform a necessary task (Davidson et al., 2006). The ability to inhibit attention to task irrelevant or distracting stimuli is central to the ability to sustain attention and allow control over one’s actions. Such abilities exhibit protracted development relative to other cognitive processes (Diamond, 2006).
The brain regions supporting inhibitory processes include the prefrontal (including the anterior cingulate cortex and lateral prefrontal cortex), temporal, and parietal cortices (Bush, Luu, and Posner, 2000; Carter, Mintun, and Cohen, 1995; Taylor, Kornblum, Lauber, Minoshima, and Koeppe, 1997). This network of brain regions has been shown to exhibit a more protracted development relative to other networks and likely relates to the delayed developmental trajectory of the cognitive processes mediated by it (Bunge, Dudukovic, Thomason, Vaidya, and Gabrieli, 2002). Despite the delayed maturation of this network, young children have been observed to perform inhibitory tasks with reasonable levels of success. Children as young as age four exhibit the goal directed, self-regulatory behaviors required to inhibit their attention and actions when necessary (Davidson et al., 2006; Mezzacappa, 2004). The relevance of the development of such behavior has been made obvious through research that provided a cognitive control intervention to preschool children and demonstrated gains in academic performance (Diamond, Barnett, Thomas, and Munro, 2007). Mathematics and reading performance have also been linked to the prefrontal and parietal network (Maguire, Frith, and Morris, 1999). Specifically, mathematical calculations and numerical magnitude processing has been found to activate bilateral regions of the intraparietal sulcus in children and adults (Ansari and Dhital, 2006; Gobel, Johansen-Berg, Behrens, and Rushworth, 2004; Rivera, Reiss, Eckert, and Menon, 2005), as well as the right dorsolateral prefrontal cortex in children (Ansari and Dhital, 2006). Thus, similarities exist in the neural networks that underlie cognitive control and academic achievement.
Interestingly, in adult populations physical activity effects on cognition have been found to exhibit a disproportionately larger benefit to tasks or task components requiring extensive amounts of cognitive control (Angevaren, Aufdemkampe, Verhaar, Aleman, and Vanhees, 2008; Colcombe and Kramer, 2003), with corroborating evidence from imaging studies indicating selective increases in gray and white matter volume in the prefrontal, temporal, and parietal cortices (Colcombe et al., 2004, 2006; Erickson et al. in press), suggesting that these regions are particularly susceptible to intervention. Further, selective changes in the P3 component to an inhibitory control task following acute exercise suggest that single bouts of exercise may increase attentional resource allocation and improve cognitive processing speed in adults (Hillman et al., 2003). To date, no such relationship has been established in the literature in preadolescent populations; thus the extension of the findings within adult populations to children is speculative. However, given the protracted development of -- and the observed physical activity benefits to -- the neural network underlying attentional inhibition, an acute exercise effect on inhibitory control would be expected.
Accordingly, the effect of a single, acute bout of exercise on preadolescent children’s neurocognitive function was examined during performance on a modified Eriksen flanker task (Eriksen and Eriksen, 1974) requiring variable amounts of inhibitory control, and on a series of standardized achievement tests related to reading, spelling, and mathematics. The examination of acute exercise on inhibitory control in children extends prior research in adults (e.g., Hillman et al., 2003). Thus, it was predicted that relative to a baseline condition, children would exhibit larger P3 amplitude following acute exercise, indicative of enhance attentional allocation, and shorter P3 latency, indicative of faster cognitive processing speed. These acute exercise effects were expected to be larger during task components placing larger demands upon the attentional control network. Additionally, the focus on academic achievement extends prior preadolescent studies examining changes in scholastic performance (Caterino & Polak, 1999; Gabbard, & Barton, 1979; McNaughten & Gabbard, 1993) following participation in structured physical activities. It was predicted that children would exhibit increased performance on academic achievement tests following acute exercise due to the established relationship between single bouts of physical activity and enhanced performance on tasks requiring cognitive and attentional control.
Experimental Procedures
Participants
Table 1 provides the demographic and fitness data for all participants. Twenty right-handed preadolescent children from the east-central Illinois region were recruited to serve as participants. All participants provided written assent and their legal guardians provided written informed consent in accordance with the Institutional Review Board of the University of Illinois at Urbana-Champaign. Prior to testing, legal guardians’ completed a health history and demographics questionnaire, reported that their child was free of neurological diseases, attentional disorders (as indexed by scores below 14 and 22 for females and males, respectively on the ADHD Rating Scale IV), or physical disabilities, and indicated normal or corrected to normal vision based on the minimal 20/20 standard. They further reported that their child did not have any condition that would be exacerbated by physical exercise using the Physical Activity Readiness Questionnaire (Thomas, Reading, and Shephard, 1992), and socioeconomic status (SES) was determined using a trichotomous index based on: a) participation in free or reduced-price lunch program at school, b) the highest level of education obtained by the mother and father, and c) number of parents who worked full-time (Birnbaum et al., 2002). Participants, in collaboration with their legal guardian, completed the Tanner Staging System (Tanner, 1962), indicating that their pubertal status was at or below a score of 2 (i.e., prepubescent) on the 4-point scale. Additionally, children were administered the Kaufman Brief Intelligence Test (K-BIT; Kaufman and Kaufman, 1990) by a trained experimenter to assess intelligence quotient, and completed the Edinburgh Handedness Inventory (Oldfield, 1971) to determine hand dominance.
Table 1.
