Abstract
Research in songbirds shows that singing behavior is regulated by both brain areas involved in vocal behavior as well as those involved in social behavior. Interestingly, the precise role of these regions in song can vary as a function of the social, environmental and breeding context. To date, little is known about the neurotransmitters underlying such context-dependent regulation of song. Dopamine (DA) modulates highly motivated, goal-directed behaviors (including sexually motivated song) and emerging data implicate DA in the context-dependent regulation of singing behavior. This study was performed to begin to examine whether differences in DA receptors may underlie, in part, context-dependent differences in song production. We used autoradiographic procedures to label D1-like and D2-like DA receptors to examine the relationship between DA receptor density and singing behavior in multiple contexts in male European starlings (Sturnus vulgaris). Within a breeding context (when testosterone (T) was high), D1-like receptor density in the medial preoptic nucleus (POM) and midbrain central gray (GCt) negatively correlated with song used to attract a female. Additionally in this context, D1-like receptor density in POM, GCt, medial bed nucleus of the stria terminalis (BSTm), and lateral septum (LS) negatively correlated with song likely used to defend a nestbox. In contrast, in a non-breeding context (when T was low), D1-like receptor density in POM and LS positively correlated with song used to maintain social flocks. No relationships were identified between song in any context and D2-like receptor densities. Differences in the brain regions and directional relationships between D1-like receptor binding and song suggest that dopaminergic systems play a region and context-specific role in song. These data also suggest that individual variation in singing behavior may, in part, be explained by individual differences in D1-like receptor density in brain regions implicated in social behavior.
Keywords: medial preoptic nucleus, midbrain central gray, medial bed nucleus of the stria terminalis, lateral septum, songbird, birdsong
Animals exhibit vocal behavior in a variety of contexts in response to many different social, physiological, and environmental stimuli. The specific stimuli that elicit such communication signals depend upon many factors such as the presence or absence of potential mates or rivals, dominance status, and season. Furthermore, there is typically pronounced individual variation in the degree to which animals respond to these various stimuli with vocal signals. To date, the neurotransmitters regulating context-appropriate vocal communication and the neural correlates of individual variation in vocal behavior have not been well-studied.
Male European starlings (Sturnus vulgaris) provide an excellent model in which to examine the neural regulation of context-appropriate vocal communication. Except for a brief period during molt, male starlings sing year-round but the function of song and the social and physiological cues that stimulate song production differ depending upon the context in which it occurs (Eens, 1997). Within the context of breeding (i.e. the spring breeding season), circulating levels of testosterone (T) are elevated (Ball and Wingfield, 1987, Riters et al., 2002) and song plays an important role in mate attraction and nest site defense (Eens, 1997). When approached by an opposite-sex conspecific, unpaired males typically fly to nest sites and produce high levels of intersexually motivated, courtship song (Cuthill and Hindmarsh, 1985, Pinxten and Eens, 1990, Eens et al., 1993, Eens et al., 1994, Riters et al., 2000). Males also respond to same-sex conspecifics by flying to nest sites and displaying intrasexually motivated song (Eens, 1997). Agonistic encounters between males at a nest site are often accompanied by singing behavior (Eens, 1997), and prospecting males tend to avoid nestboxes broadcasting breeding context-typical male conspecific song (Mountjoy and Lemon, 1991).
In a non-breeding context (i.e. fall and winter), male starlings sing at high rates when circulating levels of T are basal (Ball and Wingfield, 1987, Riters et al., 2002). In this context, males do not respond to opposite-sex conspecifics by altering their rate of song production (Riters et al., 2000) and song is not sexually motivated (nonsexually motivated), at least for purposes of immediate copulation. At this time, males abandon nest sites and form large, mixed-sex flocks (Feare, 1984) where song appears to maintain flock cohesion (Eens, 1997). Non-breeding context song may also be important in establishing and maintaining dominance hierarchies within the flock (Summers et al., 1987, Hausberger et al., 1995) but in most cases, seems to be used in non-aggressive situations (Eens, 1997). For example, males are often observed sitting in close proximity on the same perch while singing (Eens, 1997). Taken together, in contrast with breeding context song, non-breeding context song functions in social affiliation and appears to be unrelated to immediate mate attraction or nest site defense (Eens, 1997, Riters et al., 2000).
Several lines of evidence suggest the songbird brain differentially regulates singing behavior depending upon the context in which it occurs. In songbirds, song learning, production and perception are controlled by the song control system including HVC (used as a proper name), robust nucleus of the arcopallium (RA), and Area X (among several others) (Brenowitz et al., 1997, Margoliash, 1997, Wild, 1997, Mello, 2002). Studies utilizing immunocytochemistry and in situ hybridization for immediate early genes (IEGs) and electrophysiology suggest neural activity in HVC, RA, and Area X associated with singing behavior differs depending upon whether song is sexually or nonsexually motivated (Heimovics and Riters, 2005), sung on or away from a nest site (Riters et al., 2004a), or directed towards or away from a conspecific (Jarvis et al., 1998, Hessler and Doupe, 1999). Brain areas outside of the song control system, known to regulate multiple forms of social behavior (including communication) (Newman, 1999, Goodson, 2005) are also implicated in the neural regulation of singing behavior (e.g. Goodson, 1998, Riters and Ball, 1999, Maney and Ball, 2003). Immunocytochemistry for IEGs, lesion, and electrophysiology studies suggest that these ‘social behavior network’ nuclei (including the medial preoptic nucleus (POM), ventral tegmental area (VTA), midbrain central gray (GCt; the avian homolog of the mammalian periaqueductal gray), lateral septum (LS), medial bed nucleus of the stria terminalis (BSTm), anterior hypothalamus (AH), and ventromedial nucleus of the hypothalamus (VMH)) differentially regulate song production depending upon whether it occurs within or outside of the breeding season (Heimovics and Riters, 2005, Alger and Riters, 2006, Heimovics and Riters, 2006, Heimovics and Riters, 2007), on or away from a nest site (Riters et al., 2004b), in colonial or territorial species (Goodson et al., 1999), or directed towards or away from a conspecific (Yanagihara and Hessler, 2006, Hara et al., 2007). Taken together, these data suggest that regions implicated in song control and regions involved in social behavior interact to regulate singing behavior context-dependently.
To date, the neurotransmitter systems underlying the context-dependent regulation of birdsong remain unclear. The neurotransmitter dopamine (DA) has been implicated in reinforcement and reward (Wise, 2005). More recently, rather than underlying reward itself, DA has been suggested to underlie highly motivated, goal-directed or anticipatory responses to stimuli associated with reward, including opposite sex conspecifics, food, and drugs of abuse (Blackburn et al., 1992, Hull et al., 1995, Berridge and Robinson, 1998, Salamone et al., 2003). A growing body of research also implicates DA in the neural regulation of vocal communication directed towards opposite-sex conspecifics. Peripheral pharmacological manipulations of breeding context male starlings show that DA agonists facilitate and DA antagonists inhibit singing behavior in response to the introduction of a female conspecific (Schroeder and Riters, 2006). DA antagonists also inhibit courtship singing in male zebra finches (Rauceo et al., 2007). Additionally, DA stimulates vocal behavior associated with the anticipation of social reward in rats (Burgdorf et al., 2001, Wintink and Brudzynski, 2001), suggesting the role of DA in goal-directed vocal communication extends to mammalian species as well.
