Abstract
Purpose
Relapse-free survival (RFS) is a powerful measure of treatment efficacy. We describe the sensitivity of standard surveillance studies for detecting relapse of neuroblastoma (NB).
Patients and Methods
The patients were in complete/very good partial remission of high-risk NB; routine monitoring revealed asymptomatic and, therefore, unsuspected relapses in 113 patients, whereas 41 patients had symptoms prompting urgent evaluations. Assessments every 2 to 4 months included computed tomography, iodine-131–metaiodobenzylguanidine 131I-MIBG; through November 1999) or iodine-123–metaiodobenzylguanidine (123I-MIBG) scan, urine catecholamines, and bone marrow (BM) histology. Bone scan was routine through 2002.
Results
123I-MIBG scan was the most reliable study for revealing unsuspected relapse; it had an 82% detection rate, which was superior to the rates with 131I-MIBG scan (64%; P = .1), bone scan (36%; P < .001), and BM histology (34%; P < .001). Among asymptomatic patients, 123I-MIBG scan was the sole positive study indicating relapse in 25 (27%) of 91 patients compared with one (4.5%) of 22 patients for 131I-MIBG scan (P = .04) and 0% to 6% of patients for each of the other studies (P < .001). Patients whose monitoring included 123I-MIBG scan were significantly less likely than patients monitored by 131I-MIBG scan to have an extensive osteomedullary relapse and had a significantly longer survival from relapse (P < .001) and from diagnosis (P = .002). They also had significantly longer survival than patients with symptomatic relapses (P = .002).
Conclusion
123I-MIBG scan is essential for valid estimation of the duration of RFS of patients with high-risk NB. Without monitoring that includes 123I-MIBG scan, caution should be used when comparing RFS between institutions and protocols.
INTRODUCTION
Neuroblastoma (NB) arises from precursors of the sympathetic nervous system; hence, there are high urinary levels of catecholamines, such as vanillylmandelic acid, homovanillic acid, and dopamine, in more than 90% of patients. The primary sites are in the retroperitoneum (approximately 65%), posterior mediastinum (approximately 20%), pelvis (< 5%), and neck (< 5%); sometimes, no primary site is identified. Metastases in cortical bone, bone marrow (BM), lymph nodes, and/or liver are found in 50% to 60% of patients, but spread to lung parenchyma or the CNS is rare.1 Patients usually present with symptoms and signs from local effects of primary or metastatic tumor, including abdominal distention, back pain, limp, palpable bony masses, lymphadenopathy, paraparesis, irritability, headache, anemia, or thrombocytopenia.
Clearly, assessment of disease status in patients with high-risk NB requires a multitude of studies, including computed tomography (CT), technetium-99m–methylene diphosphonate bone scan, metaiodobenzylguanidine (MIBG) scan, BM examinations, and urine catecholamine levels.2 Carrying out this battery of tests in the infants and toddlers who comprise the majority of NB patients can be a laborious task for medical staff, family, and patient and is financially costly. Nevertheless, reasons for regularly performing these evaluations include gauging the efficacy of treatment, estimating prognosis, discovering relapse before a catastrophic event (eg, paralysis), and revealing relapse with a small tumor burden, which may improve the chance of long-term survival or cure. On the basis of a large experience at Memorial Sloan-Kettering Cancer Center (MSKCC), we now report on the utility of surveillance studies for detecting asymptomatic and, therefore, unsuspected relapse of high-risk NB. Iodine-123 (123I)–MIBG scan emerges as essential for accurately assessing relapse-free survival (RFS).
PATIENTS AND METHODS
The 154 patients in this report had all achieved first or second complete remission (CR)/very good partial remission (VGPR) of high-risk NB (stage 4 diagnosed at > 18 months of age or MYCN-amplified stage 3 or 4 at any age) but experienced relapse. One hundred thirteen patients were asymptomatic and had unremarkable physical examinations and blood tests but, on routine monitoring, were found to be in relapse, whereas 41 patients had new physical findings (eg, lymphadenopathy) or new complaints (eg, limp or headaches) that, by raising a suspicion of relapse, prompted urgent investigations. CR was defined by the International Neuroblastoma Response Criteria as no evidence of NB by CT, scintigraphy, BM histochemical studies, and urine catecholamine levels, whereas VGPR allowed residual abnormalities in bone scan.3 The patients were being observed in accordance with the International Neuroblastoma Response Criteria, which have been the basis for requirements of formal MSKCC protocols from 1990 to the present. Informed written consents for treatments and evaluations were obtained in compliance with institutional review board rules.