Mean (SD) Values for Participant Demographics and Fitness Data
| Variable | All Participants | Females | Males |
|---|---|---|---|
| Age (years) | 9.6 (.7) | 9.3 (.8) | 9.7 (.6) |
| Tanner Scale | 1.4 (.4) | 1.1 (.2) | 1.6 (.4) |
| K-BIT (IQ) | 120.7 (10.2) | 123.3 (8.8) | 119.2 (11.0) |
| ADHD | 7.3 (3.8) | 5.3 (3.2) | 8.4 (3.8) |
| Body Mass Index (kg/m2) | 18.5 (4.7) | 16.9 (3.1) | 19.3 (5.2) |
| VO2 max (ml/kg/min) | 40.1 (8.9) | 36.1 (7.6) | 42.3 (9.0) |
| HR max (bpm) | 191.4 (11.7) | 188.6 (15.4) | 192.8 (9.5) |
| Resting HR (bpm) | 84.7 (1.7) | 83.6 (2.4) | 85.2 (2.3) |
| Post Exercise HR (bpm) | 81.8 (1.9) | 84.1 (2.8) | 80.5 (2.6) |
| Mean Exercise HR (bpm) | 125.4 (1) | 125.5 (1.2) | 125.3 (1.4) |
| Mean Exercise Omni RPE | 2.8 (.4) | 3.3 (.5) | 2.5 (.5) |
Note: HR max = maximum heart rate achieved during cardiorespiratory fitness (VO2 max) test; Resting HR = heart rate prior to cognitive task during the seated rest session; Post Exercise HR = heart rate prior to the cognitive task during acute aerobic exercise session; Mean Exercise HR = average HR during the acute aerobic exercise session; Mean Exercise Omni RPE = the average ratings of perceived exertion during the acute aerobic exercise session.
Modified Flanker Task
Participants completed a modified flanker task (Eriksen and Eriksen, 1974; Hillman et al., 2006; Pontifex and Hillman, 2007) to assess inhibitory control. Congruent and incongruent trials require participants to press a button corresponding to the direction of the centrally presented target arrow. Congruent trials consisted of an array of five arrows facing the same direction (e.g., ≪≪< or "≫"≫>) and incongruent trials consisted of the four flanking arrows facing the opposite direction to that of the target arrow (e.g., "≫<"≫ or ≪>≪). Following the provision of task instructions, participants were afforded the opportunity to ask questions and 20 practice trials were administered prior to the start of testing. The experimenter observed participants during the practice trials and checked their performance to ensure that they understood the task. If a participant’s task performance was below 60%, another 20 practice trials were administered. All participants performed adequately following the second set of practice trials. Participants were administered two blocks of 100 trials, consisting of equiprobable congruency and directionality. Stimuli were 2.5 cm tall white arrows presented focally for 120 ms on a black background with a response window of 1000 ms and a variable inter-stimulus interval of 1100, 1300, or 1500 ms. Total task duration was approximately 7.5 minutes including a 2.5 minute rest between task blocks. This task allows not only for measures of response speed and accuracy, but also for changes in the speed and accuracy of information processing across the two conditions through the calculation of interference scores. These scores require simple subtractions across task conditions to yield the difference in performance between congruent and incongruent trials (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005).
Electroencephalogram
Electroencephalographic (EEG) activity was recorded from 64 electrode sites labeled according to the international 10–10 system (Chatrian, Lettich, and Nelson, 1985) using a Neuroscan Quik-cap referenced to average mastoids, with AFz serving as the ground electrode. Electro-oculographic (EOG) activity was recorded using additional electrodes placed above and below the left orbit and on the outer canthus of each eye. Continuous data were digitized at a sampling rate of 500 Hz, amplified 500× with a DC to 70 Hz filter, and a 60 Hz notch filter was applied using a Neuroscan Synamps amplifier. Prior to testing all electrodes had an impedance < 10kΩ.
Offline data reduction included EOG correction using a spatial filter (Compumedics Neuroscan, 2003) and rejection of trials with a response error or artifact exceeding ±75 μV. Stimulus-locked epochs were created from −100 to 1000 ms around the stimuli. Baseline correction occurred using the 100 ms pre-stimulus period and the data were filtered using a zero phase shift 30 Hz (24 dB/octave) low pass filter. The P3 component was quantified as the maximum positive deflections occurring within a 300–600 ms latency window. Peak data were outputted in ASCII format and analyzed using SPSS 15.0.
Academic Achievement Assessment
Participants completed the Wide Range Achievement Test - 3rd edition (WRAT3; Wide Range, Inc., Wilmington, DE) to assess academic achievement in the content areas of reading (i.e., the number of words correctly pronounced aloud), spelling (i.e., the number of words correctly spelled), and arithmetic (i.e., the number of mathematical problems correctly solved). The WRAT3 is a paper and pencil based academic achievement assessment that has been age-normed referenced and has been strongly correlated with the California Achievement Test – Form E and the Stanford Achievement Test (Wilkinson, 1993). The WRAT3 allows for repeated administration through the use of two equivalent forms designed specifically for pre- and post- intervention testing (Wilkinson, 1993). Administration of the WRAT3 was conducted individually by trained experimenters with the duration of the assessment taking 15–20 minutes.
Cardiorespiratory fitness assessment
Cardiorespiratory fitness data is listed in Table 1. Maximal oxygen consumption (V̇O2max) was measured using a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages for oxygen uptake (V̇O2) and respiratory exchange ratio (RER) assessed every 30 seconds. A modified Balke protocol (ACSM, 2005) was employed using a motor-driven treadmill at a constant speed with increases in grade increments of 2.5% every two minutes until volitional exhaustion occurred. A Polar heart rate monitor (Model A1, Polar Electro, Finland) was used to measure HR throughout the test and ratings of perceived exertion (RPE) were assessed every two minutes using the children’s OMNI scale (Utter et al., 2002). The children’s OMNI scale for RPE uses a numerical scale from 0 to 10, with a score of 2 indicating “a little tired” and a score of 9 indicating “very, very tired”, with associated pictographs to represent perceived physical effort. Relative peak oxygen consumption was expressed in ml/kg/min and was based upon maximal effort as evidenced by 1) a peak heart rate ≥ 185 bpm (ACSM, 2005) and a heart rate plateau (Freedson and Goodman, 1993); 2) RER ≥ 1.0 (Bar-Or, 1983); and/or 3) ratings on the children’s OMNI scale of perceived exertion ≥ 8 (Utter et al., 2002). Cardiorespiratory fitness data is listed in Table 1.