Recent data also suggest that DA may be responsible for the context-dependent modulation of singing behavior. In zebra finches, in vivo microdialysis reveals that, relative to song that is not directed towards a conspecific, levels of DA in Area X are higher during directed singing (Sasaki et al., 2006). In starlings, immunoreactive tyrosine hydroxylase (TH, the rate-limiting enzyme in DA synthesis) in Area X, POM, and VTA correlates with intersexually motivated, but not nonsexually motivated singing behavior (Heimovics and Riters, 2008). While these data do not preclude a role for DA in the regulation of song in other contexts they do suggest that DA synthesis in Area X, POM, and VTA may be more tightly linked to female-directed vocal communication. Although the effects of DA manipulations on intrasexually and nonsexually motivated singing behavior have not been examined in songbirds, it is plausible that dopaminergic modulation of vocal behavior is context-dependent.
DA (Barclay and Harding, 1988, Sakaguchi and Saito, 1989, Barclay and Harding, 1990) and TH-immunoreactive fibers (Bottjer, 1993, Soha et al., 1996) are found throughout the song control system and the boundaries of Area X can be defined based on a high density of DA receptors in a manner that is consistent with Nissl-defined boundaries (Casto and Ball, 1994). Tract-tracing studies identify VTA and GCt as the primary sources of dopaminergic inputs to Area X, HVC, and RA (Lewis et al., 1981, Appeltants et al., 2000, Appeltants et al., 2002). In mammals, mesolimbic and incertohypothalamic DA systems implicated in motivation and reward include projections from VTA to septum and BST and projections from zona incerta to the preoptic area (Feldman et al., 1997). In male starlings, POM and VTA share reciprocal neuroanatomical connections (Riters and Alger, 2004). In songbirds, DA receptors and DA-related proteins are dense within POM and VTA (Barclay and Harding, 1988, 1990, Ball et al., 1995, Absil et al., 2001, Bharati and Goodson, 2006) and present in other brain regions implicated in social behavior (Bailhache and Balthazart, 1993, Bottjer, 1993, Heimovics and Riters, 2008). Taken together, DA neural circuits within song control and social behavior nuclei are well-positioned to modulate song context-dependently.
DA systems are sensitive to changes in circulating steroid hormone levels. High concentrations of mRNA coding for gonadal steroid hormone receptors are found in VTA and GCt (Maney et al., 2001), and an abundance of androgen and estrogen receptors are found throughout the song control system (see Ball et al., 2002 for review). Furthermore, in songbirds, treatments with exogenous steroids 1) increase TH immunoreactivity in HVC and RA, 2) increase DA levels in the preoptic area, lateral magnocellular nucleus of the anterior nidopallium (lMAN; another song control nucleus), and Area X, and 3) alter DA turnover in the preoptic area, Area X and RA (Barclay and Harding, 1990, Appeltants et al., 2003). The link between steroid hormones and DA further suggests that DA activity may differentially regulate song within a breeding context (when T is high) and a non-breeding context (when T is low).
If DA systems modulate singing behavior context-dependently or underlie individual differences in singing behavior, one candidate component of dopaminergic transmission that might mediate such effects would be the DA receptor system. Changes in dopamine receptor density can be indicative of changes in the physiological responsiveness to dopamine. Localizing such changes to specific brain areas would provide insight into the modulation of dopaminergic neurotransmission in defined neural circuits. We hypothesize that context-dependent effects on singing and/or individual differences in song behavior could be reflected in differences in the abundance and/or distribution of DA receptors in brain areas known to be involved in the context-dependent regulation of song production (i.e. regions involved in song control and social behavior). To examine this possibility, we used receptor autoradiography methods to label D1-like and D2-like dopamine receptors in order to examine relationships between DA receptor density in song control and social behavior nuclei and intersexually, nonsexually, and likely intrasexually motivated song.
EXPERIMENTAL PROCEDURES
Capture and housing
Forty adult male and ten adult female European starlings were captured from December 2005 through February 2006 on a single farm northwest of Madison, Wisconsin using fly-in traps. After capture, birds were housed indoors in the University of Wisconsin-Madison Department of Zoology animal facilities in single sex cages (91cm × 47cm × 47cm) on a light cycle mimicking the natural outdoor photoperiod. Twelve weeks prior to the beginning of behavioral observations, the photoperiod was manipulated to induce the state of “photosensitivity”; a condition characteristic of the time period just prior to the breeding season in which a bird is ready to respond to increasing photoperiods with marked gonadal growth, increases in plasma steroid hormone concentrations and sexual behavior. Specifically, birds were placed on 18L:6D for six weeks to render them photorefractory and then shifted to 6L:18D for six weeks to induce photosensitivity (Dawson et al., 2001). Photosensitive birds then either served as non-breeding context birds or were photoperiod and hormone manipulated to induce a breeding context-typical endocrine state and associated behaviors (see below). Protocols used for bird acquisition, surgery, and behavioral testing were in adherence to guidelines approved by the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996 as well as the University of Wisconsin-Madison Research Animal Resource Committee (RARC). Every effort was made to minimize the number of animals used and their suffering.
Hormone implants
Four weeks prior to the beginning of behavioral testing, males were randomly assigned to either breeding (n=30) or non-breeding (n=10) context flocks. Females were randomly assigned to serve as either breeding (n=5) or non-breeding (n=5) context stimulus birds. Breeding context males were given subcutaneous implants of testosterone (two, 14mm lengths of silastic tubing [Dow Corning, i.d., 1.47mm; o.d. 1.96mm] packed for 10mm with crystalline testosterone proprionate and sealed with silastic glue [Sigma]). Breeding context females were given subcutaneous implants of estrogen (two, 17mm lengths of silastic tubing packed for 13mm with 17β-estradiol sealed and with silastic glue [Sigma]). Breeding context animals were hormone implanted to facilitate courtship behavior. As a control, non-breeding context males and females were given blank implants. Birds were lightly anesthetized using isoflurane gas anesthesia and a small incision was made in the skin over the breast muscle. Implants were placed under the skin, the incision was sutured and birds were allowed to recover on a heated pad. Once fully recovered, birds were placed back into single-sex cages. Breeding context birds were shifted to 11L:13D (a photoperiod which induces gonadal growth characteristic of the early breeding season, (Dawson et al., 2001)) and non-breeding context birds remained on 6L:18D. They remained in these conditions for the remainder of the experiment.
Behavioral observations
One to two days after implant surgery, three flocks of ten breeding context males and one flock of ten non-breeding context males were introduced into four separate indoor behavioral observation aviaries. Birds were behaviorally tested in flocks because we were most interested in examining individual differences in singing behavior as it occurs within a dynamic social system (i.e. flock). Multiple breeding context flocks were used because, within a breeding context, our focus was song produced from nest boxes (see below) and we wanted to ensure our sample included adequate numbers of males singing from next boxes for analysis. Observation aviaries contained five nestboxes and branches for perching. Food and water were provided ad libitum. Behavioral observations began after a four week habituation period. For all flocks, behavioral observations took place between 0900 and 1200.