For ≥ 3 years from the start of induction or retrieval therapy, disease status was assessed every 2 to 4 months, usually over 2 to 5 days, by physical examination; CT of chest, abdomen, and pelvis; iodine-131 (131I)–MIBG (January 1990 to November 1999) or 123I-MIBG scan; urine catecholamine levels; and BM histochemical examinations (aspirates and biopsies from bilateral posterior iliac crests and aspirates ± biopsies from bilateral anterior iliac crests). CT of head/orbits was initially performed only if patients had prior evidence of NB in those sites but became a part of the standard monitoring program in the mid-1990s. Bone scan was routine through 2002. MIBG scans, bone scans, and CTs were performed as previously described.4 Disease status was unknown to those evaluating BM or radiographic studies.
Statistical comparisons between groups of patients were performed with χ2 tests using Yates' correction for continuity.5 Survival was estimated using the Kaplan-Meier method.6 All patients died of disease except for two patients.
RESULTS
Patient Characteristics and Tests
The 113 patients with asymptomatic/unsuspected relapse included 78 patients in first CR/VGPR and 35 patients in second CR/VGPR (Table 1). Abdominal primary sites predominated, and at the start of induction or retrieval therapy, 26% of the patients had calvarial masses, and 21% had distant nodal disease. Only five patients had no previously documented cortical bone or BM involvement; one patient in first CR/VGPR and three patients in second CR/VGPR had stage 4 NB by virtue of distant lymph node metastases, and one patient in second CR/VGPR had MYCN-amplified stage 3 NB. Four patients never had documented MIBG avidity of disease, including two (9%) of 22 patients studied by 131I-MIBG scan and two (2%) of 91 patients studied by 123I-MIBG scan. Six patients (all with MIBG-avid NB) never had documented elevation of urine catecholamines. Tests at unsuspected relapse included MIBG scan in all patients, BM studies in all but two patients, and CT of chest/abdomen/pelvis in all but one patient (Table 2). Bone scan was performed in all 22 patients studied by 131I-MIBG scan and in 44 of 91 patients studied by 123I-MIBG scan.
Table 1.
Clinical Profile of Patients With Unsuspected Relapse
| Characteristic | Patients in First CR/VGPR (n = 78) |
Patients in Second or Later CR/VGPR (n = 35) |
All Patients (N = 113) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 48 | 22 | 70 | |||
| Female | 30 | 13 | 43 | |||
| Male to female ratio | 1.6 | 1.7 | 1.6 | |||
| Adolescent | 8 | 0 | 8 | |||
| Prior sites of disease | ||||||
| Primary site in abdomen | 67 | 86 | 27 | 77 | 94 | 83 |
| Bone marrow | 76 | 97 | 28 | 80 | 104 | 92 |
| Cortical bone | 71 | 91 | 27 | 77 | 98 | 87 |
| Skull/orbital mass | 25 | 32 | 5 | 14 | 30 | 26 |
| Distant nodes | 16 | 21 | 8 | 23 | 24 | 21 |
| Liver | 7 | 9 | 3 | 9 | 10 | 9 |
| Lungs | 2 | 3 | 0 | 0 | 2 | 2 |
| Prior high urine catecholamines | 75 | 96 | 32 | 91 | 107 | 95 |
| Months to relapse | ||||||
| Median | 19 | 15* | 18 | |||
| Range | 8–53† | 5–45* | 5–53 | |||
Abbreviation: CR/VGPR, complete remission/very good partial remission.
Calculated from start of retrieval therapy.
Calculated from start of induction therapy.
Table 2.