Procedure
A within-subjects design had participants visit the laboratory on two separate days (10.6 ± 9.4 days between sessions) in which they had not participated in physical education or other structured physical activity (e.g., youth sport involvement). Both visits occurred at the same time of day. During the first visit, participants and their legal guardians completed all paperwork including written informed assent/consent as described above. They were then outfitted with a heart rate monitor (Model A1, Polar Electro, Finland) and their resting heart rate was recorded after 5 minutes of seated rest. Participants were counterbalanced into two different experimental conditions such that half of the participants received the resting session on the first day and the aerobic exercise session on the second day. The other half received the aerobic exercise session on the first day and the resting session on the second day. The resting session consisted of 20 minutes of seated rest, during which time the legal guardian was present and conversation was kept to a minimum. Following the seated rest period, participants were outfitted with the electrode cap prior to administration of the modified flanker task (M = 24.1 ± 6.0 minutes post seated rest). After the completion of this task, the electrode cap was removed and the WRAT3 was administered. Upon completion of all cognitive testing participants performed a cardiorespiratory fitness assessment to determine aerobic fitness. The aerobic exercise session consisted of 20 minutes of aerobic exercise on a motor-driven treadmill at 60% of their estimated maximum heart rate (HRmax; 220-age). Mean HR for this intensity was 125.4 ± 1.0 bpm which equated to 65.7 ± 0.9 % of actual HRmax. Following the completion of the acute exercise bout, participants were outfitted with an electrode cap and once their heart rate (HR) returned to within 10% of their pre-exercise levels (M = 25.4 ± 6.7 minutes post exercise) the modified flanker task was performed. Following completion of the flanker task, the electrode cap was removed and the WRAT3 was administered. Upon completion of both visits, participants and their legal guardians were briefed on the purpose of the experiment and receive $40 remuneration ($10/hour) for their involvement in the experiment.
Statistical Analysis
Preliminary analyses were conducted to examine the order in which the sessions occurred to ensure that the observed effects were not due to the specific order in which participants received the exercise and rest conditions. These analyses employed an additional between-subjects variable with two levels (Session Order: Rest, Aerobic Exercise vs. Aerobic Exercise, Rest) to the analyses described below for each dependent measure.
Academic achievement was tested using paired sample t tests of Session for each academic achievement subject (i.e., reading, spelling, mathematics). Analyses were conducted separately for task performance measures (RT and response accuracy) using a 2 (Session: Exercise, Rest) × 2 (Task Congruency: Congruent, Incongruent) repeated measures ANOVA. Secondary analyses examined interference scores using paired sample t tests of Session, which calculate the difference in speed or accuracy between flanker congruent and incongruent trials.
P3 analyses were conducted using 45 electrode sites (nine coronal sites within each of five regions). P3 values (amplitude, latency) were submitted to a 2 (Session) × 2 (Task Congruency) × 5 (Region: Frontal, Fronto-Central, Central, Centro-Parietal, Parietal) × 9 (Site: 7, 5, 3, 1, z, 2, 4, 6, 8) repeated measures ANOVA. Analyses with three or more within-subject levels used the Greenhouse-Geisser statistic with subsidiary univariate ANOVAs and Bonferroni corrected t tests used for post hoc comparisons. The family-wise alpha level was set at .05.
Results
Session Order
Preliminary analyses were performed to test whether the Session Order, which was counterbalanced across participants, had an effect on any of the dependent variables. Findings revealed no significant main effect or interaction involving Session Order for any variable, F’s (1, 18) ≤ 2.2, p ≥ .15, η2 ≤.11. Thus, all further analyses were collapsed across Session Order.
Achievement Test Performance
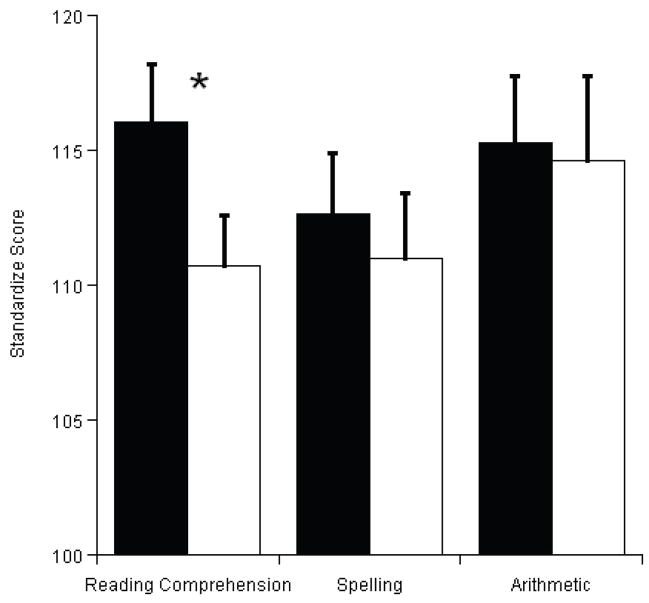
Analyses conducted on the three subtests of the WRAT3 indicated significantly better performance for reading comprehension following acute exercise relative to rest, t(1, 19) = 2.6, p = .016 (see Figure 1). No such effect was observed for spelling or arithmetic (p’s > .39).
Figure 1.
Mean (SEM) comparison of acute exercise (black bars) and resting (white bars) sessions for performance on the three subtests of the Wide Range Achievement Test 3.
Task Performance
RT. The omnibus analysis for RT indicated a Congruency effect, F(1, 19) = 139.1, p < .001, η2 = .88, with shorter RT for congruent (M = 493.1, SE = 12.8) relative to incongruent trials (M = 552.6, SE = 12.0). No effects of Session were observed, p > .24 (see Figure 2). A secondary analysis of the interference effects (incongruent-congruent) for each Session also yielded non-significant differences between acute exercise (M = 55.0, SE = 5.1) and rest (M = 64.1, SE = 7.3), p = .24.
Figure 2.