Breeding context flocks
Within breeding context flocks we focused our analyses selectively on song produced from nest boxes. As mentioned in the introduction, within a breeding context song sung from nest boxes serves both to attract females and to repel male competitors. The function of song from other locations is not as clear. Given our specific interest in examining the role of DA in song produced within functionally distinct contexts, we considered song from nest boxes to be the least ambiguous in terms of function. We consider song from a nest box in the presence of a female to most strongly reflect intersexually motivated song. In contrast, we considered song in the absence of a female to be less intersexually motivated and, because several males were present and a limited number of nestboxes were available in the aviary, more likely associated with male defense of nest boxes. Thus we refer to song from a nestbox when no female was present as intrasexually motivated song. In sum, intersexually motivated singing behavior was operationally defined as song sung from on top of, on the perch of, or inside of a nestbox with a female conspecific present in the aviary, and intrasexually motivated singing behavior was operationally defined as song produced from the same locations without a female conspecific present in the aviary.
In all three breeding context flocks, both inter- and intrasexually motivated song were quantified in the same individuals. In other words, measures of song sung from a nestbox (both with and without a female present) were taken for every male on every test day. Specifically, flocks were observed on five consecutive days for one hour each day (thirty minutes with and thirty minutes without a breeding context female present in the aviary) and a point sampling technique was used to determine the proportion of time any member of the flock spent singing from a nestbox (i.e. during each thirty minute observation period it was noted at sixty second intervals whether any member of the flock was singing from a nestbox (Eens and Pinxten, 1990)). The order of thirty minute observations (with versus without a female present) was counterbalanced across days and a novel female conspecific was used each day.
Non-breeding context flock
In a non-breeding context, males abandon nest sites, form large flocks and song is not sexually motivated but functions in social affiliation (referred to here as nonsexually motivated). Given that there is no evidence that song produced from or away from nest boxes differs functionally in a non-breeding context, nonsexually motivated song was operationally defined as song sung from any location in the aviary. Past research from our laboratory has shown that non-breeding context males do not significantly alter song production in response to the introduction of a female (e.g. Riters et al., 2000). However, to be able to compare breeding and non-breeding context flocks we controlled for the effect of exposure to a novel female conspecific. Each day a non-breeding context female was introduced to the non-breeding context flock for thirty minutes (behavioral data from these sessions were not used for analysis). A non-breeding context female was used to maintain ecological validity (i.e. in the wild a non-breeding context male would never encounter a breeding context female). Nonsexually motivated song production was quantified as described above for the breeding context flocks.
Tissue Processing
Because inter- and intrasexually motivated song were operationally defined as song sung from on top of, on the perch of, or inside of a nestbox only a subset of sixteen breeding context males (i.e. only those observed singing from nestboxes) were included in the present analysis. All ten non-breeding context males were included. One day after the last day of behavioral observations, males were sacrificed via rapid decapitation. Brains were removed, frozen immediately on dry ice, and stored at −80°C until sectioning. Brains were sectioned into 16μm-thick sections using a cryostat and thaw mounted onto gel-coated microscope slides. Six series of slides were collected so that, on each slide, consecutive sections were 80μm apart. Series one through four were used for D1-like and D2-like receptor autoradiography (one total binding and one non-specific binding for each receptor subtype), series five was used for Nissl, and series six was used for an autoradiography not discussed here. The slides were dried and stored at −20°C until use.
Autoradiography
After drying at room temperature, slides were pre-incubated in buffer (50 mM Tris HCl, 120mM NaCl, 5mM KCl, 2mM CaCl2, 1mM MgCl2, pH 7.4) for 30 min at room temperature. Slides were then incubated for one hour at room temperature in 2nM [3H]-SCH-23390 (for D1-like receptors; Perkin-Helmer, Specific activity = 85 Ci/mmol) or 0.4nM [3H]-Spiperone (for D2-like receptors; Amersham, Specific activity = 101 Ci/mmol) buffer with no competitor (total binding, series 1). These concentrations were selected based on concentration response curves run during a pilot study which indicated that they yield the best signal to noise ratio. Non-specific binding was determined by the addition of a cold competitor ((±)Butaclamol 10μM for D1-like receptors; Haloperidol 10μM for D2-like receptors. Both drugs were purchased from Sigma. One hour later, the slides were washed twice for five min in ice-cold buffer followed by a quick dip in ice-cold distilled water. Sections were fan dried, placed in X-ray cassettes and exposed to BioMax® MR films (Kodak) along with tritium standards containing known concentrations of tritium ranging from 0.00 to 488.1 μCi/g (ART-123; American Radiolabeled Chemicals Inc., St Louis, MO). The films were developed after 6 weeks.
Quantification
Films were scanned (8-bit, 600dpi) using an Epson Perfection 1240U bed scanner connected to a PC computer. Digitized autoradiograms were analyzed using MetaVue software (Fryer Company, Inc., Huntley, IL) following standard autoradiographical procedures (e.g. Wang et al., 1997, Chen and Lawrence, 2003). Specifically, the tritium standards were used to calibrate the intensity of the scanned images in terms of radiochemical concentrations in fmol/mg using the following equation: (X μCi / 1000mg) * (106 fmol/Y mg), where X equals the numerical values associated with the standard and Y equals the specific activity of the ligand (85 Ci/mmol for [3H]-SCH-23390 and 101 Ci/mmol for [3H]-Spiperone). The gray level value of each standard was then measured in Meta Vue and the known concentration of radioactivity (in fmol/mg) was assigned to each corresponding gray level. Values for standards spanning the range of optical density of labeled tissue were selected, and the relationship between the gray values and measures of radioactivity was fit to a 3rd degree polynomial equation, which was used to interpolate gray values between points on the standards.
Using these calibrated gray levels, the average intensity of specific binding for D1-like and D2-like was determined for POM, VTA, GCt, three zones of LS (caudal part, ventrolateral zone (LSc.vl), caudal part, ventral zone (LSc.v), and rostral part (LSr)), BSTm, AH, VMH, Area X, HVC, and RA (identified based on (Heimovics and Riters, 2007); except in the present study we collected measures from the portion of BSTm located just caudal to the anterior commissure (Figure 1 and 5)). The location of each brain area was verified based on landmarks visible on both autoradiograms and/or adjacent Nissl stained sections.