Monitoring Studies: Sensitivities for Detecting Unsuspected Relapse
| Study and Relapse |
131I-MIBG Group (n = 22) |
123I-MIBG Group (n = 91) |
All Patients (N = 113) |
|||
|---|---|---|---|---|---|---|
| No./Total No. | % | No./Total No. | % | No./Total No. | % | |
| 131I-MIBG scan | 14/22 | 64* | NA | NA | ||
| 123I-MIBG scan | NA | 75/91 | 82 | NA | ||
| Bone marrow histology | 13/22 | 59 | 25/89 | 28 | 38/111 | 34† |
| Bone scan | 12/22 | 55 | 12/44 | 27 | 24/66 | 36† |
| CT chest/abdomen/pelvis | 6/22 | 27 | 26/90 | 29 | 31/112 | 28† |
| CT head/orbits | 3/14 | 21 | 16/73 | 22 | 19/87 | 22† |
| Urine catecholamines | 9/21 | 43 | 14/76 | 18 | 23/97 | 24† |
| Months to relapse | ||||||
| First CR/VGPR group | ||||||
| Median | 19 | 19 | 19 | |||
| Range | 10.5–36 | 8–53 | 8–53 | |||
| Second CR/VGPR group | ||||||
| Median | 14 | 15 | 15 | |||
| Range | 13–44‡ | 5–45 | 5–45 | |||
Abbreviations: 131I-MIBG, iodine-131–metaiodobenzylguanidine; 123I-MIBG, iodine-123–metaiodobenzylguanidine; NA, not applicable; CT, computed tomography; CR/VGPR, complete remission/very good partial remission.
P = .1 when compared with 123I-MIBG scan.
P < .001 when compared with 123I-MIBG scan.
Only three patients.
The 41 patients with symptomatic relapse included 31 in first CR/VGPR and 10 in second CR/VGPR; 39 patients had stage 4 disease with bone and/or BM metastases, one patient had stage 4 disease by virtue of distant lymph node metastases, and one patient had MYCN-amplified stage 3 NB. The median time to relapse was 16 months (range, 10 to 86 months) for the 31 patients in first CR/VGPR and 13 months (range, 5 to 51 months), measured from start of retrieval therapy, for the 10 patients in second CR/VGPR.
Sensitivities for Detection of Unsuspected Relapse
123I-MIBG scan was the most reliable study for detecting relapse (Table 2). Thus, 123I-MIBG scan had an 82% detection rate, which was better than 131I-MIBG scan (64%; P = .1) and significantly superior to bone scan (36%; P < .001) and BM histology (34%; P < .001).
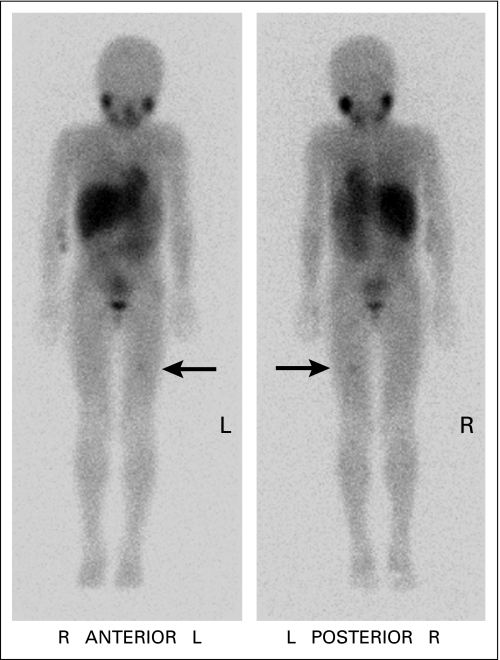
Patients whose monitoring included 123I-MIBG scans were less likely than patients who underwent 131I-MIBG scans to have an extensive osteomedullary relapse, as measured by significantly lower rates of concurrent newly involved BM (28% v 59%, respectively; P = .01), newly abnormal bone scan (27% v 55%, respectively; P = .05), and newly elevated urine catecholamines (18% v 43%, respectively; P = .04; Table 2). Other findings also showed detection of relapse at a less advanced state of disease in patients monitored by 123I-MIBG scan. Thus, 123I-MIBG scan was the only positive study that revealed the unsuspected relapse in 25 (27%) of 91 patients compared with one (4.5%) of 22 patients for 131I-MIBG scan (P = .04), five (4.5%) of 111 patients for BM histology (P < .001), one (1.5%) of 66 patients for bone scan (P < .001), seven (6%) of 112 patients for CT of chest/abdomen/pelvis (P < .001), three (3%) of 87 patients for CT of head/orbits (P < .001), and zero of 97 patients for urine catecholamine levels (P < .001; Table 3). Furthermore, among the 25 patients whose relapse was detected solely by 123I-MIBG scan, 22 patients had only one focus (Fig 1), one patient had two foci, and two patients had three distinct foci of abnormal radiotracer uptake.