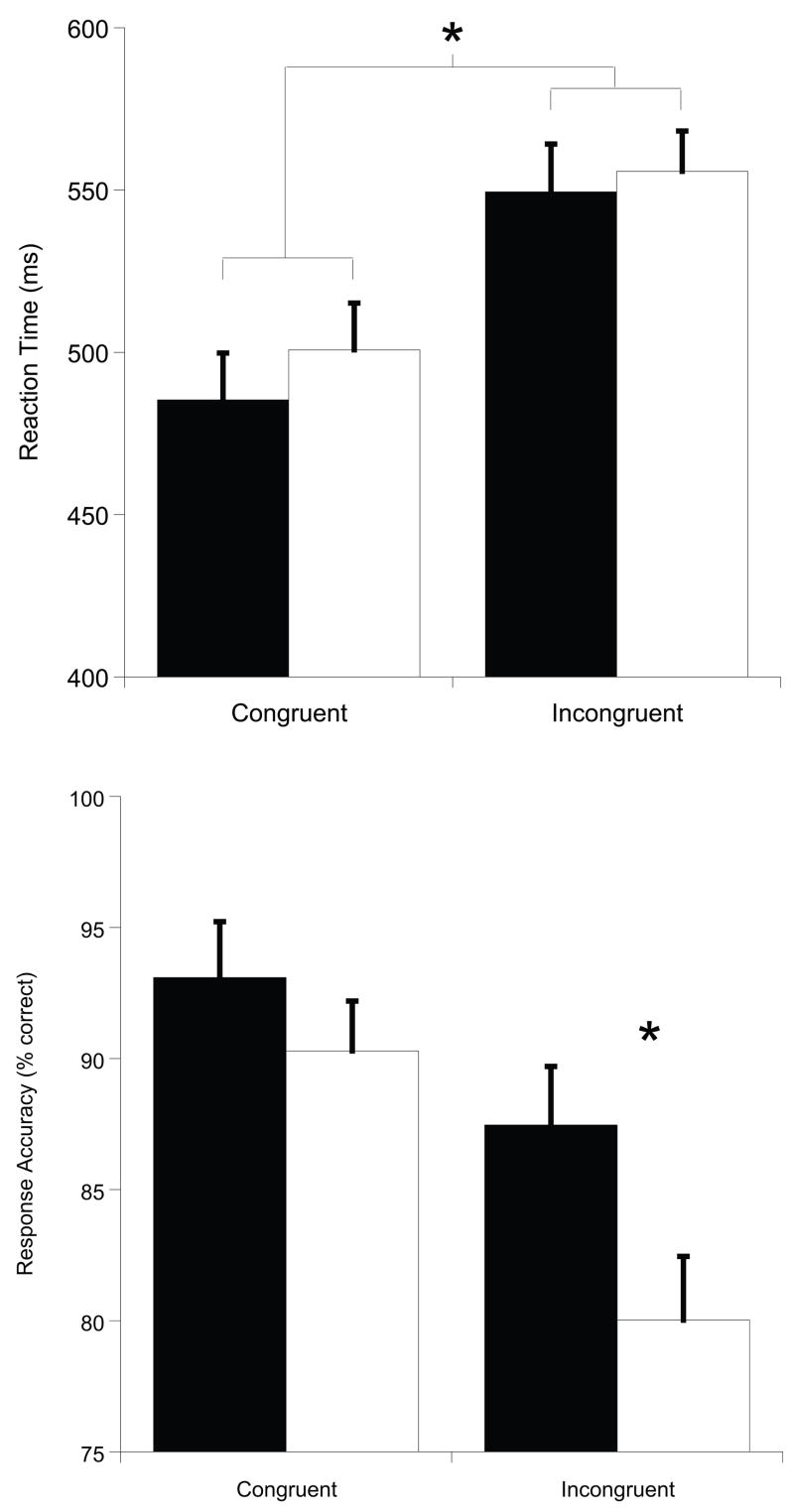
Mean (SEM) reaction time and response accuracy for congruent and incongruent trials of the flanker task as a function of acute exercise (black bars) and resting (white bars) sessions.
Response Accuracy
The omnibus analysis indicated effects of Session, F(1, 19) = 8.8, p < .01, η2 = .32, and Congruency, F(1, 19) = 53.3, p < .001, η2 = .74, that were superseded by a Session × Congruency interaction, F(1, 19) = 5.1, p < .05, η2 = .21. Decomposition of the significant 2-way interaction yielded increased response accuracy following acute exercise relative to rest only during incongruent trials, t(1, 19) = 3.0, p = .008. Although a similar effect was observed for the congruent condition, it was considered non-significant following the Bonferroni correction, t(1, 19) = 2.1, p = .05 (see Figure 2). The secondary analysis comparing the interference effect (congruent – incongruent) indicated a significantly smaller difference between flanker task congruencies following acute exercise (M = 5.6, SE = 1.0) relative to rest (M = 10.3, SE = 1.9), t(1, 19) = 2.3, p < .05.
Event-Related Brain Potentials
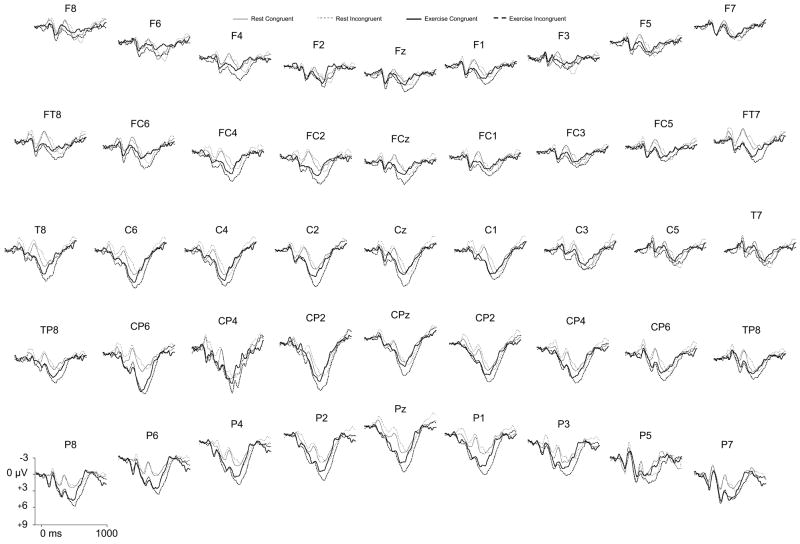
Figure 3 depicts the stimulus-locked ERP waveforms across session and task congruency.