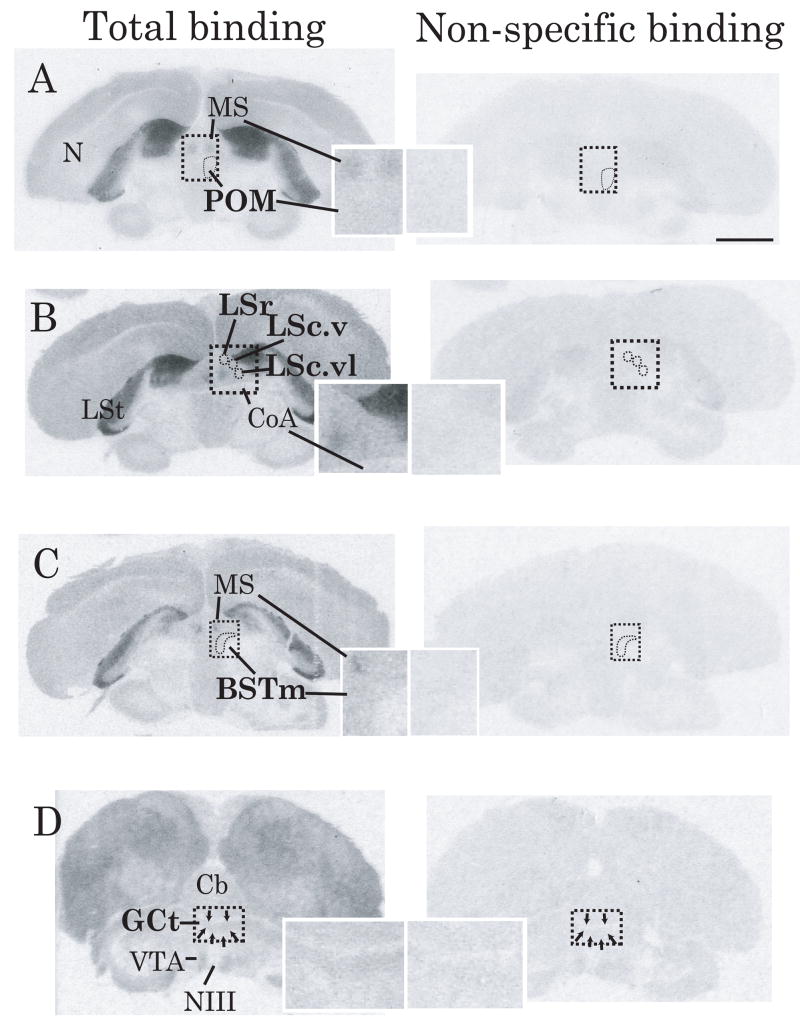
Figure 1.
A) Representative autoradiograms illustrating differences between total (left column) and non-specific (right column) D1-like receptor binding in sections containing regions in which binding related to song; A) POM, B) Subdivisions of LS, C) BSTm, and D) GCt. The boundaries of POM, subdivisions of LS, BSTm and GCt are indicated within the dashed boxes. Overlays contain magnified images (500%) of the area blocked off in the images at lower magnification. In all brain regions examined in all animals, total binding was higher than non-specific binding. Thus, to determine specific binding, non-specific binding values were subtracted from total binding values. Abbreviations: N = nidopallium, MS = medial septum, LSt = lateral striatum, CoA = anterior commissure, Cb = cerebellum, NIII = 3rd cranial nerve. See text for additional abbreviations. Contrast was adjusted identically using Adobe Photoshop Elements 6.0 for all images. Scale bar in top right figure = approximately 3 mm.
Figure 5.
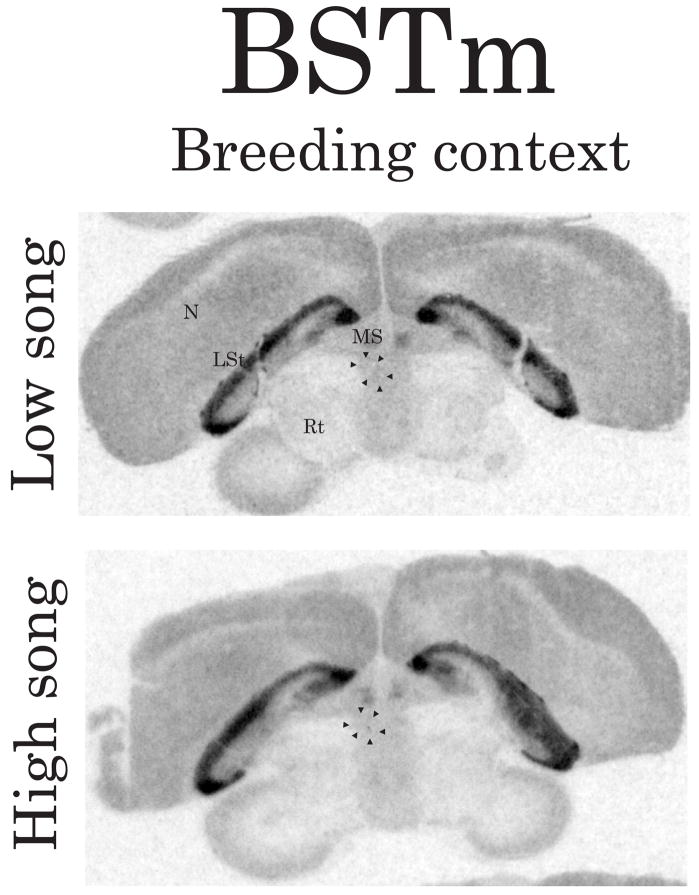
Representative autoradiograms illustrating the density/distribution of D1-like receptors in coronal brain sections containing BSTm. Arrows indicate the approximate boundaries of BSTm (verified in adjacent Nissl-stained sections) in the left hemisphere. The top image is from a breeding context male observed singing at a low rate and the bottom image is from a breeding context male observed singing at a high rate. Abbreviations: LSt, lateral striatum; MS medial septum; N, nidopallium; Rt, nucleus rotundus. See Figure 2 for scale bar.
Specific binding was determined by subtracting non-specific binding values from total binding values. In all regions, total binding was higher than non-specific binding (Figure 1). Within each region, total binding was quantified by measuring the average intensity of calibrated gray levels within a circular region located within the boundaries of each nucleus (visible in adjacent Nissl-stained tissue) on three serial sections bilaterally. In cases of tissue damage or uneven exposure of autoradiograms, average intensity was measured on a fourth section. If tissue damage and/or uneven exposure was extensive, that individual was dropped from quantification for that brain area. For all regions except Area X, nucleus boundaries were not visible on non-specific binding autoradiograms and non-specific binding did not appear to differ between individuals. The latter was verified by quantifying the average intensity of non-specific binding in these regions (the location of each was verified using adjacent Nissl-stained sections) in six randomly selected individuals and finding these values did not differ significantly across individuals. However, non-specific binding did vary across regions (Figure 1). Thus, to determine specific binding in POM, VTA, GCt, LS, BSTm, AH, VMH, and RA, we calculated a mean average intensity of non-specific binding for each region from these six individuals and subtracted that value from all total binding values. In Area X, nucleus boundaries were visible on autoradiograms, and measures could be taken on adjacent non-specifically labeled section of Area X easily without the use of Nissl stained tissue. Thus, to determine specific binding in Area X we quantified the average intensity of non-specific binding for every individual on adjacent sections and subtracted those values from total binding. For all individuals, specific binding values were averaged separately for each region and receptor subtype and the mean used for statistical analysis.
Statistics
Point sampled intersexually, intrasexually, and nonsexually motivated singing behavior was summed across the five days of behavioral observations. Because the song measures were proportion data, they were arcsine transformed to improve normality (Lehner, 1996). Specifically, the number of point samples with song out of one hundred and fifty (i.e. five days with thirty point samples each day) was transformed using the following equation: 2*arcsine √ ( # observed songs/150). Data were analyzed using Statistica 6.0 software (Stat Soft Inc., Tulsa, OK). Pearson correlation analysis was used to determine the extent to which specific binding for D1-like and D2-like receptors in POM, VTA, GCt, LS, BSTm, AH, VMH, Area X, RA, and HVC correlated with intersexually, intrasexually and nonsexually motivated song. T-tests were used to compare specific binding for D1-like and D2-like receptors in POM, VTA, GCt, LS, BSTm, AH, VMH, Area X, RA, and HVC between breeding context and non-breeding context males. When specific binding data did not meet the assumptions of parametric statistics they were log transformed. In all cases log transformed data met the assumptions of parametric statistics.