Table 3.
Single Study As Sole Indicator of Unsuspected Relapse
| Study |
131I-MIBG Group (n = 22) |
123I-MIBG Group (n = 91) |
All Patients (N = 113) |
|||
|---|---|---|---|---|---|---|
| No./Total No. | % | No./Total No. | % | No./Total No. | % | |
| 131I-MIBG scan | 1/22 | 4.5* | NA | NA | ||
| 123I-MIBG scan | NA | 25/91 | 27 | NA | ||
| Bone marrow histology | 0/22 | 0 | 5/89 | 6 | 5/111 | 4.5† |
| Bone scan | 0/22 | 0 | 1/44 | 2 | 1/66 | 1.5† |
| CT chest/abdomen/pelvis | 1/22 | 4.5 | 6/90 | 7 | 7/112 | 6† |
| CT head/orbits | 1/14 | 7 | 2/73 | 3 | 3/87 | 3† |
| Catecholamines | 0/21 | 0 | 0/76 | 0 | 0/97 | 0† |
Abbreviations: 131I-MIBG, iodine-131–metaiodobenzylguanidine; 123I-MIBG, iodine-123–metaiodobenzylguanidine; NA, not applicable; CT, computed tomography.
P = .04 when compared with 123I-MIBG scan.
P < .001 when compared with 123I-MIBG scan.
Fig 1.
Iodine-123–metaiodobenzylguanidine scan showing focal tracer activity in left distal femur of an asymptomatic 7.2-year-old girl who was 34 months from diagnosis. Concurrent routine surveillance studies, including bone marrow histology and urine catecholamines, showed no evidence of neuroblastoma. Magnetic resonance imaging demonstrated an intramedullary lesion. She is in second complete remission 17+ months later. L, left; R, right.
Among the 16 patients with a normal (ie, falsely negative) 123I-MIBG scan at the time of unsuspected relapse (Table 2), two patients had NB that, based on prior and subsequent 123I-MIBG scans, was not MIBG avid (one focal relapse in dura and one relapse in retroperitoneum); one patient experienced relapse in BM and calvarium; isolated relapses were noted in BM (n = 6), soft tissue (n = 3), liver (n = 2), brain (n = 1), and vertebral body (n = 1); and urine catecholamines were elevated in one of 12 patients tested. Thus, overall, these data, combined with the data in Table 2, show that, in patients with MIBG-avid NB, 123I-MIBG scan failed to detect relapse in BM in seven (25%) of 28 patients, in extracranial soft tissue (including liver) in five (21%) of 24 patients, and in head/orbits in two (13%) of 15 patients.
Survival
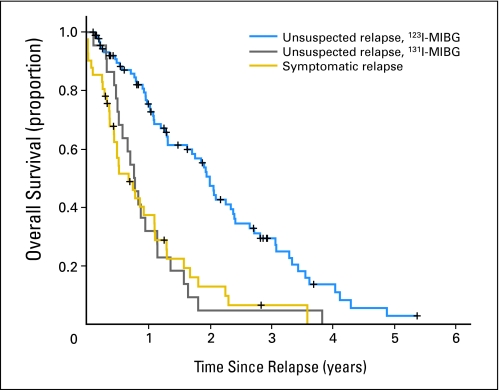
For the patients with an asymptomatic/unsuspected relapse whose surveillance studies included 123I-MIBG scans, survival after relapse was significantly longer than for both the patients with an asymptomatic/unsuspected relapse whose surveillance studies included 131I-MIBG scans and the patients who had a symptomatic relapse (P < .001; Fig 2). In these three groups, the median survival times were 2.0 years (95% CI, 1.8 to 2.2 years), 0.8 years (95% CI, 0.6 to 1.0 year), and 0.7 years (95% CI, 0.3 to 1.0 year), respectively.