Figure 3.
Grand averaged ERP waveforms for session and task congruency at each of the electrode sites.
P3 Amplitude
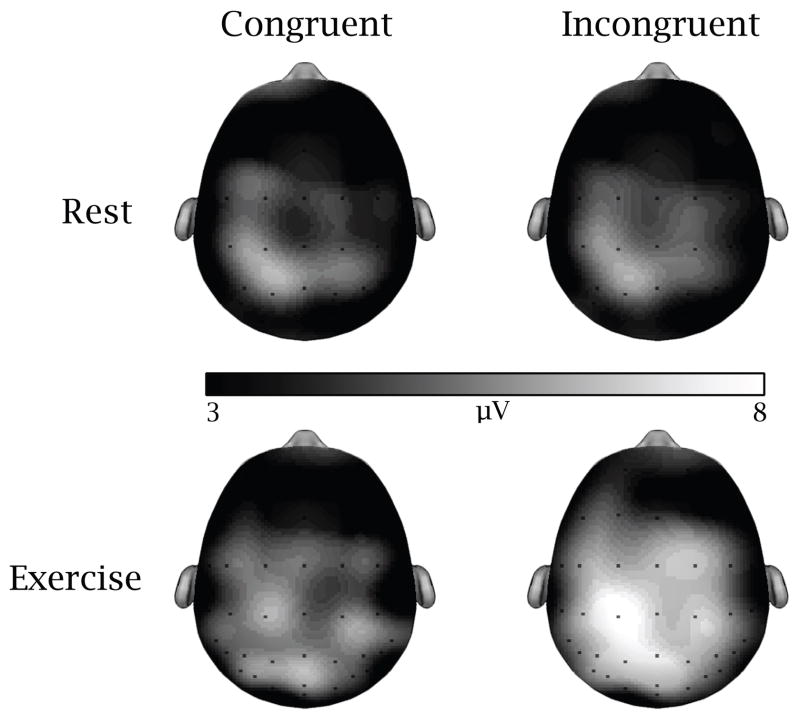
The omnibus analysis evidenced main effects of Session, F(1, 19) = 8.0, p = .01, η2 = .30, Region, F(1.9, 36.1) = 39.1, p < .001, η2 = .67, and Site, F(3.4, 63.6) = 13.6, p < .001, η2 = .42, and 2-way interactions of Congruency × Region, F(2.9, 55.1) = 5.0, p < .005, η2 = .21, and Region × Site, F(6.5, 124.2) = 2.7, p = .01, η2 = .13. However, these analyses were superseded by 3-way interactions of Session × Congruency × Region, F(2.1, 39.2) = 3.1, p = .05, η2 = .14, and Session × Congruency × Site, F(3.4, 65.1) = 4.2, p < .01, η2 = .18. Decomposition of the Session × Congruency × Region interaction examined Session × Congruency within each of the five regions. The subsidiary ANOVA for the frontal region yielded a Congruency effect, F(1, 19) = 5.1, p < .05, η2 = .21, with increased amplitude for incongruent (M = 4.7, SE = .49) relative to congruent (M = 3.9, SE = .56) trials. The fronto-central region revealed effects of Session, F(1, 19) = 5.8, p < .03, η2 = .23, and Congruency, F(1, 19) = 7.5, p = .01, η2 = .28, with greater amplitude exhibited following acute exercise (M = 5.5, SE = .68) and for incongruent trials (M = 5.1, SE = .53) relative to rest (M = 3.7, SE = .58) and congruent trials (M = 4.1, SE = .55), respectively. The central region yielded a Session main effect, F(1, 19) = 4.9, p < .04, η2 = .21, with greater amplitude following acute exercise (M = 6.5, SE = .60) relative to rest (M = 4.8, SE = .72). The centro-parietal region yielded a Session effect, F(1, 19) = 8.0, p = .01, η2 = .30, that was superseded by a Session × Congruency interaction, F(1, 19) = 4.3, p = .04, η2 = .20. Post hoc t tests indicated larger amplitude following acute exercise relative to rest for incongruent trials, t(1, 19) = 3.0, p = .007. A similar effect was observed for congruent trials, but was considered non-significant following Bonferroni correction, t(1, 19) = 2.4, p > .025 (see Figure 4). Lastly, for the parietal region, a Session effect was observed, F(1, 19) = 10.0, p = .005, η2 = .34, with increased amplitude following acute exercise (M = 8.6, SE = .82) relative to rest (M = 5.5, SE = .93).
Figure 4.
Topographic maps of the P3 component as a function of session and task congruency.
Decomposition of the Session × Congruency × Site interaction examined Session × Congruency within each of the nine sites. A main effect of Session was observed at the left lateral (7, 5), left medial (3, 1), midline (z), and right medial (2, 4) sites, F(1, 19) > 5.3, p ≤ .03, η2 ≥ .22, indicating increased P3 amplitude following acute exercise relative to rest. At the left medial sites (3, 1), the Session main effect was superseded by and Session × Congruency interaction, F(1, 19) > 6.9, p < .04, η2 > .21. Decomposition of this interaction indicated increased amplitude following acute exercise relative to rest only during incongruent trials, t(1, 19) ≥ 2.9, p ≤ .01 (see Figure 4). No such effect was observed during congruent trials following Bonferroni correction (p > .035). In addition, main effects of Congruency were observed along midline (z) and right medial (2) sites, F(1, 19) ≥ 4.6, p < .05, η2 ≥ .20, with increased amplitude for incongruent relative to congruent trials. No significant effects were observed at the right lateral sites (6, 8).