RESULTS
DA receptor density in males singing within a breeding context
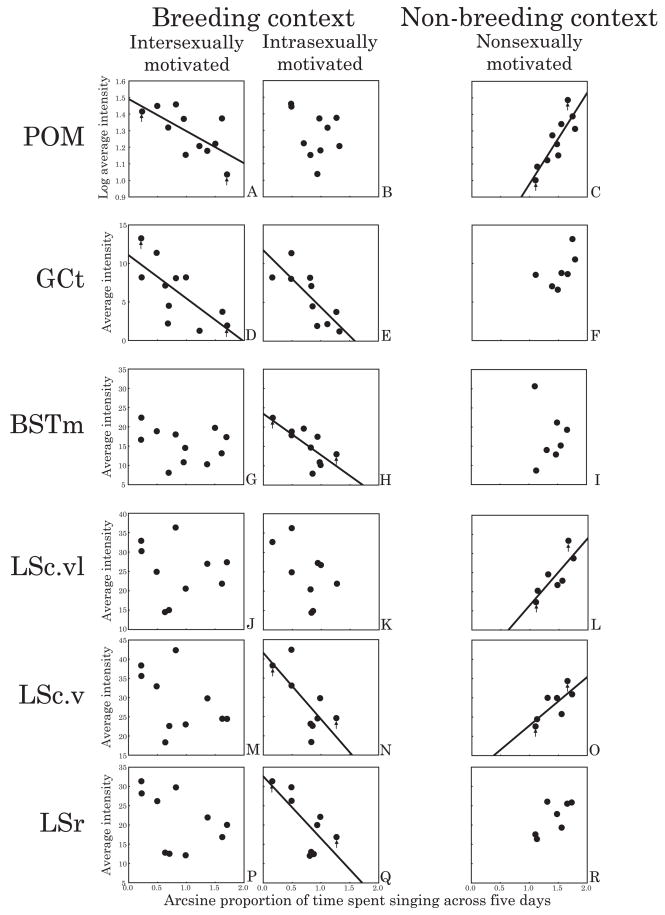
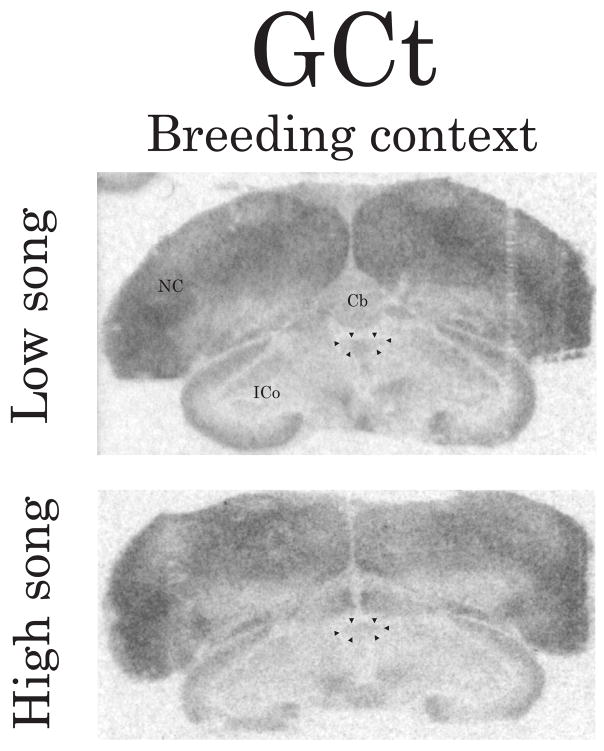
Results of correlations between song and D1-like receptor density for males from the three breeding context flocks were similar for each brain region. Thus males from all three flocks were combined for analyses. Pearson correlation analyses revealed negative linear relationships between intersexually motivated singing behavior and D1-like receptor density in POM and GCt (POM: Figure 2A and Figure 3, left images, r = −0.66, p = 0.03; GCt: Figure 2D and Figure 4, r = −0.71, p = 0.01). D1 receptor density did not significantly correlate with intersexually motivated song in any of the other brain regions examined (p≥0.21 in all cases), including VTA, AH, VMH, Area X (which was densely labeled and also defined by D1-like binding as has been previously reported (Casto and Ball, 1994), and HVC and RA (which were also distinguishable from surrounding areas based on the pattern of D1-like receptor binding).
Figure 2.
Scatterplots showing the relationship between singing behavior and D1-like receptor density in (A–C) POM, (D–F) GCt, (G–I) BSTm, (J–L) LSc.vl, (M–O) LSc.v, and (P–R) LSr. Left panels are males from breeding context flocks that were observed singing from a nestbox in the presence of a female (intersexually motivated song), middle panels are males observed singing from a nestbox without a female present (presumed intrasexually motivated song), and right panels are non-breeding context males observed singing without a female present. Breeding context singing behavior with and without a female present was quantified in the same individuals and each point represents one individual. Differences in sample sizes between inter-and intrasexually motivated song are due to individuals not displaying vocal behavior in both contexts. Differences in sample sizes between regions within a context are due to tissue damage and/or uneven exposure of autoradiograms. Presence of regression line indicates significant (p<0.05) linear relationships. Arrows indicate the low and high singers represented in Figures 3–6.
Figure 3.
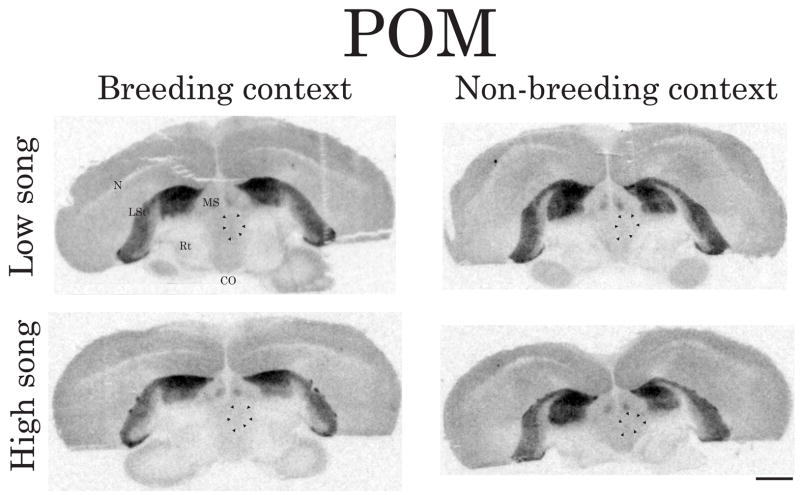
Representative autoradiograms illustrating the density/distribution of D1-like receptors in coronal brain sections containing POM. Arrows indicate the approximate boundaries of POM (verified in adjacent Nissl-stained sections) in the right hemisphere. The top images are from males singing at low rates and the bottom images are from males singing at high rates. The left images are from breeding context males and the right images are from non-breeding context males. Abbreviations: CO, optic chiasm; LSt, lateral striatum; MS medial septum; N, nidopallium; Rt, nucleus rotundus. The contrast for each image (in this figure and Figures 4–6) was enhanced in an identical fashion for all individuals being compared using Adobe Photoshop Elements 6.0. Scale bar = 2mm
Figure 4.