Fig 2.
Survival from time of relapse of patients with unsuspected relapse detected by monitoring that included iodine-123–metaiodobenzylguanidine (123I-MIBG) scans (blue line), unsuspected relapse detected by monitoring that included iodine-131–metaiodobenzylguanidine (131I-MIBG) scans (gray line), and symptomatic relapse (gold line; P < .001).
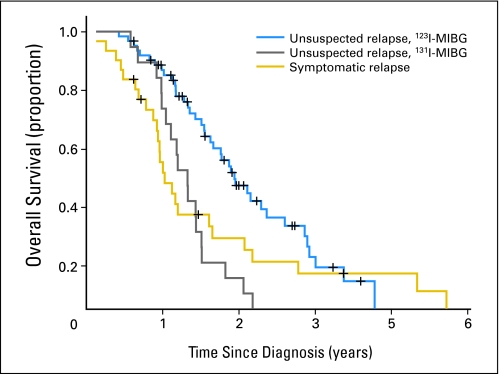
Among all 108 patients in first CR/VGPR, the 59 patients with an asymptomatic/unsuspected relapse whose monitoring included 123I-MIBG scans had significantly longer survival calculated from diagnosis than the 18 patients with an unsuspected relapse whose monitoring included 131I-MIBG scans and the 31 patients who had a symptomatic relapse (P = .002; Fig 3). In these three groups, the median survival times were 3.8 years (95% CI, 3.3 to 4.3 years), 2.4 years (95% CI, 1.8 to 3.0 years), and 2.0 years (95% CI, 1.6 to 2.4 years), respectively.
Fig 3.
Survival from diagnosis of patients in first complete remission/very good partial remission with unsuspected relapse detected by monitoring that included iodine-123–metaiodobenzylguanidine (123I-MIBG) scans (blue line), unsuspected relapse detected by monitoring that included iodine-131–metaiodobenzylguanidine (131I-MIBG) scans (gray line), and symptomatic relapse (gold line; P = .002).
All patients studied with 131I-MIBG have died. To date, 14 of the 51 patients in first CR/VGPR who had unsuspected relapses while being monitored by 123I-MIBG are in second CR/VGPR 5+ to 67+ months (median, 15+ months) after relapse.
DISCUSSION
Many reports describing the sensitivity and specificity of 131I- or 123I-MIBG scans for detecting NB concerned patients treated with moderate-dose chemotherapy; the MIBG findings confirmed the widespread residual NB seen with other staging modalities.2 In contrast, more recently, we4 and others7–9 reported on the utility of comprehensive evaluations for defining response of high-risk NB to contemporary dose-intensive induction chemotherapy; one conclusion was that MIBG scintigraphy and BM testing were prerequisites for accurate determination of disease status,4 although MIBG uptake may also be seen in mature tumor. We now present a complementary report on the sensitivity of standard staging studies for detecting an unsuspected relapse in patients with high-risk NB who had previously achieved CR/VGPR. Other reports have highlighted the utility of MIBG scans in relapse but did not present comparisons with results of comprehensive concurrent staging studies7–13 or covered a heterogeneous patient population (eg, all stages, CR or resistant NB, symptomatic or unsuspected relapse).13,14 Hence, those reports did not identify which test might be best for monitoring.
Through ≥ 3 years from enrollment onto formal treatment protocols, MSKCC patients with high-risk NB undergo a battery of tests to assess disease status every 2 to 4 months. The principal aim is to define the anti-NB activity of the treatment using RFS. That monitoring policy has resulted in the large experience with detecting unsuspected relapse presented in this report. The salient finding is the significantly superior sensitivity of 123I-MIBG scan compared with 131I-MIBG scan, BM histology, bone scan, CT, and urine catecholamine levels.