P3 Latency
The omnibus analysis revealed main effects of Congruency, F(1, 19) = 6.3, p = .02, η2 = .25, and Region, F(1.8, 34.6) = 13.3, p < .001, η2 = .41, which were superseded by two 2-way interactions of Congruency × Region, F(1.8, 34.1) = 4.2, p < .03, η2 = .18, and Region × Site, F(9.7, 184.2) = 2.0, p < .04, η2 = .10. Decomposition of the Congruency × Region interaction used Bonferroni corrected t tests to compare task congruency within each of the five regions. Results indicated significantly longer P3 latencies for incongruent trials at the central (M = 541.7 ms, SE = 13.5 ms) and centro-parietal (M = 526.9 ms, SE = 12.5 ms) regions relative to congruent trials (central: M = 497.4 ms, SE = 12.1 ms; centro-parietal: M = 492.6, SE = 12.2 ms), t(1, 19) > 3.0, p < .01. Decomposition of the Region × Site interaction compared sites within each region and yielded significant Site effects at the frontal and fronto-central regions, F(5.4, 103.1) > 2.5, p < .03, η2 = .12. Given that this interaction is peripheral to the focus of the paper, and the number of t tests (i.e., 36) required to compare nine sites against one another matched with the stringent Bonferroni-corrected criterion value (p < .0014), Site effects for the frontal and fronto-central region are simply characterized by shorter P3 latency at medial relative to lateral sites.
Discussion
Overall, the findings revealed that a single, acute bout of moderately-intense aerobic exercise facilitated children’s cognitive performance and influenced ERP components elicited in a task requiring cognitive control. Specifically, following acute exercise, children exhibited increases in response accuracy and P3 amplitude during incongruent trials. Acute exercise also benefited performance on an academic achievement test of reading. Accordingly, these data indicate that acute exercise might serve as a cost-effective means for improving specific aspects of academic achievement and enhancing cognitive control during preadolescent childhood.
Task Performance
Flanker task performance replicated the robust finding of prolonged RT and decreased response accuracy during incongruent relative to congruent trials (Eriksen and Eriksen, 1974); an effect that has been observed in preadolescent children (Bunge et al., 2002; Hillman et al., 2009; Mezzacappa, 2004) and adult populations (Kramer et al., 1999; Hillman et al., 2006) alike. Novel to this report are the effects of acute exercise on preadolescent children’s task performance. Results indicated general improvements in response accuracy following exercise relative to rest, with selectively better performance during incongruent trials requiring greater amounts of inhibition. Despite a significant trend for congruent trials, the effect of acute exercise was not quite robust enough during this task condition requiring lesser amounts of inhibitory control. Secondary analyses of the interference effect, which examine the cost of conflict engendered by the presence of incongruent flanker stimuli, indicated significantly better accuracy following acute exercise relative to rest. The absolute means for this comparison indicated that task performance improved by almost 5% (d = .5), suggesting that an acute bout of exercise served to enhance the cognitive control of attention through the capability to manage conflict in the visual environment.
RT data indicated that an acute bout of exercise was ineffective in modulating the speed of preadolescent children’s responses. This finding does not stand alone, as prior work with young adults (Hillman et al., 2003) also found no differences in flanker RT following acute exercise. Previous studies focused on the same relationship using other stimulus discrimination tasks have also reported that RT latency appears insensitive to the effects of acute exercise (Grego et al., 2004; Kamijo et al., 2004; Magnié et al., 2000). Regardless, response accuracy may be a more informative measure in this population, given that previous reports have suggested that preadolescent children respond more impulsively than adults with less modulation of their RT (Davidson et al., 2006). As such, the improvements in task performance indicate that acute exercise may be a viable treatment for enhancing cognitive control of inhibition in preadolescent children.
Event-Related Brain Potentials
The neuroelectric changes underlying children’s goal-directed behaviors may provide insight into specific component processes involved in the information processing stream that are influenced by acute aerobic exercise participation. That is, ERPs, and the P3 potential in particular, have been useful in elucidating exercise-induced changes in processes occurring between stimulus engagement and response execution. The present findings contribute to the understanding of this relationship in that the amplitude of the P3 potential increased following exercise relative to rest. Specifically, increases in P3 amplitude were observed following exercise, replicating previous reports with young adults (Hillman et al., 2003). However, a more fine-grain analysis revealed a general amplitude increase in the fronto-central, central, and parietal regions. At the central-parietal region, where maximal P3 amplitude was achieved, a more selective effect was noted, with increased amplitude observed following exercise only for incongruent trials; the trials requiring the most cognitive control. Similar to the response accuracy findings described above, only a trend for congruent trials was observed.
Examination of the P3 topography from a coronal scalp perspective yielded similar findings. Greater exercise-induced P3 amplitude was observed at electrodes overlying the left lateral and medial sites, the midline sites, and the right medial sites. No such modulation was observed over right lateral sites. Additionally, selective increases in P3 amplitude for incongruent trials following exercise were observed at left medial sites, with only a trend noted for congruent trials. Accordingly, similar to the response accuracy findings, acute exercise appears to generally enhance P3 amplitude across the majority of the scalp recording sites, with selective effects at the left medial electrodes overlying the central-parietal region. This finding stands in contrast to specific congruency effects, which exhibited increased amplitude for incongruent trials at midline and right medial electrodes overlying the frontal region.
The observation of a general exercise-induced enhancement in cognitive function with selectively larger effects for task components requiring greater amounts of cognitive control observed herein corroborates prior acute findings in adults (Hillman et al., 2003). For instance, Hillman et al. (2003) observed shorter P3 latency following acute exercise only during incongruent flanker trials. However, to the best of our knowledge the current data are the first to demonstrate this relationship in preadolescent children, providing support for the tenant that acute exercise may serve as a viable treatment for enhancing cognitive function in this population. Given that P3 amplitude is thought to reflect the allocation of attentional resources towards a particular aspect of the stimulus environment (Polich, 2007), and the flanker task has been found to activate the frontal-parietal attentional network (Colcombe et al., 2004), the topography of the exercise-induced P3 modulation appears well-situated. That is, despite the fact that the P3 is believed to be the result of a neural network involving multiple regions (Polich, 2007), it has been repeatedly found to achieve its maxima over the parietal scalp region. Further, neuroimaging findings have revealed increased activation of the superior parietal lobule with higher fitness in older adults (Colcombe et al., 2004), indicating that this particular region appears amenable to exercise intervention. The current results support and extend these prior neuroimaging findings and suggest that preadolescent populations may also derive benefits to the attentional networks underlying cognitive control during inhibitory tasks.