Representative autoradiograms illustrating the density/distribution of D1-like receptors in coronal brain sections containing GCt. Arrows indicate the approximate boundaries of GCt (verified in adjacent Nissl-stained sections). The top image is from a breeding context male observed singing at a low rate and the bottom image is from a breeding context male observed singing at a high rate. Abbreviations: Cb, cerebellum; ICo, nucleus intercollicularis; NC, caudal nidopallium. See Figure 2 for scale bar.
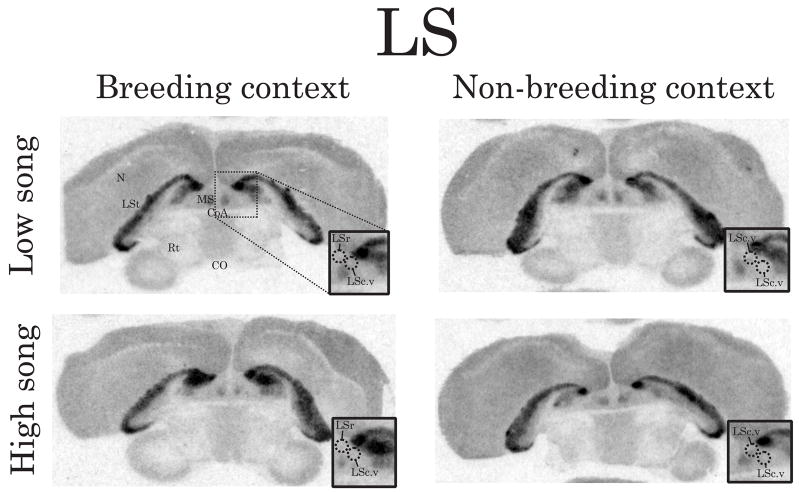
Correlation analyses also revealed negative linear relationships between intrasexually motivated singing behavior and D1-like receptor density in GCt, BSTm, LSc.v and LSr (GCt: Figure 2E and Figure 4, r = −0.80, p = 0.006; BSTm: Figure 2H and Figure 5, r = −0.73, p = 0.02; LSc.v: Figure 2N and Figure 6, left images, r = −0.71, p = 0.03; LSr: Figure 2Q and Figure 6, left images, r = −0.71, p = 0.03). In all other brain regions examined, D1-like receptor density did not significantly correlate with intrasexually motivated song (p≥0.13 in all cases).
Figure 6.
Representative autoradiograms illustrating the density/distribution of D1-like receptors in coronal brain sections containing LS. Inset box is a magnified version of the autoradiogram centered on LS in the right hemisphere. Dashed circles indicate the zones within LS in which significant relationships between D1-like receptor density and song were observed. The top images are from males singing at low rates and the bottom images are from males singing at high rates. The left images are from breeding context males and the right images are from non-breeding context males. Abbreviations: CoA, anterior commissure; CO, optic chiasm; LSt, lateral striatum; MS medial septum; N, nidopallium; Rt, nucleus rotundus. See Figure 2 for scale bar.
In all brain regions examined, D2-like receptor density did not significantly correlate with either inter- or intrasexually motivated song (although a trend for a negative relationship between D2-like receptor density in GCt and intrasexually motivated song was observed (p=0.08). In all other regions p≥0.28 ).
DA receptor density in males singing within a non-breeding context
Correlation analyses revealed positive linear relationships between nonsexually motivated singing behavior and D1-like receptor density in POM, LSc.vl, and LSc.v (POM: Figure 2C and Figure 3, right images, r = 0.86, p = 0.001; LSc.vl: Figure 2L and Figure 6, right images, r = 0.82, p = 0.03; LSc.v: Figure 2O and Figure 6, right images, r = 0.77, p = 0.04). In all other brain regions examined, D1-like receptor density did not significantly correlate with nonsexually motivated song (p≥0.26 in all cases). In all brain regions examined, D2-like receptor density did not significantly correlate with nonsexually motivated song (p≥0.30 in all cases). As was the case for breeding context birds, Area X was densely labeled and defined by D1-like binding and HVC and RA were also distinguishable from surrounding areas based on the pattern of D1-like receptor binding.
Breeding versus non-breeding context males
T-tests revealed no significant differences between breeding and non-breeding context males in either D1-like or D2-like receptor density in any of the brain regions examined.
DISCUSSION
The data presented here suggest that D1-like receptor binding in brain regions involved in social behavior correlates with singing behavior in multiple contexts. Moreover, our findings suggest that the specific regions involved and the direction of the correlations between D1-like receptor density and song differ between nonsexually, intersexually, and presumed intrasexually motivated contexts. No significant relationships were observed between singing behavior in any context and D2-like receptor binding density in any of the brain areas investigated. This suggests a primary role for DA acting on D1-like receptors in the context-dependent regulation of song.
D1-like receptor density differentially correlates with inter- and presumed intrasexually motivated song
The present findings are the first to demonstrate a linear association between singing behavior and D1-like receptor density in POM, GCt, BSTm and LS within a breeding context. Interestingly, the brain regions in which D1-like receptor binding was associated with intersexually (female present) and intrasexually (female absent) motivated song only partially overlapped. This pattern is consistent with research in mammals showing that neural circuits activated during sexually motivated and agonistically motivated behaviors can be distinct (Kollack-Walker and Newman, 1995).
Correlations were identified between intersexually motivated song and D1-like receptor density in POM and GCt, regions implicated in sexually motivated male behavior in multiple vertebrate species (Hull et al., 1997, Putnam et al., 2001, Schulz et al., 2003, Ball and Balthazart, 2004, Charlier et al., 2005). Recent data from our laboratory also show that TH (the rate-limiting enzyme in DA synthesis) in POM significantly correlates with intersexually motivated singing behavior (Heimovics and Riters, 2008). Given that intersexually motivated song plays a critical role in mate attraction, it is possible that the present data reflect a role for DA in POM and GCt in regulating sexually motivated aspects of breeding context singing behavior.
Correlations were identified between intrasexually motivated song and D1-like receptor density in GCt, BSTm, and LS. In addition to sexual behavior, GCt is also involved in regulating agonistic behavior, including territorial singing behavior (Kollack-Walker and Newman, 1995, Maney and Ball, 2003, Adams, 2006). BST and LS are also involved in the neural regulation of agonistic behavior associated with dominance status in multiple species (Irvin et al., 1990, Jasnow et al., 2004, Maney et al., 2005). To date, few studies have examined the role of DA in GCt, BST and LS in the regulation of communication related to dominance or aggression (but see (Korzan et al., 2002, Wommack and Delville, 2002). Given that intrasexually motivated song plays a critical role in nest box defense, it is likely the present data reflect a role for DA in GCt, BSTm, and LS in regulating agonistically motivated aspects of breeding context singing behavior.