A vital secondary consideration in the strict monitoring policy is to detect relapse early, when the tumor burden is still small. The hypothesis is that prolonged survival and possibly even cure would be less likely if relapse involves extensive or bulky disease. In fact, the MSKCC experience shows that asymptomatic patients whose monitoring includes 123I-MIBG rather than 131I-MIBG scans have less extensive relapse, which might partly account for their significantly longer survival from relapse (Fig 2) and from diagnosis (Fig 3). The availability of better treatments may also play a role in the longer survival. A localized relapse (Fig 1) can be treated with focal radiotherapy and recently devised chemotherapy regimens (eg, ABT-75115 and irinotecan-temozolomide16) that allow good quality of life; the resulting CR can be consolidated with emerging biologic therapies, including retinoids and immunotherapy. In contrast, achieving CR is far less likely with an extensive relapse, even using aggressive, and perforce toxic, treatments.
State of the art 123I-MIBG scintigraphy, including single-photon emission CT imaging, vastly enhances NB detection, although it is still possible that small lesions can be missed. Thus, among our 91 asymptomatic patients whose monitoring included 123I-MIBG scan, 25 patients (27%) would have been categorized as being in CR/VGPR had they not undergone that scan (Table 3), and 22 of these 25 patients had only a single focus of abnormal uptake (Fig 1). The outstanding advantage of 123I-MIBG scan is in earlier detection of osteomedullary relapse. These results suggest that periodic 123I-MIBG scans may be essential for valid estimation of the duration of RFS. It seems that caution should be used when referring to CR/VGPR rates of, and RFS rates after, treatments in the 1990s, before MIBG scans became part of the standard evaluation of response and when 131I-MIBG (rather than 123I-MIBG) scan was widely used. In one large national study, for example, MIBG scan was included in the monitoring of only 18% of patients.17
Bone scan remains useful at diagnosis for assessing metastatic involvement of cortical bone,18–20 which may have prognostic importance and is, therefore, of interest regarding the efficacy of treatments. For patients who achieve CR, however, bone scan is no longer indicated as part of the routine monitoring work-up because of its significantly lower sensitivity for detecting asymptomatic and unsuspected relapse compared with 123I-MIBG scan (P < .001).
Several drawbacks to 123I-MIBG scans for detecting unsuspected relapse merit attention. First, failure to detect BM relapse in seven (25%) of 28 patients studied by 123I-MIBG scan supports retaining BM studies for confirming remission status. Second, lack of MIBG avidity, which was noted in two (2%) of 91 patients studied by 123I-MIBG scan at MSKCC and has been noted in up to 6% to 10% of NB patients,13,14,21,22 should prompt use of positron emission tomography for monitoring in such patients.23,24 Third, suboptimal visualization by 123I-MIBG scan of small lesions in liver or brain means that special attention should be paid to those sites,25–27 especially in patients who may have risk factors (eg, history of lumbar puncture) for relapse in those organs.
Among the patients with false-negative 123I-MIBG scans at asymptomatic/unsuspected relapse, urine catecholamine elevations were found in only one of 12 patients. Explanations for this unsatisfactory detection rate include small tumor burden at relapse, a correlation between poor MIBG avidity and low catecholamine production, and decreased catecholamine production in some treated NBs.13 High urine catecholamine excretion was not the sole indicator of relapse in any of our patients, but it served as a helpful confirmatory purpose. Others have also found catecholamine levels to be less than optimal for monitoring NB patients,13,14 suggesting that normal catecholamine levels are associated with a limited extent of recurrent disease.
Concerns about radiographic carcinogenicity, especially in children,28 prompt reservations about follow-up imaging of pediatric cancer patients. Magnetic resonance imaging or ultrasonography should replace CT in monitoring patients with low- or intermediate-risk NB treated by surgery ± chemotherapy. In contrast, for patients with high-risk NB, the relatively small amount of radiation from CT, added to that already received with local radiotherapy (routinely used to assure local control), may not justify the financial cost of magnetic resonance imaging. Regarding scintigraphy, bone scans can be dispensed with in routine follow-up, and the radiation absorbed dose with 123I-MIBG (which is one tenth of the dose with 131I-MIBG scan29) is less than the low levels associated with the minor subcellular damage that is readily repaired by the body's physiologic protective mechanisms.30
In conclusion, just as they proved mandatory for accurate classification of response to contemporary dose-intensive induction chemotherapy,4 123I-MIBG scan and BM testing should be performed regularly to assure the most meaningful data on duration of RFS, which constitutes an important end point in comparing the efficacy of different treatment programs. Furthermore, comparisons of RFS results with those of past treatment programs may be of limited value if the latter did not include regular monitoring with 123I-MIBG scans and BM testing. After 3 years from the start of treatment, in the off-therapy setting, a reasonable approach might be to tailor surveillance evaluations to the likelihood of relapse (based on clinical and biologic features) of each individual patient, while taking into account the risk-benefit ratio and financial costs of the tests; for example, more evaluations might be warranted in patients whose NB was refractory to induction therapy.