It should be noted that the observed P3 findings are intriguing given that they were observed approximately 25 minutes after the cessation of exercise. Prior work (Hillman et al., 2003) has found similar effects of acute exercise on P3 in young adults 48 minutes after the completion of exercise. These findings raise questions regarding the durability of acute exercise effects on neuroelectric and task performance indices of inhibitory control. Clearly, one avenue for future research is to further characterize the duration of the potential benefits of acute exercise on neurocognitive function.
Academic Achievement Test Performance
The application of exercise-induced effects on brain and cognition is of interest to maximizing cognitive health and function during development. Gaining an understanding of this relationship during tasks that are directly relevant to scholastic performance is crucial toward providing efficacious recommendations for interventions with a focus on enacting public policy and current practice aimed at improving academic standards. The current findings provide a beginning point with which to understand the real-world implications of providing physical activity opportunities during the school day. Specifically, results indicated better reading achievement following acute-exercise, while spelling and mathematics achievement were unaffected. Normative data provided by Wilkinson (1993) suggests that acute exercise might provide meaningful differences in test performance with the observed improvements being indicative of a full grade level increase in reading achievement.
Surprising however, was the lack of exercise-related findings in arithmetic given the computational nature of problem solving requiring the increased recruitment of cognitive control. It is possible that acute exercise, or the dose provided herein, was not sufficient to enhance this aspect of cognition. It is also possible that the beneficial influence of the acute exercise bout subsided by the time this subtest was administered. That is, the WRAT was administered in a fixed order; such that the reading subtest was administered first, followed by the spelling and arithmetic subtests, respectively. Despite that the means for the three subtests are in the hypothesized direction, only reading was robust enough to yield significance. Such a finding is supported by the observed effect sizes (Reading: d = .59, Spelling: d = .16, Arithmetic: d = .06) and future research should consider administering the WRAT closer to the cessation of the acute exercise bout to better determine the relationship to all subject matters. Alternatively, prior research has observed chronic physical activity and increases in aerobic fitness relate to better performance on mathematical achievement (Castelli et al., 2007; Coe et al., 2006), suggesting that the instrument used to measure this aspect of achievement may not be sensitive enough to detect the subtle improvement conferred by acute exercise. Still other research has indicated an equivocal relation between exercise and performance on mathematics tasks (see Trudeau and Shephard, 2008 for review).
It should be noted that this experiment did not assess differences in participation motivation for exercise or academics. It is possible that this factor may also play a role in the relationship between acute exercise and cognition. Future research will need to investigate these factors further to better understand the utility of acute exercise in enhancing scholastic performance.
Lastly, a variety of mechanisms have been posited to account for the underlying changes in cognition following acute bouts of exercise. Non-human animal studies have observed positive associations between aerobic exercise and biochemicals known to increase neuronal proliferation and survival (e.g., brain derived neurotrophic factor, insulin like growth factor 1, serotonin; Brezun & Daszuta, 2000; Russo-Neustadt, Ha, Ramirez, & Kesslak, 2001; van Praag, Kempermann, & Gage, 1999; Vaynman & Gomez-Pinilla, 2005). Other studies suggest that increases in cerebral blood flow may underlie exercise-induced changes in the brain (Delp, et al., 2001; but see Querido & Sheel, 2007 for review), which in turn alter cognitive function. Acute exercise has been found to increase cerebral blood flow due to increases in brain metabolism, which alters the regulation of oxygen, carbon dioxide, glucose, and lactate to neural tissue (Dempsey, Hanson, and Henderson, 1984; Jorgensen et al., 2000). Lastly, others have proposed a more general arousal mechanism whereby acute bouts of exercise arouse the organism in a general fashion leading to changes in cognitive function (Magnié et al., 2000; Kamijo et al., 2004; but see Polich and Kok, 1995 for review). Clearly, future research will need to address these potential mechanisms to better determine the underlying causes of acute exercise-induced changes in cognition.
The development of interventions toward improving school-based performance has become a recent priority. One recent approach, which has proven successful, has been through the implementation of cognitive control training. Diamond and her colleagues (2007) demonstrated the importance of curricula in the development of cognitive control using a randomized control design that randomly assigned teachers and preschool children to a curriculum aimed at either enhancing executive functions or at balanced literacy. Their results indicated improvements in performance in 4–5 year old students assigned to the executive control intervention. Importantly, the tasks involved in the executive control intervention were highly correlated with standardized academic achievement measures (Diamond et al., 2007). Given the capability to develop and deliver cognitive control intervention to preadolescent children in school, as well as the relationship of acute exercise to cognitive control described herein, it seems promising that effective acute exercise interventions may be developed in schools, perhaps in conjunction with cognitive control training, to promote enhanced academic achievement in school age children.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (RO1 HD060385) to Charles Hillman and the National Institute on Aging (RO1 AG25667 and RO1 AG25302) to Arthur Kramer.