Negative relationships between D1-like receptor density and breeding context song may reflect receptor down-regulation
In all brain regions where D1-like receptor density significantly correlated with breeding context song, the direction of the relationship was negative. This was somewhat surprising given that peripherally administered DA agonists stimulate (and DA antagonists inhibit) male song in response to female conspecifics (starlings: (Schroeder and Riters, 2006), zebra finches: (Rauceo et al., 2007)). Furthermore, across vertebrate taxa, DA activity is generally elevated in mesolimbic and incertohypothalamic nuclei in association with sociosexual behaviors (Miczek and Tornatzky, 1996, Ferrari et al., 2003, Schulz et al., 2003, Korzan et al., 2006). Importantly, studies in mammals show that chronic treatment with a D1 agonist leads to D1 receptor down-regulation (Neisewander et al., 1991). Thus one interpretation of the present results is that DA activity is elevated in POM, GCt, BSTm, and LS in males singing high levels of breeding context song resulting in receptor down-regulation. In contrast, DA activity in POM, GCt, BSTm and LS may be lower in males singing at low rates thus attenuating receptor down-regulation in these individuals.
D1-like receptor density in POM and LS also correlates with nonsexually motivated song
D1-like receptor density in POM and LS also significantly correlated with non-breeding context song. This suggests that D1-like receptor binding in POM and LS is not exclusively associated with sexually motivated vocal communication. In starlings, lesions to POM facilitate (Alger and Riters, 2006) and immunolabeling for the opioid neuropeptide met-enkephalin in POM correlates with (Riters et al., 2005) non-breeding context singing behavior. Also in starlings, IEG immunolabeling in LS positively correlates with non-breeding context song (Heimovics and Riters, 2006, 2007). Thus the present findings are consistent with past work implicating POM and LS in the regulation of nonsexually motivated vocal communication and suggest that DA in POM and LS may regulate song used in the context of flock cohesion.
Inverse relationships observed for breeding and non-breeding context song
Interestingly, D1-like receptor density in POM and LS was positively correlated with non-breeding context song but was negatively correlated with breeding context song. This pattern is identical to past work in starlings demonstrating that the numbers of IEG-labeled cells in LS is positively correlated with non-breeding context song but negatively correlated with breeding context song (Heimovics and Riters, 2007). Not much is known about the dynamics of DA release in POM and LS outside of sexually-relevant contexts. In songbirds DA levels and turnover are significantly lower in control animals as compared to those treated with steroid hormones (Barclay and Harding, 1990). Also, DA release in at least one brain region (Area X) is significantly lower in males singing undirected song as compared to males singing directed courtship song (Sasaki et al., 2006) and DA neurons in VTA appear to be more strongly activated during courtship singing as compared to non-courtship singing (Huang and Hessler, 2008). In the present study, non-breeding context males had basal levels of steroid hormones and were not directing song towards females. Thus, the positive relationship between D1-like receptor binding in POM and LS and non-breeding context song may be due to DA activity in these two areas not being elevated to the point that would lead to receptor down-regulation. In other words, D1-like receptor binding in POM and LS may facilitate non-breeding context singing behavior but, it may be that due to lower dopaminergic tone, D1-like receptor density is highest in the highest singers. Pharmacological manipulations of dopaminergic neurotransmission in non-breeding context males are needed to more fully interpret the significance of these findings.
Zones of LS may be functionally distinct
The present data are consistent with past work showing regional specificity within LS in the regulation of singing behavior (Goodson, et al., 2005, Heimovics and Riters, 2006, Heimovics and Riters, 2007). Here, D1-like receptor density within LSc.v and LSr (but not LSc.vl) significantly correlated with intrasexually motivated, breeding context song. In contrast, D1-like receptor density within LSc.vl and LSc.v (but not LSr) significantly correlated with nonsexually motivated, non-breeding context song. These findings suggest that the zones within LS in which DA regulates song may shift seasonally.
DA receptor density and endocrine state
In songbirds indices of DA synthesis and turnover are modulated by exogenous steroid hormones (Barclay and Harding, 1988, 1990, Appeltants et al., 2003). However, to date, the effect of exogenous steroid hormones on DA receptors in songbirds has not been examined extensively. Here we found no significant difference between breeding (T-implanted) and non-breeding (blank-implanted) context males in either D1-like or D2-like receptor density in any of the regions we examined. These findings are consistent with past work in starlings showing no significant sex difference in absolute D1 receptor density in Area X (Casto and Ball, 1994) and research in hens showing no effect of reproductive cycle on either D1 or D2 mRNA in the hypothalamus (Schnell et al., 1999).
D2-like receptor density did not significantly correlate with song in any context
In the present study, we found no significant relationship between D2-like receptor density in any of the brain regions examined and either breeding or non-breeding context song. D2-like (and D1-like) DA receptors can be post-synaptic and are present in the projection areas of midbrain DA neurons (Cooper et al., 2003). D2-like receptors are also located pre-synaptically and function as a DA autoreceptor, inhibiting DA synthesis and release (Cooper et al., 2003). Given the known functions of D2-like receptors, it is somewhat surprising that they do not appear to correlate with song in either context. However, the autoradiographic procedures used in this study do not provide the anatomical resolution needed to differentiate between pre- and post-synaptic D2-like receptors. Thus, failure to detect relationships between D2-like receptor densities and singing behavior does not preclude a role of D2-like receptors in the regulation of singing behavior. Additional studies are needed to determine the role of D2-like receptors in the context-dependent regulation of song.
Conclusions
Peripheral pharmacological manipulations of male songbirds show that dopaminergic neurotransmission stimulates vocal communication (Schroeder and Riters, 2006, Rauceo et al., 2007). The present data highlight POM, GCt, BSTm and LS as potential sites where DA agonists or antagonists may act (in addition to Area X (Sasaki et al., 2006)) to modulate singing behavior. The present data also demonstrate that individual variation in singing behavior may be explained by individual variation in D1-like receptor density in POM, GCt, BSTm and LS.
DA has a long history of being associated with reward (Wise, 2005) and, more recently, has also been associated with motivated, goal-directed behaviors associated with reward (Pfaus and Phillips, 1991, Blackburn et al., 1992, Hull et al., 1995, Moses et al., 1995, van Furth et al., 1995, Koob, 1996, Berridge and Robinson, 1998, Salamone et al., 2003). Thus the linear relationships between D1-like receptors and song observed in this study may relate to individual differences in hedonic value associated with song or individual differences in the motivation to communicate. Given that breeding context song is often rewarded with copulation and non-breeding context song has no obvious immediate external reward, our data may reflect context-dependent differences in the role of DA in the neural regulation of vocal behavior.
Acknowledgments
The data presented in this paper are based upon work supported by grants from NIMH (R01MH080225) to LVR, from NINDS (R01 NS35467) to GFB and a graduate research fellowship from NSF to SAH. CAC is a F.N.R.S. Postdoctoral Researcher. We thank George and Virginia Emlen (the Emlen Award for research in the behavioral sciences at UW-Madison) for providing funding for this collaboration. We gratefully acknowledge Kate Skogen, Jeff Alexander, Chris Elliot, and John Irwin for help with starling capture and animal care; Ben Pawlish, Sharlene Sue, and Hayley Kleitz for their help with tissue processing; and Bill Feeny for assistance with illustrations.