Prolonged survival despite persistence or relapse of NB is an emerging phenomenon.31–34 Although one reason might be the detection of less extensive relapses through improved surveillance, as with 123I-MIBG rather than 131I-MIBG scan, another reason might be the identification of better treatments in recent years, including novel therapies with modest toxicity. Not only do the latter allow good quality of life, but it is plausible that in some patients, they will help achieve cure.
Acknowledgment
For their services assessing the various studies in this report, we thank the numerous members of the Memorial Sloan-Kettering Cancer Center Departments of Medical Imaging and Pathology.
Footnotes
Supported in part by Grants No. CA61017 and CA72868 from the National Cancer Institute, Bethesda, MD; Hope Street Kids, Alexandria, VA; the Justin Zahn Fund, New York, NY; the Katie's Find a Cure Fund, New York, NY; and the Robert Steel Foundation, New York, NY.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brian H. Kushner, Nai-Kong V. Cheung
Financial support: Nai-Kong V. Cheung
Administrative support: Nai-Kong V. Cheung
Provision of study materials or patients: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Collection and assembly of data: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Data analysis and interpretation: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Manuscript writing: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
Final approval of manuscript: Brian H. Kushner, Kim Kramer, Shakeel Modak, Nai-Kong V. Cheung
REFERENCES
- 1.Dubois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Kushner BH. Neuroblastoma: A disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–1188. [PubMed] [Google Scholar]
- 3.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 4.Kushner BH, Yeh SDJ, Kramer K, et al. Impact of MIBG scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–1086. doi: 10.1200/JCO.2003.07.142. [DOI] [PubMed] [Google Scholar]
- 5.Preacher KJ. Calculation for the chi-square test: An interactive calculation tool for chi-square tests of goodness of fit and independence. http://www.quantpsy.org.
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 8.Katzenstein HM, Cohn SL, Shore RM, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. doi: 10.1200/JCO.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with 123I-MIBG in patients with stage 4 neuroblastoma > 1 year of age on a high-risk treatment protocol: Results of the German Neuroblastoma Trial NB97. Eur J Cancer. 2008;44:1552–1558. doi: 10.1016/j.ejca.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Maurea S, Lastoria S, Caracò C, et al. Iodine-131-MIBG imaging to monitor chemotherapy response in advanced neuroblastoma: Comparison with laboratory analysis. J Nucl Med. 1994;35:1429–1435. [PubMed] [Google Scholar]
- 11.Hadj-Djilani NL, Lebtahi NE, Delaloye AB, et al. Diagnosis and follow-up of neuroblastoma by means of iodine-123 metaiodobenzylguanidine scintigraphy and bone scan, and the influence of histology. Eur J Nucl Med. 1995;22:322–329. doi: 10.1007/BF00941848. [DOI] [PubMed] [Google Scholar]
- 12.Kohnert K, Lerch H, Thelen M, et al. Follow-up of neuroblastoma stage IV by 123-I-mIBG-scintigraphy, bone scintigraphy and catecholamine metabolites. Nuklearmedizin. 1996;35:220–224. [PubMed] [Google Scholar]
- 13.Okuyama C, Ushijima Y, Kubota T, et al. Utility of follow-up studies using meta-[123 I]iodobenzylguanidine scintigraphy for detecting recurrent neuroblastoma. Nucl Med Commun. 2002;23:663–672. doi: 10.1097/00006231-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Simon T, Hero B, Hunneman DH, et al. Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer. 2003;39:1899–1903. doi: 10.1016/s0959-8049(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 15.Fox E, Maris JM, Widemann BC, et al. A phase 1 study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 7 days in pediatric patients with solid tumors. Clin Cancer Res. 2006;12:4882–4887. doi: 10.1158/1078-0432.CCR-06-0534. [DOI] [PubMed] [Google Scholar]