Comprehensive List of Abbreviations
- ADHD
attention deficit hyperactivity disorder
- bpm
beats per minute
- DC
direct current
- dB
decibel
- EEG
electroencephalogram
- EOG
electrooculogram
- ERP
event-related brain potential
- HR
heart rate
- Hz
Hertz
- K-BIT
Kaufman brief intelligence test
- kg
kilogram
- kΩ
kilohm
- μV
microvolt
- mL
milliliter
- ms
millisecond
- min
minute
- RER
Respiratory exchange ratio
- RPE
ratings of perceived exertion
- RT
reaction time
- SES
socioeconomic status
- VO2max
maximal oxygen uptake
- WRAT
wide range achievement test
Footnotes
Competing Interest Statement
The authors declare no competing or conflicting interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. New York: Lippincott Williams Wilkins; 2006. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. The Cochrane Collaboration. West Sussex: Wiley & Sons; 2008. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment (Review) pp. 1–98. [DOI] [PubMed] [Google Scholar]
- Ansari D, Dhital B. Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. J Cogn Neurosci. 2006;18:1820–1828. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000;12:391–6. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN. Survey development for assessing correlates of young adolescents’ eating. Am J Health Behav. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Buck SM, Hillman CH, Castelli DM. Aerobic fitness influences on Stroop task performance in preadolescent children. Med Sci Sports Exerc. 2008;4:166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- Budde H, Voelcker-Rehage C, Pietraßyk-Kendziorra S, Ribeiro P, Tidow G. Acute coordinative exercise improves attentional performance in adolescents. Neurosci Lett. 2008;441:219–223. doi: 10.1016/j.neulet.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: An H2150 PET study of Stroop task performance. Neuroimage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin H. Physical fitness and academic achievement in 3rd 5th Grade Students. J Sport Exerc Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Caterino MC, Polack ED. Effects of two types of activity on the performance of second-, third-, and fourth-grade students on a test of concentration. Percep Motor Skills. 1999;89:245–248. doi: 10.2466/pms.1999.89.1.245. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- Coe DP, Pivarnik JM, Womack CJ, Reeves MJ, Malina RM. Effect of physical education and activity levels on academic achievement in children. Med Sci Sports Exerc. 2006;38:1515–1519. doi: 10.1249/01.mss.0000227537.13175.1b. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol: Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan. Software Manual. El Paso, TX: 2003. Offline analysis of acquired data (SCAN 4.3 – Vol. II, EDIT 4.3) [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, Gregoski M. Effects of aerobic exercise on overweight children’s cognitive functioning: A randomized controlled trial. Res Q Exerc Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol. 2001;533:849–859. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol. 1984;355:161–75. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. New York: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan-Johnson CC. P3 latency: A new metric of information processing. Psychophysiology. 1981;18:207–215. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CE. Effects of noise letters in the identification of target letters in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. doi: 10.1002/hipo.20547. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fields T, Diego M, Sanders CE. Exercise is positively related to adolescents’ relationships and academics. Adolescence. 2001;36:105–110. [PubMed] [Google Scholar]
- Freedson PS, Goodman TL. Measurement of oxygen consumption. In: Rowland TW, editor. Pediatric laboratory exercise testing: Clinical guidelines. Champaign: Human Kinetics; 1993. pp. 91–113. [Google Scholar]
- Gabbard C, Barton J. Effects of physical activity on mathematical computation among young children. J Psychol. 1979;103:287–288. [Google Scholar]
- Gobel SM, Johansen-Berg H, Behrens T, Rushworth MF. Response-selection-related parietal activation during number comparison. J Cogn Neurosci. 2004;16:1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- Grego F, Vallier J-M, Collardeau M, Bermon S, Ferrari P, Candito M, et al. Effects of long duration exercise on cognitive function, blood glucose, and counterregulatory hormones in male cyclists. Neurosci Lett. 2004;364:76–80. doi: 10.1016/j.neulet.2004.03.085. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JT, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev Psych. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med Sci Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Motl RW, Pontifex MB, Posthuma D, Stubbe JH, Boomsma DI, de Geus EJC. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psych. 2006;25:678–687. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Snook EM, Jerome GJ. Acute cardiovascular exercise and executive control function. Int J Psychophysiol. 2003;48:307–314. doi: 10.1016/s0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Jorgensen LG, Nowak M, Ide K, Secher NH. Cerebral blood flow and metabolism. In: Saltin B, Boushel R, Secher N, Mitchell J, editors. Exercise and circulation in health and disease. Champaign: Human Kinetics; 2000. pp. 113–236. [Google Scholar]
- Kamijo K, Nishihira Y, Hatta A, Kaneda T, Wasaka T, Kida T, Kuroiwa K. Differential influences of exercise intensity on information processing in the central nervous system. Euro J Appl Physiol. 2004;92:305–311. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kim EY, Iwaki N, Imashioya H, Uno H, Fujita T. Error-related negativity in a visual Go/No-Go task: children vs. adults. Dev Neuropsych. 2007;31:181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lindner KJ. The physical activity participation-academic performance relationship revisited: perceived and actual performance and the effect of banding (academic tracking) Pediat Exerc Sci. 2002;14:155–169. [Google Scholar]
- Magnié MN, Bermon S, Martin F, Madany-Lounis M, Suisse G, Muhammad W, Dolisi C. P300, N400, aerobic fitness and maximal aerobic exercise. Psychophysiology. 2000;37:369–377. [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Morris RGM. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122:1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Mahar MT, Murphy SK, Rowe DA, Golden J, Shields AT, Raedeke TD. Effects of a classroom-based program on physical activity and on-task behavior. Med Sci Sports Exerc. 2006;38:2086–2094. doi: 10.1249/01.mss.0000235359.16685.a3. [DOI] [PubMed] [Google Scholar]
- McNaughten D, Gabbard C. Physical exertion and the immediate mental performance of sixth-grade children. Percept Motor Skills. 1993;77:1155–1159. doi: 10.2466/pms.1993.77.3f.1155. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Polich J. Task difficulty, probability and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1987;63:251–259. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Hillman CH. Neuroelectric and behavioral indices of interference control during acute cycling. Clin Neurophysiol. 2007;118:570–580. doi: 10.1016/j.clinph.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–785. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005;15:1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediat Exerc Sci. 2003;15:243–256. [Google Scholar]
- Tanner JM. Growth at adolescence: With a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe SA. Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003a;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Cognitive and behavioral responses to acute exercise in youth: a review. Ped Exerc Sci. 2003b;15:348–359. [Google Scholar]
- Tomporowski PD, Davis CL, Lambourne K, Gregoski M, Tkacz J. Task switching in overweight children: Effects of acute exercise and age. J Sport Exerc Psychol. 2008;30:497–511. doi: 10.1123/jsep.30.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomporowski PD, Davis CL, Miller PH, Naglieri JA. Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau F, Shephard RJ. Physical education, school physical activity, school sports and academic performance. Int J Behav Nutrit Physl Activ. 2008;5:5–10. doi: 10.1186/1479-5868-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Roberson RJ, Nieman DC, Kang J. Children’s OMNI scale of perceived exertion: Walking/running evaluation. Med Sci Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: Exercise impacts functional plasticity in the intact ad injured central nervous system by using neurotrophins. Neurorehabilitation Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3 Administration manual. Wilmington, DE: Jastak Associates; 1993. [Google Scholar]