Comprehensive list of abbreviations
(Listed in the order in which they appear in the manuscript)
- DA
dopamine
- T
testosterone
- HVC
(used as a proper name)
- RA
robust nucleus of the arcopallium
- POM
medial preoptic nucleus
- VTA
ventral tegmental area
- GCt
midbrain central gray
- LS
lateral septum (including subdivisions, caudal part, ventrolateral zone (LSc.vl), caudal part, ventral zone (LSc.v), and rostral part (LSr))
- BSTm
medial bed nucleus of the stria terminalis
- AH
anterior hypothalamus
- VMH
ventromedial nucleus of the hypothalamus
- TH
tyrosine hydroxylase
- lMAN
lateral magnocellular nucleus of the anterior nidopallium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Foidart A, Hemmings HC, Jr, Steinbusch HW, Ball GF, Balthazart J. Distribution of DARPP-32 immunoreactive structures in the quail brain: anatomical relationship with dopamine and aromatase. J Chem Neuroanat. 2001;21:23–39. doi: 10.1016/s0891-0618(00)00094-6. [DOI] [PubMed] [Google Scholar]
- Adams DB. Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev. 2006;30:304–318. doi: 10.1016/j.neubiorev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Bailhache T, Balthazart J. The catecholaminergic system of the quail brain: immunocytochemical studies of dopamine beta-hydroxylase and tyrosine hydroxylase. J Comp Neurol. 1993;329:230–256. doi: 10.1002/cne.903290206. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9:121–133. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Ball GF, Wingfield JC. Changes in Plasma-Levels of Luteinizing-Hormone and Sex Steroid-Hormones in Relation to Multiple-Broodedness and Nest-Site Density in Male Starlings. Physiological Zoology. 1987;60:191–199. [Google Scholar]
- Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Res. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Chen F, Lawrence AJ. The effects of antidepressant treatment on serotonergic and dopaminergic systems in Fawn-Hooded rats: a quantitative autoradiography study. Brain Res. 2003;976:22–29. doi: 10.1016/s0006-8993(03)02598-8. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. New York: Oxford University Press; 2003. [Google Scholar]
- Cuthill I, Hindmarsh AM. Increase in starling song activity with removal of mate. Animal Behaviour. 1985;33:326–328. [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: An integrated approach. Adv Study Beh. 1997;26:355–434. [Google Scholar]
- Eens M, Pinxten R. Extra-Pair Courtship in the Starling Sturnus-Vulgaris. Ibis. 1990;132:618–619. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the song and song repertoire in the European starling (Sturnus vulgaris): An aviary experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Variation in singing activity during the breeding cycle of the European starling Sturnus vulgaris. Belgian Journal of Zoology. 1994;124:167–174. [Google Scholar]
- Feare C. The starling. Oxford [Oxfordshire] ; New York: Oxford University Press; 1984. [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of neuropsychopharmacology. Sunderland, Mass: Sinauer Associates; 1997. [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;101:167–180. [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberger M, Richardyris MA, Henry L, Lepage L, Schmidt I. Song Sharing Reflects the Social-Organization in a Captive Group of European Starlings (Sturnus-Vulgaris) Journal of Comparative Psychology. 1995;109:222–241. [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–735. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. ZENK labeling within social behavior brain regions reveals breeding context-dependent patterns of neural activity associated with song in male European starlings (Sturnus vulgaris) Behav Brain Res. 2007;176:333–343. doi: 10.1016/j.bbr.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Testosterone, preoptic dopamine, and copulation in male rats. Brain Res Bull. 1997;44:327–333. doi: 10.1016/s0361-9230(97)00211-6. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- Korzan WJ, Forster GL, Watt MJ, Summers CH. Dopaminergic activity modulation via aggression, status, and a visual social signal. Behav Neurosci. 2006;120:93–102. doi: 10.1037/0735-7044.120.1.93. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers TR, Summers CH. Manipulation of visual sympathetic sign stimulus modifies social status and plasma catecholamines. Gen Comp Endocrinol. 2002;128:153–161. doi: 10.1016/s0016-6480(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Lehner PN. Handbook of Ethological Methods. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–693. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Tornatzky W. Ethopharmacology of aggression: impact on autonomic and mesocorticolimbic activity. Ann N Y Acad Sci. 1996;794:60–77. doi: 10.1111/j.1749-6632.1996.tb32509.x. [DOI] [PubMed] [Google Scholar]
- Moses J, Loucks JA, Watson HL, Matuszewich L, Hull EM. Dopaminergic drugs in the medial preoptic area and nucleus accumbens: effects on motor activity, sexual motivation, and sexual performance. Pharmacol Biochem Behav. 1995;51:681–686. doi: 10.1016/0091-3057(94)00437-n. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Song as an Attractant for Male and Female European Starlings, and the Influence of Song Complexity on Their Response. Behavioral Ecology and Sociobiology. 1991;28:97–100. [Google Scholar]
- Neisewander JL, Lucki I, McGonigle P. Behavioral and neurochemical effects of chronic administration of reserpine and SKF-38393 in rats. J Pharmacol Exp Ther. 1991;257:850–860. [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pinxten R, Eens M. Polygyny in the European Starling - Effect on Female Reproductive Success. Animal Behaviour. 1990;40:1035–1047. [Google Scholar]
- Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Horm Behav. 2001;39:216–224. doi: 10.1006/hbeh.2001.1648. [DOI] [PubMed] [Google Scholar]
- Rauceo S, Harding CF, Maldonado A, Gaysinkaya L, Tulloch I, Rodriguez E. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2)-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. Journal of Comparative Neurology. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for Opioid Involvement in the Regulation of Song Production in Male European Starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behavioural Brain Research. 2004a;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004b;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Saito N. The acetylcholine and catecholamine contents in song control nuclei of zebra finch during song ontogeny. Brain Res Dev Brain Res. 1989;47:313–317. doi: 10.1016/0165-3806(89)90189-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell SA, You S, El Halawani ME. D1 and D2 dopamine receptor messenger ribonucleic acid in brain and pituitary during the reproductive cycle of the turkey hen. Biol Reprod. 1999;60:1378–1383. doi: 10.1095/biolreprod60.6.1378. [DOI] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol Behav. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Romeo RD, Morris JA, Lookingland KJ, Sisk CL. Medial preoptic area dopaminergic responses to female pheromones develop during puberty in the male Syrian hamster. Brain Res. 2003;988:139–145. doi: 10.1016/s0006-8993(03)03358-4. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Summers RW, Westlake GE, Feare CJ. Differences in the Ages, Sexes and Physical Condition of Starlings Sturnus-Vulgaris at the Center and Periphery of Roosts. Ibis. 1987;129:96–102. [Google Scholar]
- van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res Brain Res Rev. 1995;21:162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ, Liu Y, Insel TR. Species differences in vasopressin receptor binding are evident early in development: comparative anatomic studies in prairie and montane voles. J Comp Neurol. 1997;378:535–546. [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack JC, Delville Y. Chronic social stress during puberty enhances tyrosine hydroxylase immunoreactivity within the limbic system in golden hamsters. Brain Res. 2002;933:139–143. doi: 10.1016/s0006-8993(02)02311-9. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]