- 16.Kushner BH, Kramer K, Modak S, et al. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24:5271–5276. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 17.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 18.Gordon I, Peters AM, Gutman A, et al. Skeletal assessment in neuroblastoma: The pitfalls of iodine-123-MIBG scans. J Nucl Med. 1990;31:129–134. [PubMed] [Google Scholar]
- 19.Turba E, Fagioli G, Mancini AF, et al. Evaluation of stage 4 neuroblastoma patients by means of MIBG and 99mTc-MDP scintigraphy. J Nucl Biol Med. 1993;37:107–114. [PubMed] [Google Scholar]
- 20.Perel Y, Conway J, Kletzel M, et al. Clinical impact and prognostic value of metaiodobenzylguanidine imaging in children with metastatic neuroblastoma. J Pediatr Hematol Oncol. 1999;21:13–18. doi: 10.1097/00043426-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Biasotti S, Garavanta A, Villavecchia GP, et al. False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med Pediatr Oncol. 2000;35:153–155. doi: 10.1002/1096-911x(200008)35:2<153::aid-mpo18>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Hero B, Hunneman DH, Gahr M, et al. Evaluation of catecholamine metabolites, mIBG scan, and bone marrow cytology as response markers in stage 4 neuroblastoma. Med Pediatr Oncol. 2001;36:220–223. doi: 10.1002/1096-911X(20010101)36:1<220::AID-MPO1053>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Shulkin BL, Hutchingson RJ, Castle VP, et al. Neuroblastoma: Positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology. 1996;199:743–750. doi: 10.1148/radiology.199.3.8637999. [DOI] [PubMed] [Google Scholar]
- 24.Kushner BH, Yeung HWD, Larson SM, et al. Extending PET scan utility to high-risk neuroblastoma: 18F-fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol. 2001;19:3397–3405. doi: 10.1200/JCO.2001.19.14.3397. [DOI] [PubMed] [Google Scholar]
- 25.Bonnin F, Lumbroso J, Tenenbaum F, et al. Refining interpretation of MIBG scans in children. J Nucl Med. 1994;35:803–810. [PubMed] [Google Scholar]
- 26.Kramer K, Kushner BH, Heller G, et al. Neuroblastoma metastatic to the central nervous system: The Memorial Sloan-Kettering Cancer Center experience and a literature review. Cancer. 2001;91:1510–1519. [PubMed] [Google Scholar]
- 27.Matthay KK, Brisse H, Couanet D, et al. Central nervous system metastases in neuroblastoma: Radiologic, clinical, and biologic features in 23 patients. Cancer. 2003;98:155–165. doi: 10.1002/cncr.11448. [DOI] [PubMed] [Google Scholar]
- 28.Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 29.Bombardieri E, Aktolun C, Baum RP, et al. 131I/123I-metaiodobenzylguanidine (MIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30:BP132–BP139. doi: 10.1007/s00259-003-1357-0. [DOI] [PubMed] [Google Scholar]
- 30.Tubiana M, Arengo A, Averbeck D, et al. Low-dose risk assessment: Comments on the summary of the international workshop. Radiat Res. 2007;167:742–744. doi: 10.1667/RR0917.1. [DOI] [PubMed] [Google Scholar]
- 31.Cotterill SJ, Pearson ADJ, Pritchard J, et al. Late relapse and prognosis for neuroblastoma patients surviving 5 years or more: A report from the European Neuroblastoma Study Group “Survey.”. Med Pediatr Oncol. 2001;36:235–238. doi: 10.1002/1096-911X(20010101)36:1<235::AID-MPO1057>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Kushner BH, Kramer K, Cheung N-KV. Chronic neuroblastoma: Indolent stage 4 disease in children. Cancer. 2002;95:1366–1375. doi: 10.1002/cncr.10800. [DOI] [PubMed] [Google Scholar]
- 33.Lau L, Tai D, Weitzman S, et al. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–232. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Santana V, Furman WL, McGregor LM, et al. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. doi: 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]